Summary
The extracellular matrix glycoprotein, fibronectin, is a potent promoter of peripheral neurite outgrowth. Interactions of peripheral neurons with fibronectin have been shown to be primarily mediated by the β1 class of integrin heterodimers. In the present study, we have examined the expression and regulation of fibronectin and its integrin receptor, α5β1, in developing and regenerating chick peripheral nerve. We show that fibronectin and α5β1 are expressed at comparatively high levels in developing nerve with α5β1 expression on axons and non-neuronal cells. With nerve maturation, both proteins are less prominently expressed and the cellular pattern of α5β1 expression becomes more restricted. Following lesion of mature nerve, both fibronectin and α5β1 are strongly induced with prominent expression of (α5β1 on regenerating neurites and Schwann cells. The elevation in fibronectin levels in the regenerating nerve is highest in the vicinity of the lesion, an area undergoing extensive cellular remodeling including Schwann cell migration and growth cone extension. Our results suggest that fibronectin and its receptor, α5β1, may mediate functionally important interactions in the development and regeneration of peripheral nerve.
Keywords: fibronectin, integrin, peripheral nerve, chick
Introduction
In the formation of the peripheral nervous system, neural crest cells migrate and neurons extend axons through areas rich in extracellular matrix (ECM; for review, Sanes, 1989). Studies in vitro have demonstrated that ECM constituents, in particular fibronectin (FN), support the attachment, spreading and migration of neural crest cells and potently promote peripheral neurite outgrowth (Rogers et al., 1983; Tomaselli et al., 1986; Humphries et al., 1988; Dufour et al., 1988). FN has been shown to be localized along the pathways of migrating neural crest cells (Newgreen and Thiery, 1980; Krotoski et al., 1986) and reagents, such as RGDS-containing peptides, which disrupt interactions of cells with fibronectin, inhibit the migration of neural crest cells (Boucaut et al., 1984).
The primary class of cellular FN receptors identified thus far are members of the integrin family of heterodimers (for review see Hynes, 1992; Hemler, 1990; Reichardt and Tomaselli, 1991). Each integrin heterodimer is composed of an α and β subunit with the ligand specificity determined by the particular combination of subunits. Four heterodimers containing the β1 subunit: α5β1, α3β1, α4β1 and αVβ1, have been identified as FN receptors (Pytela et al., 1985; Elices et al., 1990; Takada et al., 1988; Wayner et al., 1988; Vogel et al., 1990)). α5β1, αVβ1 and possibly α3β1 interact with the RGD-sensitive major cell attachment site of FN (Pierschbacher and Ruoslahti, 1984; Elices et al., 1991) while α4β1 appears to interact with several sites in the C terminus of FN, including two heparin-binding regions and the alternatively spliced CS1 domain (Wayner et al., 1989; Guan and Hynes, 1990; Mould and Humphries, 1991).
Dorsal root ganglion neurons in vitro have been demonstrated to interact with both of these domains, extending neurites on both the C-terminal heparin-binding fragment and the RGD-containing 75×103 Mr fragment (Humphries et al., 1988; Rogers et al., 1985). Similarly neural crest cells are known to interact with each domain (Dufour et al., 1988). The interactions of both neural crest cells and peripheral neurons with FN are mediated by integrins containing the β1 subunit (Bronner-Fraser, 1985; Bozyczko and Horwitz, 1986; Tomaselli et al., 1986; Duband et al., 1986). These results suggest that the two cell populations utilize α5β1, αVβ1 or α3β1 to interact with the RGD-sensitive cell binding domain as well as α4β1 to interact with the C-terminal binding sites.
Fibronectin expression is regulated both during embryogenesis (Roman and McDonald, 1992) and in wound repair in adult mammalian skin (ffrench-Constant and Hynes, 1989; ffrench-Constant et al., 1989; Clark, 1990). During embryogenesis, the pattern of alternative splicing of FN is spatially and temporally regulated with inclusion of the alternatively spliced EIIIA and EIIIB regions only during the early stages of embryogenesis (ffrench-Constant and Hynes, 1989). During cutaneous wound healing in adult skin, these two embryonic splice forms are reexpressed by the cells at the wound base (ffrench-Constant et al., 1989). The pronounced elevation in FN expression following skin injury is thought to be a critical component of the wound response as it provides a provisional matrix that facilitates the migration of several cell types into the wound region (for review, see Clark, 1990).
Previous work has shown that the responsiveness of sensory neurons to FN, assayed in vitro, is down regulated during embryogenesis (Kawasaki et al., 1986; Millaruelo et al., 1988). Similarly, recent work suggests that the tissue distribution of the α5β1 integrin receptor becomes more restricted during embryognenesis (Muschler and Horwitz, 1991; Roman and McDonald, 1992). However, the expression and function of FN receptors has been shown to increase in epidermal cells isolated from healing wounds (Takashima et al., 1986; Grinnell et al., 1987). Several neuronal cell surface adhesion molecules whose expression decreases during development have been shown to be upregulated following nerve injury (Daniloff et al., 1986; Martini and Schachter, 1988).
The aim of the present study was to characterize the expression and regulation of FN and one of its putative neuronal integrin receptors, α5β1, in the peripheral nerve. We have focused on the α5β1 heterodimer because preliminary examinations of the other β1-containing FN receptors did not reveal appropriate cellular expression (αv), or obvious expression changes (α3) or adequate reagents (α4) to prompt their further study (data not shown). Our results show that FN and α5β1 are more prominently expressed in the developing chick peripheral nerve than in the mature nerve and, following transection of a mature nerve, the levels of both fibronectin and the α5 integrin subunit are strongly increased.
Materials and methods
Surgery
Three-week-old White Leghorn chickens were anaesthetized with ketamine/xylazine and the medial-ulnar nerve innervating the right wing was transected. The proximal and distal nerve stumps were juxtaposed minimizing their separation to facilitate regeneration; the maximal gap distance between the two stumps was no greater than 1 mm. At various time points following nerve transection (3 days, 1 week, 2 weeks, 4 weeks), the animals were killed by an overdose of the same anaesthetics followed by CO2 asphyxiation. The regenerated nerve was removed from the animal and divided into equal length segments (2-3 mm) proximal and distal to the transection site (Fig. 1). An equal length segment from the contralateral unoperated nerve was removed and served as control. All tissues were frozen immediately and stored at −80°C usually overnight before homogenization. For each experiment, nerves from 4-7 animals were transected and homogenized together. This procedure was repeated 2-4 times for each experimental time point.
Fig. 1.
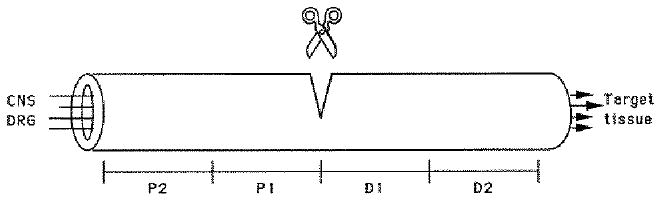
At various time points following medial-ulnar nerve transection, nerve segments of equal length (2-3 mm) proximal (P1, P2) and distal (D1, D2) to the transection site were removed and processed as described.
Antibodies
The anti-fibronectin-CS1 monoclonal antibody (mAb FN-kv1) was generated using E10 cultured chick brain glial cells as the immunogen (Harlow and Lane, 1988). Cultured glial cells, scraped off in phosphate-buffered saline (PBS: 0.2g/l KCl, 0.2g/l KH2PO4, 2.16 g/l Na2HPO4, 8 g/l NaCl; 0.1mM CaCl2, 0.1mM MgCl2) with 1 mM PMSF, chymotrypsin, leupeptin, antipain and pepstatin were injected with RIBI adjuvant (Hamilton, Montana) into Balb/C mice. Hybridoma supernatants were screened for their ability to block chick E6 retinal cell adhesion to the ‘CS1’ domain of fibronectin (peptide sequence: DELPQLVTLPHPHPNLHGPEILDVPSTC). The hybridoma secreting FN-kv1 was subcloned by limiting dilution, grown in RPMI with 4% fetal calf serum and 1% Nutridoma (Boehringer Manheim Biochemicals, Indianapolis, IN) and injected into Balb-c mice to obtain ascites. The allotype of the FN-kv1 mAb is mouse IgG1 (Calbiochem Hybridoma Subtyping kit, San Diego, CA). To purify the ascites Trizma (pH 8-9) was added to 100 mM and NaCl to 2.5 M. The ascites was then fractionated on protein-A Sepharose-CL-4B (Pharmacia, Uppsala Sweden) according to Harlow and Lane (1988). The IgG was dialyzed against PBS (Ca2+- and Mg2+-free) and stored at −20°C. A second monoclonal antibody against fibronectin, VA13, was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, and the Department of Biology, University of Iowa, Iowa City, IA (under contract NO1-HD-6-2915 from the NICHD). VA13 recognizes intact FN as well as the 140/160×103 Mr cell-binding fragments generated by elastase digestion. Both mAbs gave identical staining patterns on immunoblots and immunocytochemistry and were thus used interchangeably.
