Abstract
Background and Aims
Seeds of Grevillea linearifolia germinate following fire, and have seed-coat dormancy broken by smoke and heat shock. Smoke breaks seed coat dormancy in Emmenanthe penduliflora by altering the permeability of the seed coat to an internal germination inhibitor, which subsequently escapes. This model was tested for in G. linearifolia by investigating the permeability of the seed coat to diffusion of high-molecular-weight compounds, and whether this changed after exposure to fire cues.
Methods
Germination response of the seeds to heat shock, smoke or heat + smoke was tested. Penetration of Lucifer Yellow dye into intact seeds was examined after 24 and 48 h of exposure, and penetration of the dye from the inside of the seed coat outwards was examined after 24 h. Histochemical staining with Nile Red and Acridine Orange was used to locate cuticles, suberin and lignin.
Key Results
Twenty-three per cent of untreated seeds germinated; heat shock and smoke increased germination additively up to approx. 80 % for both cues combined. Lucifer Yellow did not penetrate fully through the seed coat of untreated seeds, whether diffusing inwards or outwards. Three barriers to diffusion were identified. Treatment with heat or smoke slightly increased penetration of the dye, but did not completely remove the barriers. Suberin was identified in secondary walls of exotestal and mesotestal cells, and was absent from primary cell walls. Movement of Lucifer Yellow occurred through the middle lamella and primary cell wall of suberized cells; movement of the dye was impeded where suberin was absent.
Conclusions
Fire cues did not significantly decrease barriers to diffusion of high-molecular-weight compounds in the seed coat of Grevillea, and must be breaking dormancy by another mechanism.
Key words: Fire, germination, smoke, heat shock, Lucifer Yellow, seed coat dormancy, apoplastic tracing, confocal, Grevillea linearifolia, suberin
INTRODUCTION
For many plant species in fire-prone vegetation, the optimum time for germination and establishment is after a fire. For seeds in the soil seed bank, a common way of limiting readiness to germinate to the post-fire period is for the seeds to be dormant when shed from the parent plant, and for this dormancy to be broken by cues from the fire. Seeds of east Australian Grevillea species show this pattern. Seeds returned to the laboratory, while showing some germination if allowed to imbibe, respond to fire-related germination cues such as smoke or heat; the greatest response is to the combination of the two (Kenny, 2000; Morris, 2000). These seeds also germinate if scarified (Edwards and Whelan, 1995; Morris, 2000). Investigation of Grevillea linearifolia showed that dormancy is mediated by the seed coat, as its removal resulted in full germination of all dissected embryos (Morris et al., 2000).
Some progress has been made in uncovering the mechanism of seed coat dormancy in Grevillea species. The known models of seed coat dormancy are (1) a mechanical restriction of germination, (2) preventing the exit of inhibitors from the embryo, (3) the presence of chemical inhibitors in the seed coat, (4) the restriction of water uptake and (5) the restriction of oxygen uptake (Bewley and Black, 1994). The seed coat of Grevillea is not impermeable to water: seeds imbibe freely when exposed to water (Morris, 2000; Morris et al., 2000) and the relative water content of dissected embryos from imbibed seeds was 30 times that of embryos from air-dry seeds (Briggs et al., 2005). Furthermore, the seed coat inhibitor model was not supported by the experiments of Morris et al. (2000); dissected embryos still germinated if re-inserted into the seed coat.
Seeds of some smoke-responsive chaparral species have a sub-dermal barrier that is semi-permeable, allowing water to enter but excluding high-molecular-weight solutes, e.g. Emmenanthe penduliflora, Phacelia grandiflora, Romneya coulteri and Dicentra chrysantha. The barrier in these species was located between the testa and the endosperm; exposure to smoke removed the barrier to diffusion of high-molecular-weight compounds (Keeley and Fotheringham, 1998). This was confirmed in some detail for Emmenanthe penduliflora by Egerton-Warburton (1998), who showed that the barrier was an internal cuticle in which pores opened up after smoke treatment. It was hypothesized that the embryo of unsmoked seeds contained a high-molecular-weight germination inhibitor; smoke, by altering the permeability of the internal cuticle, would allow this inhibitor to diffuse out of the seed, and germination to occur (Keeley and Fotheringham, 1998; Egerton-Warburon, 1998). This mechanism was confirmed, and the inhibitor was isolated and identified (Egerton-Warburton and Ghisalberti, 2001). Whether a similar mechanism applies to Grevillea dormancy is unknown.
As part of an investigation into the dormancy mechanisms in Grevillea spp., the anatomy and histochemistry of the seed coat of untreated seeds from three species (G. linearifolia, G. buxifolia and G. sericea) have been detailed (Briggs et al., 2005). These three species produce the oat-type seed, which has an oil-filled eliosome at the chalazal end (Olde and Marriott, 1995; Auld and Denham, 1999). The bitegmic seed coat was found to be similar in all three species. The outer integument or testa was three-layered, with an exotesta (suberized), mesotesta and lignified endotesta (reminiscent of the palisade layer in the hard coated seeds of the Fabaceae). The inner integument or tegmen consisted of bi-and sometimes tri-layered non-lignified yet sclerenchymatous elongated cells. A crushed cell layer was found between the tegmen and the embryo. Between the eliosome and the embryo was a multilayered hypostase. Two internal cuticles were identified by histochemical staining with Nile Red and Sudan Black B (the thickest between the endotesta and the tegmen and a thinner cuticle over the inner surface of the endotegmal layer). Suberized secondary walls were found in the exotesta and hypostase. These anatomical features suggested two possible mechanisms for seed coat dormancy: (1) there may be internal barriers to the movement of high-molecular-weight molecules as in Emmenanthe; and (2) the endotesta and tegmen may act as a mechanical constraint to germination.
To test the hypothesis of internal barriers to diffusion of high-molecular-weight compounds, the movement of Lucifer Yellow CH into a limited number of unheated, unsmoked seeds of G. linearifolia has been investigated (Briggs et al., 2005). Lucifer Yellow CH (FW 457·2) is a double negatively charged molecule that has frequently been used as an apoplastic tracer and has already been used in a study of seed coat dormancy in Emmenanthe penduliflora. The inwards movement of Lucifer Yellow (LY) was halted in the exotestal layer (or if the seed coat surface was damaged, the dye penetrated a short distance into the mesotesta); the dye easily penetrated the eliosome tissue but was halted at the hypostase and in the outer layer of a bi-layered lignified endotesta (Briggs et al., 2005). These barriers to diffusion meant that LY did not penetrate into the embryo. These results were consistent with the hypothesis of internal barriers to diffusion of high-molecular-weight compounds in the seed coat of unheated, unsmoked Grevillea seeds. Whether smoke and/or heat changes the permeability of these barriers, and whether germination inhibitors are located in the embryo, is unknown.
