Abstract
MLL leukemias are characterized cytogenetically by reciprocal translocations of the MLL gene at 11q23 and clinically by unfavorable outcomes. Evidence indicating that MLL leukemias are resistant to apoptosis encourages the identification of agents that induce cell death by other mechanisms. The AF4-mimetic peptide PFWT induces necrosis in the t(4;11) leukemia cell line, MV4-11. Treatment of MV4-11 cells with PFWT in combination with four chemotherapeutic compounds results in sequence-dependent synergy, induction of both apoptotic and necrotic cell death, and inhibition of MV4-11 clonogenicity. Therefore, PFWT holds promise as a therapy for MLL leukemias that augments effects of several clinically available chemotherapeutic agents.
Keywords: drug synergy, peptide therapeutics, apoptosis, necrosis, MLL
1. Introduction
The prognosis for patients with specific sub-types of leukemia, including those involving rearrangements of the Mixed Lineage Leukemia (MLL) gene at the 11q23 locus, remains poor. Leukemias with MLL gene rearrangements account for approximately 5–10% of all cases of acute leukemia and in infants the incidence of MLL-associated leukemias rises to 80% [1,2]. Current treatment protocols have been successful in increasing remission rates, however, when treated with aggressive multi-agent chemotherapy, remission duration in infants with MLL leukemia is typically brief [3]. Identification of the cellular functions of MLL fusion proteins may provide a new avenue for therapeutic interventions to substantially alter the natural history of the disease. In its native state, MLL is a critical component in chromatin remodeling complexes that maintain the expression of a large number of genes [4–8]. Several studies have begun to characterize the cellular role of MLL fusion proteins. Two of the most commonly encountered fusion proteins, MLL-AF4 and MLL-AF9, have been shown to alter the cell cycle or cell cycle proteins possibly rendering leukemias more resistant to cell cycle-specific antineoplastic drugs [9,10,11]. Expression of the fusion proteins in a controlled setting has also been shown to interfere with p53-mediated response to DNA damage providing another possible mechanism for resistance to chemotherapy [12]. The expression of many putative oncogenes is dysregulated both in clinical specimens and in experimental systems expressing MLL fusion proteins. Best studied are the Hox genes, several of which are highly expressed in MLL leukemias, but abnormal regulation of other genes that may contribute to the malignant phenotype has also been described [13–16]. In sum, MLL fusion proteins arising from MLL gene rearrangements may not only contribute directly to leukemogenesis but may impart resistance to cytotoxic chemotherapeutics.
Our understanding of the normal function of MLL fusion partners is also expanding. AF4, the most commonly encountered MLL fusion partner in acute lymphoblastic leukemia (ALL), is a member of the AF4/FMR2/LAF4/AF5 family of proteins [17]. AF4 has roles in neuronal and early lymphoid development [18, 19]. Recently, AF4 was identified as an integral part of a complex containing the RNA polymerase II (Pol II) transcription elongation factor b (P-TEFb). AF4, in complex with other recognized MLL fusion partners, AF9, ENL and AF10, mediates histone H3-K79 methylation via recruitment of Dot1 to the Pol II elongation machinery [20, 21].
Previously, our laboratory identified a direct physical interaction between AF4 and AF9. The interaction site between AF4 and AF9 is maintained in MLL-AF4 fusion proteins, and the MLL-AF4 protein is capable of redirecting nuclear localization of AF9 [22]. While the functional significance of the AF4-AF9 interaction in MLL-related leukemias is currently unknown, a peptide capable of blocking the interaction of AF4 and AF9 both in vitro and in vivo demonstrated cytotoxicity toward leukemia cell lines exhibiting t(4;11) translocations. Moreover, the activity of the peptide is correlated with its ability to disrupt AF4-AF9 complexes [23]. More recent studies show that PFWT induces necrotic cell death in the MLL leukemia cell lines MV4-11 and MOLM-13. Cell death is unaffected by caspase inhibitors but can be blocked by the protease inhibitor TLCK suggesting that serine proteases contribute to cytotoxicity [24]. Given that MLL leukemias are relatively resistant to currently available pro-apoptotic therapies, we wished to determine the effect of combinations of PFWT and several important chemotherapeutic agents currently used in clinical studies. These include etoposide, an inhibitor of topoisomerase II, cytarabine, a nucleoside analog, an inhibitor of HSP-90, 17-(Allylamino)-17-demethoxygeldanamycin (17AAG), and an ATP-competitive thienylcarboxamide FMS-like tyrosine (Flt-3) kinase inhibitor [25-28]. In this report, we demonstrate that combination treatment of MV4-11 cells with PFWT and these chemotherapeutic agents results in synergistic cytotoxicity that is dependent upon the order of exposure. Furthermore, combination treatment decreased clonogenic proliferation of MV4-11 cells. Interestingly, characteristics of both necrotic and apoptotic cell death were observed in MV4-11 cells treated with PFWT and etoposide. The findings suggest that compounds that interfere with AF4-AF9 interactions may improve the clinical response of MLL leukemias to currently available drugs.
2. Materials and Methods
2.1 Reagents
Reagents were purchased from suppliers as indicated: Thiazolyl blue tetrazolium bromide (MTT) and beta-actin antibody, Sigma, St. Louis, MO; 17AAG and etoposide, A.G. Scientific, San Diego, CA; cytarabine and 5-Phenyl-thiazol-2-yl)-(4-(2-pyrrolidin-1-ylethoxy)-phenyl)-amine (hereafter designated Flt-3 inhibitor), EMD Biosciences, La Jolla, CA; RPMI-1640, ATCC, Manassas, VA; fetal bovine serum (FBS), and penicillin/streptomycin (P/S), Invitrogen, Carlsbad, CA; caspase-3 antibody, Cell Signaling, Boston, MA; MethoCult medium, Stem Cell Technologies, Vancouver, British Colombia. PFWT-13 (Penetratin-LWVKIDLDLLSRV) and PFmut (Penetratin-LWEKSDLDLLSRV) were synthesized by Global Peptide, Fort Collins, CO, and purified to >80% by HPLC. Peptides were dissolved 25 mg/ml in DMSO. Cytarabine was dissolved in H20; all other chemotherapeutic drugs were dissolved in DMSO.
