SUMMARY
Cell death in the developing retina is regulated, but so far little is known about what factors regulate the cell death. Several neurotrophic factors and receptors, including the neurotrophins and Trk receptors, are expressed during the critical time. We have studied the developing avian retina with respect to the role of nerve growth factor (NGF) in these processes. Our starting point for the work was that NGF and its receptor TrkA are expressed in a partially overlapping pattern in the inner nuclear layer of the developing retina. Our results show that TrkA and NGF-expressing cells are postmitotic. The first NGF-expressing cells were found on the vitreal side of the central region of E5.5–E6 retina. This pattern changed and NGF-expressing cells identified as horizontal cells were later confined to the external inner nuclear layer. We show that these horizontal cells co-express TrkA and NGF, unlike a subpopulation of amacrine cells that only expresses TrkA. In contrast to the horizontal cells, which survive, the majority of the TrkA-expressing amacrine cells die during a period of cell death in the inner nuclear layer. Intraocular injections of NGF protein rescued the dying amacrine cells and injection of antisense oligonucleotides for NGF that block its synthesis, caused death among the TrkA-expressing horizontal cells, which normally would survive. Our results suggest that NGF supports the survival of TrkA expressing avian horizontal cells in an autocrine mode of action in the retina of E10-E12 chicks. The cells co-express TrkA and NGF and the role for NGF is to maintain the TrkA-expressing horizontal cells. The TrkA-expressing amacrine cells are not supported by NGF and subsequently die. In addition to the effect on survival, our results suggest that NGF plays a role in horizontal cell plasticity.
Keywords: Retina, Cell death, Horizontal cell, Amacrine cell, NGF, TrkA, Chick, BrdU
INTRODUCTION
Cells that undergo naturally occurring death has been found in the early and late developing retina (Hughes and McLoon, 1979; Rager and Rager, 1978; Ulshafer and Clavert, 1979). Early cell death in chick embryo occurs during formation of the optic cup (Cuadros and Rios, 1988; Martín-Partido et al., 1988) and late death occurs after the time at which retinal neurones have started to differentiate (Cook et al., 1998). Cells that die during the early period (embryonic day (E)2–E4.5) are proliferating (Diaz et al., 1999), and are located in defined regions of the neuroepithelium that are associated with bending and fusion of the lips of the coroid fissure. The later period (E8–E16) starts in the central retina and proceeds towards the peripheral parts following the general pattern of retinal development (Prada et al., 1991). The distribution of dying cells is layer specific and the different classes of retinal neurones die during specific and restricted times of this period (Cook et al., 1998). The specific timecourses and patterns show that the extent of death and survival is strictly regulated.
Several neurotrophic factors and receptors including the neurotrophins and Trk receptors (Lewin and Barde, 1996) are expressed in specific temporal and cellular patterns in the retina during this period (Hallböök et al., 1996; von Bartheld, 1998). The nerve growth factor (NGF) receptors TrkA and p75 are expressed in the developing retina: TrkA is expressed by horizontal and amacrine cells (Karlsson et al., 1998), whereas p75 is expressed by several cell types in the early and late retina (Hallböök et al., 1990; Karlsson et al., 1998; Large et al., 1989; von Bartheld et al., 1991). Frade et al. have suggested that NGF can induce cell death via p75 during the early cell death. NGF was suggested to be synthesised by microglia cells that migrate to the early retina and present NGF to neurones that die (Frade and Barde, 1998; Frade and Barde, 1999; Frade et al., 1996). The cells that die do not express TrkA receptors. We have previously shown that NGF mRNA levels increase after E6, and that the expression is located in the external part of the inner nuclear layer (INL) at E18 (Hallböök et al., 1996). The increase of NGF expression is coincident with the late cell death, and the localisation of NGF synthesis corresponds to persisting TrkA-expressing horizontal cells. This pattern suggests that the NGF synthesis supports the survival of the remaining TrkA-expressing horizontal cells.
In this work we have experimentally tested this hypothesis by blocking the endogenous synthesis of NGF. We have characterised the cellular origin of NGF and TrkA expression, and have studied whether the start of expression precedes or follows neuronal differentiation.
MATERIALS AND METHODS
Embryos and embryo culture
Fertilised White Leghorn eggs were incubated in a humidified incubator and embryos were staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1951). Retinas from embryos older than E6 were dissected, whereas younger specimens were analysed as total embryos. Embryos for injection (see below) were treated as petri dish cultures or were injected in ovo (Selleck, 1996). For the embryo cultures, fertilised eggs incubated for 2.5 days were cracked open aseptically and poured into sterile high wall petri dishes. The cultures were kept at 37°C in a humidified incubator. The development of embryos in cultures can be slower than that of embryos developing in ovo, so they were staged just before injections and analyses. The stages were back translated to embryonic days (as indicated in the text).
In situ hybridisation analysis
Nonradioactive in situ hybridisation was performed on dissected whole retina. The procedure was essentially performed as previously described (Nieto et al., 1996; Wilkinson, 1992). The probes were digoxigenin- (DIG) and fluorescein-labelled antisense or sense riboprobes corresponding to nucleotides 1009–1440 of the chicken TrkA receptor (Bäckström et al., 1996), nucleotides 98–1054 of the chicken prepro-NGF gene (Ebendal et al., 1986) and nucleotides 1266–2321 of the genomic sequence of chicken Brn3a (Lindeberg et al., 1997). The TrkA probe spanned trans- and juxtamembrane regions. Tissue was fixed for 2–3 hours in 4% paraformaldehyde in PBS (PFA), bleached in 3% H2O2 and treated with 20 μg/ml proteinase-K for 20 minutes. Retinas were then hybridised for 40 hours at 65–70°C. Probe concentration was approximately 1 μg/ml for each probe. Alkaline phosphatase (AP)-conjugated anti-DIG and anti-fluorescein antibodies were used to detect the probes. The AP substrates nitroblue tetrazolium chloride (NBT, blue colour)/5-bromo-4-chloro-3-indolyl-phosphate, 4-toluidine salt (BCIP) and 2-[4-iodophenyl]-3-[4-nitrophenyl]-5-phenyl tetrazolium chloride (INT, red colour)/BCIP (Boehringer-Mannheim) were used and the tissue was incubated for 1 to 4 days until desired reaction product intensity was achieved. When using a single probe, NBT/BCIP was preferred and retinas were fixed before embedded in paraffin wax and sectioned in a microtome. When two probes were hybridised, NBT/BCIP was used with the first probe (DIG) and INT/BCIP with the second (fluorescein). The AP activity of the DIG-antibody was blocked with 0.1 M glycine-HCl pH 2.2, 0.1% Tween-20 for 10 minutes before addition of the AP-fluorescein antibody. The reaction product from INT/BCIP is not stable in organic solvents and therefore the retinas were fixed, cryoprotected in 15–30% sucrose, mounted in Tissue-Tek (Sakura, Tokyo, Japan) and cut using a cryostat (14 μm). These sections were mounted in PBS before viewing in bright-field illumination microscopy.
