Abstract
The formation of the Aspergillus nidulans fruiting body is affected by a number of genetic and environmental factors. Here, the nsdC (never in sexual development) gene—encoding a putative transcription factor carrying a novel type of zinc-finger DNA-binding domain consisting of two C2H2's and a C2HC motif that are highly conserved in most fungi but not in plants or animals—was investigated. Two distinct transcripts of 2.6 and 3.0 kb were generated from nsdC. The 2.6-kb mRNA accumulated differentially in various stages of growth and development, while the level of the 3.0-kb mRNA remained relatively constant throughout the life cycle. While the deletion of nsdC resulted in the complete loss of fruiting body formation under all conditions favoring sexual development, overexpression of nsdC not only enhanced formation of fruiting bodies (cleistothecia) but also overcame inhibitory effects of certain stresses on cleistothecial development, implying that NsdC is a key positive regulator of sexual development. Deletion of nsdC also retarded vegetative growth and hyperactive asexual sporulation, suggesting that NsdC is necessary not only for sexual development but also for regulating asexual sporulation negatively. Overexpression of veA or nsdD does not rescue the failure of fruiting body formation caused by nsdC deletion. Furthermore, nsdC expression is not affected by either VeA or NsdD, and vice versa, indicating that NsdC regulates sexual development independently of VeA or NsdD.
MANY fungi produce spores through sexual or asexual development to resist unfavorable conditions or to propagate to other habitats. The homothallic ascomycete Aspergillus nidulans develops both sexual and asexual spores through complicated morphogenic processes. After a certain period of vegetative growth, asexual sporulation begins by production of spore-bearing structures called conidiophores from foot cells of aerial hyphae. Sequential development of metula and phialides takes place and is followed by formation of conidia by budding at the tips of phialides (Smith et al. 1977). A number of genes have been identified that are associated with asexual development and the regulatory network of these genes is well established (Clutterbuck 1969; Adams et al. 1998; Fischer 2002).
A. nidulans also undergoes sexual reproduction and produces sexual spores called ascospores, eight of which are formed in one of the asci that develop in closed fruiting bodies known as cleistothecia. Sexual development begins with the formation of primordia from ascogenous hyphae in the nest-like structure made of a number of thick-walled Hülle cells. The primordia mature to cleistothecia in which many mycelia grow and develop into crozier, where nuclear fusion and subsequent meiosis take place (Sohn and Yoon 2002; Han et al. 2007).
After growing to a certain age, mycelia acquire competence for development after which differentiation is normally induced by air exposure (Axelrod et al. 1973). Environmental factors that affect the growth of mycelia—including nutritional status, culture conditions, and some environmental stresses—are responsible for developmental decisions determining sexual or asexual reproduction (Zonneveld 1977; Han et al. 1990, 2003, 2007). A well-nourished condition without any environmental stresses favors sexual development, while stresses, including carbon or nitrogen source starvation, oxidative stress, high osmolarity, or intense visible light, block or inhibit fruiting body formation. Concomitantly, such stresses promote asexual development. Possibly, many genes are involved in the recognition of environmental conditions or factors, which is critical in decisions pertaining to development and regulation of the expression of developmental genes. A number of genes involved in these stages have been identified, although detailed knowledge of their functions at each stage is incomplete. They include signaling components such as the G-protein-coupled receptor system, mitogen-activated protein kinase cascades, and cAMP pathways (Han and Prade 2002; Kawasaki et al. 2002; Wei et al. 2003; Han et al. 2004; Seo et al. 2004) and also several putative transcription factors including StuA, SteA, NsdD, and RosA (Miller et al. 1992; Pascon and Miller 2000; Han et al. 2001; Vienken et al. 2005).
Some signaling components and transcription factors function negatively on sexual development. One of the seven membrane-bound G-protein-coupled receptors, GprD, has been suggested to be involved in the negative regulation of sexual development (Han et al. 2004). A putative Zn(II)2Cys6 transcription factor, RosA, is also a negative regulator (Vienken et al. 2005). Deletion of the rosA gene results in the reduction of fruiting body formation under standard culture conditions, but not under unfavorable environmental conditions such as carbon starvation or high osmolarity. Thus, RosA has been proposed to repress sexual development upon integration of several environmental signals.
The homeodomain C2H2 zinc-finger transcription factor, SteA, a homolog of Saccharomyces cerevisiae Ste12, is one of the transcription factors that positively regulate cleistothecia development (Vallim et al. 2000). Deletion of the steA gene blocks sexual development at the Hülle cell formation stage. Another well-known positive regulator, VeA, may mediate light-responsive conidiation (Mooney and Yager 1990). Deletion of the veA gene completely blocks sexual development, while veA overexpression enhances fruiting body formation in standard conditions and induces sexual reproduction even in non-inducible conditions such as high osmolarity or in submerged culture (Kim et al. 2002). These observations suggest that VeA is influential in the positive regulation of sexual development.
Sexual fruiting body formation in A. nidulans is influenced favorably or unfavorably by a variety of environmental factors, a situation that offers opportunities for screening the mutants of developmental genes involved in the signaling cascades and the regulation of gene expression in response to those factors. A low level of aerobic respiration caused by either plate-sealing or treatment with various inhibitors favors sexual development (Han et al. 1990, 2003). Imposing hypoxia during development allows the discrimination of mutants defective in cleistothecia development from wild type, which enables the massive screening of mutants. By using this strategy, NSD mutants have been isolated and arranged in four complementation groups (Han et al. 1994). Among them, the nsdD gene complementing the NSD19 mutation has been isolated and characterized. The nsdD gene encodes a putative GATA-type transcription factor that also positively regulates sexual reproduction (Chae et al. 1995; Han et al. 2001). nsdD mutants that lack a zinc-finger motif and overexpression strains show typical mutant phenotypes of positive regulators, which suggests that NsdD is a key regulator for cellular commitment to sexual development.
Although many genes involved in sexual development have been identified and knowledge of the signaling components and transcription factors continues to accumulate, the connection or links among them are still unclear. Since the conditions and the processes of sexual development are complex, a number of regulators likely remain to be identified. Previously, we isolated the nsdC gene and found it to be a novel putative transcription factor carrying a fungal-specific zinc-finger motif (Kim and Han 2006). Here, we present evidence that NsdC acts as a positive regulator of sexual development that is closely related to hypoxic conditions and carbon sources favorable for fruiting body formation.
MATERIALS AND METHODS
Strains and culture conditions:
A. nidulans strains used in this study are listed in Table 1. LDB115 was obtained from the cross between VER7 (ΔargB, veA+), which was previously used as a transformation recipient strain (Han et al. 2001) and A786 to eliminate an extra mutation in VER7 that affected sexual development. HSY2 was isolated from the cross between LDB115 and A4 and was used as a recipient strain for transformation with argB+ vectors. HSY2-P1 and HSY2-P2 were positive control strains obtained by transformation of HSY2 with pJYargB and pRB2-1, respectively. Genotypes of recombinants carrying the nsdC deletion mutation or the nsdC overexpression gene with various genetic markers are shown in Table 1. KOD2 and NDP5 are ΔnsdD recombinants free of same unidentified mutation that was found in VER7. OED1 was obtained by transformation of HSY2 with the nsdD overexpression vector (Han et al. 2001). KCOD41 [ΔnsdC, niiA(p)∷nsdD], KDOC51 [ΔnsdD, niiA(p)∷nsdC], and KVOC6 [ΔveA, niiA(p)∷nsdC] were generated by crosses of KNC64 and OED1, NDP5 and ONC65, and ΔveA28 and ONC65, respectively. KCOV21 [ΔnsdC, niiA(p)∷veA] was obtained by transformation of KNC6418 with pNQ-pyroA-veA14. The ΔveA28 strain in which the entire open reading frame (ORF) is deleted and pNQ-pyroA-veA14, which is the veA overexpression vector carrying the pyroA+ gene were gifts of S. K. Chae (Paichai University).