The aim of the present study was therefore to confirm the existence of putative barriers to diffusion of high-molecular-weight molecules for a larger number of unsmoked, unheated seeds, and to determine whether smoke, heat or the combination of the two affected this permeability.
MATERIALS AND METHODS
Plant material and treatments
Seeds of Grevillea linearifolia were collected during November, 2006 from Killarney Heights and Manly Dam, Sydney, NSW, Australia, and stored in paper bags at room temperature (RT). Three treatments were used: (1) heat, (2) smoke and (3) heat and smoke. To apply heat shock, seeds in open glass Petri dishes were heated at 80 °C for 10 min in a fan-oven, and this process was repeated separately for each replicate container. Seeds were smoked in a glass chamber, each replicate container being smoked individually (Morrison and Morris, 2000). Smoke was produced by burning dry, fine fuel litter from a eucalypt forest in a bee-keeper's burner forced out by an electric air-pump through a condensing tube, which both cooled and dried the smoke. For seeds that received both treatments, seeds were smoked within 1–2 min after heating. Treated seeds were stored in paper envelopes at RT until examination. Treatments were applied independently four times, with 7–10 seeds in each application.
Test for germinability of seeds
In March–April, 2007, a sub-set of the seed lot was treated with the fire cues to determine the germination response of the seeds. For each treatment, six replicates of five seeds each were separately treated with heat, smoke or heat and smoke (see above). Controls were unheated and unsmoked. Treated and control seeds were transferred to plastic 9 cm Petri dishes on one layer of Whatman no. 1 filter paper, moistened with aqueous fungicide (0·1 % Fongarid®) and incubated at a constant 20 °C under a 12/12-h light/darkn cycle. Seeds were regularly checked (approximately weekly) for germination. A seed with a 1-mm-long radicle was scored as a germinant and removed. The experiment was ended when the rate of germination was very low/no germination had been recorded for 14 d (8 weeks). Germination was calculated as a percentage of initial seeds per Petri dish. Data were analysed as a two-way factorial ANOVA, with smoke and heat as fixed effects. Tests of assumptions indicated that transformation of percentage germination data was not required.
Apoplastic tracing with Lucifer Yellow CH
Inward diffusion through whole seeds
Whole untreated (i.e. control) and treated seeds were placed into 1·5-mL micro-Eppendorf tubes containing 200 µL of 0·1 % Lucifer Yellow CH dilithium salt (ProSciTech C122, CAS no. 67769-47-5) and placed in the dark for 24 h at RT. Examination of a small number of replicates indicated that after 24 h the dye had not reached the embryo, and therefore the diffusion experiment was repeated with the diffusion time doubled to 48 h. LY was replaced with fixative and seeds were fixed (see below).
Outward diffusion through halved seeds
Control and treated seeds were imbibed on moistened filter paper (1 h), blotted, stuck onto microscope slides with double-sided sticky tape and the top part of the seed coat was dissected off. The exposed embryo was carefully prised out avoiding damage to the tegmen. Microscope slides with attached seed coat shells were placed into Petri dishes on top of moistened filter paper. Lucifer Yellow CH (0·1 %) was applied to the inner surface of the seed coat, the dish was covered and placed in the dark at RT for 24 h. After 24 h LY was removed and the seed coats were fixed (see below). This experiment was carried out twice, first using seeds 3 months old (three seeds from each treatment) and again when seeds were 12 months old (ten seeds from each treatment). Germination of untreated seeds was greater in the older seed lot than in the original experiment (data not shown).
Fixation of tissue
All seed material was fixed for 24 h at 4 °C in 1 mL of 2·5 % glutaraldehyde/3 % paraformaldehyde in phosphate-buffered saline. Whole seeds traced with LY were rinsed with fixative, cut under fresh fixative into four segments (2–3 mm in length) and transferred to clean vials; empty shells were rinsed with fixative then placed into fresh fixative without further cutting. To determine aldehyde-induced autofluorescence, control and treatment seeds were imbibed for 20 min, sectioned under fixative into four segments and then fixed as above. Following fixation, seeds were rinsed with three changes of distilled water, gradually dehydrated with 40 or 50 % ethanol and then stored at 4 °C until examination.
Histochemical staining
Hand-cut transverse and longitudinal sections were taken from imbibed, unfixed seeds or fixed LY-traced seeds (control and treated) using one half of a double-sided razor blade. LY-traced sections were first examined without counterstaining, and the same section was then stained with Nile Red (see below).
For cuticles and neutral lipids, sections were covered by freshly diluted Nile Red (40 µg mL−1) (Oparka and Read, 1994), stained for 40–60 min in the dark, rinsed with distilled water then mounted in glycerol or distilled water.
For cutin, lignin and negatively charged walls, sections were covered with freshly made Acridine Orange (0·1 % aq.) stained for 5 min in the dark (Oparka and Read, 1994), rinsed and mounted in distilled water.
Autofluorescence
To determine any contribution to emission due to autofluorescence, unfixed and fixed control and treatment seeds were also examined at the various wavelengths and gain settings used in this study. Dry seeds were imbibed for 20 min with distilled water and then hand-sectioned (see above); fixed seed segments were re-hydrated before sectioning. Sections were mounted in water or glycerol for examination.
Microscopy
All sections were examined on a Leica TCS SP5 laser scanning confocal inverted microscope using 10× or 20× (water/glycerol) objectives. Confocal imaging was through the Leica Application Suite Advanced Fluorescence Software (1·5·1 Build 869). Sections were first examined using epifluorescence microscopy to determine the presence and gross distribution of LY then confocally with an argon laser and the following excitation wavelengths and photomultiplier tube (PMT) combinations: Nile Red and LY – 488-nm laser line, PMT1 (500–585 nm) and PMT2 (590–730 nm) or 488-nm and 543-nm laser lines, PMT1 (500–560 nm) and PMT2 (610–740 nm); LY and Acridine Orange – 458-nm and 514-nm laser lines, PMT1 (450–510 nm) and PMT2 (580–650 nm); LY alone – 458-nm laser line, PMT1 (460–570 nm) and PMT2 (572–670 nm); Acridine Orange alone – 514-nm laser line, PMT1 (520–555 nm) and PMT2 (558–630 nm). Images were collected in 1024 × 1024-pixel format with eight or 16 times line averaging. Gains were adjusted to give maximum definition, clarity and trueness of colour (i.e. LY to appear yellow, cuticles to appear orange-gold as seen under epifluorescence). Images were exported in .tiff format.
