SUMMARY
To identify potentially important extracellular matrix adhesive molecules in neural crest cell migration, the possible role of vitronectin and its corresponding integrin receptors was examined in the adhesion and migration of avian neural crest cells in vitro. Adhesion and migration on vitronectin were comparable to those found on fibronectin and could be almost entirely abolished by antibodies against vitronectin and by RGD peptides. Immunoprecipitation and immunocytochemistry analyses revealed that neural crest cells expressed primarily the αVβ1, αVβ3 and αVβ5 integrins as possible vitronectin receptors. Inhibition assays of cellular adhesion and migration with functionperturbing antibodies demonstrated that adhesion of neural crest cells to vitronectin was mediated essentially by one or more of the different αV integrins, with a possible preeminence of αVβ1, whereas cell migration involved mostly the αVβ3 and αVβ5 integrins. Immunofluorescence labeling of cultured motile neural crest cells revealed that the αV integrins are differentially distributed on the cell surface. The β1 and αV subunits were both diffuse on the surface of cells and in focal adhesion sites in association with vinculin, talin and α-actinin, whereas the αVβ3 and αVβ5 integrins were essentially diffuse on the cell surface. Finally, vitronectin could be detected by immunoblotting and immunohistochemistry in the early embryo during the ontogeny of the neural crest. It was in particular closely associated with the surface of migrating neural crest cells. In conclusion, our study indicates that neural crest cells can adhere to and migrate on vitronectin in vitro by an RGDdependent mechanism involving at least the αVβ1, αVβ3 and αVβ5 integrins and that these integrins may have specific roles in the control of cell adhesion and migration.
Keywords: neural crest, vitronectin, integrins, quail, cell adhesion, cell migration
INTRODUCTION
During early embryonic development, certain groups of cells, like neural crest cells, can transiently express locomotory properties that allow them to migrate long distances from their sites of origin and populate other areas of the embryo where they undergo differentiation (Le Douarin, 1982; Newgreen and Erickson, 1986; Levi et al., 1990; Erickson and Perris, 1993). During migration to their final destination, neural crest cells penetrate extracellular matrices that are known to contain fibronectin, collagens, laminin, tenascin and a variety of proteoglycans (Thiery et al., 1982; Krotoski et al., 1986; Duband and Thiery, 1987; Tan et al., 1987; Mackie et al., 1988; Perris et al., 1991a,b). The role of these matrix components in migration has been analyzed in detail in the avian embryo essentially in in vitro approaches. Neural crest cells cultured in vitro adhere to and migrate efficiently on fibronectin, laminin, and type I, IV and VI collagens (Newgreen et al., 1982; Rovasio et al., 1983; Tucker and Erickson, 1984; Perris et al., 1989, 1991a, 1993a). In addition, antibodies to fibronectin or to the integrin β1 subunit and RGD peptides can impair neural crest cell migration on fibronectin substrata (Rovasio et al., 1983; Boucaut et al., 1984; Bronner-Fraser, 1985; Duband et al., 1986). Likewise, antibodies to the β1 or to the α1 subunit of integrins can affect neural crest cell adhesion to laminin or collagens (Lallier and Bronner-Fraser, 1992; Perris et al., 1993b). These studies thus provide strong evidence that avian neural crest cells can adhere and migrate in vitro on a variety of extracellular matrix molecules through β1 integrins.
In vivo, injection of RGD-containing peptides or antibodies to fibronectin, to a laminin-proteoglycan complex or to the integrin β1 subunit into the cranial region of avian embryos cause severe deficencies in neural crest cell migration (Boucaut et al., 1984; Bronner-Fraser, 1985; Poole and Thiery, 1986; Bronner-Fraser and Lallier, 1991). However, the same antibodies fail to perturb neural crest cell migration in trunk regions although they are able to inhibit strongly myoblast migration (Jaffredo et al., 1988; Bronner-Fraser, 1993). This indicates that, while cranial neural crest cells are likely to migrate in vivo primarily on fibronectin and laminin using β1 integrins, truncal neural crest cells may be able to interact with additional extracellular matrix molecules for migration using non-β1 integrins, allowing them to overcome the inhibitory effect of the antibodies. Consistent with this, it has been shown recently that cranial and trunk neural crest cells may differ in their mechanisms of adhesion to selected extracellular matrix components in vitro (Lallier et al., 1992).
Therefore, additional extracellular matrix components that promote truncal neural crest cell locomotion have to be determined. A possible candidate is vitronectin, a multifunctional adhesive glycoprotein of Mr of about 70×103 (70K) found in the circulation and in the extracellular matrix of various tissues and which interacts with the surface of cells primarily through the αVβ3 integrin, also called vitronectin receptor (for reviews, see Preissner, 1991; Felding-Habermann and Cheresh, 1993). Owing to its multidomain structure with binding sites for various integrins, heparin, collagen, plasminogen and plasminogen activator inhibitor 1, vitronectin plays a critical role in the substratum adhesion of a large variety of cell types, in hemostasis and in immune defense. Surprisingly, although considerable information has accumulated regarding its structural and adhesive properties, the involvement of vitronectin as a possible regulatory extracellular matrix molecule during development has been poorly investigated. In the present report, we examine in vitro the adhesive and migratory response of avian trunk neural crest cells to vitronectin. We also describe the distribution of vitronectin in neural crest cell migratory pathways and characterize the repertoire and functions of the vitronectin-binding integrins in these cells.
MATERIALS AND METHODS
Adhesive proteins and antibodies
Vitronectin was purified from bovine serum by affinity chromatography on a heparin-Sepharose column as described by Yatohgo et al. (1988). Bovine plasma fibronectin was purified on a gelatin-Sepharose column as described previously (Rovasio et al., 1983). Rabbit polyclonal antibodies to chicken and bovine vitronectin were kindly provided by Dr M. Hayashi (Ochanomizu University, Tokyo, Japan) or have been described elsewhere (Neugebauer et al., 1991). A monoclonal antibody (mAb) to human vitronectin (clone VIT-2), rabbit antibodies to chicken α-actinin and RGDS peptides were purchased from Sigma. GRDGS peptides that are known to lack inhibitory activity on vitronectin or on fibronectin were a kind gift of Dr K. M. Yamada (NIH, Bethesda, USA). Polyclonal antibodies to chicken fibronectin have been described previously (Duband and Thiery, 1982). Polyclonal antibodies (2992) and the rat mAb ES46-8 both directed against the chicken β1-integrin subunit were kindly provided by Dr K. M. Yamada. The CSAT hybridoma (anti-chicken β1 integrin subunit) was kindly donated by Dr C. Buck (The Wistar Institute, Philadelphia, USA). The mAb Chav-1 (anti-chicken αV integrin subunit) has been described previously (Neugebauer et al., 1991). Rabbit polyclonal antibodies (VNR-C3) raised against a peptide corresponding to the cytoplasmic domain of the chick αV subunit were described previously (Bossy and Reichardt, 1990). Rabbit polyclonal antibodies (344 and 4377) raised against peptides corresponding to the cytoplasmic domains of the human β3 and β5 subunits and cross-reacting with the avian β3 and β5 chains were a kind gift of Dr K. Venstrom (University of California, San Francisco, USA). The mAbs LM609 and P3G2 to the human αVβ3 and αVβ5 integrins, respectively, were described elsewhere (Cheresh and Spiro, 1987; Wayner et al., 1991). Polyclonal antibodies to chicken vinculin were a kind gift of Dr B. Geiger (The Weizmann Institute, Rehovot, Israel). Polyclonal antibodies to chicken talin were a gift of Dr S. Saga (Nagoya University School of Medicine, Nagoya, Japan). A mouse monoclonal antibody, called NC-1, identical to HNK-1 and specifically recognizing neural crest cells has been described elsewhere (Tucker et al., 1984).
Embryos and cell cultures
Japanese quail (Coturnix coturnix japonica) embryos were used throughout the study. Eggs were incubated at 38±1°C and staged according to the number of somite pairs and to the duration of incubation. Trunk neural crest cell cultures were generated as described previously (Duband et al., 1988a). Cells were cultured at 37°C in a humidified 5% CO2 atmosphere in DMEM supplemented with 5% calf serum depleted in fibronectin. In a number of experiments though, cells were cultured in DMEM supplemented with 0.1% ovalbumin, transferrin and insulin, each at 10 μg/ml.
Assays for cellular adhesion
Cellular adhesion assays were performed in 10 cm bacteriological Petri dishes. The use of Petri dishes designed for cell culture was avoided because they gave high non-specific cell adhesion. Small areas of the dishes were incubated at 37°C for 1 hour with 50 μl of vitronectin or fibronectin at various concentrations from 0.05 to 100 μg/ml in calcium- and magnesium-free phosphate-buffered saline (PBS), followed by incubation with bovine serum albumin (BSA) in PBS at 10 mg/ml for 30 minutes and extensive washes in PBS. Neural crest cells were obtained from at least 50 neural tube explants cultured on fibronectin or vitronectin for 18 hours. Cells were harvested using treatment for 3 minutes at 37°C with an enzyme-free cell dissociation buffer (Gibco-BRL). After addition of prewarmed DMEM, cells were collected, sedimented at 1,000 revs/minute for 10 minutes and resuspended in serum-free DMEM. Cells were counted and a 50 μl aliquot of cell suspension containing approximately 5×103 cells was deposited on the areas precoated with the proteins to be tested. The dishes were then incubated at 37°C for 1 hour, rinsed in PBS to remove the non-adherent cells and fixed in a 3.7% formaldehyde solution in PBS. Attached and spread cells were counted under a Nikon inverted phase contrast microscope.
