Abstract
A monoclonal antibody (3A3) raised against a rat neural cell line (PC12) was shown previously to bind to the surfaces of these cells, inhibiting substratum adhesion. Immunochemical and other data indicated that the heterodimer recognized by 3A3 was a member of the integrin family of adhesive receptors and had a β1 subunit. The relationship of the α subunit to other integrins was unknown. Here we show that 3A3 recognizes in rat tissues a heterodimer (~185 kDa, ~110 kDa; unreduced) that is electrophoretically and immunochemically indistinguishable from the antigen in PC12 cells. Immunoaffinity purification of the heterodimer from neonatal rats and protein microsequencing indicate that the α subunit is identical at 11 or 13 N-terminal residues with VLA-1, an integrin on human hematopoietic cells. Monoclonal antibody 3A3 inhibits the attachment of rat astrocytes to laminin or collagen but not to fibronectin or polylysine. These data suggest strongly that the integrin recognized by 3A3 is the rat homologue of VLA-1, i.e., α1β1, and that α1β1 is a dual laminin/collagen receptor.
Previous reports described attachment and neurite outgrowth of a neural cell line (PC12) on laminin, collagen, and other adhesive proteins (Turner et al., 1987; Tomaselli et al., 1987, 1988). These functional studies suggested that PC12 cells possess a dual laminin/collagen receptor (Turner et al., 1987). Subsequently, monoclonal antibody (mab)1 3A3 generated against PC12 cells was found to inhibit adhesion to and neurite outgrowth on laminin and collagen (Turner et al., 1989). mab 3A3 immunoprecipitates two proteins of ~ 110 and ~ 185 kDa. First, functional (Turner et al., 1989) and, later, immunochemical data suggested these proteins were members of the integrin family (Hynes, 1987; Buck & Horwitz, 1987; Ruoslahti & Pierschbacher, 1987) of heterodimeric adhesive receptors. Tomaselli et al. (1987, 1988) had shown earlier that PC12 cells express primarily two integrins, each with a β1 subunit. A mixture of the two integrins incorporated into liposomes bound to laminin and collagen (Tomaselli et al., 1988). More recently, we have shown that one of these is identical with the integrin recognized by mab 3A3 (K. J. Tomaselli, D. E. Hall, L. F. Reichardt, L. A. Flier, D. C. Turner, and S. Carbonetto, unpublished experiments). Since mab 3A3 recognizes a single heterodimer, it appears that this integrin is a dual receptor for laminin and collagen (Turner et al., 1989). While the β subunit of this receptor has been identified as β1, the identity of the α subunit has not been determined. Since the α subunits in the β1 integrin family largely determine the ligand binding specificity of each heterodimer (Hynes, 1987; Ruoslahti & Pierschbacher, 1987), it was important to ascertain whether the α subunit had a homologue among the several integrins identified in other species to which its functional properties could be related.
Here we show that the heterodimer in PC12 cells recognized by mab 3A3 is expressed in rat tissues where it is electrophoretically and immunologically indistinguishable from that in PC12 cells. Having this rich source of receptor has permitted purication and microsequencing of the larger subunit. The amino acid sequence shows unambiguously that this is an integrin α subunit identical at 11 of the first 13 N-terminal amino acid residues with the α1 subunit of human VLA-1, an integrin in human hematopoietic cells (Hemler, 1988). These data, together with those of others (Kramer & Marks, 1989; Ignatius & Reichardt, 1988; discussed below), argue strongly that the α1β1 integrin is a dual laminin/collagen integrin is hematopoietic cells and neural cells where it mediates neurite outgrowth in culture (Turner et al., 1989) and nerve regeneration in vivo (Toyota et al., 1990).
Experimental Procedures
Cell Culture and Immunocytochemistry
PC12 cells were maintained in culture in RPMI medium (Gibco) plus 5% fetal calf serum and 10% horse serum as described previously (Turner et al., 1988; Greene & Tischler, 1982). Primary cultures highly enriched for astrocytes (80–95%) were prepared by the methods of McCarthy and deVellis (1980) and rat dorsal root ganglion cultures by methods similar to those reported previously (Carbonetto & Stach, 1982). Primary cultures were maintained in Dulbecco’s minimum essential medium (DMEM; Gibco) plus 10% fetal calf serum, gentamicin or penicillin/streptomycin (l%), and, for dorsal root ganglion cultures, nerve growth factor (βNGF; 100 ng/mL).
