Abstract
Focal adhesion kinase (FAK) is a critical component in transducing signals downstream of both integrins and growth factor receptors. To determine how the loss of FAK affects the epidermis in vivo, we have generated a mouse model with a keratinocyte-restricted deletion of fak(FAKK5 KO mice). FAKK5 KO mice displayed three major phenotypes – irregularities of hair cycle, sebaceous glands hypoplasia, and a thinner epidermis – pointing to defects in the proliferative capacity of multipotent stem cells found in the bulge. FAK-null keratinocytes in conventional primary culture undergo massive apoptosis hindering further analyses, whereas the defects observed in vivo do not shorten the mouse lifespan. These results suggest that the structure and the signaling environment of the native tissue may overcome the lack of signaling through FAK. Our findings point to the importance of in vivo and threedimensional in vitro models in analyses of cell migration, proliferation, and survival. Surprisingly, the difference between FAKloxP/+ and FAKK5 KO mice in wound closure was not statistically significant, suggesting that in vivo loss of FAK does not affect migration/proliferation of basal keratinocytes in the same way as it affects multipotent stem cells of the skin.
Keywords: FAK, wound healing, hair, migration, proliferation, keratinocytes, stem cells
Introduction
Signals from extracellular matrix (ECM) and growth factors, transduced by integrin family adhesion receptors and growth factor receptors, play critical roles in regulating keratinocyte function by activating intracellular signaling cascades. Integrins, which have no intrinsic kinase activity, function by activating nonreceptor tyrosine kinases, such as focal adhesion kinase (FAK), and mobilizing the cytoskeleton. In our previous studies, we knocked out fak by homologous recombination in mouse embryonic stem cells. Mutant embryos died during late stages of gastrulation with the most striking defects in the morphogenesis of mesodermal tissues (Furuta et al., 1995; Ilic et al., 1995a). Using this animal model and cells of mesodermal origin derived from FAK-null embryos (fibroblasts, endothelial cells), we and others have determined that FAK plays an indispensable role in regulating cell migration, cell proliferation, and morphogenesis (Abbi and Guan, 2002; Damsky and Ilic, 2002; Hanks et al., 2003; McLean et al., 2003; Parsons, 2003; Schlaepfer et al., 2004; Mitra et al., 2005). FAK has also recently been shown to integrate signals from adhesion receptors and growth factor receptors (Sieg et al., 2000).
Much less is known about the role of FAK in epithelial tissues. However, given the important signaling functions determined for FAK in other cell types, we hypothesized that FAK plays critical roles in keratinocyte proliferation and migration. To address this hypothesis, we have generated a mouse model with a keratinocyte-restricted deletion of FAK by crossing mice carrying a floxed fak gene (Beggs et al., 2003) with transgenic mice expressing Cre recombinase under the control of the K5 promoter (Ramirez et al., 2004). In the skin, this promoter is active in basal cells of stratified epithelia and the outer root sheath cells of hair follicles. The mutant mice displayed three major phenotypes in the hair cycle, sebaceous glands, and epidermis, suggesting that proliferation and/or migration of multipotent stem cells in the bulge might be impaired in the absence of signaling through FAK.
Keratinocyte proliferation and migration are also important features during the second phase of wound healing when resident skin cell types proliferate and migrate from the proximal intact tissue to the wound site. Among the 50 structural and regulatory genes whose expression is significantly higher in keratinocytes and other resident cells surrounding the wound compared to cells away from the wound (Coulombe, 1997), more than half are linked either directly or indirectly to activation and function of FAK (Schlaepfer et al., 2004). However, comparative studies of skin wound healing kinetics in normal (FAKloxP/+) and mice with a keratinocyte-restricted deletion of FAK (FAKK5 KO) had a surprising outcome. Whereas in vivo wound healing assays failed to demonstrate a requirement of FAK for wound closure, isolated FAK-null keratinocytes were unable to survive in the culture.
Results
FAK is expressed in mouse epidermis
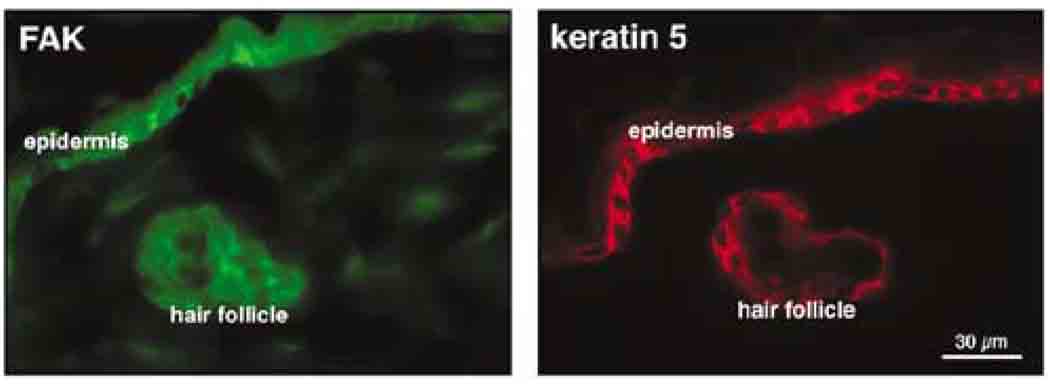
Initially, we used immunolocalization to evaluate FAK expression in the adult mouse skin. Keratin 5-positive cells of mouse epidermis and hair follicle also stained positively for FAK on an adjacent section in a pattern that was primarily plasma membrane associated (Figure 1).