2.2 Cell Lines
The MV4-11, KOPN-8 and RAJI cell lines were obtained from ATCC, and the MOLM-13 cell line was obtained from from DSMZ, Braunschweig, Germany. IMCD3 cells were provided by Dr. Samir El-Dahr, Tulane University. Leukemia cell lines were maintained in RPMI-1640 with 10% FBS, 2% P/S and IMCD3 cells were grown in DMEM with 10% FBS, 2% P/S. Cells were incubated at 37°C/5% CO2 in a humidified incubator.
2.3 Cell Viability Assays
Assays were performed at a final cell number of (1) 2.5×105 in 500 μL for MV4-11, MOLM-13 and RAJI cell lines; (2) 5.0×105 for the KOPN-8 cell line, and (3) 1.25×105 in 500 μl for the IMCD3 kidney ductal cell line. Cell lines were treated with various concentrations of PFWT (μg/mL), 17AAG (nM), Flt-3 inhibitor (nM), etoposide (nM) and cytarabine (nM) to determine IC50 values. The final DMSO concentration was 0.5% for all assays performed.
Samples were plated in quadruplicate in 96 well plates in a final volume of 100 μL and incubated for 72 h at 37°C/5% CO2 in a humidified incubator. Viability was assayed using 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide detection at 550 nm after incubation of samples for 6 h at 37°C with MTT at a concentration of 0.5 mg/mL. All experiments were performed in quadruplicate and individually repeated three times.
2.4 Synergy Assays and Analysis
Cells were incubated in RPMI 1640 with 10% FBS and P/S for 72 h at 37°C/5% CO2 in a humidified incubator. Cell numbers were as described in Section 2.3. Culture medium contained drugs at concentrations of 0.5X, X, 2X, where “X” is the IC50 for each drug as determined by cell viability assays. For drugs that did not exhibit a dose response, doses at the higher end of the titration were utilized. The experimental design for multiple drug combination studies is as follows: (1) simultaneous treatment for 72 h, (2) pre-treatment with PFWT for 24 h followed by addition of chemotherapeutic agent for 48 h or (3) pre-treatment with chemotherapeutic agent for 24 h followed by addition of PFWT for 48 h. Cell viability was determined by MTT assay as described above and median effect analysis performed using the CalcuSyn program (Biosoft) based upon previously described methods [29,30]. All experiments were performed in quadruplicate and individually repeated three times. The combination index values (CI) values >1.0 are antagonistic, equal to 1.0 are additive and <1.0 are synergistic.
2.5 Cell Cycle Analysis
MV4-11 cells at 5.0×105 cells/mL were treated for 6 h or for 24 h in medium containing DMSO, PFWT (25 μg/mL), or PFmut (25 μg/mL) and were kept in a 37°C/5% CO2 humidified incubator. Following drug exposure, samples were rinsed with PBS and fixed in 70% ethanol 4h to overnight at −20°C. Cells were subsequently prepared for analysis by further washes with cold PBS + 1% FBS + 0.09% NaN3, pH 7.20, followed by staining for 15 min. at 37 °C in the dark with 50 μg/mL propidium iodide (PI) + 100 μg/mL RNase A. Samples were processed on a BD FACS Canto using FACS DIVA software and further analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.6 Western blotting
MV4-11 cells were treated with etoposide and PFWT alone or in combination for a total of 48 h, at dosages corresponding to the respective IC50 values for both agents. Western blotting for caspase-3 was performed on whole cell lysates of treated cells, using an antibody capable of detecting both the proenzyme and active forms of caspase-3. Beta-actin was probed as a loading control.
2.7 Ultrastructural Studies
Electron microscopic studies were performed on 1.0×107 MV4-11 cells. Cells were incubated in medium containing etoposide (or controls) for 24 h after which PFWT (or controls) was added and incubation was continued for an additional 24 h. After treatment, samples were washed in PBS and processed by Professional Histology Services (New Orleans, LA) as previously described [24]. Samples were visualized on a Philips CM-10 transmission electron microscope and images captured using Advanced Microscopy Techniques Corp imaging program and a XR111 (11 Megapixel) Bottom Mount, CCD Camera System.
2.8 Clonogenic Proliferation Assays
A total of 5.0×105 MV4-11 cells were treated with PFWT and cytarabine, etoposide, 17AAG, or Flt-3 inhibitor alone or in combination at respective IC50 values determined by MTT assays. Cells were incubated for a total of 72 h at 37°C/5% CO2 in a humidified incubator. Cells were pre-treated with cytarabine or etoposide for 24 h prior to the addition of PFWT for the final 48 h of incubation. Co-treatment of MV4-11 cells with PFWT and 17AAG or Flt-3 inhibitor was performed simultaneously for the entire 72 h treatment. After incubation, treated cells were rinsed in PBS, resuspended in 5.0 mL RPMI-1640 and 30 μL of cell suspensions was diluted into a final volume of 400 μL RPMI. The 400 μL cell suspensions were then added to 4.0 mL of MethoCult H4320, plated in triplicate, and incubated at 37°C/5% CO2 in a humidified environment for 10 d prior to counting. Colonies with ≥ 50 cells were enumerated.
3. Results
3.1 Co-treatment of t(4;11) and t(9;11) leukemia cells with PFWT and antineoplastic agents results in synergistic cytotoxicity
Prior analysis of MV4-11 and MOLM-13 cells demonstrated susceptibility to the peptide PFWT by a necrotic death pathway [24]. Given that most antineoplastic agents induce apoptosis, we reasoned that the combination of clinically available chemotherapeutic compounds with PFWT is unlikely to be associated with cross resistance. We chose to examine the interaction between PFWT and cytarabine, etoposide, 17AAG, and 5-Phenyl-thiazol-2-yl)-(4-(2-pyrrolidin-1-yl-ethoxy)-phenyl)-amine (a Flt-3 kinase inhibitor). These drugs represent a wide spectrum of clinically relevant anti-leukemic compounds with differing mechanisms of action, but which all cause cell death through apoptosis (see Discussion).
For in vitro cytotoxicity assays of drug combinations, we first determined whether different sequences of drug exposure influenced cell killing. A sequence-dependent increase in cytotoxicity was observed for combination treatment of MV4-11 cells with PFWT plus cytarabine and etoposide. This effect was not seen for combination treatments of PFWT plus 17AAG and Flt-3 inhibitor. Based on these results, combinations of PFWT and 17AAG or Flt-3 inhibitor were performed by simultaneous drug addition, whereas MV4-11 cells were pre-treated with either cytarabine or etoposide for 24 h prior to addition of PFWT. Pilot studies also established the IC50 for each of the drugs. Drug interaction studies were then performed using three fixed dose combinations-0.5X the IC50, 1X the IC50, and 2X the IC50.
