Summary
To study the development of the cerebellum, we generated a transgenicmouse line Tg(mα6-cre)B1LFR that expresses CRE recombinase under the GABAA receptor α6 subunit promoter. In this line, recombination of an R26R reporter allele occurred postnatally in granule cells of the cerebellum and dorsal cochlear nucleus, as well as in a subset of precerebellar nuclei in the brainstem. All neurons in which recombination occurred originated during embryogenesis from the rhombic lip. This might be explained by a very early specification event at the rhombic lip that primes cells derived from this structure to express the transgene during neuronal maturation. As no recombination occurred in the inferior olive, it may be derived from a distinct subset of precursors at the rhombic lip. No recombination occurred in any of the interneurons in the cerebellum (stellate cells, basket cells, and Golgi cells), consistent with the hypothesis that they are not derived from the same embryonic tissue as the granule cells. genesis 33:160–169, 2002.
Keywords: cre-mediated, lip derivatives, recombination
The cerebellum is an ancient part of the vertebrate brain that is particularly well developed in mammals. It is involved in fine motor and balance control, and in motor learning (Mauk et al., 2000). It receives two main inputs: excitatory climbing fibers originating from the inferior olive, which synapse directly onto Purkinje cells, and excitatory mossy fibers originating from a set of precerebellar nuclei, including the external cuneate nucleus, lateral reticular nucleus, vestibular nuclei, pontine gray nucleus, and pontine reticulotegmental nucleus. Mossy fibers synapse onto cerebellar granule cells, which in turn provide excitatory input to Purkinje cells in the form of parallel fibers. Purkinje cells are inhibitory neurons that control the main cerebellar output system, the deep cerebellar nuclei.
The development of the cerebellum and the precerebellar system has been well studied using careful anatomical observations (Cajal, 1911; His, 1891), combined with 3H-thymidine birthdating studies (Altman and Bayer, 1997; Miale and Sidman, 1961), and by transplantation experiments using quail-chicken chimeras (Hallonet et al., 1990; Wingate and Hatten, 1999). The rhombic lip, a proliferative zone at the dorsal rim of the embryonic rhombencephalic neural tube, has been shown to be the source of several classes of neurons associated with the cerebellum and the precerebellar system. The first neurons born at the rhombic lip migrate via an intramural route to the inferior olive. The second wave of neurons migrates posteriorly via an extramural route to form the external cuneate nucleus and the lateral reticular nucleus, and anteriorly to the pontine gray nucleus and pontine reticulotegmental nucleus. The last cells generated at the rhombic lip are the granule cell precursors. They migrate across the entire surface of the cerebellum to form at birth a thin layer of precursors, the external germinal layer. During the first 2–3 postnatal weeks, these precursors divide rapidly, and newborn granule cells descend through the molecular layer of the cerebellum, past the Purkinje cell layer to their final location in the internal granule cell layer. Purkinje cells and the neurons of the deep nuclei are not derived from the rhombic lip but rather from a deeper part of the rhombencephalic alar plate, from where they migrate into the emerging cerebellum (Altman and Bayer, 1997). Golgi cells are generated perinatally, followed by stellate and basket cells in the first two postnatal weeks. Their site of origin is under dispute and has been proposed to be either the external granule cell layer (Altman and Bayer, 1980; Hausmann et al., 1985) or precursors originating from the ventricular neuroepithelium (Hallonet et al., 1990; Zhang and Goldman, 1996).
In the past decade, several early transcription factors and patterning genes have been described that regulate the induction of cerebellar or precerebellar structures (reviewed in Hatten et al., 1997). Fibroblast growth factor FGF-8 can induce ectopic midbrain and cerebellar structures in the chick (Martínez et al., 1999). Mice with mutations in the genes for the secreted signaling molecule Wnt-1 (McMahon and Bradley, 1990; Thomas and Capecchi, 1990), the transcription factor EN-1 (Wurst et al., 1994), or compound mutations of the transcription factors Pax2 and Pax5 (Urbanek et al., 1997) completely lack the midbrain and the cerebellum. Other transcription factors such as MATH-1 are only required for the generation of a subset of neurons, suggesting that sub-divisions of the rhombic lip that give rise to different cell types correlate with gene expression domains. Mice carrying a deletion of Math-1 lack derivatives of the upper rhombic lip (granule cell precursors and pontine nuclei), whereas generation of neurons that originate from the lower rhombic lip (olivary neurons) is unaffected (Akazawa et al., 1995; Ben-Arie et al., 1997, 2000).
Mice expressing site-specific recombinases, such as CRE or FLP, under the control of cell type-specific promoters have been useful for studies of cell lineage (Lewandoski, 2001). When they are crossed to a mouse line that carries a recombination-dependent reporter gene, they mediate activation of the reporter gene in a cell-type-specific manner. Once recombination has occurred, reporter expression persists independently of the recombinase expression, and the activated reporter allele is transmitted to all daughter cells. Mouse lines expressing CRE-recombinase late in development in specific subpopulations of cells are also very valuable for the study of conditional alleles of essential genes.