Figure 1.
FAK is expressed in keratin 5-positive cells of adult mouse skin. Immunostaining for FAK was carried out with the anti-FAK antibody from LabVision. A similar staining pattern was obtained with the anti-FAK antibodies from Santa Cruz Biotechnology (data not shown).
Generating mice lacking FAK in keratin-5-expressing cells
We generated a conditional deletion of FAK using CreloxP technology. loxP sites flanking the second exon of the FAK kinase domain were introduced into embryonic stem cells (Beggs et al., 2003). Homozygous FAKoxP/loxP mice and all heterozygous mice – FAKoxP/+, FAKK5loxP/+, FAKoxP/− mice carrying one FAK-loxP allele and one either wild-type (FAKoxP/+) or one FAK-loxP and one FAK-null allele (FAKoxP/−) from the original knockout (Ilic et al., 1995b) – as well as mice with one recombined and one wild-type allele (FAKK5 loxP/+) were viable, fertile and showed no obvious phenotype. Specific deletion of FAK in the epidermis was accomplished by mating FAKoxP/− female mice to FAK heterozygous male mice that also expressed Cre recombinase driven by K5 promoter (Ramirez et al., 2004). K5 expression is first detected in ectodermal cells at embryonic day (E) l1.5. From El3.5 onwards, K5 is detected in the precursors of most of the epithelia and organs that express K5 at adult stages, including the skin. At birth, the mice designed to disrupt the expression of fak in K5-expressing epithelia (FAKK5 KO) did not differ obviously from their littermates.
FAKK5 KO mice do not express full-length FAK, FAK fragments, or FRNK in epidermis
The first step in evaluating offspring of the mating described above was to confirm that expression of fulllength FAK was indeed disrupted at the DNA as well as RNA and protein levels. Genomic DNA was prepared from tail biopsies of 7-day-old mice and their genotypes were determined by PCR (Figure 2a). In crossing of male K5Cre+FAK+/− and female K5Cre−FAKoxP/− mice, one out of six newborn pups should have a conditional deletion of FAK in K5-positive cells. Genotype analysis of the first 286 pups showed a Mendelian distribution, suggesting that deletion of FAK in K5-positive cells does not result in either embryonic or perinatal lethality (Figure 2b).
Figure 2.
DNA, RNA, and protein analyses confirmed disruption of full-length FAK in FAK conditional knockout mice. (a) Possible genotypes from crossing of male K5Cre+FAK+/− and female K5Cre−FAKloxP/− mouse. WT, wild type; HE, heterozygote; KO, knockout. (b) Distribution of the wild-type (18.2%), heterozygote (64.7%), and knockout (17.1%) genotype among first 286 pups is according to the Mendelian law (16.7, 66.6, and 16.7%, respectively). (c) RNA quality and quantity were analysed using the Agilent 2100 bioanalyser and RNA 6000 Nano LabChip Kit (Agilent Technologies). Only samples with the highest quality of RNA (i.e. 3, 4, and 5) were used for real-time PCR analyses. RNA was of comparable quality in both whole skin and epidermal prep from the same animal. (d) Real-time PCR analyses of FAK expression. Only samples from FAKK5 KO and FAKloxP/+ littermates that had similar mRNA levels of E-cadherin (E-cad), desmoglein 3 (Dgl3), and K5, genes expressed specifically in epidermis but not in dermis, were compared for FAK mRNA levels. Data are shown as fold increase/decrease of mRNA levels in samples from FAKK5 KO animals using log l0 scale. The differences are less pronounced and error bars are bigger in samples prepared from whole skin because of the presence of dermal fibroblasts, which do not have deleted FAK. Scheme of FAK, outlining in red the deleted exon, which encodes amino acids 445–472, and the location of primers for real-time PCR (arrows) are shown. (e) Northern blot analyses of FAK expression. Northern blots with both FAK N- and C-terminal probes demonstrated that the epidermis of FAKK5 KO mice has greatly reduced intensity of bends corresponding to full-length FAK mRNA. As a positive control for FRNK expression, we used RNA isolated from primary mouse embryonic fibroblasts transduced with FRNK-expressing adenovirus. Although the total amount of loaded RNA (20 µg/lane) was the same in all samples (see ethidium bromide staining of the gel), levels of GAPDH mRNA and FAK were higher in fibroblasts. Therefore, image of the blot with a C-terminal probe is a combination of shorter and longer exposure. FRNK was not detected in mouse epidermis at the age examined (2–4-day-old animals). (f) Western blot analyses of FAK expression. Whole lysates from the epidermis of newborn pups were blotted with antibodies against FAK N-terminal, kinase, and C-terminal domains. Lower molecular weight bands in FAKloxP/+ lysates are likely products of FAK degradation/cleavage by calpain or caspases (Mitra et al., 2005). UBI, N-terminal monoclonal 4.41 antibody from Upstate Biotechnologies; TL, monoclonal antibody from BD Transduction Laboratories; LV, polyclonal antibody from Lab Vision.
