SUMMARY
Mice lacking the POU domain-containing transcription factor Brn-3a have several neuronal deficits. In the present paper, we show that Brn-3a plays two distinct roles during development of the trigeminal ganglion. In this ganglion, neurons expressing the neurotrophin receptors, TrkB and TrkC, are born between E9.5 and E11.5. In the absence of Brn-3a, very few neurons ever express TrkC, but TrkB-expressing neurons are present at E12.5 in elevated numbers, suggesting that Brn-3a may be a constituent of a regulatory circuit determining which Trk receptor is expressed by these early-born neurons. Most neurons expressing the neurotrophin receptor TrkA are generated between E11.5 and E13.5 in this ganglion and their initial generation is not prevented by absence of Brn-3a. However, after E12.5, absence of Brn-3a results in a progressive loss in neuronal TrkA and TrkB expression, which leads to a massive wave of apoptosis that peaks at E15.5. Despite complete absence of the Trk receptors at E17.5 and P0, approximately 30% of the normal complement of neurons survive to birth in Brn-3a mutants. Approximately 70% of these express the GDNF receptor subunit, c-ret; many can be sustained by GDNF, but not by NGF in culture. Thus, the vast majority of surviving neurons are probably sustained in vivo by trophic factor(s) whose receptors are not regulated by Brn-3a. In conclusion, our data indicate the specific functions of Brn-3a in controlling the survival and differentiation of trigeminal neurons by regulating expression of each of the three Trk receptors.
Keywords: Brn-3a, POU domain factor, Knockout mice, Trk receptor, Trigeminal ganglion
INTRODUCTION
Vertebrate sensory neurons form a distinct functional and structural unit in charge of processing external stimuli. Given their roles as conveyors for different modalities of sensory information, it is crucial to understand the molecular mechanisms that regulate the development and maintenance of sensory neurons. The development of murine sensory neurons is initiated by neural crest cells which migrate out from the neural tube between E8 and E9. Between E9.5 and E10.5, a discrete group of cells form the coalesced sensory ganglia primordia (Serbedzija et al., 1992, 1994; reviewed in Le Douarin et al., 1992). At the early stages of sensory ganglia formation, precursors within the ganglia have the ability to proliferate and the potential to differentiate into neurons. The process generating sensory neurons occurs rapidly between E10.5 and E13.5, after which the number of neurons within the ganglia remains rather constant. According to anatomical location, murine sensory ganglia can be further divided into cranial ganglia and dorsal root ganglia. While the cells of origin for dorsal root ganglia are entirely derived from the neural crest, different cranial ganglia receive contributions from the neural crest, neural placodes, or both (Serbedzija et al., 1992, 1994; Le Douarin et al., 1992).
Critical for development of sensory neurons is access to neurotrophic factors. The essential roles of neurotrophins and their receptors for sensory neurons are well-established by data from in vitro cultures and by analyses of mice with null mutations in these genes (reviewed in Fariñas and Reichardt, 1997). In the trigeminal ganglion, for example, absence of NGF or its receptor TrkA results in loss of approximately 75% of the normal complement of neurons, while absence of BDNF or its receptor TrkB results in loss of approximately 30% of the neurons (Crowley et al., 1994; Jones et al., 1994; Klein et al., 1993; Smeyne et al., 1994; Fariñas and Reichardt, 1997). Interestingly, absence of NT-3 results in loss of 60% of the trigeminal neurons, whereas mutation in its receptor TrkC only results in loss of 20% of these neurons (Tessarollo et al., 1997; Wilkinson et al., 1996). The stronger phenotype of the NT-3 mutant is due to the fact that NT-3 is required in vivo for the survival of some TrkA and TrkB neurons, in addition to all TrkC neurons (Huang et al., 1999). Similar to the phenotypes of mice with null mutations in neurotrophins, mutations of Trk receptors also lead to losses of neurons in the trigeminal ganglia due to apoptotic cell death which occurs at different stages during embryogenesis (Piñon et al., 1996). Specific subpopulations of neurons are lost in different mutants. For instance, virtually all sensory neurons modulating nociception are lost in NGF or TrkA mutants, whereas proprioceptive neurons are selectively eliminated in NT-3 or TrkC mutants (Klein et al., 1993; Crowley et al., 1994; Smeyne et al., 1994; Fariñas et al., 1994). Together, these data underscore the importance of neurotrophins and Trk receptors in regulating survival and differentiation of sensory neurons.
Despite the advance in understanding the functions of neurotrophins and Trk receptors in the development of sensory neurons, much less is known about how transcription factors can regulate this process. Proteins in the basic helix-loop-helix (bHLH) family have been shown to play important roles in cell fate determination in various organisms (reviewed in Jan and Jan, 1994). Two novel members of the basic helix-loop-helix family transcription factors, neurogenin 1 and neurogenin 2, distantly related to the Drosophila atonal gene, have been shown to be required for development of proximal and distal cranial ganglia, respectively (Fode et al., 1998; Gradwohl et al., 1996; Ma et al., 1996, 1998). Neurogenin 1 is present in the trigeminal placode as early as E8.5 and, by E9, neurogenin 1 is abundant in the primordia of the trigeminal and the vestibulococchlear ganglia (Ma et al., 1998). Consistent with the roles of basic helix-loop-helix genes in cell fate determination (Jan and Jan, 1994), mice with a targeted deletion in neurogenin 1 show severe defects in trigeminal and vestibulococchlear ganglia (Ma et al., 1998). This deficit presumably is due to disruption of a cascade of genes activated by neurogenin 1. Interestingly, one of the notable findings in mice with a null mutation in neurogenin 1 is the presence of extensive apoptotic cell death in the trigeminal ganglia around E11.5–12.5, suggesting that neurogenin 1 may regulate cell survival via unknown mechanisms (Ma et al., 1998).
A second class of transcription factors that has been implicated in regulating sensory neuron development is the POU domain factor family. Historically, members in the POU domain factor family have been shown to be important for cell type specification (Treacy and Rosenfeld, 1992). The Brn-3 genes, including Brn-3a, Brn-3b and Brn-3c, belong to the POU IV subfamily and have been shown to be required for proper development of various sensory nervous systems (Xiang et al., 1997a). Among these factors, Brn-3a shows an intense expression in the developing sensory ganglia preceding that of Brn-3b and Brn-3c (Xiang et al., 1995, 1996). Furthermore, in early sensory ganglia, Brn-3a expression has been seen in both proliferating precursors and differentiating neurons (Fedtsova and Turner, 1995; E. J. H., unpublished observations). After E12.5–E13.5, expression of Brn-3a is only present in sensory neurons, not precursors. The importance of Brn-3a has been demonstrated by generation of mice lacking this factor. These mice die perinatally due to defects in sensory ganglia, the inferior olivary nucleus, the habenula nucleus and the red nucleus (McEvilly et al., 1996; Xiang et al., 1996). Although it has been shown that the expression of neurotrophin receptors may be reduced in the sensory ganglia of Brn-3a mutant mice (McEvilly et al., 1996), it is unclear as to how and when these deficits occur. Since Brn-3a is expressed in both the proliferating precursors and the differentiated neurons, the observed deficits may reflect essential roles for Brn-3a on either cell type.
In the current study, we investigated the deficits in trigeminal ganglia resulting from absence of Brn-3a, focusing on regulation of neurotrophin receptors. Using antibodies specific for various neurotrophin receptors and neuronal markers, we compared the generation and the maintenance of trigeminal neurons in wild-type and Brn-3a mutant ganglia. We show that, despite its expression in proliferating precursors, absence of Brn-3a does not appear to reduce the number of these precursors in early trigeminal ganglia. Instead, Brn-3a plays two essential roles in trigeminal ganglion development, which together can account for the final neuronal deficit. In its absence, TrkC-expressing neurons never appear in the ganglion. Without Brn-3a, expression of TrkA and TrkB receptors begins normally, but is not maintained. Consequently, neurons dependent on signals conveyed through these receptors die after E13.5. Interestingly, mutation of Brn-3a does not inhibit the expression of another neurotrophin receptor p75NTR or prevent development of responsiveness to GDNF, which requires c-ret. A vast majority of trigeminal sensory neurons that survive in absence of Brn-3a express c-ret and thus are likely to be sustained in vivo by GDNF or related ligands.
MATERIALS AND METHODS
Animals husbandry and histological preparations
Mice with a targeted deletion in Brn-3a were maintained in C57/BL6 background as described previously (Xiang et al., 1996). Timed pregnant female were killed at various stages and embryos were fixed in Carnoy’s fixative (60% ethanol, 30% chloroform and 10% acetic acid), embedded in paraffin and sectioned at 7 μm. For c-ret immunohistochemistry, embryos or trigeminal ganglia were fixed in 3% paraformaldehyde/15% picric acid, cryoprotected in 40% sucrose and sectioned at 7 μm in a cryostat. For whole-mount immunostaining, embryos were fixed in 80% methanol and 20% dimethyl sulfoxide (DMSO) at 4°C for 4 hours or overnight, bleached in 10% H2O2 for 5 hours at room temperature and washed in Tris-buffered saline (TBS; 50 mM Tris, pH 7.5, 0.85% NaCl). Embryos were then incubated with primary antibody at room temperature overnight in blocking solution (80% calf serum, 20% DMSO, 0.1% thimerosol), washed five times with TBS, 1 hour each, incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase Fab fragment (Sigma, St. Louis, MO), 1:300 dilution, washed with TBS and developed with DAB/0.02% H2O2.