Two antibodies were used to recognize the integrin α5 subunit: (1) A2F7, a monoclonal antibody from Drs J. Muschler and A. F. Horwitz (University of Illinois, Urbana; Muschler and Horwitz, 1991) and (2) α5-47, a polyclonal antibody (affinity-purified IgG) generated against a 20mer peptide corresponding to the C terminus of the human α5 sequence (LPYGTAMEKAQLKPPATSDA; prepared as in Roman et al., 1989). Since the polyclonal antibody, α5-47, provided a much stronger signal for immunocytochemistry than the A2F7 monoclonal antibody, it alone was used for all immunocytochemistry.
To identify axons, two neural specific mAbs were used: (1) a mAb generated against a β-tubulin isoform (cβ4) specific for neurons (Tuji, from Dr A. Frankfurter, University of Virginia; Yaginuma et al., 1990)) and (2) a cocktail of mAbs against the 68, 160, 200×103 Mr neurofilament subunits (Boehringer-Manheim). To label Schwann cells, a polyclonal antibody against the S-100 protein (Dakopatts) was used. A polyclonal affinity-purified antibody raised against mouse laminin (LN) was used to visualize LN isoforms (JW2; Lander et al., 1985).
Immunoblots
Nerve segments were homogenized in 10 mM Hepes, pH 7.4 in 0.15 M NaCl, 0.32 M sucrose, 2 mM PMSF, 1 mM chymotrypsin, leupeptin, aprotinin and pepstatin, and centrifuged at 10,000 revs/minute for 30 minutes. The pellets were resuspended in PBS (pH 7.4) with 1% Triton X-100 and protease inhibitors, vortexed several times over 30 minutes, and incubated on ice. After addition of SDS sample buffer (final concentration of 3% SDS; Laemli, 1970), the homogenates were incubated on ice for 30 minutes followed by boiling for 3 minutes. The extracts were then centrifuged for 10 minutes at 10,000 revs/minute and protein determinations were made on the supernatants by the Amido Schwartz method. After loading equal protein concentrations, samples were electrophoresed on a 6.5% non-reducing polyacrylamide gel (Laemmli, 1970). The gels were then transferred electrophoretically to nitrocellulose for 1 hour at 500 mA. The blots were blocked in 5% Blotto (5% dry milk powder, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl) for 45 minutes and then incubated in primary antibody for 1 hour (polyclonal antibody) at room temperature or overnight at 4°C (monoclonal antibody). After washing four times for 10 minutes each, the blots were incubated for 1 hour in a secondary antibody, goat anti-rabbit or goat anti-mouse (as appropriate) IgG coupled to alkaline phosphatase (Promega, WI). After further washing, the alkaline phosphate reaction product was generated by developing at pH 9.5 with the substrates, nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Sigma). To measure changes in α5 subunit expression, the immunoblots were scanned on a Biorad densitometer (Brugg and Matus, 1988). Immunoprecipitations were performed according to Neugebauer et al., 1991.
Immunocytochemistry
Regenerated and control nerves were removed from the animal and either frozen immediately in liquid nitrogen or fixed in 3% paraformaldehyde for 2 hours followed by overnight incubation in 15% sucrose. After embedding in Tissue-Tek OCT (Miles, Inc.), the nerve segments were sectioned at 10-14 μm and the slides stored at −20°C. Both the contralateral control nerve and regenerating nerves were embedded in the same block of OCT; thus each slide contained sections of both nerves. To examine developing nerve, medial nerves were removed from 1-day-old chicks and treated similarly. The sections taken from nerves that had not been previously fixed were then fixed in either 3% paraformaldehyde for 20 minutes or −20°C methanol for 10 minutes. Sections were blocked for 30 minutes in either 5% milk powder or 5% normal goat serum in Tris-buffered saline plus 0.2% Triton X-100 for 30 minutes followed by overnight incubation at 4°C in primary antibody (10 μg/ml). Secondary antibodies included goat anti-rabbit fluorescein (Cappel, 1:100), goat anti-mouse rhodamine (Accurate, 1:100), goat anti-rabbit biotin (Vectastain, 1;200), strepavidin fluoroscein (Amersham, 1:100). Sections were then mounted in gelvatol with 2% n-propyl gallate (Sigma) added as an antioxidant and observed on a Zeiss Axiophot or photomicroscope. All photographs were taken with either hypersensitized Kodak Technical pan 2415 (Lumicon; Livermore, CA) or Kodak TMAX 100. To verify the specificity of the antibody labeling, controls included sections incubated in secondary antibody alone and for the anti-α5 cytoplasmic IgG, the antibody was incubated for 1 hour with 10 μg/ml of a 20mer peptide from the C terminus of the human α5 sequence before application to sections. Additional experiments were performed to insure that there was no cross-reactivity to the cytoplasmic domains of other integrin α subunits, focusing on three with highest sequence conservation in the cytoplasmic domain (α8, αv, α6). Preincubation with peptides from the C termini of both the chick α8 integrin subunit (Bossy et al., 1991) and the human α6a subunit, did not interfere with the α5-47 labeling of nerve. Polyclonal antibodies against the C termini of the α6 and αv integrin subunits generated very different staining patterns from that of the anti-α5 IgG in chick peripheral nerve (not shown).
For immunocytochemical comparisons of expression levels of a particular antigen, sections of the two nerves being compared (e.g. immature vs. mature nerve; control vs. regenerating nerve) were collected on the same slide. After immunolabeling, photographic exposures of equal duration were taken and all negatives were processed equally.
Results
To identify the chicken α5 subunit, we used two different antibodies: an affinity-purified polyconal antibody generated against the C terminus of the human α5 sequence (α5-47) and a monoclonal antibody (A2F7) generated against the 150×103 Mr band (‘band 1’; Hynes et al., 1989) immunoprecipitated by the CSAT antibody (Bozyczko and Horwitz, 1986). Previous work has confirmed that this 150×103 Mr protein from chick fibroblasts binds to a FN column and is a chick homolog of the human α5 subunit (Hynes et al., 1989; Muschler and Horwitz, 1991). Both antibodies recognized a single band of 150×103 Mr in extracts of chick peripheral nerve (Fig. 2A, lane 1 and 2). Since it was essential to determine that the α5-47 polyclonal antibody was specifically recognizing a chick homolog of the human α5 subunit, we tested whether A2F7 would recognize the 150×103 Mr protein immunoprecipitated from a chick peripheral nerve extract by the α5-47 affinity-purified IgG. A single band was recognized by the mAb A2F7 (Fig. 2B, lane 1, which was not recognized in a control immunoprecipation, Fig. 2B, lane 2) indicating that the two antibodies interact with the same antigen and that the α5-47 IgG recognizes a chick homolog of the human α5 integrin subunit. Additional evidence that the α5-47 polyclonal antibody recognizes a chick fibronectin receptor is that α5-47 immunohistochemically stains fibrillar apparent ECM contact sites and focal contacts (Roman et al., 1989) in chick fibroblasts cultured overnight on fibronectin (data not shown; see Methods for details on additional control experiments).
Fig. 2.
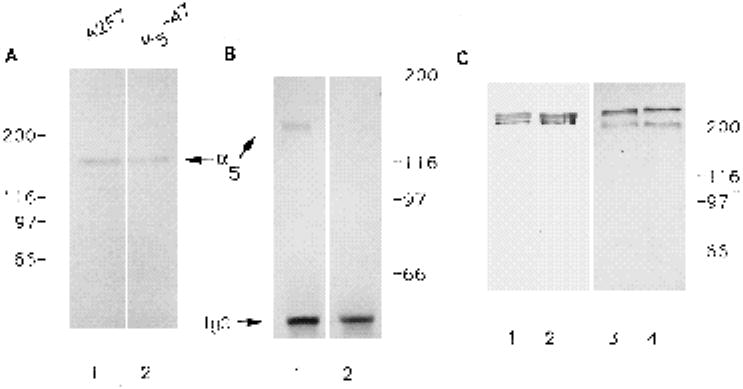
Characterization of antibodies directed against fibronectin and the α5 subunit. (A) Both the mAb A2F7 (lane 1) and the polyclonal antibody α5-47 (lane 2) recognize a 150×103 Mr band in extracts of chick peripheral nerve. (B) The mAb A2F7 recognizes a protein ~150×103 Mr immunoprecipitated by the human α5-47 polyclonal antibody (1) but does not recognize any bands immunoprecipated from the same chick peripheral nerve extract with a polyclonal antibody against the human β5 subunit (2) (C) Both mAb VA13 and FN-kv1 recognize purified bovine FN (lanes 1 and 2 respectively). Similarly, both antibodies recognize chicken FN in an extract from chick medial nerve (lane 3, Va1-3; lane 4, FN-kv1).
Two different monoclonal antibodies against FN were used in this study: mAb Va13, which recognizes intact FN and the RGD-cell binding domain, and mAb FN-kv1, which recognizes the CS1 domain and intact FN. Both antibodies recognized purified bovine FN on a reducing gel (Fig.2C, lanes 1 and 2) and recognized the same bands, around 220×103 Mr, on an immunoblot of chick peripheral nerve extract (Fig. 2C, lanes 3 and 4).