Assays for cellular migration
Cellular migration assays were performed either in 10 cm bacteriological Petri dishes or in Terasaki plates previously coated with adhesion-promoting proteins as for assays for cellular adhesion. Cultures were incubated in DMEM supplemented with either 5% calf serum or 0.1% ovalbumin and observed periodically with a Nikon phase contrast inverted microscope. The extent of migration of neural crest cells was estimated by measuring the linear distance between the neural tube explant and the front of migration of neural crest cells. This parameter was found to reflect accurately the degree of motility of individual neural crest cells (Duband et al., 1991). For time-lapse videomicroscopy, cultures were performed in Terasaki plates as described previously (Dufour et al., 1988) and were observed with a Nikon Diaphot inverted microscope in a heated plexiglass chamber equipped with a video camera (Hamamatsu, Japan) connected to a TV monitor (Hitachi, Japan) and a time-lapse recorder (Mitsubishi, Japan). The total distance migrated by the selected cells was measured and the speed of locomotion calculated. The degree of persistence of movement was defined as the ratio between the linear distance and the total distance covered by the cells.
Histological sections and immunofluorescent stainings
Embryos were routinely fixed at 4°C in 3.7% formaldehyde in PBS for at least 12 hours, depending on the size of the embryos. After several extensive washes in PBS, embryos were embedded in a sucrose solution in PBS (15% wt/vol) and subsequently frozen in Cryo-M-Bed compound (Bright Instrument, Huntington, England) in liquid nitrogen. Sections were cut at 10-12 μm on a cryostat (Bright Instrument) and mounted on Superfrost/Plus slides (CML, Nemours, France). For immunofluorescent labeling of cell cultures, neural tubes or embryonic fibroblasts were explanted onto vitronectin-coated glass coverslips in Petri dishes and cultured in DMEM supplemented with 5% fibronectin-depleted serum. After washes in serum-free DMEM, cultures were fixed using different procedures: (i) in cold methanol for 5 minutes, followed by cold acetone for 1 minute, (ii) in 3.7% formaldehyde in PBS for 1 hour at room temperature, (iii) in 3.7% formaldehyde-0.5% Triton X-100-5% sucrose in PBS for 5 minutes followed by a 1 hour incubation in 3.7% formaldehyde in PBS. Cultures were subsequently rinsed in PBS and, in some cases, they were subjected to permeabilization with 0.5% Triton X-100 in PBS for 3 minutes. Sections and cultures were subjected to immunofluorescent staining using biotinylated secondary antibodies and fluorescein-conjugated streptavidin (Amersham). For control experiments, sections were treated with non-immune rabbit antibodies or unrelated mAbs in place of the primary antibody, or with anti-vitronectin antibodies previously incubated with an excess of purified vitronectin. Preparations were observed with a Leica epifluorescence microscope and photographed using TMX-400 Kodak film.
Immunoblottings and immunoprecipitations
For immunoblotting, samples of embryonic tissues or cultured cells were briefly homogenized and extracted at 90°C with SDS-sample buffer under reducing or non-reducing conditions. The extracts were clarified by centrifugation and subjected to SDS-PAGE followed by immunoblotting analysis. PAGE was performed in Laemmli buffer system on 7.5% polyacrylamide slab minigels. The protein bands were electroblotted for 1 hour onto nitrocellulose in 50 mM Trisglycine, 20% methanol buffer. The nitrocellulose membranes were then incubated with a 4% bovine serum albumin solution in PBS for 1 hour at ambient temperature, followed by incubation with antibody solution for 12 hours at 4°C. The sheets were rinsed in PBS supplemented with 1% Tween 20 and incubated first with biotinylated secondary antibodies for 2 hours at room temperature, then with 125I-labelled streptavidin (Amersham) for 1 hour at room temperature. After rinsing, the blot was subjected to autoradiography.
For immunoprecipitation experiments, cells were metabolically labeled with [35S]methionine for at least 8 hours, extracted in 2% Triton X-100 and processed for immunoprecipitation using protein-A-Sepharose as described previously (Duband et al., 1988b). Samples were subjected to SDS-PAGE on 7.5%-acrylamide gels under non-reducing conditions for detection of integrin complexes and on 10%-acrylamide gels under reducing conditions for detection of vitronectin followed by fluorography.
RESULTS
Distribution of vitronectin at the time of neural crest cell migration
We have at first investigated whether vitronectin is expressed in the embryo at the time of neural crest cell migration and, more specifically, if it is associated with migrating neural crest cells, using immunoblotting and immunohistochemistry. For this purpose, we used three different antibodies directed against vitronectin: two polyclonal antibodies against chicken vitronectin and characterized previously (Kitagaki-Ogawa et al., 1990; Neugebauer et al., 1991; Nagano et al., 1992) and the mAb VIT-2 to human vitronectin from Sigma. All three antibodies specifically recognized chicken serum vitronectin in immunoblots (data not shown) and gave identical results in immunohistochemical experiments. Fig. 1 shows that, in immunoblotting experiments, the antibodies recognized primarily a band of about Mr of 68K and, occasionally, a minor band of 78K in extracts of embryos at day 2.5 of development (E2.5) as well as in E3.5 and E4.5 embryos. These bands corresponded to the molecular masses expected for the two well-characterized forms of chicken vitronectin (Kitagaki-Ogawa et al., 1990). Another band of Mr of about 43K could also be detected in all extracts of embryonic tissues and corresponded either to one of the recently characterized forms of yolk vitronectin (Nagano et al., 1992) or to a degradation product of vitronectin, this molecule being very susceptible to proteolytic cleavage.
Fig. 1.

Immunoblotting analysis of vitronectin expression in embryos at the time of neural crest cell development. Extracts of truncal and cranial regions of embryos at E2.5 (lanes 1 and 2, respectively) and of truncal regions of embryos at E3.5 and E4.5 (lanes 3 and 4, respectively) were subjected to immunoblotting using a monoclonal antibody to human vitronectin. Approximately the same amounts of material were loaded in the different lanes. Two major bands of about Mr of 68K and 43K are found at very similar amounts in all embryo extracts. The upper band corresponds to vitronectin and the lower band corresponds either to a degradation product of vitronectin or to one of the forms of yolk vitronectin (Nagano et al., 1992). The band of Mr of 78K that can be seen in lane 4 is likely can be attributed to the high molecular mass form of vitronectin (Kitagaki-Ogawa et al., 1990). Molecular mass standards are indicated on the left.
The distribution of vitronectin was analyzed in detail in the trunk region of embryos at the time of neural crest cell migration and differentiation, using immunofluorescence labeling of frozen sections of formaldehyde-fixed embryos. At the onset of neural crest cell migration in 25-somite embryos, vitronectin immunoreactivity was found in all tissues of the embryo, including the somites, the lateral mesoderm, the neural tube, the notochord, the ectoderm and the endoderm (Fig. 2A). Early migrating neural crest cells showed an intense staining for vitronectin on their surface. Interestingly, vitronectin exhibited a very distinct pattern of distribution from fibronectin (Fig. 2B). In particular, it was intimately associated with the surface of both epithelial and mesenchymal cells and was not abundant in the acellular areas in between tissues and in basement membranes of epithelia.
Fig. 2.

Immunofluorescence analysis of the distribution of vitronectin at the time of neural crest cell migration. (A,B) Consecutive transverse sections through the brachial level of a 25-somite-stage embryo (E2.5) stained for vitronectin and fibronectin, respectively. Vitronectin immunoreactivity is present on the surface of all cells in the embryo. Staining is also prominent on neural crest cells (indicated by arrows). (C,D) Consecutive transverse sections through the rostral part of a sclerotome at the brachial level of a 32-somite stage embryo (E3-3.5) stained for vitronectin and NC-1, respectively. Both neural crest cells accumulating along the neural tube to form the spinal ganglion and those migrating through the sclerotome (indicated by arrows) show a strong reactivity for vitronectin. a, aorta; d, dermamyotome; e, ectoderm; n, notochord; nc, neural crest cells; nt, neural tube; s, somite; sc, sclerotome. (A,B, ×230; C,D, ×220).
At stages of active neural crest cells migration in 30- to 35-somite embryos, all tissues were reactive for anti-vitronectin antibodies. Neural crest cells themselves, either accumulating along the neural tube or invading the rostral sclerotome, showed a strong staining (Fig. 2C,D). It should be noted that we did not detect any difference in staining intensity in the rostral and caudal halves of the sclerotome prior to and during neural crest invasion. Staining for vitronectin increased in intensity on the surface of neural crest cells as they differentiated into the dorsal root and sympathetic ganglia (Fig. 3A). Staining was also particularly prominent along neurites extending from the ganglia and from motoneurons. Vitronectin staining persisted on the surface of cells of the peripheral ganglia and along nerves until E7 after which it declined progressively and disappeared almost entirely after E10 (Fig. 3B).
Fig. 3.