Replication
Overall, 102 seeds were examined by confocal microscopy; 22 seeds for the 24-h LY inward diffusion experiment (four each for controls, heat-treated, smoke-treated and ten heat + smoke-treated); 24 seeds for the 48-h LY inward diffusion experiment (six seeds from each of the treatments and six from the controls); 52 seeds for the 24-h LY outward diffusion (13 from each treatment and controls); and the remaining seeds were unfixed or fixed for autofluorescence.
RESULTS
Germination response to treatments
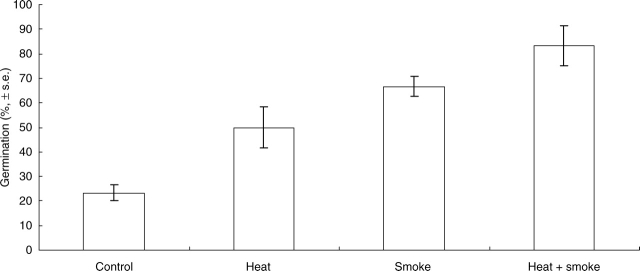
Untreated seeds showed some germination (23 % of the seed lot, Fig. 1). The fire cues increased germination independently and additively, heat increasing germination to 50 % and smoke to 67 % (Fig. 1; heat main effect, F1,20 = 11·27, P = 0·003; smoke main effect, F1,20 = 35·27, P < 0·001). Seeds that received both smoke and heat showed approx. 80 % germination (Fig. 1).
Fig. 1.
Mean germination of Grevillea linearifolia seeds that were untreated (control), or exposed to heat shock, smoke or heat and smoke combined.
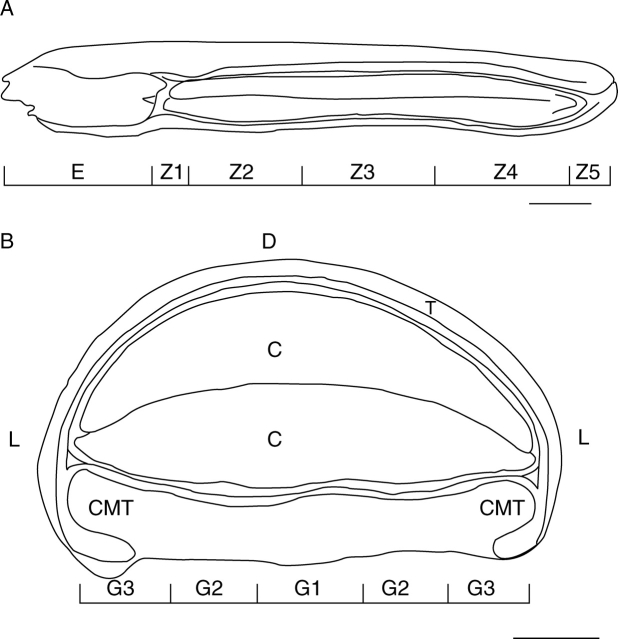
Zonation of the seed coat
During the initial LY tracing experiments, characteristic differences in depth of diffusion of the dye into the seed coat were detected. This occurred both longitudinally along the seed (termed zones, Fig. 2A) and transversely in the ventral groove (termed regions, Fig. 2B). In thin-section view, region G1 in zones 1, 2 and 4 had two layers of columnar cells in the exotesta increasing to three or four layers in zone 3, while regions G2 and G3 usually only had one; exotestal cells of G3 were curved rather than straight (Fig. 3A). The cells forming the innermost layer of the mesotesta were distinct from those forming the other mesotestal layers. They were small with thin, tightly appressed radial walls and were filled with a brown pigment.
Fig. 2.
(A) Map diagram showing the longitudinal zonation of the seed. Scale bar = 1 mm. (B) Map diagram showing the regions of the seed in transverse section. Scale bar = 0·5 mm.
Fig. 3.
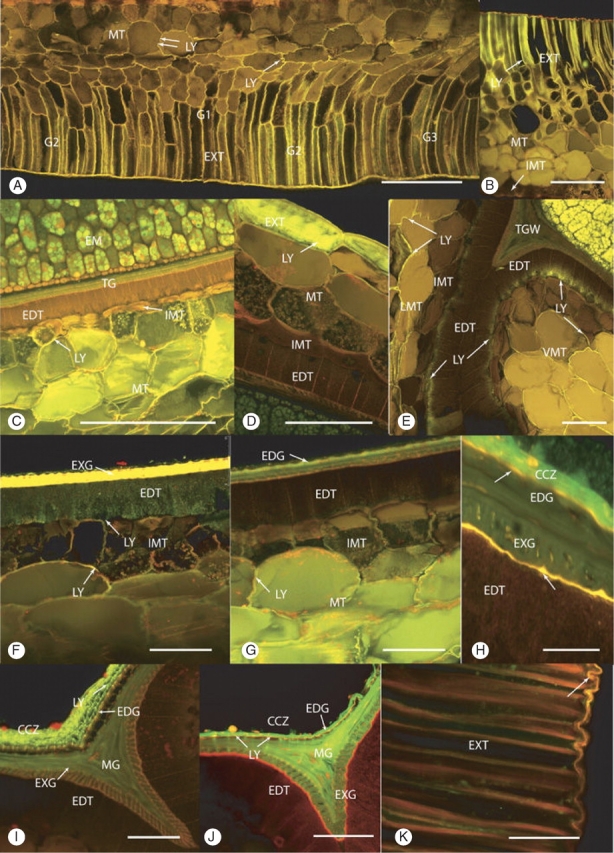
(A–C) Cross-sections showing the movement of Lucifer Yellow (LY) into the seed following 24-h immersion. (A) Heat- and smoke-treated seed, zone 2, ventral. LY had penetrated up to the innermost layer of the mesotesta (double arrows) in the G1 and G2 regions. There are two to three layers of columnar-shaped cells in the G1 region. These cells are probably exotestal in origin. The intensity of LY-induced fluorescence is greatest in the columnar exotestal cells, decreasing in the outer squat-shaped mesotestal cells (single arrow) and is weakest further inwards (double arrows). Scale bar = 250 µm. (B) Control seed, ventral. LY-induced fluorescence was found halfway into the mesotestal layer of the testa. Scale bar = 180 µm. (C) Heat- and smoke-treated seed, zone 3, ventral, merged image. LY had penetrated through the mesotesta reaching but not entering the innermost mesotestal layer (IMT). Embryo (EM), endotesta (EDT), tegmen (TG), mesotesta (MT). Scale bar = 250 µm. (D–G) Cross-sections through whole, intact seeds that were immersed in Lucifer Yellow for 48 h. (D) Control seed, zone 3, dorsal. LY has diffused beyond the exotesta into the outer mesotestal layer. Endotesta (EDT), exotesta (EXT), innermost mesotestal layer (IMT), mesotesta (MT). Scale bar = 50 µm. (E) Control seed, zone 2, ventral G3. LY penetrated through the lateral mesotesta (LMT) into the outer part of the endotesta cells, forming the neck region of the endotesta wing (WEDT). LY has also diffused through the curved area of the ventral groove into the endotesta. Endotesta (EDT), innermost mesotestal layer (IMT), tegmal wedge (TGW). Scale bar = 100 µm. (F) Smoke-treated seed, zone 3, ventral, G1. LY penetrated through the mesotesta and into the endotesta (EDT). Innermost mesotestal layer (IMT), exotegmen (EXG). Scale bar = 50 µm. (G) Smoke-treated seed, zone 3, ventral, G1. LY has diffused through the mesotesta (MT) and entered the radial walls of the innermost layer of the mesotesta (IMT), but has not progressed into the abutting endotesta (EDT). Scale bar = 50 µm. (H–K) Cross-section of seeds that were halved, the embryo carefully removed and Lucifer Yellow applied to the cavity