To study the postnatal development of the cerebellum, we generated a transgenic mouse line Tg(mα6-cre)B1LFR that expresses CRE recombinase under the control of the promoter of the γ-aminobutyric acid receptor GABAA subunit α6. This promoter has previously been used successfully to drive lacZ-expression in post-mitotic, postmigratory cerebellar granule cells (Bahn et al., 1997). As expected, this line mediated recombination of a reporter allele postnatally in cerebellar granule cells. In addition, postnatal recombination was detected in granule cells of the dorsal cochlear nucleus and in a specific subpopulation of precerebellar nuclei—the pontine gray nucleus, the reticulotegmental nucleus of the pons, the external cuneate nucleus, and the lateral reticular nucleus. No recombination was detected in the vestibular nuclei, the inferior olive, or any neurons of the cerebellum other than granule cells. This pattern suggests that the transgene is activated in the entire population of neurons derived from rhombic lip, with the exception of the neurons of the inferior olive. These data are consistent with the hypothesis that the neurons of the inferior olive are derived from a distinct subset of precursors at the rhombic lip as it has been proposed from the transgenic expression pattern in a Wnt1::FLP mouse (Rodriguez and Dymecki, 2000). The observed expression pattern is also in good agreement with the proposal that granule cells are the only neurons derived from the external germinal layer and that specifically the stellate cells, basket cell, and Golgi cells are not derived from the same precursors as the granule cells.
RESULTS
Generation of the Mouse Line Tg(mα6-cre)B1LFR
GABAA receptors are pentameric ligand-gated chloride channels with a developmentally regulated subunit composition. Expression of the GABAA receptor α6 subunit is very restricted, limited in the cerebellum to postmitotic, postmigratory cerebellar granule cells. Expression is also detected in granule cells of the dorsal cochlear nucleus, fiber tracts in the superior colliculus, spinal cord, retina, and axons of the olfactory nerve (Gutiérrez et al., 1996). A 7-kb fragment of the murine GABAA receptor α6 subunit promoter has been successfully used to drive the lacZ reporter gene in transgenic mice, faithfully replicating the endogenous expression in the cerebellum (Bahn et al., 1997). To generate a transgenic mouse line expressing the CRE recombinase under the control of the GABAA receptor α6 sub-unit promoter, we applied a strategy analogous to the approach taken by Bahn and colleagues (1997) using an internal ribosome entry site (IRES) followed by Cre, instead of LacZ (Fig. 1a). Transgenic founders were screened by PCR and Southern (data not shown). To examine the pattern of transgene expression, positive strains were crossed to the R26R reporter mouse line, in which cre-mediated recombination permanently activates the transcription of a lacZ reporter (Soriano, 1999). Recombination at the level of the DNA is irreversible and is transmitted to the entire progeny of a cell. For the purpose of this paper, “recombination” will be defined as expression of β-galactosidase from the R26R reporter locus. Of the 10 lines analyzed, two showed basically no recombination, four showed partially or completely nonspecific recombination throughout the entire brain, and four lines resulted in an identical and specific pattern of recombination. One of the specific lines, Tg(mα6-cre)B1LFR, was chosen for further analysis.
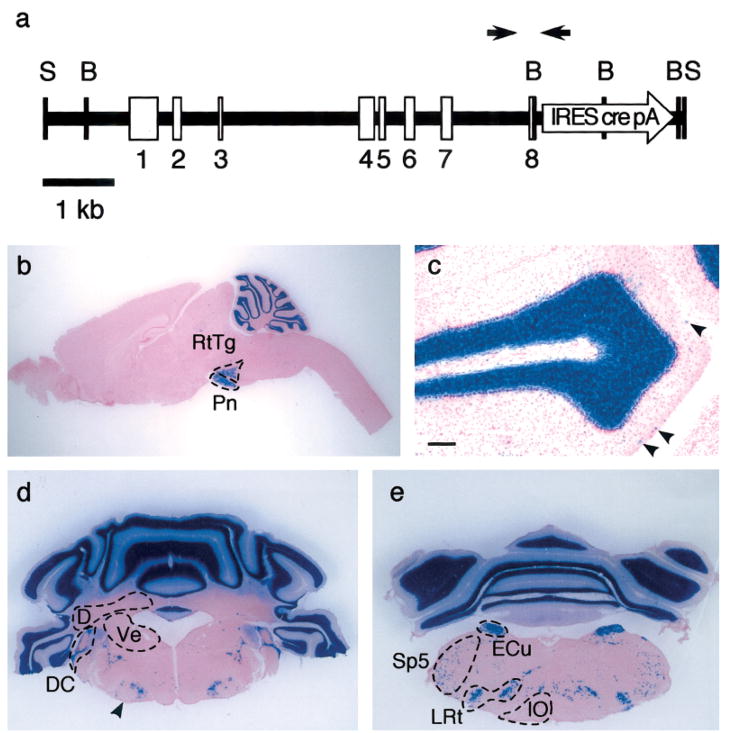
FIG. 1.
(a) Transgenic construct: an open reading frame for CRE recombinase, flanked by an internal ribosome entry site and an SV40 polyadenylation site (white arrow “IRES cre pA”), was inserted into exon 8 of a 7 kb mouse GABAA receptor α6 subunit promoter. Exons are indicated by numbered boxes. Black arrows indicate the position of the primers used for genotyping (not drawn to scale). S: SphI sites; B: BamHI sites. (b– e) Recombination in Tg(mα6-cre)B1LFR as revealed by recombination of the R26R allele: 40 μm sections were stained for β-galactosidase activity with X-Gal (blue stain) and counterstained with a general nuclear marker (pink stain). (b) Sagittal section of an entire brain showing staining in the cerebellar granule cell layer, pontine nuclei (Pn), and reticulotegmental nucleus of the pons (RtTg). (c) Sagittal section through lobule 3 of the cerebellum. Arrowheads indicate rare blue cells in the molecular layer. Scale bar = 100 μm (d, e) Coronal sections through the cerebellum and brainstem. Recombination was detected in the dorsal cochlear nucleus (DC), external cuneate nucleus (ECu), lateral reticular nucleus (LRt), scattered cells in the spinal 5 nucleus (Sp5) (spinal trigeminal sensory nucleus), and surrounding the facial motor nucleus (arrowhead). No recombination was found in the vestibular nuclei (Ve), inferior olive (IO), or the deep nuclei (D).