Immunofluorescence analysis of the distribution of vitronectin at the time of formation of peripheral ganglia. (A,B) Transverse sections through the dorsal root ganglion at the brachial level of embryos at E5 and E9 stained for vitronectin. The spinal cord, motor nerves and neurons of the dorsal root ganglion show a strong immunoreactivity for vitronectin at E5, but are faintly labeled at E9, i.e. after neuronal differentiation has occurred. g, dorsal root ganglion; n, motor nerve; s, spinal cord. (A, ×180; B, ×160).
Adhesion and migration of neural crest cells on vitronectin
The results presented above establish that, during migration, neural crest cells are in tight contact with vitronectin. The ability of neural crest cells to adhere to and migrate on vitronectin substrata was then analyzed in in vitro assays and was compared with results obtained on fibronectin. Since vitronectin is present at a high concentration in serum (0.25-0.45 mg/ml), migration assays were performed under serum-free conditions in order to avoid any possible contamination by serum vitronectin, particularly at low coating concentrations. As shown on Fig. 4, the profiles of the dose-response curve of neural crest cell attachment, spreading and migration on vitronectin were very similar to those obtained with fibronectin. Attachment and spreading were dose-dependent at concentrations below 5 μg/ml and were maximal at higher concentrations. Maximal values for attachment and spreading on vitronectin were 85% and 60% of the cells, respectively, and, on fibronectin, values were 100% and 75% of the cells, respectively. Outward migration of neural crest cells on vitronectin was dose-dependent within the range of 0.05-10 μg/ml. Raising the coating concentration of vitronectin above 10 μg/ml did not significantly increase the expansion of the neural crest population. Migration was also evaluated in the presence of 5% serum and values were found to be very similar to those obtained in the absence of serum, except for the very low coating concentrations of the molecule (below 0.1 μg/ml) for which the extent of migration was significantly higher than without serum (data not shown). In further experiments, migration assays were performed on vitronectin at 5 μg/ml in the presence of DMEM supplemented with 5% serum.
Fig. 4.

Comparative attachment (A), spreading (B) and migration (C) of neural crest cells on vitronectin (closed squares) and fibronectin (open circles) adsorbed onto bacteriological Petri dishes at concentrations ranging from 0.05 μg/ml to 100 μg/ml. Migration assays were performed in serum-free medium. In A and B, results are expressed as the percentage of attached and spread cells in relation to the total number of cells deposited on the substratum. In C, results are expressed as the linear distance in μm between the periphery of the neural crest outgrowth and the edge of the neural tube explant after 18 hours of culture. Each value represents, in A and B, the mean ± s.d. of at least 6 different measurements in at least 3 independent experiments and in C the mean ± s.d. of at least 20 different measurements in at least 3 independent experiments.
The number of migrating neural crest cells and their cell shape on vitronectin substrata were also found to be virtually identical to those exhibited by neural crest cells cultured onto fibronectin (Fig. 5; see also Duband et al., 1991). When neural tubes were deposited on substrata coated with vitronectin at high concentrations (≥10 μg/ml), neural crest cells migrated out within 2-4 hours and actively moved about on the substratum. After 15 hours in culture (Fig. 5A,B), the neural crest cell population organized into a large halo around the neural tube and individual cells exhibited a well-spread morphology with several processes per cell. At low coating concentrations of vitronectin (≤1 μg/ml), the cell population was sparse and individual cells were less spread and more elongated in shape (Fig. 5C,D). A significant proportion of cells remained round.
Fig. 5.

Migration of neural crest cells on vitronectin adsorbed to the substratum at the concentrations of 50 μg/ml (A,B) and 0.5 μg/ml (C,D). (B,D) Higher magnifications of A and C, respectively, showing the morphology of cells. Neural tube explants were deposited onto vitronectin-coated bacteriological Petri dishes and neural crest cells were allowed to migrate on the substratum for 15 hours in serum-free medium and then photographed. At high coating concentrations, vitronectin yields a large number of well-spread cells that migrate long distances from the neural tube. At low concentrations, vitronectin also produces significant migration of cells, though most of them are poorly spread. nt, neural tube.
The migratory behavior of neural crest cells cultured on vitronectin was further studied using time-lapse videomicroscopy. As described previously for neural crest cells cultured on fibronectin (Dufour et al., 1988; Duband et al., 1991), neural crest cells on vitronectin exhibited an intense lobopodial activity and changed shape very rapidly from flattened and multipolar to bipolar and elongated. In addition, cells frequently exchanged neighbors and when a cell collided with an adjacent one, it immediately modified its trajectory. Values of speed of locomotion (52±10 μm/hour) and persistence of movement (0.70±0.06) were comparable to those found on fibronectin substrata.
Effect of anti-vitronectin antibodies and RGD peptides on the adhesion and migration of neural crest cells on vitronectin
Adhesion and migration assays on vitronectin were performed in the presence of anti-vitronectin antibodies at varying concentrations. As shown on Fig. 6, polyclonal antibodies to vitronectin affected adhesion and migration of neural crest cells on vitronectin in a dose-dependent manner. At 1 mg/ml, the antibodies totally abolished both attachment and spreading of neural crest cells on vitronectin and severely perturbed outward migration of neural crest cells from neural tube explants. The distance covered by the neural crest outgrowth after 12 hours of culture in the presence of the antibodies was only 18% of the control and corresponded to a cell velocity below 10 μm/hour. In addition, the antibodies produced extensive rounding up of spread neural crest cells which ultimately regrouped into clusters (Fig. 7A). This indicates that adhesion and migration of neural crest cells on vitronectin result from direct binding between cells and vitronectin and is not mediated by other adhesion molecules that might be secreted and deposited by neural crest cells themselves. In contrast, antibodies to fibronectin at the same concentrations did not significantly affect adhesion and migration of neural crest cells and did not cause rounding up of migratory cells (Figs 6, 7B). Conversely, antibodies to vitronectin were ineffective on the adhesion and migration of neural crest cells on fibronectin (data not shown).
Fig. 6.

Neural crest cell attachment (A), spreading (B) and migration (C) on vitronectin at 5-10 μg/ml in the presence of antibodies to vitronectin at the concentrations of 1 mg/ml and 0.1 mg/ml or to fibronectin at 1 mg/ml, and of RGDS peptides at the concentrations of 1 mg/ml and 0.1 mg/ml. In A and B, results are expressed as the percentage of attachment and spreading in control experiments. In control assays, at least 50% of the cells deposited on the substratum were adherent. In C, neural crest cells were allowed to emigrate from the neural tube explants for 5 hours, and antibodies or peptides were then applied to the culture for 15 hours in DMEM supplemented with 5% serum. Results are expressed as the linear distance in μm between the periphery of the neural crest outgrowth and the edge of the neural tube explant after 15 hours of culture in the presence of the antibodies or peptides. Each value represents in A and B the mean ± s.d. of at least six different measurements in at least three independent experiments and in C the mean ± s.d. of at least 20 different measurements in at least 3 independent experiments.
Fig. 7.

Migration of neural crest cells on vitronectin in the presence of antibodies to vitronectin at the concentration of 1 mg/ml (A), of antibodies to fibronectin at 1 mg/ml (B) and of RGDS peptides at 1 mg/ml (C). Neural crest cells were allowed to emigrate from the neural tube explants on vitronectin at 10 μg/ml for 5 hours. Antibodies or peptides were then applied to the culture for 4 hours. Both the antibodies to vitronectin and the RGDS peptides produce extensive rounding up of neural crest cells followed by cell aggregation. nt, neural tube. Bar, 100 μm.
The adhesive properties of vitronectin result primarily from the presence of a cell-binding domain located toward the amino-terminus of the molecule and that contains an RGD sequence (Suzuki et al., 1984). We have therefore tested whether soluble synthetic RGDS peptides are able to block adhesion and migration of neural crest cells on vitronectin substrata. We found that these peptides could affect both adhesion and migration of neural crest cells in a dose-dependent manner (Fig. 6). High doses of peptides (1 mg/ml) totally abolished cell adhesion and reduced migration to about 20% of the control. Lower doses of peptides (0.1 mg/ml) abolished almost entirely cell spreading and reduced cell attachment by 70% and cell migration by at least 60%. Like antibodies to vitronectin, RGDS peptides were able to induce complete and rapid rounding up and aggregation of migrating neural crest cells on vitronectin (Fig. 7C). In contrast, scrambled peptides (GRDGS) did not affect cell adhesion and migration on vitronectin at all concentrations tested (0.1-1 mg/ml; data not shown). In addition to the RGD sequence, vitronectin contains an heparin-binding domain which may also participate in cellular adhesiveness (Barnes et al., 1985). In order to test whether this domain is involved in neural crest cell migration on vitronectin, cells were incubated in the presence of soluble heparin at concentrations ranging from 1 μg/ml to 100 μg/ml. Heparin showed only a weak effect on neural crest cell migration at all concentrations tested (data not shown).
