Abstract
The present investigation used an antibody directed against the extracellular domain of the signal transducing nerve growth factor receptor, trkA, to reveal immunoreactive perikarya or fibers within the olfactory bulb and tubercle, cingulate cortex, nucleus accumbens, striatum, endopiriform nucleus, septal/diagonal band complex, nucleus basalis, hippocampal complex, thalamic paraventricular and reunions nuclei, periventricular hypothalamus, interpeduncular nucleus, mesencephalic nucleus of the fifth nerve, dorsal nucleus of the lateral lemniscus, prepositus hypoglossal nucleus, ventral cochlear nucleus, ventral lateral tegmentum, medial vestibular nucleus, spinal trigeminal nucleus oralis, nucleus of the solitary tract, raphe nuclei, and spinal cord. Colocalization experiments revealed that virtually all striatal trkA-immunoreactive neurons (> 99%) coexpressed choline acetyltransferase (ChAT) but not p75 nerve growth factor receptor (NGFR). Within the septal/diagonal band complex virtually all trkA neurons (>95%) coexpressed both ChAT and p75 NGFR. More caudally, dual stained sections revealed numerous trkA/ChAT (> 80%) and trkA/p75 NGFR (> 95%) immunoreactive neurons within the nucleus basalis. In the brainstem, raphe serotonergic neurons (45%) coexpressed trkA. Sections stained with a pan-trk antibody that recognizes primarily trkA, as well as trkB and trkC, labeled neurons within all of these regions as well as within the hypothalamic arcuate, supramammilary, and supraoptic nuclei, hippocampus, inferior and superior colliculus, substantia nigra, ventral tegmental area of T’sai, and cerebellar Purkinje cells. Virtually all of these other regions with the exception of the cerebellum also expressed pan-trk immunoreactivity in the monkey. The widespread expression of trkA throughout the central neural axis suggests that this receptor may play a role in signal transduction mechanisms linked to NGF-related substances in cholinergic basal forebrain and non-cholinergic systems. These findings suggest that pharmacological use of ligands for trkA could have beneficial effects on the multiple neuronal systems that are affected in such disorders as Alzheimer’s disease.
Keywords: tyrosine kinase receptors, nerve growth factor, basal forebrain, rat, monkey
The classic research of Levi-Montalcini and coworkers (for review, see Levi-Montalcini and Angeletti 1968; Thoenen and Barde, 1980) first demonstrated that nerve growth factor (NGF) was an important trophic substance for the development and maintenance of noradrenergic peripheral sympathetic neurons. A potential role for NFG within the central nervous system (CNS) was first identified in the adult rat brain following injection of radiolabeled NGF into the cortex and hippocampus. These investigations did not reveal retrogradely labeled perikarya within the noradrenergic locus coeruleus as expected, but instead exclusively labeled neurons within the septal/diagonal band complex and the nucleus basalis magnocellularis (Schwab et al., 1979; Seiler and Schwab, 1984). These transport studies, combined with additional converging lines of evidence, indicate that NGF modulates the development, survival, and maintenance of cholinergic basal forebrain neurons. NGF levels are, for instance, found in highest concentrations within basal forebrain neuron target regions such as the cerebral cortex, hippocampus, and olfactory bulb (e.g., Whittemore et al., 1986; Large et al., 1986). Autoradiographic investigations have shown that neurons which bind radiolabeled NGF codistribute with basal forebrain cholinergic neurons (Richardson et al., 1986; Raivich and Kreutzberg, 1987). Furthermore, immunohistochemical and in situ hybridization studies in rats (Yan and Johnson, 1988; Batchelor et al., 1989; Koh et al., 1989), monkeys (Kordower et al., 1988), and humans (Hefti et al., 1986; Hefti and Mash, 1989; Higgins and Mufson, 1989; Kordower et al., 1989a,b; Mufson et al., 1989) have demonstrated a high degree of codistribution and colocalization between the low-affinity p75 NGF receptor (NGFR) and the specific cholinergic marker, choline acetyltransferase (ChAT). Recently, NGF immunoreactivity also has been found within the cholinergic basal forebrain (CBF) neurons of rats (Conner and Varon, 1992; Conner et al., 1992), monkeys, and humans (Mufson et al., 1994).
Functionally, NGF has neurotrophic effects upon CBF neurons both in vitro and in vivo (see Hefti et al., 1989). In this regard, NGF increases ChAT activity and enhances neurite outgrowth of septal neurons in culture (Hefti et al., 1989). In vivo, intraventricular administration of NGF reverses the age-related atrophy of cholinergic basal forebrain neurons (Fischer et al., 1987) and prevents the degeneration of cholinergic septal/diagonal band neurons following fimbria fornix transection in rats (Williams et al., 1986; Kromer, 1987; Hefti et al., 1989) and nonhuman primates (Koliatsos et al., 1990; Tuszynski et al., 1991). Similarly, intracerebral grafts of NGF-secreting cells can rescue axotomized basal forebrain neurons in rodents (Rosenberg et al., 1988; Kawaja et al., 1992; Winn et al., 1994) and young (Emerich et al., in press) and aged non-human primates (Kordower et al., 1994 and in press a and b.
NGF is one member of a superfamily of neurotrophins which includes brain-derived neurotrophic factor (BDNF; e.g., Hohn et al., 1990), neurotrophin-3 (NT-3; e.g., Maison-pierre et al., 1990), and neurotrophin-4/5 (NT-4/5; e.g., Ip et al., 1993; Berkmeier et al., 1991). These neurotrophins all share significant structural homology and some overlapping functional effects. Although regions of nonidentity confer neurotrophin specificity, each binds to the low-affinity p75 NGFR (Chao et al., 1986; Bothwell, 1991; Ebendal, 1992). While the p75 NGFR may, in some instances, play a functional role in neurotrophin activity (e.g., Hempstead et al., 1989), signal transduction appears to require an interaction with a distinct tyrosine kinase (trk) receptor (see review: Parada et al., 1992). NGF binding stimulates the tyrosine kinase activity of trkA (Bothwell, 1991; Kaplan et al., 1991; Klein et al., 1991; Ebendal, 1992; Jing et al., 1992; Parada et al., 1992). Inactivation, removal, or functional inhibition of the tyrosine kinase domain (Ferrari et al., 1992; Jing et al., 1992; Knusel and Hefti, 1992; Nye et al., 1992; Ohmichi et al., 1992; Tapley et al., 1992) results in the loss of NGF actions. Although the precise CNS cell groups containing trkA is not clearly defined, it is known that cholinergic neurons of the striatum and basal forebrain express trkA mRNA (Lu et al., 1989; Kokaia et al., 1993; Miranda et al., 1993) and protein (Steininger et al., 1993). A recent in situ hybridization study revealed a more widespread distribution for trkA message throughout the rat CNS including many non-basal forebrain cholinergic cell groups (Gibbs and Pfaff, 1994). However, there are no systematic immunocytochemical investigations of the distribution of trkA protein containing neurons and fibers and their relation to neurotransmitters other than choline acetyltransferase (Steininger et al., 1993).
Therefore, the purpose of the present study was to utilize a polyclonal antibody, directed against the rat extracellular domain of the trkA receptor (RTA; Clary et al., 1994), to characterize the distribution of this signal transducing receptor throughout the adult rat CNS. Dual immunohistochemical staining procedures were used to assess the extent to which trkA-immunoreactive (ir) neurons colocalized with choline acetyltransferase in the basal forebrain and serotonin in the brainstem raphe nuclear complex. Moreover, the immunohistochemical distribution of the trkA protein was compared to that seen using an antibody directed against the low-affinity p75 NGFR and a pan-trk antisera which primarily recognizes trkA (Steininger et al., 1993; Kordower et al., in press), although it potentially displays some crossreactivity with trkB and trkC. Finally, we compared the distribution of the trkA protein seen within the rodent and monkey brainstem and spinal cord.
MATERIALS AND METHODS
Subjects
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN; weighing 250–300 g; n = 6) were housed one per cage at 20°C on a 12:12-hour light-dark cycle. Food and water were available ad libitum. Tissue from the brainstem and spinal cord of three New World Cebus apella monkeys and three Macaca mulatta monkeys of both genders were also examined. These animals were part of a previously reported investigation of trk immunostaining within the forebrain (Kordower et al., 1994 and in press a and b. All animal related procedures were conducted in strict compliance with approved institutional animal care protocols and in accordance with NIH guidelines (Guide for the Care and Use of Laboratory Animals, NIH publication No. 86-23, 1985).
Tissue preparation
Each rat was anesthetized with sodium pentobarbital (25 mg/kg body weight, i.p.), perfused transcardially with 0.1 M phosphate-buffered saline (PBS) followed by 250 ml of 4% paraformaldehyde in phosphate buffer (pH 7.4), and cryoprotected in 30% sucrose/PBS. Prior to killing, monkeys were pretreated with ketamine (10 mg/kg, i.m.) and then deeply anesthetized with sodium pentobarbital (25 mg/kg, i.v.). Prior to perfusion, monkeys were injected with 1 ml of heparin (20,000 IU) into the left ventricle of the heart. Each animal was then perfused transcardially with 0.9% saline and 4% paraformaldehyde. For both rats and monkeys, the brains were removed from the calvaria and cryoprotected in 30% sucrose in 0.1 M phosphate buffer at 4°C. All brains were cut frozen at 40 μm thickness on a sliding knife microtome and stored at 0°C in a cryoprotectant solution prior to processing.
Antibodies
A series of sections from each rat brain and spinal cord was processed for the visualization of the rat trkA receptor. The production and specificity of this antibody (RTA) has been described previously (Clary et al., 1994). Briefly, a polyclonal antiserum was prepared that recognizes the extracellular domain of the trkA receptor. A truncated portion of the full-length rat trkA coding sequence containing the extracellular domain was generated by polymerase chain reaction. The expression cassette directed production of a truncated trkA protein in baculovirus-infected Spodoptera frugiperda cells. The RTA antibody does not recognize either trkB or trkC (Clary et al., 1994). The RTA antibody was used at a dilution of 1:10,000 in the present study. Adjacent sections were immunohistochemically stained with a pan-trk antibody (1:500; polyclonal rabbit anti-trk; Oncogene Science Inc., Cambridge, MA), which is directed against the 77–90 amino acid sequence of the trk protein and crossreacts principally, although not exclusively, with the trkA receptor (Steininger et al., 1993; Kordower et al., 1994 and in press a and b). Additional sections were processed for the low-affinity p75 NGFR receptor (1:5,000; monoclonal mouse anti-p75 NGFR; Oncogene Science Inc., Cambridge, MA), choline acetyltransferase (1:80; ChAT; monoclonal mouse anti-ChAT; Boehringer Mannheim Corporation, Indianapolis, IN), and serotonin (1:10,000; polyclonal goat anti-serotonin; IncStar, Stillwater, MN). Additionally, selected trkA-immunostained sections were concurrently immunolabeled for p75 NGFR, and ChAT or serotonin.