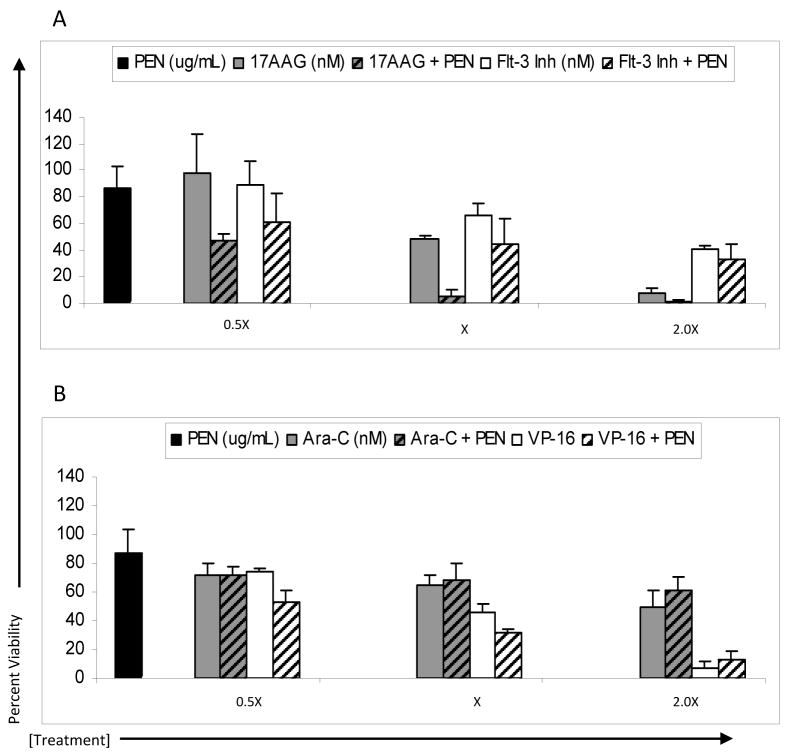
Combined treatment of MV4-11 cells resulted in an increase in cytotoxicity for all drug combinations tested when compared to individual drugs alone (Figure 1). Combined drug effects were analyzed according to the methods of Chou and Talalay and combination indices (CI) were determined for each drug pair and dose. Using a CI threshold of <1.0 as an indicator of synergy, a synergistic cytotoxic effect was observed for MV4-11 cells treated with PFWT and all four chemotherapeutic drugs (Figure 2).
Figure 1.
Treatment of MV4-11 and MOLM-13 cells with PFWT in combination with chemotherapeutic drugs results in an overall decrease in cell viability compared to treatment with PFWT or chemotherapeutic drug alone. MV4-11 cells were treated simultaneously (SIMT) with PFWT (μg/mL) and either 17AAG (nM) or a Flt-3 Inhibitor (nM) for 72 h or sequentially (SQT) with PFWT (μg/mL) and either cytarabine (nM) or etoposide (nM) for 72 h. In the latter case (SQT), cells were pretreated with either cytarabine or etoposide for 24 h prior to the addition of PFWT. MOLM-13 cells were treated sequentially with PFWT (μg/mL) and either 17AAG (nM), cytarabine (nM), a Flt3 inhibitor (nM) or etoposide (nM) for 72 h. For combined drug treatment of MOLM-13 cells, cells were pre-treated with individual drugs 24 h prior to the addition of PFWT. Data is a combination of at least three experiments and standard error of the mean (SEM) is represented by error bars. AraC = cytarabine; VP-16 = etoposide.
Figure 2.
Combination indices (CI) for drug interactions were calculated from cytotoxicity assays. CI values are plotted as a function of drug concentration for each of the respective cell lines and drug combinations. CI>1: antagonistic; CI=1: additive; CI<1: synergistic. Drugs were administered in fixed dose combinations where X = IC50 for each drug. AraC = cytarabine; VP-16 = etoposide. The CI values were generated using Calcusyn software as described in Materials and Methods.
To extend our analysis to other leukemia cell lines with MLL rearrangements, the t(9;11) cell line MOLM-13 was tested with combinations of PFWT and cytarabine, etoposide, 17AAG, and Flt-3 inhibitor. MOLM-13 cells express the MLL-AF9 chimeric oncoprotein, and PFWT was previously demonstrated to induce necrotic cell death in this cell line [24]. Combination treatment of MOLM-13 cells resulted in an increase in cytotoxicity for all combinations compared to individual treatment alone (Figure 1). Dependence on the sequence of treatment was again observed and required initial exposure to 17AAG, Flt-3 inhibitor, cytarabine or etoposide prior to the addition of PFWT to achieve maximum cytotoxicity (data not shown). Combination treatments of PFWT and cytarabine or etoposide resulted in synergistic cytotoxicity at 0.5X and 1X concentrations (Figure 2). However, for MOLM-13 cells, the combination of PFWT and 17AAG or Flt-3 inhibitor resulted in cytotoxicity that was only additive or slightly antagonistic (with the exception of 17AAG and PFWT at 1X IC50) (Figure 2). These results support the potential utility of combination treatment with PFWT and cytarabine or etoposide for leukemias with t(9;11) translocations.
3.2 Combination treatment of t(11;19) and a non MLL-rearranged cell lines results in additive/antagonistic cytotoxicity
The MLL rearranged leukemia cell line KOPN-8 and B-cell lymphoma cell line RAJI were chosen for analysis to more broadly assess the effect of combination treatment using PFWT. KOPN-8 expresses the MLL-ENL fusion gene as a consequence of a t(11;19) translocation. Importantly, ENL is a close homolog of AF9 [31]. Initial cytotoxicity experiments demonstrated that KOPN-8 cells did not exhibit a dependence upon the order of addition of PFWT and cytarabine, etoposide, 17AAG, or Flt-3 inhibitor. Treatment of KOPN-8 cells with PFWT resulted in cytotoxicity at concentrations similar to other MLL-rearranged cell lines. However, synergistic cytotoxicity was not observed with combination treatment of KOPN-8 cells with PFWT and chemotherapeutic agents. Further analysis of these effects illustrated that the majority of drug combinations resulted in additive to antagonistic cytotoxicity compared to PFWT treatment alone. Only combination treatment of PFWT and cytarabine at the 0.5X concentration induced a synergistic effect upon cytotoxicity (Figures 2 and 3). The KOPN-8 cell line was relatively resistant to treatment with Flt-3 Inhibitor in contrast to other MLL-rearranged cell lines. In the absence of a dose-response to the Flt-3 inhibitor, synergy analysis could not be performed.
