Abstract
Background and Aims
In andromonoecious taxa with separate floral types along the inflorescence, architectural or plastic effects can simulate floral sexual dimorphism. Both the primary and secondary sexual characteristics of the cyathia of the protogynous andromonoecious species Euphorbia nicaeensis were analysed according to their sex and arrangement on the inflorescence.
Methods
The production of male and hermaphrodite cyathia at each inflorescence level was surveyed in two natural populations. The longevity, size, pollen production and viability, and nectar secretion of both types of cyathia were checked between inflorescence levels and between sexes at the only level at which they occur together. This sampling method makes it possible to know whether differences between cyathia types are based on sex or are attributable to inflorescence development.
Key Results
Male cyathia were produced predominantly at the first and second inflorescence levels, whereas at levels 3–5, the cyathia were almost exclusively hermaphrodite. Viable pollen production by male cyathia at the second inflorescence level was higher than that of hermaphrodite cyathia at the third level but, when males and hermaphrodites at the same level were compared, their pollen production was similar. Male and hermaphrodite cyathia were similar in size, irrespective of the inflorescence level, although the exclusively hermaphrodite cyathia of the last level were smaller. Both cyathium types produced similar amounts of sugar. However, male cyathia produced nectar during their whole lifespans, whereas hermaphrodites produced it exclusively during their male phase. Moreover, the nectary activity of male cyathia started earlier in the day than that of hermaphrodites.
Conclusions
An apparent floral dimorphism exists in the primary sexual characteristics of Euphorbia nicaeensis because differences in pollen production between cyathium types are due to theirs positions. Similarly, differences affecting most secondary sexual characteristics are only apparent between the two cyathium types. However, E. nicaeensis shows a true but slight floral dimorphism in some of the secondary sex characters related to nectar secretion. The lack of nectar production by the female phase of the hermaphrodite cyathia of E. nicaeensis indicates that this is a deceit-pollinated species.
Key words: Andromonoecy, cyathia longevity, deceit pollination, Euphorbia nicaeensis, Euphorbiaceae, inflorescence architecture, nectar secretion, positional effects, protogyny, viable pollen production, sexual dimorphism
INTRODUCTION
Andromonoecy is a sexual system of plants in which an individual bears both staminate and perfect flowers (Yampolsky, 1920). This is a rare sexual system, which appears in only 1·7 % of all angiosperms (Yampolsky and Yampolsky, 1922), but it is widespread in particular groups (Lovett Doust, 1980; Arroyo, 1981; Whalen and Costich, 1986; Anderson and Symon, 1989; Koul et al., 1993; Narbona et al., 2002). Bertin (1982) suggested several hypotheses to explain the evolution of andromonoecy. The best-supported involves an allocation of resources by which staminate flowers prevent the outlay of resources for initial fruit development, because in hermaphrodite flowers, many fruits abort during development (Primack and Lloyd, 1980). Furthermore, the development of staminate flowers may have two selective advantages. First, they provide a pollen surplus that enhances male fitness (Bertin, 1982; Anderson and Symon, 1989; Podolsky, 1993; Connolly and Anderson, 2003). Thus, in the andromonoecious Solanum carolinense, male reproductive success was enhanced by an increase in the proportion of male flowers (Elle and Meagher, 2000). Secondly, staminate flowers increase floral display that improves pollinator attendance, which in turn benefits female fitness (Bertin, 1982; Anderson and Symon, 1989; Spalik, 1991).