BrdU incorporation in combination with in situ hybridisation and immunohistochemistry analyses
Incorporation of 5-bromo-2′-deoxy-uridine (BrdU), was used to visualise cells passing the S-phase of the cell cycle and was combined with in situ hybridisation for NGF mRNA. 100 μl of 12 mg/ml BrdU (Sigma, St Louis, MO) was injected into the yolk sac of an embryo in culture or in ovo. The shortest incubation time for effective and reproducible BrdU incorporation after injection was titrated and found to be 12 hours. After BrdU incorporation, E6, E6.5, E7 and E9 retinas were dissected, fixed in PFA and processed for in situ hybridisation. After colour reaction with NBT/BCIP and fixation, the retinas were cryoprotected, cut using a cryostat and processed for BrdU immunohistochemistry using a monoclonal anti-BrdU antibody (B-2531, Sigma) diluted 1:500 in PBS; 3% of Triton-X100; 1% BSA and 5% horse serum. The sections were incubated with a horseradish peroxidase-conjugated anti-mouse IgG (A-9309, Sigma) diluted 1/200 in PBS. To visualise the secondary antibody, we used the peroxidase-3–3′-diaminobenzidine reaction according to the manufacturer’s instructions (Vector Labs, Burlingame, CA). The NBT/BCIP reaction precipitate was stable throughout the latter steps.
Retinas from BrdU-injected E5-E9 embryos were dissected, fixed in PFA, cryo-protected and sectioned using a cryostat. The sections were processed for BrdU and TrkA double immunohistochemistry. A polyclonal 1μg/ml anti-TrkA antibody (Lefcort et al., 1996; Oakley et al., 1997) and the monoclonal BrdU antibody were diluted in PBS, 0.3% Triton X-100 and 5% normal serum to detect TrkA and BrdU. Secondary antibodies conjugated to fluorescein isothiocyanate or Texas Red (Vector, Burlingame, CA) were diluted 1/100 and detected by epifluorescence microscopy.
TUNEL analysis and TrkA immunohistochemistry
Normal retinas and retinas from injected embryos were fixed with PFA, cryoprotected with 30% sucrose, frozen and cut in a cryostat. Sections parallel to the optic axis, were processed for terminal deoxynucleotidyl transferase TdT dUTP nick end labelling (TUNEL) according to the manufacturer’s recommendations (Apoptosis detection system, Promega, Madison WI). The anti-TrkA antibody was diluted 1/500 in equilibration buffer and was added during the incubation with TdT transferase. Epifluorescence using Texas Red- and fluorescein isothiocyanate-conjugated secondary antibodies, as well as the diaminobenzidine reaction was used to detect the TrkA antibody. TUNEL-positive cells and TrkA-immunoreactive cells were visualised directly with epifluorescence microscopy.
Intraocular injections of NGF protein and antisense oligonucleotides
Intravitreal injections of NGF protein or phosphorothioate oligodeoxynucleotides (PONs) were made into chicken embryo petri dish cultures (Selleck, 1996) or in ovo using a fine Hamilton syringe. Petri dish cultures were used for injections of E10/E11 embryos, and E15/E16 embryos were injected in ovo. Injected volume was 2 μl or 4 μl. Injections can lead to retinal detachment and these were discarded from the series. 5 μg NGF protein in PBS was injected into the E10/E11 and E15/E16 embryos, and analyses were made 1 or 2 days after injection. Vehicle was injected as a control. Five antisense chicken NGF PONs, AS1-AS5, and two non-sense, NS1 and NS2, were previously designed and tested for their capacity to inhibit NGF expression in COS cells (Hallböök et al., 1997; Hallböök et al., 2000). The AS1 PON that target the translational initiation signal in the NGF mRNA was efficient in inhibiting NGF expression. From our previous experiments we know that an effective concentration of PONs AS1 is 25 μM. To achieve this concentration in the in vivo retina, AS1 was labelled with 35S to a specific activity of 1.5×107 CPM/μg and different amounts of AS1 were injected intraocularly. 24 hours after injection, the embryos were dissected and the retina of the injected eye was rinsed and homogenised before a sample was measured using a scintillation counter. Approximately 0.5 nmoles of AS1, NS1 or NS2 was injected at E10/E11, and 3 nmoles at E15/E16, to give a retinal concentration of 25 μM. The efficacy of the injections was assessed by measuring NGF protein levels in injected retinas (described below). PON penetration into the retina was analysed using fluorescein-conjugated PONS as shown previously (Catsicas et al., 1995; Osen- Sand et al., 1993). Unspecific tissue effects were tested by studying cell and fibre layers using Nissl staining, TUNEL and SNAP25 immunohistochemistry after injection of antisense and non-sense PONs at increasing amounts. No such effects were seen when injecting PONs giving a retinal concentration of 25 μM. Minor effects such as vacuolisation and regional retinal detachment could be seen when amounts of PONs corresponding to retinal concentration more than 140 μM were injected. Eyes were dissected 1 or 2 days after injection and sections were processed for TrkA and TUNEL analyses.
Enzyme immunoassay for NGF
NGF protein levels were measured in normal and PON-injected E11–E12 retinas using a fluorimetric two-site sandwich enzyme immunoassay with anti-βNGF antibody conjugated with β-galactosidase (Korsching and Thoenen, 1987). 96-well microtiter plates for fluorimetry were coated with 0.5 μg/ml monoclonal anti-βNGF antibody (Boehringer-Mannheim, 1008 226, clone 27/21) in 0.05 M NaHCO3 buffer pH 9.6, blocked with 1% BSA in the same buffer and washed in Tris-buffered saline with 0.5% Tween-20 pH 7.4, as previously described (Söderström et al., 1990). Control-wells were coated with 0.5 μg/ml normal mouse IgG in the same buffer. Dissected retinas were homogenised in Tris-buffered saline pH 7.4, 0.5% Tween-20, 0.1% BSA, 10 mM EDTA, 20 kallekrein units/ml aprotinin (Sigma, A1153) and 0.1 mM phenyl-methyl-sulfonyl-fluorid (Boehringer-Mannheim). Twofold sample dilutions in Tris-buffered saline with 0.1% BSA and 0.5%Tween-20 were applied onto the prepared plates and incubated at 4°C overnight. Mouse NGF diluted in the same buffer served as a standard (Söderström et al., 1990). 2.5 ng/ml β-galactosidase-conjugated anti-NGF antibody in Tris-buffered saline, 2 mM MgCl2, 0.05% Tween-20 and 0.1% BSA was added to the plates, which were incubated over night at 4°C. The next day they were washed in Tris-buffered saline with 0.5% Tween-20 as previously described. 200 μl/well of 97.5 μM methylumbelliferyl-β-galactoside (Sigma, M1633) in 0.05 M phosphate buffer pH 7.2 with 1 mM MgSO4, 0.2 mM MnSO4, 2 mM EDTA and 0.05% azide was used as a substrate for β-galactosidase. Fluorescence was detected in a MicroFluor™ Reader (Dynatech Laboratories, VI 22021) after 1 hour and after 24 hours. Data were processed and analysed as previously described in detail (Söderström et al., 1990).