for 24 h of outward diffusion. (H) Heat- and smoke-treated seed, zone 3, G1, counterstained with Nile Red. LY was confined to the crushed cell zone (CCZ). The two inner cuticles (arrows) fluoresced gold. Endotegmen (EDG), exotegmen (EXG), endotesta (EDT). Scale bar = 25 µm. (I) Control seed, zone 3, tegmal wedge. LY is retained in the crushed cell zone (CCZ) with no passage through the inner cuticles. Endotegmen (EDG), exotegmen (EXG), endotesta (EDT), mesotegmen (MG). Scale bar = 50 µm. (J) Heat- and smoke-treated seed counterstained with Nile Red, zone 3, tegmal wedge. LY was present in the crushed cell zone (CCZ) and within the tegmal layers. Stronger lines of LY indicated where the dye has moved into the tegmen via cuticular pores (arrows). A break in the CCZ and the inner cuticle also permitted movement of the dye into the tegmen. The thicker inner cuticle (large arrow) between the endotesta and the exotegmen fluoresces red rather than orange. Endotesta (EDT), endotegmen (EDG), mesotegmen (MTG), exotegmen Scale bar = 50 µm. (K) Control seed, unfixed, zone 1, ventral, G1, stained with Nile Red. The suberized walls of the exotestal (EXT) cells strongly fluoresce red. The overlying outer cuticle (arrow) fluoresce orange gold. Scale bar = 50 µm.
Autofluorescence
The intensity of autofluorescence depended upon the laser power and gains selected, but when reduced to the setting used to collect the images, autofluorescence was negligible and/or clearly different from the emission colour induced by the dye. There was no difference in autofluorescence colours between fixed and unfixed material.
Distribution of Lucifer Yellow CH following 24 and 48 h inwards diffusion into intact seeds
After 24 h immersion in LY, the dye had not passed beyond the middle layer of the mesotesta in any of the control or treated seeds. At the eliosome end, the dye was found in the walls of the oil-filled cells occupying the centre of the eliosome, and in the walls of the adjacent inner tegmal cells. The dye did not penetrate beyond the hypostase. In the dorsal and lateral parts of the seed, it fluoresced intensely in the walls of the squat-shaped exotestal cells; where this layer was broken it was also present in the walls of a few adjacent mesotestal cells. In the ventral groove, the walls of the columnar-shaped exotestal cells exhibited intense LY-induced fluorescence, and in the treated (Fig. 3A) and control seeds (Fig. 3B), walls in the outer one to three layers of mesotestal cells also fluoresced. The depth of diffusion by LY was not consistent between the treatments nor between seeds from the same treatment, nor along the length of the same seed nor along the ventral groove (Fig. 3A). The greatest penetration into the mesotesta usually occurred in zones 2 and 3 (Fig. 3C).
After 48 h inward diffusion, LY still had not reached the embryo or tegmen layers in either the control or the treated seeds. In the control seeds, there had been further inward movement of LY through the dorsal and lateral surfaces (Fig. 3D), but again it was variable along the length of the seed and between seeds. In the ventral groove, the depth of penetration by the dye was variable along the groove. In only one seed were traces of the dye found in the endotestal layer and then only in the outer third of the cell (Fig. 3E).
In seeds from the three treatments, LY had also penetrated further into the testa, but again there was variation between seeds from the same treatment and, furthermore, there was no clear difference between the three treatments. Of all of the seeds examined, in only one heat-treated and one smoke-treated seed (20 min smoke treatment) was LY present in the ventral endotesta overlying regions G1 and G2 (Fig. 3F), but traces were found in the radial walls of the innermost layer of the mesotesta (i.e. layer 5) in one smoke-treated (Fig. 3G) and two heat + smoke (20 min smoke)-treated seeds. In one heat-treated seed, LY had diffused throughout the curved part of the G3 region and into the abutting endotestal cells; it had also diffused through the adjacent lateral mesotestal cells and entered the abutting endotestal cells (not shown). The longitudinal zonation pattern of LY diffusion found in the control seeds was repeated in each of the three treatments.