Vitronectin synthesis by neural crest cells
As vitronectin was found closely associated with the surface of neural crest cells in vivo and as it was able to support cell motility in vitro, we analyzed whether neural crest cells produce and deposit their own vitronectin on their surface in vitro. Neural crest cells were metabolically labeled with [35S]methionine and culture supernatant and membrane extracts were subjected to immunoprecipitation with polyclonal antibodies to chicken vitronectin. As shown on Fig. 8A, proteins of Mr of about 78K and 68K, corresponding to the two forms of vitronectin, were immunoprecipitated from membrane extracts but were not found in culture supernatants, indicating that neural crest cells produce vitronectin, but that it is retained on the cell surface and not secreted in the culture medium. Other bands of Mr lower than 68K were also found in cell extracts. These bands corresponded probably either to degradation products of vitronectin or to other proteins associated with vitronectin and coprecipitated by the antibodies.
Fig. 8.

(A) Vitronectin synthesis by neural crest cells. Cell extract (lane 1) and culture supernatant (lane 2) of metabolically labeled neural crest cells were subjected to immunoprecipitation with antibodies to vitronectin. (B) Integrin receptors for vitronectin on neural crest cells. Neural crest cells cultured on vitronectin were metabolically labeled, extracted in detergent and the lysates were subjected to immunoprecipitation using the VNR-C3 polyclonal antibody to the cytoplasmic domain of the chicken αV integrin subunit (lane 1), the 2992 polyclonal antibody to the chicken β1 integrin subunit (lane 2), the LM609 monoclonal antibody to the human αVβ3 integrin complex (lane 3) and the 4377 polyclonal antibody to the cytoplasmic domain of the human β5 integrin subunit (lane 4). Samples were resolved by SDS-PAGE on 10% acrylamide gels under reducing conditions in A and on 7.5% acrylamide gels under nonreducing conditions in B and radiolabeled bands were visualized by fluorography. Molecular mass markers are indicated on the left of panels A and B.
Integrin receptors for vitronectin on neural crest cells
Vitronectin interacts with the cells’ surface primarily through integrins containing the αV subunit (Felding-Habermann and Cheresh, 1993). This subunit has been found to combine to multiple β chains, including β1, β3, β5, β6 and β8. Among these heterodimers, the αVβ1, αVβ3 and αVβ5 integrins are the most abundant and have each been shown to mediate cellular adhesion to vitronectin (Pytela et al., 1985; Suzuki et al., 1986; Cheresh and Spiro, 1987; Freed et al., 1989; Cheresh et al., 1989; Bodary and McLean, 1990; Smith et al., 1990; Krissansen et al., 1990), although they may also bind other ligands. For example, αVβ1 binds fibronectin (Vogel et al., 1990) and αVβ3 is highly promiscuous recognizing the RGD sequence in a wide array of matrix proteins. In contrast, the αVβ6 heterodimer has been found so far to constitute only a fibronectin receptor while the ligand specificity of αVβ8 remains still to be defined (Moyle et al., 1991; Busk et al., 1992). To characterize the vitronectin-binding integrins that are expressed by neural crest cells, cells were grown on vitronectin substrata and metabolically labelled. Membrane extracts were then subjected to immunoprecipitation with antibodies raised against the cytoplasmic domain of the chicken αV subunit (polyclonal antibody VNR-C3), the chicken β1 integrin subunit (polyclonal antibody 2992), the human αVβ3 integrin (mAb LM609) and the cytoplasmic domains of the human β3 and β5 integrin subunits (polyclonal antibodies 344 and 4377).
As shown on Fig. 8B, lane 1, the antibody VNR-C3 to the αV chain immunoprecipitated a set of proteins of Mr of about 150K, 140K, 110K, 95K and 85K in non-reducing conditions. The Mr 150K and 140K bands corresponded to the αV subunit and its biosynthetic precursor, respectively (Cheresh and Spiro, 1987; Bossy and Reichardt, 1990). The Mr 110K band comigrated with precisely the same mobility as the β1 chain immunoprecipitated with the anti-β1 antibody 2992 (Fig. 8B, lane 2), and the Mr 95K and 85K bands comigrated with the β3 and β5 subunits immunoprecipitated with the mAb LM609 (anti-αVβ3) and the antibody 4377 (anti-β5), respectively (Fig. 8B, lanes 3, 4). However, the presence of other αV-associated β chains comigrating with the β3 subunit (i.e. β6 and β8) cannot be excluded (Moyle et al., 1991; Busk et al., 1992). It should be noted that immunoprecipitations of the αV chain from neural crest cell extracts produced bands in apparent amounts significantly higher than any other α subunits that are presumably expressed by neural crest cells (i.e. α1 and α5), suggesting that αV integrins are major integrins on these cells (data not shown). As described previously (Akiyama et al., 1986; Duband et al., 1988b), the antibody 2992 (anti-β1) immunoprecipitated a series of proteins of Mr 160K, 150K, 140K, 110K and 100K corresponding to various α chains, the β1 subunit and its biosynthetic precursor, respectively (Fig. 8B, lane 2). The mAb LM609 (anti-αVβ3) was found to immunoprecipitate two proteins of Mr 140K and 95K corresponding to the αV and β3 subunits (Fig. 8B, lane 3; Cheresh and Spiro, 1987). The antibody 344 to the cytoplasmic domain of the β3 integrin subunit immunoprecipitated the same proteins as the mAb LM609 (not shown). Finally, the antibody 4377 (anti-β5) immunoprecipitated two proteins of Mr 140K and 85K most likely corresponding to the αV and β5 subunits (Fig. 8B, lane 4; Busk et al., 1992).
In order to further characterize the vitronectin-binding integrins expressed by neural crest cells and, more specifically, to detect any β3 and β5 integrins that do not contain the αV chain, extracts of metabolically labelled of neural crest cells were first immunodepleted with the antibody VNR-C3 to remove the αV integrins and were subsequently subjected to immunoprecipitation with the antibody 344 (anti-β3) or the antibody 4377 (anti-β5). No band migrating at Mr 95K and 85K could be detected in these immune precipitates, indicating that virtually all the β3 and β5 chains are associated with the αV subunit (data not shown).
These results then indicate that neural crest cells express multiple αV integrins in high amounts, including αVβ1, αVβ3 and αVβ5 as presumptive vitronectin receptors, as well as a number of β1 integrins.
Role of the vitronectin-binding integrins in neural crest cell adhesion and migration on vitronectin
Since a number of potential vitronectin receptors appeared to be expressed by neural crest cells, we have performed a series of experiments to determine their possible involvement in neural crest cell adhesion to and migration on vitronectin using a variety of function-blocking antibodies to integrins, either separately or in combination. These antibodies were the polyclonal antibody 2992 to the β1 subunit and the mAbs CSAT, Chav-1, LM609 and P3G2 known to inhibit β1 integrins, αV integrins, the αVβ3 integrin and the αVβ5 integrin, respectively (Neff et al., 1982; Chen et al., 1985; Horwitz et al., 1985; Duband et al., 1986; Cheresh and Spiro, 1987; Cheresh, 1987; Neugebauer et al., 1991; Wayner et al., 1991). All mAbs were tested at concentrations ranging from 5 μg/ml to 50 μg/ml and, when tested in combination, they were applied at the lowest concentration that gave the maximal inhibitory activity. The polyclonal antibody 2992 was tested at concentrations from 0.1 mg/ml to 1 mg/ml.
In adhesion assays (Fig. 9A), the greatest effect of any of the antibodies was obtained with the mAb Chav-1 to αV, which reduced neural crest cell spreading to about 40% of the control. The mAb CSAT and polyclonal antibody 2992 to β1 had significant, though much weaker, inhibitory effects on cell spreading (about 80% of the control). In contrast, the mAb P3G2 to αVβ5 did not affect cell spreading while the mAb LM609 to αVβ3 caused a significant enhancement in neural crest cell spreading to vitronectin (120% of the control). When used in combination, these two antibodies failed to perturb cell adhesion. Likewise, they did not potentiate the effect of the mAb CSAT. In contrast, the mAbs CSAT and Chav-1 showed a significant additive effect and considerably perturbed neural crest cell spreading to less than 20% of the control. Finally, combination of the four mAbs also produced a dramatic inhibition of cell spreading, but to values not greater than the mAbs CSAT and Chav-1 used in combination.
Fig. 9.

Neural crest cell spreading (A) and migration (B) on vitronectin adsorbed onto bacteriological Petri dishes at 5 μg/ml in the presence of various function-perturbing antibodies to integrins. The polyclonal antibody 2992 (anti-β1), mAb CSAT (anti-β1), mAb Chav-1 (anti-αV), mAb LM609 (anti-αVβ3) and mAb P3G2 (anti-αVβ5) were used in adhesion assays at 1 mg/ml, 100 μg/ml, 25 μg/ml, 10 μg/ml and 10 μg/ml, respectively. In migration assays, the polyclonal antibody 2992 was at 1 mg/ml and the various mAbs were at 50 μg/ml. Results are expressed as the percentage of spreading and migration in control experiments. In control assays for cellular adhesion, at least 50% of the cells deposited on the substratum were adherent. In assays for cellular migration, neural crest cells were allowed to emigrate from the neural tube explants for 5 hours and antibodies were then added to the culture medium for 15 hours. Each value represents, in A, the mean ± s.d. of at least 6 different measurements in at least 3 independent experiments and, in B, the mean ± s.d. of at least 20 different measurements in at least 3 independent experiments.