Determination of cell number and density
The number of cells was counted in the central region of a retinal section using microscopy at high magnification in four microscopic fields, two on both sides of the optic nerve exit. Four sections from one retina were counted and an average number was calculated for a particular retina. Cell density was used when retinas from different ages were compared. The density was calculated by dividing the cell number with the retinal area of the counted region. The method was modified from (Dutting et al., 1983). At least four different animals (n=4), subjected to the same experimental treatment, were analysed, and the mean values and standard deviations were calculated. Significance was calculated using the unpaired t-test.
RESULTS
NGF- and TrkA-expressing cells are postmitotic
We have used nonradioactive in situ hybridisation analysis to study expression of NGF and TrkA mRNA in the developing avian retina. In situ hybridisation was used to be certain that the sites of synthesis were studied. Immunohistochemistry for NGF on avian tissue does not give reproducible results with any anti-NGF antibody that we have tested. Immunohistochemistry for TrkA using an anti-avian TrkA antibody gives reproducible results that correlate with in situ data. The very first cells labelled for NGF mRNA were detected in the central part of the E5.5–E6 (stage 28) retina. These cells were located close to the vitreal side of the neuroepithelium (Fig. 1A–F). The pattern changed with time and at E6–6.5 the cells were dispersed over the neuroepithelium. At E7, labelled cells were found lined up in the external part of the neuroepithelium, now identifiable as external part of the INL. This change proceeded peripherally in the retina as shown in Fig. 1C. Analysis of BrdU incorporation and NGF mRNA expression was used to study whether the early NGF-expressing cells were postmitotic or not. Cells that were co-labelled for NGF mRNA and BrdU could not be found in E6 or E7 retinas (Fig. 1E, I), suggesting that NGF synthesis commences after the completion of the last S-phase and cell division. The location of the NGF-expressing cells in the external part of the E7 INL suggested that these cells be of the horizontal cell type.
Fig. 1.
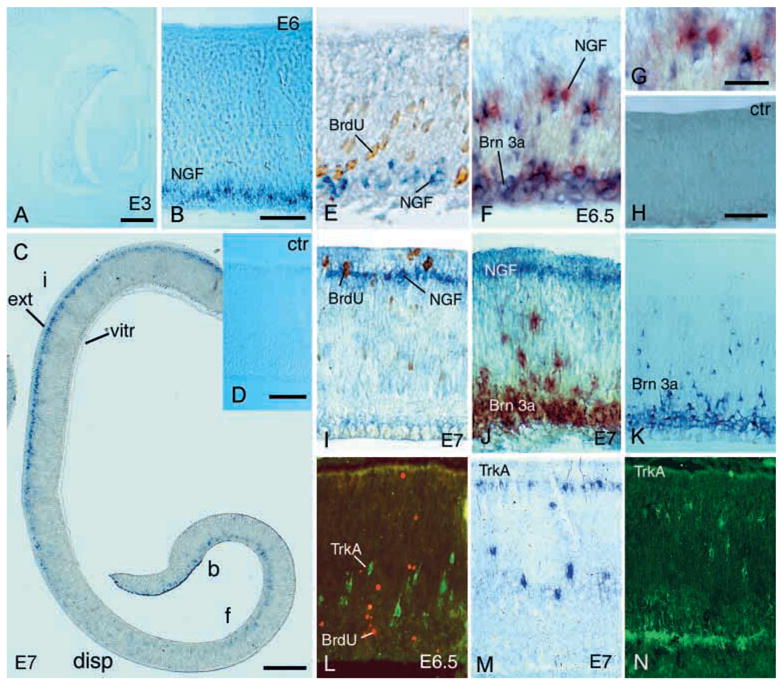
Analysis of early NGF and TrkA expression in embryonic retina. mRNA expression was analysed using in situ hybridisation analysis. Micrographs show cross sections of central region retina and are oriented with vitreal side facing downwards. (A) Analysis of E3 retina. No labelling was found. (B) Analysis of NGF mRNA expression in E5.5–E6 central retina. (C) Overview of a section of an E7 retina with labelling for NGF mRNA. Ventricular/external side (ext), vitreal side (vitr) and area of retina with dispersed NGF cells (disp). Note that the pigment epithelium is not included. b, f and i indicate regions of the retina that are representative for B, F and I, respectively. (D) E7 control (ctr) retina hybridised with sense probe for NGF mRNA. (E) Combination of NGF in situ hybridisation and BrdU immunohistochemistry analyses of E6 retina. (F) Analysis of NGF and Brn3a mRNA expression in the E6.5 retina using double in situ hybridisation. (G) High magnification of cells shown in F (NGF, red and Brn3a, blue). (H) E6.5 control retina hybridised with sense probes for NGF and Brn3a mRNA. (I) Combination of NGF in situ hybridisation and BrdU immunohistochemistry analyses of E7 retina. (J) Analysis of NGF and Brn3a mRNA expression in E7 retina. (K) Analysis of Brn3a mRNA expression in E7 retina. (L) Analysis of TrkA (fluorescein) and BrdU immunoreactivity (Texas Red) in the E6.5 retina. (M) In situ hybridisation and (N) immunohistochemical analyses of TrkA expression in E7 retina. Scale bar in A, 75 μm for A; in B, 80 μm for B, D–F, I–N; in C, 200 μm for C; in G, 25 μm for G; in H, 50 μm for H.
In an attempt to identify the early NGF-expressing cells, we labelled retinas for NGF and Brn3a mRNA expression using a double in situ hybridisation protocol. Brn3a is a transcription factor belonging to the Brn3 subfamily of POU class IV transcription factors and is expressed by ganglion cells in the developing chicken retina (Lindeberg et al., 1997). Cells that seemed to be labelled only for NGF or possibly co-labelled for NGF and Brn3a (Fig. 1F, G) could be found on the vitreal side of the E5.5–E6 neuroepithelium. These results were not fully conclusive because of the high density of Brn3a-labelled cells in the prospective ganglion cell layer, together with the relatively thick sections that were used. However, the dispersed NGF-labelled cells in the E6–E6.5 neuroepithelium were not co-labelled for Brn3a mRNA (Fig. 1F, G) and by E7, the patterns of NGF and Brn3a labelling were completely segregated (Fig. 1J, K).
We have previously shown that TrkA is expressed in the retina and the first cells are found in the central region of the E5.5–E6 embryonic retina (Hallböök et al., 1996; Karlsson et al., 1998). We combined BrdU incorporation with immunohistochemistry for TrkA and BrdU and the results showed that TrkA cells were not co-labelled for BrdU (Fig. 1L).