Distribution of Lucifer Yellow CH following 24 h outwards diffusion from the embryo cavity
Outward diffusion by LY was stopped either in the crushed cell zone (Fig. 3H) or in the tegmal layer (Fig. 3I, J); no dye was found outside of the outer of the two inner cuticles nor within the abutting endotestal cells. There was a fairly consistent pattern exhibited by all seeds examined from each of the three treatments and the control. In the control seeds, LY was largely confined to the crushed cell layer lying along the ventral innermost inner cuticle (Fig. 3I). Transverse sections from zones 3 and 4 of treated seeds revealed that the dye was located solely within the crushed cell zone in regions G1 and G2 (Fig. 3H), but in region G3 it was also found within the mesotegmal part of the wedge tissue; it was not present in the exotegmen or abutting endotesta (Fig. 3J). In these seeds, the dye had crossed the innermost cuticle either where it was broken or through cuticular pores (Fig. 3J). Longitudinal sections incorporating zones 3–5 revealed that the dye gradually penetrated further outward into the exotegmal layer (zone 4) until, in zone 5, it had penetrated through the entire tegmen except at the micropyle. Medial LS through the micropyle showed no LY in the exotegmen, micropyle passage or abutting endotestal cells (not shown).
Staining with Nile Red for cuticles, suberin and neutral lipids
Nile Red stained the outer cuticle overlying the ventral groove (Fig. 3K) and the two inner cuticles (Fig. 3H, J) an intense gold, orange or red, depending on the excitation wavelengths used. The external cuticle overlying the lateral and dorsal surfaces was minimal, fragmentary or absent in the controls (Fig. 4A) and treated seeds (Fig. 4B). The thickened suberized walls of the squat (Fig. 4A, B) and columnar (Fig. 3K) exotestal cells fluoresced red. As in the earlier study (Briggs et al., 2005), one inner cuticle was located between the testa and tegmen, the other on the inner surface of the tegmen (Fig. 3H).
Fig. 4.
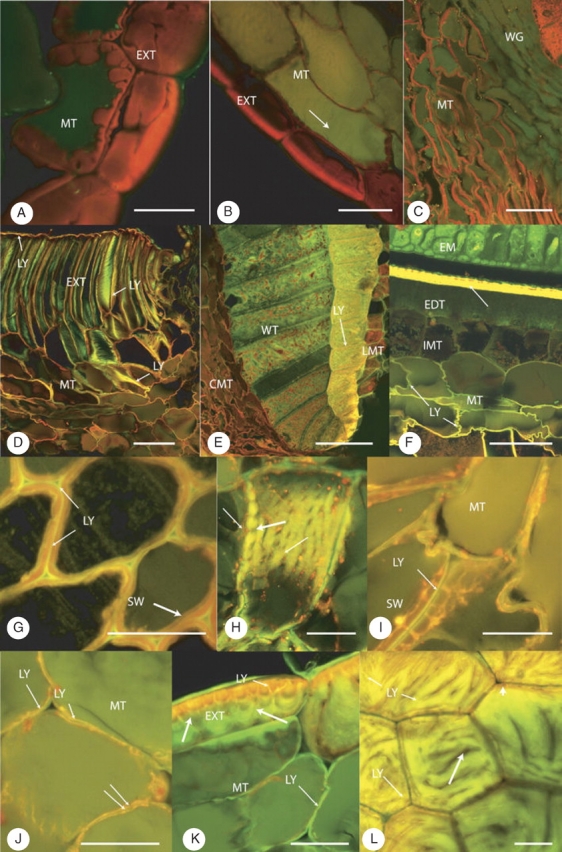
(A) Control seed, fixed, hypostase zone 1, dorsal, stained with Nile Red. Strong red fluorescence from the suberized walls of the squat-shaped exotestal cells (EXT) and underlying outer mesotestal cells (MT). Unsuberized walls show no fluorescence. There is no sign of an overlying cuticle. Scale bar = 25 µm. (B) Heat- and smoke-treated seed, fixed, zone 2, lateral, stained with Nile Red. Strong red fluorescence from the thick walls of the exotestal (EXT) cells, weaker from the thinner suberized walls of the adjacent mesotestal cells (MT). No outer cuticle is present. The unsuberized walls are not fluorescent but there is aldehyde-induced fluorescence from the vacuolar contents (arrow). Scale bar = 50 µm. (C) Control seed, fixed, zone 1 hypostase, ventral, G3, stained with Nile Red. Suberized mesotestal (MT) walls stained red, but unsuberized mesotestal walls are unstained. Strong red fluorescence from the wing endotesta cells (WG). Scale bar = 75 µm. (D–F) Seeds immersed in Lucifer Yellow for 48 h, rinsed, fixed and sections counterstained with Acridine Orange. (D) Control seed, zone 4/5, ventral, G3. Laser line, 488 nm; PMT1, 500–598 nm; PMT2, 590–740 nm. Lucifer Yellow (LY) is trapped between the outer cuticle and the suberized outer tangential walls (red/brown/orange) of the exotestal cells (arrow). When LY is seen in face view against the suberized walls, it appears more green than yellow (arrows). Under these settings the unsuberized walls of the mesotesta show an orange-brown colour. Scale bar = 100 µm. (E) Heat-treated seed, zone 4/5, G3. Laser line, 488 nm; PMT1, 500–589 nm; PMT2, 590–740 nm; Z projection, 34 steps, 0·75-μm step width. Wing endotesta (WT) adjacent to the raphe vascular trace. Lucifer Yellow (LY) has diffused through the walls of the lateral mesotesta (LMT) into the radial and tangential walls of the outer layer of cells forming the endotestal wing. No diffusion of Lucifer Yellow occurred beyond these cells into the remaining wing cells. The red-brown fluorescence of the cells in the curved mesotestal (CMT) region indicates a lack of penetration by LY beyond the ventral exotesta. Under these settings, where Lucifer Yellow was absent in the wing region, the lignin component of the internal lacework wall system and radial walls fluoresced green; pigment in the wing cells and mesotestal cells fluoresced red-brown. Scale bar = 75 µm. (F) Smoke-treated seed, zone 3, G1. Inner half of the testa and abutting tegmen. Lucifer Yellow (LY) had diffused through the mesotesta reaching, but not entering, the innermost mesotestal layer (IMT). The counterstaining by Acridine Orange of thin-walled, unsuberized mesotestal cells has altered the usual yellow colour of Lucifer Yellow to pale orange-brown in some parts of the wall. Mesotesta (MT), endotesta (EDT), embryo (EM), tegmen (TG). Scale bar = 50 µm. (G–L) Immersed in Lucifer Yellow for 24 h, fixed, cross-sectioned and sections counterstained with Nile Red. (G) Smoke-treated seed, zone 3. Tangential view through the columnar exotestal cells. Lucifer Yellow (LY) was only found in the outer wall layer overlying the suberized secondary wall (SW), which fluoresces red following staining with Nile Red. No Lucifer Yellow was preserved in the intercellular spaces by fixation. Scale bar = 25 µm. (H) Smoke-treated seed, zone 3, G1. Columnar-shaped mesotestal cell showing the presence of Lucifer Yellow in the primary wall but not in the plasmodesmata (large arrow). The red autofluorescent material (double arrows) present in the peripheral layer of the cytoplasm can be seen associated with some of the plasmodesmata. Scale bar = 25 µm. (I) Control seed, zone 2, G2. In suberized mesotestal cells, Lucifer Yellow (LY) is only present in the outer, thin primary wall overlying the thickened secondary wall (SW), which fluoresced red with Nile Red. No Lucifer Yellow occurred in the intercellular spaces (arrow). Scale bar = 25 µm. (J) Heat-treated seed, zone 2/3, G3. Unsuberized mesotestal cell (MT). Lucifer Yellow (LY) was found in the inner part of the thin primary wall but not in