Abstract
Sensory ganglia that innervate taste buds and gustatory papillae (geniculate and petrosal) are reduced in volume by about 40% in mice with a targeted deletion of the gene for brain-derived neurotrophic factor (BDNF). In contrast, the trigeminal ganglion, which innervates papillae but not taste buds on the anterior tongue, is reduced by only about 18%. These specific alterations in ganglia that innervate taste organs make possible a test for roles of lingual innervation in the development of appropriate number, morphology, and spatial pattern of fungiform and circumvallate papillae and associated taste buds. We studied tongues of BDNF null mutant and wild-type littermates and made quantitative analyses of all fungiform papillae on the anterior tongue, the single circumvallate papilla on the posterior tongue, and all taste buds in both papilla types. Fungiform papillae and taste buds were reduced in number by about 60% and were substantially smaller in diameter in mutant mice 15–25 days postnatal. Remaining fungiform papillae were selectively concentrated in the tongue tip region. The circumvallate papilla was reduced in diameter and length by about 40%, and papilla morphology was disrupted. Taste bud number in the circumvallate was reduced by about 70% in mutant tongues, and the remaining taste buds were smaller than those on wild-type tongues. Our results demonstrate a selective dependence of taste organs on a full complement of appropriate innervation for normal growth and morphogenesis. Effects on papillae are not random but are more pronounced in specific lingual regions. Although the geniculate and petrosal ganglia sustain at least half of their normal complement of cell number in BDNF −/− mice, remaining ganglion cells do not substitute for lost neurons to rescue taste organs at control numbers. Whereas gustatory ganglia and the taste papillae initially form independently, our results suggest interdependence in later development because ganglia derive BDNF support from target organs and papillae require sensory innervation for morphogenesis.
Indexing terms: fungiform papilla, circumvallate papilla, geniculate ganglion, petrosal ganglion, trigeminal ganglion
The molecular mechanisms that operate in nerve–taste organ interactions during the development of the gustatory system are not known. However, evidence is accumulating to indicate that taste organs produce neurotrophins that sustain ganglion cells, which in turn innervate and maintain developing gustatory papillae and taste buds (Mistretta, 1998). We studied the taste system in mice with a targeted gene deletion for one of the neurotrophins, brain-derived neurotrophic factor (BDNF). Because the BDNF null mutants have selective, substantial reductions in taste ganglion cells, they can be used to determine the roles of sensory innervation in taste organ development and differentiation.
Taste organs on the mammalian tongue are complex structures called papillae, which are comprised of an epithelial covering over a broad core of connective tissue, and taste buds, discrete collections of about 40–60 cells within the papilla epithelium. The three gustatory papilla types, fungiform, circumvallate, and foliate, are distributed on the tongue in distinctive spatial patterns (Mistretta, 1991). Furthermore, there is a characteristic number and location of taste buds within the three papilla types that is species dependent. The gustatory papillae and taste buds, therefore, form a patterned organ system that is well suited to detect and localize chemicals on the tongue.
During development, papillae and taste buds acquire an extensive sensory innervation. Whereas induction of the gustatory papillae is not neurally dependent (Farbman and Mbiene, 1991; Mbiene et al., 1997), the papillae require innervation for sustained morphogenesis, growth, and maintenance (Nagato et al., 1995). Similarly, although the chemosensory field has long assumed that lingual taste buds require sensory innervation for induction (Farbman, 1965; Farbman and Mbiene, 1991; Mistretta, 1991; Mistretta and Hill, 1995), recent studies have suggested that mammalian (Fritzsch et al., 1997b) and axolotl (Barlow et al., 1996) taste buds may also be induced independently of innervation. However, it is clear that, for morphologic and functional differentiation and for acquisition of full numerical complement, taste buds depend on sensory innervation (Hosley et al., 1987; Mistretta et al., 1988; Nagai et al., 1988; Krimm and Hill, 1998).
Innervation for the gustatory papillae and taste buds derives from three sensory ganglia: the geniculate ganglion that innervates taste buds in fungiform papillae, which are located primarily on the anterior tongue, via the chorda tympani nerve; the trigeminal ganglion that innervates anterior lingual epithelium and the fungiform papillae, but not taste buds, via the lingual branch of the trigeminal nerve; and the petrosal ganglion, innervating taste buds and papilla epithelium in circumvallate and foliate papillae on the posterior tongue, via the glossopharyngeal nerve (Miller, 1974; Mistretta, 1991; Mistretta and Hill, 1995). Thus, to understand interactions between nerves and peripheral target organs in taste development, knowledge about sensory ganglion development is essential. Because substantial portions of the geniculate and petrosal ganglia remain in BDNF null mutants, these mice do not present a useful model for studying the role of nerves in taste bud induction. However, evaluation of knockout mice provides an opportunity to test quantitative relations between ganglia and taste organs during development and thereby learn about neural regulation of taste papillae and buds.
Mice with a targeted mutation of the gene for BDNF have demonstrated that this neurotrophin is essential for normal cell survival in the cranial ganglia innervating taste organs (Jones et al., 1994; Liu et al., 1995). In null mutant mice, the geniculate ganglion is reduced in volume by about 40–50% compared with wild-type controls. The petrosal ganglion is reduced by about 44%. In contrast, the trigeminal ganglion, providing somatosensory innervation to nongustatory portions of the fungiform papilla epithelium but not to taste buds, is reduced by only about 16%. Obviously, these reductions in ganglion cells reduce total innervation available to peripheral target organs.
We studied the tongues of BDNF null mutant mice and wild-type littermates (Jones et al., 1994) to test developmental requirements for an intact sensory innervation in regulating (1) development of the typical number and pattern of fungiform papillae, (2) growth and morphogenesis of fungiform and circumvallate papillae, and (3) development and maintenance of the typical number of taste buds in a papilla-specific manner.
Preliminary results from these studies have appeared in abstract form (Mistretta et al., 1996). Two other studies have reported on alterations in the taste system in another strain of BDNF −/− mice (Nosrat et al., 1997; Oakley et al., 1998). The present article extends the results of these other reports by providing quantification of papilla size, distribution, and taste bud number in both fungiform and circumvallate papillae. In the present article, we report on papilla pattern and on papilla and taste bud size and number in fungiform and circumvallate papillae in BDNF mutant mice as old as 25 days postnatal.
MATERIALS AND METHODS
Tongue dissection and histology
Tongues were dissected from heads of postnatal mice with mutations of the gene for BDNF (BDNF −/−) and their wild-type littermates (BDNF +/+) that had been perfused with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (Jones et al., 1994). All animal procedures were in compliance with approved institutional animal care and use protocols and according to National Institutes of Health guidelines. Mice ranged in age from 15 to 25 days postnatal (P15–P25, P0 = day of birth; Table 1). Skin was dissected from the mouse head, and the distance from the external auditory canal to the snout was measured. The entire tongue was then dissected from the lower jaw, and oral tongue length was measured, from the tongue tip to the border between the oral and pharyngeal tongue, just caudal to the circumvallate papilla (Fig. 1A).