Distribution of α5 and FN in normal adult nerve
The α5 subunit associates with the integrin β1 subunit to form the functional FN receptor, α5β1; it has not been observed associated with any β integrin subunit other than β1. To obtain evidence for the presence of the α5β1 heterodimer in chick peripheral nerve, we determined by immunocytochemistry that all cells expressing the α5 subunit also expressed the β1 subunit (data not shown); co-precipitation from chick nerve extracts with the α5-47 polyclonal antibody of an 110×103 Mr protein recognized specifically by anti-β1 antibodies provided direct biochemical evidence for the association of the two integrin subunits in peripheral nerve (data not shown). In cross sections of mature (4 week) peripheral nerve, both α5 (Fig. 3A) and FN (Fig. 3B) were prominently expressed within the perineurium and endoneurium. Overlapping expression of the two antigens was evident within both compartments. Examination with higher magnification showed strong expression of the α5 subunit on myelinated axons and myelinating Schwann cells (Fig. 3C). Non-myelinated axons and non-myelinating Schwann cells expressed lower levels of the α5 subunit. Non-neuronal cells, including capillary endothelial cells and fibroblasts also expressed the α5 subunit. Fibronectin was localized in rings surrounding the Schwann cell endoneurial tubes (Fig. 3B,D) corresponding to the site of basal lamina deposition (Peters et al., 1976). To confirm the localization of FN along Schwann cell endoneurial tubes, longitudinal sections of peripheral nerve were double labeled with anti-FN and a known Schwann cell marker, S-100 (Fig. 3E,F). The anti-S100 IgG labels Schwann cell cytoplasm (Fig. 3E). FN was distributed just outside and running along part of the length of the same endoneurial tube (Fig. 3F). The expression on axons of the integrin α5 subunit was also clearly demonstrated in longitudinal sections of peripheral nerve (Fig. 3G,H); being particularly evident under Nomarski optics (Fig. 3H) and revealing a punctate distribution at higher magnification with epi-fluorescence optics (Fig. 3G). In developing peripheral nerve, the α5 subunit (Fig. 3I) was prominently expressed on axons, identified with the neuronal-specific anti-neurofilament antibody (Fig. 3J), and non-neuronal cells.
Fig. 3.
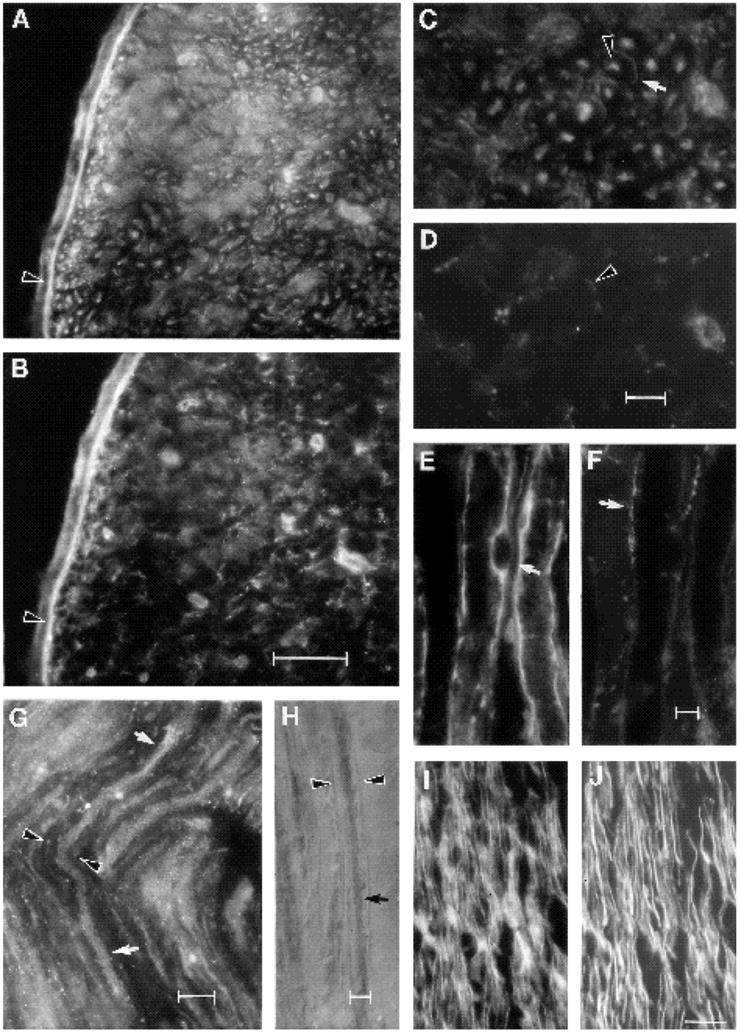
Distribution of the integrin subunit, α5, and fibronectin in unoperated peripheral nerve. (A-D) Transverse section of a nerve from a 4-week animal double labeled with anti-α 5-47 (A,C) and anti-fibronectin IgG (B,D). (A,B) Note the overlapping localization of the α5 subunit and FN in perineurium (arrowhead). (C,D) Same field as above, but with higher magnification view of endoneurium. (C) Note that myelinated axons are recognized by the anti-α5 antibody (C; arrowhead) as are their myelinating Schwann cells (arrow). (D) Same field as C under optics to visualize the FN localization surrounding myelinating Schwann cell endoneurial tubes (arrowhead). (E,F) Longitudinal section from a 4-week adult nerve double labeled with S-100 (E) and FN antibodies (F). Note that FN is localized (F, arrow) adjacent to the myelinating Schwann cell (E, arrow). (G,H) Longitudinal sections of normal adult nerve labeled with anti-α5 IgG and (G) with a fluorescently linked secondary antibody; note punctate staining of myelinated axon (between arrows) and of surrounding myelinated Schwann cell (arrowhead). (H) Here, the secondary antibody was linked to horseradish peroxidase and the section visualized with Nomarski optics. Note intense labeling of a myelinated axon (arrow) inside its myelin sheath (arrowhead). (I,J) Longitudinal section of an immature nerve from a 1-day-old chick, double labeled with anti-α5 (I) and anti-neurofilament (J). Note that axons are amongst the cells at this stage that express α5. Bar (A,B) 50 μm; (C,D) 10 μm; (E,F) 5 μm; (G)10 μm; (H)5 μm; (I,J) 25 μm.
Changes in expression of FN and the α5 subunit during peripheral nerve maturation
Several molecules that promote neurite outgrowth and their receptors have been shown to be down regulated as development proceeds (Martini and Schachner, 1988; Dodd et al., 1988). Reduced expression of these molecules often correlates with the arrival of axons at their targets (Cohen et al., 1986; 1989; Hall et al., 1987; de Curtis et al., 1991).
To determine whether expression of FN and the α5 subunit might also be developmentally regulated within the peripheral nerve, we compared their expression levels in hatchlings (day 1) to 4-week-old chicks by immunocytochemistry (Fig. 4). We found the distribution of FN to be more sparse and dispersed in the older nerve (Fig. 4D) compared to its more prominent expression in the younger nerve (Fig. 4C) where it was broadly expressed along axon bundies. The pattern of the α5 subunit expression changed considerably when comparing longitudinal sections from the two ages (Fig. 4A,B). In the day 1 nerve (Fig. 4A), all axons were brightly labeled with the anti-α5 antibody while in the 4 week nerve (Fig. 4B) brightly labeled myelinated axons were separated by bundles of more faintly labeled non-myelinated axons. While at both ages, α5 was expressed on non-neuronal cells, the proportion of such cells expressing α5 appeared to be greater in the younger nerve (see Fig. 3I,J). Between these two timepoints, the nerve undergoes a period of extensive cellular remodelling and differentiation, including myelination (Saxod and Bouvet, 1982). The visible decrease in α5 subunit expression within the more mature nerve corresponds primarily to a decreased expression on non-myelinated axons and partially to a diminished expression or presence of non-neuronal cells.
Fig. 4.
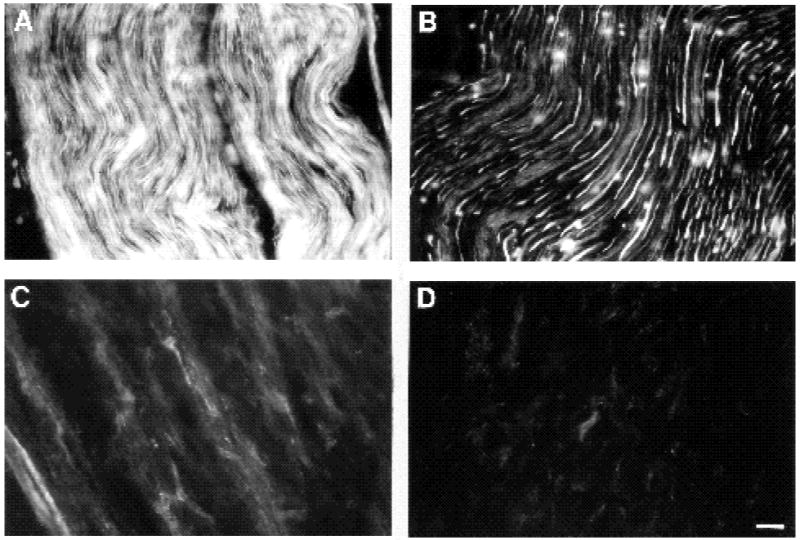
Immunohistochemical comparison of expression levels of α5 and FN in nerves from a 1-day-old (A,C) versus 4-week-old (B,D) chick. (A,B) Anti-α5 labeling; (C,D) anti-FN labeling. All exposure times and development times (for the same given antigen) were of equal duration in order to compare fairly the levels of fluorescence intensity between the two ages. Note that for both antigens, their levels decrease with nerve maturation. Bar, 35 μm.