the middle lamella (double arrows) or intercellular spaces (arrow). Scale bar = 25 µm. (K) Smoke-treated seed, zone 3, lateral position. The thickened secondary walls of the exotestal cells (EXT) is clearly lobed with the lobes separated by crevasses (arrows). Within the wall are narrow channels, unsuberized areas that stain for pectin. These ‘channels’ are filled with Lucifer Yellow (LY) and allow penetration by Lucifer Yellow to non-suberized parts of the secondary wall. Interaction between the Nile Red-induced red fluorescence and the yellow from that induced by Lucifer Yellow resulted in an orange fluorescence where Lucifer Yellow and suberin coincide. In this image, the presence of Lucifer Yellow in the walls of the abutting, non-suberized mesotestal cells (MT) fluoresces green. Scale bar = 25 µm. (L) Smoke-treated seed, zone 5, tangential scraping. Face view of outer tangential walls of several squat exotestal cells showing various levels through the secondary wall. The ‘channels’ (small arrow) are filled with Lucifer Yellow but the ‘crevasses’ (large arrow) are not. Some walls show the location of the suberin component that stains with Nile Red (arrowhead). Scale bar = 25 µm.
In some control and treated seeds, small groups of mesotestal cells in the dorsal (Fig. 4A, B) or lateral regions had thickened, red-fluorescent walls; in the ventral groove, mesotestal cells in the outer layers often had thickened, red-fluorescent walls (Fig. 4C). However, the extent of thick-walled mesotestal cells in the ventral groove varied between seeds and even within the various zones of the same seed.
Staining with Acridine Orange for cutin, lignin and negatively charged material
The fluorescence emission spectra induced by Acridine Orange depended upon the excitation wavelength and PMT ranges used. In unfixed control material excited with the 514-nm laser line, the suberized walls of the exotesta fluoresced yellow- red and the lignified walls of the wing endotesta fluoresced yellow; the unsuberized walls of the mesotestal, exotegmen and embryo also fluoresced yellow. However, when excited with the 488-nm laser line, the lignified walls fluoresced emerald-green. When LY-traced sections were counterstained with 0·1 % Acridine Orange and excited at 488 nm, the LY-induced yellow fluorescence could be clearly seen amongst the Acridine Orange-induced orange-red suberized walls of the exotesta (Fig. 4D) and the emerald-green fluorescent lignified walls and interior lacework of the wing endotesta (Fig. 4E). Where LY was present in the non-suberized walls of the mesotesta, the yellow fluorescence was often masked and the walls fluoresced orange-brown (Fig. 4F).
Location of LY in the apoplast and relationship to the suberized wall layer
LY-induced fluorescence was only found in the primary wall layer of the columnar exotestal cells, i.e. outside the suberized secondary wall (Fig. 4G, H). In the ventral mesotestal cells, LY-induced fluorescence occurred as a solid continuous line along the outer surface (or primary cell wall) of cells with a suberized secondary wall layer (Fig. 4I), but in non-suberized cells, it occupied the entire wall (Fig. 4J). In the lateral and dorsal mesotesta LY-induced fluorescence was again in the primary wall of non-suberized cells (Fig. 4K). In the squat-shaped exotestal cells, LY-induced fluorescence was in the thin outer primary wall layer and also within the non-suberized parts of the lamellated, suberized secondary wall (Fig. 4K, L).
DISCUSSION
The present study has confirmed that there are barrier(s) to the diffusion of high-molecular-weight compounds in the coat of untreated Grevillea linearifolia seeds, making them similar to dormant seeds of chaparral species such as Emmenanthe penduliflora. In E. penduliflora, this barrier retains a germination inhibitor inside the seed (Egerton-Warburton and Ghisalberti, 2001). There are other similarities between seeds of Grevillea and chaparral species: the external cuticle does not restrict entry of apoplastic dyes, and the seed coat is water-permeable (Keeley and Fotheringham, 1998; Briggs et al., 2005).
Seeds of G. linearifolia, however, differed from those of E. penduliflora in the structural location of the barrier(s) within the seed coat, and whether fire cues removed the barrier(s). The seed coat of E. penduliflora has a single integument and the barrier to diffusion was an inner cuticle, located on the inner surface of the seed coat adjacent to the endosperm (Egerton-Warburton, 1998). The seed coat of Grevillea, by contrast, had multiple barriers to diffusion of high-molecular-weight compounds; of particular importance were the two inner cuticles resulting from the two integuments of the seed coat. In G. linearifolia, the diffusion of LY outwards from the crushed cell layer through the seed coat was mostly stopped by the cuticle lining the inner surface of the tegmen; the dye appeared in the tegmen only where there were noticeable breaks in the inner cuticle or through cuticular pores near the tegmen wedge. It did not penetrate beyond the outer cuticle lying between the tegmen and endotesta. When LY diffused from the outside of the seed inwards, the barrier to diffusion lay further out in the seed coat than either internal cuticle. The innermost layer of the mesotesta was the greatest distance inwards that LY penetrated in most seeds; occasionally, the dye penetrated past this layer and into the outermost part of the endotesta. The distances for the dye to traverse were similar in the two species (approx. 200 µm across the seed coat of Emmenanthe; approx. 200–250 µm in the dorsal region of Grevillea seeds, and 550–600 µm in the ventral region). The lack of penetration of the dye into the seed coat of Grevillea was due to structural features, rather than differences in the distance of diffusion. The exception to the presence of multiple barriers in Grevillea occurs where the top ends of the cotyledons are adjacent to the hypostase, which at this location forms a single barrier to diffusion of LY to or from the embryo. The two integuments are not continuous around the embryo here (Briggs et al., 2005).