In migration assays (Figs 9B, 10), the mAb CSAT and the polyclonal antibody 2992 to the β1 chain had a weak, but significant, effect on the outward migration of neural crest cells at all concentrations tested (about 70-80% of the control). However, the two antibodies displayed different effects on the cellular morphology. In the presence of the mAb CSAT even at high concentrations, cells remained spread onto the substratum, but were mostly bipolar and elongated instead of multipolar as observed in controls (Fig. 10A,B). In contrast, in the presence of the antibody 2992, cells were poorly flattened, scattered and often aggregated into multicellular clusters (Fig. 10C). The weaker effect of the mAb CSAT on the cell morphology as compared to the antibody 2992 is probably due to a lower affinity for integrin receptors. The mAb Chav-1 to αV reduced the outgrowth of the neural crest population to about 70% of the control at concentrations above 25 μg/ml. Cells in the outgrowth were generally much less spread than in the absence of the antibody, and a significant number of round cells regrouped into clusters (Fig. 10D). In contrast to what was observed for cell spreading, the mAbs Chav-1 and CSAT did not show any additive effect on neural crest cell migration. At low concentrations (≤10 μg/ml), the mAb LM609 to αVβ3 caused a reduction in the outward migration of neural crest cells to about 80% of the control, but did not affect the cells’ morphology and the general aspect of the halo. At higher concentrations, however, it exhibited a strong effect on the expansion of the migrating cell population (45% of the control at 25 μg/ml and 33% at 50 μg/ml; Fig. 9B). Such a reduction of the outgrowth of the cell population corresponded to a decrease in the speed of locomotion of cells from more than 50 μm/hour to less than 10 μm/hour. Most cells at the periphery of the outgrowth were round, detached from the substratum and highly compacted, while those situated close to the neural tube explant showed a more flattened aspect (Fig. 10e). The mAb P3G2 to αVβ5 also affected the outward migration of neural crest cells (Fig. 9B). Its effect was weak at concentrations below 25 μg/ml (94% and 78% of the control at 10 μg/ml and 25 μg/ml) to become considerable at high concentrations (55% of the control at 50 μg/ml). However, in contrast to mAb anti-αVβ3, it did not induce considerable rounding up of the cells even at high concentrations (Fig. 10F). Various combinations of the mAbs LM609, P3G2, CSAT and Chav-1 also caused almost complete inhibition of neural crest cell migration (about 20% of the control or less; Fig. 9B). In most cases, cells were round and formed clusters along the neural tube explants (Fig. 10G).
Fig. 10.
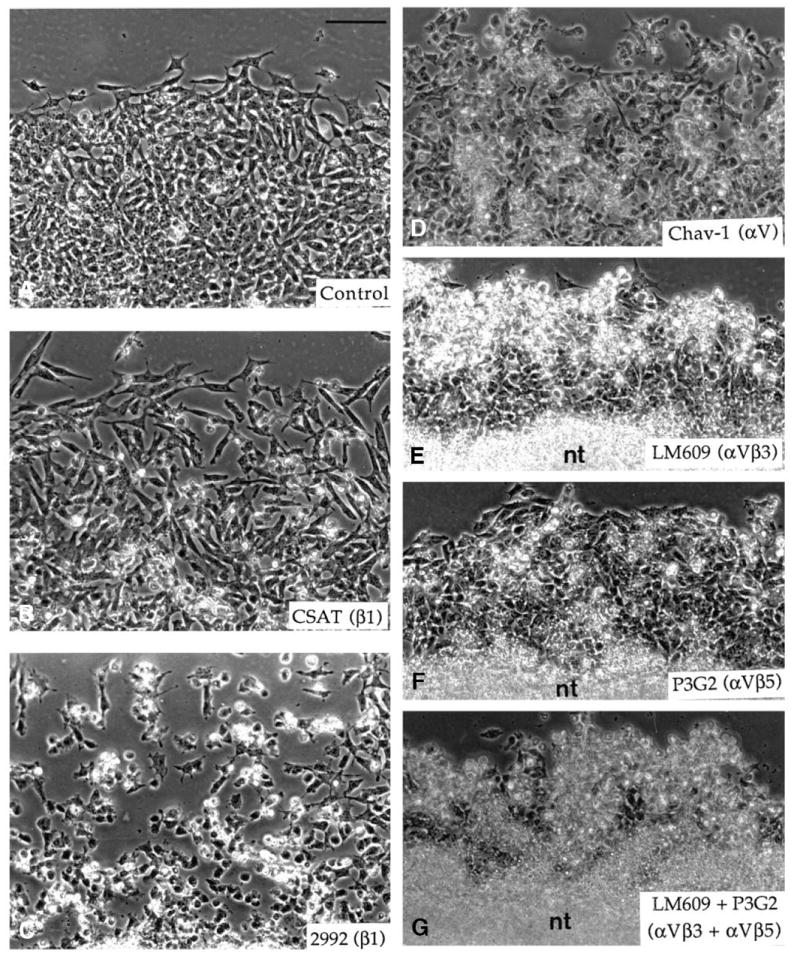
Migration of neural crest cells on vitronectin in the presence of various function-perturbing antibodies to integrins. Neural crest cells were allowed to emigrate from the neural tube explants on vitronectin at the concentration of 10 μg/ml for 5 hours. Antibodies were then applied to the culture for 15 hours. (A) Control without antibody, (B) mAb CSAT (anti-β1) at 250 μg/ml, (C) polyclonal antibody 2992 (anti-β1) at 0.5 mg/ml, (D) mAb Chav-1 (anti-αV) at 50 μg/ml, (E) mAb LM609 (anti-αVβ3) at 50 μg/ml, (F) mAb P3G2 (anti-αVβ5) at 50 μg/ml and (G) mAbs LM609 and P3G2 both at 50 μg/ml. nt, neural tube. Bar, 100 μm.
Surface distribution of integrins on neural crest cells cultured on vitronectin
The surface distribution of integrins on the surface of neural crest cells was examined by immunofluorescence using antibodies to the β1, β3, β5 and αV subunits and to the αVβ3 and αVβ5 integrins. Because integrins bound to their ligand may be inaccessible to antibodies in certain regions of the cell, particularly to those with function-blocking activity, several fixation protocoles were tested for each antibody (see Materials and Methods). These treatments were adapted either for visualization of diffusibly distributed integrins or for detection of integrins that are concentrated in focal adhesion sites. When neural crest cells were fixed with formaldehyde followed or not by permeabilization treatment, staining for the β1 subunit was exclusively uniform and diffuse on the cell surface. No focal distribution of the receptor could be observed (not shown, but see Duband et al., 1986). In contrast, treatment of cells with formaldehyde combined with Triton X-100 allowed visualization of the β1 subunit both as a diffuse staining on the cell surface and in focal contacts (Fig. 11A). This staining in focal contacts corresponded to the pattern obtained with antibodies to vinculin (Fig. 11E), talin (not shown) and α-actinin (Fig. 11F). However, it is noteworthy that focal sites were mostly detectable in cells that exhibited a well-spread morphology and were transiently immobile, but were less numerous in elongated, actively moving cells. Staining for the αV subunit was prominent in focal contacts when cold methanol-acetone fixation was used (Fig. 11B), but was also found out of focal contacts when other fixation procedures were employed. For mAbs LM609 and P3G2 to αVβ3 and αVβ5, all fixation procedures gave essentially a uniform, diffuse staining on the cell surface, which showed little colocalization with focal contacts (Fig. 11C,D). The only difference which could be observed between the various fixation procedures was in the nucleus area for which permeabilization increased staining intensity. The antibodies 344 and 4377 to the β3 and β5 subunits, respectively, gave virtually identical patterns of immunofluorescence staining to the mAbs LM609 and P3G2 (data not shown).
Fig. 11.

Immunofluorescence detection of the β1 (A) and αV (B) integrin subunits, the αVβ3 (C) and αVβ5 (D) integrins, vinculin (E) and α-actinin (F) on neural crest cells cultured on vitronectin. Cells were cultured for 18 hours and fixed using different procedures before immunofluorescence staining. For the β1 subunit, cells were fixed in PBS containing 3.7% formaldehyde, 0.5% Triton X-100 and 5% sucrose for 5 minutes and postfixed in 3.7% formaldehyde for 1 hour. For the αV subunit, cells were fixed in cold methanol for 5 minutes followed by cold acetone for 1 minute. For vinculin and α-actinin, cells were first fixed in 3.7% formaldehyde for 1 hour and permeabilized with 0.5% Triton X-100 during 5 minutes. For the αVβ3 and αVβ5 integrins, cells were first fixed in 3.7% formaldehyde for 1 hour. Neural crest cells exhibit β1 and αV integrin subunits both in focal adhesion sites and as a diffuse pattern on their surface. The αVβ3 and αVβ5 integrin receptors display essentially a uniform, diffuse pattern of staining on the cell surface and are not concentrated in adhesion plaques. Vinculin is concentrated in focal contacts at the tips of cell processes and α-actinin is also in focal contacts and along actin microfilaments. Bar, 25 μm.
DISCUSSION
In the present study, in order to identify additional extracellular matrix molecules that may participate in the control of trunk neural crest cell migration, we have examined the adhesive and locomotory response of avian trunk neural crest cells to vitronectin in vitro. Our major findings are the following: (i) vitronectin is widely expressed in the developing embryo and is found on the surface of migrating neural crest cells; (ii) vitronectin promotes adhesion and migration of neural crest cells through its RGD sequence; (iii) neural crest cells express at least the αVβ1, αVβ3 and αVβ5 integrins as vitronectin receptors; (iv) adhesion of neural crest cells to vitronectin can be affected by antibodies to the αV integrin subunit, whereas migration is almost totally abolished by antibodies to the αVβ3 and αVβ5 integrins; (v) the various αV integrins are differentially distributed on the surface of neural crest cells.
