Abstract
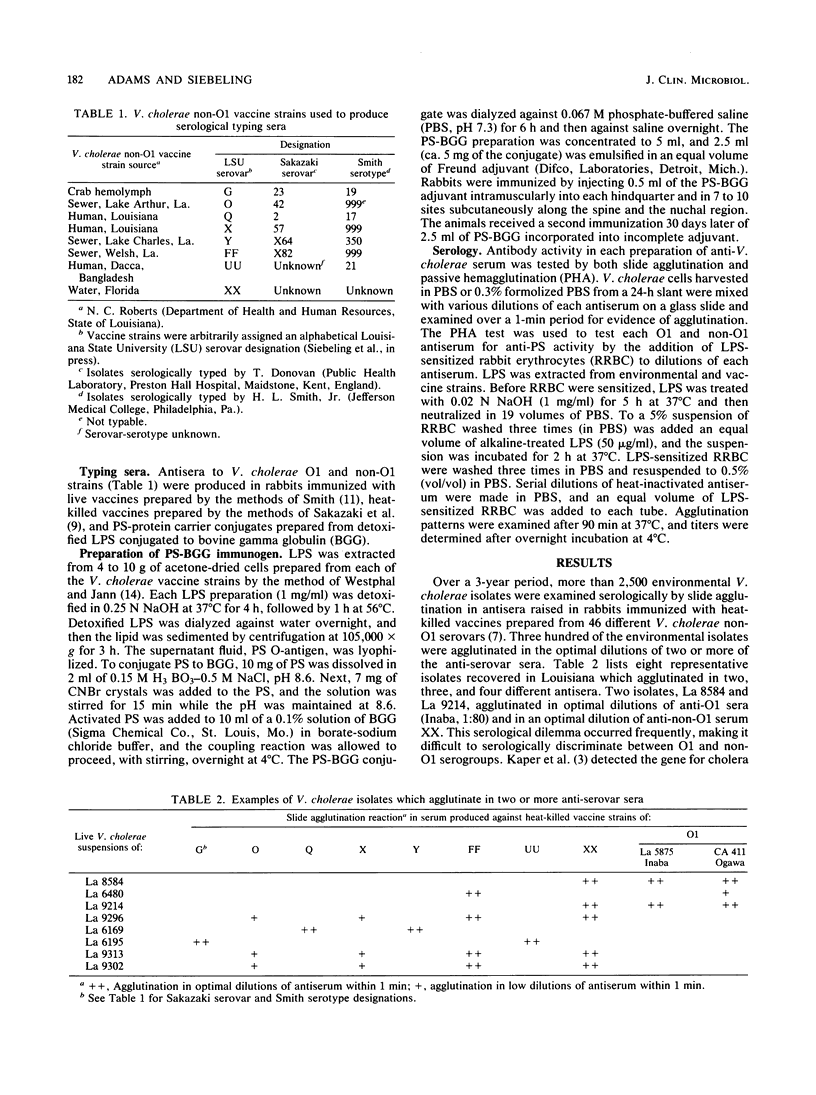
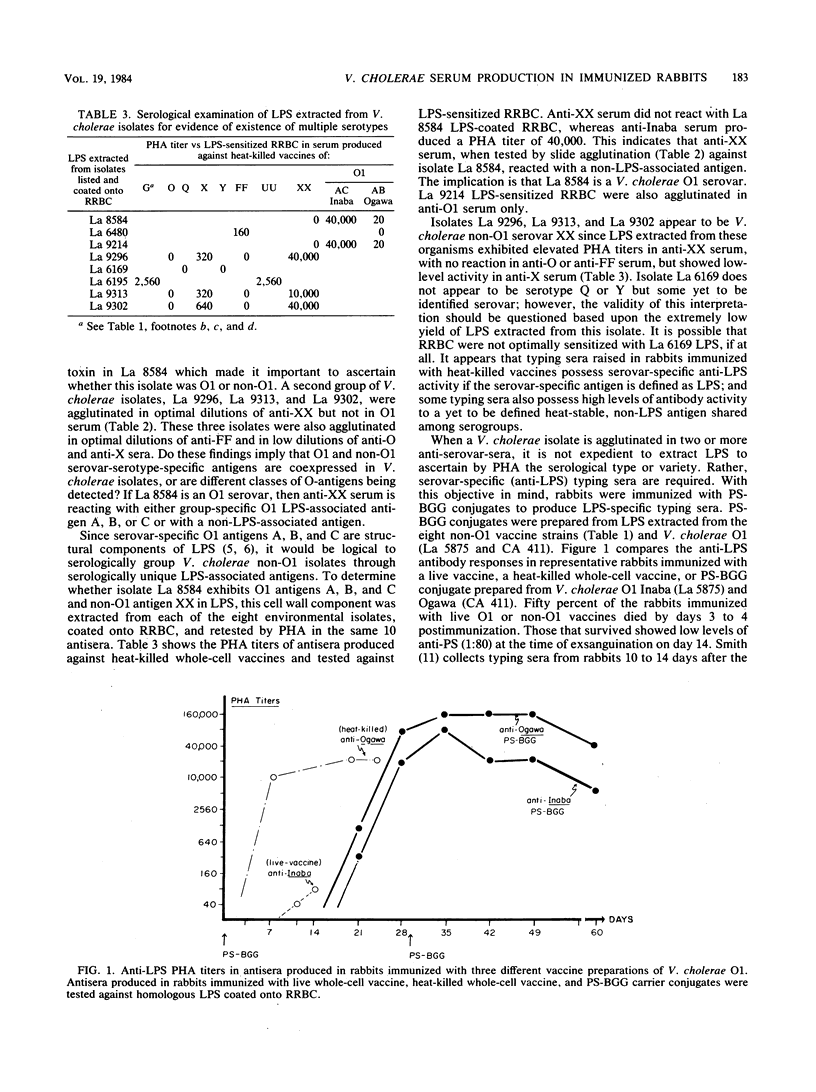
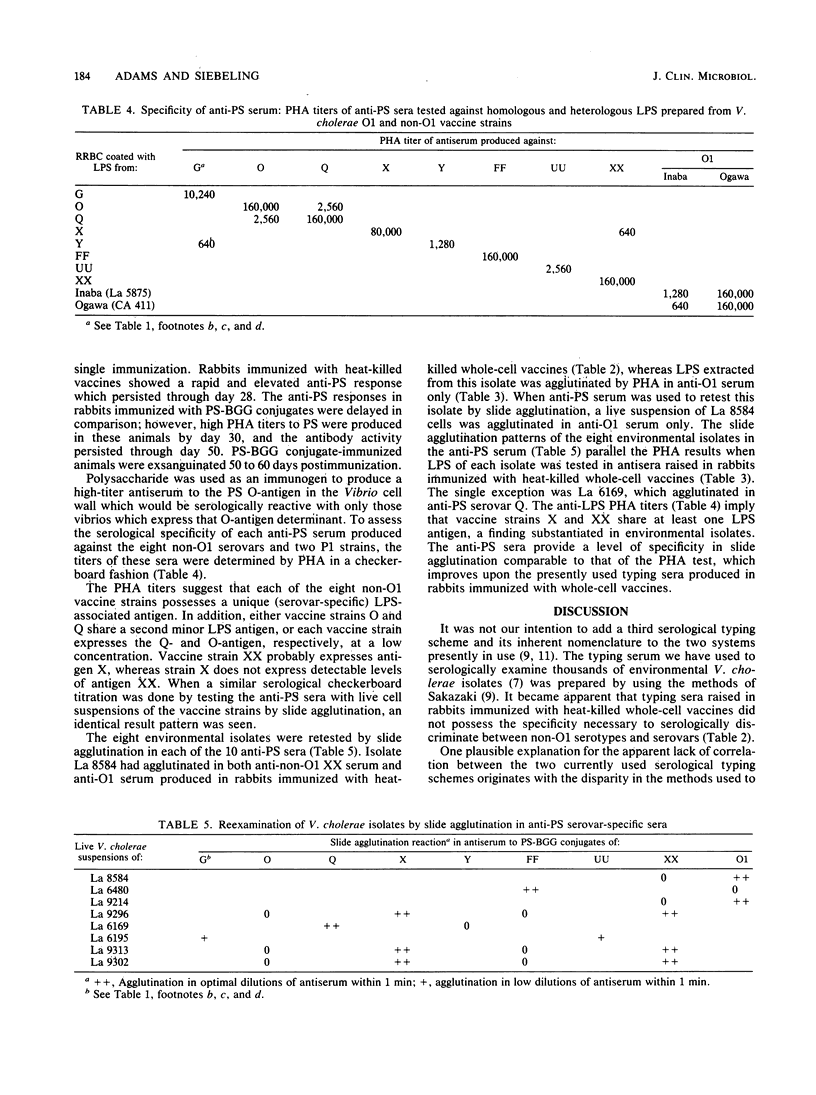
Two systems are currently used to serologically type Vibrio cholerae O1 and non-O1 isolates. Antiserovar-serotype serum in the Smith system is produced in rabbits immunized with live whole-cell vaccines, and that in the Sakazaki system is produced in rabbits immunized with heat-killed vaccines. In neither system is the serovar-serotype-specific antigen clearly defined. During the course of a serological survey, ca. 10% of more than 2,500 V. cholerae isolates examined agglutinated in the optimal dilutions of two, three, or four different anti-serovar sera prepared by the methods of Sakazaki. An occasional isolate agglutinated in both anti-O1 and non-O1 sera. Lipopolysaccharide was extracted from eight of these possible multiple serovars, coated onto rabbit erythrocytes, and retested in these same antisera by passive hemagglutination. With one exception the lipopolysaccharide-rabbit