For immunocytochemistry, astrocytes and neurons were labeled with primary and fluorescently labeled secondary antibodies by conventional methods (Turner et al., 1989). Cultures of rat myotubes and fibroblasts where prepared from neonatal rat hind limb (Bloch, 1979) and were treated with the detergent saponin (0.2% in PBS supplemented with 10 mM MgCl2, and 1 mM EGTA). After gentle agitation for 5–10 min, the bulk of the cellular material is removed from the culture substratum, leaving behind regions of the cell firmly adherent to the substratum (Bloch, 1984). This substratum-attached material was fixed with 2% paraformaldehyde in PBS for 15 min, blocked with 0.1 M glycine in PBS, and processed for immunocytochemistry.
Cell Attachment Assays
Culture dishes (24 wells) were coated with collagen (Sigma Type III), laminin (purified from Engelbreth–Holm–Swarm sarcoma; Timpl et al., 1982), fibronectin (purified from horse serum; Chiquet et al., 1979), or polylysine (300 kDa; Sigma) in PBS overnight at 4 °C. A series of parametric studies were done to ascertain the concentrations of these proteins/poly(amino acids) that gave just maximal attachment of rat astrocytes. At these concentrations (50 μg/mL for fibronectin; 10 μg/mL for collagen, laminin, and polylysine), the sensitivity of the assay for inhibition of attachment is optimal. Following coating, the substrata were washed and were equilibrated with 200 μL of solution containing mab 3A3 in PBS (100 μg/mL). Primary cultures of rat astrocytes were detached from their substrata with EDTA (1 mM) in Ca2+/Mg2+-free Hanks’ balanced salt solution and centrifuged, the Hanks’ solution was replaced with culture medium, and the cells were triturated gently to give a suspension of single cells. The number of cells in suspension was determined by counting a small volume in a hemocytometer, and aliquots (200 μL) of cells were pipeted into individual culture wells to assess attachment to the coated substrata. The dishes were gently shaken to distribute the cells uniformly within each well. The number of cells attached were assayed as described previously (Turner et al., 1989).
Receptor Purification
mab 3A3 (IgG1) was partially purified from ascites fluid on an Affi-Gel Blue column (Bio-Rad) and was covalently coupled to Affi-Gel 10 (Bio-Rad) at 8 mg of IgG/mL of beads. Thirty neonatal rats (4–6 days) were homogenized in ice-cold phosphate-buffered saline (PBS; 500 mL) plus phenylmethanesulfonyl fluoride (1 mM), N-ethylmaleimide (1 mM), soybean trypsin inhibitor (50 μg/mL), and NP-40 (1 %). The crude extract was centrifuged at l000g (10 min) followed by 100000g (30 min), and the pellets were discarded in each case. The supernatants were equilibrated overnight at 4 °C with Affi-Gel 10 beads (25 mL) coupled to bovine serum albumin, and the remaining extract (400 mL) was similarly equilibrated with antibody-coupled Affi-Gel IO. The beads (25 mL) were washed with PBS containing 0.1% NP-40 and eluted with glycine buffer (0.3 M, pH 2.0) whereupon the eluant was immediately neutralized with Tris base. The eluant was dialyzed against distilled water containing protease inhibitors, lyophilized, and subjected to SDS-PAGE using recrystallized SDS (Hunkapiller & Hood, 1983).
Immunoblotting
Partially purified 3A3 antigen was immunoblotted by the methods of Towbin et al. (1979). Briefly, equal aliquots of partially purified 3A3 antigen were electrophoresed in separate lanes of a polyacrylamide gel (5%) and the proteins transferred electrophoretically to nitrocellulose paper. The lane with molecular weight standards was stained with Coomassie blue, and the rest of the blot was blocked with 3% bovine serum albumin in PBS overnight at 15 °C. Next, the blot was washed five times for 10 min each with 0.1% Tween-20 in PBS and then cut into separate lanes. Each lane was incubated with primary and secondary antibodies for 1–2 h with shaking at 23 °C and washing between antibody changes. After a final wash with 0.1% Tween-20 in PBS, binding of the horseradish peroxidase conjugated secondary antibody was revealed by reaction with diaminobenzidine.