Immunohistochemistry
Immunohistochemistry was carried out according to a modification of a previously reported procedure (Kordower et al., 1989a,b; Mufson et al., 1989, 1993a,b). Briefly, following several rinses in Tris-buffered saline solution (TBS), tissue sections were incubated for 20 minutes in a TBS solution containing 0.1 m sodium periodate to inhibit endogenous peroxidase activity. After three rinses in TBS with 0.25% Triton X-100 (TBS-Tx), sections were soaked for 1 hour in a TBS-Tx and 10% normal blocking serum (goat serum for trkA and pan-trk; horse serum for p75 NGFR, ChAT, and serotonin) and 2% bovine serum albumin. After decanting the blocking solution, the primary antibody at the appropriate dilution (see above) was applied for 24 hours at room temperature with constant agitation in a medium containing 0.4% Triton X-100 and 3% blocking serum. After decanting the primary antibody solution and washing the tissue (3 × 10 minutes), sections were incubated in a 1:500 dilution of biotinylated IgG secondary anti-serum (goat anti-rabbit for trkA and pan-trk; horse anti-mouse for ChAT; horse anti-goat for serotonin; Vector Laboratories) for 1 hour. Following 3 × 10-minute rinses, processed sections were incubated for 60 minutes in an avidin-biotin complex (“Elite Kit,” Vector Laboratories; 1:200). Sections were washed (3 × 10 minutes) in a 0.2 M sodium acetate, 1.0 M imidazole buffer (pH 7.4). The chromogen solution which completed the reaction consisted of 2.5% nickel II sulfate (Fisher), 0.05% 3,3′ diaminobenzidine (DAB), and 0.005% H2O2 mixed in this buffer. The reaction was terminated with washes in acetate-imidazole buffer. Sections were mounted on gelatin-coated slides, dehydrated through graded alcohols (70%, 95%, 100%), cleared in xylenes, and coverslipped with Permount. Additional sections were stained with cresyl violet acetate (1% in H2O, pH 3.3) for the demonstration of Nissl substance.
Double-immunohistochemical staining procedure
We used a previously described dual-immunohistochemical procedure (Levey et al., 1986; Mufson et al., 1991a; Benzing et al., 1993) to determine whether trkA-ir neurons within the basal forebrain and brainstem raphe colocalized with cholinergic (ChAT and p75 NGFR) and serotonergic neurons, respectively. The immunohistochemical protocol described above was employed with the exception that DAB (brown reaction product) was used as the first chromogen to visualize trkA-containing neurons followed either by antisera directed against ChAT or p75 NGFR. In each case benzidine dihydrochloride (BDHC) was used as the second chromogen which produces a granular dark blue reaction product. The order of antigen presentation was reversed for the dual localization of serotonin and trkA. This dual procedure allows easy visual discrimination of colocalized antigens within the same neurons (Levey et al., 1986; Mufson et al., 1991a; Benzing et al., 1993). Tissue sections processed for dual immunohistochemistry with BDHC were dehydrated quickly through graded alcohols and covered with Permount.
Immunohistochemical controls
Control sections were processed in a manner identical with that described above except that the primary antibody was deleted or an irrelevant IgG matched for protein concentration was substituted for the primary antibody. In the colocalization experiments, for the control sections the tissue was processed in an identical manner as described above except that the antibody solvent or irrelevant IgG was substituted in place of either the first or second primary antibody. It should be noted that the specificity for trkA, pan-trk, NGFR, ChAT, or serotonin (or other antigens) is not absolute. Therefore, the potential for antisera to crossreact with other structurally related proteins cannot be excluded. Because of this caution, which is inherent to any immunohistochemical procedure, the term immunohistochemical reaction (or -immunoreactivity, -ir) in this study refers to “-like” immunoreactivity. As an additional control, experiments were carried out in which the pan-trk antibody was preadsorbed with a tenfold excess of the pan-trk peptide (Oncogene Inc.) for 48 hours prior to tissue incubation (for details, see Kordower et al., 1994 and in press a and b).
Data analysis
The distribution of the trkA-ir perikarya and fibers as well as cell counts of single versus double immunopositive neurons was evaluated with the aid of a Hewlett-Packard X-Y plotter interfaced with an IBM driven Microplotter 4000+ computer system (Dilog Instruments, Tallahassee, FL) coupled to the mechanical stage of a Nikon microscope. Data analysis was performed under brightfield and dark-field illumination. The terminology used for structures of the rat brain was derived from the atlas of Paxinos and Watson (1986).
RESULTS
General characteristics
TrkA-ir cell bodies and fibers appeared dark blue-black throughout the rodent CNS when reacted with nickel intensification. TrkA-ir neurons typically displayed bipolar and multipolar morphological phenotypes within different brain regions (Figs. 1–6). Neuronal trkA-ir was located mainly in the cytoplasm. Single-labeled sections immunohistochemically stained for trkA revealed positive neurons within the olfactory tubercle, nucleus accumbens, striatum, basal forebrain subfields (Chl–Ch4; Mesulam et al., 1983), thalamus, hypothalamus, and brainstem (Figs. 1–7). TrkA-ir fibers were found scattered in many of these regions as well as within the cortex, hippocampus, and spinal cord (Figs. 7–12). Dual-labeled experiments colocalizing ChAT and trkA revealed a concordance of these proteins within striatal (>99%) and magnocellular basal forebrain complex neurons (80–95%). In contrast, sections dual stained for trkA and p75 NGFR revealed a virtually 100% colocalization only within the basal forebrain cholinergic neurons. In the brainstem we observed a subpopulation of serotonergic raphe neurons (45%) colocalized with trkA. Control sections incubated in the vehicle solution, an irrelevant IgG, or with preadsorbed antisera in lieu of the primary antibody did not display either trkA, pan-trk, ChAT, p75 NGFR, or serotonin immunoreactivity. Dual-immunostained sections in which one primary antibody was omitted revealed staining for only the appropriate antigens.
Fig. 1.
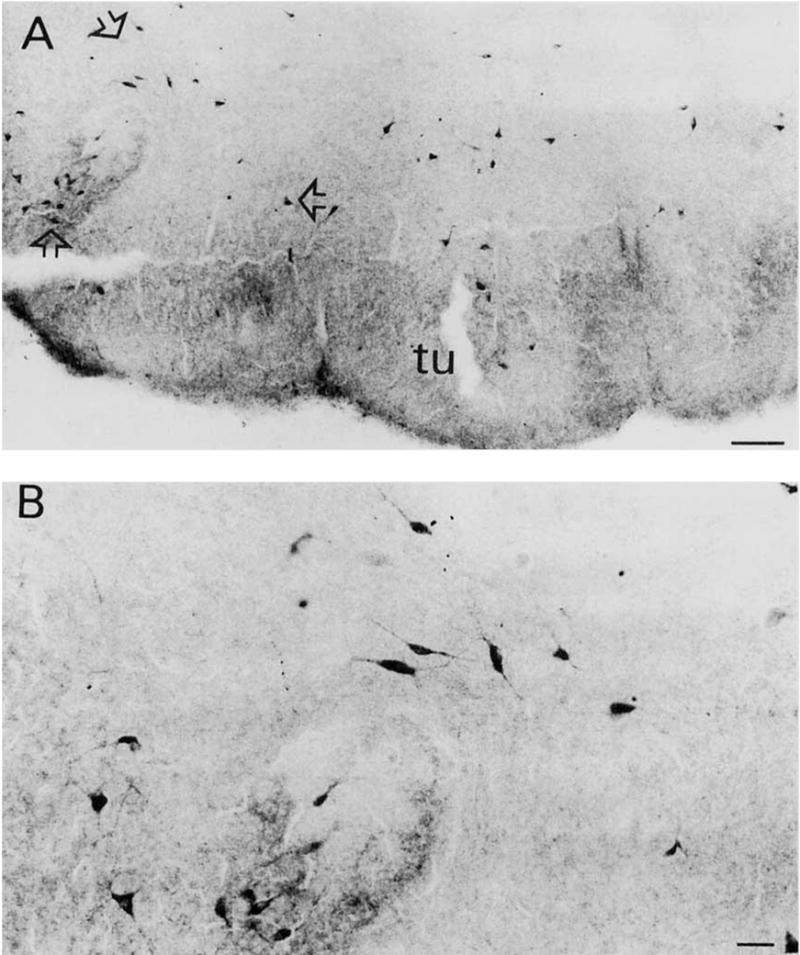
A: TrkA-immunoreactive (ir) neurons in rat anterior olfactory tubercle. Note the intensely immunostained neuropil. B: Higher-magnification photomicrograph of trkA-ir bipolar and multipolar neurons within the area outlined by the open arrows seen in A. Scale bars = 100 μm in A, 30 μm in B.
Fig. 6.

A: TrkA-ir neurons and neuropil staining within the ventral cochlear nucleus. B: High-magnification photomicrograph of the area of the cochlear nucleus delineated by arrows in A. Scale bars = 100 μm in A, 20 μm in B.
Fig. 7.

Schematic drawings showing the widespread distribution of trkA-immunoreactive fibers (short lines, left hemisphere) and neurons (black dots, right hemisphere) throughout the rat central nervous system. Coronal brain sections are arranged from rostral to caudal (A–I). Spinal cord cervical (J), thoracic (K), and lumbar (L) levels are also displayed. Each dot represents three trk-ir neurons.
Fig. 12.

Low- (A) and higher- (B) brightfield photomicrographs of a transversal section of the rat cervical spinal cord showing trkA-ir fibers within the substantia gelatinosa (laminae I and II). Scale bars = 100 μm in A, 30 μm in B.
TrkA-immunoreactive neurons within rat telencephalon
In the telencephalon, trkA-ir neurons were observed in the olfactory tubercle, nucleus accumbens, striatum, and basal forebrain subfields (Figs. 1–4, 7; Table 1). The neuropil of the olfactory tubercle was stained intensely for trkA (Fig. 1). Numerous trkA-ir neurons were scattered within this region with a preponderance of immunostained perikarya located in its more dorsal aspect. The morphology of trkA-ir neurons within the olfactory tubercle was bipolar and multipolar (85–208 μm2). The nucleus accumbens exhibited several trkA-ir neurons mainly within its more medial aspect. Numerous trkA-ir neurons were also seen scattered throughout the striatum. The distribution and morphology of trkA-ir neurons within the striatum was similar to that seen for ChAT, and these proteins colocalized greater than 99% within this structure (Fig. 13A). Striatal trkA-ir neurons were medium sized (186–280 μm2) with short neurites extending from the cell soma and exhibiting the morphology indicative of interneurons (Figs. 2, 13A). TrkA-ir within the striatum gave rise to modest neuropil staining. A patch-matrix organization of trkA-ir was not evident.