To confirm the absence of full-length FAK mRNA in the epidermis of FAKK5 KO mice, we used the real-time PCR technique. More than 10 mice each of the FAKK5 KO and FAKloxP/+ genotypes were analysed. RNA samples were prepared for each mouse from intact skin and from isolated epidermis. Only the samples of high RNA quality were used for further analyses (Figure 2c). FAK mRNA levels were much lower in samples from FAKK5 KO mice (Figure 2d). Traces of FAK mRNA detected in epidermal samples from FAKK5 KO mice are likely from nonkeratinocyte contaminants that are unavoidable in any epidermal prep.
The FAK gene gives rise to two independent transcripts, one encoding full-length FAK (125kDa) and the other encoding the much shorter FAK-related non-kinase (FRNK). FAK and FRNK transcriptions are controlled by two independent promoters. The promoter for FRNK is located within the intron of FAK kinase domain (Nolan et al., 1999). Since the FAK exon that is deleted in our system is upstream of the FRNK promoter, both the promoter and coding regions of FRNK remained intact. It has also been reported that in neural tissue, the FAK gene gives several transcripts encoding only the N-terminal part of the protein, all of which are predicted to end up upstream of our deletion (Andre and Becker-Andre, 1993). To determine whether FRNK or any truncated N-terminal products are either normally expressed in epidermis or are induced in epidermis as a result of our deletion of full-length FAK, we performed a series of Northern and Western blot analyses using probes and antibodies specific for the N- or C-terminal parts of the FAK transcript/protein. Both approaches revealed the complete absence of FAK, FRNK, or truncated N-terminal products in both normal and mutant epidermis (Figure 2e and f).
Hair cycle irregularities in FAKK5 KO mice
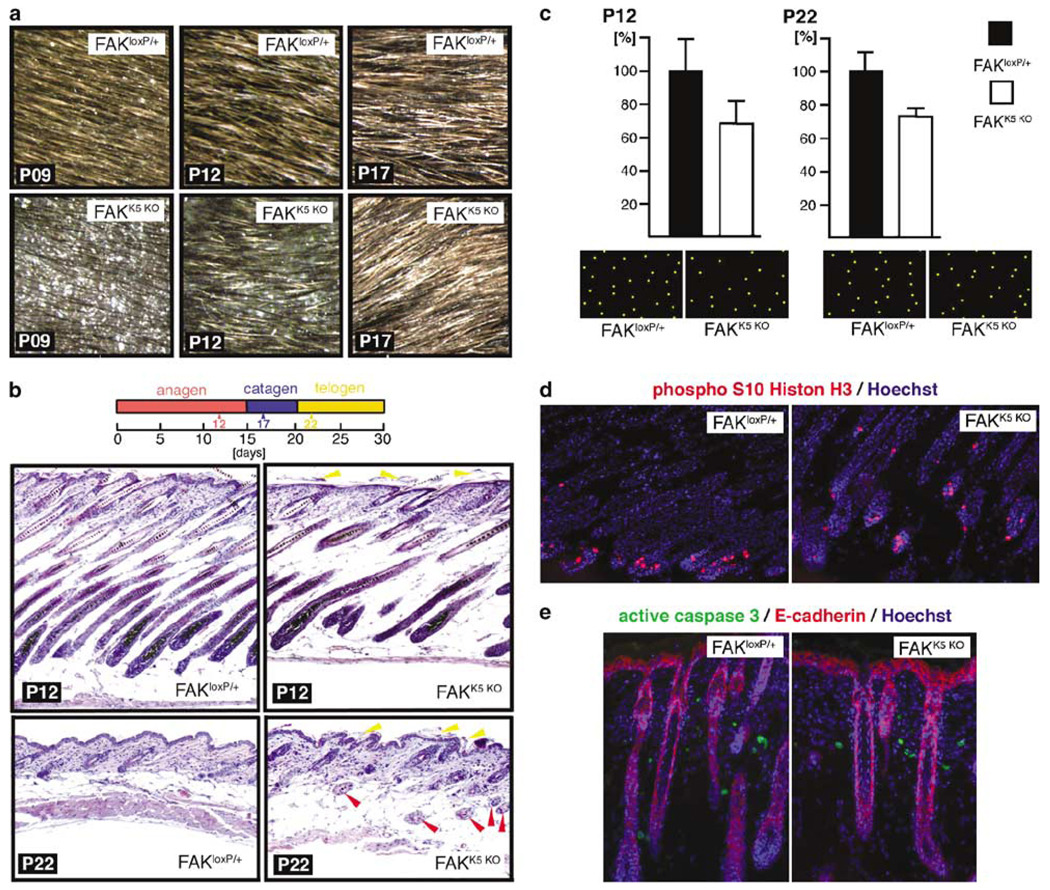
The appearance of newborn FAKK5 KO pups was indistinguishable from their FAKloxP/+ littermates. A phenotype became clear a few days later, when hair started to grow. From approximately postnatal day (P) 7 until P17, initial hair growth was sparse in the FAKK5 KO mice. By P17, the pelage of the FAKK5 KO mice appeared identical to the pelage of their normal littermates (Figure 3a). To investigate whether the transient difference in the appearance of the pelage resulted from altered phasing of the hair cycle, we examined middorsal skin histology at the level of the thoracolumbar junction in FAKloxP/+ and FAKK5 KO littermates at P12, P17, and P22. These days were chosen to represent each of the three phases in the first postnatal hair cycle (Paus et al., 1999; Stenn and Paus, 2001). The presence of hair follicles in the hypodermis of P22 FAKK5 KO mice suggested an irregular hair cycle (Figure 3b). To determine which part of the hair cycle might be affected, we counted the number of hair follicles per surface area on hematoxylin/eosin-stained histological sections cut parallel to the skin surface. At all days examined (P12, P17, P22), FAKK5 KO mice have consistently about 25% fewer hair follicles than FAKloxP/+ littermates (Figure 3c; data for P17 are not shown). Since FAKK5 KO mice at P12 have a less dense pelage than at P17 (Figure 3a), these results suggest that not all follicles in FAKK5 KO mice have an emerging hair shaft at P12, and that the process is delayed. Moreover, hair follicles in the FAKK5 KO mice are orientated randomly, rather than being arrayed in a hexagonal pattern as they are in FAKloxP/+ mice. Hair follicle number and spacing in heterozygous mice are comparable to FAKloxP/+ at all three time points examined, P12, P17, and P22 (not shown). Adult FAKK5 KO mice periodically showed patches of receding hair, which lasted for several days, disappeared, and then appeared again at another location, suggesting that subtle abnormalities in the hair cycle persist throughout the life of the FAKK5 KO mice.