Expression of FN and α5 strongly increases during nerve regeneration
Nerve injury triggers a state of active growth both in terms of neurite extension and Schwann cell differentiation (for review see Fawcett and Keynes, 1990). To test whether there were changes in expression of FN or α5β1 in response to such an injury, we transected the medial-ulnar nerve in 3 week chickens. To examine regulation of the integrin α5 subunit, the relative amounts of α5 expression in both control and experimental nerves were compared on immunoblots by densitometry. We measured a sixfold increase (Fig. 5) in the expression of the α5 subunit in regenerating nerve relative to contralateral control nerve, peaking 1 week following nerve transection. This increase occurred both in the segment immediately proximal and distal to the transection site.
Fig. 5.
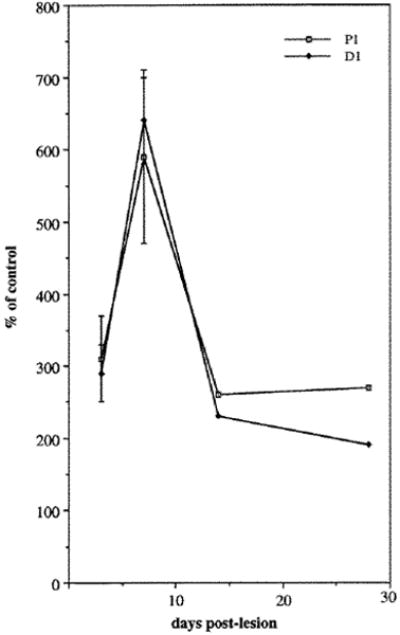
Quantification of changes in α5 expression in the regenerating nerve compared to the contralateral control nerve (=100% on y-axis). For each experiment, 4-7 nerves were homogenized together. Values expressed for the first two timepoints are the mean (± s.e.m.) of 2-4 experiments.
To determine the cell types in which these changes occurred, longitudinal sections of regenerating and control nerve at 1 week following nerve transection were labeled with the anti-α5 and FN antibodies as well as neuronal and Schwann cell markers (Fig. 6). In agreement with our densitometry results, we found that nerve injury strongly induced expression of the integrin α5 subunit. Compared to the contralateral control nerve (Fig. 6A), the α5 subunit was more highly expressed in the proximal (P2 and P1) and distal (D2 and D1) regions of the regenerating nerve as well as in the site of injury (Fig. 6H). In this nerve, the distal front of the regenerating axons had not yet reached the D1 region (see Fig. 8). Thus the expression of the α5 subunit observed distal to the transection site (D1 and D2 regions) must reflect expression on non-neuronal cells. In the more distal D2 region, the distribution and morphology of the α5 labeled cells closely resembled proliferating Schwann cells that align to form the Bands of Bungner during Wallerian degeneration (Allt, 1976; for review see Fawcett and Keynes, 1990). These cells could not be positively identified with anti-S100 antibodies since immature proliferating Schwann cells express little of this antigen (Neuberger and Cornbrooks, 1989; Jessen et al., 1989). Proliferating fibroblasts and endothelial cells may also contribute to the staining pattern. At the site of injury, the integrin α5 subunit co-localized with axons and sprouting growth cones as indicated by the overlap of neurofilament (Fig. 6I) and α5 expression (Fig. 6H). Since there were also cells in this region that expressed α5 but not the neuronal markers (neurofilaments or (β-tubulin), the non-neuronal cells with which the axonal sprouts and growth cones were associated (most likely Schwann cells, see Hall, 1986; Fawcett and Keynes, 1990) must also express the α5 subunit. Thus, it appears that the number of cells expressing α5 increased in the regenerating nerve, both proximal and distal to the transection site.
Fig. 6.
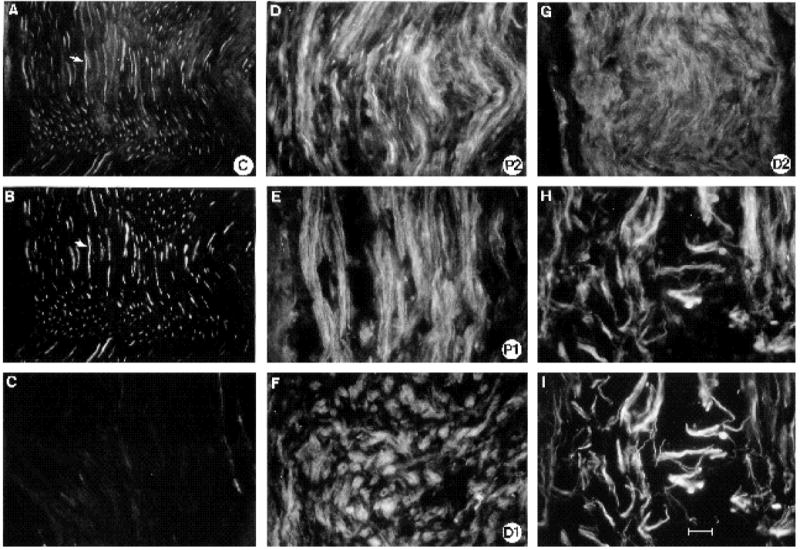
α5 expression in regenerating nerve one week following nerve transection. (A-C) Control nerve; (D-H) regenerating nerve labeled with anti-α5 antibody. To compare levels of α5 expression, all photographic exposures and development times were of equal length (see text). (A,B) Same section of contralateral control nerve double labeled with α5 (A) and neurofilament antibodies (B). Note how all axons express α5 (arrow). (C) Adjacent section of control nerve which was incubated with the C-terminal α5 peptide and the α5 ab. The staining is considerably reduced indicating that this peptide can specifically compete with the endogenous ligand for recognition by the α5 polyclonal antibody. (D) P2 region of regenerating nerve, (E) P1 region, (F) D1 region, (G) D2 region, where Schwann cells are dividing and starting to align to form the Bands of Bungner. (H,I) Double label with anti-α5 (H) and anti-neurofilament antibodies (I) at transection site to indicate that axonal sprouts (as labeled by neurofilament abs) also express α5; note how the antigens overlap. Bar, 25 μm.
Fig. 8.
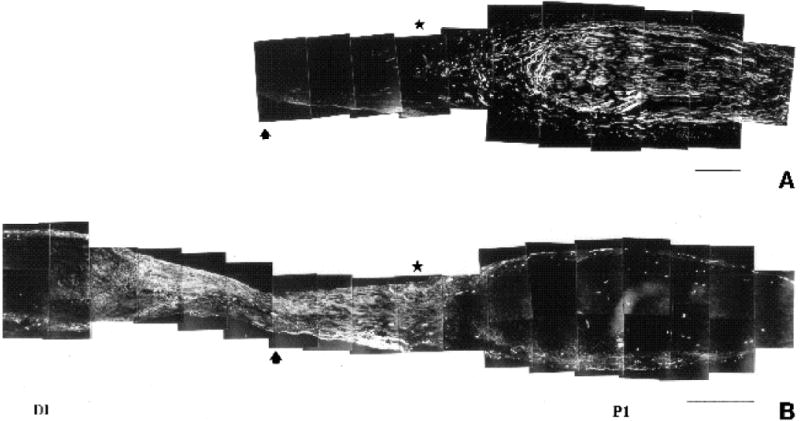
Adjacent sections of regenerating nerve one week following nerve transection, labelled with (A) anti-β-tubulin, a neuronal marker, to illustrate position of regenerating growth cones and (B) anti-FN to demonstrate discrete pattern of FN expression in the regenerating nerve. Note how FN expression is highest in the ‘bridge’ region connecting the proximal (P1) and distal (D1) stumps. Arrow marks the site of transection. Note how the most distally extended growth cones (star) have penetrated into the region of intense FN expression. Bar (A) 300 μm; (B) 450 μm.
The distribution of fibronectin was also examined in the regenerating nerve (Fig. 7). Compared to the contralateral control nerve (Fig. 7A), one week after nerve transection a dramatic increase in the level of fibronectin expression was observed in the regenerating nerve (Fig. 7B-F). Interestingly, while the P2 and D2 regions showed a slight increase compared to the control section, the area surrounding and including the transection site (P1, P1-D1, D1; Fig. 7C-D) showed a very strong increase compared to control nerve. In fact, the region with the highest expression was very discrete: it began in the area surrounding the nerve transection site in the vicinity of the distal front of the regenerating growth cones (distal edge of P1) and increased dramatically in the region connecting P1 to D1 (P-D), terminating in the proximal portion of the D1 region. This very circumscribed pattern of FN expression with respect to the disposition of the regenerating growth cones is more clearly illustrated in Fig. 8, where several mm of the regenerating nerve are reproduced. The region of highest FN intensity (Fig. 8B) coincided with the ‘bridge’ region connecting the proximal and distal stumps (Longo et al., 1984; Martini et al., 1990). The growth cones of the regenerating neurites (labelled in Fig. 8A with anti-β-tubulin antibody) had penetrated into the region of elevated FN expression, which extended distally in advance of the growth cones.