The fire cues did not remove the barriers to diffusion of high-molecular-weight compounds in the seed coat of Grevillea. In smoked seeds of E. penduliflora, LY appeared in the embryo after 2 h of exposure to the dye, as smoke scarified the external cuticle and opened up pores within the internal cuticle (Egerton-Warburton, 1998), allowing the germination inhibitor in the embryo to escape (Egerton-Warburton and Ghisalberti, 2001). In G. linearifolia, even after 48 h immersion in the dye, no LY penetrated through the seed coat to reach the embryo in smoked or heated seeds. While the fire cues did increase the penetration of the dye into the seed coat, so that the dye was found in the endotesta of several seeds, the barrier to diffusion remained. Nor did the permeability of the two internal cuticles in G. linearifolia change after exposure to smoke, as revealed in the outwards diffusion experiment. Furthermore, no penetration through the hypostase was detected after any of the three treatments. The germinability test of the Grevillea seeds showed that the heat and smoke treatments used increased germination, as observed in earlier experiments (Morris, 2000); thus, if the fire cues were breaking dormancy by a version of the Emmenanthe mechanism, this should have been detectable in the dye diffusion experiments. Neither smoke, nor heat nor their combination removed any of the barriers to diffusion in the seed coat of G. linearifolia.
There are other differences between the seeds of Grevillea and chaparral species (Keeley and Fotheringham, 1998; Egerton-Warburton, 1998). Seeds of Grevillea respond to both heat and smoke, whilst chaparral species respond to smoke only. A palisade-like layer (endotesta) is present in Grevillea, but is absent in the chaparral smoke-responders. A further difference between G. linearifolia and E. penduliflora is that the penetration by the dye through the seed coat was not uniform in the former, either along the seed or from any of the sides. This variability suggested a pattern of ‘zones’ and ‘regions’ in the seed coat structure. These penetration patterns appear to be related to (1) the distribution and location of suberized cells and (2) the variation in the number of layers in the ventral groove, particularly in zones 2 and 3 where the central corridor of cells (i.e. G1) often consisted of several layers of suberized, columnar-shaped cells. In E. penduliflora, such a zonation pattern was lacking and no suberized cells were reported by Egerton-Warburton (1998).
Suberin has been found in many plant organs as part of their normal development (Esau, 1977; Barnabas and Arnott, 1987; Barnabas, 1989; Franke et al., 2005) or as a consequence of wounding (Esau, 1977; Lulai and Corsini, 1998). Apart from the findings of Briggs et al. (2005) there are only a few reports of suberized tissues within the seed coat, and in these, suberin was deposited in the chalazal region to seal off the vascular trace (Zee and O'Brien, 1970; Espelie et al., 1980; Cochrane, 1983). Suberin is often deposited as lamellae, either throughout the entire wall or concentrated into bands in the radial walls (i.e. Casparian strips). In leaves of the sea grass Thalassodendron ciliatum, suberin is found throughout the cell wall and in the middle lamella between contiguous bundle sheath cells (Barnabas, 1989). In the seed coat of G. linearifolia, suberin was only deposited as layers in the secondary wall and not in the primary wall or middle lamella.
Suberized cell walls have three characteristics, i.e. they are tissue specific, and have a poly(aliphatic) (lipid) domain and a unique lignin-like poly(phenolic) domain (Kolattukudy, 1980; Bernards and Razem, 2001). The term ‘suberin’ has most frequently been used to define the poly(aliphatic) matrix, but most suberized tissues contain small amounts of covalently linked lignin (monolignols) as well as a significant amount of associated hydroxycinnamic acids. In our present study, the partitioning of Nile Red into the thickened mesotestal and exotestal walls clearly revealed the polyaliphatic lipid domain while similar localization of Acridine Orange revealed the negatively charged lignin-like polyphenolic domain. The abilities of the confocal laser scanning microscope were instrumental in separating both components, and together with these two dyes allows a clear, fluorescence-based method for the identification of suberin in plant tissues.
The multi-layered aliphatic polymer, with its associated waxes, is thought to provide suberized tissues with their water impermeability (Kolattukudy, 1980), and thus the permeability of tracer dyes through the wall would depend upon the extent of suberization within the wall (Barnabas, 1989, 1994). In Grevillea, the suberized secondary cell walls appeared to facilitate the inward movement of LY as non-suberized mesotestal cells retarded the inward movement during the first 24 h. In the ventral groove, the suberized exotestal and adjacent suberized mesotestal cells allowed LY to move rapidly into the testa but only to the extent of suberization of the tissue. In the lateral and dorsal sides, usually only the squat-shaped exotestal cells were suberized and hence the dye was restricted to this layer. However, as the squat-shaped exotestal cells had incomplete suberization of the secondary walls, LY was able to penetrate into the secondary wall layers and accumulate in the non-suberized, pectic portions, resulting in the intense fluorescence seen in these cells. Impedance to the dye was only overcome after a further 24 h of immersion (i.e. 48 h in total), which resulted in accumulation of the dye into the apoplast of the non-suberized mesotestal cells.
The slow movement by LY throughout the seed coat of Grevillea, especially in the thin-walled inner mesotestal layers, may be due to strong negative charges in the primary wall and middle lamella, as indicated by Acridine Orange staining and previous histochemical study (Briggs et al., 2005). Acridine Orange is a cationic metachromatic dye that binds to negatively charged groups such as found in DNA, RNA (Hepler and Gunning, 1998), lignin and cutin (Oparka and Read, 1994).
For Grevillea, the current study does not support a change in the permeability of the seed coat to high-molecular-weight compounds after exposure to either fire cue. Whether the embryo of Grevillea contains a germination inhibitor (as in Emmenanthe) is unknown, and looking for one was beyond the scope of the present study. The increased germination following heat, smoke or heat followed by smoke treatments may result from physical effects on the seed coat, or from physiological effects on the embryo (van Staden et al., 2000). Smoke can substitute for light requirements for germination in Grand Rapids lettuce seed (Drewes et al., 1995), and interact with phytohormones to affect germination (van Staden et al., 1995; Strydom et al., 1996). There is debate about whether smoke acts via hormones, with some evidence against this hypothesis; van Staden et al. (2000) argue that the widespread occurrence of the smoke response suggests that it emerged early in the evolution of seed plants, and acts on a trigger for germination at a fundamental stage to provide energy for the process. For Grevillea, we are currently investigating whether the fire cues affect the physical properties of the cell walls in the seed coat, and whether such changes are consistent with the ‘mechanical constraint’ model of seed coat dormancy.