Immunohistochemistry
Primary antibodies were used at the following concentrations in blocking solution (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.4% Triton X-100, 3% bovine serum albumin, and 10% normal serum from the host species of the secondary antibody to be used): rabbit anti-TrkA IgG (RTA), 1 μg/ml (Clary et al., 1994), affinity-purified rabbit anti-TrkB (RTB), 5 μg/ml (Fariñas et al., 1998; Huang et al., 1999), affinity-purified anti-TrkC (RTC), 5 μg/ml (Fariñas et al., 1998; Huang et al., 1999), rabbit anti-p75NTR IgG (Rex), 5 μg/ml (Weskamp and Reichardt, 1991), rabbit anti-Brn-3a, 1 μg/ml (E. J. H., unpublished data), rabbit anti-neurofilament 150 kDa, 1:1000 (Chemicon, Temecula, CA), anti-parvalbumin antibody (Swant, Switzerland) and rabbit anti-c-ret, 2 μg/ml (Immuno-Biological Laboratories, Tokyo, Japan). For detection of these antibodies, biotinylated goat anti-rabbit IgG or rabbit anti-goat IgG, and the ABC complex from the Vectastain kit (Vector Laboratories, Burlingame, CA) were used following the manufacturer’s instructions. The DAB solution for c-ret antibody was used at 5 ng/ml. In experiments comparing Trk receptors, expression in wild-type and Brn-3a mutant mice, tissue sections of both genotypes were always processed, incubated with primary or secondary antibodies and developed simultaneously. Methods for cell counting and for BrdU injection and labeling have been described previously (Wilkinson et al., 1996; Huang et al., 1999). Briefly, the numbers of immunoreactive cells were determined in every ten sections for most developmental stages, except for E10.5 and E11.5 where cells in every five sections were counted. For double-labeling experiments (Fig. 1A,B), Brn-3a immunoreactivity was first developed using DAB/nickel ammonium sulfate (black reaction) and the p75NTR immunoreactivity was then detected using alkaline phosphatase reaction (red reaction).
Fig. 1.
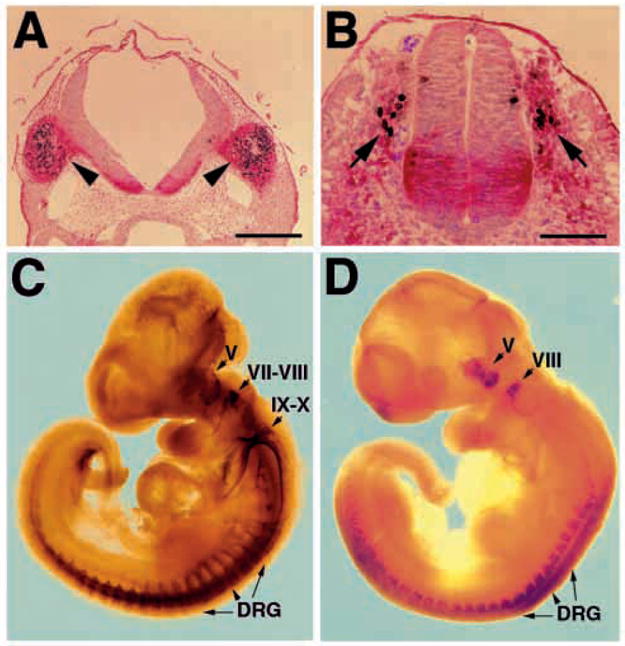
Brn-3a is expressed in migrating neural crest and in sensory ganglia at early embryonic stages. (A,B) Detection of Brn-3a in a subset of migrating trigeminal (A) and trunk (B) neural crest cells in E9-9.5 embryos. Brn-3a immunoreactivity is in black and localizes in the nucleus, and p75NTR is in red and localizes in the cytoplasm. (C,D) Comparison of neurofilament (C) and Brn-3a (D) expression in sensory ganglia of E10.5 embryos. While neurofilament is present in all cranial ganglia and trunk sensory ganglia, Brn-3a is only detected in trigeminal (V), vestibulococchlear (VIII) and dorsal root ganglia (DRG). Scale bars, 100 μm (A) and 50 μm (B).
In vitro culture of trigeminal neurons
Dissociated trigeminal neurons were cultured using a published protocol (Buchman and Davies, 1993). Briefly, trigeminal ganglia were dissected from E12.5 embryos or P0 newborn mice, trypsinized and triturated. Dissociated neurons were then plated on 16-well chamber slides coated with poly(DL)ornithine (0.5 mg/ml) and laminin (10 μg/ml) in triplicate, at a density of 2000 cells/well in defined F-14 medium, containing thyroxine (400 ng/ml), triiodothyronine (340 ng/ml), progesterone (60 ng/ml), sodium selenite (38 ng/ml), 0.35% bovine serum albumin and N2 supplement (Life Technologies, Gaithesburg, MD). Various concentrations of NGF were used in E12.5 neuron cultures to obtain a dose-response relationship, whereas for P0 cultures, NGF and GDNF were used at 10 and 50 ng/ml, respectively, since these concentrations have been shown to be optimal for neurons at this stage. Surviving neurons with distinct refractile cell bodies and elaborated neurites were scored at 24 or 48 hours after culture (Buchman and Davies, 1993).
RESULTS
Loss of Brn-3a has no effects on precursors, but results in abnormal elimination of neurons via apoptotic cell death
Several previous reports have documented the expression of Brn-3a in the developing sensory ganglia (Gerrero et al., 1993; Fedtsova and Turner, 1995; Xiang et al., 1995, 1996; McEvilly et al., 1996). We extended these observations to the time intervals during embryogenesis when Brn-3a expression is first detected. Comparing expression of Brn-3a to that of the neurotrophin receptor p75NTR (Weskamp and Reichardt, 1991), a neural crest marker, we found that Brn-3a protein is first detected in a subset of migrating cranial and trunk neural crest cells as early as E9-E9.5 (Fig. 1A,B; red, p75NTR; black, Brn-3a). As the development of sensory ganglia progresses, the number of Brn-3a-immunoreactive cells increases and Brn-3a is observed in both proliferating cells and differentiating neurons (Fedtsova and Turner, 1995) (data not shown). By stages E10.5 and E11.5, the trigeminal (V), vestibulococchlear (VIII) and dorsal root ganglia (DRG) show intense Brn-3a expression, whereas the geniculate (VII) and nodose-petrosal ganglia of cranial ganglia IX–X complex show no evidence of Brn-3a expression (Fig. 1D). The superior-jugular ganglia of the IX–X complex, however, do contain a small number of Brn-3a-immunoreactive cells (not shown). The expression pattern of Brn-3a and the phenotype of mice lacking Brn-3a indicate an important role for Brn-3a in sensory ganglia development. In this paper, we focus on the roles of Brn-3a in development of the trigeminal ganglion.
Because Brn-3a is present in proliferating precursors, it seemed possible that absence of Brn-3a may affect the precursors and consequently ganglion development prior to neurogenesis. To test this hypothesis, we assessed the number of BrdU-incorporating precursors in trigeminal ganglia at E10.5 and E11.5. Results in Fig. 2A show no differences between wild-type and mutant ganglia in the number of proliferating precursors that have incorporated BrdU during a 2 hour pulse. To assess the effect of the Brn-3a mutation on the neuronal population, we compared the morphologies of wild-type and mutant ganglia at various stages using a Cresyl violet (Nissl) stain (Fig. 3). Interestingly, although the morphologies of cells in the mutant ganglia did not appear to differ from those in wild-type ganglia at E11.5 and E12.5, the E11.5 mutant ganglia showed fewer apoptotic profiles (Figs 2B, 3A,B). Unlike the wild-type ganglia, the mutant ganglia showed a sharp increase in apoptotic cell death at E15.5 (Figs 2B, 3C,D), which continued through E17.5 to P0. By P0, the mutant ganglia appeared to be smaller and contained many fewer neurons than were present in wild-type ganglia (Figs 2C, 3E,F; Table 1).
Fig. 2.
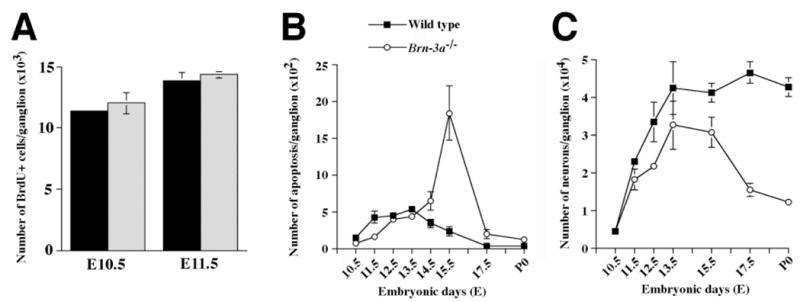
Loss of Brn-3a does not affect the number of proliferating cells in trigeminal ganglia, but results in two phases of neuronal deficit. (A) The numbers of BrdU-incorporating cells in trigeminal ganglia at E10.5 and E11.5 do not differ between the wild-type (solid bars) and Brn-3a mutant (hatched bars) mice. (B) In wild-type trigeminal ganglia, there is a continuous presence of apoptotic cell death from E11.5 to E13.5. In contrast, the mutant ganglia show much reduced apoptotic profiles at E11.5 and sharply elevated cell death at E15.5. (C) The number of neurons, determined by neurofilament-immunoreactive cells from E10.5 to E15.5 and cells with Nissl substance and prominent nucleoli from E17.5 to P0, shows two phases of deficit. From E11.5 to E15.5, there is a loss of a fraction of neurons in mutant ganglia. Interestingly, starting from E15.5, the mutant ganglia show a dramatic decrease in neuron number such that by P0, only about 30% of the normal complement of wild-type neurons remain in the ganglia. Data are shown as mean ± s.e.m. and are analyzed using two-tailed Student’s t-test.