Fig. 4.
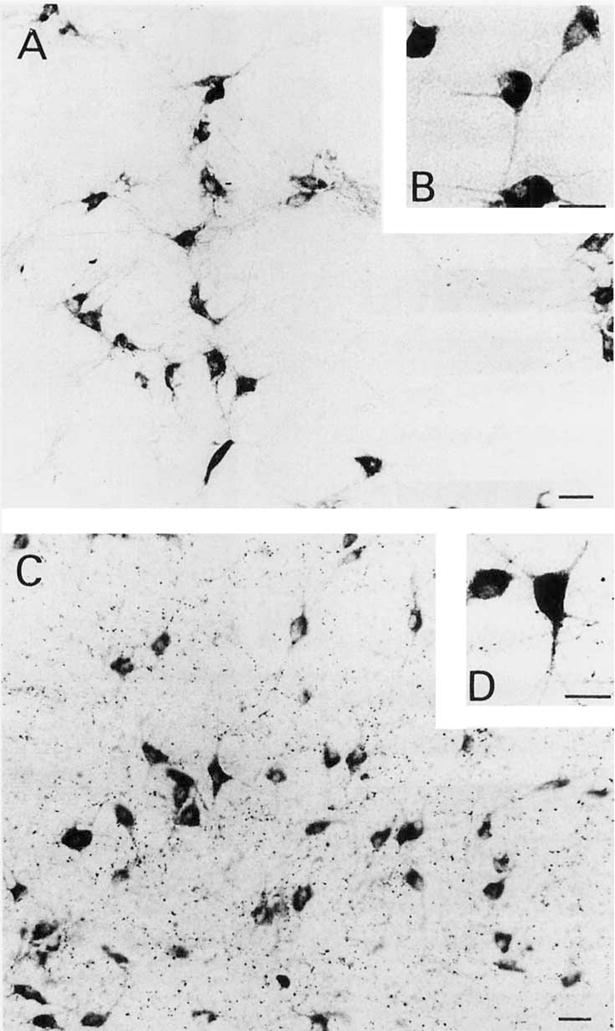
Low- (A) and high- (B) power photomicrographs of magnocellular trkA-ir neurons within the nucleus basalis. Low- (C) and high- (D) power photomicrographs of trkA-ir neurons within the horizontal limb of the diagonal band nucleus. Note in C the dense particulate trkA immunoreactivity within the neuropil. Scale bars = 30 μm in A,C; 20 μm in B,D.
TABLE 1.
Distribution of trkA Neurons and Colocalization With p75 NGFR, ChAT, and Serotonin
| trkA | Pan-trk | trkA/p75 NGFR | trkA/ChAT | trkA/serotonin | |
| Telencephalon | |||||
| Olfactory tubercle | + | + | + | ||
| Accumbens n. | + | + | |||
| Striatum | + | + | + | ||
| Medial septum | + | + | + | + | |
| Diagonal band | + | + | + | + | |
| Basal nucleus of Meynert | + | + | + | + | |
| Diencephalon | |||||
| Paraventricular thalaraic n. | + | + | |||
| Reuniens thalamic n. | + | + | |||
| Periventricular hypothalamic n. | + | + | |||
| Arcuate hypothalamic n. | + | ||||
| Supraoptic n. | + | ||||
| Supramammillary n. | + | ||||
| Hippocampus | + | ||||
| Mesencephalon | |||||
| Inferior colliculus | + | ||||
| Superior colliculus | + | ||||
| Interpeduncular n. | + | + | |||
| Substantia nigra | + | ||||
| Ventral tegmental area | + | ||||
| Mesencephalic trigeminal n. | + | + | + | ||
| Spinal trigeminal n., oral part | + | + | |||
| Dorsal n. of the lateral lemniscus | + | + | |||
| Myelencephalon | |||||
| Prepositus hypoglossal n. | + | + | |||
| Raphe n. | + | + | + | ||
| nucleus of the solitary tract | + | + | |||
| Ventral lateral tegmentum | + | + | |||
| Ventral cochlear n. | + | + | |||
| Cerebellum | |||||
| Cerebellar Purkinje cells | + | ||||
Fig. 13.

Color photomicrographs showing colocalization of trkA with choline acetyltransferase (ChAT), p75 nerve growth factor receptor (NGFR), and serotonin. A–D: Colocalization of the high-affinity trkA (smooth brown product) and ChAT (granular blue-black) reaction product within striatal (A), nucleus basalis (B), and vertical limb of the diagonal band neurons (C,D). The arrow in C points to a single-labeled ChAT-ir neuron. E: Colocalization of high-affinity trkA (brown) and low-affinity p75 NGFR (granular blue-black) reaction product within the nucleus basalis. Arrow indicates a p75 NGFR-immunoreactive blood vessel. F: High-power photomicrograph illustrating a double-labeled basal forebrain neuron for both trkA and p75 NGFR. G: Colocalization of serotonin (brown) and trkA immunoreactivity (granular blue black) in the raphe magnus. Arrows indicate neurons single labeled for serotonin. H: High-power photomicrograph of a single serotonin (brown reaction product; arrow) and double-labeled neuron (serotonin brown and trk A granular blue-black staining) within the median raphe. Scale bars = 15 μm in A,D; 30 μm in B,C,E; 20 μm in F–G.
Fig. 2.

A: TrkA-ir bipolar and polymorphic neurons in the rat striatum. Note the light trkA-ir within the neuropil. B,C: Higher-power photomicrographs illustrating striatal trkA-ir bipolar neurons. Their morphology is indicative of interneurons. Scale bars = 30 μm in A, 20 μm in B,C.
Proceeding caudally, a continuum of trkA-ir neurons was seen extending throughout the subfields of the rodent basal forebrain (Table 1). Large and medium-sized trkA-ir neurons (129–268 μm2) were seen within the medial septum and diagonal band complex (Figs. 3, 4, 7). These cells were located along the midline and paramidline within the septum. Ventrally, numerous large magnocellular trkA-ir neurons (161–323 μm2) were seen within the vertical limb of the diagonal band (Figs. 3, 7). More caudally, trkA-ir neurons were found within the horizontal limb of the diagonal band (Figs. 4C,D, 7). At this level, numerous magnocellular trkA-ir perikarya were also observed scattered within the rodent nucleus basalis (Figs. 4A,B, 7). Clusters of these cells were embedded within the internal capsule and a few were dispersed within the ventral portion of the globus pallidus. Morphologically these neurons appeared mainly as large multipolar perikarya (64–263 μm2) exhibiting either triangular or fusiform shapes with long neurites emanating from the soma. No trkA-ir neuronal cell somata were detected in the hippocampal formation or the amygdala.
Fig. 3.
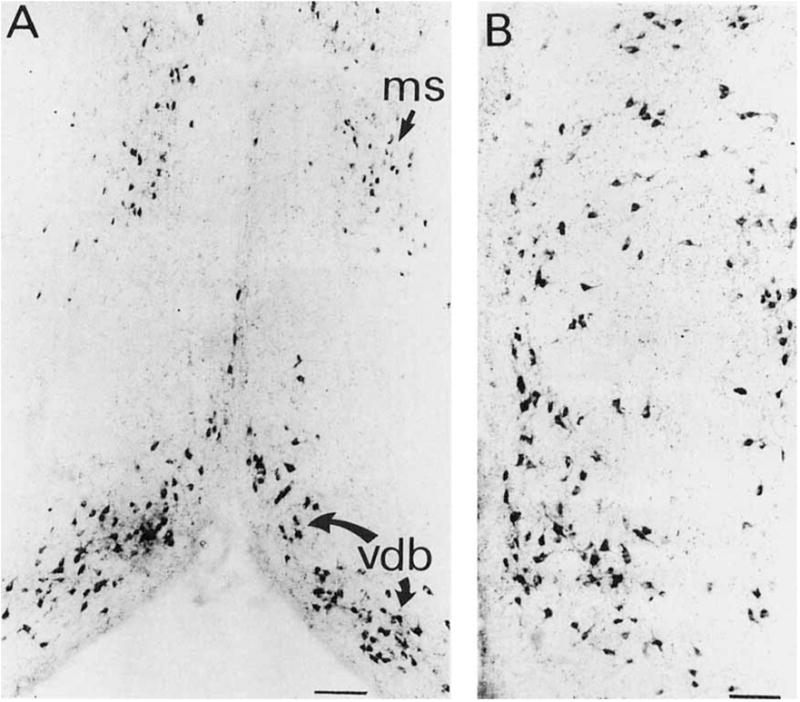
A: TrkA-ir neurons within the medial septum and vertical limb of the diagonal band nucleus. B: Higher-magnification photomicrograph of the region of the vertical limb of the diagonal band. Scale bars = 200 μm in A, 100 μm in B.
TrkA-immunoreactive neurons within the rat diencephalon
Within the diencephalon, both darkly and lightly labeled trkA-ir neurons were found only within the thalamic paraventricular (75–117 μm2; Figs. 5A and 7) and reuniens (93–129 μm2) nuclei (Fig. 7) as well as within the periventricular hypothalamic region (Fig. 7; Table 1). Morphologically the trkA-ir neurons within these nuclei appeared round in shape with a modest dendritic arborization.
Fig. 5.
