Abstract
Neurotrophins regulate survival, axonal growth, and target innervation of sensory and other neurons. Neurotrophin-3 (NT-3) is expressed specifically in cells adjacent to extending axons of dorsal root ganglia neurons, and its absence results in loss of most of these neurons before their axons reach their targets. However, axons are not required for NT-3 expression in limbs; instead, local signals from ectoderm induce NT-3 expression in adjacent mesenchyme. Wnt factors expressed in limb ectoderm induce NT-3 in the underlying mesenchyme. Thus, epithelial-mesenchymal interactions mediated by Wnt factors control NT-3 expression and may regulate axonal growth and guidance.
Neurotrophins are a family of proteins expressed in target tissues that regulate neuronal survival, differentiation, and function (1). These trophic factors mainly signal through the Trk family of receptor tyrosine kinases that are found on neuronal cell bodies and projections. Gene-targeting experiments have shown that neurotrophins and Trk receptors are required for survival of specific neuronal populations within the peripheral nervous system (1). NT-3 is essential for the survival of most sensory neurons early in development before they reach their final target, at which point they may gain access to target-derived trophic factors (2, 3). Expression of NT-3 in early embryos correlates with extending tips of sensory axons en route to their final targets (Fig. 1A) (2). Here, we investigate mechanisms responsible for controlling NT-3 expression. NT-3 expression may be induced by axonal tips passing nearby (4), or may be induced through local interactions, independent of neurons and their axonal projections. In this latter model, NT-3 may have an instructive role in sensory neuron differentiation and pathfinding in vivo (5).
Fig. 1.
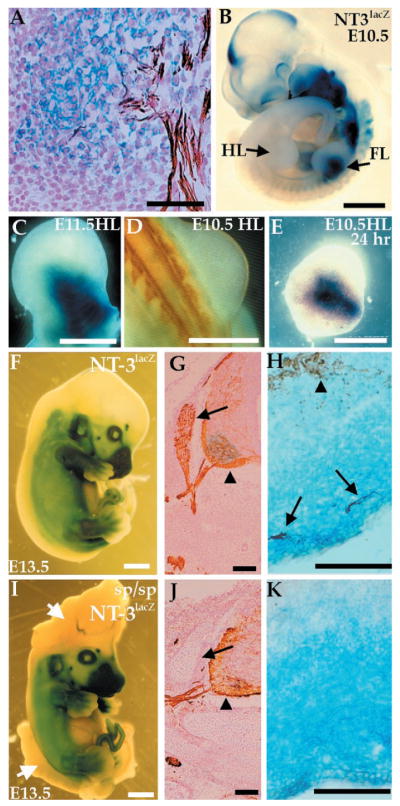
Expression of NT-3 in the limb does not depend on innervation. Expression of NT-3 was detected by a lacZ reporter gene stained with X-Gal (blue), and axons were visualized with a neurofilament antibody (NF, brown). (A) E11.5 NT-3lacZ embryo section shows colocalization of X-Gal staining with ends of sensory neuron projections. (B) E10.5 NT-3lacZ heterozygous embryo shows lacZ expression in forelimb (FL) but not hindlimb (HL). (C) E11.5 hindlimb stained for lacZ expression. (D) Dorsal view of an E10.5 embryo stained with NF. No axons are present in the hindlimb at this time. (E) E10.5 hindlimb cultured for 24 hours and stained for lacZ expression. (F to K) Expression of lacZ or NF (or both) in E13.5 NT-3lacZ embryos in a wild-type (F to H) or splotch background (I to K). Embryos were stained for lacZ expression (F and I). The splotch embryo exhibits severe defects in the cranial and lumbosacral region (arrows, I). No significant difference in lacZ expression in wild-type and mutant embryos is detected. (G and J) NF staining of caudal neural tube sections. DRGs (arrows) are absent in splotch mutants, although motor neurons (arrowheads) are present. (H and K) lacZ/NF costaining of the distal hindlimb. Sensory (arrows) but not motor (arrowhead) axons correlate with NT3 expression as detected by lacZ expression (H). (K) NT-3 expression is normal in the absence of NF staining in splotch mice. Bars, 50 μm (A, H, and K); 1 mm (B, F, and I); 0.5 mm (C to E); 100 μm (G and J).