TABLE 1.
Strains used in this study
| Strain | Genotypea | Source |
|---|---|---|
| A4 | Wild type | FGSCb |
| A773 | pyrG89; wA3; pyroA4; veA1 | FGSC |
| HSY2 | anA1; ΔargB∷trpC | This study |
| NSD206 | biA1; nsdC6; sB3; chaA1 | Kim and Han (2006) |
| HSY2-P1 | anA1; ΔargB∷trpC; pJYargB (argB+) | This study |
| KNC64 | anA1; ΔnsdC∷argB; ΔargB∷trpC | This study |
| KNC641 | ΔnsdC∷argB; argB∷trpC | This study |
| KNC6418 | pyrG89; ΔnsdC∷argB; ΔargB∷trpC (?); pyroA4; veA1 | KNC64 × A773 |
| HSY2-P2 | anA1; ΔargB∷trpC; pRB2-1 (argB+) | This study |
| ONC65 | anA1; niiA(p)∷nsdC∷argB; ΔargB∷trpC | This study |
| KOD2 | ΔargB∷trpC; ΔnsdD∷argB; choA1 | This study |
| OED1 | anA1; ΔargB∷trpC; niiA(p)∷nsdD∷argB | This study |
| ΔveA28 | pabaA1; argB2; pyroA4; chaA1 ΔveA∷argB | S. K. Chae |
| OVAR5 | pabaA1 yA2; ΔargB∷trpC; trpC801 veA1 niiA(p)∷veA∷argB | Kim et al. (2002) |
| KDOC51 | anA1; ΔargB∷trpC; niiA(p)∷nsdC∷argB; ΔnsdD∷argB | ONC65 × KOD2 |
| KCOD41 | ΔnsdC∷argB; ΔargB∷trpC; niiA(p)∷nsdD∷argB | KNC64 × OED1 |
| KVOC6 | pabaA1; argB2(?) niiA(p)∷nsdC∷argB; ΔveA∷argB | ONC65 × ΔveA28 |
| KCOV21 | pyrG89; ΔnsdC∷argB; pyroA4; ΔargB∷trpC(?); veA1 niiA(p)∷veA∷pyroA | Transformed by pNQ-pyroA-veA14 |
All strains contain the wild-type veA allele unless otherwise indicated.
FGSC, Fungal Genetics Stock Center.
Minimal medium (MM) and complete medium (CM) were prepared as described previously (Han et al. 2001). Standard fungal techniques for culture, observation, and genetic analyses were carried out as described previously (Käfer 1977; Han et al. 2001). Sexual development was induced by sealing culture plates with parafilm for 24 hr. For observation of salt or osmotic stress, 0.6 m KCl or 1.2 m sorbitol were added on MM. Light illumination was performed in a growth chamber equipped with white fluorescent and metal SP lamps (maximum 20,000 Lux) and a temperature control system. The rate of conidiation was estimated every 2 hr by the ratio of conidial-headed colonies per 40 observed colonies arising from a conidium on solid MM.
Construction of vectors:
The 4-kb SpeI–EcoRI fragment containing the whole nsdC gene from the genomic library was cloned into the pBluescript SK(−) cloning site to generate pNSD61. The knockout vector pKNC64 was constructed by replacing the SmaI–SmaI segment in pNSD61 by the argB gene of the pJYargB plasmid (a gift from J.-H. Yu, University of Wisconsin-Madison). The nsdC ORF amplified with oligonucleotides PC101 and PC102 was cloned into T vector (Promega, Madison, WI), yielding pTNSD671. pONC67 was created by fusing the nsdC ORF digested from pTNSD671 with NheI and filled using Klenow polymerase (Takara Bio, Shiga, Japan) downward from the XhoI-digested and Klenow-filled niiA promoter in pRB2-1, a modified vector of pRB2 (Han et al. 2001), of which an ATG sequence between the niiA promoter and XhoI cloning site was removed.
Molecular techniques:
For genomic DNA preparation, mycelium cultured for 18 hr was dried for 3 hr at 65° and ground using a mortar. The mycelial powder was suspended in 400 μl lysis buffer (50 mm Tris–HCl, pH 8.0, 50 mm EDTA, 3% sodium dodecyl sulfate and 1% 2-mercaptoethanol) and incubated for 1 hr at 65° in a heating block, prior to the addition of an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1). The supernatant was separated by centrifugation, and genomic DNA was harvested by ethanol precipitation. Total RNA of A. nidulans was prepared from the mycelia frozen with liquid nitrogen and ground using a mortar with Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Southern and Northern blots were performed as previously described (Han et al. 2001). Probes were labeled with [α-32P]dCTP using a random priming kit (Takara Bio, Shiga, Japan) and purified using a PROBER kit (iNtRON Biotechnology, Seoul, South Korea).
Microscope and photography:
Photomicrographs were taken using an Olympus C-5060 digital camera through an Olympus BX50 microscope. Photographs were taken using a Samsung STW-NV7 digital camera.
RESULTS
nsdC encodes a novel C2H2-type zinc-finger protein:
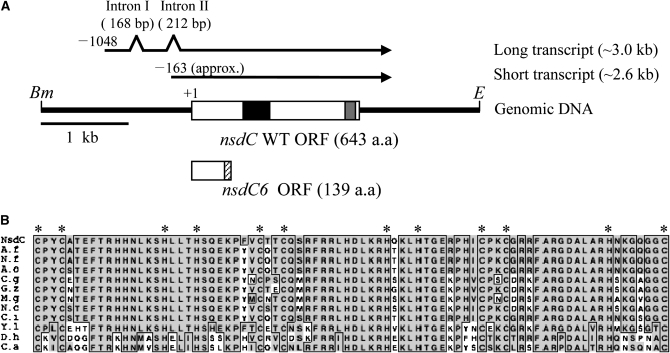
We screened a number of NSD mutants that failed to develop cleistothecia even under the conditions that forced mycelia to undergo sexual development (Han et al. 1990). Genetic analysis of the mutants revealed four complementation groups: nsdA, nsdB, nsdC, and nsdD (Han et al. 1994, 2001). nsdC6, the only mutant allele identified in the nsdC locus, caused complete failure of fruiting body formation and markedly reduced the growth rate. The nsdC gene was isolated from the AMA1-NotI genomic library that complements the nsdC6 mutation (Kim and Han 2006). Nucleotide sequence determination of nsdC revealed an ORF of 1929 bp with no introns, which was predicted to encode a protein consisted of 643 amino acids. The same gene designated AN4263.3 in the Broad Institute Aspergillus genome database carries a 668-amino-acid ORF. Reverse transcription–polymerase chain reaction (RT–PCR) and cDNA sequencing data revealed two introns at the 5′-untranslated region (UTR), and a nsdC start codon was predicted by the annotation of the genome database located within an intron just upstream of the ORF (Figure 1A). The NsdC polypeptide was determined to harbor a novel type of C2H2 zinc-finger domain consisting of two classic C2H2 motifs and a C2HC in the midst of the polypeptide (Figure 1). The intervals between the zinc-binding residues of two C2H2 fingers were the same and could be described as C-X2-C-X12-H-X3-H, which is very common in other C2H2 fingers found in A. nidulans. The C2HC possessed a maximum interval between C and H, which is rarely found in most C2HC-type fingers. The NsdC-type zinc-finger domain consists of 80 amino acids, which are highly conserved not only among filamentous fungi but also among various yeast-type fungi (Figure 1B). The orthologs of Aspergillus fumigatus and Neurospora crassa displayed amino acid sequence identity >90%. But no apparent orthologs were found in S. cerevisiae and Schizosaccharomyces pombe.