Analysis of Reporter Gene Expression
In adult mice, recombination was detected as expected throughout the granule cell layer with very few blue cells in the rest of the cerebellum (Fig. 1b, c, and Table 1). Strong recombination was also detected in the granule cells of the dorsal and ventral cochlear nuclei (Fig. 1d), consistent with the reported expression of a lacZ reporter gene from the endogenous GABAA receptor α6 locus (Jones et al., 1996). Surprisingly, strong recombination was also observed in most precerebellar brain stem nuclei, including the pontine gray nucleus, pontine reticulotegmental nucleus, lateral reticular nucleus, and external cuneate nucleus. Only scattered cells were found in the trigeminal sensory nuclei, around the facial motor nucleus, and in the inferior and superior colliculus (Fig. 1b, d, e). A subpopulation of cells was recombined in layer 1 throughout the cerebral cortex, including the corresponding layer of the hippocampus (Fig. 2a). Very little recombination was observed in the spinal cord (Fig. 1b and data not shown). Significantly, recombination was absent in two precerebellar nuclei, the vestibular nuclei and the inferior olive (Fig. 1d, e).
Table 1.
Recombination in the Cerebellum of Tg(mα6-cre)B1LFR Crossed to R26R
| Recombined cells of total scored | Recombined cells/mm3 mean ± STD | Recombination | |
|---|---|---|---|
| Granule cellsa | 447/488 | 92% | |
| Purkinje cellsb | 0/6396 | 0% | |
| Golgi cellsc | 0/198 | 0% | |
| Molecular layerd | 590 ± 110 | 1%e | |
| Deep nucleid | 84 ± 40 | 0.2%f |
Double immunofluorescence staining against β-galactosidase and GABAA receptor α6 subunit.
Anti-parvalbumin staining on X-Gal stained sections.
Double immunofluorescence staining against β-galactosidase and GABA.
X-Gal staining and fast nuclear red counterstain.
Assuming a cell density of 77,000 cells/mm3 as described for the rat molecular layer (Korbo et al., 1993).
Assuming a cell density of 34.000 cells/mm3 as described for the rat dentate nucleus (Chan-Palay, 1977).
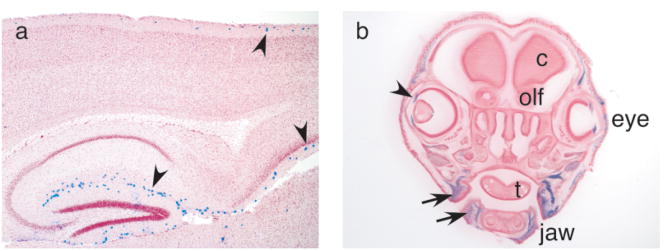
FIG. 2.
(a) Recombination in the adult forebrain. Sagittal 40 μm sections were treated as described in Figure 1. Arrowheads point at blue cells in layer 1 of the cortex and the corresponding layer of the hippocampus. (b) Recombination in cranial neural crest derivatives at birth. 100 μm coronal section through the head of a newborn pup showing blue stain in muscle (arrows) and the iris (arrowhead). c: cortex; olf: olfactory bulb; t: tongue.
In addition to the recombination in the brain, labeled cells were detected in muscle and connective tissue, primarily in the head and neck area. Figure 2b depicts a coronal section of the head of a newborn pup stained with X-Gal, showing that recombination had occurred in muscle around the upper and lower jaw, underneath the skin, and in the iris, but not in the muscle of the tongue. It was particularly striking that many labeled structures are neural crest derivatives, including facial and masticatory muscles, the iris, and scattered cells in the inner ear ossicles and tracheal rings (Fig. 2b and data not shown).
Recombination in the Cerebellum
We next analyzed the recombination pattern in the cerebellum at the cellular level (Fig. 3 and Table 1). In X-Gal stained sections, the entire granule cell layer appeared uniformly blue because the blue product of the X-Gal reaction diffuses slightly in the tissue. Very few blue cells were detected in the molecular layer (arrowheads in Fig. 1c) and the deep nuclei. Quantitative analysis showed that 590 ± 110 cells/mm3 (mean ± SD) were recombined in the molecular layer, which corresponds to 1% of the density of neurons present in the molecular layer as estimated from the rat (Korbo et al., 1993). Most of these cells probably correspond to “displaced granule cells” as has been proposed (Jones et al., 1997). To verify this hypothesis, we immunostained sections with rabbit antibodies against the granule cell marker GABAA receptor α6 subunit. To identify recombined cells, we colabeled the sections with a mouse monoclonal antibody against β-galactosidase that had previously been shown to result in a punctate staining pattern when used with the R26R reporter mouse (Rico et al., 2002). Indeed, double-labeled cells in the molecular layer resembled granule cells in size and morphology (Fig. 3a). Thus essentially no recombination occurred in interneurons (stellate cells and basket cells) in the molecular layer. In the deep nuclei, 84 ± 40 cells/mm3 (mean ± SD) were recombined, which corresponds to 0.2% of the density of neurons as estimated from the rat dentate nucleus (Chan-Palay, 1977), suggesting that recombination in the deep nuclei is almost neglectable.