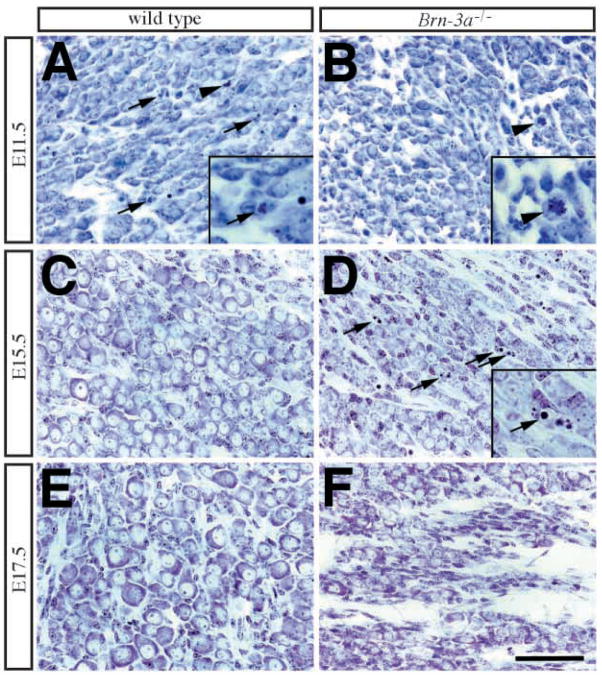
Fig. 3.
Morphological abnormalities in Brn-3a mutant ganglia at different embryonic stages. Cresyl violet (Nissl) stain of trigeminal ganglia from wild-type (WT) and Brn-3a mutant (Brn-3a−/−) mice at E11.5, E15.5, and E17.5. (A,B) At E11.5, cells in wildtype ganglia undergo apoptotic cell death (arrows) and mitosis (arrowhead), whereas in mutant ganglia, apoptotic profiles are fewer but mitosis is still present (arrowhead). (C,D) In contrast to the presence of well-developed sensory neurons with Nissl substance in wild-type ganglia (C), there are numerous apoptotic profiles in the mutant ganglia (D, arrows). The insets in A, B and D represent two-fold magnification of pyknotic nuclei (arrows in A,D) and mitosis (arrowhead in B). (E,F) By E17.5 and P0, the mutant ganglia contains much less neurons compared with that in the wild type. Scale bar, 50 μm.
Table 1.
Number of neurons and neurons expressing different Trk receptor in developing trigeminal ganglia of wild-type and Brn-3a mutant mice
| Embryonic stage | Wild type | Brn-3a−/− | % of wild type |
|---|---|---|---|
| E10.5 - Neurofilament | 4284±897 (3) | 4439±1087 (3)* | n.s. |
| TrkB | 538±18 (2) | 508±168 (2)* | n.s. |
| TrkC | 2697±672 (3) | 224±103 (3)§ | 8 |
| E11.5 - Neurofilament | 22977±744 (3) | 18032±2755 (3)‡ | 78 |
| TrkA | 9530±1420 (3) | 9130±1807 (3)* | n.s. |
| TrkB | 7075±155 (3) | 6723±583 (3)* | n.s. |
| TrkC | 10097±654 (3) | 912±335 (4)§ | 9 |
| E12.5 - Neurofilament | 33303±5240 (3) | 21555±783 (3)§ | 65 |
| TrkA | 25178±2996 (4) | 16890±4870 (4)‡ | 67 |
| TrkB | 6210±127 (3) | 9073±940 (3)§ | 146 |
| TrkC | 6477±474 (3) | 109±48 (3)§ | 2 |
| E13.5 - Neurofilament | 42263±6060 (3) | 32510±5369 (3)¶ | 77 |
| TrkA | 36180±3433 (3) | 26707±3280 (3)§ | 74 |
| TrkB | 4915±244 (4) | 6855±3035 (4)* | 139 |
| TrkC | 6605±55 (3) | 630±20 (3)§ | 9 |
| E15.5 - Neurofilament | 41129±2461 (2) | 30650±4086 (2)‡ | 74 |
| TrkA | 26768±2843 (3) | 11819±3852 (3)§ | 44 |
| TrkB | 5207±1007 (3) | 2151±791 (3)§ | 41 |
| TrkC | 7277±883 (2) | 375±26 (2)§ | 5 |
| E17.5 - Neuron | 46468±2976 (3) | 15452±1766 (3)§ | 33 |
| TrkA | 26080 (1) | 320 (1) | 1 |
| TrkB | 4320 (1) | 200 (1) | 5 |
| TrkC | n.d. | n.d. | n.d. |
| P0 - Neuron | 42592±2427 (3) | 12224±685 (3)§ | 29 |
| TrkA | 26360 (1) | 220 (1) | 1 |
| TrkB | 4195±45 (2) | 220±30 (2)§ | 5 |
| TrkC | n.d. | n.d. | n.d. |
Note: For the analyses at E17.5 and P0, cells with distinct features, such as Nissl’s substance, clear nucleus and prominent nucleolus, were considered as neurons.
Two-tailed Student’s t test was used for statistical analyses.
Not significant;
0.01<P<0.05;
P<0.01;
0.05<P<0.1.
Parentheses indicate the number of animals examined for each stage.
Data were presented as mean±s.e.m.
Note that the staining for TrkA receptor was in general weak and diffuse at E10.5, therefore the number for TrkA-immunoreactive neurons at this stage is hard to determine.
n.d., not done; n.s., not significant.
Although the morphologies of sensory neurons can be easily distinguished from those of other cellular populations at E17.5 and P0, it is more difficult to identify neurons at earlier stages. In order to determine if the abnormal loss of cells is due to elimination of the neurons and, if so, to identify the stages at which this occurs, we used the neurofilament 150 kDa subunit as a neuronal marker (Wilkinson et al., 1996) and determined the total number of neurons from E10.5 to E15.5 (Table 1; Fig. 2C). Similar to neurons in the wild-type ganglia, neurons in the mutant ganglia show intense neurofilament staining at all embryonic stages, indicating that the absence of Brn-3a does not affect the expression of neurofilament in trigeminal neurons (Xiang et al., 1996 and data not shown). This enabled us to determine neuronal numbers in both wild-type and mutant ganglia (Fig. 2C). Although there was no difference in neuronal numbers between wild-type and mutant ganglia at E10.5, between E11.5 and E15.5, Brn-3a mutants appeared to have 20–35% fewer neurons (Table 1; Fig. 2C). Following a peak of apoptosis at E15.5, though, the numbers of neurons in mutant ganglia precipitously and continuously decrease. By P0, only 30% of the normal complement of neurons survive in the mutant trigeminal ganglia (Table 1; Fig. 2C).
Failure to express TrkC in Brn-3a mutant ganglia
Quantitation of neuronal number in wild-type ganglia suggests that the vast bulk of neurogenesis occurs between E10.5 and E13.5 (Table 1; Fig. 2C). After E13.5, neuronal numbers remain essentially constant. Sensory neurons in developing ganglia require neurotrophins, and null mutations in neurotrophins or neurotrophin receptors lead to loss of certain neuronal populations (Fariñas and Reichardt, 1997). Based on the expression of different Trk receptors, sensory neurons can be further subdivided into distinct groups, which have been shown in adults to convey different modalities of sensory information. Using antibodies specific for each receptor, results in Fig. 4A show the time courses of generation of neurons expressing each Trk receptor (see also Table 1). For example, the numbers of neurons expressing TrkB or TrkC reach maxima at E11.5, after which numbers of each decline. In contrast, the vast majority of TrkA neurons are generated between E11.5 and E13.5. After reaching a maximum at E13.5, numbers of TrkA-expressing neurons also decline.
Fig. 4.
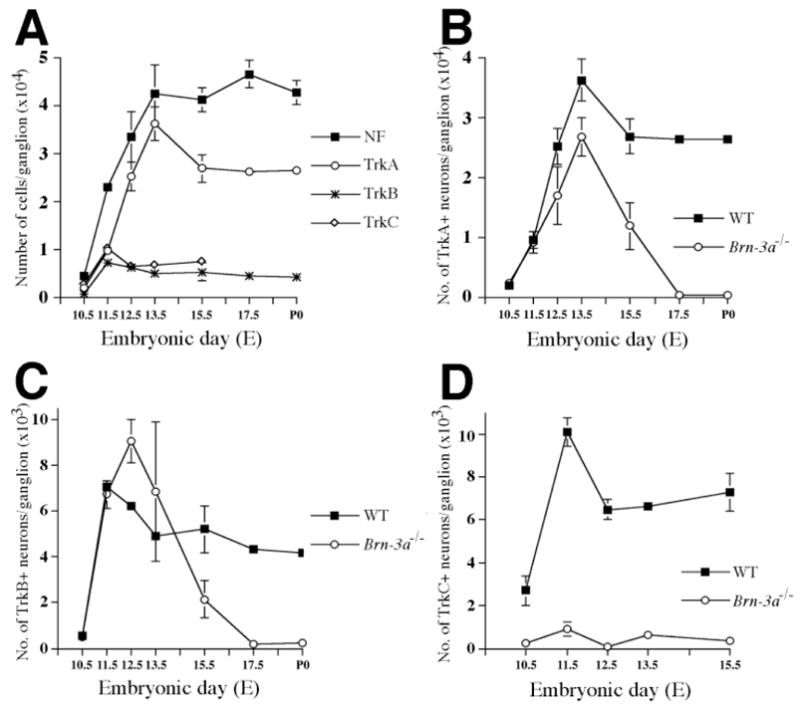
Dynamic expression of Trk receptors in wild-type ganglia and abnormal loss of Trk receptor expression in mutant ganglia. (A) Neurons expressing different Trk receptors are generated at different stages during the formation of trigeminal ganglia. The numbers for TrkB and TrkC neurons reach maxima at E11.5 and that for TrkA neurons at E13.5, each follows by a slight decrease in 1–2 days. Because most neurons express only one Trk receptor, it is most likely that neurons are born expressing one Trk receptor. (B–D) In Brn-3a mutant ganglia, the number of TrkC-immunoreactive neurons is dramatically reduced to less than 10% of that in the wild type at all stages (D). Interestingly, the number of TrkB-expressing neurons increases at E12.5 in the mutant. Although the initial expression of TrkA and TrkB receptor is not affected by loss of Brn-3a, maintenance of TrkA and TrkB expression appears to be impaired in the mutant ganglia (B,C). The progressive decrease of the expression of TrkA and TrkB occurs between E13.5 and E15.5, such that by E17.5, none of the remaining neurons express either receptor.