Figure 3.
Hair phenotype in FAKK5 KO mice. (a) At initial stages, pelage of FAKK5 KO mice seems to have less hair (P9 and P12). By P17, there is no difference in the appearance of FAKloxP/+ and FAKK5 KO littermates. Images of the same area of the same FAKloxP/+ and FAKK5 KO mice were taken over a period of 9 days. (b) Hematoxylin/eosin-stained sections of FAKloxP/+ and FAKK5 KO mice at P12 and P22. Deeper follicles of P12 FAKK5 KO mice are misaligned and their skin is thinner than the skin of their FAKloxP/+ littermates. At P22, however, the skin is somewhat thicker, and this could be explained by the abnormal presence of hair follicles in the hypodermis (red arrowheads). Deposits of keratinous material are more abundant at the surface of the FAKK5 KO epidermis (yellow arrowheads). (c) Diagrammatic reconstruction of the hair follicle distribution on histological sections parallel with the skin surface hair count in FAKloxP/+ and FAKK5 KO littermates at P12 and P22 shows that FAKK5 KO mice, when compared with their littermates, have constantly about 25% less hair even though the appearance is different before and after PI7. (d) Similar mitosis level in hair follicles of P12 FAKloxP/+ and FAKK5 KO littermates. (e) Lack of FAK does not induce epithelial cell apoptosis in vivo in P07 mice. Comparable mitosis and almost nonexisting apoptosis were observed in all samples throughout the period analysed (P07–P22).
Retarded hair growth in FAKK5 KO mice implied that the lack of FAK might impair proliferation and/or survival of multipotent stem cells that give rise to hair follicle. We compared rates of mitosis and apoptosis in hair follicles of FAKloxP/+ and FAKK5 KO littermates, through the period between P07 and P22, and could not find a difference (Figure 3d and e). These results suggest that the time required to accomplish all stages of cell cycle is not the same in cells that do and do not have FAK. The abnormality is most obvious in the first hair cycle because it is synchronized all over the body.
Sebaceous gland hypoplasia and thinner epidermis in FAKK5 KO mice
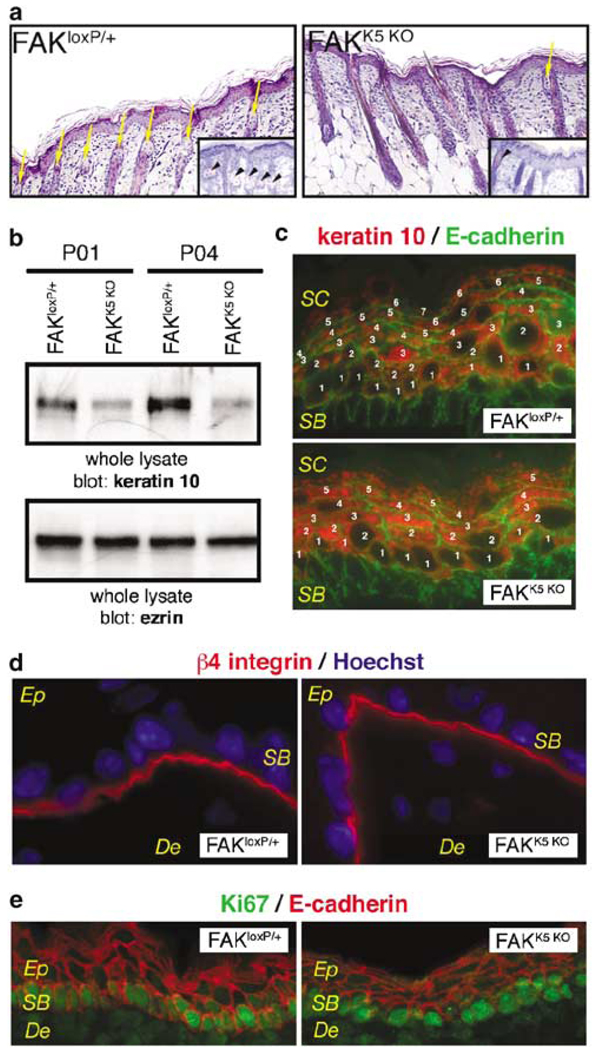
Hair growth is dependent in significant part on the proliferation and migration capacity of multipotent stem cells in the bulge, located on the hair follicle. These cells also give rise to sebaceous glands and epidermis (Alonso and Fuchs, 2003). If proliferation and/or migration of cells in the bulge are impaired, sebaceous glands and epidermis should also show some defects. Indeed, histological analysis of skin sections of 2–4-month-old mice revealed a significant deficit in sebaceous glands (Figure 4a). In addition, a thinner epidermis was evident in FAKK5 KO mice at all ages examined (E16.5–P14). We demonstrated the thinner epidermis in FAKK5 KO mice by Western blot, which showed a lower level of keratin 10 (Figure 4b), and by immunostaining, which showed fewer keratin 10-positive layers (Figure 4c). Keratin 10 is a marker of differentiated keratinocytes. Since FAK has a role in migration and it is localized on leading edge of migrating cell (Schlaepfer et al., 2004), the thinner epidermis in FAKK5 KO mice might be caused by impaired migration of cells from basal to upper layers due to loss of polarity. However, β4 integrin expression was restricted to the dermal–epidermal junction, suggesting that a polarity of keratinocytes in the epidermis of FAKK5 KO mice is not impaired (Figure 4d). FAK is also involved in regulation of cell proliferation (Oktay et al., 1999) and cell survival (Ilic et al., 1998). Based on Ki67 immunostaining, we found no difference between FAKloxP/+ and FAKK5 KO mice in proliferating cells restricted to the basal layer of epidermis (Figure 4e).