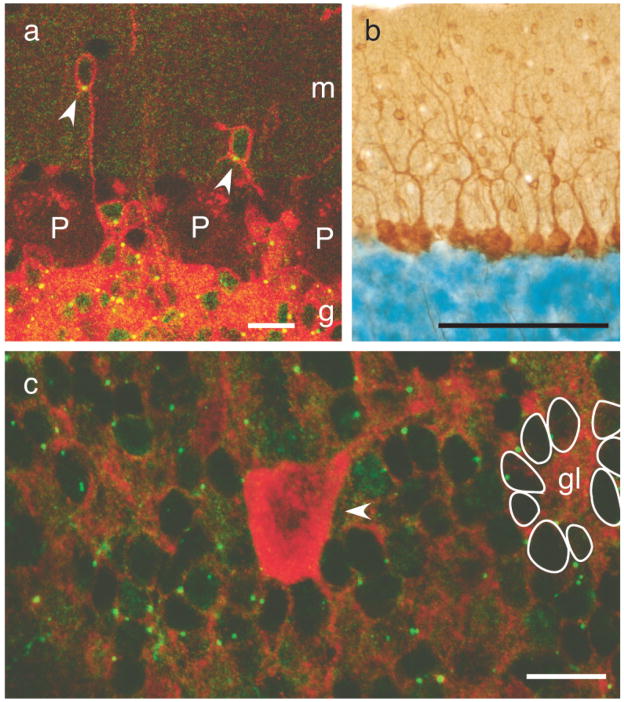
FIG. 3.
Analysis of recombined cell types in the adult cerebellum. (a) Displaced, β-galactosidase-positive granule cells were found in the molecular layer (arrow heads). Projection of six confocal sections (0.32 μm apart) of double-immunofluorescence staining against β-galactosidase (green punctate label) and GABAA receptor α6 subunit (red label). g: granule cell layer; m: molecular layer; P: Purkinje cell. Scale bar = 10 μm. (b) Recombination did not occur in Purkinje cells or interneurons in the molecular layer: X-Gal stained (blue) 40 μm section labeled with immunohistochemistry against parvalbumin (brown stain). Scale bar = 100 μm. (c) Recombination occurred in granule cells but not in Golgi cells: projection of four confocal sections (0.5 μm apart) of double-immunofluorescence staining against β-galactosidase (green punctate label) and GABA (red label). Granule cells are visible as unlabeled “holes” with green puncta in the periphery. Nine representative granule cells are traced in white, surrounding a glomerulus (gl). A GABA-positive Golgi cell is marked with an arrowhead. Scale bar = 10 μm.
To study Purkinje cells, we labeled X-Gal stained sections with an antiserum against parvalbumin, which stains Purkinje cells as well as interneurons in the molecular layer (Fig. 3b). None of 6,396 Purkinje cells tested were double labeled. Anti-GABA staining was used to identify Golgi cells in the granule cell layer. Colabeling with the monoclonal antibody against β-galactosidase showed that none of 198 Golgi cells tested expressed β-galactosidase (Fig. 3c). Note the uniform pattern of β-galactosidase-positive puncta around the granule cell nuclei, which are visible as densely packed “dark holes” in Figure 3b. Nine representative granule cells are traced surrounding a glomerulus, the central unit of the mossy fiber-granule cell synapses. Because of the very thin rim of cytoplasm surrounding a granule cell, it is technically difficult to assay double labeling with the cytoplasmic β-galactosidase-positive puncta. In sections double labeled for the granule cell marker GABAA receptor α6 subunit and β-galactosidase, 92 ± 3 % of the granule cells were unambiguously positive for both markers. In summary we conclude that granule cells are the only neurons recombined in the cerebellum.
Developmental Time Course
To determine when the recombination occurred during development, we studied animals from P0 to adult. At birth, recombination had already occurred in the muscles in the face (Fig. 2b), but the only cells in the brain expressing β-galactosidase were located in layer 1 of the cortex (data not shown). Prior to postnatal day 2 (P2), no recombination was observed in the midbrain, brain stem, or cerebellum (Fig. 4a). By P4.5, a few scattered recombined cells were detected in the brain stem and midbrain as well as in the external germinal layer and the internal granule cell layer of the cerebellum (Fig. 4b). Recombined cells continued to accumulate until P12.5 in the four precerebellar nuclei that showed recombination in the adult, namely the pontine gray nucleus, pontine reticulotegmental nucleus, lateral reticular nucleus, and external cuneate nucleus. At this time, almost adult levels of recombination were reached (Fig. 4a–f and data not shown).
FIG. 4.