Given the roles of Trk receptors in supporting the survival of sensory neurons (Piñon et al., 1996), we examined the effect of Brn-3a absence on these different sensory neuron populations. Since the expression of TrkC is most intense at E10.5–11.5 and TrkC-expressing neurons represent approximately 40–50% of total neurons at these stages (Table 1; Fig. 4A), we first examined TrkC expression in the mutant ganglia. To collect the data in a convincing way, immunohistochemical staining for Trk receptors in wild-type and mutant animals was performed in parallel and reactions were carried out to completion. To our surprise, unlike the intense TrkC expression in numerous neurons in wild-type ganglia, neurons within Brn-3a mutant ganglia expressed little or no TrkC receptor from E10.5 to E15.5 (Figs 4D, 5). In contrast to the almost complete absence of TrkC in trigeminal ganglia, the expression of TrkC receptor in neurons of dorsal root ganglia is similar to that of wild-type animals (data not shown), indicating that the striking effect on TrkC expression in the trigeminal ganglia is specific. At stages between E10.5 and E15.5, few or no neurons express TrkC (Table 1; Fig. 4D), though TrkC is expressed at normal levels in the vasculature (Fig. 5F, arrowhead).
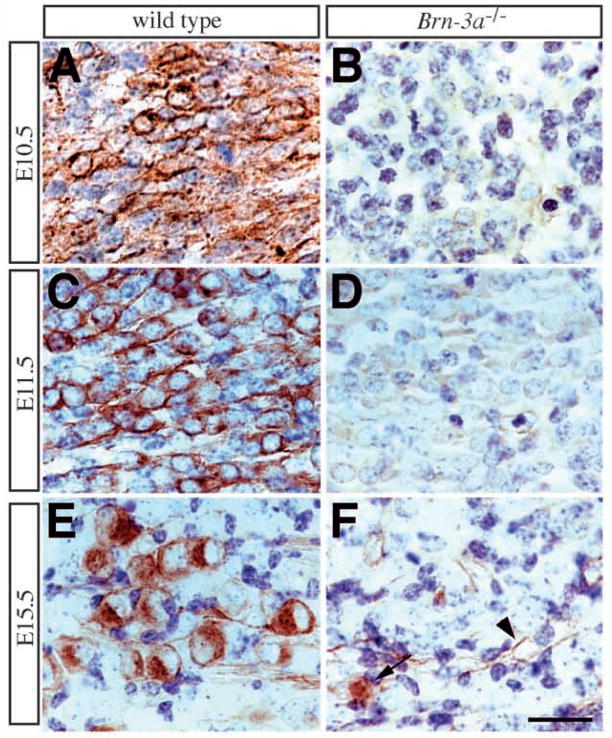
Fig. 5.
Failure to express TrkC in Brn-3a mutant ganglia. (A,C,E) Expression of TrkC receptor in wild-type ganglia shows a dynamic pattern during embryogenesis. TrkC expression is apparent in a small number of neurons at E10.5 (A). At E11.5, the number of TrkC-immunoreactive neurons becomes abundant and accounts for about 40–50% of neurons (C). However, this number decreases at E12.5 and remains unchanged after E13.5 (Table 1; Fig. 4D). At later stages, TrkC receptor is only present in a small number of neurons (E). (B,D,F) In contrast, there is essentially no TrkC receptor antigen detected at all stages. The light brown staining in mutant ganglia at E11.5 represents background color (D). Although rare TrkC-immunoreactive neurons can be identified at E15.5 (arrow, F), they constitute less than 10% of the wild-type TrkC neuron number (Table 1). TrkC expression in the vasculature is not affected in the mutant ganglia (arrowhead, F). Scale bar, 25 μm.
Progressive loss of TrkA and TrkB receptor expression in mutant ganglia
Results in Fig. 2C show that extensive neurogenesis occurs in Brn-3a mutants from E11.5 to E13.5. To determine which Trk receptors are expressed by these neurons, we examined expression of TrkA and TrkB. The results presented in Table 1 and Fig. 4 show that, at E10.5 and E11.5, there is no difference in TrkB receptor expression between the wild-type and Brn-3a mutant ganglia, either in staining intensity or in the number of TrkB-expressing neurons (Table 1; Fig. 4C). However, at E12.5, when neurons expressing TrkB tend to skew toward the anterior part of the ganglia and are less abundant inside the ganglia (not shown), there were more TrkB-expressing neurons in the mutant than in the wild-type ganglia, and this increase was observed throughout the entire ganglia (Fig. 6A,B). The anterior-to-posterior gradient of TrkB-expressing neurons, however, is still observed in the mutant ganglia (not shown). One day later at E13.5, the number of TrkB neurons in different mutant ganglia was variable (Table 1). Half of the mutant ganglia contained elevated numbers of TrkB neurons that were similar to the numbers present in E12.5 embryos, whereas the other half contained reduced numbers that were similar to those present in E15.5 embryos. By E15.5, all mutant ganglia showed a dramatic decrease in the number of TrkB-expressing neurons and, by E17.5 and P0, none of the surviving neurons expressed TrkB receptor (Fig. 6C–F). Interestingly, however, the expression of TrkB receptor in both vasculature and mesenchyme surrounding the sensory ganglia did not appear to be affected in the mutant ganglia (Fig. 6C–F, arrows).
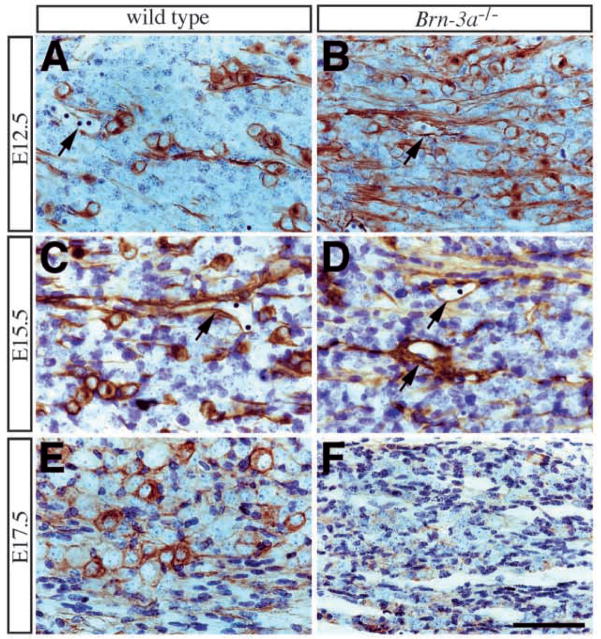
Fig. 6.
Progressive loss of TrkB expression in mutant ganglia. (A,B) The initial expression of TrkB receptor in trigeminal ganglia is not affected by absence of Brn-3a (not shown). However, at E12.5 when the number of TrkB-immunoreactive neurons decreases in the wild-type ganglia, the number of these neurons in mutant ganglia increases. Individual TrkB neurons in mutant ganglia contains staining characteristics similar to those in wild-type ganglia. At E15.5 (C,D) the number of TrkB-immunoreactive neurons reduces dramatically and by E17.5 (E,F) none of the remaining neurons express TrkB receptor. In contrast to the progressive loss of TrkB expression in neurons, TrkB immunoreactivity in the vasculature (arrows, A–D) is not affected in mutant ganglia. Scale bar, 50 μm.
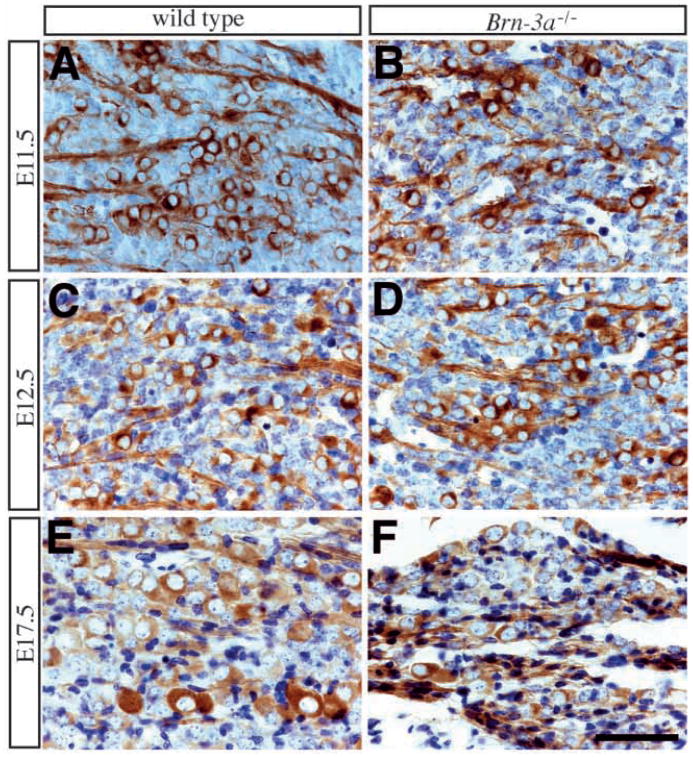
Similar to the TrkB receptor, the expression of the TrkA receptor in the early stages of embryogenesis at E10.5 and E11.5 showed no differences between wild-type and mutant ganglia (not shown). The intensity of TrkA immunoreactivity was weak at E10.5 and became more prominent at E11.5. The number of TrkA-expressing neurons was identical in ganglia of both genotypes at E11.5 (Table 1; Fig. 4B). Beginning from E12.5, however, neurons in the Brn-3a mutant ganglia showed a slightly reduced intensity of TrkA receptor expression accompanied by a small reduction in their number (Table 1; Figs 4B, 7A,B). This reduction in level of TrkA receptor expression and in number of TrkA-expressing neurons became more prominent at E13.5 and E15.5 (Fig. 7C–F), so that by E17.5 and P0, none of the remaining neurons expressed the TrkA receptor (Fig. 7G,H).