TABLE 1.
Mouse Data1
| Age (n) | Genotype | Procedure | Analysis |
|---|---|---|---|
| P15 (3) | +/+ | Sections | fg and/or cv; tb |
| P15 (3) | −/− | Sections | fg and/or cv; tb |
| P16 (1) | +/+ | Whole tongue | fg counts |
| P16 (1) | −/− | Whole tongue | fg counts |
| P17 (2) | +/+ | Whole tongue | fg counts |
| P17 (3) | −/− | Whole tongue | fg counts |
| P20 (1) | +/+ | Sections | fg and/or cv; tb |
| P20 (1) | −/− | Sections | fg and/or cv; tb |
| P25 (1) | +/+ | Sections | fg and/or cv; tb |
| P25 (1) | −/− | Sections | fg and/or cv; tb |
n, number of mice, in parentheses; fg, fungiform papilla number and size; cv, circumvallate papilla size; tb, number and size of taste buds in each papilla; P, postnatal day.
Fig. 1.
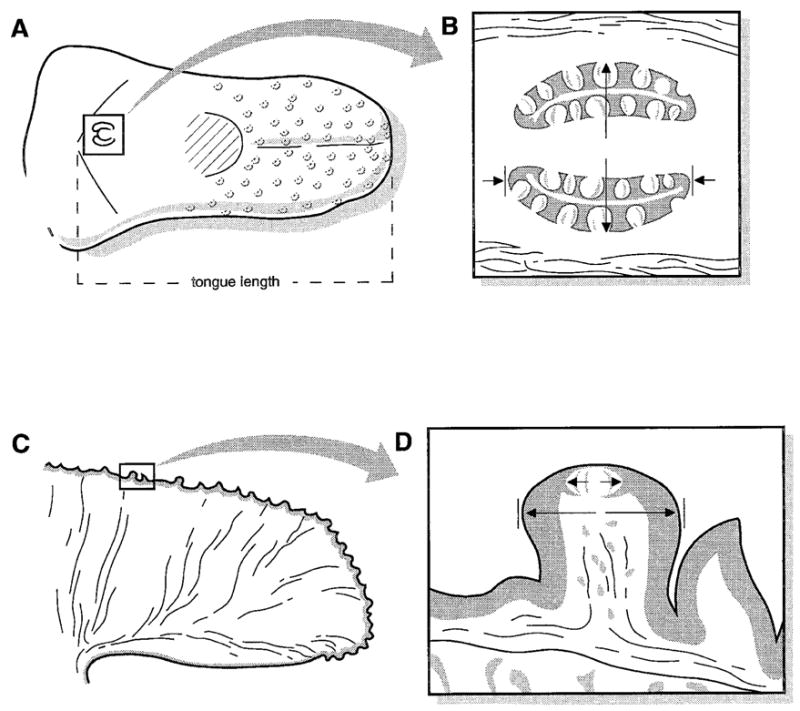
Diagrams of postnatal mouse tongue illustrate planes of tissue dissection and areas used to quantify tongue and taste organ size. A: Dorsal view of the entire tongue, with fungiform papillae diagrammed on the anterior tongue and the single, midline circumvallate papilla in the boxed region on the posterior tongue. Oral tongue length was measured from the tongue tip to the border between oral and pharyngeal tongue, which is just caudal to the circumvallate papilla. The hatched region near the middle of the tongue represents the intermolar eminence. B: Diagram of circumvallate papilla and taste buds. The papilla was dissected and sectioned in a plane parallel to the surface of the tongue to provide clear sections through all taste buds. Papilla diameter was measured at the widest point, from the external epithelial wall to the external wall, indicated by long arrows. Papilla length was measured by averaging measures of the trench walls, indicated by short arrows. C: Diagram of the sagittal section of anterior tongue, dissected just anterior to the intermolar eminence and then cut down the midline. One fungiform papilla is in a boxed region on the dorsal surface. D: Fungiform papilla and single taste bud in papilla apex in a sagittal section. Papilla and taste bud diameters were measured at the widest point, indicated by two sets of arrows.
A tissue block was dissected from the posterior tongue to include the complete circumvallate papilla and some surrounding tissue (Fig. 1A). Blocks were placed in molds containing embedding compound (OCT, Miles Scientific, Elkhart, IN) and were oriented for transverse sections, parallel to the surface of the tongue. This orientation produces sections through the complete papilla walls and inner connective tissue, from the dorsal to the ventral extent, and through all taste buds (Mistretta and Baum, 1984; Fig. 1B).
The remaining tongue piece was cut just at the anterior border of the intermolar eminence and then hemisected at the midline to provide two anterior half tongue pieces (Fig. 1A,C). Each half tongue was placed in molds with OCT embedding compound and oriented for sagittal sections. The sagittal orientation for the anterior tongue with fungiform papillae provides an orientation that optimizes potential for complete cross sections through most papillae and taste buds (Fig. 1D).
Embedded circumvallate papillae and anterior tongue halves were frozen in hexane cooled by an acetone and solid carbon dioxide bath and stored at −80°C until used for sectioning. Serial sections were cut at 8 μm on a cryostat, mounted on gelatin-coated slides, and stained with hematoxylin and eosin for light microscopy.
Sections were viewed with a Leitz diaplan microscope for tracing with a drawing tube attachment and photomicroscopy. Photomicrographs were scanned from color slides, stored as digital images, and arranged in plates by transporting images to Adobe Photoshop (Adobe, Mountain View, CA).
In addition to sectioned tongues, four BDNF −/− and three +/+ tongues at P16 or P17 were dissected and placed briefly in methylene blue or green food dye to stain the dorsal surface for study of tongue topography (Table 1). In these tongues, fungiform papillae are easily distinguished because they stain more lightly than the surrounding, nongustatory filiform papillae that cover much of the tongue (Fig. 2). In addition, the dyes intensely stain the taste pore within the single taste bud of each fungiform papilla.
Fig. 2.