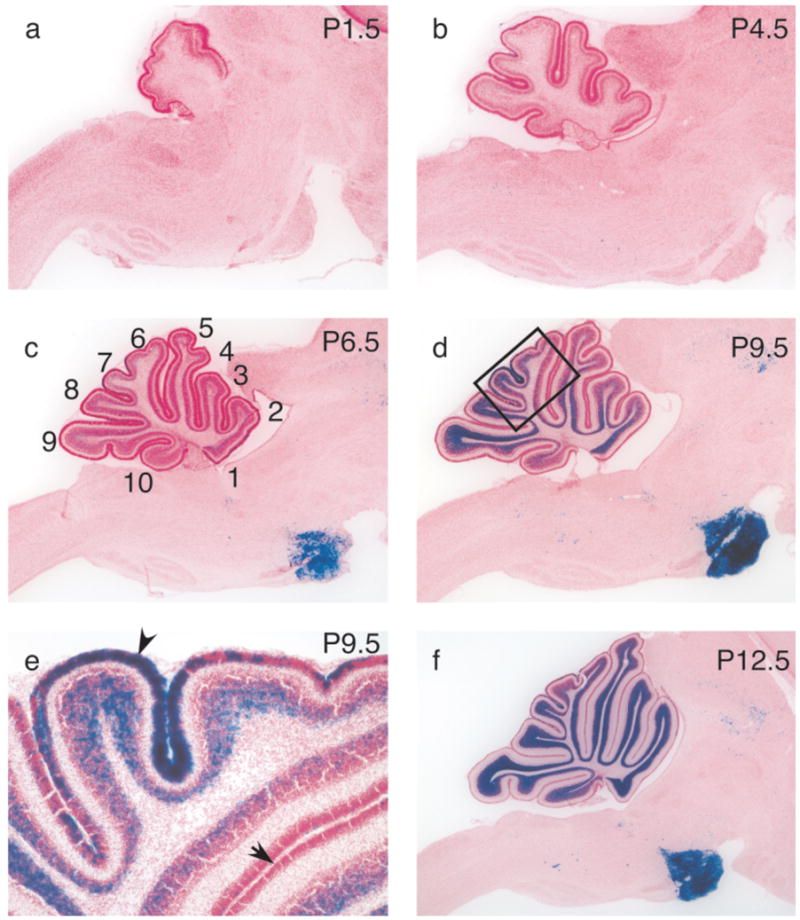
(a–f) Time course of recombination in the cerebellum and the brain stem from P1.5 to P12.5. 40 μm sagittal sections were treated as described in Figure 1. The age of the animals is indicated in the upper right corner. Numbers in (c) indicate the cerebellar lobules. The box in (d) indicates the area blown up in (e). Note blue cells in the external germinal layer in lobule 7 (arrowhead in e) but less so in other lobules (arrow in e).
As the granule cell layer continued to expand from P4.5 to P20, the percentage of recombined granule cells increased continuously, with lobules 2, 3, 8, and 9 progressing slightly faster than the others (nomenclature of lobules as in Paxinos and Franklin, 2001). By P15, almost adult levels of recombination were reached. Within a given lobule, the earlier born, more mature granule cells closer to the white matter expressed β-galactosidase before the later born granule cells closer to the Purkinje cells (Fig. 4e). Only a few cells in the external germinal layer expressed the reporter gene, except in lobules 6 and 7, where recombination was observed in a substantial fraction of the cells as early as P4.5 but especially around P9 (Fig. 4e). Interestingly, granule cells in lobules 6 and 7 are among the last cells born in the vermis of the rat cerebellum (Altman, 1969). The observed temporal expression pattern is similar to the pattern reported for a mouse line where a lacZ reporter gene was knocked directly into the GABAA receptor α6 subunit locus (Mellor et al., 1998). Mellor and colleagues detected lacZ expression starting at P5.5 in lobule 10, and covering most lobules by P11. They never observed expression in the external germinal layer. In Tg(mα6-cre)B1LFR, expression of the R26R reporter starts about a day earlier, occurs in the external germinal layer in lobules 5 and 7, and lobule 10 is the last lobule to express β-galactosidase. The granule cells of the dorsal cochlear nucleus started to express β-galactosidase very late at P9, and reached adult levels around P14 (data not show).
DISCUSSION
We generated a transgenic mouse line that expresses CRE recombinase under the control of the GABAA receptor α6 subunit promoter. When crossed to a reporter mouse that expresses lacZ upon cre-mediated recombination, β-galactosidase was detected postnatally in a well-defined set of precerebellar nuclei (pontine gray nucleus, reticulotegmental nucleus of the pons, external cuneate nucleus, and lateral reticular nucleus), as well as in cerebellar granule cells and granule cells of the dorsal cochlear nucleus. Recombination was also detected in some cranial neural crest derivatives. All neurons that expressed the reporter are known to be derived from a common germinal zone, the rhombic lip, during embryogenesis (Altman and Bayer, 1997; Pierce, 1967). However, recombination is only detected several days after the cells emerge from the rhombic lip, suggesting that a very early specification event in the rhombic lip may influence transgene expression in a late phase of neuronal maturation.
Interestingly, no recombination was detected ion the inferior olive, which is also derived from the rhombic lip. Olivary neurons differ fromother rhombic lip derivatives in several ways: (1) they are born earlier, (2) they migrate by an intramural route instead of on the surface of the brain (Altman and Bayer, 1997), (3) they are the source of climbing fibers, and (4) they were shown to be derived from a different pool of precursors as determined by a Wnt1::FLP transgenic mouse that mediated recombination in precursors for large parts of the midbrain and the cerebellar system but not for the inferior olive (Rodriguez and Dymecki, 2000). Tg(mα6-cre)B1LFR thus appears to mediate recombination in a subset of rhombic lip derivatives that might correspond to an anatomically distinct pool of neuronal precursors.