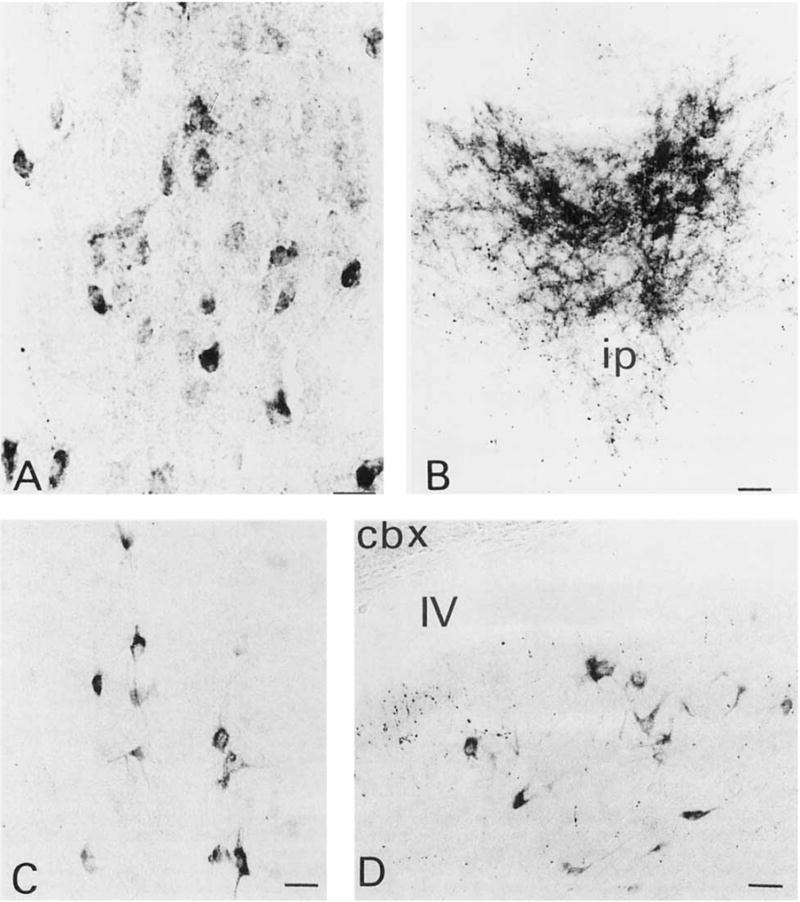
A: Lightly and darkly labeled trkA-ir neurons in the paraventricular thalamic nucleus. These neurons were characterized by their small round shape lacking distinct dendritic processes. B: TrkA-ir fibers located within the apical division of the interpeduncular nucleus. C: TrkA-ir neurons scattered within the nucleus of the raphe obscurus. D: TrkA-ir pleomorphic neurons in the medial vestibular nucleus. Scale bars = 20 μm in A, 30 μm in B–D.
TrkA-immunoreactive neurons within the rat mesencephalon
TrkA-ir neurons were seen within the interpeduncular nucleus (40–69 μm2), the mesencephalic and oralis portion of the spinal nucleus of the fifth nerve (107–172 μm2), and the dorsal nucleus of the lateral lemniscus (127–161 μm2) (Fig. 7). No trkA-ir neurons were seen in the central gray matter, pedunculopontine nucleus, lateral tegmental nucleus, or red nucleus (Table 1).
TrkA-immunoreactive neurons within the rat myelencephalon
Many trkA-ir cells were found within the prepositus hypoglossal nucleus (77–147 μm2; Figs. 7, 11) and ventral cochlear nucleus (Figs. 6, 7). The later region also displayed intense trkA immunoreactivity within the neuropil (Fig. 6). Several trkA-ir neurons were also seen within the ventral lateral tegmentum, medial vestibular nucleus (98–224 μm2), and raphe nuclei dorsal, median, obscurus, and magnus (98–250 μm2; Figs. 5C, 7, 13G,H), whereas only a very few were seen within the solitarius tract nucleus (Fig. 7; Table 1).
Fig. 11.

Low- (A) and higher- (B) power darkfield photomicrographs showing numerous trkA-ir cells and fibers in the prepositus hypoglossal nucleus. Scale bars = 100 μm in A, 30 μm in B.
TrkA-immunoreactive fibers within the rat central nervous system
A dense accumulation of trkA-ir fibers that formed distinct patches were observed within the glomerular layer of the olfactory bulb (Figs. 7, 8A). These fibers were mainly seen within the medial aspect of the olfactory bulb. A few fine trkA-ir fibers were found coursing medial to the nucleus accumbens (Figs. 7, 8B). Other trkA-ir fibers were seen throughout the rostrocaudal extent of the cingulate cortex (Fig. 7). These fibers were primarily located within layers 3 and 5. At the level of the septal/diagonal band complex, numerous varicose trkA-ir fibers were seen coursing within this region (Figs. 7, 9A,B). A few trkA-ir fibers were observed in the lateral aspect of the septum adjacent to the ventricle (Fig. 7). More laterally, trkA-ir fibers were seen within the endopiriform nucleus (Figs. 7, 10A) which did not contain trkA-ir perikarya. These fibers were thick with numerous varicosities. Some trkA-ir fibers were also located ventral to the anterior commissure (Fig. 7). Dense accumulations of trkA-ir fibers were observed within the diencephalon (Fig. 7). Numerous beaded trkA-ir fibers were found embedded within the horizontal limb of the diagonal band (Fig. 7, 8C). In the coronal plane, these fibers gave the appearance of a fine particulate scattered throughout this region (Fig. 4C). Emanating from this fiber plexus was a dorsally directed fascicle which divided into a lateral and medial bundle. The lateral component traveled within the internal capsule but did not interdigitate with the trkA-ir neurons located within the nucleus basalis (Fig. 7). The medial component coursed around the fornix enroute to the paratenial and paraventricular midline thalamic nuclei (Fig. 7). The thalamic paraventricular nucleus contained the heaviest accumulation of trkA-ir fibers within the thalamus. At the level of the lateral hypothalamic area a few trkA-ir fibers were seen within this region (Fig. 7). Furthermore, numerous trkA-ir fibers were also found distributed within the piriform cortex which were followed toward the amygdaloid complex (Fig. 7). More caudally, trkA-ir fibers were seen within the hippocampal CA4 field (Figs. 7, 10B). These fibers were arranged in a loose disorganized pattern within the hilum of the dentate gyrus and for the most part did not enter the granular cell layer of the dentate gyrus. TrkA-ir fibers also innervated CA1 as well as the subicular complex and entorhinal cortex (Fig. 7). TrkA fibers were observed within the dorsal aspect of the interpenducular nucleus (Figs. 5B, 7), periaqueductal gray, prepositus hypoglossal nucleus (Figs. 7, 11), raphe nuclei, and pyramids (Fig. 7). At all levels of the spinal cord a dense collection of trkA-ir fibers was observed within layers I and II of the substantia gelatinosa (Figs. 7, 12). Only a few lightly immunoreactive trkA fibers were seen within the deeper layers of the spinal cord.
Fig. 8.

Darkfield photomicrographs of trkA-ir stained fibers. A: TrkA-ir fibers and dense patches of immunoreactivity within the glomerular layer of the olfactory bulb. B: TrkA-ir fibers coursing medial to the nucleus accumbens. C: TrkA-ir fibers embedded within the nucleus of horizontal limb of the diagonal band. Darkfield artifacts were artistically eliminated on original figure. Scale bars = 30 in A, μm in A, 100 μm in B,C.
Fig. 9.

Darkfield microphotographs illustrating fine trkA-ir fibers in (A) the medial septum and (B) the vertical limb of the diagonal band nucleus. Scale bar = 100 μm for A and B.
Fig. 10.

Darkfield photomicrographs of trkA-ir fibers. A: Thick trkA-ir fibers distributed within the dorsal endopiriform nucleus. B: TrkA-ir fibers arranged in a loose, disorganized pattern within the hilus of the dentate gyrus of the hippocampus. Note that these fibers do not enter the granular layer of the dentate gyrus (white arrows). Scale bar = 30 μm for A and B.
DUAL IMMUNOHISTOCHEMICAL INVESTIGATIONS
Colocalization of TrkA/ChAT and p75 NGFR
Colocalization of trkA with either ChAT or p75 NGFR immunoreactivity revealed trkA as a brown reaction product and ChAT and p75 NGFR as a granular blue-black precipitate within the neuronal soma, proximal dendrites, and distal processes (Fig. 13A–F). Table 1 compares regions with Colocalization of trkA, ChAT, and p75 NGFR. Within the striatum virtually all trkA-ir neurons (> 99%) colocalized with ChAT (Fig. 13A). In striatal sections dual stained for trkA and p75 NGFR, only trkA-ir neurons were observed. Occasionally, one or two striatal neurons displayed both trk and p75 NGFR. These cells were located in the ventral medial aspect of the caudal portion of the striatum and may be displaced basal forebrain neurons. Within the septal/diagonal band complex we observed that virtually all trkA neurons (>95%) coexpressed both ChAT and p75 NGFR (Fig. 13C,D). More caudally, dual-stained sections revealed numerous trkA/ChAT-ir (>80%) (Fig. 13B) and trkA/p75 NGFR-ir (>95%) (Fig. 13E,F) neurons within the nucleus basalis. In the brainstem, the cholinergic neurons of the pedunculopontine and lateral dorsal tegmental nuclei were trkA and p75 NGFR immunonegative. However, trkA and p75 NGFR colocalized within the neurons of the mesencephalic nucleus of the fifth nerve.
Colocalization of trkA and serotonin
As described above, numerous small round trkA-ir neurons were visualized within the brainstem raphe nuclei. In order to determine the transmitter phenotype of these perikarya, we performed dual-immunohistochemical experiments using DAB as a chromogen for serotonin (brown reaction product) and BDHC for trkA (granular blue-black precipitate). Analysis of the dual-stained sections revealed that of the serotonergic neurons of the raphe nuclei 45% coexpressed trkA immunoreactivity (Fig. 13G,H; Table 1). The majority of these dual-labeled neurons were seen in the raphe median, obscurus, and magnus nuclei (Fig. 13G,H).
Pan-trk–immunoreactive neurons in rat and monkey central nervous system
Sections immunostained with the pan-trk antibody revealed labeled neurons within all regions which displayed trkA immunoreactivity in the rat (Table 1). In addition, this pan-trk antibody also stained neurons within the hypothalamic arcuate and supraoptic nuclei (Fig. 14), inferior and superior colliculus, ventral tegmental area of T’sai, substantia nigra, and the cerebellar Purkinje cells (Fig. 15). We have previously shown that this pan-trk antibody stains neurons within the monkey striatum and cholinergic basal forebrain (Kordower et al., 1994 and in press a and b) similar to that seen in the present study. In addition, brainstem and spinal cord sections from these monkeys stained with the pan-trk antibody displayed a pattern of immunoreactivity similar to that described in the rat (present study). For example, numerous pan-trk–ir neurons were seen within the rat and monkey raphe nuclear complex (Fig. 16), and the substantia gelatinosa of the spinal cord exhibited pan-trk–ir fibers (Fig. 17).
Fig. 14.

A: Numerous pan-trk—immunoreactive neurons in the supraoptic nucleus. B: Higher-power photomicrograph of neurons seen in A. Scale bars = 30 μm in A, 20 μm in B.
Fig. 15.