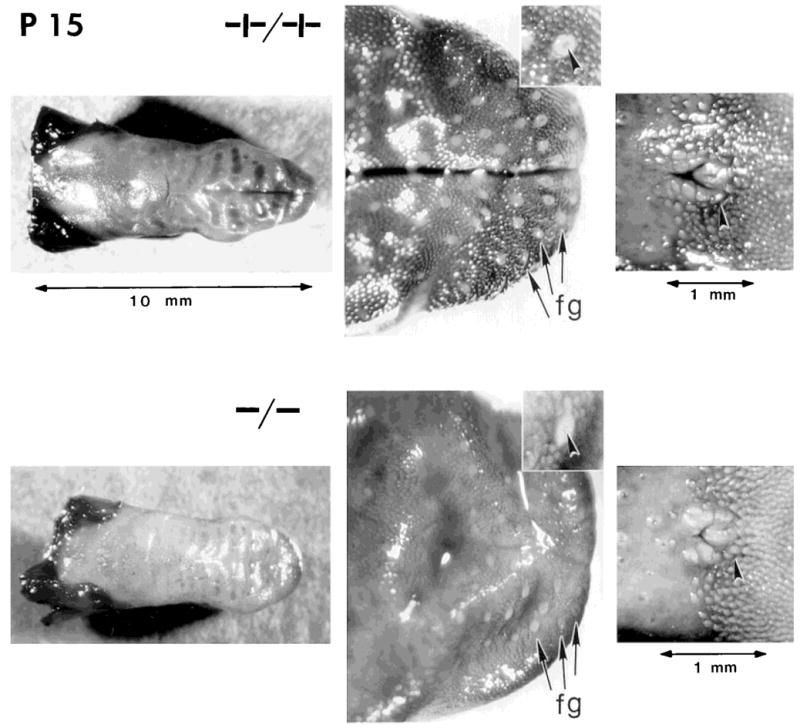
Whole tongues from postnatal day 15 (P15) wild-type (+/+) and null mutant (−/−) littermates, stained with dye to enhance visibility of fungiform papillae and taste pores and the circumvallate papilla. Tongues from +/+ and −/− mice were similar in general topography (left panels; scale at top applies to +/+ and −/− tongues). However, higher magnification of the anterior tongues in the middle panels illustrates the more numerous and distinctive fungiform papillae (fg) on +/+ tongues; in the center of each papilla, a stained taste pore is seen (arrowheads in insets). Panels on the right illustrate the circumvallate papilla (arrowheads) from +/+ and −/− tongues.
Fungiform papillae and taste buds
Measures were made of the number and size of fungiform papillae and those of associated taste buds. Measurements were made from either intact tongues or from serially sectioned tongue tissues by using the most appropriate preparation to obtain accurate data.
Papilla number was determined in intact, stained tongues examined under a dissecting microscope (Fig. 2). Each fungiform papilla was counted, and the location was marked on a drawing of the tongue. Counts were made on two separate occasions by the same investigator; the difference in counts averaged 7%. This method of surface observation is more accurate for counting papillae than using histological sections because the surface-stained tongue can be rotated by hand at all angles to view fungiform papillae on the tongue dorsum and edge. Each papilla is unambiguously identified, in contrast to sectioned tongues, where papillae on the lingual edge are cut at odd angles and cannot always be clearly identified.
Papilla size was determined from tracings of serial sections of anterior tongue halves at P15, P20, and P25. Each tongue section was traced by camera lucida, and then individual papillae were identified and labeled on tracings. The diameter of each papilla was measured at the widest point (Fig. 1D).
Taste bud number was determined from intact tongues after first establishing the validity of this method through comparisons of data from intact and sectioned tongues. The fungiform papilla on the rodent tongue (e.g., mouse, rat, gerbil, hamster) contains one taste bud in the apical papilla epithelium. In surface-stained tongues, each fungiform papilla was examined, and the presence or absence of a taste pore was recorded. In addition, in sectioned mouse tongues, the presence of a taste bud was microscopically determined and noted on the serial tracings of half tongues and papillae. Thus, we determined whether any observable papillae lacked a taste bud. In fact, no papillae were identified that did not have a taste bud. Taste bud size was assessed from serial sections of the anterior tongue. The diameter of each taste bud was measured at the widest point (Fig. 1D).
Circumvallate papilla and taste buds
The tongues of mouse and rat contain one circumvallate papilla, located in the midline of the posterior tongue, just in front of the border between the oral and pharyngeal tongue (Mistretta, 1991). The papilla contains a few hundred taste buds. Camera lucida tracings were made from serial sections of the circumvallate papilla. Papilla size was determined by measuring the papilla diameter at its widest point, from external wall to external wall (Fig. 1B). The length of papilla epithelium was determined by measuring both left and right trenches in the middle 50% of sections, where trench length is maximal, and averaging these values. The measures of width and length provide an indication of complete papilla size including the connective tissue core and the gustatory epithelium alone, respectively.
To quantify taste buds, every profile of a taste bud in each serial section of the circumvallate papilla was first counted (Mistretta and Baum, 1984). Because papillae were sectioned parallel to the tongue surface, taste buds were not cut at spurious angles in the curved papilla walls, as can occur in coronal or sagittal sections. Once a total count of all taste bud profiles was made for one papilla, then the average number of sections occupied by a single taste bud was determined. For this average, 10 individual taste buds were selected and traced to learn whether they occupied two, three, or four sections, for example. The mean number of these sections for the 10 taste buds was then divided into the total number of taste bud profiles by using the formula: total number of taste bud profiles in all sections/average number of profiles for ten single taste buds = total number of taste buds per circumvallate papilla (Mistretta and Baum, 1984). Taste bud diameter was measured from the serial tracings at the widest point for each bud.
Data analysis
We initially analyzed data in two age groups, P15 (ages P15–P17) and P20 (P20–P25). However, because of the relatively small number of animals at the oldest ages (Table 1), we pooled all ages and analyzed data for BDNF −/− and +/+ mice by using Student’s t-test (significant at P ≤ 0.05). None of the general conclusions was altered by pooling all data versus using two age groups. Although the overall number of animals is small for this study, quantification of fungiform papilla size and number and of taste bud size and number in both fungiform and circumvallate papillae includes hundreds of data points.
RESULTS
Tongue length and head size
Tongues from BDNF null mutant mice were similar in overall topography to those from wild-type littermates (Fig. 2). Tongue length differed between mutant and wild-type mice by only 17% (mean = 10.3 mm in +/+; 8.6 mm in −/−; Table 2). Head length averaged 16.1 mm in wild-type mice versus 13.7 mm in null mutants, a difference of 15%. These reductions of 17% and 15% in tongue and head lengths, respectively, presumably reflect the smaller body size of BDNF −/− mice. Neither difference is of sufficient magnitude to account for observed alterations in taste organs.
TABLE 2.
Differences in Gustatory Papilla and Taste Bud Size and Number in Mice Aged 15–25 Days Postnatal
| Mean (S.D.) [n]1 |
||||
|---|---|---|---|---|
| +/+ | −/− | % Reduction | P | |
| Head length (mm) | 16.1 (2.6) [6] | 13.7 (1.9) [9] | 15 | 0.03 |
| Tongue length (mm) | 10.3 (1.4) [6] | 8.6 (0.7) [9] | 17 | 0.03 |
| Fungiform papillae Number2 | 90 (27) [3] | 39 (10) [4] | 57 | 0.05 |
| Diameter (μm) | 81 (18) [142] | 54 (12) [55] | 33 | <0.001 |
| Number of taste buds3 | 90 (27) [3] | 39 (10) [4] | 57 | 0.05 |
| Taste bud diameter (μm) | 42 (6) [142] | 35 (8) [55] | 18 | <0.001 |
| Circumvallate papilla Diameter (μm) | 476 (18) [4] | 289 (24) [3] | 39 | 0.002 |
| Trench length (μm)4 | 503 (98) [8] | 301 (47) [6] | 40 | <0.001 |
| Number of taste buds | 197 (27) [4] | 53 (28) [3] | 73 | 0.002 |
| Taste bud diameter (μm) | 53 (11) [785] | 43 (9) [158] | 19 | <0.001 |
Data are presented as means (S.D.), with number of tongues or organs (papilla or taste bud) examined in brackets.