We examined whether sensory neurons are required for NT-3 expression in one of the main targets of dorsal root ganglia (DRGs), the limb. We developed a whole limb organ culture system using mice heterozygous for the NT-3lacZ allele (6). In such mice, the NT-3 coding region is replaced by the lacZ marker gene (7), and X-Gal staining of these mice accurately reflects the endogenous expression of NT-3 (2). In hindlimb mesenchyme, expression of the lacZ marker in NT-3lacZ mice begins at embryonic day 11.5 (E11.5), which corresponds to the time when sensory and motor axons invade the limb (Fig. 1, B and C) (2). In hindlimbs of E10.5 mice, X-Gal (Fig. 1B) or neurofilament staining (Fig. 1D) could not be detected. In the absence of neurons, isolated E10.5 hindlimbs cultured for 1 day expressed lacZ in a pattern that was spatially indistinguishable from expression in an E11.5 hindlimb (Fig. 1, C and E). Limbs were routinely cultured in 10% fetal bovine serum, but induction of lacZ was also observed in the complete absence of serum (8). These results suggest that motor and DRG axons are not required for proper NT-3 induction in vitro.
To more stringently test the role of sensory neurons in inducing NT-3, we crossed the NT-3lacZ allele to splotch mice (6), which carry a mutation in the Pax-3 transcription factor (9). Splotch mice have, among other deficits, a depletion of neural crest cell derivatives, including DRGs (10). In splotch embryos, NT-3 expression at E10 through E14, as assessed by X-Gal staining, appears normal despite the absence of DRG neurons (Fig. 1, F to K) (8). At earlier time points (E10 and E11), when sensory and motor axons are mostly fasciculated, it could be argued that signals from motor neuron axons, which are disorganized but present in splotch embryos, can induce NT-3. However, by E14, the endings of sensory and motor axons are separated, and the expression of NT-3 within the dermis, a tissue not invaded by motor neurons, appeared normal in the absence of sensory axons (Fig. 1, H and K). Therefore, these results and those from organ culture argue that NT-3 induction does not depend on the presence of sensory neurons.
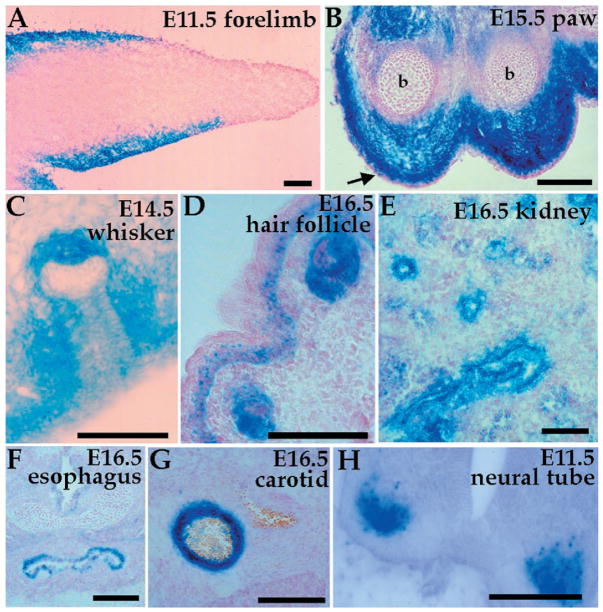
Our data suggest that local interactions, independent of neuronal innervation, are necessary to establish the pattern of NT-3 expression. To identify the signals that regulate NT-3 expression, we characterized lacZ expression in NT-3lacZ mice during embryogenesis. Many of the tissues expressing NT-3, apart from being targets of axons, are also sites of epithelial-mesenchymal boundaries. These domains of expression include limbs, whiskers, hair follicles, kidneys, the digestive system, and arteries (Fig. 2) (2, 11, 12). In most of these cases, NT-3 expression was observed in the mesenchyme; however, in the kidney NT-3 is expressed in the epithelial compartment (Fig. 2E). In the limb, the expression is mainly mesenchymal (Fig. 2, A and B). Embryonic expression of NT-3 is not confined to sites of epithelial-mesenchymal interactions. For example, within the spinal cord NT-3 is expressed in motor neurons, a target of central projections of NT-3–dependent sensory neurons (Fig. 2H).