Figure 1.—
The nsdC gene structure and conserved putative zinc-finger domain of NsdC. (A) Schematic of the 5-kb BamHI–EcoRI fragment containing the nsdC gene and the gene structure of wild-type nsdC and nsdC6 mutant alleles are shown. The open box displays the nsdC ORF, the solid box indicates the C2H2C2H2C2HC zinc-finger domain, and the shaded box shows the coiled-coil region. The size and structure of the nsdC transcript was verified by RT–PCR, which revealed intron I (168 bp) and intron II (212 bp) at the 5′-UTR. The nsdC6 mutation was caused by insertion of a thymine residue at ORF 407 bp, which generated a frameshift resulting in production of a 139-amino-acid C-terminal truncated polypeptide. The stripped box shows the amino acid of nsdC6 created by the frameshift. Bm, BamHI; E, EcoRI. (B) Multiple alignment of a putative C2H2C2H2C2HC zinc-finger domain found in NsdC. NsdC, A. nidulans NsdC (AY577544); N.f, Neosartorya fischeri C2H2 zinc-finger protein (XP_001261312); A.f, A. fumigatus C2H2 zinc-finger protein (XP_749117); A.o, Aspergillus oryzae hypothetical protein (XP_001822225); C.g, Chaetomium globosum hypothetical protein (XP_001229358); G.z, Giberella zeae hypothetical protein (XP_381526); N.c, N. crassa hypothetical protein (XP_965439); M.g, Magnaporthe grisea hypothetical protein (XP_368740); C.i; Coccidioides immitis hypothetical protein (XP_001240347); Y.l, Yarrowya lipolytica hypothetical protein (XP_504657); D.h, Debaryomyces hansenii hypothetical protein (XP_458294); C.a, Candida albicans hypothetical protein (XP_722294). Multiple alignment was performed by ClustalW and visualized by SeqVu 1.1.
In the nsdC6 allele, an additional base pair, “T,” was inserted next to base 407 in the ORF, which caused a reading frameshift resulting in early termination of translation. The NsdC6 polypeptide was predicted to have only 139 amino acids lacking the zinc-finger domain (Figure 1A).
Two different-sized mRNAs are transcribed from nsdC:
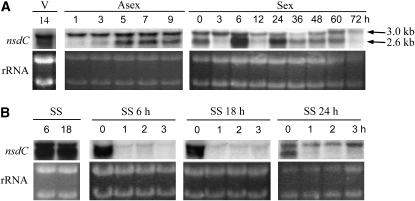
We examined the steady-state mRNA levels of nsdC throughout the life cycle by Northern blot analysis using a PCR-amplified 940-bp genomic DNA fragment (PC1 and PC26; Table 2) as a nsdC-specific probe. Two different mRNAs of ∼2.6 and 3.0 kb were detected from vegetative mycelia formed in submerged cultures (Figure 2A). 5′-RACE predicted the 5′-ends of 3.0- and 2.6-kb transcripts to be 1048 and 163 nucleotides upstream of the ATG of the putative ORF, respectively (Figure 1A). The short mRNA accumulated at certain periods of development while the level of the longer mRNA remained relatively constant throughout the life cycle. The amount of the 2.6-kb mRNA was reduced just after induction of conidiation and increased after 5 hr. The increased level was maintained for 5 hr and, after 10 hr, declined to the level of the early stage of conidiation. The period during which the 2.6-kb mRNA was accumulated coincided with sterigmata development. The stage-specific accumulation of the small mRNA was also seen during sexual development. When the mycelia were cultured in sealing conditions for 20 hr to induce sexual development, the level of the 2.6-kb mRNA increased as soon as the seal was removed from the culture plate. However, this was followed by a marked decrease to the level present during early asexual development. The mRNA level transiently increased at 3–4, 24–28, and 60 hr after induction of sexual development. These periods coincided with Hülle cell proliferation, crozier formation, and ascospore maturation, respectively. These periodic and orchestrated increases of the 2.6-kb mRNA indicate that the alternative expression of nsdC has some significant relationship with a common physiological event during certain stages of sexual or asexual development.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence | Description |
|---|---|---|
| PC1 | 5′-GCC GAG CAT AAA GAA GCA-3′ | Forward primer of nsdC |
| PC26 | 5′-CGA ACA GTC CCC TAC AGG-3′ | Reverse primer of nsdC |
| PC101a | 5′-CGC TAg CAT GAC TTC GAT CTA T-3′ | Base changed forward primer of nsdC with NheI site |
| PC102a | 5′-GTG GCC GCT AGc AAC ATA TTA C-3′ | Base changed reverse primer of nsdC with NheI site |
| veAup1 | 5′-CGT ACT CCT ATC CAT CGC-3′ | Forward primer of veA |
| veAlow1 | 5′-GTC GAA CGT ATA GGC AGG-3′ | Reverse primer of veA |
Underlined sequences are NheI restriction enzyme sites. Lowercase letters indicate in vitro-mutagenized nucleotides.
Figure 2.—
Pattern of the nsdC gene expression during developmental processes. (A) Northern blot analysis showing the nsdC mRNA accumulation patterns in wild-type strain A4 throughout the life cycle. Vegetative mycelia cultured for 14 hr (V) were transferred onto solid MM and incubated for a given time as indicated above each lane (Asex). The same vegetative mycelia were transferred onto MM, and the plate was sealed with parafilm and aluminum foil, incubated for 24 hr to induce sexual development, and incubated further for a given time as indicated above each lane after removal of the seal (Sex). Two transcripts of ∼2.6 and 3.0 kb were detected at specific time points. Approximate equal loading of RNA samples was evaluated by ethidium-bromide-stained rRNA bands. (B) The expression of the 2.6-kb nsdC transcript was increased at the sealing state (SS) for inducing sexual development. This increase rapidly disappeared when the seals were removed after 6, 18, and 24 hr (SS 6, SS 18, and SS 24 hr, respectively).
Accumulation of the 2.6-kb mRNA is increased at low oxygen levels:
The amount of the 2.6-kb mRNA was greatly increased when mycelia generated from submerged cultures were transferred to solid media that were sealed and cultured for 24 hr to induce sexual development (Figure 2A). This mRNA increase was also observed in mycelia cultured for 6 or 18 hr in the hypoxic condition (Figure 2B). Because the 6-hr incubation of mycelial balls in a sealed plate is insufficient for full induction of sexual development (Han et al. 1990), it is likely that the increased level of the 2.6-kb mRNA was not due simply to the induction of sexual development, but rather was a consequence of hypoxic growth. The accumulation of the 2.6-kb mRNA that was also observed in mycelia cultured in an anaerobic jar supports this view (data not shown). The 2.6-kb mRNA was reduced within 1 hr after removal of the seal, regardless of the length of the sealing period (Figure 2B), indicating that the accumulation of the 2.6-kb mRNA was sensitive to air exposure.