erythrocytes were now agglutinated in a single antiserum. Antipolysaccharide sera were produced in rabbits immunized with the polysaccharide moiety extracted from eight non-O1 and two O1 vaccine strains conjugated to bovine gamma globulin protein carrier. The antipolysaccharide sera showed passive hemagglutination titers versus lipopolysaccharide-rabbit erythrocytes comparable to those achieved in antisera from rabbits immunized with heat-killed whole-cell vaccines. In the slide agglutination test antipolysaccharide sera serologically discriminated between two O1 isolates that were previously agglutinated in both anti-O1 and anti-non-O1 whole-cell sera. It is recommended that serological types or varieties of V. cholerae non-O1 be based upon serologically recognizable differences in lipopolysaccharide-associated antigens as are antigens A, B, and C in the O1 group.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner D. J., Davis B. R., Kudoh Y., Ohashi M., Sakazaki R., Shimada T., Smith H. L., Jr Serological comparison of two collections of Vibrio cholerae non O1. J Clin Microbiol. 1982 Aug;16(2):319–323. doi: 10.1128/jcm.16.2.319-323.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond J. W. The structure of the O-antigenic side chain of the lipopolysaccharide of Vibrio cholerae 569B (Inaba). Biochim Biophys Acta. 1979 May 1;584(2):346–352. doi: 10.1016/0304-4165(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Sakazaki R., Shimada T. Serovars of Vibrio cholerae identified during 1970-1975. Jpn J Med Sci Biol. 1977 Oct;30(5):279–282. doi: 10.7883/yoken1952.30.279. [DOI] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K., Gomez C. Z., Sen R. Serological studies on the cholera group of vibrios. Jpn J Med Sci Biol. 1970 Feb;23(1):13–20. doi: 10.7883/yoken1952.23.13. [DOI] [PubMed] [Google Scholar]
- Shimada T., Sakazaki R. Additional serovars and inter-O antigenic relationships of Vibrio cholerae. Jpn J Med Sci Biol. 1977 Oct;30(5):275–277. doi: 10.7883/yoken1952.30.275. [DOI] [PubMed] [Google Scholar]