Figure 3.
Treatment of KOPN-8 and RAJI cells with PFWT in combination with chemotherapeutic drugs induces a modest decrease in cell viability compared to treatment with PFWT or drugs alone. KOPN-8 cells were treated simultaneously (SIMT) with PFWT (μg/mL) and either 17AAG (nM), a Flt-3 Inhibitor (nM), cytarabine (nM) or etoposide (nM) for 72 h. RAJI cells were treated simultaneously (SIMT) with PFWT (μg/mL) and either 17AAG (nM) or a Flt-3 Inhibitor (nM) for 72 h. They were treated sequentially (SEQ) with PFWT (μg/mL) and either cytarabine (nM) or etoposide (nM) for 72 h. In sequentially treated cells (SEQ), cells were pretreated with either cytarabine or etoposide for 24 h followed by the addition of PFWT. Standard error of the mean (SEM) is represented by error bars. AraC = cytarabine; VP-16 = etoposide.
The non MLL-rearranged cell line, RAJI, exhibited slight sensitivity to PFWT treatment that was not dose-dependant. Without a relationship between dose and response to PFWT, drug synergy could not be analyzed. A dependence upon the sequence of addition of PFWT and chemotherapeutic agents was observed for RAJI cells exposed to the combination of PFWT and both cytarabine and etoposide. The results of cells exposed sequentially to PFWT and cytarabine or etoposide are shown (Figure 3).
While the data imply that PFWT exhibits minimal toxicity toward cells lacking MLL rearrangements, we wanted to more broadly define the activity of PFWT in other cell types. We examined the effects of combination treatment with PFWT and both etoposide and cytarabine upon the inner medullary collecting duct kidney cell line, IMCD3. Initial studies to determine IC50 values for PFWT in IMCD3 cells revealed a plateau with greater than 50% cell viability at higher drug concentrations precluding formal calculation of this parameter (data not shown). An arbitrary value of 50 μg/mL PFWT was therefore assigned for combination experiments with etoposide and cytarabine. It is noteworthy that over the PFWT concentration range 25–100 μg/ml, a measurable dose response was observed. In combination with etoposide or cytarabine, no dependence upon order of addition was observed for PFWT in IMCD3 cells (Figure 4A). Therefore cells were treated simultaneously with drugs for 72 h. Analysis of cytotoxicity using Calcusyn program demonstrated moderate synergy to antagonism for PFWT and etoposide as well as PFWT and cytarabine (Figure 4B). Although PFWT is only minimally toxic to IMCD3 cells in vitro, it has the potential to augment the cytotoxic effects of other drugs for these particular cells.
Figure 4.
Treatment of IMCD3 cells with PFWT in combination with chemotherapeutic drugs induces a modest decrease in cell viability compared to treatment with PFWT or drugs alone. (A) IMCD3 cells were treated simultaneously (SIMT) with PFWT (μg/mL) and either cytarabine (nM) or etoposide (nM) for 72 h. Standard error of the mean (SEM) is represented by error bars. AraC = cytarabine; VP-16 = etoposide. (B) CI values are plotted as a function of drug concentration and drug combinations. CI>1: antagonistic; CI=1: additive; CI<1: synergistic. Drugs were administered in fixed dose combinations where X = IC50 for each drug. AraC = cytarabine; VP-16 = etoposide. The CI values were generated using Calcusyn software as described in Materials and Methods.
3.3 The penetratin transport sequence does not contribute to drug synergy
The effect of the penetratin peptide alone and in combination with 17AAG, Flt-3 Inh, cytarabine and etoposide was also examined to determine the potential influence of the protein transduction domain (PTD) on the combined cytotoxicity with these chemotherapeutic agents. MV4-11 cells were treated as described previously with the exception that penetratin alone (50 μg/mL) was substituted for PFWT in all combination treatments. Specifically, MV4-11 cells were treated with penetratin and either 17AAG or Flt-3 Inh simultaneously for 72 h while cells were treated with cytarabine or etoposide for 24 h prior to addition of penetratin peptide for a total of 72 h. With the exception of the Hsp90 inhibitor 17AAG, there was no increased cytoxicity when penetratin was combined with the chemotherapeutic drugs (Figure 5A–B). Surprisingly, penetratin did augment the toxicity of 17AAG. Therefore it is possible that the synergy observed for the combination of PFWT and 17AAG in MV4-11 cells is attributable to the activity of the penetratin PTD. However, for Flt-3 Inh, cytarabine, and etoposide, the penetratin PTD contained within PFWT peptide likely contributes little to the observed drug synergy.
Figure 5.
Treatment of MV4-11 with penetratin transport sequence in combination with chemotherapeutic drugs did not contribute to drug cytotoxicity. MV4-11 cells were treated simultaneously (SIMT) with penetratin (μg/mL) and either 17AAG (nM) or a Flt-3 Inhibitor (nM) for 72 h or sequentially (SQT) with penetratin (μg/mL) and either cytarabine (nM) or etoposide (nM) for 72 h. In the latter case (SQT), cells were pretreated with either cytarabine or etoposide for 24 h prior to the addition of PFWT. Data is a combination of at least three experiments and standard error of the mean (SEM) is represented by error bars. AraC = cytarabine; VP-16 = etoposide.
3.4 PFWT induces accumulation of MV4-11 cells in G1
Many cytotoxic agents target rapidly dividing cells. Etoposide and cytarabine, in particular, act on cells during the S/S-G2 phases of the cell cycle [32,33]. When combined with drugs whose actions lead to arrest of cells in a specific phase of the cell cycle, these cell cycle-specific antineoplastic drugs may be rendered less potent. The sequence dependence required to achieve maximal cell killing in drug combination studies led us to hypothesize that PFWT induces cell cycle arrest that interferes with the activity of etoposide and cytarabine. To test this, MV4-11 cells were exposed to 25 μg/ml PFWT (or control) and flow cytometric cell cycle analyses of PI-stained cells were performed (Figure 6). When compared to cells exposed to PFmut peptide or DMSO controls, PFWT treated cells exhibit an increase in the sub-G1 population. Excluding the sub-G1 population of dead and dying cells, the fraction of cells in G1 increases from approximately 60% to 70% for cells exposed to PFWT for 24 h. The effect on cell cycle distribution is even more pronounced at 6 h with 84% of cells exposed to PFWT in G1 and a reduction of cells in S phase from 26.6% (DMSO-treated) to 5.7% (PFWT-treated). The reduction of cells in S phase following PFWT treatment may account, in part, for a diminution in cell killing by cytarabine and etoposide. However these findings are descriptive rather than mechanistic and it is unclear whether PFWT-induced alterations in cell cycle control affect the toxicity of cytarabine and etoposide.