NsdC is a key positive regulator in sexual development:
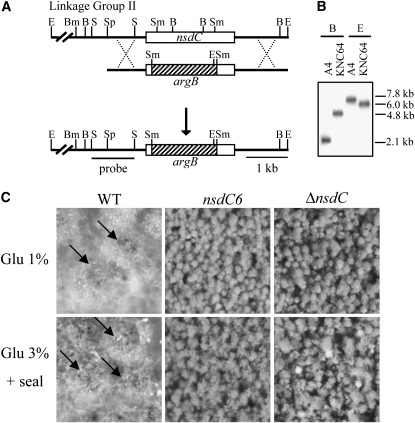
To characterize the function of NsdC, the responsible gene was replaced with the selective marker gene argB. More than 10 transformed strains showing a sterile phenotype were isolated, and deletion of the nsdC gene was verified by Southern blot analysis (Figure 3B). A nsdC deletion mutant, KNC64 (ΔnsdC, veA+), was crossed with HSY2 carrying the argB deletion mutation, and the meiotic progenies were analyzed to confirm that the true nsdC gene was deleted and that the strain did not harbor any extra mutation that might affect the phenotype. All argB+ progenies exhibited the nsdC deletion phenotype, while all argB− progenies were wild type, indicating that the sterile phenotype of KCN64 was due to the deletion of the true nsdC gene. Similar results were found in the cross between KNC64 and A4, the wild-type strain, in which half of the meiotic progenies displayed the nsdC deletion phenotype and the remaining half displayed the wild-type phenotype, without any other phenotype evident.
Figure 3.—
Construction and phenotype of the nsdC deletion mutant. (A) Schematic of the nsdC deletion construct. The construct was generated by replacing nsdC with a selection marker, argB, at the SmaI–SmaI site of nsdC ORF. B, BstXI; Bm, BamHI; E, EcoRI; S, SacII; Sm, SmaI; Sp, SpeI. (B) Southern blot analysis of the nsdC deletion mutant. Genomic DNA of each wild type (A4) and nsdC deletion strain (KNC64), which was digested with BstXI (B) and EcoRI (E), was separated on a 0.8% agarose gel, blotted, and hybridized with the SacII–SacII DNA fragment at the 5′ flanking region of the nsdC gene as the nsdC-specific probe. (C) The phenotypes of wild type (WT), the nsdC allele mutation (nsdC6), and the nsdC deletion mutant (ΔnsdC) under normal conditions (CM) and under sexually induced conditions (3% glucose and sealing for 24 hr). The wild-type strain produced cleistothecia and conidia on CM and on CM with 3% glucose + sealing conditions. But nsdC6 and ΔnsdC strains never formed cleistothecia under normal and sexually induced conditions.
We selected a nsdC deletion progeny (KNC641) that did not carry any auxotrophic mutation among the progenies of the cross between KNC64 and A4 for a more precise analysis of the developmental phenotype. The nsdC deletion mutant was unable to produce cleistothecia and Hülle cells under standard culture, even after prolonged incubation (Figure 3C). Furthermore, no sexual development was induced under conditions favorable for sexual development, including high-glucose concentration or restricted aeration (data not shown) and even where the two factors were combined (Figure 3C). These results suggest that NsdC was necessary for the induction of sexual development regardless of the environmental conditions. The phenotypes of the deletion mutants were the same as those of the nsdC6 allelic mutant, which was predicted to produce a truncated polypeptide lacking the zinc-finger motif, indicating that the zinc-finger motif plays an important role in the control of sexual development.
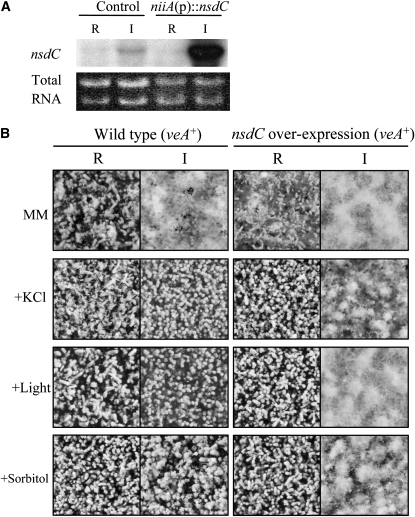
To determine whether nsdC could activate sexual development, we also examined the effect of forced expression of the nsdC gene on the developmental pattern. The nsdC ORF was fused downstream of the nitrate-inducible niiA promoter [niiA(p)] and was introduced as a single copy into a wild-type recipient strain (HSY2). As shown in Figure 4A, the nsdC transcript was greatly increased in a transformant, ONC65, but not in a control strain, HSY2-P2, when cultured on sodium nitrate as the sole nitrogen source. In the presence of ammonium tartrate, however, mRNA was maintained at the control level, indicating that transcription of nsdC in ONC65 was inducible by sodium nitrate. Under repressing conditions (growth on 0.2% ammonium tartrate), no phenotypic changes in development were observed compared with wild type. However, induction of the nsdC gene with 0.6% sodium nitrate resulted in preferential development of sexual organs, while asexual sporulation was greatly reduced (Figure 4B). Strain ONC65 developed cleistothecia at almost the normal level in the presence of 0.6 m KCl or ∼10 W/m2 white light, conditions that abrogated cleistothecia formation in a wild-type strain (Figure 4B). These nsdC forced-expression phenotypes were very similar to those of nsdD and veA, which positively control sexual development (Han et al. 2001; Kim et al. 2002). However, forced expression of nsdC could not induce any of the sexual structures in submerged cultures (data not shown), in contrast to nsdD or veA overexpression, which resulted in the development of Hülle cells or the entire sexual structures, respectively (Han et al. 2001; Kim et al. 2002). The phenotypic changes resulting from deletion or overexpression of the nsdC gene suggest that NsdC functions as a positive regulator of sexual development.
Figure 4.—
Phenotype of nsdC overexpression under various stress conditions. (A) Northern blot analysis depicting the induced overexpression of nsdC under the control of the niiA promotor [niiA(p)] by 0.6% sodium nitrate as a nitrogen source (induced condition, I), which resulted in high accumulation of the nsdC transcript compared to the repression of the promoter by 0.2% ammonium tartrate (repressed condition, R) in the overexpressing strain. (B) Phenotype of wild-type and nsdC overexpression strains under various stress conditions. The wild-type strain never produced cleistothecia under the stresses of salt (0.6 m KCl), osmotic pressure (1.2 m sorbitol), and light, while the nsdC-overexpressing strain formed sexual structures even under the stressed condition.