Because staminate and perfect flowers have different functions in andromonoecious species, it can be asked whether both flower types make different male investments. If staminate flowers are important in pollinator attraction, more staminate flowers with fewer stamens or less pollen would be expected (Bertin, 1982; Spalik and Woodell, 1994). However, on the contrary, staminate flowers could be expected to show specialization in male function, investing in higher pollen production (Anderson and Symon, 1989; Spalik and Woodell, 1994). Some studies have shown that staminate flowers yield less pollen than perfect ones (e.g. Emms, 1993; Podolsky, 1993; Spalik and Woodell, 1994) but, in others, the reverse pattern has been observed (e.g. Anderson and Symon, 1989; Huan, 2003; Ortiz et al., 2003; Narbona et al., 2005), and similar pollen production between flower types has also been reported (e.g. Anderson and Symon, 1989; Diggle, 1993; Schlessman and Graceffa, 2002; Cuevas and Polito, 2004). In the few cases in which pollen viability has been measured, the pollen of staminate flowers was more viable than that of perfect flowers (Traveset, 1995; Narbona et al., 2005) or both flower types showed similar pollen viability (Cuevas and Polito, 2004). The existence of these differences based on sex between flowers of the same species is termed ‘floral dimorphism’. However, this phenomenon can also affect secondary sexual characteristics, such as nectar production and corolla size (Yampolsky, 1920; Delph, 1996).
Sexual dimorphism is a frequent phenomenon, which has traditionally been explained by both a ‘non-functional’ hypothesis, which presupposes developmental correlations between the stamens and corolla (Plack, 1957; Weiss and Halevy, 1989), and a ‘functional’ hypothesis, which postulates that selection for male function or for the protection of the developing reproductive organs drives the evolution of the sexual dimorphism (Assouad et al., 1978; Uno, 1982; Bell, 1985; Eckhart, 1991; Delph, 1996; Delph et al., 1996). Recently two new and alternative hypotheses have been proposed, which include the effects of inflorescence architecture (Diggle, 2003) and the effects of phenotypic plasticity during development (Diggle, 1993). Both the flower position in the inflorescence and the inflorescence position in the plant directly affect the floral characteristics (Diggle, 1995). Thus, an apparent sexual dimorphism could arise if floral types occur at different positions in the inflorescence (Diggle, 2003; Miller and Diggle, 2003). Furthermore, if floral types are produced at different times, one of them may be more subject to plastic variation because of an exhaustion of resources (Diggle, 1997; Gibbs et al., 1999; Ashman and Hitchens, 2000; Buide, 2004), and this variation could cause an apparent sexual dimorphism (Diggle and Miller, 2004). Alternatively, placement of floral types at different positions along the inflorescence could be interpreted as an evolutionary mechanism to promote floral dimorphism (Meagher, 2007).
The genus Euphorbia has unisexual flowers grouped into characteristic pseudanthia called cyathia. The cyathium is composed by an involucre, which contains a central pistillate flower surrounded by five groups of staminate flowers (Prenner and Rudall, 2007). The pistillate flower of the cyathium develops before the male ones; thus each cyathium is functionally a protogynous hermaphrodite flower (Prenner and Rudall, 2007). The involucre is generally surrounded by four marginal nectaries, which produce highly concentrated nectar (Ehrenfeld, 1976; Narbona et al., 2005). The cyathia are arranged in compound pleiochasia – each pleiochasial branch forms several pleiochasia or dichasia, which bloom sequentially (Weberling, 1986). Some species of Euphorbia are functionally andromonoecious in that they produce both male and hermaphrodite cyathia (Eichberger, 2001; Narbona et al., 2002, 2005). The male cyathia are produced at the beginning and, in some cases, at the end of the flowering period, causing interfloral dichogamy because the inflorescence has an acropetal development (Narbona et al., 2002, 2005). The simultaneous presence of functional andromonoecy and intra- and inter-floral dichogamy establishes secondary sexual differentiation at two levels: between the cyathia of different sexes, and between the sexual phases of the hermaphrodite cyathia. In E. boetica, a species that develops up to eight inflorescence levels, a true dimorphism affecting its primary sexual characteristics and related to sexual function appears at the lower levels of the inflorescence, whereas an apparent size dimorphism arising from positional effects occurs in the upper positions (Narbona et al., 2005).