Figure 4.
Sebaceous gland hypoplasia and thinner epidermis in FAKK5 KO mice. (a) Sebaceous glands are less in number and hypoplastic in FAKK5 KO mice (yellow arrows). Insets: Oil Red staining of sebaceous glands (black arrowheads). (b) Western blot analyses of epidermis from P1 and P4 littermates show less keratin 10 in lysates from FAKK5 KO mice. Ezrin is a loading control. (c) Thinner epidermis in FAKK5 KO mice. There are fewer keratin 10-positive suprabasal layers in the epidermis of E18.5-day-old FAKK5 KO embryos. A similar difference in thickness was observed in all samples throughout the period analysed (E16.5–P14). (d) β4 integrin is confined to the dermal–epidermal junction in both FAKloxP/+ and FAKK5 KO mice. (e) Ki67-positive cycling cells are present at a similar level in the basal layer (SB) of epidermis in both FAKloxP/+ and FAKK5 KO mice. De, dermis; Ep, epidermis; SB, stratum basale; SC, stratum corneum.
Lack of FAK does not affect wound healing in vivo, although FAK is essential for keratinocyte survival in vitro
To determine how the loss of FAK affects keratinocyte migration/proliferation in vivo, we conducted woundhealing assays using FAKK5 KO mice. Full-thickness, 6-mm-diameter, punch wounds were made, one on each flank, on 8–12-week-old FAKloxP/+ and FAKK5 KO female mice and the rate of wound closure was monitored. Surprisingly, data analysis using a Wilcoxon’s rank sum test for random effects, with autocorrelation over time, did not find a statistically significant difference in wound closure (Figure 5a and b). Integrin β1 was present and active at comparable levels in the basal layer of FAKloxP/+ and FAKK5 KO mouse epidermis (Figure 5c), suggesting that signals from β1 integrin that regulate keratinocyte migration do not require FAK. Next, we attempted to follow the migration rate of FAK-null keratinocytes in vitro. However, primary keratinocytes isolated from FAKK5 KO animals would not proliferate in culture, and died within 48 h, whereas, under the same conditions, isolated FAKloxP/+ keratinocytes survived and proliferated (Figure 5d).
Figure 5.
Lack of FAK does not affect wound healing in vivo, although it is essential for keratinocyte survival in vitro. (a) Wound closure over 10 days; mm-scale is at the left. (b) Similar rate of wound healing in FAKloxP/+ and FAKK5 KO mice. (c) Hematoxylin and eosin staining of sections from 4-day wounds reveals similar histology of wound edge in FAKloxP/+ and FAKK5 KO mice. Es, eschar; HE, hyperproliferative epithelium. (d) Activated β1 integrin, recognized by immunostaining of fresh frozen unfixed tissue sections by 9EG7 antibody, is localized in the basal layer (SB) of epidermis in both FAKloxP/+ and FAKK5 KO mice. De, dermis; Ep, epidermis. (e) FAKK5 KO mouse keratinocytes could not be maintained in vitro. They neither proliferate nor survive in primary culture.