Fig. 2.
NT-3 is expressed in many sites of epithelial-mesenchymal interactions during embryogenesis. X-Gal staining of NT-3lacZ heterozygous embryo sections shows expression in (A) E11.5 forelimb mesenchyme, (B) E15.5 forelimb paw [note the absence of expression in ectoderm (arrow) and bone (b)], (C) E14.5 whisker pad, (D) E16.5 hair follicle and skin dermis, (E) E16.5 epithelial cells of the metanephric kidney, (F) E16.5 upper esophagus, (G) E16.5 carotid artery, and (H) motor neurons of E11.5 neural tube. Bars, 100 μm.
To determine whether epithelial signals are important for the induction of NT-3 in limb mesenchyme, we used the limb organ culture system described above (6). E10.5 hindlimbs from NT-3lacZ mice were cultured for 24 to 48 hours with or without ectoderm (13). Our results show that limb ectoderm is necessary for proper mesenchymal expression of lacZ in limbs (Fig. 3, A and B). To verify that the lacZ induction we observe reflects endogenous NT-3 expression, we prepared mRNA from limbs cultured for 24 hours with or without ectoderm and analyzed the expression of NT-3 and γ-actin on a Northern (RNA) blot. Whole limbs showed sixfold higher levels of NT-3 expression normalized to γ-actin compared with ectodermless limbs (Fig. 3C). The reduced γ-actin level in the ectodermless sample reflects the fact that limbs without ectoderm were smaller than whole limbs after the culturing period, because of the importance of ectodermal signals for limb outgrowth (14). To ensure that limb mesenchyme was not damaged by ectoderm removal, we replaced the ectoderm of a NT-3lacZ limb with wild-type limb ectoderm. When cocultured, the wild-type ectoderm was able to induce lacZ expression in the limb mesenchyme after 24 hours (8), and to very robust levels after 48 hours (Fig. 3, D and E). To determine whether lacZ/NT-3 expression in ectodermless hindlimbs is due to an arrest of limb development under our culture conditions, we removed ectoderm from E10.5 forelimbs, which normally already express lacZ (Fig. 1B). After culturing for 24 hours, such limbs did not express lacZ (Fig. 3, F and G), suggesting that ectoderm is not simply providing a maturation signal for the limb but is necessary for the induction and maintenance of NT-3 expression.
Fig. 3.
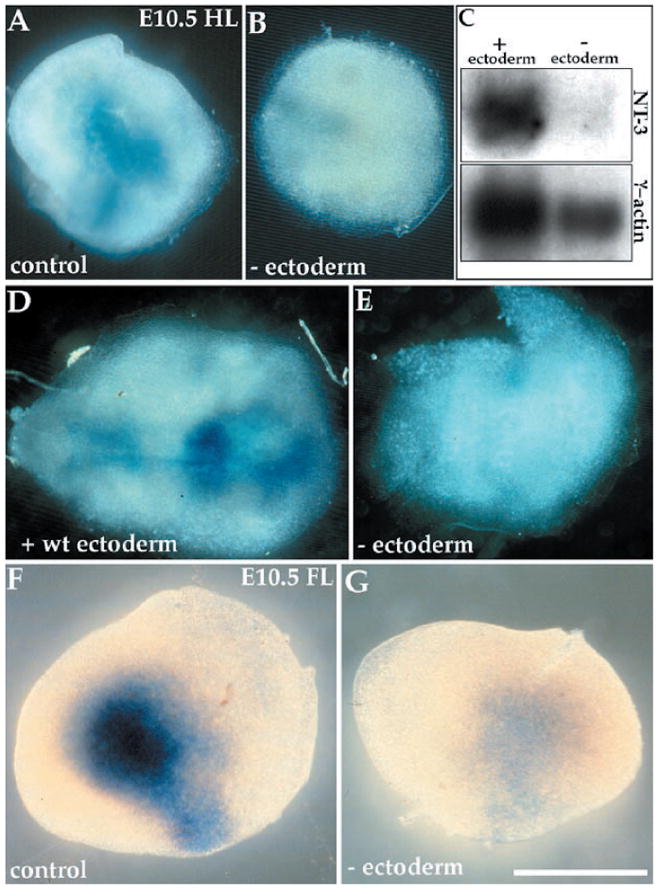
Ectoderm induces NT-3 expression in limb mesenchyme. Limbs were isolated from E10.5 NT-3lacZ heterozygotes and cultured in vitro. (A) An intact hindlimb expresses NT-3, as assessed by X-Gal staining, but (B) a hindlimb lacking ectoderm does not. (C) A Northern blot containing mRNA isolated from limbs cultured with or without ectoderm (10 limbs per lane), probed for NT-3 and γ-actin. (D) NT-3lacZ hindlimb mesenchyme reconstituted with wild-type ectoderm exhibits lacZ expression, but (E) a hindlimb lacking ectoderm does not. (F) Forelimb shows strong lacZ expression, (G) whereas a forelimb lacking ectoderm does not. All limbs were cultured for 24 hours with the exception of (D) and (E), which were cultured for 48 hours. Bar, 0.5 mm.