Fig. 7.
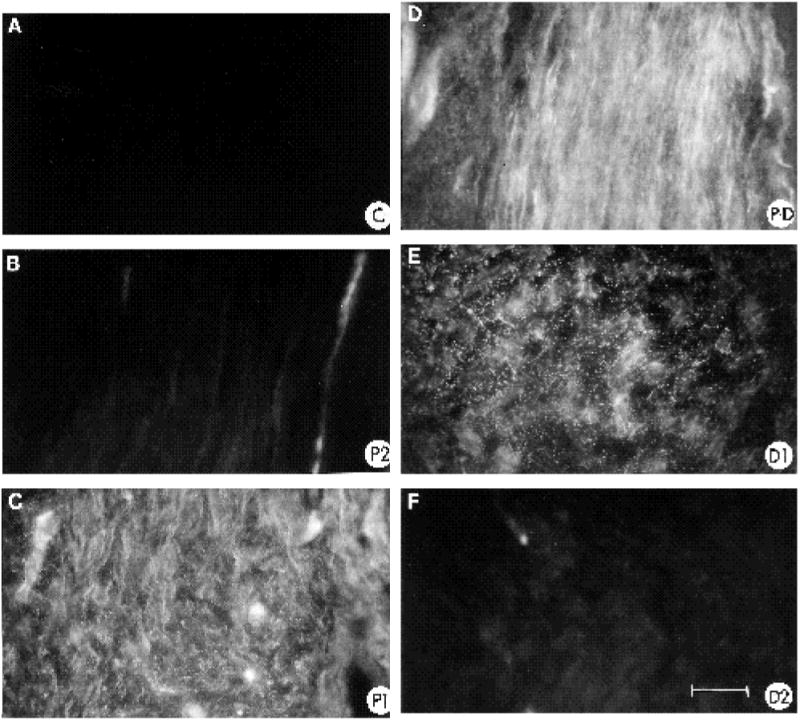
Fibronectin expression in regenerating nerve one week post-transection. All photographic exposure times were equivalent and all negatives were processed equally. (A) Contralateral control nerve stained with anti-fibronectin antibody. (B-F) Regenerating nerve stained with anti-fibronectin antibody; (B) P2 region; (C) P1 region; (D) the ‘bridge’ region connecting P1 and D1 regions of nerve; (E) D1 region; (F) D2 region. Bar, 45 μm.
To determine whether there was a general increase in expression of ECM molecules in the bridge region, we examined the distribution of laminin, a representative constituent of basal lamina, in regenerating nerve (Fig. 9). Based on antibody perturbation studies, laminin isoforms have been implicated functionally in peripheral nerve regeneration (Sandrock and Mathews, 1987a,b). Interestingly, while laminin immunoreactivity was very prominently distributed proximally and distal to the cut site, mostly on the surface of Schwann cells (Fig. 9B,D) or in the endoneurial basal lamina (Fig. 9A; see also Sanes et al., 1990), there was little detectable laminin expression in the bridge region (Fig. 9C).
Fig. 9.
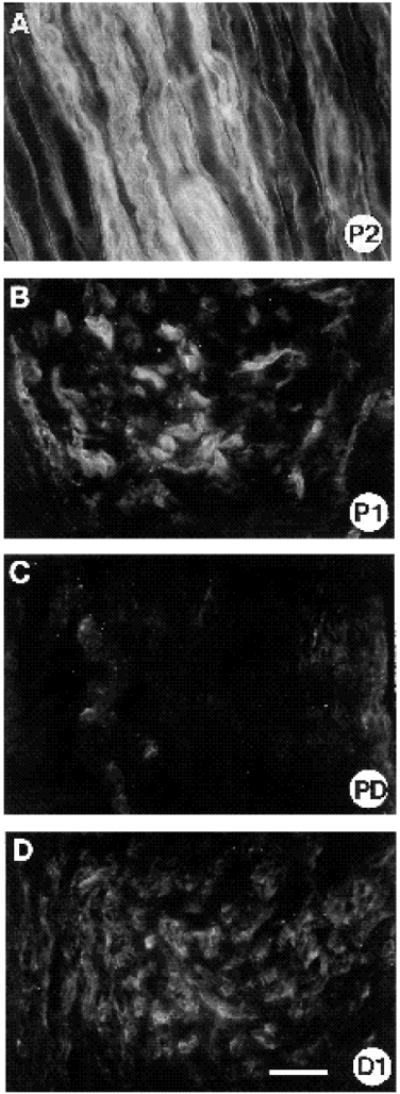
Anti-laminin labelling of section adjacent to one depicted in Fig. 8. Note how while laminin is expressed both proximally (A,B) and distally (D) to the transection site, it is virtually absent from the ‘bridge’ region (C:PD). In the P2 region (A), laminin is located surrounding the endoneurial tubes. Bar, 25 μm.
Two weeks following nerve transection, results illustrated in Figs 10 and 11 show that the expression of FN was still elevated in the regenerating nerve (compare Fig. 10A to panels B,C,E and G). The region of most intense expression still corresponded to the original transection site. Compared to one week after transection, the elevated FN expression extended further distally down the nerve into segment D2 (Fig. 10G). In addition, the regenerating axons had now traversed the bridge region and extended distally for several mm into the D2 region (Figs 10, 11). Both in the vicinity of the transection (Figs 10D,F, 11A,C,E) and further distally (D2 region, Figs 10H, 11B,D,F), α5 labeled regenerating axons were observed in regions with elevated FN expression. In the D2 segment, axons grew with straight trajectories, indicating that they were growing through endoneurial tubes (Ide et al., 1983; Longo et al., 1984; Fawcett and Keynes, 1990). However, in the bridge region, neurites were observed in several orientations (Figs 10F, 11C,E), an indication that they had not yet reached the parallel array of endoneurial tubes. Since regenerating neurites expressed α5 (Figs 6, 11), growth cones appear able to interact with FN in both the bridge and more distal regions.
Fig. 10.
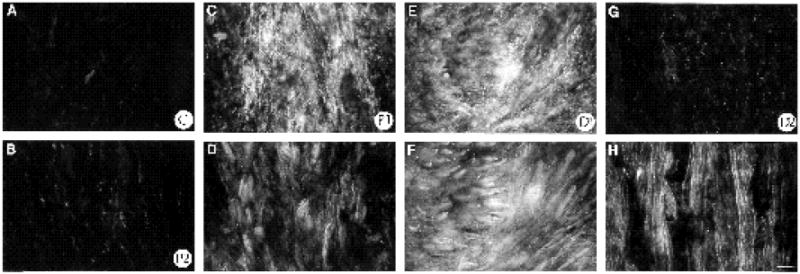
Double labeling of nerve 2 weeks following transection with anti-α5 and anti-FN antibodies. All negatives were exposed and processed equally. FN labeling depicted in (A,B,C,E,G) and α5 labeling depicted in (D,F,H). (A) Control nerve. (B) P2 region of regenerating nerve. (C,D) P1 region, note elevated expression of FN in area containing (D) α5 labeled cells. (E) D1 region, high FN expression has extended further distally by 2 weeks. In same field numerous α5 labeled cells are observed (F). Elevated FN expression is now seen in D2 region by this time (G; compare to Fig. 7F), a region where regenerating axons are growing straight through the endoneurial tubes; note numerous α5 labeled cells, most of them being axons (H). Bar, 25 μm.
Fig. 11.
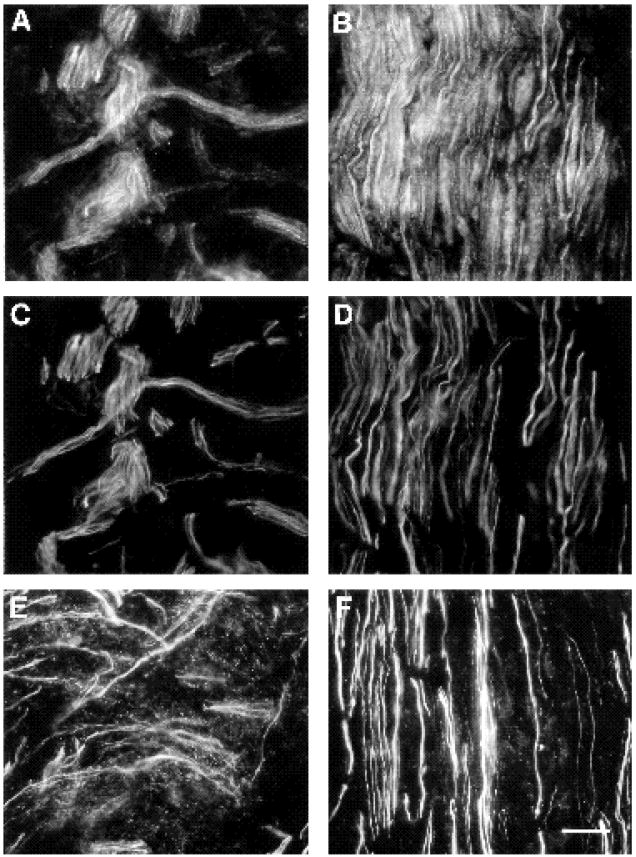
2 weeks post-transection, regenerating axons express α5 and grow in regions of elevated FN expression. Double-labeled sections of regenerating nerve 2 weeks post-transection with anti-α5 (A,B) and anti-neurofilament abs (C,D). In a proximal portion of the D1 region (A,C) just distal to the site of transection, regenerating neurites labeled with neurofilament abs (C) all express the α5 subunit (A). The curvilinear growth is typical of the morphology of regenerating neurites in this region near the site of transection. Further distally (B,D) in the D2 region, axons have aligned themselves along the endoneurial tubes and hence grow relatively straight. Note how here too, all axons (D) express α5 (B). (E,F) Double labeling of an adjacent section with anti-neurofilament and anti-FN antibodies reacted with the same secondary antibody so that both antigens could be photographed on the same negative and yet distinguished by the relatively cylindrical neurofilament staining versus the punctate FN distribution. These photographs illustrate that FN is expressed in the same area as regenerating neurites both in the D1 region (E) and further distally in the D2 region (F), although FN expression is higher in the D1 region than in the D2 region. Bar, 20 μm.