Discussion
The role of FAK in epithelial cells has not been well studied. We used mice expressing the keratin 5 promoter driving Cre recombinase, and mice with FAKloxP/loxPto generate mice lacking FAK in all layers of the epidermis. Mice lacking FAK in keratin 5-expressing cells are viable; there was no increased mortality of FAKK5 KO animals under given housing conditions. Here, we report the following: (a) Expression of full-length FAK was absent or greatly reduced in the epidermis of FAKK5 KO mice. Furthermore, neither FRNK nor any N-terminal FAK fragments were detected in the epidermis of either FAKloxP/+ or FAKK5 KO mice. (b) FAKK5 KO mice have a defective hair cycle, sebaceous gland hypoplasia, and a thinner epidermis, suggesting a reduced stem cell compartment. (c) Surprisingly, wound closure occurred at similar rates in FAKK5 KO mice and littermates with FAK expressed in keratinocytes. These findings suggest that the loss of FAK might affect migration/proliferation differently in the differentiated basal keratinocyte layer vs the multipotent skin stem cell compartment. (d) Primary keratinocytes isolated from FAKK5 KO animals failed to proliferate in culture, and died within 48 h, whereas, under the same conditions, isolated FAK-expressing keratinocytes (FAKloxP/+) survived and proliferated. These data suggest that the structural organization of and/or molecular environment in the native tissue – both the basal lamina and underlying connective tissue compartment – may overcome the lack of signaling through FAK in FAKK5 KO epidermal cells.
Epidermal stem cells found in the bulge are thought to contribute to all lineages of the hair follicle, sebaceous gland, and epidermis (Liu et al., 2003; Tumbar et al., 2004). Although the specific surface markers are not yet known, one of the potential markers is β1 integrin (Adams and Watt, 1989; Alonso and Fuchs, 2003; Ma et al., 2004; Morasso and Tomic-Canic, 2005). It has been thought that signaling via β1 integrins and mitogen-activated protein kinases (MAPKs) cooperate to maintain the epidermal stem cell compartment (Zhu et al., 1999). Activation of FAK by integrin clustering or by cell binding to ECM proteins leads to association with Src and subsequent Src-mediated phosphorylation of Grb2-binding tyrosine 925 on FAK. This links integrins to the MAPK pathway (Schlaepfer et al., 1994). However, within the last several years, it became evident that FAK is not involved in all integrinmediated MAPK activation signaling events (e.g. Wary et al., 1998). In this regard, it is interesting to compare phenotypes of K5Cre-mediated knockout of floxed β1 integrin (Brakebusch et al., 2000) and FAK. Initial hair growth is reduced in both β1K5 KO and FAKK5 KO mice. Whereas the phenomenon is transient in FAKK5 KO mice, β1K5 KO lose their hair completely by age 7 weeks. Whereas in β1K5 KO mice, the epidermis of the back skin becomes hyperthickened and the basal keratinocytes show reduced levels of α6β4 integrin, FAKK5 KO animals have a thinner epidermis and normal α6β4 integrin expression. In vivo, proliferation of β1K5 KO cells is reduced in nonwounded but not in wounded skin. Although FAKK5 KO cells proliferated equally well in both nonwounded and wounded skin, a delay in the initial hair growth suggested that the time required to accomplish the cell cycle seems to be altered. In vivo, K5Cre-mediated deletion of either β1 or FAK had no effect on cell survival. However, FAKK5 KO cells were unable to survive in vitro, whereas survival of β1K5 KO cells in vitro was unaffected. All these difference clearly point out that although β1 integrins and FAK are often part of the same pathway, they also have distinct functions. β1 integrin is paired with several different α subunits and they serve as receptors for a number of ECM proteins.
On the other hand, FAK also participates either directly or indirectly in signaling pathways that do not involve integrins, for example, epidermal, plateletderived, vascular endothelial, as well as fibroblast growth factor receptor signaling (Schlaepfer et al., 2004; Mitra et al., 2005). Impaired signaling of profollicular fibroblast growth factors may easily account for hair phenotype of mice lacking FAK in the skin and it is a subject of our future studies.
Previous studies have provided direct evidence for a role of FAK in cell migration (Ilic et al., 1995a; Cary et al., 1996; Fincham and Frame, 1998; Klemke et al., 1998), protection from apoptotic cell death (Frisch et al., 1996; Ilic et al., 1998), and in cell proliferation (Oktay et al., 1999). The common element connecting all these cellular processes is actin cytoskeleton remodeling. Indeed, the absence of FAK is known to affect actin cytoskeleton and cell shape in fibroblasts (Ilic et al., 1995a, 2004).
The role of actin cytoskeleton and ECM signaling during skin maturation is not well understood. Recent studies with the mice expressing green fluorescent protein (GFP)-actin in their keratinocytes suggest a role of both ECM signals and actin fibers in the process of epidermal stratification (Vaezi et al., 2002). To understand whether FAK has a role in this process in keratinocytes and whether this could explain the effect of FAK deficiency on thickness of epidermis, we would have to turn to in vitro work. However, inability to culture primary keratinocytes lacking FAK is a major problem, and to answer these questions an appropriate in vitro system has to be generated. One approach would be to introduce a p53-null background into FAKK5 KO mice (Ilic et al., 1995a, 1998). A more complex solution that is closer to the in vivo setting would involve culturing skin explants from El8.5 FAK-expressing and FAKK5 KO mice.
Wound closure is dependent basically on two processes: keratinocyte migration into the wound site and contraction of fibroblasts within the granulation tissue underneath. It is known from knockout experiments and cells other than keratinocytes that interference with FAK interferes with cell migration and spreading, key features of keratinocyte activation. Our finding that loss of FAK in keratinocytes does not retard wound closure cannot be explained by the fact that the crucial component of wound closure is contraction of the wound bed mediated by (myo)fibroblasts, cells present in the wound bed in large quantities and unaffected by the keratinocyte-specific deletion of FAK. In a similar type of experiments, keratinocytes lacking β1 integrin were unable to re-epithelialize the wound (Grose et al., 2002). This suggests that FAK does not have a crucial role in keratinocyte migration. A similar lack of effect on wound healing was observed in an independent study on the role of FAK in skin carcinogenesis in which FAK with a different floxed region was deleted in keratinocytes using mice in which Cre is driven by the K14, not the K5, promoter (McLean et al., 2004). The discrepancy between these two studies and the large number of other reports describing in vitro experiments points to the importance of using in vivo and three-dimensional in vitro models in analyses of cell migration, proliferation, and survival.