Euphorbia nicaeensis is an andromonoecious perennial spurge that produces male cyathia exclusively at the lower levels of the inflorescence (Narbona et al., 2002). Here, both the primary and secondary sexual characteristics of the cyathia of this species are investigated, according to their sex and their arrangement on the inflorescences, to determine whether a sexual dimorphism exists. The specific objectives are as follows: (1) to confirm the pattern of production of male cyathia in the inflorescence; (2) to assess the longevity of the male and hermaphrodite cyathia; (3) to determine whether there are differences in the involucre and nectary sizes of both types of cyathia; (4) to analyse pollen production, viability and presentation in the two types of cyathia; and (5) to compare nectar secretion between both types of cyathia and between the sexual phases of the hermaphrodite cyathia.
MATERIALS AND METHODS
Study species and study areas
Euphorbia nicaeensis All. subsp. nicaeensis is a perennial, herbaceous spurge with a circum-Mediterranean distribution; it prefers calcareous soils (Benedí et al., 1997). This species grows in dry, sunny places, in degraded stages of Mediterranean Quercus forests (Benedí et al., 1997). Flowering takes place in early summer, and dispersion in late summer. Euphorbia nicaeensis is functionally andromonoecious and presents cyathia arranged in compound pleiochasia with several levels of branching (Narbona et al., 2002).
Two populations of E. nicaeensis were studied in south-western Spain: La Camilla and Aracena. The La Camilla population is in the Sierra de Grazalema Natural Park (Province of Cadiz, 36 °47′N, 5 °24′W), at an altitude of 800 m on Jurassic dolomites. The vegetation consists of sparse scrub with very scattered trees. Most of the trees are Quercus ilex and Ceratonia siliqua; the scrub layer comprises Juniperus oxycedrus, J. phoenicea, Pistacia lentiscus and Ulex baeticus. The Aracena population is situated in the province of Huelva (37 °53′N, 6 °33′W), at an altitude of 700 m on Cambrian decalcified limestones. The vegetation consists of a cultivated woodland of Castanea sativa with shrub species such as Viburnum tinus, Daphne gnidium and Phlomis purpurea. The two localities have a typical dry-summer Mediterranean climate, with average annual precipitation of 966 mm and 1025 mm (La Camilla and Aracena, respectively).
Methods
Inflorescence development and the proportion of male and hermaphrodite cyathia were monitored in three branches from 23–29 plants from each population during June–July, 1999. Each branch developed a compound pleiochasium, and ‘level 1’ was assigned to the terminal cyathium (i.e. the first to be produced) of the inflorescence and consecutive numbers to the successive levels of branching (for more details, see Narbona et al., 2002, 2005). Because E. nicaeensis reproduces vegetatively, plants separated by >2 m, along two 100-m transects in each population were selected. Each plant was marked at the beginning of flowering and was visited every 7–10 d, when the numbers of male and hermaphrodite cyathia at each level of the inflorescence were noted.
All the following studies were made only in the La Camilla population. In ten plants, the main axes (length and width) of both the cyathium involucre and one of the nectaries were measured, noting the sexuality of the cyathia and their positions on the inflorescence. The duration of anthesis in the hermaphrodite and male cyathia was monitored for 111 marked cyathia on 15 plants. These cyathia were examined daily, and their floral phase and, where applicable, the number of stamens exposed was noted. The receptivity of the stigma was determined in flowers at different stages of their development using the hydrogen peroxide technique (Kearns and Inouye, 1993).
The daily cycle of pollen presentation was studied in 113 cyathia of eight plants, which were examined every 2 h from 0800 h to 2000 h, and the numbers of exposed stamens with pollen were noted. Thirty cyathia that had finished flowering were also collected and dissected to determine how many stamens had remained unexposed. A cyathium was considered to have finished flowering when no stamen had been exposed for three consecutive days.
Because all the cyathia at an inflorescence level in each plant were the same sex, the comparison of pollen production between sexes and within plants was only possible across different levels. Thus, in every plant, pollen production was estimated separately in male and hermaphrodite cyathia from inflorescence levels 2 and 3, respectively. To compare pollen production between male and hermaphrodite cyathia at the same inflorescence level, different plants were used, collecting cyathia from level 2, the only level at which both types of cyathia occur. Pollen production per cyathium was estimated from the number of stamens per cyathium and the number of pollen grains per stamen. The number of stamens was counted in 20 cyathia of each sex on ten plants, and in ten hermaphrodite cyathia from inflorescence level 2 of another five plants. In each of these cyathia, the pollen grains of two stamens were counted. Each stamen was dissected on a slide, and all its pollen grains were counted under a microscope.