Interestingly, recombination was detected in a subset of cranial neural crest derivatives. Examination of quailchicken chimeras has shown that the neural crest arises from the dorsal most part of the neural tube adjacent to the rhombic lip (Wingate and Hatten, 1999), suggesting that the same early specification event might affect both populations of precursors. It has to be noted that not all neural crest derivatives were recombined to the same extent. Recombination was frequently observed in muscle cells but rarely in bone cells, and not at all in the trigeminal ganglion (Fig. 2a and data not shown).
Granule cells were the only neurons recombined in the cerebellum. Because Purkinje cells and neurons of the deep nuclei are not derived from the rhombic lip, it is not surprising that recombination was not observed in these cells. However, because of their late postnatal birth dates, it has been suggested that stellate and basket cells originate in the external germinal layer, which is derived from the rhombic lip (Altman and Bayer, 1978). Similarly, it has been suggested that Golgi cells originate in the external germinal layer based on their presence in drug-induced ectopic clusters of granule cells (Hausmann et al., 1985). Studies using quail-chicken chimeras (Hallonet et al., 1990), retroviruses in rat (Zhang and Goldman, 1996), and genetic studies in mice (Maricich and Herrup, 1999) have shown, however, that cerebellar interneurons as well as Purkinje cells more likely originate in the ventral epithelium. Our failure to observe recombination in these interneurons, even in lobules where extensive recombination had occurred in the external germinal layer (Fig. 4e), is more consistent with the latter hypothesis, although the late onset of recombination precludes direct conclusions about lineage relationships.
The mouse line Tg(mα6-cre)B1LFR mediates recombination of a reporter gene about a day earlier and in more cell types than expected from studies of the GABAA receptor α6 subunit promoter, which had found reporter expression mainly in postmigratory granule cells of the cerebellum and the dorsal cochlear nucleus (Bahn et al., 1997; Jones et al., 1997). This might be explained by the high sensitivity of the reporter system: even low levels of CRE recombinase are sufficient to recombine the reporter allele, which then expresses the reporter gene at high levels from the constitutive R26R promoter. Low levels of gene expression from the GABAA receptor α6 subunit promoter at early stages or outside the cerebellum might have escaped detection in previous studies. Interestingly, when Cre is knocked into the GABAA receptor α6 subunit locus, it is not expressed in the brain stem or nonneuronal tissues (I. Aller, A. Jones, D. Merlo, and W. Wisden, unpublished research), indicating that the sensitivity of CRE recombinase cannot fully explain the extended spatial expression pattern. Four independent transgenic lines showed an identical pattern in all areas tested, strongly arguing against an integration site effect. We therefore speculate that the transgene lacks regulatory sequence elements that usually restrict expression from the genomic locus.
The late onset of recombination in the hindbrain around P4 is very striking. With the exception of the cerebellar and dorsal cochlear granule cells, which are born postnatally, all of the recombined neurons were born prenatally and had migrated to their final location in the brain stem several days before the onset of recombination. These neurons were not recombined as precursors but activated the transgenic GABAA receptor α6 subunit promoter independently in their final positions. A number of studies have shown that granule precursors are specified early, before migration from the rhombic lip. First, RU49, a Zn2+ finger transcription factor specific for the granule cell lineage is expressed at the rhombic lip as early as E14.5 in the mouse (Yang et al., 1996). Second, E14.5 rhombic lip cells can differentiate into granule cells when transplanted to P6 cerebella in vivo or when plated on postnatal granule cells in vitro, indicating that E14.5 rhombic lip cells are able to respond to instructive cues by differentiating into granule cells (Alder et al., 1996). Third, P0 external germinal layer cells activate the genomic GABAA receptor α6 subunit promoter when transplanted into the adult hippocampus or amygdala, thus activating and maintaining a cerebellar granule cell marker ectopically (Bahn et al., 1999). Our results suggest the hypothesis that among the cells originating at the rhombic lip, not only granule cell precursors but also cells of the precerebellar system undergo an early specification event that primes them to express the transgene upon neuronal maturation.
Tg(mα6-cre)B1LFR will be useful tool to study the maturation of the precerebellar system and the cerebellum. It will be of special interest to cross this transgenic mouse line with conditional alleles of essential genes, because the late onset of expression allows early development to proceed undisturbed.
MATERIALS AND METHODS
Generation of Transgenic Mice
The plasmid pmalpha6IRES-lacZ6 was generously provided by W. Wisden (Bahn et al., 1997). The 7-kb promoter including exons 1 through 8 was excised with SphI and BamHI (partial digest) and ligated into pBluescript KS (Stratagene) with a modified poly-linker site to generate plasmid SB-16. An IRES-cre-pA cassette was excised with SalI and NarI from pSP40D (Qi Wang and Nigel Killeen, in preparation) and ligated behind the mα6 promoter using SalI and BstBI (partial digest) to generate the plasmid mα6IREScre3. The transgenic construct was excised with SphI and injected into fertilized oocytes from a C57/Bl6xDBA/2 mixed background. The resulting transgenic lines were screened by amplifying a 586 bp band using polymerase chain reaction (PCR) primers ma6-3F (TAGAGCATTAGGGTGGGAG) and IRES2-R (TGCCGCCTTTGCAGGTGTGTCTTAC). Genotypes were confirmed by Southern blot, using a probe spanning the IRES-cre junction or a probe recognizing the GABAA receptor subunit α6 promoter. Positive lines were further analyzed by crossing to the R26R reporter mouse (on a CD-1 background) and analysis of lacZ expression (Soriano, 1999). Crosses to an alternative reporter mouse line CAG-CAT-EGFP (Kawamoto et al., 2000) yielded very similar results, showing that R26R reveals the complete pattern of transgene expression (data not shown).