NsdC functions in the control of mycelial growth and asexual sporulation:
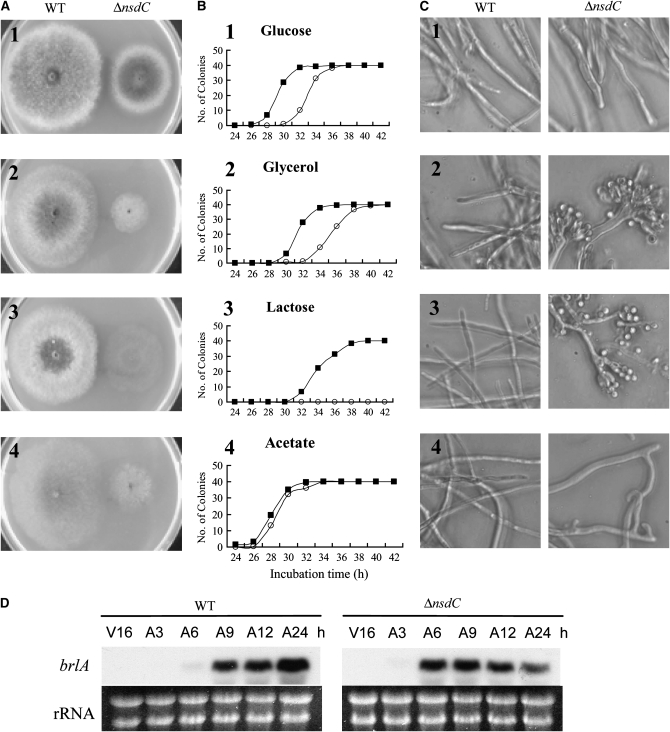
Most NSD mutants share the common phenotype of retarded growth as compared with the wild type (Han et al. 2001). The nsdC deletion was also associated with a reduced growth rate on CM, which is almost identical to that of the nsdC6 mutation (Figure 5A and Table 1). On MM containing glucose as the sole carbon source, the apical growth rate of the nsdC deletion mutant was reduced to ∼70% of the wild type. A further reduction of the growth rate was observed in culture using medium containing a less favorable carbon source such as glycerol, lactose, or acetate (Figure 5A). Loss of function of nsdC also caused an earlier development of asexual spores. The time course of asexual sporulation on media containing various carbon sources is summarized in Figure 5B. Wild-type colonies that arose from inoculation of a single conidium began to develop conidial heads at 26, 30, and 32 hr on acetate, glucose, and glycerol minimal media, respectively. The conidial head development of the nsdC deletion mutant on acetate MM occurred at almost the same time as for the wild type, but 3–4 hr earlier on MM containing glucose or glycerol as the carbon source. Furthermore, the mutant developed many conidia on lactose MM, whereas the wild type barely did. This earlier and preferential development of asexual spores was likely not due to the blockade of sexual development by nsdC mutations. Rather, NsdC may have participated in the negative regulation of asexual development. Surprisingly, conidia were formed from the mycelia of the nsdC deletion mutant cultured in liquid MM containing glycerol or lactose as the sole carbon source, but not in MM containing glucose or acetate (Figure 5C). These additional phenotypes of the loss-of-function mutation of nsdC strongly suggest that NsdC not only was able to control sexual development positively but also was able to negatively control asexual sporulation, especially when lactose or glycerol was provided as a sole carbon source, conditions where sexual development is preferentially induced. The lack of NsdC function did not influence the transcription level of brlA (Figure 5D), a key regulator of asexual sporulation. However, in the nsdC deletion mutant, the brlA mRNA was detected 3 hr earlier than a wild type. The earlier expression of brlA in the deletion mutant coincided with the earlier progression of asexual development. The result strongly suggests that NsdC represses the brlA expression at an early stage of asexual sporulation after induction.
Figure 5.—
Growth of the nsdC deletion mutant on various carbon sources. (A) Wild type (WT) and KNC641 (ΔnsdC) strains were point-inoculated on MM containing 1% glucose (1), 2% glycerol (2), 1% lactose (3), or 2% acetate (4) as the carbon source and incubated at 37° for 3 days. Growth rate of ΔnsdC was clearly delayed compared to WT. (B) The sporulation rate of ΔnsdC and WT was measured by counting the number of colonies that formed conidial heads at a given time on solid MM containing the various carbon sources. Open circles and solid squares represent A4 and KNC641, respectively. (C) The nsdC deletion strain (ΔnsdC) produced conidiophores and viable conidia under submerged culture where 2% glycerol or 1% lactose was used as the carbon source. (D) Northern blot analysis of WT and ΔnsdC strains with the brlA gene-specific probe (Lee and Adams 1994). Total RNAs were isolated from asexually induced mycelia grown in CM broth at 37° for 16 hr and transferred onto MM solid medium. Induction of the brlA gene expression during asexual growth is accelerated in the ΔnsdC strain. Numbers indicate time points after incubation of vegetative growth (V) or asexual growth (A).
Expression of nsdC is not significantly affected by nsdD or veA, and vice versa:
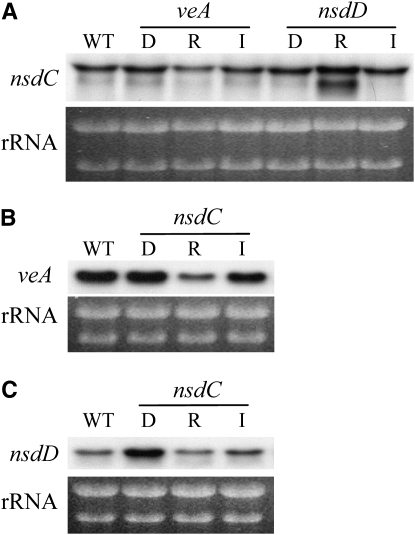
In previous studies, two positive regulators of sexual development, NsdD and VeA, were identified and characterized (Han et al. 2001; Kim et al. 2002). To ascertain whether any interrelationship among NsdD, VeA, and NsdC existed in the regulation of sexual development, the effect of the deletion or the overexpression of one gene on the transcription of the other two genes was examined by Northern blot analysis. Figure 6 shows that both deletion and overexpression of nsdD or veA did not significantly influence the expression of nsdC, indicating that the transcription of nsdC was not under the direct control of either of the two positive regulators. The transcription level of veA was also unchanged by either the absence or the excess of NsdC. A slight increase of nsdD transcript level was observed in the nsdC deletion mutant. However, nsdC overexpression did not affect the transcription of nsdD (Figure 6). Taken together, the results suggest that there is no direct regulatory interaction between nsdC and either nsdD or veA at the transcription level.
Figure 6.—
Transcriptional regulation among nsdC, nsdD, and veA. The nsdC gene expression was not affected by deletion or overexpression of veA or nsdD. The transcription level of nsdC (A), veA (B), and nsdD (C) was examined by Northern blot analysis in the deletion or overexpression strains of the other genes (D, deletion; R, repressed condition; I, induced condition). Total RNAs of wild type, KNC641 (ΔnsdC), KOD2 (ΔnsdD), and ΔveA28 (ΔveA) were prepared from vegetative mycelia cultured in liquid MM. The veA- and nsdD-specific probes were prepared as described previously (Han et al. 2001; Kim et al. 2002). WT, wild type.