Pollen viability was analysed in six plants, and the male (level 2 on the inflorescence) and hermaphrodite cyathia (level 3) of each plant were considered separately. Pollen viability was estimated by sowing the pollen on a solid nutritive medium in Petri dishes (Bar-Shalon and Mattson, 1977). For each plant, all the stamens available at the moment of sowing were used. After sowing, the dishes were kept for 6 h at 22–24 °C. A preliminary experiment showed that a longer incubation did not increase the rate of germination. Three or four samples (replicates) were taken from each dish, and the proportion of germinated pollen grains was determined as those in which the pollen tube was longer than the diameter of the grain. All the pollen grains in each replicate were counted. The average number was 221.
Nectar production was measured in 39 male and 46 hermaphrodite cyathia from level 2 of the inflorescence (four and five plants, respectively). Inflorescences were bagged in situ for 24 h using plastic bags to exclude insects. The volume and concentration of nectar were used to estimate the weight of sugar produced per cyathium (Cruden and Hermann, 1983). In the bagged inflorescences, it was observed that almost none of the hermaphrodite cyathia in the female phase produced nectar. To confirm this observation, a 100-m linear transect was made, along which the presence of nectar in the nectaries of the hermaphrodite cyathia in the female and male phases was observed, as well as in the male cyathia of three inflorescences chosen at random on 32 plants. The number of cyathia secreting nectar was counted at 1300 h on a sunny day in July. Because of the scarcity of hermaphrodite cyathia in the female phase that secreted nectar, the weight of sugar produced per cyathium was measured in only six cyathia (two plants). The activity of the nectaries throughout the day was monitored visually in 79 male and 110 hermaphrodite cyathia from level 2 of the inflorescences (five and four plants, respectively). These cyathia were examined at regular intervals from 0800 to 2000 h on two sunny days in June, and the numbers of nectaries presenting nectar on their surfaces were counted.
Statistical analysis
The numbers of inflorescence levels in the two study populations were compared using the Mann–Whitney test because data normalization with the usual transformation was not achieved (Zar, 1999). For the same reason, the comparison of the proportions of male and hermaphrodite cyathia between inflorescence levels was tested using the Kruskal–Wallis test, and differences between categories were resolved with the Nemenyi test for means separation (Zar, 1999). Differences in cyathia production per inflorescence between populations were analysed using one-way analysis of variance (ANOVA). The Mann–Whitney test was performed to determine differences in the duration of anthesis of the male cyathia and in the male phase of the hermaphrodite cyathia, and to determine differences in the stamen issue rate of both types of cyathia.
Differences in number of stamens, pollen production per stamen, and total number of pollen grains between male and hermaphrodite cyathia of inflorescence level 2 were tested using mixed-model ANOVAs where the factor ‘sex’ was fixed and the factor ‘plant’ was considered random and nested in ‘sex’; the data were logarithm transformed. The number of stamens and the total number of pollen grains of the male (level 2) and the hermaphrodite (level 3) cyathia of the same plant were compared using mixed-model ANOVAs, with ‘plant’ as random factor and ‘sex’ as fixed. Differences in pollen production per stamen between cyathia, sex and plants were analysed using mixed ANOVAs, in which the factor ‘plant’ was random, the factor ‘sex’ was fixed, and the factor ‘cyathium’ was nested in ‘plant’ and ‘sex’. Differences in pollen viability between male (level 2) and hermaphrodite (level 3) cyathia were tested using a mixed-model ANOVA where the factor ‘plant’ was considered random and the factor ‘sex’ was fixed; the data were transformed using arcsine.