Low- (A) and higher- (B) power photomicrographs illustrating pan-trk immunoreactivity in rat cerebellar Purkinje cells. Scale bars = 200 μm in A, 20 μm in B.
Fig. 16.

Low- (A) and higher- (B) power photomicrographs illustrating numerous pan-trk–immunoreactive neurons in the monkey raphe nuclei. Scale bars = 250 μm in A, 100 μm in B.
Fig. 17.
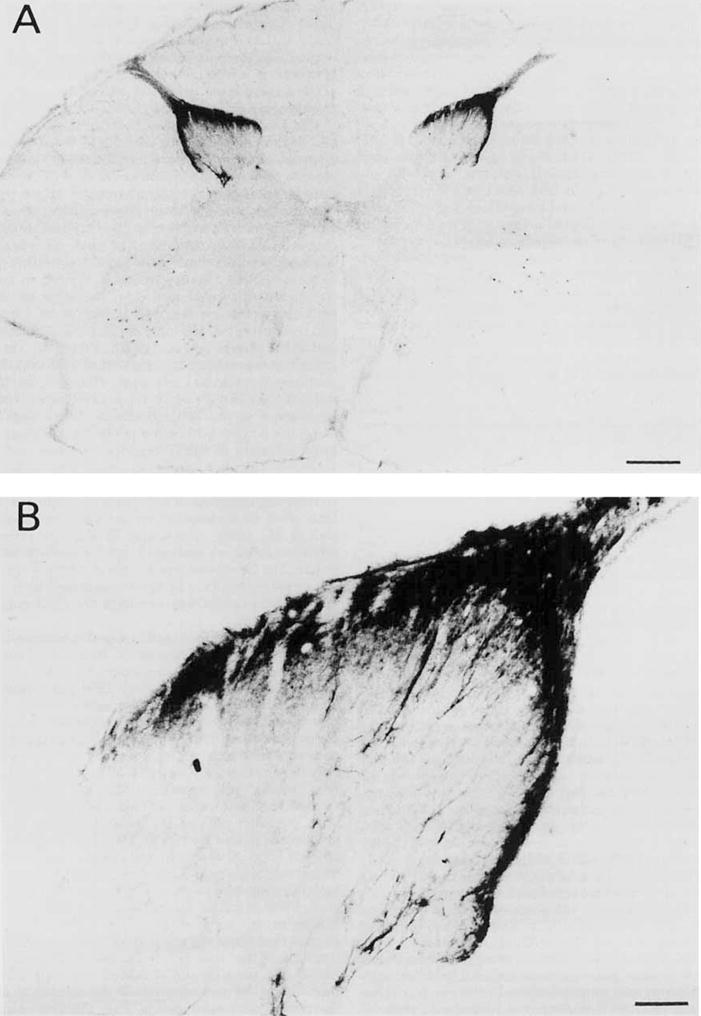
Low- (A) and higher- (B) power photomicrographs showing pan-trk-immunoreactive fibers in the monkey cervical spinal cord. This staining is mainly restricted to the substantia gelatinosa (laminae I and II), although a few fibers reached deeper layers. The immunostaining in the anterior horn is artifactual. Scale bars = 750 μm in A, 100 μm in B.
DISCUSSION
The present investigation employing an antibody directed against the extracellular domain of trkA, a signal transducing NGF receptor, revealed a widespread distribution of immunoreactive perikarya and fibers throughout the rat central nervous system. TrkA-immunoreactive neurons were observed within the olfactory tubercle, nucleus accumbens, striatum, septal/diagonal band complex, nucleus basalis, thalamic paraventricular and reunions nuclei, periventricular hypothalamus, interpeduncular nucleus, mesencephalic and oralis portion of the spinal trigemimal nucleus of the fifth nerve, dorsal nucleus of the lateral lemniscus, prepositus hypoglossal nucleus ventral cochlear nucleus, ventral lateral tegmentum, medial vestibular nucleus, solitarius tract nucleus, and raphe nuclei. Although several in situ hybridization studies have reported that neurons within these regions express mRNA for trkA (Holtzman et al., 1992; Merlio et al., 1992; Gibbs and Pfaff, 1994), localization of the trkA protein using immunohistochemistry is limited to the cholinergic basal forebrain neurons of rats (Steininger et al., 1993) and monkeys (Kordower et al., 1994 and in press a andb). Sections stained with the pan-trk antibody reveal immunoreactive neurons in all nuclear regions labeled with the RTA antisera (present study) as well as within the arcuate, supraoptic and supramammilary hypothalamic nuclei, inferior and superior colliculi, substantia nigra, and ventral tegmental area of T’sai. Although trkA mRNA has not been reported in these brainstem areas (Gibbs and Pfaff, 1994), several studies indicate that these regions express either trkB or trkC mRNA (Merlio et al., 1992; Seroogy and Gall, 1994; Seroogy et al., 1994; Altar et al., in press).
Dual immunohistochemical experiments demonstrated that within the septal/diagonal band complex virtually all trkA neurons (>95%) coexpressed both ChAT and p75 NGFR. In contrast, within the nucleus basalis trkA colocalized only with 80% of the cholinergic perikarya while greater than 95% of these trkA neurons coexpressed the p75 NGFR (see Steininger et al., 1993). A recent study combining in situ hybridization detection of trkA mRNA with immunohistochemical staining for the p75 NGFR revealed a similar degree of colocalization within the rat basal forebrain (Gibbs and Pfaff, 1994). The close correspondence between the expression of the trkA and low-affinity p75 NGFR within the basal forebrain is not an unexpected finding since multiple lines of evidence have demonstrated that NGF provides trophic influences for cholinergic basal forebrain neurons (see review in Hefti et al., 1989). As part of this process, both the trkA and p75 NGF receptors are believed to be synthesized within the perikarya of cholinergic basal forebrain neurons. Our trkA-immunohistochemical findings together with the detection of trkA mRNA (Gibbs and Pfaff, 1994) within these neurons confirm this process in the rodent brain. In fact, retrogradely transported NGF has been immunohistochemically visualized within basal forebrain consumer neurons in rats, monkeys, and humans (Conner et al., 1992; Conner and Varon, 1992; Mufson et al., 1994). It has been postulated that both trkA and p75 NGF receptors are transported in an anterograde fashion to reach target regions of basal forebrain neurons (see Jing et al., 1992, for discussion) such as the hippocampus and cerebral cortex (Mesulam et al., 1983). Then the endogenous neurotrophin binds to the trkA receptor, and is retrogradely transported to basal forebrain somata where the activated protein tyrosine kinase exerts trophic actions. Potentially, the low-affinity p75 receptor facilitates kinases of NGF to the trkA and/or the retrograde transport of the NGF/trkA complex from basal forebrain target regions to the NGF consumer perikarya within the basal forebrain (Jing et al., 1992). It is interesting to note that although virtually all ChAT-containing neurons were immunopositive for p75 NGFR, only about 80% coexpressed trkA within the nucleus basalis. This suggests that a subpopulation of cholinergic basal forebrain neurons may potentially not be responsive to the trophic effects of NGF, but may respond instead to another neurotrophin (Hempstead et al., 1989; Jing et al., 1992).
TrkA immunoreactivity was also observed in many non-basal forebrain regions. In this regard, numerous trkA-ir neurons were observed within the olfactory tubercle, nucleus accumbens, striatum, thalamic paraventricular and reuniens nuclei, periventricular hypothalamus, interpeduncular nucleus, mesencephalic and oralis portion of the spinal trigeminal nucleus of the fifth nerve, dorsal nucleus of the lateral lemniscus, prepositus hypoglossal nucleus ventral cochlear nucleus, ventral lateral tegmentum, medial vestibular nucleus, solitarius tract nucleus, and raphe nuclei. As mentioned previously, these regions also express trkA mRNA (Gibbs and Pfaff, 1994). Moreover, virtually all of these non-basal forebrain trkA-ir regions expressed p75 mRNA or protein under normal or altered physiological conditions (present study data not shown: Kordower et al., 1988; Yan and Johnson, 1988; Pioro and Cuello, 1990a,b; Mufson et al., 1992). Thus, there appears to be a good correlation between the regional coexpression of trkA and p75 NGFR, suggesting that both NGF receptors may play unique roles underlying the biological activity of NGF in the brain. However, biological evidence for a physiological effect of NGF on any of these regions has yet to be clearly defined. It is interesting to note that there are regions such as the thalamic paraventricular and reuniens nuclei which express the protein (present study) and mRNA for trkA (Gibbs and Pfaff, 1994) but do not display the p75 NGFR or high-affinity NGF-binding sites. It is possible that these regions contain extremely low levels of p75 NGFR which are undetectable under normal conditions. However, during stressful physiological events these trkA-ir neurons can express increased levels of p75 NGFR. For example, it is known that following peripheral nerve damage that neurons of the hypoglossal, facial, and spinal cord express high levels of p75 NGFR (Armstrong et al., 1991; Saika et al., 1991; Koliatsos et al., 1991; Hayes et al., 1992; Rende et al., 1992; Kordower et al., 1992). It remains to be determined whether NGF has any effect on these thalamic trkA-ir neurons.
Alternatively, the presence of trkA immunoreactivity within cholinergic striatal neurons (present study; Kordower et al., 1994 and in press a and b) lends support to the notion that neurotrophins such as NGF can act in a local manner (Lu et al., 1989; Mufson et al., 1991b; Miranda et al., 1993) in addition to the classic target-derived fashion proposed for basal forebrain function (see review: Eide et al., 1993). Since the trkA receptor mediates the biological activity of NGF (see reviews: Bothwell, 1991; Ebendal, 1992; Parada et al., 1992), the presence of trkA receptors alone within neurons suggests a functional role for NGF within these cells. Indeed, several studies have demonstrated that cholinergic striatal neurons are sensitive to the trophic effects of NGF. Infusion of NGF into neonatal rat pups increases ChAT activity within the striatum (Mobley et al., 1985). Striatal neurons during development express the low-affinity NGF receptor in rodents (Yan and Johnson, 1988; Lu et al., 1989) and humans (Kordower and Mufson, 1993), and striatal neurons express the high-affinity trkA receptor during rodent development (Miranda et al., 1993; Ringstedt et al., 1993) and in adulthood (Steininger et al., 1993). In the case of the striatum, it is likely that NGF acts within the striatum through interactions with the trkA receptor. In this regard, infusions of NGF (Davies and Beardsall, 1992; Kordower et al., in press a-c) or grafting of NGF secreting cells into the striatum (Schumacher et al., 1991; Frim et al., 1993) protect cholinergic interneurons from the destructive effects of mitochondria] dysfunction or excitatory amino acid administration. Furthermore, cholinergic striatal neurons hypertrophy in response to grafts of NGF secreting cells in normal rats (Chen et al., in press). Interestingly, trkA-ir striatal cholinergic neurons in the rat (present study), adult nonhuman primate (Kordower et al., 1988,1994 and in press a and b), and human (Mufson et al., 1989) do not express the low-affinity p75 NGF receptor under normal physiological conditions. Therefore, it appears that the trophic effects of NGF in vivo can occur without the presence of the low-affinity p75 NGF receptor. However, it is also possible that the p75 NGF receptor is present at low levels in the striatum and thus contributes to the biological action of NGF.