Overexpression of nsdD rescues growth and submerged conidiation but not sexual development of the nsdC deletion mutant:
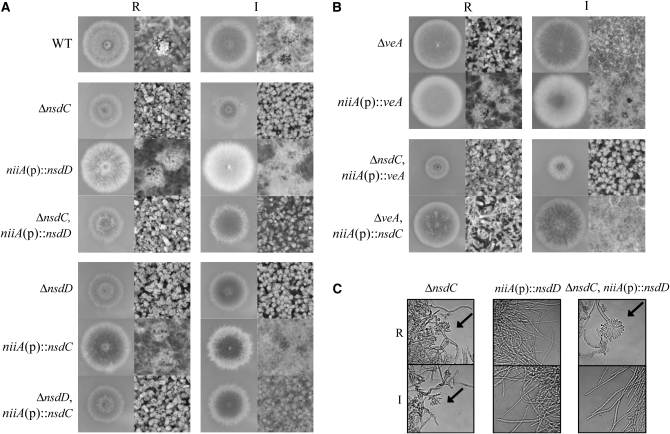
Since overexpression of any of these genes enhances sexual development, overexpression may affect the phenotype of a deletion mutant of any other gene. Recombinants carrying nsdC overexpression and nsdD deletion, or vice versa, were isolated in meiotic progenies obtained by crosses between the mutant strains. Deletion mutations and overexpressions were confirmed by PCR and Northern blot analysis (data not shown). The growth and developmental phenotypes of the strains are shown in Figure 7. The overexpression of nsdC had no influence on the complete sterility of the nsdD deletion and vice versa (Figure 7A), which strongly suggests that NsdD and NsdC function independently in the commitment to sexual development. Overexpression of nsdC did not recover the reduced growth rate in the nsdD deletion mutant (Figure 7A). However, impaired growth and conidiation in the submerged state that are caused by the nsdC deletion were recovered by overexpression of nsdD (Figure 7, A and C). However, no fruiting bodies were produced in spite of nsdD overexpression, indicating that the failure of sexual development in nsdC mutants bore no direct relationship with a growth defect or preferential asexual development. The interrelationship between veA and nsdC was also examined by similar experiments. No effect of overexpression of either gene on the deletion mutation of the other gene was evident, except that overexpression of nsdC reduced the asexual sporulation of the veA deletion mutant (Figure 7B). The result suggested that VeA and NsdC also function independently of sexual development and that VeA is not influential in the negative regulation of asexual development. In contrast, the conidiation of the nsdD deletion mutant was not affected by overexpression of nsdC (Figure 7A), indicating the necessity of NsdD in the repression of asexual development.
Figure 7.—
Genetic interaction among nsdC, nsdD, and veA. Phenotypes of the double mutants KCOD1 [ΔnsdC, niiA(p)∷nsdD], KDOC51 [ΔnsdD, niiA(p)∷nsdC], KCOV2 [ΔnsdC, niiA(p)∷veA], and KVOC9 [ΔveA, niiA(p)∷nsdC] are shown. (A) Sexual development as well as growth and conidia development of A4 (WT, wild type), KNC641 (ΔnsdC), KOD2 (ΔnsdD), ΔveA28 (ΔveA), ONC65 [niiA(p)∷nsdC], OED1 [niiA(p)∷nsdD], KCOD1, and KDOC51 strains were point-inoculated and observed under repressed (R) and induced (I) culture conditions. The growth rate of KCOD1 was recovered while that of KDOC51 remained unchanged. (B) Growth and developmental patterns were also observed in OVAR5 [niiA(p)∷veA], KCOV2, and KVOC9 strains in repressed (R) and induced (I) conditions. (C) KNC641, OED1, and KCOD1 were cultured under submerged conditions using lactose as a carbon source for 96 hr. KNC641 and KCOD1 in the repressed condition displayed asexual structures, but KCOD1 in the induced condition did not.
DISCUSSION
Triple C2H2 zinc-finger motifs are well-known DNA-binding domains found in many transcription factors of higher eukaryotic cells (Iuchi 2001). According to the annotation of the A. nidulans genome sequence, ∼40 proteins are predicted to carry the C2H2 zinc-finger domain (Broad Institute; http://www.broad.mit.edu/). Several transcription factors involved in the regulation of metabolism or differentiation, including CreA, AmdX, BrlA, FlbA, and SteA, are known to carry double C2H2 zinc-finger motifs (Dowzer and Kelly 1991; Lee and Adams 1994; Murphy et al. 1997; Vallim et al. 2000). NsdC, a putative transcription factor identified as a regulator of development, also carries C2H2 zinc fingers. However, unlike other known transcription factors carrying the C2H2 zinc finger, NsdC possesses an additional C2HC motif comprising 80 amino acid residues that is highly conserved in various fungal species (Figure 1B). The C2HC motif is found in various eukaryotic proteins that have diverse functions such as chromatin acetylation (Akhtar and Becker 2001; Smith et al. 2005), DNA repair (Jones et al. 1988), and development control (Raabe et al. 2004; Xie et al. 2005). Many C2HC motifs bind to DNA (Lee et al. 1998; Simpson et al. 2003) or interact with motifs of other transcription factors (Sadowski et al. 2003). Some motifs can combine with C2H2- or C2C2 GATA-type motifs (McLaughlin et al. 1994; Haenlin et al. 1997; Hahm et al. 1998). Furthermore, other motifs interact with the GATA zinc finger in a fashion that is functionally complementary to GATA (Fox et al. 1999). The C2HC zinc-finger motif in NsdC combines with two C2H2 fingers that are likely to bind to DNA as three C2H2 fingers do in higher eukaryotes. Alternatively, since most of the C2H2 transcription factors of A. nidulans carry double-finger motifs, which seem to be sufficient for DNA binding, the additional C2HC finger may interact with some other protein factor. In this regard, a binding assay using a yeast two-hybrid system was carried out but failed to show that the two transcription factors bound each other (data not shown). The role of the C2HC zinc-finger motif in NsdC awaits further study.
Deletion of nsdC resulted in the complete loss of fruiting body formation under all conditions favoring sexual development (Figure 3), and overexpression not only enhanced formation of fruiting bodies but also overcame inhibitory effects of certain stresses on cleistothecial development (Figure 4). These observations suggest that NsdC participates as a positive regulator of sexual development. Several transcription factors that positively regulate sexual development have been identified in A. nidulans. They include SteA, a homeodomain protein carrying two tandem C2H2 zinc-finger domains (Vallim et al. 2000), and NsdD, a putative GATA-type transcription factor carrying a type IVb C2C2 zinc finger (Han et al. 2001). Although deletion mutants or overexpression strains of those transcription factor genes share common phenotypes concerning sexual development, no evidence for direct interaction among those regulators has yet been presented. We also found no apparent evidence for any direct regulatory interaction among veA, nsdD, and nsdC at the transcriptional level (Figure 6). Furthermore, the failure of cleistothecia development through the deletion of one of two genes was not recovered by overexpression of the other gene (Figure 7). These results suggest that all three regulators are necessary for sexual reproduction and that any of them alone is not sufficient even if it is expressed in an excess amount. Although the three regulators are not directly involved in the genetic regulation of the expression of other regulator genes, they may be functionally connected with each other through some other mediators in the control of sexual development.