NGF and TrkA mRNA is co-expressed in horizontal cells but not amacrine cells
TrkA expression is confined to both horizontal and amacrine cells in the E7–E13 retina (Figs 1M, N, 2B). At later ages, mainly horizontal cells express TrkA (Fig. 2F). The patterns of TrkA and NGF expression overlap on the horizontal cells, but not on the amacrine cells, suggesting that NGF and TrkA are co-expressed in horizontal cells (Fig. 2A, B, E, F). We used the double labelling in situ hybridisation protocol to study NGF and TrkA co-expression. The results from analysis of E10, E12 and E17 retinas showed that labelling for NGF and TrkA mRNA could be found over the same horizontal cell. Thus, NGF and TrkA mRNA are co-expressed by horizontal cells (Fig. 2C, D, G, H) but not amacrine cells.
Fig. 2.
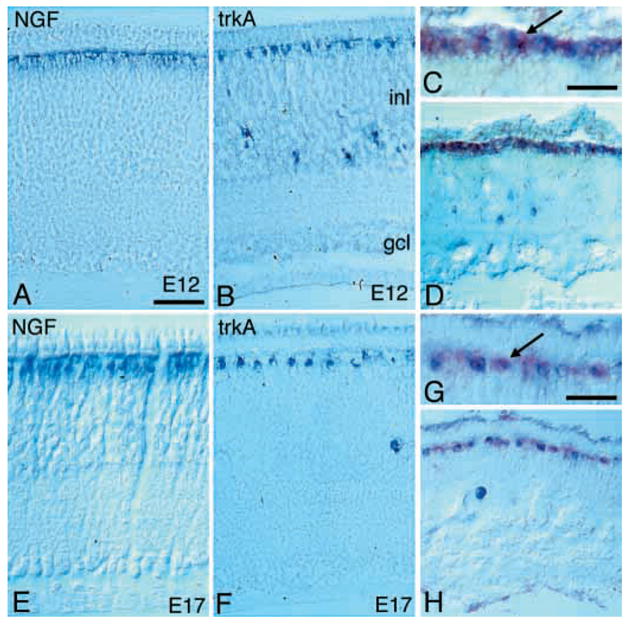
NGF and TrkA mRNA expression in the E12 and E17 developing chicken retina. In situ hybridisation analysis of NGF and TrkA mRNA expression in E12 (A–D) and E17 retina (E–H). Micrographs show cross sections of central region retina and sections are oriented with vitreal side facing down. (A, E) Analysis of NGF mRNA expression. (B, F) Analysis of TrkA mRNA expression. (C, D, G, H) Double-in situ hybridisation analysis of NGF (red) and TrkA (blue) mRNA expression. (C, G) High-power magnification of the regions shown in D, H, respectively. Arrows indicate double-labelled cells. gcl, ganglion cell layer; inl, inner nuclear layer. Scale bar in A, 50 μm for A, B, D–F, H; in C and G, 25 μm.
Many TrkA-expressing amacrine cells are TUNEL positive in the E11–E13 embryonic retina
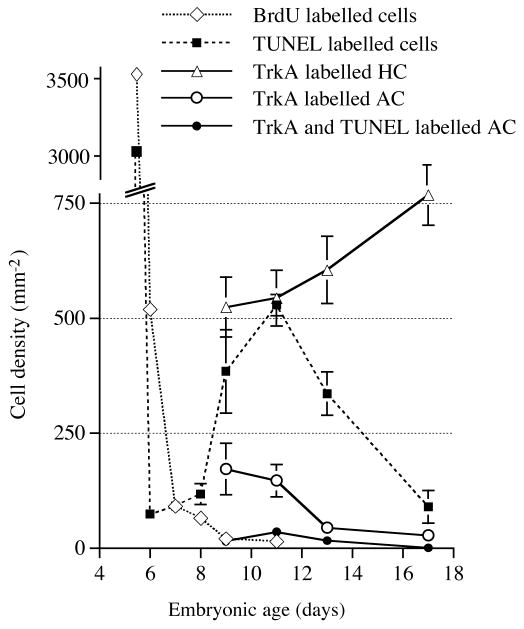
Analysis of the density of TrkA-expressing horizontal and amacrine cells in E9–E17 retinas, showed a decrease of TrkA-expressing amacrine cells around E10–E12, in contrast to the TrkA-expressing horizontal cells that remained (Fig. 3). As a result of reduced thickness of the INL during these stages, the density of horizontal cells increased (Fig. 3), even though the actual number of TrkA-positive horizontal cells remained constant. TUNEL was used to monitor cell death in the INL. There is a clear peak in the density of TUNEL cells around E10–E12. This peak follows a period (E6–E8) with very few TUNEL cells. In addition, the peak comes after the period with extensive proliferation (BrdU incorporation) in the early retina (Fig. 3). The peak also coincides with the reduction of TrkA-expressing amacrine cells. The combination of TUNEL and TrkA immunohistochemistry was used to study if this reduction was due to cell death. At E9, no cells were co-labelled by TrkA and TUNEL (Fig. 4A). However, at E10–E13, numerous TrkA immunoreactive cells with a typical neuronal and amacrine-like morphology were labelled with TUNEL (Fig. 4B), suggesting that TrkA-expressing amacrine cells die. The density of TrkA and TUNEL-amacrine cells peaked at E11 and this timepoint coincides with the major peak of TUNEL-cells in the INL (Fig. 3). Notably, no TUNEL was found at any time on TrkA-labelled cells with horizontal cell morphology and location (Fig. 4A–C), suggesting that cell death among the TrkA-expressing horizontals is very rare or even absent.
Fig. 3.
Number of TUNEL, TrkA and BrdU immunoreactive cells in the inner nuclear layer of E5-E18 retina. Cell density plotted against the age of the developing retina. The number of cells was counted in cross sections of central retina and density was calculated as number of cells per mm2 of transverse section of inner nuclear layer. The size, including thickness of the retina, increased from E4 to E6 as a result of extensive cell proliferation, decreased cell death and increased cell size. This resulted in a drop of cell density between E5 and E6. TrkA-expressing amacrine cells (AC) and horizontal cells (HC) were counted from E9 as earliest age when those cells are clearly distinguishable. The increase in TrkA-labelled horizontal cell density seen after E10 is a result of decreased thickness of the inner nuclear layer and not a result of more cells. Error bars are s.d. (n=4).
Fig. 4.