The present immunohistochemical study, as well as in situ hybridization (Gibbs and Pfaff, 1994) experiments, demonstrate that the neurons of the raphe nuclear complex contain the protein and mRNA for trkA. These investigations revealed trkA perikarya primarily within raphe magnus, median, and obscurus. We also found trk-ir neurons within the monkey raphe complex. Our dual-immunohistochemical staining demonstrated that 45% of the total serotonin containing neuronal population within the raphe nuclear complex coexpressed trkA. Similar to neurons of the cholinergic basal forebrain, perikarya of the raphe nuclei project in a widespread fashion to the hippocampus and cerebral cortex (Nieuwenhuys, 1985) and express trkB and trkC (Merlio et al., 1992). Presently, there is no experimental evidence to suggest that NGF acts directly on serotonergic neuronal cell types. However, initial studies indicate that BDNF, which binds preferentially with trkB (Berkmeier et al., 1991; Bothwell, 1991), can influence serotonin activity within the midbrain. For example, mid-brain infusions of BDNF produce analgesia and augment serotonin turnover locally in descending and ascending pathways (Siuciak et al., 1994; Martin-Iverson et al., 1994). In contrast, infusions of NT-3 into the midbrain have very little effect on nociception and serotonin metabolism (Siuciak et al., 1994), which may be due the expression of different forms of truncated trkC receptors within this region. The functional significance of multiple expression of trk receptors within the same region and their ability to bind various neurotrophins within the CNS remain to be fully evaluated.
In the present investigation the distribution of specific trkA labeling was compared to that visualized using a pan-trk antibody which preferentially stains trkA (Steininger et al., 1993; Kordower et al., 1994 and in press a and b) and probably trkB and trkC (Oncogene Science, MA). The pan-trk antibody labeled neurons within all regions seen in the present study using the specific trkA antisera as well as perikarya within the arcuate, supramammilary, supraoptic hypothalamic nuclei, hilar and CA3 regions of the hippocampus, inferior and superior colliculus, substantia nigra, ventral tegmental area of T’sai and cerebellar Purkinje cells. Purkinje cells of the cerebellum also express the protein and mRNA for p75 NGFR (Pioro and Cuello, 1990b; Mufson et al., 1991b). In the present study, all regions which contained pan-trk but not trkA immunoreactivity have been reported to express either the trkB protein (Zhou et al., 1993) or mRNA for trkB and trkC (Merlio et al., 1992; Kokaia et al., 1993; Altar et al., 1994). These findings suggest that these regions may be responsive to the neurotrophins BDNF and NT-3. Indeed, infusions of human BDNF into the striatum of rats retrogradely labeled neurons within the pars compacta of the substantia nigra and the ventral tegmental area of T’sai (Mufson et al., 1994), which express mRNA for BDNF (Seroogy et al., 1994) and trkB (Merlio et al., 1992; Kokaia et al., 1993; Altar et al., in press). Further studies are needed to fully clarify the role that the various trk receptors play in the signal transduction of the family of NGF-related neurotrophins throughout the CNS.
TrkA-ir fibers were seen within the glomerular layer of the olfactory bulb, cingulate cortex, septum, vertical and horizontal limbs of the diagonal band, endopiriform nucleus, thalamus, hilar region of the hippocampus, subiculum, entorhinal cortex, interpeduncular nucleus, periaqueductal grey, midline raphe, and substantia gelatinosa of the spinal cord. The precise cells of origin for these trkA projection systems remain to determined. Studies utilizing retrograde transport of horseradish peroxidase combined with immunohistochemistry suggest that many of these fiber systems arise from cholinergic basal forebrain or serotonergic raphe neurons. For example, immunohistochemical staining for cholinergic cells with retrograde neuronal labeling techniques has demonstrated that the olfactory bulb is innervated by cholinergic perikarya of the horizontal limb of the diagonal band (Mesulam et al., 1983). Since we have shown that virtually all cholinergic neurons of the horizontal limb of the diagonal band colocalize trkA, then at least a portion of this projection is most likely derived from these trkA-ir neurons. The trkA-ir fibers seen within the hippocampal complex may arise, at least in part, from trkA/cholinergic-positive cells located within the septum and vertical limb of the diagonal band (Mesulam et al., 1983). TrkA-ir fibers seen within the thalamus, interpeduncular nucleus, mid-brain periaqueductal gray, and raphe complex may arise from neurons of the ventral ascending serotonergic pathway of the raphe (see review by Nieuwenhuys, 1985), which contain the protein (present study) and mRNA (Gibbs and Pfaff, 1994) for trkA. In addition, these trkA/serotonergic-immunopositive neurons may also, in part, give rise to the cells of origin for a portion of the trkA-ir fibers observed within the olfactory bulb, septum and diagonal band, cingulate and entorhinal cortex, as well as the substantia gelatinosa of the spinal cord (Bowker et al., 1981; Nieuwenhuys, 1985).
Overall, using a polyclonal antibody raised against the extracellular domain of the trkA receptor we demonstrated a widespread distribution of trkA-ir perikarya and fibers throughout the rat CNS. Thus, these regions contain neurons which can potentially transduce the signal for NGF and mediate the biological effects of this neurotrophin. Within the basal forebrain, virtually all trkA neurons also expressed the p75 low-affinity NGF receptor, whereas not all cholinergic perikarya expressed trkA. TrkA-ir neurons in any other forebrain or brainstem region, with the exception of the mesencephalic nucleus of the fifth nerve, failed to express both p75 NGFR and trkA receptors. Furthermore, the expression of trkA throughout the central neural axis suggests that this receptor may play a role in signal transduction mechanisms related to NGF (or other as yet unknown trophic factors) not only within the cholinergic basal forebrain but in non-cholinergic systems.
Finally, it has also been hypothesized that altered trophic support may underlie the pathophysiology of neurodegeneration seen in Alzheimer’s disease (AD), Parkinson’s disease, and amyotrophic lateral sclerosis (Appel, 1981; Hefti, 1986; Hefti et al., 1989; Kordower and Mufson, 1989). Preliminary investigations in our laboratory using an antibody which recognizes endogenous NGF (Conner et al., 1992; Mufson et al., 1994) have revealed a reduction in NGF-like immunoreactivity within remaining basal fore-brain neurons in AD (Mufson et al., 1993b, 1994). In AD, NGF levels in basal forebrain target regions have been reported to remain unchanged (Goedert et al., 1986) or increased (Crutcher et al., 1993) in cortex. Similarly, levels of the low-affinity p75 NGF receptor has been discribed as either stable or elevated in AD (Higgins and Mufson, 1989; Ernfors et al., 1990a,b). This suggests that there is impaired internalization and/or retrograde transport of target-derived NGF associated with a defect in the complexing of this trophin with its high-affinity signal transducing trkA receptor in AD. Perhaps, compounds could be developed that regulate either endogenous neurotrophin production or the signal transduction mechanisms under their regulation. In this regard, pharmacological use of trk receptors could have beneficial effects on the multiple neuronal systems disrupted in AD.
Acknowledgments
We thank M. Leayman and S. Jaffar for histological assistance and M. Einert for secretarial assistance. This research was supported by AG10668, AG10161, AG11482, AG09466, NS29585, MH48200, Washington Square Health Foundation, Illinois Disease Public Health Service Award and Department of Health, Navarra, Spain. L.F.R. is an investigator at the Howard Hughes Medical Institute.