Figure 6.
PFWT treatment causes accumulation of MV4-11 cells in the G0/G1 phase of the cell cycle. MV4-11 cells were treated for 6h (a) or 24h (b) with 25 μg/mL PFWT, PFmut, or equivalent volume DMSO. Percentages of the singlet population in each phase of the cell cycle are shown as insets for each graph. Note that the y-axis scales for PFWT-treated samples are different from those of control samples, due to fewer events in the live singlet gates.
3.5 MV4-11 cells exhibit features of necrotic and apoptotic cell death after combined treatment with PFWT and etoposide
In light of the synergistic cytotoxicity observed for MV4-11 cells co-treated with PFWT and pro-apoptotic chemotherapeutic agents, we were interested in determining the effects of co-treatment on the mechanism of cell death. To date, the most unambiguous method for determining cell necrosis and for distinguishing it from apoptosis is transmission electron microscopy (TEM). We utilized TEM to examine the ultrastructural features of MV4-11 cells treated with PFWT and etoposide for 24–48 h. As we have previously shown, MV4-11 cells undergo necrotic cell death characterized by loss of membrane integrity and cytoplasmic swelling following treatment with PFWT. Treatment of MV4-11 cells with etoposide resulted in a classic apoptotic phenotype including nuclear and cytoplasmic shrinkage and chromatin condensation. Following co-treatment of MV4-11 cells with PFWT and etoposide, both necrotic and apoptotic phenotypes were observed (Figure 7A). Interestingly, characteristics of apoptosis and necrosis were simultaneously apparent in the same cell.
Figure 7.
MV4-11 cells undergo both necrotic and apoptotic cell death after co-treatment with PFWT and etoposide. (A) MV4-11 cells were treated at the IC50 for PFWT and etoposide alone and in combination for a total of 48 h. Ultrastructural changes were observed by transmission electron microscopy. Note nuclear condensation (apoptosis) and membrane disintegration (necrosis) occurring simultaneously in cells treated with PFWT and etoposide in combination. Magnification is 2950x and bars represent 2 μm. (B) MV4-11 cells were treated with VP-16 and PFWT alone or in combination for a total of 48h, at dosages corresponding to the respective IC50 values for both agents. Western blotting for caspase-3 was performed on whole cell lysates of treated cells, using an antibody capable of detecting both the proenzyme (35 kDa, arrow) and active caspase-3 (12 kDa). Beta-actin was probed as a loading control.
To expand upon the TEM results, MV4-11 cells were treated in combination with PFWT and etoposide and analyzed to identify a marker of apoptosis, the activated (cleaved) form of caspase-3. Caspase-3 is a crucial mediator of apoptosis and would be anticipated to be present in MV4-11 cells treated with the pro-apoptotic compound, etoposide. As expected from the observed ultrastructural changes indicating apoptosis, the activated form of caspase-3 was identified in MV4-11 cells treated with etoposide alone. When exposed to both PFWT and etoposide, TEM analysis indicated that both apoptosis (condensed nuclei) and necrosis (cell membrane breakdown) took place. Immunoblot again showed that caspase-3 was activated. In contrast, there is no caspase-3 cleavage in cells exposed to PFWT despite disintegration of the cell membrane (Figure 7B). These results were not predicted. We had anticipated that the PFWT-etoposide drug combination would stimulate either apoptotic or necrotic cell death- but not both- within individual cells and the collective cell population.
3.6 Clonogenic proliferation of MV4-11 cells is inhibited by combination treatment with PFWT and cytarabine or 17AAG
A critical characteristic of successful anti-cancer therapies is the ability to inhibit proliferation of neoplastic progenitors. Given the synergistic cytotoxicity observed for MV4-11 cells treated with PFWT and cytarabine, etoposide, 17AAG, or Flt-3 inhibitor, the effects of these drug combinations on MV4-11 cell clonogenicity was examined using methylcellulose-based colony forming assays. MV4-11 cells were treated at the relative IC50 (1X) value for each drug alone and in fixed combinations for 72 h prior to plating. Cells were then dispersed in methylcellulose medium for 10 d. Treatment of MV4-11 cells in combination with PFWT and chemotherapeutic agents caused a reduction in colony formation greater than PFWT treatment alone (Figure 8A–B). Furthermore, combined treatment of MV4-11 with PFWT and either 17AAG or cytarabine resulted in a significant reduction in colony formation compared to treatment with either agent. In contrast, at the drug concentrations selected for these experiments, combined treatment with PFWT and Flt-3 inhibitor or etoposide did not significantly affect the number of clonogenic cells when compared to Flt-3 inhibitor or etoposide treatment alone (Figure 8A–B). We conclude not only that PFWT monotherapy inhibits MV4-11 cell clonogenicity, but also that, when combined with 17AAG or cytarabine, PFWT further suppresses colony formation. However, these assays were not designed to formally test a synergistic effect on leukemia clonogenicity.
Figure 8.
Clonogenic proliferation of MV4-11 cells is inhibited by combined treatment with PFWT and 17AAG or cytarabine. MV4-11 cells were treated with PFWT and chemotherapeutic agents alone and in combination. For these experiments, the in vitro IC50 for each individual compound was used. After 72h drug exposure, cells were plated in methylcellulose medium and incubated for 10 days, as described in Section 2.7. (A) PFWT (μg/mL) and 17AAG (nM) or Flt-3 inhibitor (nM) or (B) PFWT (μg/mL) and cytarabine (Ara-C) (nM) or etoposide (VP-16) (nM). Schematics of the sequence of treatment are shown as inlays in the upper right hand corner of graphs. One asterisk (*) indicates decreased colony number relative to PFWT alone; two asterisks (**) indicates decreased colony number relative to both PFWT and chemotherapeutic agent alone (p < 0.05). Significance was determined using Student’s t-test with two sample unequal variance and one-tailed distribution.