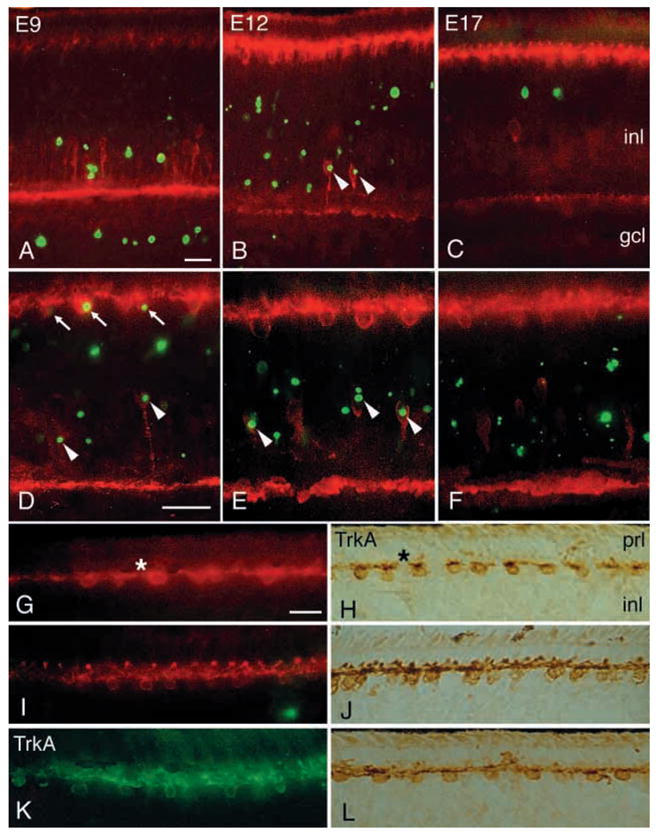
TrkA immunohistochemistry in combination with TUNEL in normal and injected retinas. TrkA immunohistochemistry was combined with TUNEL on sections of retina. (A–K) TrkA immunoreactivity is shown in red (Texas Red) and TUNEL in green (fluorescein isothiocyanate). Analysis of normal E9 (A), E12 (B) and E17 (C) retinas. (D–F) Analysis of E12 retinas after intraocular injection of AS1 antisense NGF PON (D), control PON (E) and NGF (F). (G–L) Analysis of E17 retina after intraocular injection of AS1 antisense NGF PON (G, H), control PON (I, J) and NGF (K, L). TrkA immunoreactivity is visualised using epifluorescence (G, I, K), as well as using the diaminobenzidine reaction (H, J, L). Arrowheads in B, D, E indicate TrkA amacrine; small arrows in D indicate TrkA horizontal cells that are labelled with TUNEL. Asterisks in G, H indicate the disrupted horizontal dendritic structures. gcl, ganglion cell layer; inl, inner nuclear layer; prl, photoreceptor layer. Scale bar in A, 50 μm for A–C; in D, 50 μm for D–F; in G 25 μm for G–L.
Exogenous NGF supports TrkA amacrine cell survival
NGF protein was injected into the eye of E10/E11 embryos to analyse if NGF could support the TrkA-expressing amacrine cells that would otherwise die. The number of TrkA amacrines and horizontals were counted on sections and the density of TrkA amacrine cells increased after NGF injections compared with vehicle injection (Fig. 5B). In addition, the density of cells that were positive for both TrkA immunoreactivity and TUNEL was determined. Very few TrkA and TUNEL amacrine cells could be found after NGF injection compared with normal and vehicle-treated retinas (Fig. 4B, F). The density of double-labelled amacrine cells decreased from approximately 35 to less than 5 cells/mm2 showing that NGF could counteract cell death of the TrkA immunoreactive amacrine cells (Fig. 5B). NGF was also injected into the E15/E16 eye with analysis the day after. Very few TUNEL cells can be found in any part of the INL at this age and the added NGF had no effect on the density of those cells (Fig. 4K, L). However, the added NGF protein distorted the structure of TrkA immunoreactive arceform horizontal dendrites as shown in Fig. 4K, L. Injection of vehicle did not affect the cells.
Fig. 5.
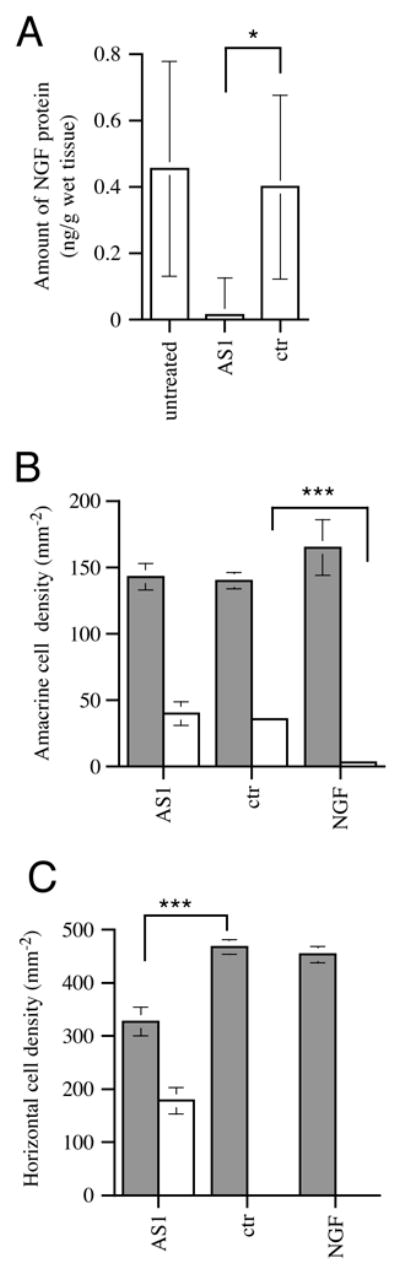
Effect on NGF protein levels and density of TrkA amacrine and horizontal cells in E12 retinas after intraocular injections of antisense NGF PON or NGF protein. (A) The level of NGF protein was analysed using an enzyme immunoassay in normal (untreated, n=4), AS1 antisense NGF PON- (AS1, n=5) and nonsense PON- (ctr, n=5) treated retinas. Density of amacrine cells (B) and horizontal cells (C) in E12 retina after treatment with AS1 (n=4), nonsense (ctr, n=4) and NGF protein (NGF, n=4). Stippled bars indicate the total number of TrkA immunoreactive cells and white bars indicate TrkA-and TUNEL-positive cells. Graphs show mean densities±s.d.
*P=0.02, ***P<0.0005 (t-test). White bars for ctr and NGF in C are zero.
Antisense NGF PON reduce NGF levels and cause death of TrkA-expressing horizontal cells
The fact that NGF is expressed only by the surviving horizontal cells, which also express TrkA receptors and that added NGF can counteract the death of TrkA amacrine cells, suggest that NGF could be a survival factor for the TrkA horizontal cells. To further test this hypothesis, we blocked the endogenously produced NGF and analysed survival of TrkA horizontal cells. We have previously shown that antisense oligonucleotides to the chicken NGF mRNA can block NGF protein synthesis (Hallböök et al., 1997; Hallböök et al., 2000). We injected antisense (AS1) and non-sense (NS1 or NS2) PONs into eyes of E10/E11 and E15/E16 embryos. E10/E11 is immediately before and E15/E16 is immediately after the cell death peak in the INL. Injections of 0.5 nmoles or 3 nmoles AS1 or NS PONs (25 μM) did not cause any unspecific tissue effects as shown by TUNEL, Nissl staining and SNAP25 immunohistochemistry (not shown). The levels of NGF protein were analysed in the normal and AS1 or NS PONs treated E11–E12 retina using an enzyme immunoassay. E12 retina was shown to contain approximately 0.4 ng NGF/g wet tissue. Treatment with AS1 PON decreased the NGF levels significantly when compared with normal and NS PON-treated retina (Fig. 5A). The histology of retinas from injected eyes were subsequently analysed in a similar way as after the NGF injections.