Abbreviations
- ac
anterior commissure
- Acb
accumbens nucleus
- ACg
anterior cingulate cortex
- amg
amygdala
- aob
accesory olfactory bulb
- av
anteroventral thalamic nucleus
- CA1
CA1 field of Ammon’s horn
- CA4
CA4 field of Ammon’s horn
- cbx
cerebellar cortex
- cc
cms cerebri
- eg
central gray
- cl
claustrum
- cm
central medial thalamic nucleus
- cp
caudate/putamen
- cu
cuneate fasciculus
- DG
dentate gyrus
- dll
dorsal nucleus of the lateral lemniscus
- en
endopiriform nucleus
- ent
entorhinal cortex
- f
fornix
- fi
fimbria of the hippocampus
- fmi
forceps minor of corpus callosum
- gl
glomerular layer of the olfactory bulb
- gp
globus pallidus
- gVII
genu of the facial nerve
- h
hippocampus
- hdb
nucleus of the horizontal limb of the diagonal band
- ic
internal capsule
- ifc
inferior colliculus
- ip
interpeduncular nucleus
- IV
IV ventricle
- Id
laterodorsal thalamic nucleus
- LV
Lateral ventricle
- md
mediodorsal thalamic nucleus
- meV
mesencephalic trigeminal nucleus
- ms
medial septal nucleus
- mve
medial vestibular nucleus
- nb
basal nucleus of Meynert
- oc
optic chiasm
- ot
optic tract
- PA
lateral preoptic area
- pas
parasubiculum
- PCg
postcingulate cortex
- pir
piriform cortex
- prh
prepositus hypoglossal nucleus
- prs
presubiculum
- pt
paratenial thalamic nucleus
- pva
paraventricular thalamic nucleus
- py
pyramidal tract
- re
reuniens thalamic nucleus
- rh
rhomboid thalamic nucleus
- rn
raphe nuclei
- RSp
retrosplenial cortex
- rt
reticular thalamus
- s
subiculum
- sc
superior colliculus
- scp
superior cerebellar peduncle
- sg
substantia gelatinosa
- sm
stria medullaris of the thalamus
- sol
nucleus of the solitary tract
- spVo
spinal trigeminal nucleus, oralis
- tu
olfactory tubercle
- vc
ventral cochlear nucleus
- vdb
nucleus of the vertical limb of the diagonal band
- vl
ventrolateral thalamic nucleus
- vll
ventral nucleus of the lateral lemniscus
- vm
ventromedial thalamus
- vpl
ventral posterolateral thalamic nucleus
LITERATURE CITED
- Altar CA, Siuciak JA, Wright P, Ip NY, Lindsay RM, Wiegand SJ. In situ hybridization of trkB and trkC receptor mRNA in rat forebrain and association with high affinity binding of [125I] BDNF, [125I] NT-4/5 and [125I] NT-3. Eur J Neurosci. 1994;6:1389–1405. doi: 10.1111/j.1460-9568.1994.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Appel SH. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, Parkinsonism, and Alzheimer’s disease. Ann Neurol. 1981;10:499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Brady R, Hersh B, Hayes RC, Wiley RG. Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motor neurons following nerve injury. J Comp Neurol. 1991;304:596–607. doi: 10.1002/cne.903040407. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Armstrong DM, Blaker SN, Gage FH. Nerve growth factor receptor and choline acetyltransferase colocalization in neurons within the rat forebrain: response to fimbria-fornix transection. J Comp Neurol. 1989;284:187–204. doi: 10.1002/cne.902840204. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Kordower JH, Mufson EJ. Galanin-immunoreactivity within the primate basal forebrain: evolutionary changes between monkeys and apes. J Comp Neurol. 1993;336:31–39. doi: 10.1002/cne.903360103. [DOI] [PubMed] [Google Scholar]
- Berkmeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Keeping track of neurotrophin receptors. Cell. 1991;65:915–918. doi: 10.1016/0092-8674(91)90540-f. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD. Serotonergic projections to the spinal cord from the midbrain in the rat: an immunocytochemical and retrograde transport study. Neurosci Lett. 1981;24:221–226. doi: 10.1016/0304-3940(81)90160-9. [DOI] [PubMed] [Google Scholar]
- Chao MV. Expression and structure of the human NGF receptor. Cell. 1986;47:5545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Chen EY, Winn SR, Baetge EE, Kordower JH. Hypertrophy of cholinergic and noncholinergic striatal neurons following intrastriatal transplants of polymer encapsulated NGF secreted cell. Soc Neurosci Abstr. 1994;20:1329. [Google Scholar]
- Clary DO, Weskamp G, Austin LR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Varon S. Distribution of nerve growth factor-like immunoreactive neurons in the adult rat brain following colchicine treatment. J Comp Neurol. 1992;326:347–362. doi: 10.1002/cne.903260304. [DOI] [PubMed] [Google Scholar]
- Conner JM, Muir D, Varon S, Hagg T, Manthorpe M. The localization of nerve growth factor-like immunoreactivity in the adult rat basal forebrain and hippocampal formation. J Comp Neurol. 1992;319:454–462. doi: 10.1002/cne.903190310. [DOI] [PubMed] [Google Scholar]
- Crutcher KA, Scott SA, Liang S, Everson WV, Weingarten J. Detection of NGF-like activity in human brain tissue: increased levels in Alzheimer’s disease. J Neurosci. 1993;13:2540–2550. doi: 10.1523/JNEUROSCI.13-06-02540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DW, Beardsall K. Nerve growth factor selectively prevents excitotoxin induced degeneration of striatal cholinergic neurons. Neurosci Lett. 1992;140:161–164. doi: 10.1016/0304-3940(92)90092-l. [DOI] [PubMed] [Google Scholar]
- Ebendal T. Function and evolution in the NGF family and its receptors. J Neurosci Res. 1992;32:461–470. doi: 10.1002/jnr.490320402. [DOI] [PubMed] [Google Scholar]
- Eide FF, Lowenstein DH, Reichardt LF. Neurotrophins and their receptors—current concepts and implications for neurological disease. Exp Neurol. 1993;121:200–214. doi: 10.1006/exnr.1993.1087. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Winn SR, Hammang JP, Baetge EE, Kordower JH. Grafts of polymer-encapsulated cells genetically modified to forebrain neurons in nonhuman primates. J Comp Neurol. doi: 10.1002/cne.903490110. (in press) [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lindefors N, Chan-Palay V, Persson H. Cholinergic neurons of the nucleus basalis express elevated levels of nerve growth factor mRNA in senile dementia of the Alzheimer’s type. Dementia. 1990a;28:138–145. [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990b;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Fabris M, Fiori MG, Gabellini N, Volonte C. Gangliosides prevent the inhibition by K-252a of NGF responses in PC12 cells. Dev Brain Res. 1992;65:35–42. doi: 10.1016/0165-3806(92)90005-h. [DOI] [PubMed] [Google Scholar]
- Fischer W, Wictorin K, Björklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987;329:65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- Frim DM, Simpson J, Uhler TA, Short MP, Bossi SR, Breakefield XO, Isacson O. Striatal degeneration induced by mitochondrial blockade is prevented by biologically delivered NGF. J Neurosci Res. 1993;35:452–458. doi: 10.1002/jnr.490350413. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW. In situ hybridization detection of trkA mRNA in brain: distribution, colocalization with p75 NGFR and up-regulation by nerve growth factor. J Comp Neurol. 1994;341:324–339. doi: 10.1002/cne.903410304. [DOI] [PubMed] [Google Scholar]
- Goedert M, Fine A, Hunt SP, Ullrich A. Nerve growth factor RNA in peripheral and rat central tissues and in the human central nervous system: lesion effects in the rat brain and levels in Alzheimer’s disease. Mol Brain Res. 1986;1:85–92. doi: 10.1016/0169-328x(86)90023-9. [DOI] [PubMed] [Google Scholar]
- Hayes RC, Wiley RG, Armstrong DM. Induction of nerve growth factor (p75NGFR) mRNA within hypoglossal motor neurons following axonal injury. Mol Brain Res. 1992;15:291–297. doi: 10.1016/0169-328x(92)90120-z. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor (NGF) promotes survival of septal cholinergic neurons after fimbria transection. J Neurosci. 1986;32:2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F, Mash DC. Localization of nerve growth factor receptors in the normal human brain and in Alzheimer’s disease. Neurobiol Aging. 1989;10:75–87. doi: 10.1016/s0197-4580(89)80014-4. [DOI] [PubMed] [Google Scholar]
- Hefti F, Hartikka J, Salvaterra P, Weiner WJ, Mash DC. Localization of nerve growth factor receptors on cholinergic neurons of the human basal forebrain. Neurosci Lett. 1986;69:275–281. doi: 10.1016/0304-3940(86)90410-6. [DOI] [PubMed] [Google Scholar]
- Hefti F, Hartikka J, Knusel B. Function of neurotrophic factors in the adult and aging brain and their possible use in the treatment of neurodegenerative diseases. Neurobiol Aging. 1989;10:515–533. doi: 10.1016/0197-4580(89)90118-8. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Schleifer LS, Chao MV. Expression of functional nerve growth factor receptors after gene transfer. Science. 1989;243:373–375. doi: 10.1126/science.2536190. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Mufson EJ. NGF receptor gene expression is decreased in the nucleus basalis in Alzheimer’s disease. Exp Neurol. 1989;106:222–236. doi: 10.1016/0014-4886(89)90155-6. [DOI] [PubMed] [Google Scholar]
- Hohn A, Leibrock J, Barley K, Barde YA. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Li Y, Parada LF, Kinsman S, Chen CK, Valletta JS, Zhou J, Long JB, Mobley WC. p140 trk mRNA marks NGF-responsive forebrain neurons: evidence that trk gene expression is induced by NGF. Neuron. 1992;9:465–478. doi: 10.1016/0896-6273(92)90184-f. [DOI] [PubMed] [Google Scholar]
- Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Fandl J, Greene LA, Barbacid M, Yancopoulos GD. Similarities and differences in the way neurotrophins interact with the trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- Jing S, Tapley P, Barbacid M. Nerve growth factor mediates signal transduction through trk homodimer receptors. Neuron. 1992;9:1067–1979. doi: 10.1016/0896-6273(92)90066-m. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Rosenberg MB, Yoshida K, Gage FH. Somatic gene transfer of nerve growth factor promotes the survival of axotomized septal neurons and the regeneration of their axons in adult rats. J Neurosci. 1992;12:2849–2864. doi: 10.1523/JNEUROSCI.12-07-02849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Jung S, Nanduri V, O’Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Knusel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- Koh S, Oyler GA, Higgins GA. Localization of nerve growth factor receptor messenger RNA and protein in the adult rat brain. Exp Neurol. 1989;106:209–221. doi: 10.1016/0014-4886(89)90154-4. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Bengzon J, Metsis M, Kokaia M, Persson H, Lindvall O. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci USA. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Nauta HJ, Clatterbuck RE, Holtzman DM, Mobley WC, Price DL. Mouse nerve growth factor prevents degeneration of axotomized basal forebrain neurons in the monkey. J Neurosci. 1990;10:3801–3813. doi: 10.1523/JNEUROSCI.10-12-03801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Crawford TO, Price DL. Axotomy induces nerve growth factor receptor immunoreactivity in spinal motor neurons. Brain Res. 1991;549:297–304. doi: 10.1016/0006-8993(91)90471-7. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Mufson EJ. NGF and Alzheimer’s disease: unfulfilled promise and untapped potential. Neurobiol Aging. 1989;10:543–544. doi: 10.1016/0197-4580(89)90123-1. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Mufson EJ. NGF receptor-immunoreactivity in the developing human basal ganglia. J Comp Neurol. 1993;327:359–374. doi: 10.1002/cne.903270305. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bartus RT, Bothwell M, Schatteman G, Gash DM. Nerve growth factor receptor immunoreactivity in the nonhuman primate (Cebus apella): distribution, morphology, and colocalization with cholinergic enzymes. J. Comp. Neurol. 1988;277:465–486. doi: 10.1002/cne.902770402. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bartus RT, Marciano FF, Gash DM. Telencephalic cholinergic system of the New World non-human primate (Cebus apella): morphological and cytoarchitectonic assessment and analysis of the projection to amygdala. J Comp Neurol. 1989a;279:528–545. doi: 10.1002/cne.902790403. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Gash DM, Bothwell M, Hersh L, Mufson EJ. Nerve growth factor receptor and choline acetyltransferase remain colocalized in the nucleus basalis (Ch4) of Alzheimer’s patients. Neurobiol Aging. 1989b;10:287–294. doi: 10.1016/s0197-4580(89)80013-2. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bankiewicz KS, Mufson EJ. NGF receptor (p75) immunoreactivity within hypoglossal motor neurons following axotomy in monkeys. Restor Neurol. 1992;4:411–417. doi: 10.3233/RNN-1992-4606. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Sladek JR, Mufson EJ. Trk-immunoreactivity in the monkey central nervous system: I. forebrain. J Comp Neurol. 1994;349:20–35. doi: 10.1002/cne.903490103. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Winn SR, Liu YT, Mufson EJ, Sladek JR, Hammang JP, Baetge EE, Emerich DF. The aged monkey basal forebrain: rescue and sprouting of degenerating cholinergic neurons following grafts of polymer-encapsulated human NGF secreting cells. Soc Neurosci Abstr (in press a) [Google Scholar]