Variations in the size of the cyathia and their nectaries according to sex (male versus hermaphrodite) and according to inflorescence levels were analysed using one-way multivariate ANOVA (MANOVA) tests, including width and length as predictor variables. A factorial MANOVA (‘sex’ and ‘inflorescence level’ as factors) was not possible because both types of cyathia do not occur at each level of the inflorescence. Alternatively, the sizes of the cyathia were compared between sexes at the only inflorescence level at which they occur together (level 2), using one-way MANOVA.
Comparison of the percentages of cyathia with active nectaries between male cyathia, hermaphrodite cyathia in the female phase, and hermaphrodite cyathia in the male phase were performed using the χ2 test for contingency table of percentages (Zar, 1999). Proportions of active nectaries throughout the day were compared between male and hermaphrodite cyathia using the χ2 test for contingency tables of percentages for more than two proportions (Zar, 1999). The characteristics of the nectar produced by male and hermaphrodite cyathia in male phase were compared using a mixed ANOVA, in which the factor ‘plant’ was random, and the factor ‘sex’ was fixed and nested in ‘plant’; the data were logarithm transformed. The amount of sugar produced by hermaphrodite cyathia in the female phase was compared with those of hermaphrodite cyathia in the male phase and with those of the male cyathia using the Mann–Whitney tests.
ANOVAs and MANOVAs were performed using STATISTICA 6·0 (GLM module) with Type III sums of squares (StatSoft, 2001). When the ANOVA/MANOVA showed significant differences, the means of groups were compared using the t-test with estimation of the separate variance (Welch test), as the variance of the groups was not equal (Day and Quinn, 1989). To control for the experiment-wise type I error produced by multiple comparisons, the sequential Bonferroni test for fitting the significance level was applied (Rice, 1989).
RESULTS
Distribution of male and hermaphrodite cyathia in the inflorescence
The number of inflorescence levels that developed in the La Camilla population ranged between three and four (median = 4, mode = 4), whereas it ranged between four and five in the Aracena population (median = 4, mode = 4). In general, plants from Aracena developed a higher number of inflorescence levels (U = 671·0, n1 = 54, n2 = 77, P < 0·0001) and higher total cyathia per inflorescence (F1,129 = 15·48, P < 0·001) than those of the plants from La Camilla (Table 1). Level 1 of the Aracena plants produces undeveloped cyathia. The maximum number of cyathia developed at levels 3 and 4 in La Camilla and at level 4 in Aracena (Table 1).
Table 1.
Cyathia production at each inflorescence level and at the whole inflorescence in the La Camilla and Aracena populations of E. nicaeensis
| Cyathia/inflorescence |
||||
|---|---|---|---|---|
| Population | Inflorescence level | Mean ± s.e. | Range | % inflorescences that produce cyathia |
| La Camilla | Level 1 | 1 ± 0·0 | 1–1 | 14 |
| Level 2 | 10·6 ± 0·23 | 3–27 | 100 | |
| Level 3 | 20·3 ± 0·57 | 2–54 | 100 | |
| Level 4 | 17·3 ± 2·15 | 7–42 | 61 | |
| Whole | 53·5 ± 4·17 | 24–118 | ||
| Aracena | Level 1 | – | – | 0 |
| Level 2 | 10·3 ± 0·35 | 7–19 | 61 | |
| Level 3 | 19·6 ± 0·55 | 8–38 | 100 | |
| Level 4 | 37·1 ± 1·40 | 7–74 | 100 | |
| Level 5 | 19·1 ± 3·57 | 3–56 | 26 | |
| Whole | 72·3 ± 5·07 | 28–144 | ||
% inflorescences that produce cyathia = percentage of total analysed inflorescences that produce cyathia in each inflorescence level.