4. Discussion
Due to the well-documented resistance of MLL-rearranged leukemias to chemotherapeutic regimens available in the clinic, there is a need for the development of new drugs and drug combinations to effect more prolonged remissions in these patients. Our laboratory has designed a synthetic peptide, PFWT, which targets the AF4-AF9 protein-protein interaction that seems to be important for the survival of t(4;11) and t(9;11) MLL leukemia cells. Through a series of experiments, we have previously demonstrated that this peptide preferentially kills t(4;11) and t(9;11) leukemias, yet shows minimal toxicity toward non-MLL leukemias and other non-transformed cell lines, and does not inhibit colony-forming potential of hematopoietic progenitors. We have further established that PFWT-mediated cell death is necrotic in character, in contrast to most standard cytotoxic agents in use today. We anticipated that combining PFWT with other useful chemotherapeutic drugs would enhance the effectiveness of these agents against MLL leukemia cells. It was this hypothesis that we tested here.
For the purposes of this study, we chose to examine the effects on t(4;11), t(9;11), and t(11;19) cell lines of combination treatments of PFWT and cytarabine, etoposide, the HSP-90 inhibitor 17AAG, or an inhibitor of the Flt-3 kinase. Cytarabine and etoposide were chosen as they are routinely included in front-line chemotherapeutic regimens applied to MLL-associated leukemias. 17AAG and Flt-3 kinase inhibitors are under clinical investigation for treatment of drug-resistant leukemia including MLL leukemias with activating mutations of Flt-3 kinase [13, 26, 34–40]. Each of the four drugs has also been demonstrated to be cytotoxic to MV4-11 cells individually and to induce synergistic cytotoxicity in combination with each other in MV4-11 and MOLM-13 cell lines [3, 28, 41–43]. Of the agents examined, 17AAG, etoposide and cytarabine have been shown to induce apoptotic cell death, while the death pathway induced by the Flt-3 inhibitor used for this study has not been characterized in MV4-11 cells [28, 44–46]. Other Flt-3 inhibitors such as CEP701 and PKC412 induce apoptosis in the MV4-11 cell line [41, 47]. However, leukemia cells with MLL translocations appear to be intrinsically resistant to pro-apoptotic agents; indeed, MLL translocations have been directly implicated in inhibiting apoptosis [12,15,16, 48–50]. Therefore, drugs that induce cell death by mechanisms other than apoptosis are of particular interest as alternative means of treating leukemias that are resistant to traditional chemotherapeutic agents.
Our findings indicate that PFWT does synergize, although to different degrees, with each of the four agents studied to kill MLL-rearranged leukemia cell lines harboring t(4;11) and t(9;11) translocations. In most cases, this synergy was sequence dependent; the cytotoxic agent had to be applied to the cells 24 h prior to addition of PFWT in order for the drug combination to have maximum toxicity. This was true for all drug combinations when tested on the t(9;11) leukemia cell line MOLM-13, while only etoposide combined with PFWT and cytarabine plus PFWT demonstrated this relationship in the t(4;11) leukemia cell line MV4-11. The t(11;19) cell line KOPN-8, expresses a MLL-ENL fusion transcript that is highly homologous to MLL-AF9. While KOPN-8 cells exhibit a dose dependent response to PFWT and an IC50 that is less than the other MLL-rearranged cell lines tested, synergy was not observed for most of the PFWT-drug combinations. Thus while MLL-rearrangements appear to predict sensitivity to the PFWT peptide, PFWT-drug synergy is a more variable feature of the specific cell lines.
One possible explanation for the enhanced cytotoxicity associated with combing PFWT and other drugs is that the penetratin PTD affects cell membrane dynamics and drug uptake. Penetratin may simply facilitate the uptake of other drugs thereby increasing potency. At least two lines of evidence indicate this is not the case. First, while all MLL-rearranged cell lines examined are sensitive to PFWT in a dose-dependant manner, PFWT-drug synergy varied among the cell lines. If PFWT acts by non-specifically increasing drug uptake, drug synergy is to be expected in all cell lines that transport PFWT across the membrane. Second, the penetratin peptide was minimally toxic to MV4-11 cells, and in combination with chemotherapeutics exhibited no increased toxicity for three of four drugs tested (17AAG was the exception).
We hypothesized that the observed sequence dependence for drug exposure is due to PFWT blocking progression through the cell cycle, since many cytotoxic agents act on actively cycling cells during the S phase of the cell cycle. Flow cytometric cell cycle analysis demonstrated that PFWT treatment resulted in an accumulation of MV4-11 cells in the G0/G1 phase of the cell cycle and depleted cells in S phase. While these results were reproducible over multiple independent analyses, we are not able to conclusively determine that peptide-induced cell cycle block renders S phase-specific drugs less active when administered simultaneously with PFWT. Nevertheless, this possibility must be considered in the design of experimental therapeutic studies.
The ability of the drug combinations to inhibit clonogenic proliferation was examined. Colony formation assays demonstrated that combined treatment of MV4-11 cells with PFWT and 17AAG or PFWT and cytarabine resulted in a significant reduction of a clonogenic cell population compared to treatment with either of the drugs alone. However, no significant reduction in colony formation was observed following combination treatment with either PFWT and Flt-3 inhibitor or PFWT and etoposide when compared to treatment with single agents. Of note, at the dosage used for our experiments, treatment of MV4-11 cells with either Flt-3 inhibitor or etoposide alone resulted in higher cytotoxicity to leukemic colony forming cells than either 17AAG or cytarabine alone. This could account for the minimal increase in cytotoxicity observed for combination treatments with PFWT and these two drugs. In sum, when measured by colony formation in methylcellulose, PFWT alone or in combination with chemotherapeutic agents effectively reduced the colony forming potential of MV4-11 cells. These results suggest a therapeutic benefit of targeting the MLL-AF4/AF9 complex in these types of MLL leukemia cells.
As each of the chemotherapeutic drugs that we studied induces apoptosis whereas PFWT causes necrotic cell death in t(4;11) and t(9;11) leukemia cell lines, we were interested in determining if either one or the other death phenotype predominated with combined treatment. Unexpectedly, both necrotic and apoptotic cells were observed in MV4-11 samples treated with PFWT and etoposide. A similar phenomenon was observed for combination treatment of MV4-11 cells with PFWT and Flt-3 inhibitor suggesting that no single cell death program predominates in response to these specific pro-necrotic and pro-apoptotic stimuli (data not shown). Ultrastructural characteristics of two types of cell death within individual cells indicate that induction of necrosis does not block apoptosis or vice versa.