The study by McLean et al. (2004) reported increased FAK-null keratinocyte cell death in vitro and in vivo, a phenomenon that we did not observe in our model using the same marker, activated caspase 3. This discrepancy may be derived from differences in genetic backgrounds and/or the age of mice under study. There is also evidence that selection of the K5 vs K14 promoter can influence phenotypic outcomes of keratinocyte-specific Cre expression. Deletion of β1 integrin affected phenotypes more dramatically when the K14 promoter was used to target kerotinocytes (Brakebusch et al., 2000; Raghavan et al., 2000). Our findings highlight the importance of using multiple experimental approaches to determine the function(s) of genes under study.
Materials and methods
Mice and genotyping
Mice were maintained and bred at the UCSF Laboratory Animal Research Center facility in accordance with institutional guidance and National Institute of Health standards. They were regularly monitored and had free access to standard mice chow and water.
FAKloxP and FAK+ bands (400 and 290 bp, respectively) are detected using 5′-GAATGCTACAGGACCCAAATAAC-3′ and 5′-GAGAATCCAGCTTTGGCTGTTG-3′ primers and the following conditions: 2 min at 95°C (one cycle), 30s at 94°C, 30s at 63°C, 45s at 72°C (35 cycles), and 15min at 72°C. The presence of FAKΔloxP band (327 bp) confirms that Cremediated recombination occurred. The band is detected using 5′-GACCTTCAACTTCTCATTTCTCC-3′ and 5′-GAATGC TACAGGACCCAAATAAC-3′ primers under the same PCR conditions. FAK band (170 bp) is detected using 5-GGCTTCT TGAAGGAACTTCTC-3′ and 5′-TGATATTGCTGAAGAG CTTGGCGG-3′ and the following conditions: 3 min at 94°C, 1 min each at 94, 60, and 72°C (35 cycles), and 1min at 72°C. K5Cre band (419bp) is detected using 5′–AACATGCTTCATCGTCGG-3′ and 5′-TTCGGATCATCAGCTACACC-3′ primers and the following conditions: 2min at 95°C (one cycle), 1 min at 95°C, 90s each at 55 and 72°C (35 cycles), and 15 min at 72°C (one cycle).
RNA isolation and Northern blot
Total RNA was isolated from the epidermis of 2–4-day-old FAKloxP/+ and FAKK5 KO mice and primary mouse embryonic fibroblasts transduced with FRNK-expressing adenovirus (Hauck et al., 2001) using the RNAeasy columns (Qiagen). Epidermal samples were processed according to the manufacturer’s recommendations for isolation of RNA from fibrous tissue. For Northern analysis, total RNA (20µg per lane) was size-fractionated on 0.8% agarose gel containing 2.2M formaldehyde, transferred to Duralon-UV membrane using Pressure Control Station (Stratagene), and fixed to the membrane using UV irradiation (Nucleic Acid Transfer Lamp, Stratagene). Probes were generated by PCR using FAK mouse cDNA and primers 5′-GGCATCATTCAGAAGATAGTG-3′ and 5′-GATCCAACTTGACCCAAGGGC-3′ for N-terminal probe, and 5′-CAGGTGCTTCC CCCTCACCTG-3′ and 5′ -GTGTGGCCGTGTCTGCCCTAG-3′ for C-terminal probe. Purified N-terminal 605 bp and C-terminal 755bp PCR products were labeled with [α-32P]dCTP using the Random Primer Labeling Kit from Stratagene. Hybridizations were performed in QuikHyb hybridization solution (Stratagene) according to the instruction of the manufacturer. Blots were later stripped and reprobed with radiolabeled glyceraldehyde phosphate dehydrogenase cDNA (GAPDH) as described by Kovacic-Milivojevic et al. (2001) to control for differences in loading and transfer of RNA among samples.
Real-time PCR
To amplify murine FAK, forward primer (GCGTGAGAGAG AAGTTCCTTCAA), reverse primer (TCACGATGTGAGG ATGGTCAA), and FAM/MGB-labeled probe (AAGCCTTA ACAATGCGTC) were designed using Primer Express software. E-cad, Dgl3, K5, and GAPDH were amplified using systems available as Assays on Demand (Applied Biosystems). Reverse transcription was carried out using the TaqMan Gold RT–PCR kit. This was followed by real-time PCR, which was performed in duplicate using the Applied Biosystems 9700HT sequence detection system. A fivefold titration of control template was included in every run, along with a minimum of three negative controls. Reactions were incubated at 50°C for 2min, then 95°C for 10min. This was followed by 40 cycles of 95°C for 15 s and 60°C for 1 min.