Protein Microsequencing
A substantial reduction in nonspecific binding of proteins from rat tissue extracts to Affi-Gel 10 columns was obtained by preabsorption of the extract (overnight at 4 °C) against Affi-Gel 10–bovine serum albumin beads. The preabsorbed extract was affinity purified and electrophoresed as described above. Bands at 185 kDa from several gels (nonreducing conditions) were cut out, the protein was electroeluted, dialyzed, and relyophilized, and the pooled protein was subjected to a second round of SDS–PAGE. The resulting single band was electroblotted onto an Immobilon membrane (Millipore) and stained with Coomassie blue (Matsudaira, 1987). The electroblotted protein band was excised from the Immobilon membrane and placed in line before a polyprene-treated glass fiber filter (Matsudaira, 1987). Edman degradation and amino acid identification were performed with an Applied Biosystems Model 477A protein sequencer on line to a Model 120A PTH analyzer (AB1 User Bulletin 32). A total of 23 amino acid residues were identified with an initial yield of 35 pmol.
Reagents
Polyclonal antisera to integrins were generated in rabbits to purified α1 and β1 subunits from rats (Houde and Carbonetto, unpublished observations) or were purchased (anti-β1, and anti-β3 antisera; Telios Pharmaceuticals, La Jolla, CA). Fluorescently labeled secondary antibodies as well as antisera to glial fibrillary acidic protein were purchased (Cappel). NGF was the generous gift of Dr. R. Stach (University of Michigan, Flint, MI).
Results and Discussion
In earlier studies (Turner et al., 1989), two mabs to PC12 cells were isolated. One, mab 3A3, was mentioned above to inhibit neurite outgrowth and attachment of PC12 cells to laminin and collagen. The other, mab 1B1, bound more avidly to PC12 cells than 3A3 but was a relatively weak inhibitor of substratum adhesion. mab 1B1 recognizes multiple polypeptides on PC12 cells (Turner et al., 1989) but labels few rat primary cells in culture. In contrast, the 3A3 antigen is found on a variety of rat cells in culture (Figure 1) including neurons, astrocytes, and fibroblasts but not myotubes (not shown). The antigen is found in a fine punctate distribution over the cell surface. Mild extraction of fibroblasts with the detergent saponin leaves behind portions of the cell firmly adherent to the substratum (Bloch, 1987) which contain linear arrays of the 3A3 antigen (Figure 1B). Thus, 3A3 labels primary cultures of rat cells, a feature our data show is not necessarily expected for mabs raised against neural cell lines. Moreover, the distribution of the labeling in regions of fibroblasts firmly adherent to the substratum is consistent with the pattern of labeling seen with other matrix receptors (Burridge, 1987) and with the functional studies, implicating the 3A3 antigen in substratum attachment.
FIGURE 1.