Data are based on counts in whole tongues.
Because each papilla that remained on tongues had a taste bud, taste bud counts are the same as papilla counts (see Materials and Methods).
Each circumvallate papilla had two trench walls; thus, data are based on eight +/+ and six −/− trenches.
Fungiform papillae and taste buds
Number of fungiform papillae and taste buds
All fungiform papillae were counted on surface-stained tongues from three wild-type and four null mutant mice at P16–P17 (Fig. 2). The papilla average was 90 in +/+ tongues versus 39 in −/− tongues (Table 2). This is a 57% reduction in total fungiform papillae on mutant tongues, which far exceeds the 17% reduction in tongue length. Because each fungiform papilla on mouse tongues has one taste bud in the papilla apex, as determined both from surface staining and serial histological sections, the papilla reduction means that taste buds on anterior tongue also were reduced by 57%. Each remaining papilla on −/− tongues had a stained taste pore in the top, indicating the presence of a taste bud.
Not only was the number of fungiform papillae substantially reduced on BDNF mutant tongues, there was also an apparent spatial distinction in the anterior tongue areas that sustained the greatest papilla loss. In Figure 3, maps of papilla distribution are presented for +/+ and −/− tongues. On null mutant tongues, the remaining fungiform papillae were primarily on the anteriormost tongue quadrant or tongue tip; fewer papillae were found laterally, and essentially no papillae were located at sides of the intermolar eminence. When this difference in papilla distribution is quantified, the average percentage of papillae on the anterior tongue quadrant is 57% in wild-type tongues versus 80% in the anterior quadrant of mutant tongues. Thus, on the remaining tongue, 43% of total papillae were distributed in wild-type mice versus 20% in null mutants. This difference in papilla distribution may reflect a selective loss in innervation in mutant tongues.
Fig. 3.
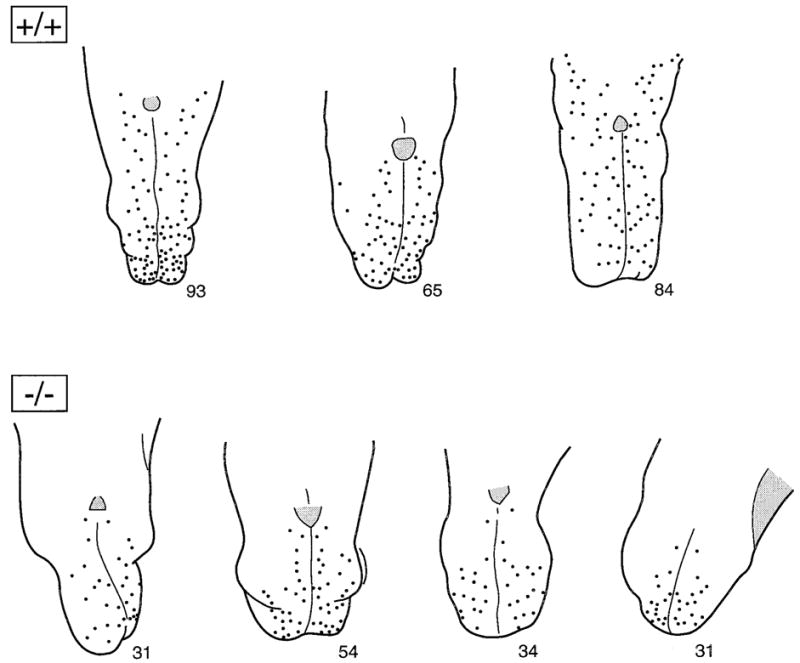
Maps of fungiform papilla counts on dorsal surfaces of three +/+ and four −/− tongues. Maps were drawn from stained tongues, illustrated in Figure 2. The total number of papillae is indicated at the tip of each tongue diagram. Not only was the number of papillae reduced on −/− tongues, but also the remaining papillae were concentrated in the anterior tongue quadrant. Numbers of papillae in this figure represent data from the second replication of papilla counts, whereas numbers of papillae in Table 2 are the average from two replications.
Size of fungiform papillae and taste buds
The maximum diameter of each fungiform papilla was determined from serial tracings of sagittal tongue sections (Fig. 1D). Papilla width averaged 81 μm in +/+ tongues versus 54 μm in −/− tongues (Table 2). This 33% reduction in BDNF mutant papilla diameter was reflected also in smaller taste bud diameter in null mutant versus wild-type mice. Taste buds averaged 42 μm in +/+ tongues versus 35 μm in −/− tongues, a reduction of 18%.
Histologic sections of mutant and wild-type papillae from P25 mouse tongues illustrate the smaller fungiform papilla diameter in BDNF −/− mice, apparently due to a reduced connective tissue core (Fig. 4). Tissue integrity is similar in mutant and wild-type papillae, and the depth of lingual epithelium appears similar. As another measure of papilla size, we measured papilla “height,” from the dorsal top of the papilla epithelium to the most ventral point. Papilla height was reduced only 6% in −/− tongues.
Fig. 4.
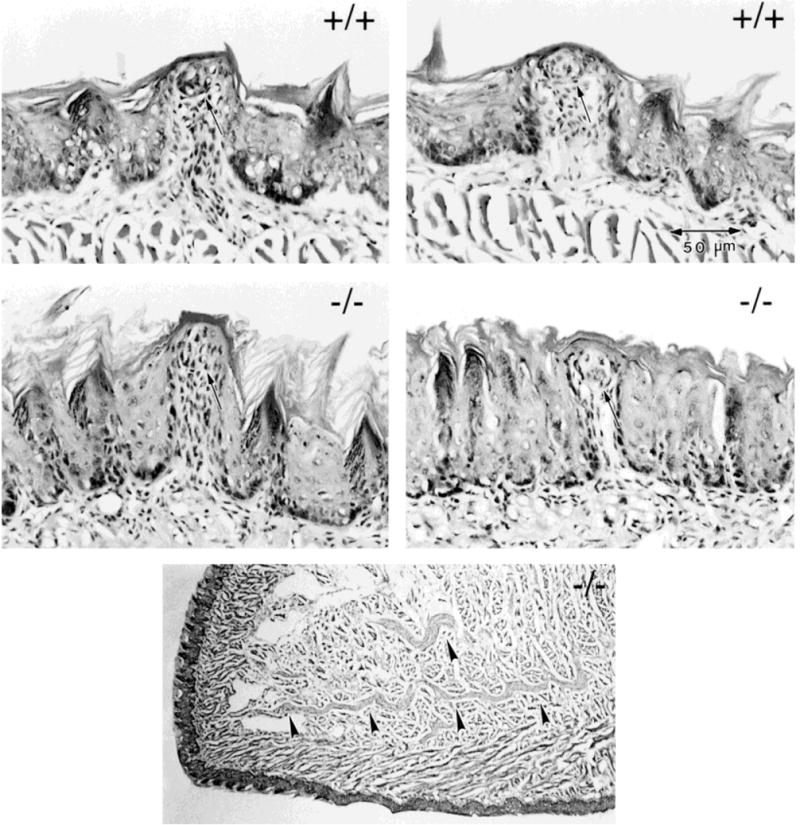
Photomicrographs of fungiform papillae from two +/+ and two −/− tongues. The bottom panel shows a low power micrograph of the anterior tongue from a −/− mouse. Fungiform papillae in null mutant tongues are smaller in average diameter than those in wild-type tongues. However, each papilla contains a taste bud, indicated with an arrow at each papilla apex. In the bottom micrograph of the anterior tongue, arrowheads indicate large bundles of the combined lingual/chorda tympani nerve. Scale bar applies to all four papillae.