ACKNOWLEDGEMENTS
We thank the University of Western Sydney Confocal and Bioimaging Facility, Hawkesbury Campus, for the use of their confocal microscopes and Dr Paul Thomas for carrying out the seed treatments and germination experiment. The support of the Australian Flora Foundation in this study is gratefully acknowledged.
LITERATURE CITED
- Auld TD, Denham AJ. The role of ants and mammals in dispersal and post-dispersal seed predation of the shrubs Grevillea (Proteaceae) Plant Ecology. 1999;144:201–213. [Google Scholar]
- Barnabas AD. Apoplastic tracer studies in the leaves of a seagrass. II. Pathways into leaf veins. Aquatic Botany. 1989;35:375–386. [Google Scholar]
- Barnabas AD. Apoplastic and symplastic pathways in leaves and roots of the seagrass Halodule uninervis (Forssk.) Aschers. Aquatic Botany. 1994;47:155–174. [Google Scholar]
- Barnabas AD, Arnott HJ. Zostera capensis Setchell: root structure in relation to function. Aquatic Botany. 1987;27:309–322. [Google Scholar]
- Bernards MA, Razem FA. The poly(phenolic) domain of potato suberin: a non-lignin cell wall bio-polymer. Phytochemistry. 2001;57:1115–1122. doi: 10.1016/s0031-9422(01)00046-2. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. 2nd edn. New York: Plenum Press; 1994. pp. 206–214. [Google Scholar]
- Briggs CL, Morris EC, Ashford AE. Investigations into seed dormancy in Grevillea linearifolia, G. buxifolia and G. sericea: anatomy and histochemistry of the seed coat. Annals of Botany. 2005;96:965–980. doi: 10.1093/aob/mci250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane MP. Morphology of the crease region in relation to assimilate uptake and water-loss during caryopsis development in barley and wheat. Australian Journal of Plant Physiology. 1983;10:473–491. [Google Scholar]
- Drewes FE, Smith MT, van Staden J. The effect of a plant-derived smoke extract on the germination of light-sensitive lettuce seed. Plant Growth Regulation. 1995;16:205–209. [Google Scholar]
- Edwards W, Whelan R. The size, distribution and germination requirements of the soil-stored seed-bank of Grevillea barklyana (Proteaceae) Australian Journal of Ecology. 1995;20:548–555. [Google Scholar]
- Egerton-Warburton L. A smoke-induced alteration of the sub-testa cuticle in seeds of the post-fire recruiter, Emmenanthe penduliflora Benth. (Hydrophyllaceae). Journal of Experimental Botany. 1998;49:1317–1327. [Google Scholar]
- Egerton-Warburton L, Ghisalberti EL. Isolation and structural identification of a germination inhibitor in fire-recruiters from the Californian chaparral. Journal of Chemical Ecology. 2001;27:371–382. doi: 10.1023/a:1005688623977. [DOI] [PubMed] [Google Scholar]
- Esau K. Anatomy of seed plants. 2nd edn. Sydney: John Wiley and Sons; 1977. [Google Scholar]
- Espelie KE, Davis RW, Kolattukudy PE. Composition, ultrastructure and function of the cutin-containing and suberin-containing layers in the leaf, fruit peel, juice-sac and inner seed coat of grapefruit (Citrus Paradisi Macfed.) Planta. 1980;149:498–511. doi: 10.1007/BF00385755. [DOI] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L. Apoplastic polyesters in Arabidopsis surface tissues – A typical suberin and a particular cutin. Phytochemistry. 2005;66:2643–2658. doi: 10.1016/j.phytochem.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Hepler PK, Gunning BES. Confocal fluorescence microscopy of plant cells. Protoplasma. 1998;201:121–157. [Google Scholar]
- Keeley JE, Fotheringham CJ. Trace gas emissions and smoke-induced seed germination. Science. 1997;276:1248–1250. [Google Scholar]
- Keeley JE, Fotheringham CJ. Smoke-induced seed germination in California chaparral. Ecology. 1998;79:2320–2336. [Google Scholar]
- Kenny B. Influence of multiple fire-related germination cues on three Sydney Grevillea (Proteaceae) species. Austral Ecology. 2000;25:664–669. [Google Scholar]
- Kolattukudy PE. Biopolyesters of plants: cutin and suberin. Science. 1980;208:990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Lulai EC, Corsini DL. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound healing. Physiological and Molecular Plant Pathology. 1998;53:209–222. [Google Scholar]
- Morris EC. Germination response of seven east Australian Grevillea species (Proteaceae) to smoke, heat exposure and scarification. Australian Journal of Botany. 2000;48:179–189. [Google Scholar]
- Morris EC, Tieu A, Dixon K. Seed coat dormancy in two species of Grevillea (Proteaceae) Annals of Botany. 2000;86:771–775. [Google Scholar]
- Morrison DA, Morris EC. Pseudoreplication and the design of seed germination experiments. Austral Ecology. 2000;25:292–296. [Google Scholar]
- Olde P, Marriott N. The Grevillea book. Vol. 1. Portland, OR: Timber Press; 1995. [Google Scholar]
- Oparka JK, Read ND. The use of fluorescent probes for studies of living plant cells. In: Harris N, Oparka KJ, editors. Plant cell biology: a practical approach. Oxford: IRL Press at Oxford University Press; 1994. pp. 27–50. [Google Scholar]
- van Staden J, Jäger AK, Strydom A. Interaction between a plant-derived smoke extract, light and phytohormones on the germination of light-sensitive lettuce seeds. Plant Growth Regulation. 1995;17:213–218. [Google Scholar]
- van Staden J, Brown NAC, Jager AK, Johnson TA. Smoke as a germination cue. Plant Species Biology. 2000;15:167–178. [Google Scholar]
- Strydom A, Jäger AK, van Staden J. Effect of a plant-derived smoke extract, N6-benzyladenine and gibberellic acid on the thermodormancy of lettuce seeds. Plant Growth Regulation. 1996;19:97–100. [Google Scholar]
- Zee SY, O'Brien TM. Studies on the ontogeny of the pigment strand in the caryopsis of wheat. Australian Journal Biological Science. 1970;23:1153–1171. [Google Scholar]