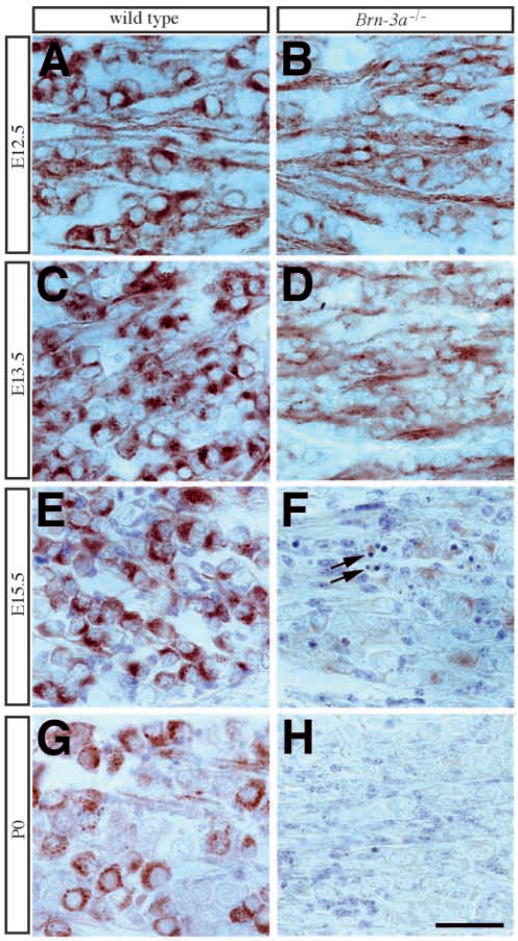
Fig. 7.
Progressive loss of TrkA expression in mutant ganglia. (A,B) In contrast to the intense TrkA expression in wild-type ganglia from E12.5 to P0, neurons in mutant ganglia show a slight reduction in TrkA staining intensity beginning at E12.5 (B). In E13.5 (C,D) mutant ganglia (D), TrkA staining in most neurons is weaker than that in the previous stage, and by E15.5 (E,F) in the mutant ganglia (F) there are very few neurons expressing TrkA. (G,H) At E17.5, the remaining neurons in mutant ganglia show no evidence of TrkA immunoreactivity. Scale bar, 50 μm.
Loss of Brn-3a does not affect the expression of p75NTR and parvalbumin
Another important neurotrophin receptor is p75NTR which, in contrast to the Trk receptors, has been shown to interact with all of the neurotrophins (Bothwell, 1995). Although the functions of p75NTR are still being characterized, it is quite clear that p75NTR is required for proper development of sensory neurons because mice with a targeted deletion in p75NTR show partial losses of members in all subclasses of sensory neurons (Bergmann et al., 1997; Stucky and Koltzenburg, 1997). Given its important functions and the fact that downregulation of p75NTR was reported in the initial characterization of Brn-3a mutant mice (McEvilly et al., 1996), we also examined the expression of p75NTR in sensory neurons (Fig. 8) (Weskamp and Reichardt, 1991). Unlike its expression in the neural crest of E8.5 to E9.5 embryos, p75NTR expression in sensory ganglia after E11.5 appears to be restricted to cells with neuronal phenotypes. p75NTR did not appear to be expressed in neural precursors although it is present in mesenchymal tissues. The expression of p75NTR in neurons becomes more apparent throughout the rest of embryonic development, with different neurons showing various intensities of p75NTR expression. In contrast to the previous report (McEvilly et al., 1996), however, we did not identify any difference in the expression of p75NTR in the trigeminal ganglia of wild-type and Brn-3a mutant (Fig. 8). Most importantly, at E17.5 when none of the remaining neurons express any Trk receptor, neurons in mutant ganglia continued to express p75NTR at levels that do not obviously differ from those in wild-type ganglia (Fig. 8E,F).
Fig. 8.
Loss of Brn-3a has no effects on the expression of p75NTR. (A,C,E) Expression of p75NTR in trigeminal ganglia at E12.5 appears to be present in cells with definitive neuronal morphology, including the presence of neuronal processes. The number of p75NTR-immunoreactive neurons increases as the development of trigeminal ganglia progresses. By E17.5, most neurons express p75NTR. (B,D,F) The expression of p75NTR in neurons of Brn-3a mutant ganglia is similar to that in the wild type. Although the number of p75NTR-immunoreactive neurons in mutant ganglia is less than that in the wild type, this probably reflects the fact that fewer neurons are present in the mutant ganglia. The staining intensity of p75NTR in wild-type and mutant ganglia does not differ at E11.5, E12.5, or E17.5. Scale bar, 50 μm.
We also examined if loss of Brn-3a affects the expression of other markers in differentiated sensory neurons. This is especially important in light of the fact that expression of specific Trk receptors, at least in the dorsal root ganglia, is associated with presence of other phenotypic properties of neurons, e.g. TrkA is associated with CGRP, substance P, and other molecules required for the function of nociceptive neurons; TrkC is associated with parvalbumin and markers of proprioceptive neurons (Klein et al., 1994; Smeyne et al., 1994; Snider and McMahon, 1998). Because absence of Brn-3a virtually eliminates TrkC expression, we examined in the mutant the expression of parvalbumin, a marker for TrkC-expressing proprioceptive sensory neurons. In all stages examined, expression of parvalbumin in the mutant ganglia had a very similar pattern and intensity to those in the wild type. At E17.5 and P0, parvalbumin-positive neurons were still present in the mutant ganglia (data not shown).
Neurons in mutant ganglia express the c-ret receptor and survive in the presence of GDNF
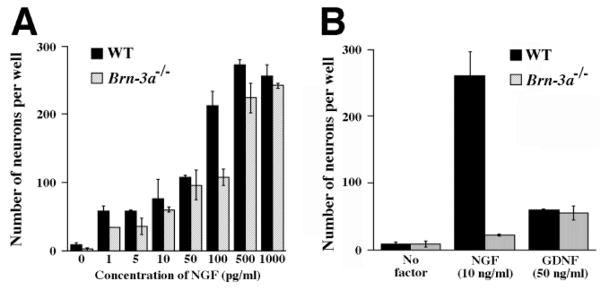
The progressive loss of TrkA receptor expression in the absence of Brn-3a predicts that NGF should support survival of many neurons at early, but not late stages. The loss of TrkB expression leads to a similar prediction for the ability of TrkB ligands, such as BDNF, to support neuronal survival at early, but not late stages. To test the predicted loss of NGF responsiveness, dissociated neurons were cultured using trigeminal ganglia from E12.5 and P0 animals. Our results show that similar numbers of neurons from E12.5 wild-type and mutant ganglia survive in the presence of various concentrations of NGF (Fig. 9A). In contrast, neurons from P0 wild type, but not mutant ganglia were supported by NGF (Fig. 9B). This confirms the prediction that, in Brn-3a mutants, loss of TrkA expression should result in loss of NGF responsiveness.
Fig. 9.
Trigeminal neurons from perinatal mutant ganglia survive in the presence of GDNF, but not NGF. (A) Trigeminal neurons from E12.5 wild-type and mutant ganglia show a similar survival response to a wide range of NGF concentrations in general. (B) While neurons from wild-type P0 ganglia survive in the presence of NGF (10 ng/ml) and GDNF (50 ng/ml), neurons from mutant ganglia at the same stage survive only in GDNF, not NGF.