Substratum-bound vitronectin has been shown to promote adhesion of a large variety of cell lines as well as of several primary cultured cells, including smooth muscles, neurons, platelets and endothelial cells (Neugebauer et al., 1991, 1992; Grabham et al., 1992; Clyman et al., 1992; and reviewed in Preissner, 1991). In addition, in in vitro assays, vitronectin was found to stimulate keratinocyte and smooth muscle cell motility (Brown et al., 1991; Clyman et al., 1992), melanoma and glioblastoma cell invasion (Seftor et al., 1991; Gladson and Cheresh, 1991), and retinal neurite outgrowth (Neugebauer et al., 1991). However, apart from a few studies in the adult animal (Hayman et al., 1983; and reviewed in Preissner, 1991), little is known about the tissular distribution of vitronectin and its possible involvement in morphogenetic events during embryonic development. The present demonstration that neural crest cells can adhere to and migrate on vitronectin substrata in vitro suggests that this molecule may play a significant role in the control of cellular adhesiveness and in cell migrations during embryonic development. This possibility is substantiated by the widespread occurence of vitronectin in the avian embryo.
A detailed analysis of the distribution of vitronectin in tissues and in cultured cells reveals that it shares some common features with fibronectin. For example, vitronectin was found in connective tissues and in cultured fibroblasts as a fibrillar pattern that overlaps frequently with fibronectin fibrils (Hayman et al., 1983; Delannet and Duband, unpublished results). These observations then suggest that fibronectin and vitronectin may play similar roles in substratum adhesion of cells, consistent with the fact that their corresponding integrin receptors, namely α5β1 and αVβ3, codistribute in focal contacts of cells cultured on a mixed matrix of vitronectin and fibronectin (Singer et al., 1988; Dejana et al., 1988). However, both molecules were found to exhibit a number of striking specificities in their distribution during early embryonic development. Unlike fibronectin, vitronectin was neither concentrated in the basement membrane of epithelial sheets nor found abundantly in acellular areas adjacent to tissues. Instead, it was closely associated with the surface of cells, even in epithelia where it was present on the lateral surfaces of the cells. These differences in the cellular distributions of fibronectin and vitronectin may reflect significant differences in the mode of action of these two molecules in the control of cellular adhesiveness during embryonic development and, more specifically, during neural crest cell migration. Interestingly, neural crest cells themselves are the source of vitronectin present on their surface, at least in vitro, whereas fibronectin which is not produced by neural crest cells is deposited ahead of them by cells lining the migration pathways (Newgreen and Thiery, 1980; ffrench-Constant and Hynes, 1988). Thus, fibronectin, in association with collagens, would serve as a scaffold onto which neural crest cells migrate. Conversely, vitronectin released by neural crest cells would be immediately trapped to the matrix due to its binding domains to collagens and heparin or would spontaneously form multimers (see for example, Preissner, 1991; Stockmann et al., 1993). Consequently, it would be immobilized in the vicinity of the cell surface, thereby generating a local environment that would facilitate cell locomotion. This particular distribution of vitronectin on the surface of locomoting cells has been described in other systems, supporting the notion that motile cells would provide their own vitronectin substratum for migration. For example, vitronectin is the sole adhesive matrix component found in glioblastoma cells and it is specifically localized to the tumor during invasion of normal brain (Gladstone and Cheresh, 1991). Likewise, it is expressed in the retina adjacent to the optic fiber layer at the time of neurite extension and disappears from this site thereafter (Neugebauer et al., 1991).
Perturbation experiments using RGDS peptides, soluble heparin and antibodies against integrins indicate that neural crest cells interact with vitronectin primarily through integrin receptors in an RGD-dependent manner. Furthermore, immunoprecipitation and immunofluorescence analyses revealed that neural crest cells express, at least in vitro, the αVβ1, αVβ3 and αVβ5 integrins that have been shown previously to constitute major vitronectin-binding receptors for a variety of cell types (Pytela et al., 1985; Suzuki et al., 1986; Cheresh and Spiro, 1987; Freed et al., 1989; Cheresh et al., 1989; Bodary and McLean, 1990; Smith et al., 1990; Krissansen et al., 1990). Consistent with these findings, experiments in progress in our laboratory indicate that, also in vivo, neural crest cells express at least the αV, β1 and β3 integrin subunits. However, as suggested by immunoprecipitation and perturbation experiments, it cannot be excluded that other integrins are expressed by neural crest cells and may participate to some extent in cell adhesion to vitronectin. These include β1 integrins containing an α chain distinct from αV and other αV integrins, e.g. αVβ6 or αVβ8.
The diversity of integrins expressed by neural crest cells raises the problem of determining their exact functional specificities. In other words, are these integrins interchangeable for promoting adhesion and migration of neural crest cells to vitronectin or do they play distinct roles in these processes? In inhibition assays of cellular adhesion using function-perturbing antibodies, we found that neural crest cell adhesion to vitronectin could be strongly impaired only in the presence of the mAb Chav-1 to the αV subunit, suggesting that adhesion is mediated primarily by αV integrins. Moreover, the mAb to the β1 subunit potentiated the mAb to the αV subunit suggesting that, in addition to αV integrins, a β1 integrin containing an α chain distinct from αV may be involved in adhesion of neural crest cells to vitronectin. However, it should be mentioned that, apart from αVβ1, none of the β1 integrins described so far have been found to bind vitronectin with significant efficiency. Therefore, if they exist, such integrins remain to be defined. Alternatively, a possible explanation to the additive effect of the mAbs to αV and β1 is that these antibodies when used separately were not able to inhibit entirely the αVβ1 integrin and that they were totally efficient only in combination. In contrast, adhesion of neural crest cells to vitronectin was not affected by mAbs LM609 and P3G2 to the αVβ3 and αVβ5 integrins and only weakly by antibodies to the β1 integrins. This observation would then suggest that the αVβ1, αVβ3 and αVβ5 integrins might play only subsidiary roles in cell adhesion to vitronectin and that this process is essentially mediated by the two other αV integrins, αVβ6 and αVβ8. However, there is no indication yet that either αVβ6 or αVβ8 can function as vitronectin receptors. The αVβ6 integrin has only been found so far to constitute a fibronectin receptor (Busk et al., 1992) whereas the ligand specificity of αVβ8 remains still to be defined. Moreover, in contrast to the other αV integrins, αVβ8 is expressed in low amounts by a very limited number of cell types, suggesting a specialized function for this integrin (Moyle et al., 1991). Therefore, a more plausible explanation to the limited effect of antibodies to individual integrins is that adhesion of neural crest cells to vitronectin is mediated primarily by the three major αV integrins (αVβ1, αVβ3 and αVβ5) and that these integrins can substitute for promoting cell adhesion.
We have observed, however, that when the antibodies were applied to migrating neural crest cells that are already spread onto vitronectin, only antibodies to the β1 chain and, in a lesser extent, to the αV chain could induce rounding up of the cells. The mAb P3G2 to αVβ5 did not affect much cell spreading and the mAb LM609 to αVβ3 provoked rounding up only after several hours. This indicates that, although these integrins may be interchangeable in adhesion assays, αVβ1 would be preeminent over αVβ3 and αVβ5 for substratum anchorage. Therefore, there might exist a hierachy among αV integrins for mediating cell adhesion, that would be apparent in locomoting cells but not in cells in suspension.
Concerning cell migration, our data demonstrate that, in contrast to β1 integrins, αVβ3 and αVβ5 appear to play a critical role in cell migration as the mAbs LM609 and P3G2 reduced considerably the outward migration of neural crest cells. In addition, the fact that cell migration could be prevented by either the mAb to αVβ3 or the mAb to αVβ5 and that these antibodies showed different effects on the cell morphology indicates that the αVβ3 and αVβ5 integrins cannot substitute for cell migration and that they may participate in different events of the locomotory process. In an apparent contradiction with these data, the mAb Chav-1 to the αV subunit showed only a weak effect on neural crest cell migration whereas it affected cell spreading with high efficiently in adhesion assays. This surprising result can be explained by a higher affinity of the antibody for αVβ1 than for αVβ3 or αVβ5. Alternatively, we have observed that the mAb Chav-1 presents a relatively low affinity for the αV subunit (data not shown). Such a low affinity would make the antibody capable of binding to integrins that are readily accessible in cells in suspension, thereby preventing their further association with vitronectin, but would make it unable to displace the preexisting bounds between integrin and vitronectin molecules in already spread cells.
Thus, it appears from our study that neural crest cells use different integrins to perform the separate functions of adhesion and migration: The αVβ1 integrin would be involved essentially in cellular adhesion while the αVβ3 and αVβ5 integrins would participate primarily in cell migration. Our data are in complete agreement with previous studies showing that different integrins that bind common ligands may differ in their functional specificities. The αVβ1 integrin, for example, has been found to function as a fibronectin receptor, yet, in contrast to α5β1, it is not able to support fibronectin matrix assembly and cell motility (Zhang et al., 1993). Likewise, the αVβ3 and αVβ5 integrins, expressed on the same cell, promote distinct cellular responses to vitronectin substrata (Leavesley et al., 1992). Finally, vascular smooth muscle cells depend exclusively on β1 integrins for adhesion to extracellular matrix components, but depend in a large extent on αVβ3 for motility on the same molecules (Clyman et al., 1992).