In each fungiform papilla in normal adult mouse tongue, there is a single taste bud, centered in the apical epithelium of the papilla. We found a taste bud in each identified fungiform papilla in histologic sections, whether in +/+ or −/− mice, thereby corroborating our observations of surface-stained tongues. Thus, even the smaller papillae in mutant tongues were able to support a taste bud (Fig. 4).
Although papillae and taste buds were reduced in number and size on anterior tongues of −/− mice, tongues maintained some innervation. In the low power micrograph shown in Figure 4, large bundles of the combined chorda tympani/lingual nerve are apparent in the anterior tongue quadrant.
In summary, measures of fungiform papillae and taste buds indicate a reduction in papilla number by 57% in BDNF null mutant tongues and a reduction in size of remaining papilla by 33%. Papilla loss was greatest posterior to the tongue tip. Because each papilla typically contains a single taste bud, the number of taste buds also was reduced by 57%. A taste bud was found in the apex of each remaining papilla in mutant tongues; however, taste buds in −/− tongues were reduced in diameter by 18%. These data demonstrate profound alterations in anterior tongue taste organs in the absence of BDNF.
Circumvallate papilla and taste buds
Circumvallate papilla size
There is a single circumvallate papilla in the center of the posterior mouse tongue, just anterior to the border of oral and pharyngeal tongue (Fig. 1A). By surface inspection, we observed a circumvallate papilla on the posterior tongue of each +/+ or −/− tongue examined (Fig. 2). However, after histologic preparation and light microscopic examination, it was apparent that the papilla on BDNF −/− tongues was much smaller and lacked morphological integrity (Fig. 5). A measure of total papilla width (Fig. 1B) indicted that +/+ papillae averaged 476 μm versus 289 μm in BDNF −/− tongues (Table 2). This is a reduction of 39% in mutant papilla size. To quantify gustatory epithelium per se, that is, the trench wall epithelium in which taste buds are located, we measured maximum left and right trench lengths (Fig. 1B). Average length in +/+ circumvallate papilla was 503 μm versus 301 μm in −/− papilla, a 40% reduction in papilla epithelium.
Fig. 5.
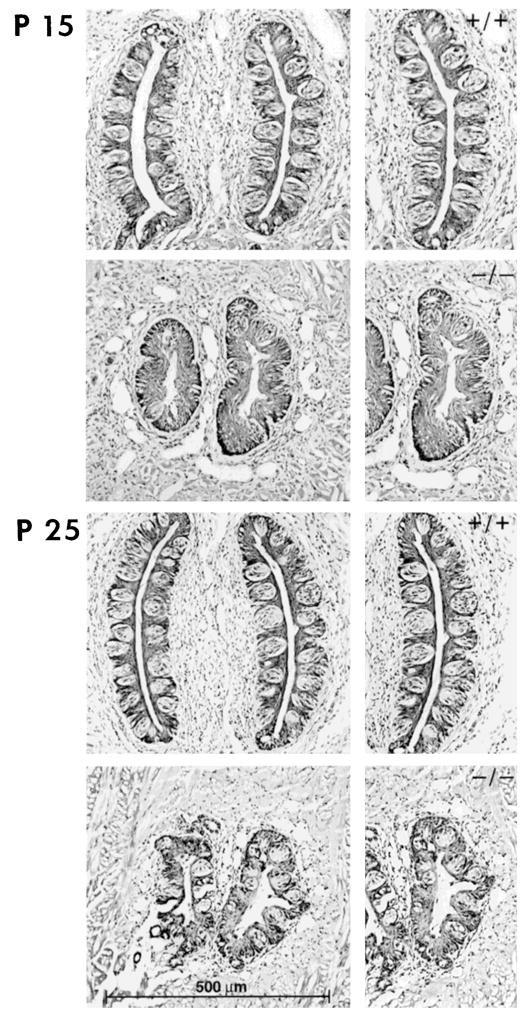
Photomicrographs of the circumvallate papilla and taste buds from +/+ and −/− tongues at postnatal day (P) 15 (top) and P25 (bottom). Compared with wild-type tongues, the circumvallate papilla in null mutants was smaller in diameter and length and lacked morphological integrity. Taste buds remained in −/− papillae but were much reduced in number. Scale bar refers to all micrographs in that column.
Number and size of circumvallate taste buds
Not only was the circumvallate papilla markedly reduced in size in BDNF −/− tongues, but taste buds also were much reduced in number (Fig. 5). There was an average of 197 taste buds in +/+ papillae versus only 53 in −/− circumvallate papillae (Table 2). This reduction of about 73% exceeds what might be predicted from the reduction in papilla size. Also, it is clear from Figure 5 that gustatory epithelium was available in −/− papillae but that taste buds were not present to occupy all potential epithelial space; this contrasts with +/+ papillae. Taste buds that remained in BDNF mutant papillae were reduced in width by 19% (mean = 53 μm ± 11 for +/+, 43 μm ± 9 for −/−).
DISCUSSION
In BDNF null mutant mice, we observed substantial reductions in gustatory papilla and taste bud size and number in addition to alterations in papilla form and distribution compared with those of wild-type animals. The sensory ganglia that innervate the tongue also are reduced in BDNF mutant mice; therefore, the results demonstrate the essential nature of sensory innervation for taste organ development. Because the gustatory papillae are the sole taste bud-bearing structures on the mammalian tongue, taste bud and papilla development are sometimes regarded as one process. However, papilla and taste bud induction and subsequent differentiation are not coincident temporal events, and it is quite likely that different regulatory mechanisms are involved in the development of papilla and taste bud (Mistretta, 1998). Therefore, in this discussion, we use separate sections to consider gustatory papillae and taste buds.