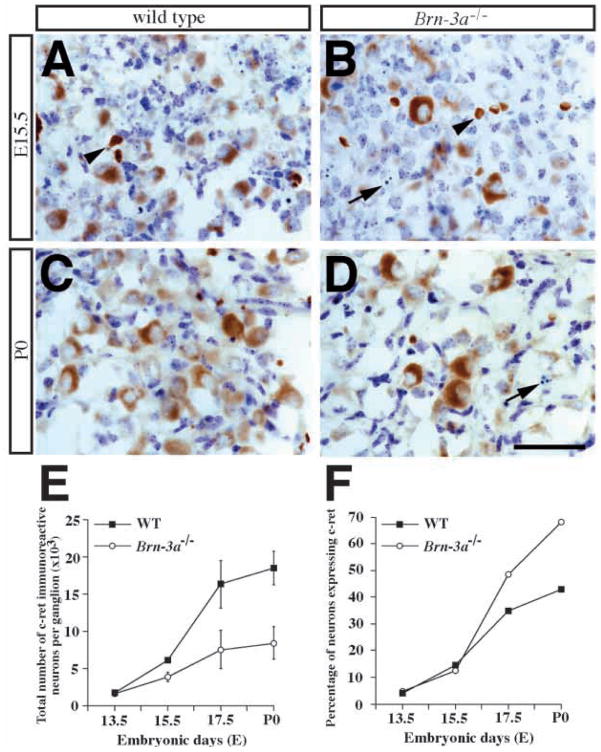
The observations that neurons in P0 mutant ganglia lack all three Trk receptors suggests that other trophic factors may sustain their survival. Previous reports, describing that GDNF as a survival factor for cranial sensory neurons (Buj-Bello et al., 1995) and the presence of the GDNF receptor constituents, such as cret, in dorsal root ganglia neurons at late embryonic and postnatal stages (Bennett et al., 1998; Molliver et al., 1997), motivated us to determine if neurons in the mutant ganglia express c-ret and are responsive to GDNF. Using dissociated cultures from P0 ganglia, results in Fig. 9B show that neurons of both wild-type and Brn-3a mutants are supported similarly by an optimal concentration of GDNF (50 ng/ml). These data suggest that GDNF may support the remaining neurons in the mutant ganglia. To examine effects of Brn-3a absence, we compared c-ret receptor expression, in trigeminal ganglia of wild-type and Brn-3a mutant mice using immunohistochemistry. Our data indicate that few neurons express c-ret at E13.5 (Fig. 10E,F). At later stages, though, increasing numbers of neurons showed a more intense c-ret immunoreactivity in wild-type ganglia (Fig. 10A,B). At E17.5 and P0, c-ret immunoreactive neurons constitute about 40% of neurons in the wild-type ganglia (Fig. 10F). Similar to the wild-type ganglia, c-ret-immunopositive neurons were also detected in the Brn-3a mutant ganglia at the corresponding embryonic stages. Although the absolute numbers of c-ret-expressing neurons were less in the mutant ganglia at all stages after E13.5, elevated percentages of neurons expressed c-ret at E17.5 and P0. At P0, about 70% of neurons in the mutant ganglia expressed c-ret compared to 40% in wild-type ganglia (Fig. 10E,F). As described previously, many apoptotic profiles were identified from E15.5 to P0 in the mutant ganglia. Interestingly, most of these profiles were negative for c-ret immunoreactivity (Fig. 10B,D).
Fig. 10.
Expression of c-ret, an important part of the GDNF receptor complex, is not affected in the mutant ganglia. (A–D) Immunocytochemistry of c-ret in the developing trigeminal ganglia show minimal c-ret expression at E13.5 (data not shown). However, the number of c-ret-immunoreactive neurons increases progressively from E15.5 to P0. Note that, in addition to labeling trigeminal neurons, c-ret antibody also reacts with red blood cells (arrowheads, A,B). Arrows in B and D indicate the presence of apoptotic profiles, immunoreactive for c-ret, in mutant ganglia at E15.5 and P0. (E,F) Quantitative analyses show a progressive increase in c-ret-immunoreactive neurons in both wild-type and mutant ganglia, albeit the number is reduced in the mutant (E). Interestingly, a higher percentage of neurons express c-ret in the mutant ganglia at E17.5 and P0, probably because of a sharp decline in neuron number at these stages (F). Scale bar, 50 μm.
DISCUSSION
The POU domain transcription factors have been shown to be important for cell type specification in different tissues and organisms (Treacy and Rosenfeld, 1992). The Brn-3 gene family belongs to the POU IV subtype and contains three members, Brn-3a, Brn-3b and Brn-3c, that are murine homologues of the C. elegans gene Unc-86 (Xiang et al., 1997a). Mutation in Unc-86 affects cell-fate commitment in several mechanosensory neuroblast lineages (Chalfie, 1993) and, interestingly, targeted deletions in Brn-3a, Brn-3b or Brn-3c lead to defects in sensory ganglia, retinal ganglion cells and inner ear hair cells, respectively (Erkman et al., 1996; Gan et al., 1996; McEvilly et al., 1996; Xiang et al., 1996, 1997b). The involvement of Brn-3a in the development of sensory ganglia is of particular interest because absence of Brn-3a has been reported to reduce the expression of neurotrophin receptors and BDNF mRNAs (McEvilly et al., 1996). In this study, we have used additional reagents to characterize in detail consequences of Brn-3a absence on development of the various populations of cells in the trigeminal ganglion. Using this information, we provide clear models for the development of trigeminal ganglion in both wild-type and Brn-3a mutant mice (Fig. 11). Our data indicate that, although Brn-3a is present in the proliferating precursors of trigeminal ganglia, absence of Brn-3a does not affect the number of precursors. Consequences appear to be restricted to neurons. Most importantly, our data indicate that the absence of Brn-3a specifically reduces expression of each of the three Trk receptors, but does not reduce the expression of p75NTR or of the GDNF receptor c-ret (Fig. 11B). The mechanism by which Brn-3a absence affects expression of TrkC clearly differs from that by which its absence affects expression of TrkA or TrkB. Presence of Brn-3a is essential for initial expression of the former, but is only essential for maintenance of expression of the latter.
Fig. 11.
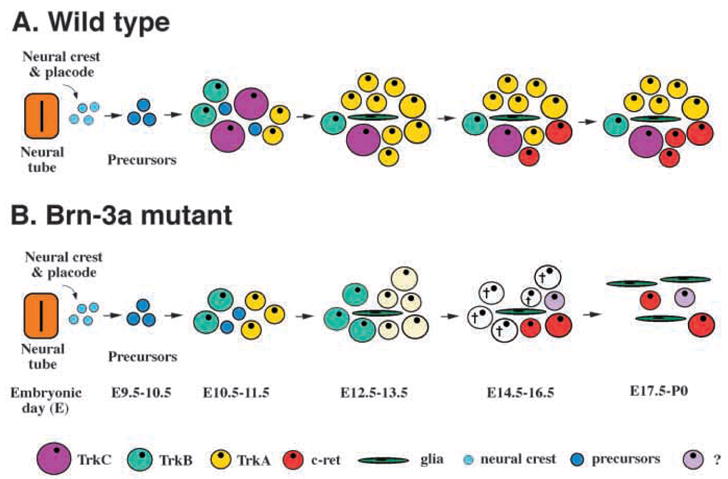
Models for trigeminal ganglion development in wild-type and Brn-3a mutant mice. (A) The development of trigeminal ganglion receives contributions from both trigeminal neural crest and neural placode. Sensory neurons in the trigeminal ganglion are generated between E10.5 and E13.5. The majority of TrkB and TrkC-expressing neurons are generated between E10.5 and E11.5, whereas the TrkA-expressing neurons are generated between E11.5 and E13.5. After which, the numbers of Trk-expressing neurons decline in 1–2 days. Between E15.5 and P0, a small number of neurons express the GDNF receptor c-ret. (B) The most dramatic effect in the absence of Brn-3a is the failure to detect TrkC-expressing neurons. In contrast, there is an increase in TrkB-expressing neurons between E12.5 and E13.5. Although the initial expression of TrkA and TrkB is not affected, there is a progressive decrease in the expression of both receptors followed by a sharp increase in apoptotic cell death that peaks at E15.5. The expression of the GDNF receptor c-ret is not affected by the absence of Brn-3a.
Generation of trigeminal neurons expressing Trk receptors
In recent studies, we characterized the expression of Trk receptors in sensory ganglia and observed that: (1) Trk receptor proteins are not present in the proliferating precursors, instead, Trk receptors are expressed exclusively in neurons (Fariñas et al., 1998; Huang et al., 1999), (2) the majority of trigeminal neurons express only one Trk receptor at E11.5 and almost none exhibit dual expression at later stages (Huang et al., 1999) and (3) generation of neurons expressing TrkB or TrkC largely occurs before E11.5, while generation of TrkA-expressing neurons primarily occurs between E11.5 and E13.5 (Figs 4, 11A). After generation, the number of each type decreases, probably because of competition for limited quantities of neurotrophins in target tissues (Fig. 4). Neuronal birth-dating experiments indicate that precursors from E9.5 and E10.5, but not those from E11.5 or later, can give rise to TrkB- or TrkC-expressing neurons. Similarly, precursors in E9.5, E10.5 and E11.5 trigeminal ganglia can give rise to TrkA-expressing neurons (Huang et al., 1999). These data strongly suggest that neurons expressing different Trk receptors are generated in two waves during the formation of trigeminal ganglia, with the first wave generating a mixture of TrkA-, TrkB- and TrkC-expressing neurons and the second wave generating only TrkA neurons (Fig. 11A). This model predicts that loss of a neurotrophin or its corresponding Trk receptor will lead to apoptotic cell death at distinct embryonic stages (Piñon et al., 1996; Huang et al., 1999).
Notwithstanding this orderly, yet dynamic, pattern of trigeminal neuron generation (Fig. 11A), there are reports that, while in dissociated cultures, mouse trigeminal neurons undergo extensive switching in neurotrophin dependence from E11.5 to E13.5, and that certain neurons supported initially by BDNF can later survive in the presence of NGF, suggesting that trigeminal neurons switch Trk receptor expression (Buchman and Davies, 1993; Paul and Davies, 1995). Furthermore, it has been shown by RT-PCR that, after dissociated culture in vitro, individual E16 rat trigeminal neurons express detectable levels of mRNAs encoding more than a single Trk receptor (Moshnyakov et al., 1996). In contrast, our data indicate that the switch of neurotrophin dependence reflects different waves of neurogenesis and that later E13.5 trigeminal neurons express only one Trk receptor (Fig. 11A; Huang et al., 1999). Although PCR may be more sensitive in detecting Trk receptor expression than antibody staining, it is possible that dissociated cultures can alter gene expression in sensory neurons, including changes in expression of genes encoding TrkA and TrkC (Friedel et al., 1997).