The functional specificities of the αV integrins is reflected by their distributions on the surface of neural crest cells and their possible modes of association with cytoskeletal elements. The αVβ3 and αVβ5 integrins were found essentially as a punctate distribution over much of the cell surface and were not particularly concentrated in focal contacts. In contrast, although it was not possible to localize the αVβ1 integrin by itself, staining for the αV and β1 subunits suggests that it is abundant in focal contacts in association with talin, vinculin and α-actinin, in addition to a diffuse distribution over the cell surface. As proposed previously (Duband et al., 1988a; Clyman et al., 1992), the diffuse location of the αVβ3 and αVβ5 integrins could favor transient cell-matrix interactions necessary for cell locomotion whereas concentration of αVβ1 integrins in focal contacts would permit anchoring of the cell to the substratum.
It has been proposed that the β1 class of integrin receptors is the prevailing category of integrins expressed by neural crest cells, based on the strong immunoreactivity of antibodies to β1 integrins on neural crest cells and on their potent effect in inhibiting cell adhesion on fibronectin and laminin (Bronner-Fraser, 1985; Duband et al., 1986; Krotoski et al., 1986). However, except for α1, the identity of the α chains associated with the β1 subunit to compose the fibronectin and laminin receptors on neural crest cells have not been defined yet (Lallier and Bronner-Fraser, 1992; Bronner-Fraser, 1993; Erickson and Perris, 1993). Our study demonstrates for the first time that, in addition to β1 integrins, non-β1 integrins, such as αVβ3 and αVβ5, may play a key role in neural crest cell migration. Moreover, as a number of the vitronectin-binding integrins bind also fibronectin (i.e. αVβ1 and αVβ3) and laminin (i.e. αVβ3), and as αV integrins are expressed in high amounts in neural crest cells as compared to α1β1 and α5β1, it may be proposed that these integrins could be also involved in adhesion and migration of neural crest cells on fibronectin and laminin.
In summary, our study demonstrates that neural crest cells can adhere to and migrate on vitronectin in vitro in a mechanism involving distinct αV integrins that are differentially distributed on the cell surface, possibly resulting from differences in their modes of association with the cytoskeleton and in the types of intracellular signals they transduce. Studying these specificities should help us to understand the process of cell motility in greater detail.
Acknowledgments
We are extremely grateful to Ken Yamada for useful discussions and advice and for providing antibodies to β1 integrins so generously. Many thanks to Masa Hayashi for providing antibodies to vitronectin and for helpful discussions, to Kristine Venstrom for the antibodies to the β5 subunit and to Clayton Buck for the CSAT hybridoma cell line. This work was supported by the Centre National de la Recherche Scientifique (Programme ATIPE), the Ministère de la Recherche et de la Technologie (91.T.0011), the Association pour la Recherche contre le Cancer (ARC-6517), the Institut National de la Santé et de la Recherche Médicale (CRE 910705), the Association Française contre les Myopathies and the Fondation pour la Recherche Médicale. M. D. is a recipient of a predoctoral fellowship of the Ministère de l’Enseignement Supérieur et de la Recherche.
References
- Akiyama SK, Yamada SS, Yamada KM. Characterization of a 140-kD avian cell surface antigen as a fibronectin-binding molecule. J Cell Biol. 1986;102:442–448. doi: 10.1083/jcb.102.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DW, Reing JE, Amos B. Heparin-binding properties of human serum spreading factor. J Biol Chem. 1985;260:9117–9122. [PubMed] [Google Scholar]
- Bodary SC, McLean JW. The integrin β1 subunit associates with the vitronectin receptor αv subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- Bossy B, Reichardt LF. Chick integrin αV subunit molecular analysis reveals high conservation of structural domains and association with multiple β subunits in embryo fibroblasts. Biochemistry. 1990;29:10191–10198. doi: 10.1021/bi00496a006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaut J-C, Darribère T, Poole TJ, Aoyama H, Yamada KM, Thiery JP. Biological active synthetic peptides as probes of embryonic development: A competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984;99:1822–1830. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Alteration in neural crest migration by a monoclonal antibody that affects cell adhesion. J Cell Biol. 1985;101:610–617. doi: 10.1083/jcb.101.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Neural crest migration in the developing embryo. Trends Cell Biol. 1993;3:392–397. doi: 10.1016/0962-8924(93)90089-j. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Lallier T. A monoclonal antibody against a laminin-heparan sulfate proteoglycan complex perturbs cranial neural crest migration in vivo. J Cell Biol. 1991;106:1321–1329. doi: 10.1083/jcb.106.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Stenn KS, Falk RJ, Woodley DT, O’Keefe EJ. Vitronectin: Effects on keratinocyte motility and inhibition of collagen-induced motility. J Invest Dermatol. 1991;96:724–728. doi: 10.1111/1523-1747.ep12470960. [DOI] [PubMed] [Google Scholar]
- Busk M, Pytela R, Sheppard D. Characterization of the integrin α5β6 as a fibronectin-binding protein. J Biol Chem. 1992;267:5790–5796. [PubMed] [Google Scholar]
- Chen W-T, Hasegawa E, Hasegawa T, Weinstock C, Yamada KM. Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol. 1985;100:1103–1114. doi: 10.1083/jcb.100.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melenoma cell attachment to vitronectin, fibrinogen and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- Cheresh DA, Smith JW, Cooper HM, Quaranta V. A novel vitronectin receptor integrin (αvβx) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989;57:59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Clyman RI, Mauray F, Kramer RH. β1 and β3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp Cell Res. 1992;200:272–284. doi: 10.1016/0014-4827(92)90173-6. [DOI] [PubMed] [Google Scholar]
- Dejana E, Colella S, Conforti G, Abbadini M, Gaboli M, Marchisio PC. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp receptors in cultured human endothelial cells. J Cell Biol. 1988;107:1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband J-L, Thiery JP. Distribution of fibronectin in the early phase of avian cephalic neural crest cell migration. Develop Biol. 1982;93:308–323. doi: 10.1016/0012-1606(82)90120-8. [DOI] [PubMed] [Google Scholar]
- Duband J-L, Thiery JP. Distribution of laminin and collagens during avian neural crest development. Development. 1987;101:461–478. doi: 10.1242/dev.101.3.461. [DOI] [PubMed] [Google Scholar]
- Duband J-L, Rocher S, Chen W-T, Yamada KM, Thiery JP. Cell adhesion and migration in the early vertebrate embryo: Location and possible role of the putative fibronectin-receptor complex. J Cell Biol. 1986;102:160–178. doi: 10.1083/jcb.102.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband J-L, Nuckolls GH, Ishihara A, Hasegawa T, Yamada KM, Thiery JP, Jacobson K. The fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol. 1988a;107:1385–1396. doi: 10.1083/jcb.107.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband J-L, Dufour S, Yamada KM, Thiery JP. The migratory behavior of avian embryonic cells does not require phosphorylation of the fibronectin-receptor complex. FEBS Lett. 1988b;230:181–185. doi: 10.1016/0014-5793(88)80667-7. [DOI] [PubMed] [Google Scholar]
- Duband J-L, Dufour S, Yamada SS, Yamada KM, Thiery JP. Neural crest cell locomotion induced by antibodies to β1 integrins: A tool for studying the roles of substratum molecular avidity and density in migration. J Cell Sci. 1991;98:517–532. doi: 10.1242/jcs.98.4.517. [DOI] [PubMed] [Google Scholar]
- Dufour S, Duband J-L, Humphries MJ, Obara M, Yamada KM, Thiery JP. Attachement, spreading, and locomotion of avian neural crest cells are mediated by multiple adhesion sites on fibronectin molecules. EMBO J. 1988;7:2661–2671. doi: 10.1002/j.1460-2075.1988.tb03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA, Perris R. The role of cell-cell and cell-matrix interactions in the morphogenesis of the neural crest. Develop Biol. 1993;159:60–74. doi: 10.1006/dbio.1993.1221. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Hynes RO. Pattern of fibronectin gene expression and splicing during cell migration in chicken embryo. Development. 1988;104:369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- Freed E, Gailit J, Van der Geer P, Ruoslahti E, Hunter T. A novel integrin β subunit is associated with the vitronectin receptor α subunit (αV) in a human osteosarcoma cell line and is a substrate for protein kinase C. EMBO J. 1989;8:2955–2965. doi: 10.1002/j.1460-2075.1989.tb08445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the αVβ3 integrin: Adhesion mechanism of transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabham PW, GaJlimore PH, Grand RJA. Vitronectin is the major serum protein essential for NGF-mediated neurite outgrowth from PC12 cells. Exp Cell Res. 1992;202:337–344. doi: 10.1016/0014-4827(92)90083-k. [DOI] [PubMed] [Google Scholar]