Histology
Mice were perfused transcardially with 25 ml of phosphate buffered saline (PBS), followed by 15–50 ml (depending on the age) of 4% paraformaldehyde in PBS, followed by 5–20 ml of 15% and 5–20 ml of 30% sucrose in PBS. Brains were dissected (newborns were processed intact, without dissecting), equilibrated in 30% sucrose overnight at 4°C, and sliced at 40–100 μm in a sliding microtome (Microm HM440E). Sections were stained while free floating in X-Gal solution for 1 h to overnight at 37°C (10 mM Tris-Cl, pH 7.3, 1 mg/ml X-Gal (Roche 745740), 0.01% Na-Desoxycholate, 0.02% Nonident P-40, 4% dimethylformamide, 2 mM MgCl2, 1 mM K3Fe(CN)6, 1 mM K4Fe(CN)6), after which they were mounted on Superfrost*Plus microscopy slides (Fisher 12-550-15), counterstained with Fast Nuclear Red (Vector H-3403), dehydrated, and coverslipped with DPX (BDH Laboratory Supplies 360294H). Anatomical structures were identified using The Mouse Brain Atlas (Paxinos and Franklin, 2001).
Immunohistochemistry
Parvalbumin-immunohistochemistry on X-Gal stained sections was performed as described (Rico et al., 2002) using rabbit anti-parvalbumin antiserum (Swant, PV-28) at a dilution of 1:2000 combined with the standard peroxidase-coupled VECTASTAIN ABC kit (Vector Laboratories, PK-4000) using diaminobenzidine as a substrate. Immunofluorescence was performed as described (Rico et al., 2002) on free-floating 40 μm sections using monoclonal anti-β-galactosidase (Promega 1:500), rabbit anti- GABA (Sigma A-2052 1:2000), affinity-purified rabbit anti- GABAA receptor α6 subunit (generously provided by F.A. Stevenson [Thompson et al., 1992] at 0.5 μm/ml). Secondary antibodies from Molecular Probes were used at a dilution of 1:400 (Alexa 488 goat anti-mouse and Texas Red goat anti-rabbit).
Quantification of Recombination in the Cerebellum
Pictures were taken on a digital SPOT camera (Diagnostic Instruments, RT Slider 2.3.1) on either a Nikon SMZ1500 dissection microscope or a Nikon Eclipse E600 microscope. Confocal pictures were taken on a MRC 1000 confocal microscope (Bio-Rad Laboratories). Three animals of 5 month of age were analyzed for each cell type. At least three sagittal sections, spaced 200 μm apart, were analyzed per animal. Granule cells were quantified by double-immunofluorescence staining of 40 μm sections for β-galactosidase and GABAA receptor α6 subunit: all granule cells were identified in a single confocal section (3,262 μm2) in the channel for GABAA receptor α6 subunit, and double-labeled cells were subsequently identified in serial confocal sections (0.18 μm apart) spanning 2.5 μm above and below the chosen plane. Golgi cells were quantified by double-immunofluorescence staining of 40 μm sections against β-galactosidase and GABA: random Golgi cells were chosen in the fluorescent channel for GABA, and subsequently pictures were taken on the SPOT camera for both channels to identify double-labeled cells. All Purkinje cells in one focal plane of an entire sagittal section were counted directly in the microscope on X-Gal-stained sections processed for immunohistochemistry against parvalbumin. Recombined cells in the entire molecular layer of 20 μm sagittal sections were identified in X-Gal-stained, fast nuclear red-counterstained sections. The volume of the molecular layer in the analyzed sections was estimated by measuring the area on low-magnification pictures using the software ImageJ (NIH), and multiplying it by the thickness of the section. Recombined cells in the deep nuclei were counted in an analogous way to the molecular layer.
Acknowledgments
Grant sponsor: U.S. Public Health Service, Grant number: NS16033.
Grant sponsors: The Swiss National Science Foundation, the Uarda Frutiger Foundation, and the Howard Hughes Medical Institute.
We thank W. Wisden for the plasmid pmalpha6IRESlacZ6, N. Killeen for the plasmid pSP40D, and F. A. Stevenson for the antibody against GABAA receptor α6 subunit. We are very grateful to A. Schmidt for the pronuclear injection; B. Rico and S. Bamji for fruitful discussions; and Y. G. Ng and B. Wyler for excellent technical assistance. U. F. was supported by a grant from the Swiss National Science Foundation, the Uarda Frutiger Foundation, and the Howard Hughes Medical Institute. L. F. R. is an investigator of the Howard Hughes Medical Institute.