Discussion
We report here on the spatiotemporal expression pattern of fibronectin and one of its receptors, the α5β1 integrin heterodimer, during development and regeneration of peripheral nerve. We and others have not found any instances where the α5 subunit is expressed in the absence of β1; in immunoprecipitations α5 is invariably associated with β1 and has not been detected in association with any other β subunit. Thus we believe in peripheral nerve the distribution of the α5 subunit reflects that of the α5β1 receptor. We have found that (1) both FN and α5β1 are prominently expressed in developing nerve with strong α5 subunit expression on neurons and non-neuronal cells, (2) the prevalence of both α5 and fibronectin decreases with maturation of the peripheral nerve, (3) in response to injury to the mature nerve, both FN and α5 are strongly induced, (4) the elevated FN expression at the site of injury suggests that it is functionally important for reestablishing connections between the severed nerve segments and (5) the distribution of the fibronectin receptor, α5β1, suggests that it is utilized by both axons and Schwann cells during nerve regeneration.
As others have noted (Palm and Furcht, 1983; Longo et al., 1984; Sanes, 1989), in mature nerve, we observed fibronectin distributed around the endoneurial tubes, the region known to correspond to the endoneurial basal lamina (Peters et al., 1976; Bunge et al., 1989a) and in the perineurium. Thus α5β1 receptors on the outer Schwann cell membrane seem likely to interact with fibronectin in the basal lamina. Since the α5β1 receptor has been demonstrated to be required for optimal FN matrix assembly (McDonald et al., 1987; Roman et al., 1989), its expression on Schwann cells is consistent with such a role in the nerve. Non-myelinated axons and non-myelinating Schwann cells, which also expressed the α5 subunit but at lower levels, have been observed in direct contact with the endoneurial basal lamina (Kuecherer-Ehret et al., 1990) and thus are also likely to interact with FN. In the mature nerve, FN would not be accessible to the α5β1 receptors on myelinated axons. It is thus possible that, in addition to FN, α5β1 may also interact with an unidentified ligand on the interior surface of myelinating Schwann cells. Many other integrins have been shown to have more than one ligand and these include integral membrane proteins (for example, see Elices et al., 1990).
In contrast to their more restricted expression in the older nerve, fibronectin and α5β1 were much more prominently expressed in a relatively immature nerve. In the hatchling nerve, FN was rather continuously expressed along axon bundles whereas in the older nerve it was sparse and more dispersed within the endoneurium. In the younger nerve, the α5 subunit seemed ubiquitously expressed throughout the nerve with strong expression on all axons. In the older nerve, strong axonal α5 expression was restricted to myelinated axons in contrast to the considerably weaker expression on non-myelinated axons. Between these two time points (day 1 versus 4 weeks), the nerve undergoes a period of extensive myelination; at hatching, fewer than 4% of axons are myelinated, while by 6 weeks, 40% of the axons are myelinated. (In the adult, the maximum number of myelinated fibers reaches 60%; Saxod and Verna, 1979.) In the nerves of hatchlings (day 1), none of the broader (myelinated) axons observed in the older nerves were evident; instead most of the axons were thin and appeared in broad bundles, characteristic of an immature nerve where several (non-myelinated) axons are often associated with an individual Schwann cell (cf. Webster and Favilla, 1984). Thus our data suggest that the diminished expression of α5 observed on the neuronal cells with nerve maturation is primarily due to a decreased expression on axons that remain non-myelinated. The prominent expression of α5β1 at this early time point also coincides with a period of pronounced Schwann cell proliferation which later ceases (Webster and Favilla, 1984; Asbury, 1967). As Schwann cells mature they alter their expression of several proteins including cell surface adhesion molecules (Daston and Ratner, 1991; Neuberger and Cornbrooks, 1989; Jessen et al., 1989).
Fibronectin and the fibronectin receptor α5β1 are induced in response to nerve injury
Our results show that FN is strongly induced in peripheral nerve in response to injury. Both one and two weeks following transection, fibronectin expression was most strongly increased in the vicinity of the site of injury. Laminin, another representative ECM constituent, is virtually absent from this region, although the expression of tenascin increases distally following nerve injury (Martini et al., 1990). Thus fibronectin, but not laminin, is distributed appropriately to play a significant role as a promotor of both Schwann cell migration and axonal regeneration through this bridge region.
The elevated FN in this area may be deposited from plasma or be synthesized locally by fibroblasts and endothelial cells in the bridge region (Longo et al., 1984; Woolley et al., 1990; Cornbrooks et al., 1983). This bridge region is not simply an acellular matrix characteristic of a fibrin clot; labelling with nuclear markers (e.g., DAPI, data not shown) demonstrates a rather uniform cellular array throughout this region connecting the two severed stumps and most likely corresponds to the fibroblasts reforming the outer nerve sheath (peri or epineurium; Bunge et al., 1989b). Since, in adult nerve, FN is localized along the external surface of Schwann cell endoneurial tubes, it is conceivable that FN might be synthesized by Schwann cells in vivo or alternatively synthesized by other neighboring non-neuronal cells and incorporated by Schwann cells into their overlying matrix (McDonald et al., 1987; Roman et al., 1989). In vitro, Schwann cell synthesis of FN has been observed in the presence of cAMP analogs and ascorbate (Baron Van Evercooren et al., 1986).
The fibronectin receptor α5β1 was also strongly induced in regenerating peripheral nerve on both axons and Schwann cells. Notable increases in α5β1 expression were observed both proximal and distal to the site of injury. Proximal to the transection, a major proportion of the increase in α5β1 appeared to be neuronal. This reflected both elevated α5β1 expression on individual axons (compare the intensity of Fig. 6H to 6A), and an increased density of neurites due to axonal sprouting, which is triggered by nerve injury (Cajal, 1928; Diamond et al., 1987). The growing tips of regenerating axons strongly expressed α5β1 (Fig. 6H,I). Further, α5 subunit was also induced in non-neuronal cells both proximal and distal to the transection site (Fig. 6F,G). This was particularly striking in the D2 region (about 3-6 mm distal to the transection site) where at one week following transection, Schwann cells were proliferating and aligning to form the bands of Bungner (Fawcett and Keynes, 1990; Allt, 1976). Thus the induction of α5 subunit in this segment must be on Schwann cells since they constitute the vast majority of cells at this time. α5β1, then, appears to be reexpressed on dedifferentiating Schwann cells as well as on regenerating axons. Overall, the expression patterns of the α5 subunit in the developing and regenerating nerve were similar; in both, expression was strong on several cell types.
Previous studies have demonstrated that fibronectin levels are greatly elevated during wound healing in adult rat skin (ffrench-Constant et al., 1989; Grinnell et al., 1981), resembling its embryonic expression pattern. The authors suggest that the reexpression of FN, whose localization during development coincides with a period of active cell migration, would facilitate the enhanced migration that occurs as part of the wound response. For successful nerve regeneration, migration of Schwann cells into the wound region is critical; in their absence axons fail to regenerate (Hall, 1986; Fawcett and Keynes, 1990). Since FN has been shown to act not only as a substratum for Schwann cell migration, but also as a chemoattractant and mitogen for Schwann cells (Baron Van-Evercoorren et al., 1982), it seems possible that the elevated FN expression in the transection region may be an integral component of successful nerve regeneration by inducing the proliferation and migration of Schwann cells to the site of injury.
Thus fibronectin might facilitate nerve regeneration via two mechanisms. (1) One mechanism could be indirect, by inducing an influx of Schwann cells into the wound region. Since Schwann cell surfaces contain several adhesion molecules that potently promote neurite outgrowth (Bixby et al., 1988; Tomaselli et al., 1986), these cells would provide an attractive substratum for axonal outgrowth. (2) A second mechanism could be direct by virtue of itself being a demonstrated attractive glycoprotein for neuronal adhesion and neurite extension (for review, see Reichardt and Tomaselli, 1991). Its elevated expression in the vicinity of the regenerating growth cones and axons strongly implicate FN as a substratum for regenerating axons. At one week following transection, FN levels were highest in the vicinity of the regenerating growth cones and decreased further distally where the axons had not yet extended. By two weeks following nerve transection, the regenerating axons had grown into the distal stump and were now regrowing through the Schwann cell endoneurial tubes. In this distal region, axons are known to grow between the Schwann cell surface and its basal lamima in contact with both (Ide et al., 1983; Fawcett and Keynes, 1990). By this time, FN expression was elevated surrounding the reformed endoneurial tubes perhaps in response to axonal contact (Bunge et al., 1989a). Significantly, at both time points, we always observed prominent expression of α5β1 on regenerating neurites. The elevated expression of α5β1 on both Schwann cells and regenerating growth cones could facilitate their motility through a FN-rich region since previous work has positively correlated enhanced motility on FN with elevated expression of the α5β1 receptor (Schreiner et al., 1989).