To identify the signaling molecules responsible for mediating local induction of NT-3 expression, we tested different secreted factors of the fibroblast growth factor (FGF), bone morphogenetic protein (BMP), and Wnt families that are expressed in the limb ectoderm (14). Beads soaked in FGF-2 or BMP-4 protein did not have detectable NT-3–inducing activity on limbs lacking ectoderm (8). To determine whether Wnt proteins can induce NT-3 expression, we cultured limb mesenchyme without ectoderm in direct contact with cell aggregates from NIH 3T3 cell lines stably transfected with cDNAs encoding different Wnt proteins or with control NIH 3T3 cells (6). Cells expressing wnt-4, which is expressed in limb ectoderm (15), induced strong NT-3 expression in limb mesenchyme (Fig. 4, A and B). Untransfected NIH 3T3 cells or cells expressing wnt-5a did not induce detectable NT-3 expression (Fig. 4, C and D). Wnt-5a is mainly expressed in the distal mesenchyme of limbs, a region where NT-3 is absent (Fig. 2A) (15). Finally, cells expressing wnt-7a and wnt-7b also induced NT-3 expression, although to a lesser extent than cells expressing wnt-4 (Fig. 4, E and F). Both wnt-7a and wnt-7b are expressed in limb ectoderm, although wnt-7a is only expressed in dorsal ectoderm (15). All the transfected cells used in this report express mRNAs encoded by the respective wnt genes, and produce Wnts (16); however, without a quantitative comparison of Wnt protein levels, differences in levels of lacZ induction cannot be interpreted as significant.
Fig. 4.
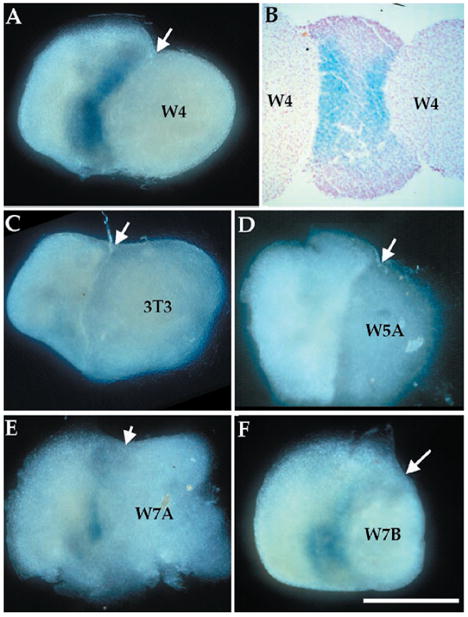
Wnt proteins are sufficient to induce NT-3 expression. Mesenchymal hindlimb tissue (without ectoderm) isolated from E10.5 NT-3lacZ heterozygous embryos was cocultured with aggregates of NIH 3T3–derived cell lines for 24 hours, after which they were stained with X-Gal. (A) Wnt-4–expressing NIH 3T3 cells induce NT-3 in limb mesenchyme. (B) A Wnt-4/limb/Wnt-4 explant stained and sectioned shows two blue stripes at the junction of the two tissues. (C) NIH 3T3 cells or (D) Wnt-5a– expressing cells do not induce NT-3, whereas (E) Wnt-7a– or (F) Wnt-7b–expressing cells induce NT-3 to a certain extent. Arrows point to junctions of limb mesenchyme and cell aggregates. Bar, 0.5 mm.