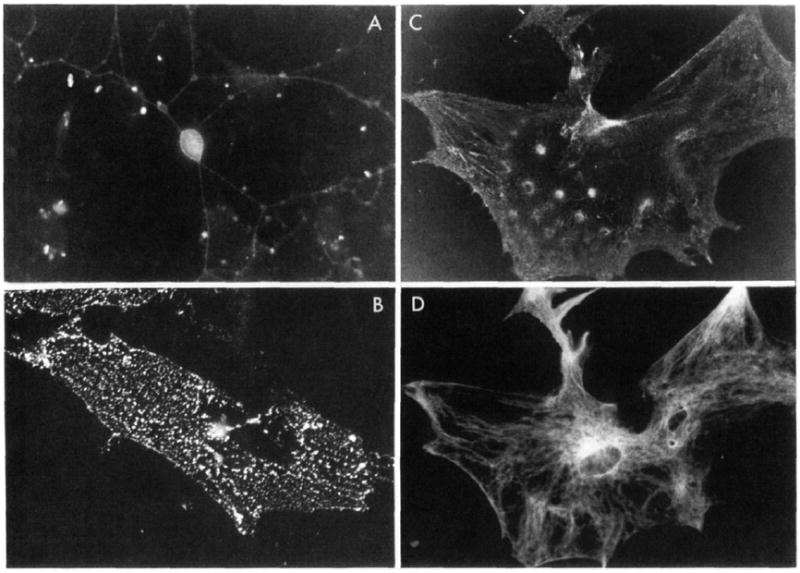
Immunocytochemical localization of the 3A3 antigen in primary cultures of rat cells. (A) Rat dorsal root ganglion cells were fixed and incubated with mab 3A3 and later with fluorescein-labeled rabbit anti-mouse antibody. A neuron (~25 μm in diameter) and its extensive neuritic arbor labeled with mab 3A3 are visible in the center of this field. (B) A culture of rat myoiubes and fibroblasts was fixed and treated with the detergent saponin (Bloch. 1984). Under these conditions (see Experimental Procedures) many cells detach, but substratum adhesion sites remain. Myotubes in these cultures do not label with mab 3A3, but in this field attachment sites of fibroblasts rich in the 3A3 antigen are visible adherent to the culture dish. (C) Cultures enriched for rat astrocytes fluoresce brightly after immunocytochemical localization of the 3A3 antigen. This cell can be identified as an astrocyte since it also reacts with an antiserum to glial fibrillary acidic protein (D).
It seemed reasonable to conclude from the immunocytochemical studies that mab 3A3 was binding to the same integrin in rat tissues as in PC12 cells. Since the former could serve as an abundant source of the antigen for purification, methods for isolation of the antigen from PC12 cells with mab 3A3 coupled to Affi-Gel 10 beads were scaled up, permitting isolation of ~100 μg of protein from 30–100 neonatal rats. However, data showing that fibronectin integrins can vary in size and immunological cross-reactivity in tissues of the same animal (Johannsen et al., 1987) led us to compare further the heterodimer from rat tissues with that from PC12 cells. The larger subunit of the heterodimer purified from neonatal rats or PC12 cells is electrophoretically the same (~185 kDa, nonreducing conditions; Figure 2A, lanes 3 and 4). The smaller subunit (~110 kDa) is found in varying amounts from preparation to preparation and is not always visible in silver-stained gels (Figure 2A, lane 3). The reason for this variability in isolation of the two subunits of the heterodimer from rat tissues and PC12 cells is unclear but may involve disxriation of the heterodimer by the mab. In Figure 2B (lane 1) is shown a preparation in which the lower band from rat tissues has been immunoblotted with an anti-β1 antiserum that similarly crass-reacts with the smaller of the bands precipitated from PC12 cells by mab 3A3 (Turner et al., 1989). This same band does not cross-react with rabbit nonimmune antisera or an anti-β3 antiserum (Figure 2B, lanes 2 and 3). Finally, an antiserum raised in rabbits to the lower band labels the surfaces of chick and rat cells in culture in a stress fiber-like pattern characteristic of antisera to integrins (data not shown). On the basis of its size and recognition by monoclonal and polyclonal antibodies, the heterodimer in rat tissues appears identical with that in PC12 cells.
FIGURE 2.