In this study, we have identified chemotherapeutic agents that exhibit synergistic cytotoxicity in conjunction with PFWT peptide in MV4-11 (MLL-AF4) and MOLM-13 (MLL-AF9) cell lines. In addition, rather than observing a predominant mechanism of cell death, we determined that combination treatment of these cell lines results in both necrotic and apoptotic cell death. The results demonstrate that PFWT can effectively augment the in vitro activity of other chemotherapeutic agents. Molecular targeting of AF4-AF9 interactions may warrant further study with the goal of developing effective new approaches to treat MLL leukemia.
Acknowledgments
This work was supported, in part, by the Louisiana Cancer Research Consortium, Loyola University Chicago Cardinal Bernardin Cancer Center, the Leukemia and Lymphoma Society (6146-05, 7372-07), and the National Institutes of Health (CA 098459, RR 020152). We appreciate the technical assistance of Eugene Suh.
Footnotes
Contributions: Cecily Bennett performed the experiments with the assistance of Amanda Winters and Nisha Barretto. Cecily Bennett, Amanda Winters and Charles Hemenway designed the experiments, analyzed, and interpreted the data. All four authors prepared the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Daser A, Rabbitts TH. Extending the repertoire of the mixed-lineage leukemia gene MLL in leukemogenesis. Genes Dev. 2004;18(9):965–974. doi: 10.1101/gad.1195504. [DOI] [PubMed] [Google Scholar]
- 2.Biondi A, Cimino G, Pieters R, Pui CH. Biological and therapeutic aspects of infant leukemia. Blood. 2000;96(1):24–33. [PubMed] [Google Scholar]
- 3.Pui CH, Gaynon PS, Boyett JM, Chessells JM, Baruchel A, Kamps W, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359(9321):1909–1915. doi: 10.1016/S0140-6736(02)08782-2. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, et al. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002;10(5):1119–1128. doi: 10.1016/s1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24(13):5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102(3):749–754. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13(8):713–719. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- 8.Guenther MG, Jenner RG, Chevalier B, Nakamura T, Croce CM, Canaani E, et al. Global and Hox-specific roles for the MLL1 methyltransferase. Proc Natl Acad Sci U S A. 2005;102:8603–8608. doi: 10.1073/pnas.0503072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caslini C, Serna A, Rossi V, Introna M, Biondi A. Modulation of cell cycle by graded expression of MLL-AF4 fusion oncoprotein. Leukemia. 2004;18(6):1064–1071. doi: 10.1038/sj.leu.2403321. [DOI] [PubMed] [Google Scholar]
- 10.Xia ZB, Popovic R, Chen J, Theisler C, Stuart T, Santillan DA, et al. The MLL fusion gene, MLL-AF4, regulates cyclin-dependent kinase inhibitor CDKN1B (p27kip1) expression. Proc Natl Acad Sci U S A. 2005;102(39):14028–14033. doi: 10.1073/pnas.0506464102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaussmann A, Wenger T, Eberle I, Bursen A, Bracharz S, Herr I, et al. Combined effects of the two reciprocal t(4;11) fusion proteins MLL.AF4 and AF4. MLL confer resistance to apoptosis, cell cycling capacity and growth transformation. Oncogene. 2007;26:3352–3363. doi: 10.1038/sj.onc.1210125. [DOI] [PubMed] [Google Scholar]
- 12.Wiederschain D, Kawai H, Shilatifard A, Yuan ZM. Multiple mixed lineage leukemia (MLL) fusion proteins suppress p53-mediated response to DNA damage. J Biol Chem. 2005;280(26):24315–24321. doi: 10.1074/jbc.M412237200. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30(1):41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 14.Milne TA, Dou Y, Martin ME, Brock HW, Roeder RG, Hess JL. MLL associates specifically with a subset of transcriptionally active target genes. Proc Natl Acad Sci U S A. 2005;102(41):14765–14770. doi: 10.1073/pnas.0503630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dell’orto MC, Banellli B, Giarin E, Accordi B, Trentin L, Romani M, et al. Down-regulation of DLX3 expression in MLL-AF4 childhood lymphoblastic leukemias is mediated by promoter region hypermethylation. Oncol Rep. 2007;18(2):417–423. [PubMed] [Google Scholar]
- 16.Nakanishi H, Nakamura T, Canaani E, Croce CM. ALL1 fusion proteins induce deregulation of EphA7 and ERK phosphorylation in human acute leukemias. Proc Natl Acad Sci U S A. 2007;104(36):14442–14447. doi: 10.1073/pnas.0703211104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilson I, Reichel M, Ennas MG, Greim R, Knörr C, Siegler G, et al. Exon/intron structure of the human AF-4 gene, a member of the AF-4/LAF-4/FMR-2 gene family coding for a nuclear protein with structural alterations in acute leukaemia. Br J Haematol. 1997;98:157–169. doi: 10.1046/j.1365-2141.1997.1522966.x. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs AM, Oliver PL, Jones EL, Jeans A, Potter A, Hovik BH, et al. A mutation in Af4 is predicted to cause cerebellar ataxia and cataracts in the robotic mouse. J Neurosci. 2003;23(5):1631–1637. doi: 10.1523/JNEUROSCI.23-05-01631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isnard P, Core N, Naquet P, Djabali M. Altered lymphoid development in mice deficient for the mAF4 proto-oncogene. Blood. 2000;96(2):705–710. [PubMed] [Google Scholar]
- 20.Bitoun E, Oliver PL, Davies KE. The mixed-lineage leukemia fusion partner AF4 stimulates RNA polymerase II transcriptional elongation and mediates coordinated chromatin remodeling. Hum Mol Genet. 2007;16(1):92–106. doi: 10.1093/hmg/ddl444. [DOI] [PubMed] [Google Scholar]
- 21.Mueller D, Bach C, Zeisig D, Garcia-Cuellar MP, Monroe S, Sreekumar A, et al. A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification. Blood. 2007;110(13):4445–4454. doi: 10.1182/blood-2007-05-090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erfurth F, Hemenway CS, de Erkenez AC, Domer PH. MLL fusion partners AF4 and AF9 interact at subnuclear foci. Leukemia. 2004;18(1):92–102. doi: 10.1038/sj.leu.2403200. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan RS, Nesbit JB, Marrero L, Erfurth F, LaRussa VF, Hemenway CS. The synthetic peptide PFWT disrupts AF4-AF9 protein complexes and induces apoptosis in t(4;11) leukemia cells. Leukemia. 2004;18(8):1364–1372. doi: 10.1038/sj.leu.2403415. [DOI] [PubMed] [Google Scholar]