The density of TrkA-expressing amacrine cells was in the same range in the AS1- or NS-treated retinas, as well as untreated E11–E12 retinas. The situation was similar for TrkA-and TUNEL-positive amacrine cells (Fig. 5B). However, when looking at the TrkA-expressing horizontal cells in AS1 treated E11/12 retina, many cells showed TUNEL. These double-labelled cells were indeed conspicuous, as no such cells were found in normal or control retina (Figs 4B, D, E, 5C). Approximately half of the TrkA-expressing horizontals were TUNEL positive. A significant reduction of the TrkA horizontal cell density was also seen in the AS1-treated retina (Fig. 5C). The absence of effect by AS1 on the TrkA amacrine cell density could be considered as a control for unspecific effects by the PON treatment.
Treatment with neither AS1 nor NS PONs caused any effect on the density of TrkA or TUNEL cells in the E16/E17 retina (Fig. 4G–L). However, treatment with AS1, but not with NS PON, caused a disruption of the TrkA immunoreactive arceform horizontal dendrites as shown in Fig. 4G, H.
DISCUSSION
In this work we have studied cells that express NGF and TrkA in the developing avian retina. The results show that horizontal cells co-express NGF and TrkA, while TrkA-expressing amacrine cells do not express NGF. The majority of the TrkA-expressing amacrine cells die during the period of cell death in the INL. Intraocular injections of NGF protein rescued the dying amacrine cells and injection of antisense NGF oligonucleotides that blocked NGF synthesis, caused death among the TrkA-expressing horizontal cells, which normally survive. Based on these results, we suggest a role for NGF in supporting the survival of horizontal cells in the developing retina.
Independent studies have shown that NGF is expressed in the avian retina (Ebendal and Persson, 1988; Frade et al., 1996; Goedert, 1986; Hallböök et al., 1996; Large et al., 1989). Frade et al. found NGF mRNA in the E4 retina using RT-PCR (Frade et al., 1996). We did not detect any NGF mRNA before E5.5–E6 (stage 28) in the developing optic cup, retina or tissue associated with the retina. The first NGF-producing cells are located on the vitreal side of the neuroepithelium. The pattern changes and labelled cells are dispersed over the neuroepithelium; by E6.5–E7 they are found lined up in the external part of the neuroepithelium (Fig. 1B–F). The change in pattern coincides with neurogenesis and migration of neuroblasts in the developing retina (Prada et al., 1991; Spence and Robson, 1989). Neuroblasts complete their last S-phase when located in the vitreal part and migrate subsequently to the ventricular (external) part of the neuroepithelium where they divide (Prada and Ramírez, 1983). No labelling for BrdU could be found in the first NGF-labelled cells, suggesting that these NGF expressing cells are postmitotic. They are likely to represent precursor cells that have completed their last division and migrate freely in the neuroepithelium. No co-labelling of BrdU and NGF could be found at later times of development. Our results show that the early dispersed NGF-expressing cells were not labelled for Brn3a mRNA, suggesting that those cells meet a different fate from the ganglion cells. However, co-labelling for Brn3a and NGF mRNA could not be dismissed in the earliest cells located on the vitreal side of the retina, and the identity of this early NGF producing cell population remains to be determined.
Similarly to NGF expression, TrkA expression commences at E5.5–E6 as shown using immunohistochemistry, in situ hybridisation and RNase protection assay (Karlsson et al., 1998). The results disagree with those by Frade et al. who could not detect any TrkA mRNA until E14 using RT-PCR (Frade et al., 1996). We know that there are two types of TrkA-expressing cells: horizontal cells and amacrine cells. Both are separately distinguishable in the E6.5–E7 retina. There are three identifiable subtypes of horizontal cells in the avian retina (Génis-Gálvez et al., 1979) and the TrkA horizontal cell is similar to the axon-less ‘candelabrum-shaped’ subtype (Karlsson et al., 1998). In this study we show that NGF and TrkA are co-expressed in these horizontal cells (Fig. 2). We could not detect any TrkA expression in the E5.5–E6 prospective ganglion cell layer where the early NGF-expressing cells was found, suggesting that NGF expression might be initiated before TrkA expression. Furthermore, the TrkA amacrine cells do not express NGF, indicating independent activation of NGF and TrkA expression. Activating signals are unknown, but both intrinsic properties and extrinsic cues can direct cell phenotypes (Cepko, 1999; Cepko et al., 1996).
The appearance of TUNEL-labelled TrkA-immunoreactive amacrine cells (Fig. 3) clearly correlates with the reduction of TrkA-expressing amacrine cells in the INL, suggesting that these cells undergo cell death. Addition of NGF protein increased the number of TrkA-immunoreactive amacrine cells and reduced the number of cells that were co-labelled for TUNEL and TrkA, showing that these cells can respond to NGF stimulation with survival (Figs 4F, 5B). Injection of AS1 PONs that reduced the levels of NGF in the retina (Fig. 5A) did not affect the TrkA amacrines. In spite of the close source of horizontal cell-produced NGF, the majority of TrkA-expressing amacrine cells die. Thus, NGF is most likely not released from the horizontal cells in a fashion that makes it available for the TrkA amacrines. This is in agreement with the low levels of NGF in the retina (0.4 ng/g, Fig. 5A) as compared to those found in (for example) the rat hippocampus, which contains 3–8 ng/g (Henriksson et al., 1992).
A few TrkA-immunoreactive amacrine cells indeed survive and are found in the adult retina (Karlsson et al., 1998; Karten et al., 1995). If their survival is supported by NGF or by other factors such as neurotrophin 3 (Bovolenta et al., 1996; Hallböök et al., 1996) is not clear. Contacts between horizontals and amacrines have not been described, but NGF from the horizontal cells could be delivered anterogradely by bipolar cells, which contact both horizontals and amacrines, as has been shown for neurotrophins in retinal ganglion cells (von Bartheld et al., 1996). However, we could not see any effects of the AS1 on the TrkA amacrine cells, which argues for involvement of other factors.
The horizontal cells co-express TrkA and NGF, suggesting an autocrine mode of action for NGF. It is not clear if co-expression of NGF and TrkA is required for NGF action, or if the NGF can be shared with neighbouring TrkA-expressing cells. A subpopulation of rat adult dorsal root ganglion neurones co-express TrkB and brain-derived neurotrophic factor, and their survival is supported by brain-derived neurotrophic factor in a similar autocrine loop (Acheson et al., 1995). We could not detect any cell death among the horizontal cells in the normal retina at any stage of development. However, if the NGF levels were reduced by AS1 PON injection in the E10–E12 retina, several of these cells died (Figs 4D, 5C). Thus, the cells are programmed for death, but they all survive. Our results clearly show that NGF that is produced by the horizontal cells themselves, supports their survival. NGF functions in this case as a survival factor, but is not synthesised and released from the horizontal cells in a fashion that leads to competition for NGF among the horizontal cells. Thus, it is not acting as a ‘classical’ neurotrophic factor (Lewin and Barde, 1996), which is produced in limited amounts. Later in development, the cells become independent of NGF for survival: injections of the inhibiting PONs at E15/E16 did not cause cell death. A conclusion from this, is that the final number of TrkA horizontal cells is determined by how many cells are produced. In contrast, cell death and selective survival determine the final number of TrkA amacrine cells.