Antibodies, immunostaining, and Western blot
Staining was carried out on either the same or adjacent 5-µm-thick sections of fresh frozen tissue. We used rabbit polyclonal antibodies against keratin 5 (Covance), phospho-S10 histone H3 (UBI), active caspase 3 (Sigma), Ki67, and FAK (LabVision), rat monoclonal against E-cadherin (Zymed), active β1 integrin and β4 integrin (BD Pharmingen), and mouse monoclonal antibodies against keratin 10 (LabVision), ezrin, and FAK (UBI, BD Transduction Laboratories). The staining procedure and Western blot have been described in detail previously (Ilic et al., 2001).
Wound healing
Two full-thickness, 6-mm-diameter, punch wounds were made, one on each flank, of 8–12-week-old FAKloxP/+ (animals: n = 8; wounds: n = 16) and FAKK5 KO (animals: n = 8; wounds: n = 16) female mice and the rate of wound closure was monitored. Mice were anesthetized using 11/min of 5% isoflurane/O2 in a rodent inhalant portable anesthesia system (Summit Medical). Bilateral full-thickness wounds were created on shaved flanks using a 6-mm-diameter Disposa-Derm punch biopsy (Cooper Surgical). Photos of wounds were taken with a SPOT RT CCD camera (Diagnostic Instruments) at days 0, 2, 4, 8, 10, and 12 under a dissecting microscope (Zeiss). To determine wound closure rates, the surface area of each wound was measured using an image analysis tool, ImageJ (National Institutes of Health). Statistical analysis was performed using Wilcoxon’s rank sum test for random effects with autocorrelation over time.
Counting hair
Hair number was counted in equivalent areas of the back skin of FAKloxP/+ and FAKK5 KO littermates as described by Cui et al. (2003). Briefly, skin samples were taken with a 6 mm dermal biopsy punch (Cooper Surgical) from three mice of each genotype, fixed in 4% paraformaldehyde overnight, and embedded in paraffin. Sections (10 µm) were cut parallel to the skin surface, and stained with hematoxylin and eosin (Sigma). Hair numbers were counted from three random areas of each biopsy at × 40 magnification.
Acknowledgements
The work is supported by grant from National Cancer Institute (CA87652) to D Ilic. We thank Drs C Damsky and D Schlaepfer (Scripps) for a critical reading of the manuscript and E Gering (SFSU) for editorial assistance. This investigation was conducted in a facility constructed with support from the Research Facilities Improvement Program Grant number C06RR16490.
Abbreviations
- E
embryonic day
- ECM
extracellular matrix
- P
postnatal day
References
- Abbi S, Guan JL. Histol Histopathol. 2002;17:1163–1171. doi: 10.14670/HH-17.1163. [DOI] [PubMed] [Google Scholar]
- Adams JC, Watt FM. Nature. 1989;340:307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- Alonso L, Fuchs E. Proc Natl Acad Sci USA. 2003;100:11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Becker-Andre M. Biochem Biophys Res Commun. 1993;190:140–147. doi: 10.1006/bbrc.1993.1022. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, et al. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, et al. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Chang JF, Guan JL. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Biochem Biophys Res Commun. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- Cui CY, Durmowicz M, Ottolenghi C, Hashimoto T, Griggs B, Srivastava AK, et al. Hum Mol Genet. 2003;12:2931–2940. doi: 10.1093/hmg/ddg325. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Ilic D. Curr Opin Cell Biol. 2002;14:594–602. doi: 10.1016/s0955-0674(02)00368-x. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, Frame MC. EMBO J. 1998;17:81–92. doi: 10.1093/emboj/17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, et al. Development. 2002;129:2303–2315. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Ryzhova L, Shin NY, Brabek J. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Sieg DJ, Hsia DA, Loftus JC, Gaarde WA, Monia BP, et al. Cancer Res. 2001;61:7079–7090. [PubMed] [Google Scholar]
- Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, et al. Nature. 1995a;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Suda T, Atsumi T, Fujimoto J, Ikawa Y, et al. Biochem Biophys Res Commun. 1995b;209:300–309. doi: 10.1006/bbrc.1995.1503. [DOI] [PubMed] [Google Scholar]
- Ilic D, Genbacev O, Jin F, Caceres E, Almeida EA, Bellingard-Dubouchaud V, et al. Am J Pathol. 2001;159:93–108. doi: 10.1016/S0002-9440(10)61677-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Kovacic B, Johkura K, Schlaepfer DD, Tomasevic N, Han Q, et al. J Cell Sci. 2004;117:177–187. doi: 10.1242/jcs.00845. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic-Milivojevic B, Roediger F, Almeida EA, Damsky CH, Gardner DG, Ilic D. Mol Biol Cell. 2001;12:2290–2307. doi: 10.1091/mbc.12.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Ma DR, Yang EN, Lee ST. Ann Acad Med Singapore. 2004;33:784–788. [PubMed] [Google Scholar]
- McLean GW, Avizienyte E, Frame MC. Expert Opin Pharmacother. 2003;4:227–234. doi: 10.1517/14656566.4.2.227. [DOI] [PubMed] [Google Scholar]
- McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, et al. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Morasso MI, Tomic-Canic M. Biol Cell. 2005;97:173–183. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan K, Lacoste J, Parsons JT. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. J Cell Biol. 1999;145:1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, et al. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A, Page A, Gandarillas A, Zanet J, Pibre S, Vidal M, et al. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, Giancotti FG. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Haase I, Watt FM. Proc Natl Acad Sci USA. 1999;96:6728–6733. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]