- Kordower JH, Bayer VCR, Bartus RT, Putney S, Walus L, Friden P. Intravenous administration of an NGF conjugate prevent neuronal degeneration in a model of Huntington’s disease. Proc Natl Acad Sci USA. doi: 10.1073/pnas.91.19.9077. (in press b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer LF. Nerve growth treatment after brain injury prevents neuronal death. Science. 1987;225:214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Large TH, Bodary SC, Clegg DO, Weskamp G, Otten U, Reichardt LF. Nerve growth factor gene expression in the developing rat brain. Science. 1986;234:214–216. doi: 10.1126/science.3764415. [DOI] [PubMed] [Google Scholar]
- Levey AI, Bolam JP, Rye DB, Hallenger AE, Demuth RM, Mesulam MM, Wainer BH. A light and electron microscopic procedure for sequential double antigen localization using diaminobenzidine dihydrochloride. J Histochem Cytochem. 1986;34:1449–1457. doi: 10.1177/34.11.2430010. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;8:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Lu B, Buck CR, Dreyfus CF, Black IE. Expression of NGF and NGF receptor mRNAs in the developing brain: evidence for local delivery and action of NGF. Exp Neurol. 1989;104:191–199. doi: 10.1016/0014-4886(89)90029-0. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Todd KG, Altar CA. Behavioral in vivo neurochemical effects of the neurotrophic factors BDNF and NT-3 and their interactions with amphetamine. J Neurosci. 1994;14:1262–1270. doi: 10.1523/JNEUROSCI.14-03-01262.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlio JP, Ernfors P, Jaber M, Persson H. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neuroscience. 1992;51:513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci USA. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley WC, Tennekoon JL, Buchannan K, Johnston MV. Choline acetyltransferase activity in the striatum of neonatal rats is increased by nerve growth factor. Science. 1985;229:284–287. doi: 10.1126/science.2861660. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Bothwell M, Hersh LB, Kordower JH. Nerve growth factor receptor immunoreactive profiles in the normal aged human basal forebrain: colocalization with cholinergic neurons. J Comp Neurol. 1989;285:196–217. doi: 10.1002/cne.902850204. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Presley LN, Kordower JH. Nerve growth factor receptor immunoreactivity within the nucleus basalis (Ch4) in Parkinson’s disease: reduced cell numbers and colocalization with cholinergic neurons. Brain Res. 1991a;531:19–30. doi: 10.1016/0006-8993(91)90682-l. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Higgins GA, Kordower JH. Nerve growth factor receptor immunoreactivity in the monkey (Cebus apella) and aged human cerebellum. J Comp Neurol. 1991b;308:555–575. doi: 10.1002/cne.903080405. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Brasher-Krug T, Kordower JH. p75 Nerve growth factor receptor immunoreactivity in the human brainstem and spinal cord. Brain Res. 1992;589:115–123. doi: 10.1016/0006-8993(92)91169-f. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Cochran E, Benzing WC, Kordower JH. Galaninergic innervation of the cholinergic vertical limb of the diagonal band (Ch2) and bed nucleus of the stria terminalis in aging. Alzheimer’s disease and Down’s syndrome Dementia. 1993a;4:237–250. doi: 10.1159/000107329. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Conner JM, Varon S, Kordower JH. Nerve growth factor immunoreactivity in the basal forebrain and hippocampal formation in primate and Alzheimer’s disease brain. Soc Neurosci Abstr. 1993b;19:260. [Google Scholar]
- Mufson EJ, Conner JM, Varon S, Kordower JH. Nerve growth factor immunoreactive profiles in the primate basal forebrain and hippocampal formation. J Comp Neurol. 1994;341:507–519. doi: 10.1002/cne.903410407. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Kroin JS, Sobreviela T, Burke MA, Kordower JH, Penn RD, Miller JA. Intrastriatal infusions of brain-derived neurotrophic factor: retrograde transport and colocalization with dopamine containing substantia nigra neurons in rat. Exp Neurol. 1994;129:15–26. doi: 10.1006/exnr.1994.1143. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. Chemoarchitecture of the Brain. Berlin: Springer-Verlag; 1985. [Google Scholar]
- Nye SH, Squinto DJ, Glass TN, Stitt P, Hanntzopoulos MJ, Macchi NS, Lindsay NS, Ip NY, Yancopoulos GD. K-252a and staurosporine selectively block autophosporylation of neurotrophin receptors and neurotrophin-mediated responses. Mol Cell Biol. 1992;3:677–686. doi: 10.1091/mbc.3.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmichi M, Seeker SJ, Pang L, Saltiel AR. Inhibition of the cellular actions of nerve growth factor by staurosporine and K252A results from the attenuation of the activity of the trk tyrosine kinase. Biochemistry. 1992;31:4034–4039. doi: 10.1021/bi00131a019. [DOI] [PubMed] [Google Scholar]
- Parada LF, Tsoulfas P, Tessarollo L, Blair J, Reid SW, Soppet D. The Trk family of tyrosine kinases: receptors for NGF-related neurotrophins. Cold Spring Harb Symp Quant Biol. 1992;57:43–51. doi: 10.1101/sqb.1992.057.01.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1986. pp. 1–236. [Google Scholar]
- Pioro EP, Cuello AC. Distribution of nerve growth factor receptor-like immunoreactivity in the adult rat central nervous system. Effects of colchicine and correlation with the cholinergic system—I. Forebrain. Neuroscience. 1990a;34:54–87. doi: 10.1016/0306-4522(90)90304-m. [DOI] [PubMed] [Google Scholar]
- Pioro EP, Cuello AC. Distribution of nerve growth factor receptor-like immunoreactivity in the adult rat central nervous system effect of colchicine and correlation with the cholinergic system—II. Brainstem, cerebellum, and spinal cord. Neuroscience. 1990b;34:89–110. doi: 10.1016/0306-4522(90)90305-n. [DOI] [PubMed] [Google Scholar]
- Raivich G, Kreutzberg GW. The localization and distribution of high affinity B NGF binding sites in the central nervous system in the adult rat. A light microscopic autoradiographic study using (125I) B NGF. Neuroscience. 1987;20:23–36. doi: 10.1016/0306-4522(87)90003-0. [DOI] [PubMed] [Google Scholar]
- Rende M, Hagg T, Manthorpe M, Varon S. Nerve growth factor receptor immunoreactivity in neurons of the normal adult rat spinal cord and its modulation after peripheral nerve lesions. J Comp Neurol. 1992;319:285–298. doi: 10.1002/cne.903190208. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Verge Issa VMK, Riopelle RJ. Distribution of neuronal receptors for nerve growth factor in the rat. J Neurosci. 1986;6:2313–2321. doi: 10.1523/JNEUROSCI.06-08-02312.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Lagercrantz H, Persson H. Expression of members of the trk family in the developing postnatal rat brain. Brain Res Dev Brain Res. 1993;72:119–131. doi: 10.1016/0165-3806(93)90165-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg MB, Friedman T, Robertson RC, Tuszynski M, Wolff JA, Breakefield XO, Gage FH. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science. 1988;242:1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- Saika T, Senba E, Noguchi K, Sato M, Yoshida S, Kubo T, Matsunaga T, Tohyama M. Effects of nerve crush and transection on mRNA levels for nerve growth factor receptor in the rat facial motor neurons. Mol Brain Res. 1991;9:157–160. doi: 10.1016/0169-328x(91)90142-k. [DOI] [PubMed] [Google Scholar]
- Schumacher JM, Short MP, Hyman BT, Breakefield XO, Isacson O. Intracerebral implantation of nerve growth factor-producing fibroblasts protects striatum against neurotoxic levels of excitatory amino acids. Neuroscience. 1991;45:561–570. doi: 10.1016/0306-4522(91)90271-o. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Otteb U, Agid Y, Thoenen H. Nerve growth factor (NGF) in the rat CNS. Absence of specific retrograde transport and tyrosine hydroxylase induction in locus coeruleus and substantia nigra. Brain Res. 1979;168:473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- Seller M, Schwab ME. Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 1984;300:33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Gall CM. Expression of neurotrophins by midbrain dopaminergic neurons. Exp Neurol. 1993;124:119–128. doi: 10.1006/exnr.1993.1182. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran T, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Altar CA, Wiegand SJ, Lindsay RM. Antinoceptive effect of brain-derived neurotrophic factor and neurotrophin-3. Brain Res. 1994;633:326–330. doi: 10.1016/0006-8993(94)91556-3. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Wainer BH, Klein R, Barbacid M, Palfrey HC. High affinity nerve growth factor receptor (trk) immunoreactivity is localized in cholinergic neurons of the basal forebrain and striatum in the adult rat. Brain Res. 1993;612:330–335. doi: 10.1016/0006-8993(93)91681-h. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- Thoenen H, Barde DYA. Physiology of nerve growth factor. Physiol Rev. 1980;60:1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Sang H, Yoshida K, Gage FH. Recombinant human nerve growth factor infusions prevent cholinergic neuronal degeneration in the primate brain. Ann Neurol. 1991;30:625–636. doi: 10.1002/ana.410300502. [DOI] [PubMed] [Google Scholar]
- Whittemore SR, Ebendal T, Larkfort L, Olson L, Seiger A, Stromberg I, Persson H. Developmental and regional expression of p nerve growth factor messenger RNA and protein in the rat central nervous system. Proc Natl Acad Sci USA. 1986;83:817–821. doi: 10.1073/pnas.83.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Varon S, Peterson G, Wictorin K, Fisher W, Bjorklund A, Gage FH. Continuous infusion of nerve growth factor prevents basal forebrain neuronal cell death after fimbria-fornix transection. Proc Natl Acad Sci USA. 1986;83:9231–9236. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn SR, Hammang JP, Emerich DP, Lee A, Palmiter RD, Baetge EE. Polymer-encapsulated cells genetically modified to secrete human nerve growth factor promote the survival of axotomized septal cholinergic neurons. Proc Natl Acad Sci USA. 1994;91:2324–2328. doi: 10.1073/pnas.91.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988;8:3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Parada LF, Soppet D, Rush RA. Distribution of trkB tyrosine immunoreactivity in the rat central nervous system. Brain Res. 1993;622:63–70. doi: 10.1016/0006-8993(93)90802-t. [DOI] [PubMed] [Google Scholar]