Horizontal cells are interneurones that form contacts with bipolar cells, photoreceptors as well as other horizontal cells. The TrkA horizontal cells develop arceform dendrites with knob-like structures in the outer plexiform layer, which are shared by neighbouring TrkA horizontal cells (Karlsson et al., 1998). The knobs are located close to the photoreceptors and are likely to be part of synaptic contacts with the photoreceptors. Horizontal cell synapses are known to undergo morphological changes in association with increased synaptic activity, with formation of spinules protruding into the photoreceptors (Wagner and Djamgoz, 1993). Injection of the inhibiting AS1 PON during horizontal dendrite formation (E10–E12), not only caused cell death but also disrupted the structure of the arceform dendrites. This effect was clearer in the E15–E17 retina. The structure of the arceform dendrites was also affected by the NGF injections, even though the effect was less severe and dendrites were distorted but not disrupted. As shown by TrkA immunohistochemistry, TrkA receptors are located on both the soma and neurites (Karlsson et al., 1998), with the most intense TrkA immunoreactivity on dendrites and knob-like structures. TrkA intensity on dendrites increased with age, suggesting that the receptors have a specific subcellular distribution. The results of the present study and our previous results, showing that NGF mRNA levels are modulated by light in the 5-day-old chick retina (Hallböök et al., 1996), are consistent with the general role for neurotrophins in synaptic plasticity (McAllister et al., 1999). Our results suggest two separate roles for NGF: support of survival and a role in horizontal cell plasticity.
Lately, attention has been focused on the role of NGF in inducing cell death via the p75 receptor. The work by Frade and Barde (Frade and Barde, 1998) suggests that NGF and p75 interact to induce early ganglion cell death in central regions of the avian retina. The cellular source of NGF was shown to be macrophages (microglial precursors) that enter the optic cup at E3 and migrate into the retina where they present NGF for certain cells that die. It is still debated if these cells actually cause the death or if they are attracted by dying cells during development (Cuadros et al., 2000; Cuadros and Navascues, 1998; Marin-Teva et al., 1999). Recently, it was shown that experimentally increased naturally occurring neuronal death in the avian isthmo-optic nucleus attracts macrophages/microglial precursors (Cuadros et al., 2000). We could not detect any NGF mRNA expression in the chick retina that was temporally or spatially associated with the early retinal cell death.
In conclusion, NGF supports the survival of TrkA-expressing avian horizontal cells in an autocrine mode of action during the late phase of cell death in the E10–E12 retina. The cells co-express TrkA and NGF, and the role for NGF is to maintain the TrkA-expressing horizontal cells. The population of TrkA-expressing amacrine cells is not supported by NGF and undergoes cell death during the late cell death phase in the INL. Our results also suggest that NGF plays a role in horizontal cell plasticity.
Acknowledgments
We are grateful to Paulina Tuvendal, Raili Engdahl, Angela Nieto and Annika Kylberg for technical advice; and to Jonas Lindeberg, Stine Söderström and Ted Ebendal for the chicken Brn3a probe and NGF EIA. Swedish Medical (12187) and Natural Research (BU 08904) Councils, Kronprinsessan Margaretas Arbetsnämnd för synskadade and Mattssons Minnesstiftelse supported this work. F. H. was supported by Göran Gustavsson’s Research Foundation and R. M. by a postdoctoral fellowship from Junta de Extremadura.
References
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Frade JM, Marti E, Rodríguez-Peña MA, Barde YA, Rodríguez-Tébar A. Neurotrophin-3 antibodies disrupt the normal development of the chick retina. J Neurosci. 1996;16:4402–4410. doi: 10.1523/JNEUROSCI.16-14-04402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström A, Söderström S, Kylberg A, Ebendal T. Molecular cloning of the chicken trkA and its expression in early peripheral ganglia. J Neurosci Res. 1996;46:67–81. doi: 10.1002/(SICI)1097-4547(19961001)46:1<67::AID-JNR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Catsicas M, Osen-Sand A, Staple J, Jones KA, Ayala G, Knowles J, Grenningloh G, Merlio-Pich E, Catsicas S. Antisense blockade of expression, SNAP-25 in vitro and in vivo. In: Agrawal S, editor. Methods in Molecular Medicine: Antisense Therapeutics. Totowa, NJ: Humana Press; 1995. pp. 57–85. [DOI] [PubMed] [Google Scholar]
- Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook B, Portera-Cailliau C, Adler R. Developmental neuronal death is not a universal phenomenon among cell types in the chick embryo retina. J Comp Neurol. 1998;396:12–19. doi: 10.1002/(sici)1096-9861(19980622)396:1<12::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Rios A. Spatial and temporal correlation between early nerve fiber growth and neuroepithelial cell death in the chick embryo retina. Anat Embryol. 1988;178:543–551. doi: 10.1007/BF00305042. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Martin D, Perez-Mendoza D, Navascues J, Clarke PG. Response of macrophage/microglial cells to experimental neuronal degeneration in the avian isthmo-optic nucleus during development. J Comp Neurol. 2000;423:659–669. doi: 10.1002/1096-9861(20000807)423:4<659::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Diaz B, Pimentel B, de Pablo F, de La Rosa EJ. Apoptotic cell death of proliferating neuroepithelial cells in the embryonic retina is prevented by insulin. Eur J Neurosci. 1999;11:1624–1632. doi: 10.1046/j.1460-9568.1999.00577.x. [DOI] [PubMed] [Google Scholar]
- Dutting D, Gierer A, Hansmann G. Self-renewal of stem cells and differentiation of nerve cells in the developing chick retina. Brain Res. 1983;312:21–32. doi: 10.1016/0165-3806(83)90117-7. [DOI] [PubMed] [Google Scholar]
- Ebendal T, Larhammar D, Persson H. Structure and expression of the chicken beta nerve growth factor gene. EMBO J. 1986;7:1483–1487. doi: 10.1002/j.1460-2075.1986.tb04386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebendal T, Persson H. Detection of nerve growth factor mRNA in the developing chicken embryo. Development. 1988;102:101–106. doi: 10.1242/dev.102.1.101. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron. 1998;20:35–41. doi: 10.1016/s0896-6273(00)80432-8. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development. 1999;126:683–690. doi: 10.1242/dev.126.4.683. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodríguez-Tébar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Génis-Gálvez JM, Prada F, Armengol JA. Evidence of three types of horizontal cells in the chicken retina. Jpn J Ophthalmol. 1979;23:378–387. [Google Scholar]