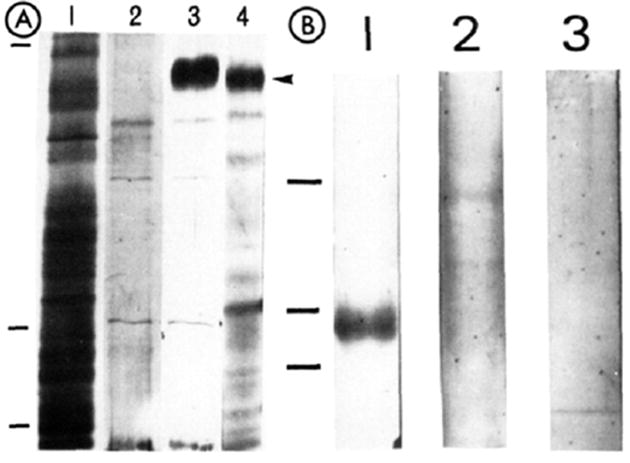
Characterization of the 3A3 antigen from neonatal rats. (A) Partially purified 3A3 antigen from neonatal rats and PC12 cells was subjected to SDS-PACE: (lane 1)crude rat extract: (lane 2) proteins eluted from a bovine serum albumin affinity column: (lane 3) neonatal rat proteins eluted from a mab 3A3 affinity column. (lane 4) PC12 protein eluted from the same 3A3 column. The arrowhead shows the position of the α subunit (185 kDa) in these silver-stained gels. (B) Western blot of the partially purified heterodimer from neonatal rats incubated with plyclonal antisera to β1 or β3 integrins or with rabbit nonimmune serum. A band at ~ 110 kDa is visible in the lane incubated with anti-β1 antisera but not in lanes incubated with either anti-β3, or rabbit nonimmune sera. The immunological reactivity identifies this lower band as a β1 subunit, and its electrophoretic mobility is the same as that reported previously for the PC12 antigen (Turner et al., 1989). The pitions of molecular mas markers arc shown to the left of the two gels in this figure and correspond to markers of 200, 116, and 92 kDa.
The integrin family has been discovered recently, hence relationships among the members are still being detailed. For the most part, these relationships have been established by immunochemical and electrophoretic criteria similar to those discussed above. While highly successful, some confusion may arise from these indirect criteria. For example, the larger subunit recognized by mab 3A3 decreases in electrophoretic mobility upon reduction (Turner et al., 1989), a feature more often associated with β rather than α subunits, several of which decrease in size when reduced (Hynes, 1987; Rouslahti & Pienchbacher. 1987; Takada et al., 1987a). This, together with the nonstoichiometric isolation of the two subunits of the heterodimer (Figure 2), delayed our earliest attempts to identify this receptor as an integrin. In the present studies, however, purification of the heterodimer recognized by mab 3A3 has allowed us to obtain protein microsequence data which confirm unambiguously that the larger subunit is an α subunit of the integrin family. This is evident from the fint four to five N-terminal amino acid residues that are highly conserved among integrin α subunits of the β1 class (Figure 3). In addition, 11 of the first 13 amino acids are identical with the α subunit of VLA-1 (Takada et al., 1987b). The variation in amino acids 7 and 10 between the 3A3 α and VLA-1 α are conservative amino acid substitutions which may result from single-base changes in the coding region of the gene. In conclusion, it appears that mab 3A3 recognizes the rat homologue of VLA-1, i.e., an integrin with α1β1 subunits.
FIGURE 3.
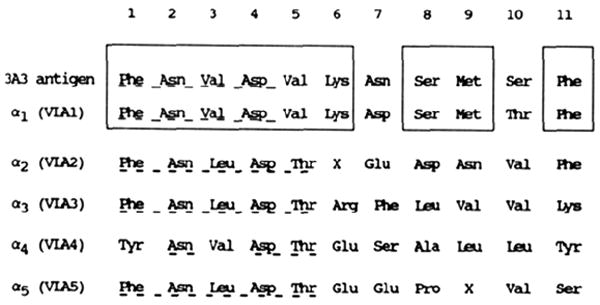
Protein microsequence data for the 185-kDa protein recognized by mab 3A3 compared with those reported for the α chains of VLAs 1–5 (Takada et al., 1987b). The rat antigen is similar through the first four residues to other α subunits of the VLAs (underlined). The homology of this α subunit to VLA-1 is evident from the boxed regions. The amino acid sequence for an additional 12 amino acid residues of the 3A3 antigen was found to be Ser-Gly-Pro-Val-Glu-Asp-Met-Phe-Gly-Tyr-Thr-Val. The sequences obtained from two separate preparations of purified protein matched perfectly, indicating no obvious polymorphisms.