Taste papillae depend on BDNF-supported innervation for development
Whereas there are substantial data to demonstrate a role for the neurotrophin family of secreted proteins in regulating development of various sensory systems (e.g., Ibanez et al., 1993; Ernfors et al., 1995; Schimmang et al., 1995; Farinas et al., 1996; Ninkina et al., 1996), knowledge about neurotrophins in development of taste is not extensive. We report that BDNF null mutant mice have severely compromised taste organs on the anterior and posterior tongue compared with their wild-type littermates. Numbers of fungiform papillae in −/− tongues are reduced by about 60%, and papilla loss is greatest on the lateral tongue and caudal to the tongue tip. Remaining papillae are about one-third smaller in diameter than those on wild-type tongues. The single circumvallate papilla on the posterior tongue also is reduced in size, by about 40% in −/− mice.
Available data on the taste system of another BDNF −/− mouse strain have indicated that fungiform papillae are reduced in number by about 35% and that the remaining papillae are “smaller” in these knockout animals (Nosrat et al., 1997). The circumvallate papilla has a “distorted” morphology (Nosrat et al., 1997). Circumvallate papilla width in P0–P12 mutant tongues is reduced in size by 31% on average (Oakley et al., 1998) compared with wild-type mice. Thus, in combination, these two studies also demonstrate smaller numbers and size of fungiform and circumvallate papillae in BDNF −/− mice, and the extent of reported reductions compares closely with that reported in the present study for both papilla types.
Our quantitative observations of alterations in the peripheral taste organs in BDNF −/− mice correlate with the 40–45% reductions in volume of geniculate and petrosal/nodose ganglia (Jones et al., 1994). In contrast, the trigeminal ganglion, which innervates fungiform papilla and lingual epithelium but not taste buds themselves, is reduced by about 15% in these BDNF −/− mice. Furthermore, the nontaste, superior, and jugular ganglia of cranial nerves IX and X are not reduced in these animals. Thus, ganglia that innervate taste organs are especially dependent on BDNF.
Because the taste papillae on mammalian tongue will form in the absence of an intact sensory innervation (Mbiene et al., 1997), the reduced papilla size and number in BDNF −/− mice presumably result from prolonged nerve depletion during developmental stages of morphogenesis. In other words, the gustatory papillae should have formed initially in mutant and in wild-type tongues but, once formed, apparently depend on innervation to sustain number, size, and shape. Thus, one might predict intact numbers and size of early papillae in embryonic null mutant tongues when fungiform and circumvallate papillae first appear at gestational days 13–14 in mouse (Kaufman, 1992).
It is interesting that fungiform papilla loss on BDNF −/− tongues is not random among the approximate 100 papilla that would typically develop. Papilla numbers remain high on the anterior tongue but are lost disproportionately on more posterior and lateral locations. A similar pattern in papilla loss has been noted in another BDNF −/− mouse strain (Nosrat et al., 1997). This finding suggests a selective loss in tongue innervation, discussed in subsequent paragraphs.
Taste buds depend on BDNF-supported innervation for development
Not only are gustatory papillae smaller in BDNF −/− tongues (≈33% reduction in size of fungiform papilla, 40% in circumvallate), but numbers of taste buds also are reduced by ≈60% (in fungiform) to 75% (circumvallate). This taste bud loss exceeds what might be predicted from the magnitude of papilla reduction. Similarly, in another BDNF −/− strain (Ernfors et al., 1994) at P0–P12, Oakley et al. (1998) reported that BDNF −/− circumvallate papillae have an average 83% reduction in numbers of taste buds versus that in wild-type mice.
On the mammalian tongue, taste buds reside only in the gustatory papillae, not in filiform papillae or interpapilla regions (Mistretta, 1991, 1998; Mistretta and Hill, 1995). Nor do taste buds develop or regenerate in extrapapilla tongue epithelium. Thus, papilla epithelium is essential for, and constrains, lingual taste bud differentiation. The small fungiform papillae that remain on BDNF −/− tongues are of sufficient diameter to sustain the single apical taste bud. However, these taste buds are also reduced in size. Presumably an interaction between the reduced innervation and extent of gustatory papilla epithelium in −/− tongues results in smaller taste buds.
In recent studies of rat taste receptive fields, a direct quantitative relation between ganglion cells and taste bud size was demonstrated (Krimm and Hill, 1998). On average, a single taste bud in a fungiform papilla is innervated by several ganglion cells. Furthermore, larger taste buds are innervated by more ganglion cells than are smaller taste buds. Thus, taste buds and their innervation are in a direct quantitative relation in adult tongue receptive fields. This quantitative relation emerges gradually during the first month of postnatal life in rat. The loss of taste ganglion cells in BDNF −/− mice would, therefore, predictably contribute to smaller taste bud size, as we have observed.
Surviving ganglion cells do not rescue lost taste organs on −/− tongues
Our results and previous data on ganglion size (Jones et al., 1994) indicate that the peripheral taste system depends on BDNF-supported innervation for the development of appropriate morphology, size, numbers, and/or spatial pattern of papillae and taste buds. Because sensory ganglia are reduced in neuronal complement, available innervation for peripheral target organs is reduced. In turn, taste papillae and taste buds are altered in a direct quantitative relation, demonstrating neural dependence of developing taste papillae and resident taste buds on gustatory nerves.
We did not assess the extent of nerves within the remaining taste papillae on tongues of BDNF −/− mice. However, other investigators have reported far fewer nerve fibers in papillae of null mutant versus wild-type mice (Nosrat et al., 1997; Oakley et al., 1998); even fewer fibers were observed in mutant mice older than P20 (Nosrat et al., 1997). Number of taste buds and density of taste bud innervation were linearly correlated in circumvallate papilla of null mutants (Oakley et al., 1998). In contrast, the filiform papillae and lingual epithelium are well innervated in mutant mice (see Fig. 5), presumably via trigeminal fibers that usually innervate nontaste tongue tissue.
Although gustatory ganglia are not totally lost in BDNF −/− mice, it is clear that the remaining geniculate and petrosal ganglion cells do not substitute for lost neurons and provide “complete” innervation for those fungiform and circumvallate papillae and resident taste buds that remain on the tongues of mutant mice. Furthermore, surviving geniculate ganglion cells do not substitute for lost neurons to sustain a normal number and pattern of fungiform papillae on −/− tongues. Nor does the trigeminal ganglion, which is much less affected in BDNF −/− mice (Jones et al., 1994), substitute for reduced innervation in the fungiform gustatory papillae on the anterior tongue. However, the trigeminal ganglion apparently does sustain general tongue innervation on the −/− tongues.
The lack of nerve rearrangement in −/− tongues to substitute for depleted ganglion cells and rescue taste organs contrasts with data on spiral ganglion cells in cochlea of neurotrophin 3 (NT-3) null mutant mice (Fritzsch et al., 1997a). In the NT-3 mutants, neuronal loss is not homogeneous along the cochlea. For example, radial fibers do not project to cochlear epithelium in the basal turn. However, some of the fibers that project toward the middle cochlea redistribute to epithelium in the basal turn in new bundles that adopt unique trajectories.