- Hayman EG, Pierschbacher MD, Ohgren Y, Ruoslahti E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc Natl Acad Sci USA. 1983;80:4003–4007. doi: 10.1073/pnas.80.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Greggs R, Decker C, Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985;101:2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffredo T, Horwitz AF, Buck CA, Rong P-M, Dieterlen-Lièvre F. Myoblast migration specifically inhibited in the chick embryo by grafted CSAT hybridoma cells secreting an anti-integrin antibody. Development. 1988;103:431–446. doi: 10.1242/dev.103.3.431. [DOI] [PubMed] [Google Scholar]
- Kitagaki-Ogawa H, Yatohgo T, Izumi M, Hayashi M, Kashiwagi H, Matsumoto I, Seno N. Diversities in animal vitronectins. Differences in molecular weight, immunoreactivity and carbohydrate chains. Biochim Biophys Acta. 1990;1033:49–56. doi: 10.1016/0304-4165(90)90193-z. [DOI] [PubMed] [Google Scholar]
- Krissansen GW, Elliott MJ, Lucas CM, Stomski FC, Berndt MC, Cheresh DA, Lopez AF, Burns GF. Identification of a novel integrin β subunit expressed on cultured monocytes (macrophages). Evidence that one α subunit can associate with multiple β subunit. J Biol Chem. 1990;265:823–830. [PubMed] [Google Scholar]
- Krotoski DM, Domingo C, Bronner-Fraser M. Distribution of a putative cell surface receptor for fibronectin and laminin in the avian embryo. J Cell Biol. 1986;103:1061–1071. doi: 10.1083/jcb.103.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallier T, Bronner-Fraser M. α1β1 integrin on neural crest cells recognizes some laminin substrata in a Ca2+-independent manner. J Cell Biol. 1992;119:1335–1345. doi: 10.1083/jcb.119.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallier T, Leblanc G, Artinger KB, Bronner-Fraser M. Cranial and trunk neural crest cells use different mechanisms for attachment to extracellular matrices. Development. 1992;116:531–541. doi: 10.1242/dev.116.3.531. [DOI] [PubMed] [Google Scholar]
- Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin β3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM. The Neural Crest. Cambridge University Press; Cambridge: 1982. [Google Scholar]
- Levi G, Duband J-L, Thiery JP. Modes of cell migration in the vertebrate embryo. Int Rev Cytol. 1990;123:201–252. doi: 10.1016/s0074-7696(08)60675-0. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Tucker RP, Halfter W, Chiquet-Ehrismann R, Epperlein HH. The distribution of tenascin coincides with pathways of neural crest cell migration. Development. 1988;102:237–250. doi: 10.1242/dev.102.1.237. [DOI] [PubMed] [Google Scholar]
- Moyle M, Napier MA, McLean JW. Cloning and expression of a divergent integrin subunit β8. J Biol Chem. 1991;266:19650–19658. [PubMed] [Google Scholar]
- Nagano Y, Hamano T, Nakashima N, Ishikawa M, Miyazaki K, Hayashi M. Yolk vitronectin: Purification and differences from its blood homologue in molecular size, heparin binding, collagen binding, and bound carbohydrate. J Biol Chem. 1992;267:24863–24870. [PubMed] [Google Scholar]
- Neff NT, Lowrey C, Decker C, Tovar A, Damsky C, Buck C, Horwitz AF. A monoclonal antibody detaches embryonic skeletal muscle from extracellular matrices. J Cell Biol. 1982;95:654–666. doi: 10.1083/jcb.95.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Emmet CJ, Venstrom KA, Reichardt LF. Vitronectin and thrombospondin promote retinal neurite outgrowth: Developmental regulation and role of integrins. Neuron. 1991;6:345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- Neugebauer KM, Venstrom KA, Reichardt LF. Adhesion of a chick myeloblast cell line to fibrinogen and vitronectin through a β1-class integrin. J Cell Biol. 1992;116:809–815. doi: 10.1083/jcb.116.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen DF, Thiery JP. Fibronectin in early avian embryos: Synthesis and distribution along the migration pathways of neural crest cells. Cell Tiss Res. 1980;211:269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- Newgreen DF, Erickson CA. The migration of neural crest cells. Int Rev Cytol. 1986;103:89–145. doi: 10.1016/s0074-7696(08)60834-7. [DOI] [PubMed] [Google Scholar]
- Newgreen DF, Gibbins IL, Sauter J, Wallenfels B, Wütz R. Ultrastructural and tissue-culture studies on the role of fibronectin, collagen and glycosaminoglycans in the migration of neural crest cells in the fowl embryo. Cell Tiss Res. 1982;221:521–549. doi: 10.1007/BF00215700. [DOI] [PubMed] [Google Scholar]
- Perris R, Paulsson M, Bronner-Fraser M. Molecular mechanisms of avian neural crest cell migration on fibronectin and laminin. Develop Biol. 1989;136:222–239. doi: 10.1016/0012-1606(89)90144-9. [DOI] [PubMed] [Google Scholar]
- Perris R, Krotoski D, Bronner-Fraser M. Collagens in avian neural crest development: Distribution in vivo and migration-promoting ability in vitro. Development. 1991a;113:969–984. doi: 10.1242/dev.113.3.969. [DOI] [PubMed] [Google Scholar]
- Perris R, Krotoski D, Lallier T, Domingo C, Sorrell JM, Bronner-Fraser M. Spatial and temporal changes in the distribution of proteoglycans during avian neural crest development. Development. 1991b;111:583–599. doi: 10.1242/dev.111.2.583. [DOI] [PubMed] [Google Scholar]
- Perris R, Kuo H-J, Glanville RW, Leibold S, Bronner-Fraser M. Neural crest cell interaction with type VI collagen is mediated by multiple cooperative binding sites within triple-helix and globular domains. Exp Cell Res. 1993a;209:103–117. doi: 10.1006/excr.1993.1290. [DOI] [PubMed] [Google Scholar]
- Perris R, Syfrig J, Paulsson M, Bronner-Fraser M. Molecular mechanisms of neural crest cell attachment and migration on types I and IV collagens. J Cell Sci. 1993b;106:1357–1368. doi: 10.1242/jcs.106.4.1357. [DOI] [PubMed] [Google Scholar]
- Poole TJ, Thiery JP. Antibodies and a synthetic peptide that block cell-fibronectin adhesion arrest neural crest cell migration in vivo. Prog Clin Biol Res. 1986;217B:235–238. [PubMed] [Google Scholar]
- Preissner KT. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci USA. 1985;82:5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovasio RA, Delouvée A, Yamada KM, Timpl R, Thiery JP. Neural crest cell migration: Requirement for exogenous fibronectin and high cell density. J Cell Biol. 1983;96:462–473. doi: 10.1083/jcb.96.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor REB, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJC. Role of the αVβ3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA. 1991;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II, Scott S, Kawka DW, Kazazis DM, Gailit J, Ruoslahti E. Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol. 1988;106:2171–2182. doi: 10.1083/jcb.106.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Vestal DJ, Irwin SV, Burke TA, Cheresh DA. Purification and functional characterization of integrin αVβ5: An adhesion receptor for vitronectin. J Biol Chem. 1990;265:11008–11013. [PubMed] [Google Scholar]
- Stockmann A, Hess S, Declerck P, Timpl R, Preissner KT. Multimeric vitronectin: Identification and characterization of conformation-dependent self-association of the adhesive protein. J Biol Chem. 1993;268:22874–22882. [PubMed] [Google Scholar]
- Suzuki S, Pierschbacher MD, Hayman EG, Nguyen K, Öhgren Y, Ruoslahti E. Domain structure of vitronectin: Alignment of active sites. J Biol Chem. 1984;259:15307–15314. [PubMed] [Google Scholar]
- Suzuki S, Argraves WS, Pytela R, Arai H, Krusius T, Pierschbacher MD, Ruoslahti E. cDNA and amino-acid sequences of the cell adhesion protein receptor recognizing vitronectin reveals a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci USA. 1986;83:8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SS, Crossin KL, Hoffman S, Edelman GM. Asymmetric expression in somites of cytotactin and its proteoglycan ligand is correlated with neural crest cell distribution. Proc Natl Acad Sci USA. 1987;84:7977–7981. doi: 10.1073/pnas.84.22.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Duband J-L, Delouvée A. Pathways and mechanism of avian trunk neural crest cell migration and localization. Develop Biol. 1982;93:324–343. doi: 10.1016/0012-1606(82)90121-x. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Aoyama H, Lipinski M, Tursz T, Thiery JP. Identical reactivity of monoclonal antibodies HNK-1 and NC-1: Conservation in vertebrates on cells derived from the neural primordium and on some leukocytes. Cell Diff. 1984;14:223–230. doi: 10.1016/0045-6039(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Erickson CA. Morphology and behavior of quail neural crest cells in artificial three-dimensional extracellular matrices. Develop Biol. 1984;104:390–405. doi: 10.1016/0012-1606(84)90094-0. [DOI] [PubMed] [Google Scholar]
- Vogel BE, Tarone G, Giancotti FG, Gailit J, Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (αvβ1) J Biol Chem. 1990;265:5934–5937. [PubMed] [Google Scholar]
- Wayner EA, Orlando RA, Cheresh DA. Integrins αVβ3 and αVβ5 contribute to cell attachement to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E. The αVβ1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol. 1993;122:235–242. doi: 10.1083/jcb.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]