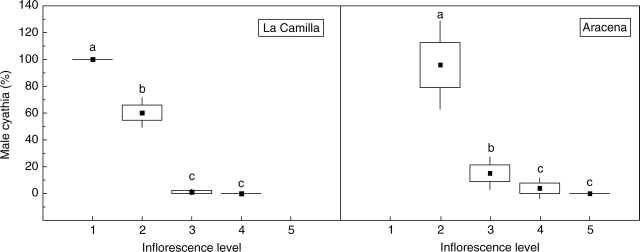
Hermaphrodite and male cyathia were found in both populations, and their proportions were statistically different between inflorescence levels (H = 80·0, P < 0·0001, d.f. = 3 in La Camilla; H = 72·5, P < 0·0001, d.f. = 3 in Aracena; Fig. 1). Female flowers were totally absent from the male cyathia. Most cyathia were male at the first level of the inflorescence that produced cyathia. At the next level, the cyathia were predominantly male in La Camilla, but predominantly hermaphrodite in Aracena. At the last two levels, the cyathia were almost exclusively hermaphrodite in both populations (Fig. 1). All cyathia at the same inflorescence level had the same sex in all studied inflorescences (e.g. at level 2 of one inflorescence, all cyathia were male or hermaphrodite, but never both). This pattern can be extended throughout the plant, i.e. all the inflorescences of a plant developed the same type of cyathia at the same inflorescence levels.
Fig. 1.
Male cyathia production in each inflorescence level in the two populations studied. Closed squares represent the mean, boxes represent s.e., and bars are 1·96 s.e. Values with different letters are significantly different at α < 0·05.
Dichogamy
The hermaphrodite cyathia were protogynous, and their female flowers were receptive for 1–5 d (mean = 2·9, mode = 3, n = 94). The female flower remained erect during its receptive period, and it later hung between two nectaries of the involucre. Once the female phase was over, the stamens issued from the cyathium involucre at a rate of 0–4 stamens daily (mean and mode = 2). Each stamen remained exposed for 1 d, usually dropping in the night or on the following morning when pushed out by the new stamen. The stamens emerged from the cyathium involucre between 0800 and 1000 h in the morning, and were open from this time to 2000 h, although little pollen remained after 1400 h. The male phase of the hermaphrodite cyathia lasted for 9–13 d (mean = 10·8, mode = 11). In the male cyathia, the duration of anthesis was 7–9 d (mean and mode = 9), and this was significantly less than that of the male phase of the hermaphrodite cyathia (U = 346, n1 = 17, n2 = 94, P = 0·0003). However, in male cyathia, the daily stamen issue rate ranged from 0 to 5 (mean = 2·4, mode = 3), and it was significantly higher than that of the hermaphrodite cyathia (U = 12418, n1 = 283, n2 = 106, P = 0·007). All the stamens produced were exposed in both types of cyathia.
Pollen production and viability
Male and hermaphrodite cyathia at level 2 of the inflorescence produced statistically similar numbers of stamens (mean ± s.e. = 19 ± 0·5 for both types; F1,15 = 0·06, P = 0·81), of pollen grains per stamen (843 ± 30 for male and 800 ± 16 for hermaphrodite; F1,10 = 0·30, P = 0·60) and, consequently, of pollen per cyathium (15288 ± 524 for male and 14844 ± 646 for hermaphrodite; F1,10 = 0·18, P = 0·68).
When male cyathia at level 2 were compared with hermaphrodite cyathia at level 3 in the same plants, the former produced an average of four stamens more than the latter (Fig. 2), and these differences were statistically significant (Table 2). The average number of stamens per cyathium was similar between plants, but there was a significant interaction between sex and plant (Table 2), indicating that this difference between the sexes was much more marked in some plants. The number of pollen grains per stamen was 835 ± 33 in male cyathia and 647 ± 21 in hermaphrodite cyathia, but this difference was only marginally significant (Table 2). Male cyathia at level 2 produced 48 % more pollen grains than hermaphrodite cyathia at level 3 (mean ± s.e. = 14995 ± 646 and 10125 ± 723, respectively; Fig. 2), and this difference was statistically significant (Table 2). Plant identity had no effect on cyathia pollen production (Table 2). The mean pollen viability per plant ranged between 40·8 ± 7·6 % and 80·3 ± 7·6 %, and the differences between plants were marginally significant (F5,32 = 4·17, P = 0·071). Pollen viability in the male (level 2) and hermaphrodite (level 3) cyathia did not differ significantly (F1,32 = 0·37, P = 0·57; Fig. 2).