Peripheral nerve transection induces an inflammatory response (Brown et al., 1991). For sensory axons, this response is thought to be an essential element for their successful regeneration. Macrophages recruited into the wound area secrete interleukin I (Il-1) which causes an elevation in NGF secretion by the non-neuronal cells in the nerve thereby trophically supporting the sensory neurons (Heumann et al., 1987; Lindholm et al., 1987; Brown et al., 1991). The increase in α5β1 in the nerve may similarly be regulated by cytokines such as Il-1. Another cytokine centrally involved in wound healing, TGF-β1 (for review see Clark, 1990), has been found to induce Schwann cell proliferation (Ridley et al., 1989). Further, levels of TGFβ1 mRNA significantly increase during nerve regeneration (Scherer and Jakolew, 1991). Interestingly, TGF-β1 has been found to upregulate expression of both FN and α5β1 on fibroblasts in vitro (Ignotz and Massague, 1987). Thus, there are several growth and differentiation factors that may directly upregulate the α5β1 and/or FN expression in regenerating peripheral nerve. In addition to regulating integrin expression levels, cytokines may also directly activate the α5β1 receptor (for review, see Hynes, 1992).
Our results suggest that one feature of the wound response induced by nerve transection, elevated FN expression, may be an integral component in the promotion of Schwann cell migration and axonal extension through a complicated terrain undergoing extensive cellular remodeling. The prominent expression of its receptor, α5β1, on Schwann cells and on regenerating and developing neurites suggests that this receptor-ligand interaction is important for successful peripheral nerve development and regeneration.
Acknowledgments
We thank T. Broekelmann for assistance in production of the α5-47 antibody, J. Muschler and A. F. Horwitz for generously providing the A2F7 monoclonal antibody, A. Frankfurter for the gift of the TuJi monoclonal antibody, M. Kirschner and T. Mitchison for the use of their microscopes, and M. Meyerson for expert typing assistance. We gratefully acknowledge D. Clary, I. de Curtis and K. Jones for critically reading the manuscript and informative discussions. Grant support included NIH#1F32NSO8451-01A1 to F. L., NS19090 to L. F. R., 2PO1HL29594 and 1RO1HL43418 to J. A. M.; L. F. R. is an investigator of the Howard Hughes Medical Institute.
References
- Allt G. Pathology of the peripheral nerve. In: Landon DN, editor. The Peripheral Nerve. London: Chapman and Hall; 1976. pp. 666–739. [Google Scholar]
- Asbury AK. Schwann cell proliferation in developing mouse sciatic nerve. J Cell Biol. 1967;34:735. doi: 10.1083/jcb.34.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Van Evercooren A, Kleinman HK, Seppa JRJ, Rentier B, Dubois-Dalcq M. Fibronectin promotes rat Schwann cell growth and motility. J Cell Biol. 1982;93:211–216. doi: 10.1083/jcb.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Van Evercooren A, Gransmuller A, Gumpel M, Baumann N, Kleinman HK. Schwann cell differentiation in vitro: Extracellular matrix deposition and interaction. Dev Neurosci. 1986;8:172–196. doi: 10.1159/000112252. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Lilien J, Reichardt LF. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J Cell Biol. 1988;107:1177–1187. doi: 10.1083/jcb.107.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy B, Bossy-Wetzel E, Reichardt LF. Characterization of the integrin α8 subunit: a new integrin β1-associated subunit which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J. 1991;10:2375–2385. doi: 10.1002/j.1460-2075.1991.tb07776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T, Poole TJ, Aoyama H, Yamada KM, Thiery JP. Biologically active synthetic peptides as probes of embryonic development: a competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest migration in avian embryos. J Cell Biol. 1984;99:1822–1830. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko D, Horwitz AF. The participation of a putative cell surface receptor for laminin and fibronectin on peripheral neurite extension. J Neurosci. 1986;6:1241–1251. doi: 10.1523/JNEUROSCI.06-05-01241.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Alterations in neural crest migration by a monoclonal antibody that affects cell adhesion. J Cell Biol. 1985;101:610–617. doi: 10.1083/jcb.101.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: Possible involvement of nerve growth factor. Neuron. 1991;6:359–370. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Brugg B, Matus A. PC12 cells express juvenile microtubule associated proteins during NGF-induced neurite outgrowth. J Cell Biol. 1988;107:643–650. doi: 10.1083/jcb.107.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB, Bunge RP, Kleitman N, Dean AC. Role of peripheral nerve extracellular matrix in Schwann cell function and in neurite regeneration. Dev Neurosci. 1989a;11:348–360. doi: 10.1159/000111911. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Wood PM, Tynan LB, Bates ML, Sanes JR. Perineurium originates from fibroblasts: Demonstration in vitro with a retroviral marker. Science. 1989b;243:229–231. doi: 10.1126/science.2492115. [DOI] [PubMed] [Google Scholar]
- Cajal S, Ramon Y. In: Degeneration and Regeneration of the Nervous System. May RM, editor. Vol. 1. Oxford University Press; 1928. [Google Scholar]
- Clark RAF. Fibronectin Matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Derm. 1990;94:128–134. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- Cohen J, Burnec JF, Winter J, Bartlett P. Retinal ganglion cells lose response to laminin with maturation. Nature. 1986;322:465–467. doi: 10.1038/322465a0. [DOI] [PubMed] [Google Scholar]
- Cohen J, Nurcombe V, Jeffrey P, Edgar D. Developmental loss of functional laminin receptors on retinal ganglion cells is regulated by their target tissue, the optic tectum. Development. 1989;107:381–387. doi: 10.1242/dev.107.2.381. [DOI] [PubMed] [Google Scholar]
- Cornbrooks CJ, Carey DJ, McDonald JA, Timpl R, Bunge RP. In vivo and in vitro observations on laminin production by Schwann cells. Neurobiol. 1983;80:3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloff JK, Levi G, Grumet M, Rieger F, Edelman GM. Altered expression of neuronal cell adhesion molecules induced by nerve injury and repair. J Cell Biol. 1986;103:929–945. doi: 10.1083/jcb.103.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daston MM, Ratner N. Expression of P30, a protein with adhesive properties, in Schwann cells and neurons of the developing and regenerating peripheral nerve. J Cell Biol. 1991;112:1229–1239. doi: 10.1083/jcb.112.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis I, Quaranta V, Tamura RN, Reichardt LF. Laminin receptors in the retina: Sequence analysis of the chick integrin α6 subunit. J Cell Biol. 1991;113:405–416. doi: 10.1083/jcb.113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Maclntyre L, Holmes M, Visheau B. Evidence that endogenous β nerve growth factor is responsible for the collateral sprouting, but not the regeneration of nociceptive axons in adult rats. Proc Natl Acad Sci USA. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Martin SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1990;1:105–110. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Duband JL, Rocher S, Chen WT, Yamada KM, Thiery JP. Cell adhesion and migration in the early vertebrate embryo: location and possible role for the putative fibronectin receptor complex. J Cell Biol. 1986;102:160–178. doi: 10.1083/jcb.102.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour S, Duband J-L, Humphries MJ, Obara M, Yamada KM, Thiery JP. Attachment, spreading and locomotion of avian neural crest cells are mediated by multiple adhesion sites on fibronectin molecules. EMBO J. 1988;7:2661–2671. doi: 10.1002/j.1460-2075.1988.tb03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices MJ, Osborn L, Takada Y, Crouse C, Luhowsky S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct form the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Elices MJ, Urry LA, Hemler ME. Receptor functions for the integrin VLA-3: Fibronectin, collagen and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J Cell Biol. 1991;112:169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Hynes RO. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989;106:375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, VanDe Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989;109:903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F, Billingham RE, Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981;76:181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Toda K, Lamke-Seymour C. Reconstitution of human epidermis in vitro is accompanied by transient activation of basal keratinocyte spreading. Exp Cell Res. 1987;172:439–449. doi: 10.1016/0014-4827(87)90402-2. [DOI] [PubMed] [Google Scholar]