Assignment of function among the integrins has relied heavily on inhibitory antibodies. Since different α subunits may be associated with the same type of β subunit (Buck & Horwitz. 1987) and vice versa (Cheresh et al., 1989), it is essential that these antibodies recognize a single heterodimer. For example, anti-β1 antibodies bind to two major integrins in PC12 cells, inhibiting them both (Tomaselli et al., 1987, 1988). In contrast, mab 3A3 recognizes a single heterodimer in PC12 cells and rat tissues. Earlier studies (Turner et al., 1989) showed that mab 3A3 inhibited PC12 attachment to laminin and collagen but not to polylysine or the plant lectin wheat germ agglutinin. The PC12 cells used for these studies attached only poorly to fibronectin, so it was not possible to assess whether mab 3A3 inhibited attachment to fibronectin. Here we show that rat astrocytes attach to plastic substrata coated with laminin, collagen, fibronectin, and polylysine (Figure 4). mab 3A3 inhibits attachment to collagen by 69% and to laminin by 28% with respect to control cells untreated with mab. The residual attachment is likely mediated by integrins other than the α1β1 that are found in astrocytes (Tawil and Carbonetto, unpublished observations). Inhibition of attachment to fibronectin and polylysine was not significant (3 and 7% respectively). This selectivity in inhibition extends previous data (Turner et al., 1989), which support the proposal that this antibody recognizes a dual laminin/collagen receptor.
FIGURE 4.
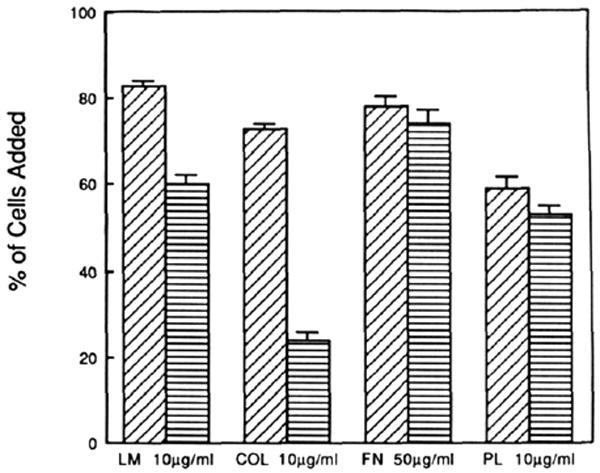
Inhibition of attachment of rat astrocytes to laminin and collagen. The concentrations of proteins/poly(amino acid) used to coat the substrata approached that which gave maximum attachment of astrocyta to optimize detection of antibody inhibition of attachment (see Experimental Procedures). mab 3A3 (100 μg/mL) inhibits attachment to laminin and collagen with no significant inhibition on fibronectin- or polylysine-coated substrata. The results are from two separate experiments with each substratum tested in triplicate. The diagonal hatched bars are without mab 3A3, and horizontally hatched bars are with mab 3A3. The error bars show the standard deviation of the mean.
Kramer and Marks (1989) utilized affinity column chromatography to show that solubilized VLA-1, a human integrin heterodimer, binds directly to collagen. The binding was indifferent to the type of collagen used. In their studies, VLA-1 did not bind to laminin. Ignatius and Reichardt (1988) have reported a rat β1 inlegrin with an α subunit of 185 kDa which binds to laminin in a cation-dependent manner, similar to the one recognized by mab 3A3. Since it is likely that the integrins in these two studies are both α1β1, the apparent discrepancy in the binding of this integrin to laminin may be resolved by our finding that the rat α1β1 functions in cells as a dual laminin/wllagen receptor. In fact. Kramer and Marks (1989) noted that functionally inhibitory antibodies would be necessary to confirm their results since affinity chromatography of integrins in detergent-containing solutions may not always reflect the function of a receptor in vivo (Wayner & Carter, 1987; Hynes et al., 1989). We conclude that the rat and human α1β1 integrins are receptors that bind directly to sites in laminin and collagen. Ultimately this must be confirmed by structure–function studies on both the human and rat α1β1 heterodimers. It seems probable, however, that the conservation in the amino acid sequence at the N-terminal region of these proteins is carried through to the ligand binding sites of these two α subunits from species which are evolutionarily closely related. Once the primary structure of the two α subunits has been determined, it may be possible to identify the region recognized by mab 3A3 and map a functionally important domain(s) of this protein.