Wnt proteins play crucial roles in development of both invertebrate and vertebrate embryos and have been implicated in human cancer (17). The intracellular components of the Wnt signaling pathway include members of the Frizzled receptor family, glycogen synthetase kinase–3 (GSK3), β-catenin, and the LEF/TCF family of transcription factors that are also highly conserved in evolution. LEF-1 is required for the development of many organs formed by epithelial-mesenchymal interactions (18), and many of these regions express NT-3, suggesting a potential direct link between Wnt signaling and NT-3 expression. NT-3 is unique among the neurotrophins for its ability to activate all three Trk receptors and to transiently support in vivo multiple classes of newly formed sensory neurons (19). Understanding NT-3 regulation may thus provide insight into the mechanisms controlling the differentiation and pathfinding of sensory neurons.
References and Notes
- 1.Reichardt LF, Fariñas I. In: Molecular Approaches to Neural Development. Cowan MW, Jessell TM, Zipurski L, editors. Oxford Univ. Press; New York: 1997. pp. 220–263. [Google Scholar]
- 2.Fariñas I, Yoshida CK, Backus C, Reichardt LF. Neuron. 1996;17:1065. doi: 10.1016/s0896-6273(00)80240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elshamy WM, Ernfors P. ibid. 1996;16:963. doi: 10.1016/s0896-6273(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 4.Verdi JM, et al. ibid. :515. [Google Scholar]
- 5.Song HJ, Ming GL, Poo MM. Nature. 1997;388:275. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 6.The generation of NT-3lacZ mice has been previously described (7). Embryos were fixed and stained for neurofilament antibody or lacZ as described (2). Some sections were counterstained with nuclear Fast Red. Splotch mice were obtained from Jackson Laboratories (Bar Harbor, ME). All explants were cultured on 8-μm polycarbonate filters in Transwell plates (Costar) at the interface of medium (15% fetal bovine serum in Dulbecco’s modified Eagle’s medium, L-glutamine, penicillin, and streptomycin). For ectoderm separation, tissues were incubated with 6% pancreatin (Gibco-BRL) for 30 min on ice, washed in 10% serum for 20 min, and peeled (13). NIH 3T3 cell lines were aggregated by culture in hanging drops (20). Explant cultures shown are representative of at least four independent experiments. Rat NT-3 and human γ-actin cDNAs were used as probes for the Northern blot, which was quantified with a multiimager (Fuji).
- 7.Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LF. Nature. 1994;369:658. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 8.Patapoutian A, Reichardt LF. unpublished observations. [Google Scholar]
- 9.Epstein DJ, Vekemans M, Gros P. Cell. 1991;67:767. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- 10.Franz T. Anat Embryol. 1993;187:371. doi: 10.1007/BF00185895. [DOI] [PubMed] [Google Scholar]
- 11.Schecterson LC, Bothwell M. Neuron. 1992;9:449. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson GA, Fariñas I, Backus C, Yoshida CK, Reichardt LF. J Neurosci. 1996;16:7661. doi: 10.1523/JNEUROSCI.16-23-07661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neubüser A, Peters H, Balling R, Martin GR. Cell. 1997;90:247. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RL, Tabin CJ. ibid. :979. [Google Scholar]
- 15.Parr BA, Shea MJ, Vassileva G, McMahon AP. Development. 1993;119:247. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 16.Kispert A, Vainio S, McMahon AP. ibid. 1998;125:4225. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- 17.Cadigan KM, Nusse R. Genes Dev. 1997;11:3286. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 18.van Genderen C, et al. ibid. 1994;8:2691. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 19.Fariñas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A. Neuron. 1998;21:325. doi: 10.1016/s0896-6273(00)80542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serafini T, et al. Cell. 1994;78:409. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 21.We thank N. Hong, U. Müller, A. Neubüser, and members of Reichardt lab for valuable comments and A. Saulys for technical help. This work was supported by research grants from the U.S. Public Health Service (National Institutes of Health grant MH48200) and the Howard Hughes Medical Institute. A.P. is a fellow of the Damon Runyon–Walter Winchell Cancer Research Foundation and L.F.R. is an investigator of the Howard Hughes Medical Institute.