- Guan J-L, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor α4β1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hall DE, Neugebauer KM, Reichardt LF. Embryonic neural retinal cell response to extracellular matrix proteins: Developmental changes and effects of the cell substratum attachment antibody (CSAT) J Cell Biol. 1987;104:623–634. doi: 10.1083/jcb.104.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM. The effect of inhibiting Schwann cell mitosis on the reinnervation of acellular autografts in the peripheral nervous system of the mouse. Neuropath and Appl Neurobiol. 1986;12:401–414. doi: 10.1111/j.1365-2990.1986.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies A Laboratory Manual. New York: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Hemler ME. VLA proteins in the integrin family: Structure, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987;104:1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM. Neurite extension of chicken peripheral nervous systems neurons on fibronectin: Relative importance of specific adhesion sites in the central cell-binding domain and the alternatively spliced type III connecting segment. J Cell Biol. 1988;106:1289–1297. doi: 10.1083/jcb.106.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Marcantonio EE, Stepp MA, Urry LA, Yee GH. Integrin heterodimer and receptor complexity in avian and mammalian cells. J Cell Biol. 1989;109:410–420. doi: 10.1083/jcb.109.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ide Z, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Research. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Cell adhesion protein receptors as targets for transforming growth factor-β action. Cell. 1987;51:189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Morgan L, Mirsky R. Schwann cell precursors and their development. J Neurochem. 1989;32:S128. doi: 10.1002/glia.440040210. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Horie H, Takenaka T. The maturation-dependent change in fibronectin receptor density of mouse dorsal root ganglion neurons. Brain Research. 1986;397:185–188. doi: 10.1016/0006-8993(86)91384-3. [DOI] [PubMed] [Google Scholar]
- Krotoski DM, Domingo C, Bronner-Fraser M. Distribution of a putative cell surface receptor for fibronectin and laminin in the avian embryo. J Cell Biol. 1986;103:1061–1071. doi: 10.1083/jcb.103.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuecherer-Ehret A, Graeber MB, Edgar D, Thoenen H, Kreutzberg GW. Immunoelectron microscopic localization of laminin in normal and regeneration mouse sciatic nerve. J Neurocytology. 1990;19:101–109. doi: 10.1007/BF01188442. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Reichardt LF. Laminin is associated with the neurite outgrowth promoting factors found in conditioned media. Proc Natl Acad Sci USA. 1985;82:2183–87. doi: 10.1073/pnas.82.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330:658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Longo FM, Hayman EG, Davis GE, Ruoslahti E, Engvall E, Manthorpe M, Varon S. Neurite-promoting factors and extracellular matrix components accumulating in vivo within nerve regeneration chambers. Brain Res. 1984;309:105–117. doi: 10.1016/0006-8993(84)91014-x. [DOI] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and myelin-association glycoprotein) in regenerating adult mouse sciatic nerve. J Cell Biol. 1988;106:1735–1746. doi: 10.1083/jcb.106.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M, Faissner A. Enhanced expression of the extracellular matrix molecule J1/tenascin in the regenerating adult mouse sciatic nerve. J Neurocytology. 1990;19:601–616. doi: 10.1007/BF01257247. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Quada BJ, Broekelmann TJ, LaChance R, Forseman K, Hasigawa K, Akiyama S. Fibronectins cell adhesive domain and an amino terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J Biol Chem. 1987;272:2957–2967. [PubMed] [Google Scholar]
- Millaruelo AI, Nieto-Sampedro M, Cotman CW. Cooperation between nerve growth factor and laminin or fibronectin in promoting sensory neuron survival and neurite outgrowth. Brain Res. 1988;466:219–228. doi: 10.1016/0165-3806(88)90047-8. [DOI] [PubMed] [Google Scholar]
- Mould AP, Humphries MJ. Identification of a novel recognition sequence for the integrin alpha 4 beta 1 in the COOH terminal heparin binding domain of fibronectin. EMBO J. 1991;10:4089–4095. doi: 10.1002/j.1460-2075.1991.tb04985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschler JL, Horwitz AF. Down regulation of the chicken α5β1 integrin fibronectin receptor during development. Development. 1991;113:327–337. doi: 10.1242/dev.113.1.327. [DOI] [PubMed] [Google Scholar]
- Neuberger TJ, Cornbrooks CJ. Transient modulation of Schwann cell antigens after peripheral nerve transection and subsequent regeneration. J Neurocytology. 1989;18:695–710. doi: 10.1007/BF01187088. [DOI] [PubMed] [Google Scholar]
- Neugebauer KM, Emmett CJ, Venstrom KA, Reichardt LF. Vitronectin and thrombospondin promote retinal neurite outgrowth: Development regulation and role of integrins. Neuron. 1991;6:345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- Newgreen D, Thiery J-P. Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res. 1980;211:269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- Palm S, Furcht L. Production of laminin and fibronectin by Schwannoma cells: cell protein interactions in vitro and protein localization in peripheral nerve in vivo. J Cell Biol. 1983;96:1218–1226. doi: 10.1083/jcb.96.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The Fine Structure of the Nervous System. Philadelphia: W. B. Saunders; 1976. [Google Scholar]
- Pierschbacher MD, Ruoslahti E. The cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Reichardt LF, Tomaselli KJ. Extracellular Matrix molecules and their receptors: Functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Davis JB, Stroobant P, Land H. Transforming frowth factors-β1 and β2 are mitogens for rat Schwann cells. J Cell Biol. 1989;109:3419–3424. doi: 10.1083/jcb.109.6.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Letourneau PC, Palm SL, McCarthy J, Furcht LT. Neurite extension by peripheral and central nervous systems neurons in response to substratum-bound fibronectin and laminin. Dev Biol. 1983;98:212–220. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Rogers SL, McCarthy JB, Palm SL, Furcht LT, Letourneau PC. Neuron-specific interactions with two neurite promoting fragments of fibronectin. J Neurosci. 1985;5:369–378. doi: 10.1523/JNEUROSCI.05-02-00369.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman J, Lachance RM, Broekelmann TJ, Kennedy JR, Wayner EA, Carter WG, McDonald JA. The fibronectin receptor is organized by extracellular matrix fibronectin: implications for oncogenic transformation and for cell recognition of fibronectin matrices. J Cell Biol. 1989;108:2529–2543. doi: 10.1083/jcb.108.6.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman J, McDonald JA. Expression of fibronectin, the integrin α5 and α-smooth muscle actin in heart and lung development. Am J Respir Cell Mol Biol. 1992;6:472–480. doi: 10.1165/ajrcmb/6.5.472. [DOI] [PubMed] [Google Scholar]
- Sandrock A, Matthew W. Identification of a peripheral nerve neurite growth-promoting activity by development and use of an in vitro bioassay. Proc Natl Acad Sci USA. 1987;84:6394–6938. doi: 10.1073/pnas.84.19.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock A, Matthew W. An in vitro neurite promoting antigen functions in axonal regeneration in vivo. Science. 1987;237:1605–1607. doi: 10.1126/science.3306923. [DOI] [PubMed] [Google Scholar]
- Sanes JR. Extracellular matrix molecules that influence neural development. Ann Rev Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: Isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990;111:1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxod R, Bouvet J. Quantitative analysis of growth and myelination of cutaneous nerve fibers in the chick. Dev Neurosci. 1982;5:143–155. doi: 10.1159/000112671. [DOI] [PubMed] [Google Scholar]
- Saxod R, Verna JN. Development of nerves and pattern of innervation in the chick skin. Ultrastructural and quantitative analysis. J Invest Derm. 1979;72:286. [Google Scholar]
- Scherer S, Jakolew SE. The expression of TGF-β1 mRNA increases during Wallerian degeneration in the PNS. Soc for Neurosci Abstr. 1991;17:562. [Google Scholar]
- Schreiner CL, Bauer JS, Danilov YN, Hussein S, Sczekan MM, Juliano RL. Isolation and characterization of CHO cell variants deficient in the expression of fibronectin receptor. J Cell Biol. 1989;109:3157–3167. doi: 10.1083/jcb.109.6.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Wayner EA, Carter WG, Hemler ME. Extracellular matrix receptors, ECMR II and ECMR I, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988;37:385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Takashima A, Bfflingham RE, Grinnell F. Activation of rabbit keratinocyte fibronectin receptor function in vivo during wound healing. J Invest Dermatol. 1986;86:585–590. doi: 10.1111/1523-1747.ep12355243. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Reichardt LF, Bixby JL. Distinct molecular interactions mediate neuronal process outgrowth on nonneuronal cell surfaces and extracellular matrices. J Cell Biol. 1986;103:2659–2672. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel BE, Tarone G, Giancotti FG, Gailit J, Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition αvβ1. J Biol Chem. 1990;265:5934–5937. [PubMed] [Google Scholar]
- Wayner EA, Carter WG, Piotrowicz RS, Kunicki TJ. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988;107:1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–30. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster H, Favilla JT. Development of peripheral nerve fibers. In: Dyck PJ, Thomas PK, Lambert EH, Bunge R, editors. Peripheral Neuropathy. I. Philadelphia: W. B. Saunders Company; 1984. pp. 329–358. [Google Scholar]
- Woolley AL, Hollowell JP, Rich KM. Fibronectin-laminin combination enhances peripheral nerve regeneration across long gaps. Otol Head Neck Surg. 1990;103:509–518. doi: 10.1177/019459989010300401. [DOI] [PubMed] [Google Scholar]
- Yaginuma H, Shiga T, Homma S, Ishihara R, Oppenheim RW. Identification of early developing axon projections from spinal interneurons in the chick embryo with a neuron specific β-tubulin antibody: evidence for a new “pioneer” pathway in the spinal cord. Development. 1990;108:705–716. doi: 10.1242/dev.108.4.705. [DOI] [PubMed] [Google Scholar]