Acknowledgments
We are grateful to Drs. Robert Bloch and George Dmytrenko for help with immunocytochemical studies of rat myotubes and fibroblasts.
Footnotes
This work was supported in part by grants to S.C. from the MRC (MA 10182) and the Spinal Cord Research Foundation, to D.C.T. from the NIH (NS 27409) and the Competitive Grants Program of the U.S. Department of Agriculture (86 CRCR 11947), and to L.F.R. from the U.S. Public Health Service. L.F.R. is an investigator at the Howard Hughes Medical Institute. S.C. is the recipient of a Chercheur Boursier from the Fonds de la Recherche en Santé du Québec; M.H. is the recipient of a postdoctoral fellowship from the Rick Hansen Association.
Abbreviations: mab, monoclonal antibody; VLA, very late antigen; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; DMEM, Dulbecco’s minimum essential medium; PBS, phosphate-buffered saline, pH 7.2.
References
- Bloch RJ. J Cell Biol. 1979;82:626–643. doi: 10.1083/jcb.82.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch RJ. J Cell Biol. 1984;99:984–993. doi: 10.1083/jcb.99.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CA, Horwitz AF. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Burridge K. Cancer Rev. 1987;4:18–78. [Google Scholar]
- Carbonetto S, Stach R. Dev Brain Res. 1982;3:364–378. doi: 10.1016/0165-3806(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Cheresh D, Smith JW, Cooper HM, Quaranta V. Cell. 1989;57:59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Chiquet ME, Puri EC, Turner DC. J Biol Chem. 1979;254:5474–5482. [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Adv Cell Neurobiol. 1982;3:373–414. [Google Scholar]
- Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH. J Cell Biol. 1990 doi: 10.1083/jcb.110.6.2175. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME. Immunol Today. 1988;41:109–113. doi: 10.1016/0167-5699(88)91280-7. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Huang C, Schwarz L. J Biol Chem. 1987;262:3300–3309. [PubMed] [Google Scholar]
- Hunkapiller MW, Hood LE. Methods Enzymol. 1983;91:486–494. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Marcantonio EE, Stepp MA, Urry LA, Yee GH. J Cell Biol. 1989;109:409–420. doi: 10.1083/jcb.109.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius MJ, Reichardt LF. Neuron. 1988;1:713–725. doi: 10.1016/0896-6273(88)90170-5. [DOI] [PubMed] [Google Scholar]
- Johansson S, Forsberg E, Lundgren B. J Biol Chem. 1987;262:7819–7824. [PubMed] [Google Scholar]
- Kramer RH, Marks N. J Biol Chem. 1989;264:4684–4688. [PubMed] [Google Scholar]
- Leptin M. Nature. 1986;321:728. doi: 10.1038/321728a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Takada Y, Huang C, Hemler ME. Nature. 1987a;326:607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- Takada Y, Strominger JL, Hemler ME. Proc Natl Acad Sci USA. 1987b;84:3239–3243. doi: 10.1073/pnas.84.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Risteli L, Ott U, Robey PG, Martin GR. Methods Enzymol. 1982;82:831–839. doi: 10.1016/0076-6879(82)82104-6. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Damsky CH, Reichardt LF. J Cell Biol. 1987;105:2347–2358. doi: 10.1083/jcb.105.5.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Damsky CH, Reichardt LF. J Cell Biol. 1988;107:1241–1252. doi: 10.1083/jcb.107.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota B, Carbonetto S, David S. Proc Natl Acad Sci USA. 1990;87:1319–1322. doi: 10.1073/pnas.87.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DC, Flier LA, Carbonetto S. Dev Biol. 1987;121:510–525. doi: 10.1016/0012-1606(87)90187-4. [DOI] [PubMed] [Google Scholar]
- Turner DC, Flier LA, Carbonetto S. J Neurosci. 1989;9:3287–3296. doi: 10.1523/JNEUROSCI.09-09-03287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner EA, Carter NG. J Cell Biol. 1987;105:1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]