- Goedert M. Molecular cloning of the chicken nerve growth factor gene: mRNA distribution in developing and adult tissues. Biochem Biophys Res Commun. 1986;141:1116–1122. doi: 10.1016/s0006-291x(86)80159-0. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Ayer-Lelièvre C, Ebendal T, Persson H. Expression of nerve growth factor receptor mRNA during early development of the chicken embryo: emphasis on cranial ganglia. Development. 1990;108:693–704. doi: 10.1242/dev.108.4.693. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Bäckström A, Kullander K, Ebendal T, Carri NG. Expression of neurotrophins and Trk receptors in the avian retina. J Comp Neurol. 1996;364:664–676. doi: 10.1002/(SICI)1096-9861(19960122)364:4<664::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Sahlen A, Catsicas S. Characterization and evaluation of NGF antisense oligonucleotides: inhibition of NGF synthesis in transfected COS cells. Antisense Nucleic Acid Drug Dev. 1997;7:89–100. doi: 10.1089/oli.1.1997.7.89. [DOI] [PubMed] [Google Scholar]
- Hallböök F, Catsicas M, Staple JK, Grenningloh G, Catsicas S. Gene functional analysis in nervous system. Methods Enzym. 2000;314:148–167. doi: 10.1016/s0076-6879(99)14101-6. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Henriksson BG, Soderstrom S, Gower AJ, Ebendal T, Winblad B, Mohammed AH. Hippocampal nerve growth factor levels are related to spatial learning ability in aged rats. Behav Brain Res. 1992;48:15–20. doi: 10.1016/s0166-4328(05)80134-2. [DOI] [PubMed] [Google Scholar]
- Hughes WF, McLoon SC. Ganglion cell death during normal retinal development in the chick: comparisons with cell death induced by early target field destruction. Exp Neurol. 1979;66:587–601. doi: 10.1016/0014-4886(79)90204-8. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Clary DO, Lefcort FB, Reichardt LF, Karten HJ, Hallböök F. Nerve growth factor receptor TrkA is expressed by horizontal and amacrine cells during chicken retinal development. J Comp Neurol. 1998;400:408–416. [PMC free article] [PubMed] [Google Scholar]
- Karten HJ, Reichardt LF, Clary DO, Keyser KT. TRK A receptors reveal a new class of non-GABAergic horizontal cells in the avian retina. ARVO abstracts Invest Ophtalmol Vis Sci. 1995;36:S256. [Google Scholar]
- Korsching S, Thoenen H. Two-site enzyme immunoassay for nerve growth factor. Methods Enzymol. 1987;147:167–185. doi: 10.1016/0076-6879(87)47108-5. [DOI] [PubMed] [Google Scholar]
- Large TH, Weskamp G, Helder JC, Radeke MJ, Misko TP, Shooter EM, Reichardt LF. Structure and developmental expression of the nerve growth factor receptor in the chicken central nervous system. Neuron. 1989;2:1123–1134. doi: 10.1016/0896-6273(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Lefcort F, Clary DO, Rusoff AC, Reichardt LF. Inhibition of the NT-3 receptor TrkC, early in chick embryogenesis, results in severe reductions in multiple neuronal subpopulations in the dorsal root ganglia. J Neurosci. 1996;16:3704–3713. doi: 10.1523/JNEUROSCI.16-11-03704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lindeberg J, Klint P, Williams R, Ebendal T. Identification of a chicken homologue in the Brn-3 subfamily of POU-transcription factors. Dev Brain Res. 1997;100:169–182. doi: 10.1016/s0165-3806(97)00038-2. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Cuadros MA, Calvente R, Almendros A, Navascues J. Naturally occurring cell death and migration of microglial precursors in the quail retina during normal development. J Comp Neurol. 1999;412:255–275. [PubMed] [Google Scholar]
- Martín-Partido G, Rodríguez-Gallardo L, Alvarez IS, Navascués J. Cell death in the ventral region of the neural retina during the early development of the chick embryo eye. Anat Rec. 1988;222:272–281. doi: 10.1002/ar.1092220308. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. In: Bronner-Fraser M, editor. Methods in Cell Biology; Methods in Avian Embryology. Vol. 51. San Diego: Academic Press; 1996. pp. 219–235. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Lefcort FB, Clary DO, Reichardt LF, Prevette D, Oppenheim RW, Frank E. Neurotrophin-3 promotes the differentiation of muscle spindle afferents in the absence of peripheral targets. J Neurosci. 1997;17:4262–4274. doi: 10.1523/JNEUROSCI.17-11-04262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, Grenningloh G, Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Prada C, Ramírez G. A Golgi study of the cell cycle and early neuron and glia differentiation in the chick retina. In: Grisolíà S, editor. Ramón y Cajal’s Contribution to the Neurosciences. Amsterdam: Elsevier Science; 1983. pp. 117–123. [Google Scholar]
- Prada C, Puga J, Pérez-Méndez L, López R, Ramírez G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Rager G, Rager U. Systems-matching by degeneration. I A quantitative electron microscopic study of the generation and degeneration of retinal ganglion cells in the chicken. Exp Brain Res. 1978;33:65–78. doi: 10.1007/BF00238795. [DOI] [PubMed] [Google Scholar]
- Selleck MA. Culture and microsurgical manipulation of the avian embryo. In: Bronner-Fraser M, editor. Methods in Avian Embryology. Vol. 51. San Diego: Academic Press; 1996. pp. 1–23. [DOI] [PubMed] [Google Scholar]
- Spence SG, Robson JA. An autoradiographic analysis of neurogenesis in the chick retina in vitro and in vivo. Neuroscience. 1989;32:801–812. doi: 10.1016/0306-4522(89)90300-x. [DOI] [PubMed] [Google Scholar]
- Söderström S, Hallböök F, Ibañez CF, Persson H, Ebendal T. Recombinant human beta-nerve growth factor (NGF): biological activities and properties in an enzyme immuno assay. J Neurosci Res. 1990;27:665–677. doi: 10.1002/jnr.490270427. [DOI] [PubMed] [Google Scholar]
- Ulshafer RJ, Clavert A. Cell death and optic fiber penetration in the optic stalk of the chick. J Morphol. 1979;162:67–76. doi: 10.1002/jmor.1051620105. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Neurotrophins in the developing and regenerating visual system. Histol Histopathol. 1998;13:437–459. doi: 10.14670/HH-13.437. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Heuer JG, Bothwell M. Expression of nerve growth factor (NGF) receptors in the brain and retina of chick embryos: comparison with cholinergic development. J Comp Neurol. 1991;310:103–129. doi: 10.1002/cne.903100110. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Byers MR, Williams R, Bothwell M. Anterograde transport of neurotrophins and axodendritic transfer in the developing visual system. Nature. 1996;379:830–833. doi: 10.1038/379830a0. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Djamgoz MB. Spinules: a case for retinal synaptic plasticity. Trends Neurosci. 1993;16:201–206. doi: 10.1016/0166-2236(93)90155-f. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization. New York: IRL Press; 1992. pp. 75–83. [Google Scholar]