The regulatory function of NsdC seems not to be restricted to sexual development but is expanded to the control of growth and asexual development, which is likely to have a relationship with carbon metabolism. A. nidulans can utilize a wide range of sugars as carbon sources, which were grouped into sources that were utilized readily, moderately, or poorly by estimating the amount of mycelia and the degree of conidiation on plates relative to growth on glucose (McCullough 1977). Glucose, fructose, and sucrose were readily utilized, while acetate, lactose, and glycerol were moderately utilized carbon sources. In the presence of readily utilized carbon sources, both sexual and asexual organs develop almost in balance (Han et al. 1990). However, the ratio of sexual to asexual development on moderately utilized carbon sources varies considerably. In the presence of acetate, conidia develop but cleistothecia do not. Conidiation occurred earliest in the presence of acetate compared with the other carbon sources (Figure 5A). In many fungi, asexual sporulation is favored by conditions under which aerobic respiration is a major energy metabolism (Galbraith and Smith 1969; Urey 1971; Ng et al. 1973). Since acetate can be utilized only via aerobic respiration in A. nidulans (Hondmann and Visser 1994), the fate of mycelia grown on an acetate-containing medium may be determined to develop only asexually (Smith et al. 1977; Han et al. 2003). On the other hand, very few conidiophores develop and their development occurs later while cleistothecia develop normally when growth occurs on lactose or glycerol (Han et al. 1990, 2003; Figure 5A). Asexual development is repressed in response to an unknown metabolic state during growth on lactose or glycerol, in which NsdC may play a role as a major regulatory component. The mutant produced conidia when growing on lactose or glycerol earlier than the wild type grown on glucose. Furthermore, conidia were developed in liquid medium when lactose or glycerol was supplied as the sole carbon source (Figure 5). These results indicate that conidiation in the nsdC deletion mutant is derepressed during growth on carbon sources favoring sexual development and strongly suggest that NsdC acts as a repressor of asexual development under those conditions. Mutants of another positive regulator of sexual development, NsdD, also produce many asexual spores (Han et al. 2001), which has raised the possibility of a negative regulatory activity of NsdD in asexual development. However, deletion of nsdD abrogates the formation of asexual spores in submerged cultures (data not shown). Thus, repression of conidiation under certain circumstances is a unique function of NsdC among the positive regulators of sexual development. It is not precisely known why conidia are not produced in liquid medium where the sole carbon source is acetate or glucose. NsdC does not seem to work on acetate, where sexual development is never induced and growth is sufficient only for asexual development. It can be supported by the fact that overexpression of nsdC influences neither asexual sporulation nor sexual development on acetate (data not shown).
Two different-sized transcripts, 2.6 and 3.0 kb, were transcribed from nsdC (Figure 2). The 2.6-kb transcript markedly accumulated in various stages of growth and development as well as under the varying cultural conditions. In A. nidulans, the stuA and brlA genes, which are necessary for normal development of conidia, produce two distinctive transcripts that are, respectively, designated α and β (Miller et al. 1992; Prade and Timberlake 1993). Their expression changes as conidiation proceeds. Transcription of brlAα is initiated in the intron of the brlAβ transcript, which results in the alternative initiation of the translation of brlAα starting from a different ATG codon from that of brlAβ. In the mycelia that acquire the competence for asexual sporulation, brlAβ is transcribed first and then transcription of brlAα is induced by the BrlAβ translated from the β transcript. The nsdC gene also has two relatively long introns in the 5′-UTR. Primer extension analysis has revealed that the small transcript initiates near the 5′ splicing site of the second intron (Figure 1A). Unless the second intron is spliced adequately, translation might initiate at an ATG located within the intron. The resulting polypeptide contains the additional 23-amino-acid residues at the N terminus, which corresponds to the putative ORF predicted by the annotation data of genomic analysis. Unlike the stuA and brlA transcripts, the level of the long 3.0-kb transcript does not change significantly when the expression of the short nsdC transcript increases to a high level. The short 2.6-kb transcript apparently accumulates in hypoxic conditions and disappears very quickly after exposure to air. This result suggests that NsdC translated from the short transcript is required for growth under low-oxygen conditions.
Increase of the 2.6-kb nsdC transcript level may also be closely related to the function of NsdC involved in the repression of asexual development and the commitment to sexual development under low-oxygen conditions. Limitation of aeration for >20 hr results in the irreversible initiation of sexual development in A. nidulans (Han et al. 1990). In contrast, the nsdC mutants initiate asexual sporulation immediately after exposure to air, implying that asexual sporulation in nsdC mutants has not been repressed during incubation in the hypoxic condition (Han et al. 1994). These results strongly suggest that NsdC plays an important role in the repression of asexual sporulation during growth in a hypoxic condition. In conclusion, the NsdC has the apparent dual functions of a positive regulator of sexual development and a negative regulator of asexual development under conditions where sexual reproduction is favored.
Acknowledgments
This work was supported by the Korea Research Foundation (grant no. KRF-2002-070-C00079).
References
- Adams, T. H., J. K. Wieser and J. H. Yu, 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar, A., and P. B. Becker, 2001. The histone H4 acetyltransferase MOF uses a C2HC zinc finger for substrate recognition. EMBO J. 2 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod, D. E., M. Gealt and M. Pastushok, 1973. Gene control of developmental competence in Aspergillus nidulans. Dev. Biol. 34 9–15. [DOI] [PubMed] [Google Scholar]
- Chae, K. S., J. H. Kim, Y. Choi, D. M. Han and K. Y. Jahng, 1995. Isolation and characterization of a genomic DNA fragment complementing an nsdD mutation of Aspergillus nidulans. Mol. Cell 5 146–150. [Google Scholar]
- Clutterbuck, A. J., 1969. A mutational analysis of conidial development in Aspergillus nidulans. Genetics 63 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowzer, C. E., and J. M. Kelly, 1991. Analysis of the creA gene, a regulator of carbon catabolite repression in Aspergillus nidulans. Mol. Cell. Biol. 11 5701–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, R., 2002. Conidiation in Aspergillus nidulans, pp. 59–86 in Molecular Biology of Fungal Development, edited by H. D. Osiewacz. Marcel Dekker, New York.
- Fox, A. H., C. Liew, M. Holmes, K. Kowalski and J. Mackay et al., 1999. Transcriptional cofactors of the FOG family interact with GATA proteins by means of multiple zinc fingers. EMBO J. 18 2812–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, J. C., and J. E. Smith, 1969. Changes in activity of certain enzymes of tricarboxylic acid cycle and the glyoxylate cycles during the initiation of conidiation of Aspergillus niger. Can. J. Microbiol. 15 1207–1212. [DOI] [PubMed] [Google Scholar]
- Haenlin, M., Y. Cubadda, F. Blondeau, P. Heitzler, Y. Lutz et al., 1997. Transcriptional activity of Pannier is regulated negatively by heterodimerization of the GATA DNA-binding domain with a cofactor encoded by the u-shaped gene of Drosophila. Genes Dev. 11 3096–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm, K., B. S. Cobb, A. S. McCarty, K. E. Brown, C. A. Klug et al., 1998. Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev. 12 782–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D. M., Y. J. Han, Y. H. Lee, K. Y. Jahng, S. H. Jahng et al., 1990. Inhibitory conditions of asexual development and their application for the screening of mutants defective in sexual development. Kor. J. Mycol. 18 225–232. [Google Scholar]
- Han, D. M., Y. J. Han, J. H. Kim, K. Y. Jahng and Y. S. Chung et al., 1994. Isolation and Characterization of NSD mutants in Aspergillus nidulans. Kor. J. Mycol. 22 1–7. [Google Scholar]
- Han, D. M., K. S. Chae and K. H. Han, 2007. Sexual development in Aspergillus nidulans, pp. 279–299 in The Aspergilli: Genomics, Medical Aspects, Biotechnology, and Research Methods, edited by G. H. Goldman and S. A. Osmani. CRC Press, New York.