Role of Brn-3a in initiation of TrkC receptor expression
The most surprising result from our current study is the virtually complete absence of TrkC receptor protein from neurons in trigeminal ganglia in Brn-3a mutants at early stages, indicating that Brn-3a is required for the induction of TrkC expression (Figs 5, 11B). This is not only demonstrated by immunohistochemistry, it is also reflected by reduced apoptotic cell death at E11.5, when TrkC-immunoreactive pyknotic profiles are frequently seen in wild-type ganglia, and by the lack of about 20–30% neurons from E11.5 to E15.5 (Fig. 2C; Table 1). The few cells that are scored as TrkC-immunopositive at E10.5 and E11.5 exhibited very weak TrkC staining intensity, not detectably above background. At E13.5 and E15.5, however, rare neurons with clear expression of TrkC are present in the mutant ganglia (Fig. 5F; Table 1). It is not clear whether the TrkC protein detected includes a tyrosine kinase domain or is instead a truncated isoform. Interestingly, even though all TrkC neurons are completely eliminated by apoptosis at E11.5 and E12.5 in the NT-3 mutants, a small number of TrkC-expressing neurons also appear at E15.5 in this mutant (I. Fariñas, personal communication). This indicates that, after the initial phase of neurogenesis, expression of Trk receptors in sensory ganglia is not static. The presence of rare TrkC-expressing neurons at later stages could be due to the expression of other TrkC receptor isoforms under the control of different transcription factors.
Role of Brn-3a in maintenance of TrkA and TrkB receptor expression
Targeted mutations in neurotrophins and neurotrophin receptors have demonstrated the critical roles of these molecules in sensory neuron development (Fariñas and Reichardt, 1997). In the trigeminal ganglion, mutations in TrkA, TrkB or TrkC lead to apoptotic cell death of subpopulations of sensory neurons (Piñon et al., 1996). For instance, loss of TrkA results in massive cell death at E13.5 and E14.5, whereas loss of TrkB leads to cell death of a smaller magnitude at E11.5 and E12.5 (Piñon et al., 1996). In contrast, expression of TrkA and TrkB in the trigeminal ganglia of Brn-3a mutants is not reduced at E11.5 and E12.5, and is only slightly reduced at E13.5 (Figs 4, 6, 7). Therefore, it is not surprising that neurons from E12.5 mutant ganglion survive in NGF (Fig. 9A) and that no apparent apoptotic cell death is present at these stages (Fig. 2B). However, the major wave of cell death in the trigeminal ganglia of Brn-3a mutants occurs at E15.5, after TrkA and TrkB receptors reduced below the critical level required to maintain neuronal survival (Fig. 11B). These data also indicate that Brn-3a can not be required for the induction of TrkA and TrkB. In contrast to its role in initiation of TrkC expression, Brn-3a is required for the maintenance, but not initiation, of TrkA and TrkB expression.
By E17.5 and P0, survival of the remaining trigeminal neurons, with no evidence of Trk receptor expression, cannot be supported by NGF in vitro (Fig. 9B). In vivo, these neurons almost certainly depend on other neurotrophic factors for their survival. However, the continued presence of pyknotic cells in the mutant ganglia suggests that these other factors may not be sufficient to support the survival of all of the remaining neurons (see below). As neurotrophin expression in target tissues that do not express Brn-3a is not expected to be affected, continued activation by these factors of p75NTR in the absence of all Trk receptors may directly induce apoptosis of these neurons. In previous work, ligand engagement of p75NTR alone in oligodendrocytes, retinal ganglion cells or sympathetic neurons has been shown to result in apoptotic cell death (Bamji et al., 1998; Casaccia-Bonnefil et al., 1996; Frade et al., 1996).
The timing of neuronal death in Brn-3a mutant ganglia coincides with the reported intervals of reduction in neuron numbers between E13.5 and birth in wild-type animals (so-called ‘enhanced apoptosis’). Although the nature of this phenomenon is unclear, the magnitude of observed reduction is quite different among reports. For example, two reports indicated that there is 45% reduction between E13.5 and P0 (Davies and Lumsden, 1984; Piñon et al., 1997), one paper reported a 20% reduction between E13.5 and P0 (Piñon et al., 1996), and one reported a 30% reduction from E14 to P0 (but no reduction from E12 to P0; ElShamy and Ernfors, 1996). In contrast, the numbers of trigeminal neurons reported by our group have consistently ranged between 42,000 and 48,000 at E13.5, and between 40,000 and 42,000 at P0 (Fariñas et al., 1994; Wilkinson et al., 1996; Moore et al., 1996; Cacalano et al., 1998). The cause of the differences among all reports is not at all clear, but is possibly attributable to differences in genetic background. Since the magnitude of neuronal loss in the Brn-3a mutants is much more severe (Fig. 2C; Table 1), we do not feel that this discrepancy diminishes the impact of our observations on Trk receptor expression in wild-type and Brn-3a mutants.
Specificity of Brn-3a functions
After completion of neurogenesis in the trigeminal ganglion at E13.5, some neurons switch trophic factor dependence from NGF to GDNF. This transition is well-documented in dorsal root ganglia in cell culture assays and by analyses of GDNF receptor expression in vivo (Molliver et al., 1997; Bennett et al., 1998). In dorsal root ganglia, a defined subpopulation of neurons expressing TrkA in early embryogenesis cease expression of this receptor and instead initiate expression of c-ret (Molliver et al., 1997; Bennett et al., 1998). In this paper, we have observed that the trigeminal ganglion also exhibits a change in expression of the GDNF receptor c-ret similar to that observed in dorsal root ganglia. Only a small number of cells express low levels of c-ret at E13.5, but the number of neurons expressing this protein increases rapidly at later stages. A similarly dramatic increase in the number of neurons expressing this protein occurs in the absence of Brn-3a, indicating that Brn-3a does not regulate this transition.
Despite the presence of c-ret in a substantial proportion of sensory neurons in vivo and the ability of GDNF to support sensory neuron survival in vitro, the embryonic development of sensory neurons is not drastically affected in the absence of GDNF, c-ret or GFRα1 (Cacalano et al., 1998; Enomoto et al., 1998; Moore et al., 1996; Schuchardt et al., 1994). For example, the trigeminal ganglion of mice lacking GDNF only exhibit a small deficiency in sensory neuron number at birth (Moore et al., 1996). Furthermore, when compared with NGF, GDNF in vitro has a less pronounced effect on supporting sensory survival. In its optimal dosage, GDNF supports the survival of fewer sensory neurons than NGF (Fig. 9B) (Buj-Bello et al., 1995; Molliver et al., 1997). Since the transition of NGF to GDNF dependence is not complete until adulthood, it is possible that GDNF may have a more pronounced effect on postnatal sensory neurons. This has not yet been addressed in vivo because mice lacking GDNF, GFRα1 or c-ret die after birth because they lack functional kidneys (Cacalano et al., 1998; Enomoto et al., 1998; Moore et al., 1996; Pichel et al., 1996; Sánchez et al., 1996). In addition to GDNF and related ligands acting through c-ret, there may be other yet unidentified trophic factors that can support the survival of sensory neurons. Indeed, a recent report indicated that ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), oncostatin M (OSM) and cardiotrophin-1 (CT-1) can support survival of trigeminal neurons at late embryonic stages (Horton et al., 1998). The fact that 30% of the trigeminal neurons in Brn-3a mutant mice do not appear to express c-ret supports this possibility.
Might Brn-3a regulate other genes that may be critical survival of trigeminal neurons? Among many genes that are implicated in mediating neuronal survival, Brn-3a has been shown to positively regulate the expression of Bcl-2 in vitro (Smith et al., 1998); however, the fact that loss of Bcl-2 only results in a 20% reduction in trigeminal neurons (Piñon et al., 1997) argues against a dramatic role of Bcl-2 in the survival of trigeminal neurons. In contrast, loss of another Bcl-2 related gene, Bcl-x, leads to a very general neuronal death at E13.5 (Motoyama et al., 1995). Could Brn-3a regulate the expression of Bcl-x? If the neuronal deficits in Brn-3a mutants were due to downregulation of Bcl-x expression, one would anticipate that the deficits should occur at a much earlier stage than E15.5. Furthermore, neurons that undergo apoptotic cell death due to loss of Bcl-x expression should express neurotrophin receptors, such as the Trk receptors and c-ret. The absence of these features argues against this possibility.
Complex transcriptional control of sensory neuron development
The elucidation of Brn-3a effects on trigeminal ganglia development suggests that Brn-3a is probably not a neuronal fate determination gene since, in the absence of Brn-3a, neurogenesis occurs and neurons are able to survive for a certain time interval. The observations that Brn-3a regulates Trk receptor expression are more consistent with the roles of a cell-type-specification gene that controls the differentiation and survival of a given cell population by regulating the key molecules required for these processes. In this aspect, the functions of Brn-3a are similar to those of many other members of the POU domain family of transcription factors (Treacy and Rosenfeld, 1992). In agreement with this, the recent characterizations of the inner ear hair cell defects in Brn-3c mutant mice also have indicated an important role for Brn-3c in supporting inner ear hair cell survival (Xiang et al., 1998). In contrast to the roles of Brn-3a, the basic helix-loop-helix (bHLH) factor neurogenin 1 appears to have a much earlier effect on trigeminal ganglion development since cells in the trigeminal ganglion are eliminated by apoptotic cell death at E11.5 to E12.5 in its absence (Ma et al., 1998).