LITERATURE CITED
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. doi: 10.1016/s0896-6273(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969;136:269–293. doi: 10.1002/cne.901360303. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Prenatal development of the cerebellar system in the rat. I. Cytogenesis and histogenesis of the deep nuclei and the cortex of the cerebellum. J Comp Neurol. 1978;179:23–48. doi: 10.1002/cne.901790104. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. I. Thymidine-radiographic study of the time of origin of neurons of the lower medulla. J Comp Neurol. 1980;194:1–35. doi: 10.1002/cne.901940102. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure, and function. New York: CRC Press; 1997. p. 783. [Google Scholar]
- Bahn S, Jones A, Wisden W. Directing gene expression to cerebellar granule cells using gamma-aminobutyric acid type A receptor alpha6 subunit transgenes. Proc Natl Acad Sci U S A. 1997;94:9417–9421. doi: 10.1073/pnas.94.17.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn S, Wisden W, Dunnett SB, Svendsen C. The intrinsic specification of gamma-aminobutyric acid type A receptor alpha6 subunit gene expression in cerebellar granule cells. Eur J Neurosci. 1999;11:2194–2198. doi: 10.1046/j.1460-9568.1999.00662.x. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, Matzuk M, Bellen HJ, Zoghbi HY. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- Cajal SRy. Histologie du Système Nerveux de l’Homme et des Vertébrés. Paris: 1911. [Google Scholar]
- Chan-Palay V. Cerebellar dentate nucleus, organization, cytology and transmitters. Berlin: Springer-Verlag; 1977. p. 548. [Google Scholar]
- Gutiérrez A, Khan ZU, De Blas AL. Immunocytochemical localization of the alpha 6 subunit of the gamma-aminobutyric acid A receptor in the rat nervous system. J Comp Neurol. 1996;365:504–510. doi: 10.1002/(SICI)1096-9861(19960212)365:3<504::AID-CNE12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hallonet ME, Teillet MA, Le Douarin NM. A new approach to the development of the cerebellum provided by the quail-chick marker system. Development. 1990;108:19–31. doi: 10.1242/dev.108.1.19. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Alder J, Zimmerman K, Heintz N. Genes involved in cerebellar cell specification and differentiation. Curr Opin Neurobiol. 1997;7:40–47. doi: 10.1016/s0959-4388(97)80118-3. [DOI] [PubMed] [Google Scholar]
- Hausmann B, Mangold U, Sievers J, Berry M. Derivation of cerebellar Golgi neurons from the external granular layer: evidence from explantation of external granule cells in vivo. J Comp Neurol. 1985;232:511–522. doi: 10.1002/cne.902320408. [DOI] [PubMed] [Google Scholar]
- His W. Die Entwicklung des menschlichen Rautenhirns vom Ende des ersten Monats bis zum Beginn des dritten Monats. I. Verlängertes Mark. Abhandlung der königlichen sächsischen Gesellschaft der Wissenschaften. Mathematisch-physikalische Klasse. 1891;29:1–74. [Google Scholar]
- Jones A, Bahn S, Grant AL, Kohler M, Wisden W. Characterization of a cerebellar granule cell-specific gene encoding the gamma-aminobutyric acid type A receptor alpha 6 subunit. J Neurochem. 1996;67:907–916. doi: 10.1046/j.1471-4159.1996.67030907.x. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Niwa H, Tashiro F, Sano S, Kondoh G, Takeda J, Tabayashi K, Miyazaki J. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 2000;470:263–268. doi: 10.1016/s0014-5793(00)01338-7. [DOI] [PubMed] [Google Scholar]
- Korbo L, Andersen BB, Ladefoged O, Moller A. Total numbers of various cell types in rat cerebellar cortex estimated using an unbiased stereological method. Brain Res. 1993;609:262–268. doi: 10.1016/0006-8993(93)90881-m. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999;41:281–294. doi: 10.1002/(sici)1097-4695(19991105)41:2<281::aid-neu10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Martínez S, Crossley PH, Cobos I, Rubenstein JL, Martin GR. FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development. 1999;126:1189–1200. doi: 10.1242/dev.126.6.1189. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Medina JF, Nores WL, Ohyama T. Cerebellar function: coordination, learning or timing? Curr Biol. 2000;10:R522–525. doi: 10.1016/s0960-9822(00)00584-4. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: electrical activity regulates the GABAA receptor alpha 6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. London: Academic Press; 2001. [Google Scholar]
- Pierce ET. Histogenesis of the dorsal and ventral cochlear nuclei in the mouse. An autoradiographic study. J Comp Neurol. 1967;131:27–54. doi: 10.1002/cne.901310104. [DOI] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002 doi: 10.1038/nn808. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Dymecki SM. Origin of the precerebellar system. Neuron. 2000;27:475–486. doi: 10.1016/s0896-6273(00)00059-3. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Bodewitz G, Stephenson FA, Turner JD. Mapping of GABAA receptor alpha 5 and alpha 6 subunit-like immunoreactivity in rat brain. Neurosci Lett. 1992;144:53–56. doi: 10.1016/0304-3940(92)90714-i. [DOI] [PubMed] [Google Scholar]
- Urbanek P, Fetka I, Meisler MH, Busslinger M. Cooperation of Pax2 and Pax5 in midbrain and cerebellum development. Proc Natl Acad Sci U S A. 1997;94:5703–5708. doi: 10.1073/pnas.94.11.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate RJ, Hatten ME. The role of the rhombic lip in avian cerebellum development. Development. 1999;126:4395–4404. doi: 10.1242/dev.126.20.4395. [DOI] [PubMed] [Google Scholar]
- Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994;120:2065–2075. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- Yang XW, Zhong R, Heintz N. Granule cell specification in the developing mouse brain as defined by expression of the zinc finger transcription factor RU49. Development. 1996;122:555–566. doi: 10.1242/dev.122.2.555. [DOI] [PubMed] [Google Scholar]
- Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron. 1996;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]