- 24.Palermo CM, Bennett CA, Winters AC, Hemenway CS. The AF4-mimetic peptide, PFWT, induces necrotic cell death in MV4-11 leukemia cells. Leukemia Res. 2008;32(4):633–642. doi: 10.1016/j.leukres.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montecucco A, Biamonti G. Cellular response to etoposide treatment. Cancer Letters. 2007;252(1):9–18. doi: 10.1016/j.canlet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Cole N, Gibson BE. High-dose cytosine arabinoside in the treatment of acute myeloid leukemia. Blood Reviews. 1997;11:39–45. doi: 10.1016/s0268-960x(97)90005-9. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsumi S, Neckers L. Extracellular heat shock protein 90: A role for a molecular chaperone in cell motility and cancer metastasis. Cancer Science. 2007;98(10):1536–1539. doi: 10.1111/j.1349-7006.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patch RJ, Baumann CA, Liu J, Gibbs AC, Ott H, Lattanze J, et al. Identification of 2-acylaminothiophene-3-carboxamides as potent inhibitors of FLT3. Bioorganic & Med Chem Lett. 2006;16(12):3282–3286. doi: 10.1016/j.bmcl.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Chou T-C, Talalay P. Generalized Equations for the Analysis of Inhibitions of Michaelis-Menten and Higher-Order Kinetic Systems with Two or More Mutually Exclusive and Nonexclusive Inhibitors. Eur J Biochem. 1981;115(1):207–216. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 31.Drexler HG, Quentmeier H, MacLeod RA. Malignant hematopoietic cell lines: in vitro models for the study of MLL gene alterations. Leukemia. 2004;18(2):227–232. doi: 10.1038/sj.leu.2403236. [DOI] [PubMed] [Google Scholar]
- 32.Potter AJ, Rabinovitch PS. The cell cycle phases of DNA damage and repair initiated by topoisomerase II-targeting chemotherapeutic drugs. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2005;572(1–2):27–44. doi: 10.1016/j.mrfmmm.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Hamada A, Kawaguchi, Takeo, Nakano, Masahiro Clinical pharmacokinetics of cytarabine formulations. Clin Pharmacokinetics. 2002;41(10):705–718. doi: 10.2165/00003088-200241100-00002. [DOI] [PubMed] [Google Scholar]
- 34.Zeleznik-Le NJ, Harden AM, Rowley JD. 11q23 translocations split the “AT-hook” cruciform DNA-binding region and the transcriptional repression domain from the activation domain of the mixed-lineage leukemia (MLL) gene. Proc Natl Acad Sci U S A. 1994;91(22):10610–10614. doi: 10.1073/pnas.91.22.10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knapper S. FLT3 inhibition in acute myeloid leukaemia. British J Haematology. 2007;138(6):687–699. doi: 10.1111/j.1365-2141.2007.06700.x. [DOI] [PubMed] [Google Scholar]
- 36.Ramanathan RK, Trump DL, Eiseman JL, Belani CP, Agarwala SS, Zuhowski EG, et al. Phase I Pharmacokinetic-Pharmacodynamic Study of 17-(Allylamino)-17-Demethoxygeldanamycin (17AAG, NSC 330507), a Novel Inhibitor of Heat Shock Protein 90, in Patients with Refractory Advanced Cancers. Clin Cancer Res. 2005;11(9):3385–3391. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 37.Banerji U, O’Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, et al. Phase I Pharmacokinetic and Pharmacodynamic Study of 17-Allylamino, 17-Demethoxygeldanamycin in Patients With Advanced Malignancies. J Clin Oncol. 2005;23(18):4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 38.DeAngelo DJ, Stone RM, Heaney ML, Nimer SD, Paquette RL, Klisovic RB, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108(12):3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 40.Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1(2):133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 41.Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104(4):1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 42.Yao Q, Nishiuchi R, Kitamura T, Kersey JH. Human leukemias with mutated FLT3 kinase are synergistically sensitive to FLT3 and Hsp90 inhibitors: the key role of the STAT5 signal transduction pathway. Leukemia. 2005;19(9):1605–1612. doi: 10.1038/sj.leu.2403881. [DOI] [PubMed] [Google Scholar]
- 43.Yao Q, Weigel B, Kersey J. Synergism between Etoposide and 17AAG in Leukemia Cells: Critical Roles for Hsp90, FLT3, Topoisomerase II, Chk1, and Rad51. Clin Cancer Res. 2007;13(5):1591–1600. doi: 10.1158/1078-0432.CCR-06-1750. [DOI] [PubMed] [Google Scholar]
- 44.Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9(12):4483–4493. [PubMed] [Google Scholar]
- 45.Grant S. Ara-C: cellular and molecular pharmacology. Adv Cancer Res. 1998;72:197–233. doi: 10.1016/s0065-230x(08)60703-4. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong SA, Kung AL, Mabon ME, Silverman LB, Stam RW, Den Boer ML, et al. Inhibition of FLT3 in MLL. Validation of a therapeutic target identified by gene expression based classification Cancer Cell. 2003;3(2):173–183. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 47.Odgerel T, Kikuchi J, Wada T, Shimizu R, Futaki K, Kano Y, et al. The FLT3 inhibitor PKC412 exerts differential cell cycle effects on leukemic cells depending on the presence of FLT3 mutations. Oncogene. 2008;27(22):3102–3110. doi: 10.1038/sj.onc.1210980. [DOI] [PubMed] [Google Scholar]
- 48.Wiederschain D, Kawai H, Gu J, Shilatifard A, Yuan ZM. Molecular basis of p53 functional inactivation by the leukemic protein MLL-ELL. Mol Cell Biol. 2003;23(12):4230–4246. doi: 10.1128/MCB.23.12.4230-4246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hotfilder M, Rottgers S, Rosemann A, Schrauder A, Schrappe M, Pieters R, et al. Leukemic Stem Cells in Childhood High-Risk ALL/t(9;22) and t(4;11) Are Present in Primitive Lymphoid-Restricted CD34+CD19− Cells. Cancer Res. 2005;65(4):1442–1449. doi: 10.1158/0008-5472.CAN-04-1356. [DOI] [PubMed] [Google Scholar]
- 50.Greaves M. A natural history for pediatric acute leukemia. Blood. 1993;82(4):1043–1051. [PubMed] [Google Scholar]