Although our results advance understanding of the roles of Brn-3a in trigeminal ganglia development, there remain several unanswered questions. For example, the mechanism for the compensatory increase in TrkB-expressing neurons in the mutant ganglion at E12.5 remains unclear. It is possible that this could be mediated through a binary regulatory circuit controlling transcription of the TrkB and TrkC genes. The phenotype of the Brn-3a mutant also illuminates a more fundamental question on the relationship between expression of Trk receptors and neuronal differentiation. As described previously, in dorsal root ganglia, parvalbumin is associated with proprioceptive neurons that express TrkC. In the absence of TrkC or NT-3, little expression of this protein is seen. Although the association of parvalbumin with TrkC-expressing neurons has not been subject to the same scrutiny in the trigeminal ganglion, the presence of parvalbumin-immunopositive neurons in Brn-3a mutant ganglia suggests that either this association is not as rigorous as in the DRG, or proprioceptive function can be assumed by neurons expressing other receptors.
In conclusion, our analyses of the Brn-3a mutant phenotype in the trigeminal ganglia indicate that Brn-3a is required for the induction of TrkC expression and for the maintenance of TrkA and TrkB expression. Because of this, loss of Brn-3a leads to two distinct phases of neuronal loss. In contrast, absence of Brn-3a does not reduce the expression of p75NTR, c-ret or parvalbumin, indicating that Brn-3a acts specifically to promote expression of the Trk receptors, but does not affect expression of several other differentiation markers.
Acknowledgments
We thank Dr Arnon Rosenthal for providing the human recombinant GDNF, Dr Jeffrey Milbrandt for providing information on c-ret antibody, Liz Copp for assistance in manuscript preparation, and members of the Reichardt laboratory for helpful comments on this work. Special thanks to Dr Jeremy Nathans for providing Brn-3a mutant mice. This work has been supported by research grants from the United States Public Health Service (NIH grant MH482000) and the Howard Hughes Medical Institute. M.X. is supported by grants from the NIH (R01 EY12020), March of Dimes Birth Defects Foundation, and Alexandrine and Alexander L. Sinsheimer Fund. E. J. H. is a recipient of the Postdoctoral Fellowship for Physicians and a Research Associate, and L. F. R. is an Investigator of the Howard Hughes Medical Institute.
References
- Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann I, Priestley JV, McMahon SB, Bröcker EB, Toyka KV, Koltzenburg M. Analysis of cutaneous sensory neurons in transgenic mice lacking the low affinity neurotrophin receptor p75. Eur J Neurosci. 1997;9:18–28. doi: 10.1111/j.1460-9568.1997.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Ann Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- Buj-Bello A, Buchman VL, Horton A, Rosenthal A, Davies AM. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;15:821–828. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Fariñas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Chalfie M. Touch receptor development and function in Caenorhabditis elegans. J Neurobiol. 1993;24:1433–1441. doi: 10.1002/neu.480241013. [DOI] [PubMed] [Google Scholar]
- Clary DO, Weskamp G, Austin LR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, MacMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Davies A, Lumsden A. Relation of target encounter and neuronal death to nerve growth factor responsiveness in the developing mouse trigeminal ganglion. J Comp Neurol. 1984;223:124–137. doi: 10.1002/cne.902230110. [DOI] [PubMed] [Google Scholar]
- ElShamy WM, Ernfors P. Requirement of neurotrophin-3 for the survival of proliferating trigeminal ganglion progenitor cells. Development. 1996;122:2405–2414. doi: 10.1242/dev.122.8.2405. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–660. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Reichardt LF. Neurotrophic factors and their receptors, Roles in neuronal development and function. In: Cowan WM, Jessell TM, Zipursky SL, editors. Molecular and Cellular Approaches to Neural Development. New York: Oxford University Press; 1997. pp. 220–263. [Google Scholar]
- Fariñas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A. Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron. 1998;21:325–334. doi: 10.1016/s0896-6273(00)80542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedtsova NG, Turner EE. Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech Dev. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Friedel RH, Schnürch H, Stubbusch J, Barde YA. Identification of genes differentially expressed by nerve growth factor- and neurotrophin-3-dependent sensory neurons. Proc Natl Acad Sci USA. 1997;94:12670–12675. doi: 10.1073/pnas.94.23.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM, Rodríguez-Tébar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci USA. 1996;93:3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrero MR, McEvilly RJ, Turner E, Lin CR, O’Connell S, Jenne KJ, Hobbs MV, Rosenfeld MG. Brn-3.0: a POU-domain protein expressed in the sensory, immune, and endocrine systems that functions on elements distinct from known octamer motifs. Proc Natl Acad Sci USA. 1993;90:10841–10845. doi: 10.1073/pnas.90.22.10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradwohl G, Fode C, Guillemot F. Restricted expression of a novel murine atonal-related bHLH protein in undifferentiated neural precursors. Dev Biol. 1996;180:227–241. doi: 10.1006/dbio.1996.0297. [DOI] [PubMed] [Google Scholar]
- Horton AR, Barlett FP, Pennica D, Davies AM. Cytokines promote the survival of mouse cranial sensory neurones at different developmental stages. Eur J Neurosci. 1998;10:673–679. doi: 10.1046/j.1460-9568.1998.00079.x. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Wilkinson GA, Fariñas I, Backus C, Zang K, Wong S, Reichardt LF. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development. 1999;126:2191–2203. doi: 10.1242/dev.126.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Genetic control of cell fate specification in Drosophila peripheral nervous system. Ann Rev Genetics. 1994;28:373–393. doi: 10.1146/annurev.ge.28.120194.002105. [DOI] [PubMed] [Google Scholar]
- Jones KR, Fariñas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C, Teillet MA. The cellular and molecular basis of early sensory ganglion development. In: Scott SA, editor. Sensory Neurons: Diversity, Development, and Plasticity. New York: Oxford University Press; 1992. pp. 143–170. [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- McEvilly RJ, Erkman L, Luo L, Sawchenko PE, Ryan AF, Rosenfeld MG. Requirement for Brn-3.0 in differentiation and survival of sensory and motor neurons. Nature. 1996;384:574–577. doi: 10.1038/384574a0. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Moshnyakov M, Arumäe U, Saarma M. mRNAs for onr, two or three members of trk receptor family are expressed in single rat trigeminal ganglion neurons. Mol Brain Res. 1996;43:141–148. doi: 10.1016/s0169-328x(96)00168-4. [DOI] [PubMed] [Google Scholar]
- Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- Paul G, Davies AM. Trigeminal sensory neurons require extrinsic signals to switch neurotrophin dependence during the early stages of target field innervation. Dev Biol. 1995;171:590–605. doi: 10.1006/dbio.1995.1307. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Piñon LG, Minichiello L, Klein R, Davies AM. Timing of neuronal death in trkA, trkB and trkC mutant embryos reveals developmental changes in sensory neuron dependence on Trk signaling. Development. 1996;122:3255–3261. doi: 10.1242/dev.122.10.3255. [DOI] [PubMed] [Google Scholar]
- Piñón LG, Middleton G, Davies AM. Bcl-2 is required for cranial sensory neuron survival at defined stages of embryonic development. Development. 1997;124:4173–4178. doi: 10.1242/dev.124.20.4173. [DOI] [PubMed] [Google Scholar]
- Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Developmental potential of trunk neural crest cells in the mouse. Development. 1994;120:1709–1718. doi: 10.1242/dev.120.7.1709. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Smith MD, Dawson SJ, Boxer LM, Latchman DS. The N-terminal domain unique to the long form of the Brn-3a transcription factor is essential to protect neuronal cells from apoptosis and for the activation of Bcl-2 gene expression. Nucleic Acid Res. 1998;26:4100–4107. doi: 10.1093/nar/26.18.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Koltzenburg M. The low-affinity neurotrophin receptor p75 regulates the function but not the selective survival of specific subpopulations of sensory neurons. J Neurosci. 1997;17:4398–4405. doi: 10.1523/JNEUROSCI.17-11-04398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Tsoulfas P, Donovan MJ, Palko ME, Blair-Flynn J, Hempstead BL, Parada LF. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci USA. 1997;94:14776–14781. doi: 10.1073/pnas.94.26.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treacy MN, Rosenfeld MG. Expression of a family of POU-domain protein regulatory genes during development of the central nervous system. Ann Rev Neurosci. 1992;15:139–165. doi: 10.1146/annurev.ne.15.030192.001035. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Wilkinson GA, Fariñas I, Backus C, Yoshida CK, Reichardt LF. Neurotrophin-3 is a survival factor in vivo for early mouse trigeminal neurons. J Neurosci. 1996;16:7661–7669. doi: 10.1523/JNEUROSCI.16-23-07661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Gao WQ, Hasson T, Shin JJ. Requirement for Brn-3c in maturation and survival, but not in fate determination for inner ear hair cells. Development. 1998;125:3935–3946. doi: 10.1242/dev.125.20.3935. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Zhou L, Chen ZY, Wagner D, O’Malley BW, Jr, Klein W, Nathans J. Role of the Brn-3 family of POU-domain genes in the development of the auditory/vestibular, somatosensory, and visual systems. Cold Spring Harbor Symp Quant Biol. 1997a;62:325–336. [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW, Jr, Klein W, Nathans J. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci USA. 1997b;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Gan L, Zhou L, Klein WH, Nathans J. Targeted deletion of the mouse POU domain gene Brn-3a causes selective loss of neurons in the brainstem and trigeminal ganglion, uncoordinated limb movement, and impaired suckling. Proc Natl Acad Sci USA. 1996;93:11950–11955. doi: 10.1073/pnas.93.21.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995;15:4762–4785. doi: 10.1523/JNEUROSCI.15-07-04762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]