- Han, K. H., and R. A. Prade, 2002. Osmotic stress-coupled maintenance of polar growth in Aspergillus nidulans. Mol. Microbiol. 43 1065–1078. [DOI] [PubMed] [Google Scholar]
- Han, K. H., K. Y. Han, J. H. Yu, K. Chae, K. Y. Jahng et al., 2001. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol. Microbiol. 41 299–309. [DOI] [PubMed] [Google Scholar]
- Han, K. H., D. B. Lee, J. H. Kim, M. S. Kim, K. Y. Han et al., 2003. Environmental factors affecting development of Aspergillus nidulans. J. Microbiol. 41 34–40. [Google Scholar]
- Han, K. H., J. A. Seo and J. H. Yu, 2004. A putative G protein-coupled receptor negatively controls sexual development in Aspergillus nidulans. Mol. Microbiol. 51 1333–1345. [DOI] [PubMed] [Google Scholar]
- Hondmann, D. H. A., and J. Visser, 1994. Carbon metabolism, pp. 61–140 in Aspergillus: 50 Years on, Progress in Industrial Microbiology, edited by S. D. Martinelli and J. R. Kinghorm. Elsevier Science, Amsterdam. [PubMed]
- Iuchi, S., 2001. Three classes of C2H2 zinc finger proteins. Cell. Mol. Life Sci. 58 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. S., S. Weber and L. Prakash, 1988. The Saccharomyces cerevisiae RAD18 gene encodes a protein that contains potential zinc finger domains for nucleic acid binding and a putative nucleotide binding sequence. Nucleic Acids Res. 16 7119–7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käfer, E., 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19 33–131. [DOI] [PubMed] [Google Scholar]
- Kawasaki, L., O. Olivia Sánchez, K. Shiozaki and J. Aguirre, 2002. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol. Microbiol. 45 1153–1163. [DOI] [PubMed] [Google Scholar]
- Kim, H. R., and D. M. Han, 2006. Isolation and characterization of the nsdC gene in sexual development of Aspergillus nidulans. Kor. J. Microbiol. 42 246–251. [Google Scholar]
- Kim, H. S., K. Y. Han, K. J. Kim, D. M. Han, K. Y. Jahng et al., 2002. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet. Biol. 37 72–80. [DOI] [PubMed] [Google Scholar]
- Lee, B. N., and T. H. Adams, 1994. Overexpression of flbA, and early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Mol. Microbiol. 14 323–334. [DOI] [PubMed] [Google Scholar]
- Lee, C. C., E. L. Beall and D. C. Rio, 1998. DNA binding by the KP repressor protein inhibits P-element transposase activity in vitro. EMBO J. 17 4166–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough, W., M. A. Payton and C. F. Roberts, 1977. Carbon metabolism in Aspergillus nidulans, pp. 97–130 in Genetics and Physiology of Aspergillus, edited by J. E. Smith and J. A. Pateman. Academic Press, New York.
- McLaughlin, C. R., Q. Tao and M. E. Abood, 1994. Isolation and developmental expression of a rat cDNA encoding a cysteine-rich zinc finger protein. Nucleic Acids Res. 22 5477–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K. Y., J. Wu and B. L. Miller, 1992. StuA is required for cell pattern formation in Aspergillus. Genes Dev. 6 1770–1782. [DOI] [PubMed] [Google Scholar]
- Mooney, J. L., and L. N. Yager, 1990. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 4 1473–1482. [DOI] [PubMed] [Google Scholar]
- Murphy, R. L, A. Andrianopoulos, M. A. Davis and M. J. Hynes, 1997. Identification of amdX, a new Cys-2-His-2 (C2H2) zinc-finger gene involved in the regulation of the amdS gene of Aspergillus nidulans. Mol. Microbiol. 23 591–602. [DOI] [PubMed] [Google Scholar]
- Ng, A. M. L., J. E. Smith and A. F. McIntosh, 1973. Changes in activity of tricarboxylic acid cycle and glyoxylate cycle enzymes during synchronous development of Aspergillus niger. Trans. Brit. Mycol. Soc. 61 13–20. [Google Scholar]
- Pascon, R. C., and B. L. Miller, 2000. Morphogenesis in Aspergillus nidulans requires Dopey (DopA), a member of a novel family of leucine zipper-like proteins conserved from yeast to humans. Mol. Microbiol. 36 1250–1264. [DOI] [PubMed] [Google Scholar]
- Prade, R. A., and W. E. Timberlake, 1993. The Aspergillus nidulans brlA regulatory locus consists of overlapping transcription units that are individually required for conidiophores development. EMBO J. 12 2439–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe, T., S. Clemens-Richter, T. Twardzik, A Ebert, G. Gramlich et al., 2004. Identification of mushroom body miniature, a zinc-finger protein implicated in brain development of Drosophila. Proc. Natl. Acad. Sci. USA 101 14276–14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski, M., B. Dichtl, W. Hübner and W. Keller, 2003. Independent functions of yeast Pcf11p in pre-mRNA 3′ end processing and in transcription termination. EMBO J. 22 2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, J. A., K. H. Han and J. H. Yu, 2004. The gprA and gprB genes encode putative G protein-coupled receptors required for self-fertilization in Aspergillus nidulans. Mol. Microbiol. 53 1611–1623. [DOI] [PubMed] [Google Scholar]
- Simpson, R. J. Y., E. D. Cram, R. Czolij, J. M. Matthews, M. Crossley et al., 2003. CCHX zinc finger derivatives retain the ability to bind Zn(II) and mediate protein-DNA interactions. J. Biol. Chem. 278 28011–28018. [DOI] [PubMed] [Google Scholar]
- Smith, A. T., S. D. Tucker-Samaras, A. H. Fairlamb and W. J. Sullivan, 2005. MYST family histone acetyltransferases in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell 4 2057–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. E., J. G. Andeson, S. G. Deans and B. Davis, 1977. Asexual development in Aspergillus, pp. 23–58 in Genetics and Physiology in Aspergillus, edited by J. E. Smith and J. A. Pateman. Academic Press, New York.
- Sohn, K. T., and K. S. Yoon, 2002. Ultrastructural study on the cleistothecium development in Aspergillus nidulans. Mycobiology 30 117–127. [Google Scholar]
- Urey, J. C., 1971. Enzyme patterns and protein synthesis during synchronous conidiation in Neurospora crassa. Dev. Biol. 26 17–27. [DOI] [PubMed] [Google Scholar]
- Vallim, M. A., K. Y. Miller and B. L. Miller, 2000. Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol. Microbiol. 36 290–301. [DOI] [PubMed] [Google Scholar]
- Vienken, K., M. Scherer and R. Fischer, 2005. The Zn(II)2Cys6 putative Aspergillus nidulans transcription factor repressor of sexual development inhibits sexual development under low-carbon conditions and in submersed culture. Genetics 169 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H., N. Requena and R. Fischer, 2003. The MAPKK kinase SteC regulates conidiophore morphology and is essential for heterokaryon formation and sexual development in the homothallic fungus Aspergillus nidulans. Mol. Microbiol. 47 1577–1588. [DOI] [PubMed] [Google Scholar]
- Xie, Z., Z. L. Zhang, X. Zou, J. Huang, P Ruas et al., 2005. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 137 176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld, B. J. M., 1977. Biochemistry and ultrastructure of sexual development of Aspergillus, pp. 59–80 in Genetics and Physiology of Aspergillus, edited by J. E. Smith and J. A. Pateman. Academic Press, London.