Comparable plasticity also might be expected in the taste system, especially given reports of neural rearrangment during receptive field development in pre- and postnatal sheep (Mistretta et al., 1988; Nagai et al., 1988). However, in embryonic rat, nerves innervating tongue and taste organs maintain discrete territories during periods of formation and morphogenesis of taste papillae and do not project throughout tongue tissues in a homogeneous manner, which requires subsequent retraction to densely innervate papillae (Mbiene and Mistretta, 1997). Rather, it appears that taste papillae, once induced via nonneural mechanisms, may actually attract nerves that subsequently innervate taste buds. This line of reasoning leads to the suggestion that lack of BDNF also has nonneuronal effects on the taste system; such effects may alter presumptive papilla epithelium and mesenchyme so that not all fungiform papillae form and the circumvallate papilla never develops its normal size. Study of embryonic BDNF −/− tongues could help to resolve these propositions.
Selective effects and source of BDNF
The data from null mutant mice demonstrate selective effects of BDNF on development of taste ganglia and in turn on the taste receptor organs. Our laboratory also has evidence for a direct effect of exogenous BDNF and another member of the neurotrophin family of secreted proteins, neurotrophin-4 (NT-4), on neurite outgrowth in cultured geniculate ganglion cells from rat embryo; nerve growth factor (NGF) and NT-3 support only very limited neurite extension in the explants (Rao et al., 1997; work in progress). In contrast, NGF and NT-3 support extensive neurite growth in cultured trigeminal ganglion, whereas BDNF is less effective. These data reaffirm that BDNF supports development of taste ganglion cells in a selective manner.
Because BDNF mRNA is expressed in fungiform and circumvallate papillae during embryonic development (Nosrat and Olson, 1995), a target-derived source of this neurotrophin is available for nerves growing into the tongue. In contrast, NT-3 is expressed in surrounding epithelial structures but not in taste papillae or buds, leading to the conclusion that BDNF and NT-3 have distinct but complementary roles in supporting development of gustatory and somatosensory innervation of the tongue (Nosrat et al., 1996). Furthermore, study of null mutant mice also supports the proposal that BDNF and NT-3 support gustatory and somatosensory innervation of the tongue, respectively, without overlap (Nosrat et al., 1997).
Although our observations have focused on innervation of peripheral taste organs, during a period well after ganglion cells initially grow out to embryonic tongue, ganglion cell loss presumably occurs earlier. Geniculate ganglion cells have been identified at 12 days of gestation in mouse (Fritzsch et al., 1997b), whereas tongue formation has been reported to begin at gestational day 11 (Kaufman, 1992), and the first fibers have been reported to reach the tongue at 12.5 days of gestation (Fritzsch et al., 1997b). Therefore, the ganglion cells are produced in advance of tongue innervation and would not be dependent on tongue factors at this stage. Other studies of mutant mice have indicated that BDNF does not affect formation of cranial ganglia or extension of nerve fibers to peripheral targets (Ernfors et al., 1994). Rather, effects apparently occur later and alter the ability of particular neuron subsets to make neural connections and maintain innervation of target cells.
BDNF mRNA expression in embryonic mouse geniculate ganglion suggests the potential for autocrine or paracrine regulation of neuron survival via BDNF (Schecterson and Bothwell, 1992) and the presence of trkB mRNA in rat geniculate ganglion at gestational day 12 (Pirvola et al., 1994) indicates early expression of BDNF receptors. Data from trkB null mutant mice has demonstrated a reduction in geniculate ganglion cells to 35% of that in wild-type animals as early as 12.5 days of gestation and a reduction to 10% of control by 13.5 days of gestation (Fritzsch et al., 1997b). Quantitative cell counts in geniculate and petrosal ganglia in embryonic BDNF mutant and wild-type mice will be necessary for a better understanding of BDNF effects on gustatory ganglia.
What taste ganglion cells remain in BDNF −/− mice and what is their role?
In BDNF −/− mice, about 40–60% of geniculate and petrosal ganglion cells are lost by the time of postnatal assessment (Jones et al., 1994; Liu et al., 1995), which indicates that half of the taste ganglion cells may not depend on BDNF for acquisition and maintenance. Because both BDNF and NT-4 are ligands for the same tryrosine kinase receptor, trkB, NT-4 could act to rescue ganglion cells that are BDNF dependent. However, in NT-4 null mutant mice, 50–60% of geniculate ganglion and petrosal cells are lost, and in BDNF −/− and NT-4 −/− double mutants, only 6–10% of the ganglian cells remain (Liu et al., 1995). This additive effect on taste ganglia suggests that BDNF and NT-4 act on separate populations of neurons.
NT-3 also has an apparent role in sustaining geniculate and petrosal ganglion cells because these ganglia have been reported to be reduced by about 45–50% in NT-3 −/− mice (Liebl et al., 1997). In BDNF/NT-3 double knockout mice, about 35% of geniculate and petrosal ganglia remain (Liebl et al., 1997). Considered with data on NT-4 and BDNF/NT-4 mutants, this again suggests an important role for NT-4 in gustatory ganglion support.
In the geniculate ganglion, neurons innervate not only taste papillae and buds on the anterior tongue via the chorda tympani nerve but also taste buds in the mucosa of the soft palate via the greater superficial nerve. These presumably are separate populations of sensory ganglion cells that may depend on separate neurotrophins for support, but data are not available to address this issue.
Although a general picture of taste organ dependence on BDNF has been presented in much of the present paper, in fact the data reflect the complexity of the taste system itself. For example, fungiform papilla loss in BDNF −/− tongues is not homogeneously or randomly distributed, but rather is localized. Fungiform papillae are lost primarily from the more caudal and lateral regions of the anterior tongue (Fig. 3), so one can propose distinct populations of geniculate ganglion cells that innervate taste organs on the anterior tongue tip as opposed to more caudal and lateral tongue regions. Miller and Preslar (1975) demonstrated that in rat more than 50% of total fungiform papillae are located on the tongue tip. Specific branches of the chorda tympani nerve distribute to various tongue regions (Miller et al., 1978), and two of these provide principal innervation for papillae on the tongue tip. The chorda tympani branches may derive from subsets of geniculate ganglion cells that differ in their dependence on specific neurotrophins.
Not only the geniculate ganglion cell population but also the petrosal ganglion has multiple sensory targets. Petrosal cells contribute via the lingual branch of the glossopharyngeal nerve to innervate the circumvallate papilla and taste buds, the posterior folds of the foliate papillae and taste buds, and nongustatory posterior tongue epithelium (Mistretta, 1991). In addition, an estimated 18% of the petrosal population innervates carotid body receptors (Katz and Black, 1986).
Based on the hetrogeneous ganglion cell populations, it is not surprising that multiple neurotrophins have been suggested as participants in gustatory neuron survival (Fritzsch et al., 1997b; Nosrat et al., 1997; Oakley et al., 1998). Clearly, however, BDNF is a major factor in the development of the taste system.
Acknowledgments
National Institute on Deafness and Other Communication Disorders NIH; Grant number: DC00456; Grant sponsor: National Institute of Mental Health; Grant number: MH48200; Grant sponsor: Howard Hughes Medical Institute.
This research was supported in part by grants from the NIH (DC00456 to C.M.M.), the National Institute of Mental Health (MH48200 to L.F.R.), and the Howard Hughes Medical Institute (L.F.R.). I.F. was a recipient of a long-term fellowship from the Human Frontier Science Organization. L.F.R. is an investigator of the Howard Hughes Medical Institute.
LITERATURE CITED
- Barlow LA, Chien C-B, Northcutt RG. Embryonic taste buds develop in the absence of innervation. Development. 1996;122:1103–1111. doi: 10.1242/dev.122.4.1103. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee K-F, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Electron microscope study of the developing taste bud in the rat fungiform papilla. Dev Biol. 1965;11:110–135. doi: 10.1016/0012-1606(65)90040-0. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Mbiene J-P. Early development and innervation of taste bud–bearing papillae on the rat tongue. J Comp Neurol. 1991;304:172–186. doi: 10.1002/cne.903040203. [DOI] [PubMed] [Google Scholar]
- Farinas I, Yoshida CK, Backus C, Reichardt LF. Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron. 1996;17:1065–1078. doi: 10.1016/s0896-6273(00)80240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997a;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int J Dev Neurosci. 1997b;15:563–576. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Hosley MA, Hughes SE, Oakley B. Neural induction of taste buds. J Comp Neurol. 1987;260:224–232. doi: 10.1002/cne.902600206. [DOI] [PubMed] [Google Scholar]
- Ibanez CF, Ernfors P, Tommusk T, Ip NY, Arenas E, Yancopoulos GD, Persson H. Neurotrophin-4 is a target-derived neurotrophic factor for neurons of the trigeminal ganglion. Development. 1993;117:1345–1353. doi: 10.1242/dev.117.4.1345. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DM, Black IB. Expression and regulation of tyrosine catechol-aminergic traits in primary sensory neurons: relationship to target innervation in vivo. J Neurosci. 1986;6:983–989. doi: 10.1523/JNEUROSCI.06-04-00983.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH. The atlas of mouse development. San Diego: Academic Press; 1992. p. 512. [Google Scholar]
- Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol. 1998;398:13–24. [PubMed] [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Mbiene J-P, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: nerves follow precise and spatially restricted pathways. Acta Anat. 1997;160:139–158. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- Mbiene J-P, MacCallum D, Mistretta CM. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J Comp Neurol. 1997;377:324–340. doi: 10.1002/(sici)1096-9861(19970120)377:3<324::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Miller IJ. Branched chorda tympani neurons and interactions among taste receptors. J Comp Neurol. 1974;158:155–165. doi: 10.1002/cne.901580204. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Jr, Preslar AJ. Spatial distribution of fungiform papillae. Anat Rec. 1975;181:679–684. doi: 10.1002/ar.1091810309. [DOI] [PubMed] [Google Scholar]
- Miller IJ, Jr, Gomez MM, Lubarsky EH. Distribution of the facial nerve to taste receptors in the rat. Chem Senses Flavour. 1978;3:397–411. [Google Scholar]
- Mistretta CM. Developmental neurobiology of the taste system. In: Getchell TV, Doty RL, Bartoshuk LM, Snow Jb, editors. Smell and taste in health and disease. New York: Raven Press; 1991. pp. 35–64. [Google Scholar]
- Mistretta CM. Symposium on the role of innervation in induction and differentiation of taste organs: introduction and background. Ann NY Acad Sci. 1998;855:1–13. doi: 10.1111/j.1749-6632.1998.tb10542.x. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Baum BJ. Quantitative study of taste buds in fungiform and circumvallate papillae of young and aged rats. J Anat. 1984;138:323–332. [PMC free article] [PubMed] [Google Scholar]
- Mistretta CM, Hill DL. Development of the taste system. Basic neurobiology. In: Doty RL, editor. Handbook of olfaction and gustation. New York: Marcel Dekker; 1995. pp. 635–668. [Google Scholar]
- Mistretta CM, Gurkan S, Bradley RM. Morphology of chorda tympani receptive fields and proposed neural rearrangements during development. J Neurosci. 1988;8:73–78. doi: 10.1523/JNEUROSCI.08-01-00073.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistretta CM, Goosens K, Farinas I, Reichardt LF. BDNF deletion alters gustatory papilla and taste bud size and number in postnatal mouse. Soc Neurosci Abstr. 1996;22:991. [Google Scholar]
- Nagai T, Mistretta CM, Bradley RM. Developmental decrease in size of peripheral receptive fields of single chorda tympani nerve fibers and relation to increasing NaCl taste sensitivity. J Neurosci. 1988;8:64–72. doi: 10.1523/JNEUROSCI.08-01-00064.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagato T, Matsumoto K, Tanioka H, Kodama J, Toh H. Effect of denervation on morphogenesis of the rat fungiform papilla. Acta Anat. 1995;153:301–309. doi: 10.1159/000147739. [DOI] [PubMed] [Google Scholar]
- Ninkina N, Adu J, Fischer A, Pinon LGP, Buchman VL, Davies AM. Expression and function of TrkB variants in developing sensory neurons. EMBO J. 1996;15:6385–6393. [PMC free article] [PubMed] [Google Scholar]
- Nosrat CA, Olson L. Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. J Comp Neurol. 1995;360:698–704. doi: 10.1002/cne.903600413. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Ebendal T, Olson L. Differential expression of brain-derived neurotophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. J Comp Neurol. 1996;376:587–602. doi: 10.1002/(SICI)1096-9861(19961223)376:4<587::AID-CNE7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Oakley B, Brandemihl A, Cooper D, Lau D, Lawton A, Zhang C. The morphogenesis of mouse vallate gustatory epithelium and taste buds requires BDNF-dependent taste neurons. Dev Brain Res. 1998;105:85–96. [PubMed] [Google Scholar]
- Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994;75:131–144. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Rao H, Xu Z, MacCallum DK, Mistretta CM. BDNF and NGF differ in promoting neurite outgrowth from cultured embryonic rat geniculate and trigeminal ganglia. Chem Senses. 1997;22:775. [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Minichiello L, Vazquez E, San Jose I, Giraldez F, Klein R, Represa J. Developing inner ear sensory neurons require TrkB and TrkC receptors for innervation of their peripheral targets. Development. 1995;121:3381–3391. doi: 10.1242/dev.121.10.3381. [DOI] [PubMed] [Google Scholar]