Fig. 2.
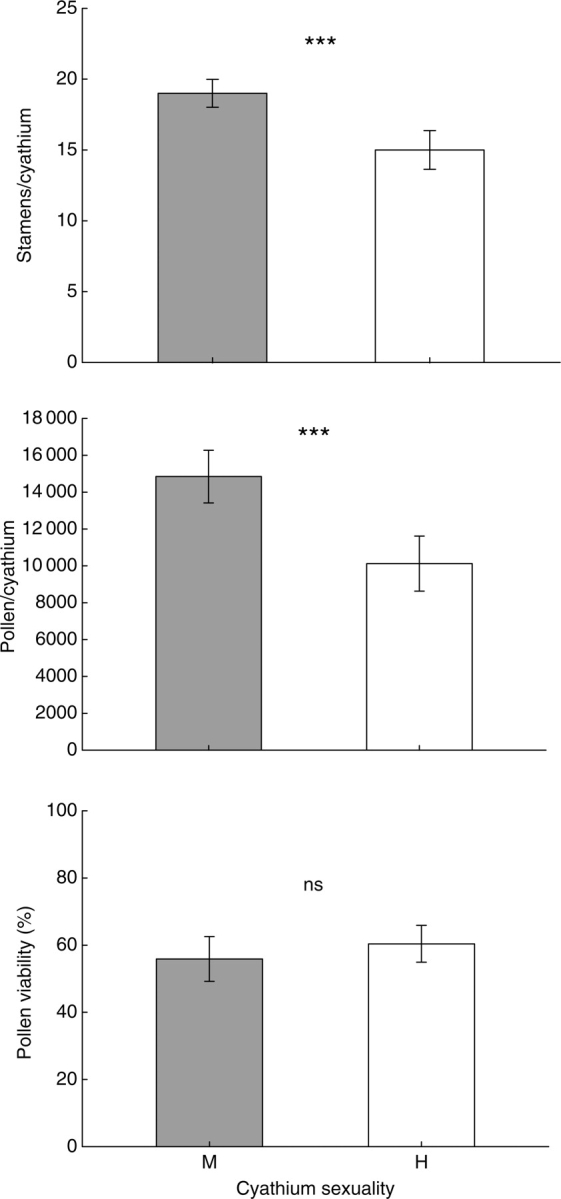
Number of stamens per cyathium, pollen grains per cyathium, and pollen viability in male (M) and hermaphrodite (H) cyathia from levels 2 and 3 of the inflorescence, respectively. Values are means ± 95% confidence intervals. Statistically significant differences between male and hermaphrodite are shown (ANOVA test, see the text and Table 2). *** P < 0·001; ns, not significant.
Table 2.
Results of mixed-model ANOVAs comparing the number stamens per cyathium, the pollen grains per stamen, and total pollen grains per cyathium between male and hermaphrodite cyathia from levels 2 and 3 of the inflorescence, respectively
| Stamens/cyathium |
Pollen/stamen |
Pollen/cyathium |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | S.S. | F | P | d.f. | S.S. | F | P | d.f. | S.S. | F | P | |
| Plant | 9 | 91 | 0·82 | 0·6089 | 4 | 261512 | 3·55 | 0·1236 | 4 | 141 × 105 | 0·69 | 0·6371 |
| Sex | 1 | 136 | 11·09 | 0·0087 | 1 | 231991 | 13·24 | 0·0209 | 1 | 1625 × 105 | 31·64 | 0·0049 |
| Plant × sex | 9 | 111 | 5·14 | 0·0011 | 4 | 73649 | 2·99 | 0·0716 | 4 | 205 × 105 | 3·61 | 0·0455 |
| Cyathium (plant × sex) | 10 | 61739 | 1·52 | 0·2170 | ||||||||
| Error | 20 | 48 | 16 | 64668 | 9 | 142 × 105 | ||||||
P values in bold are significant at Bonferroni-corrected P level (0·05/3 = 0·017).
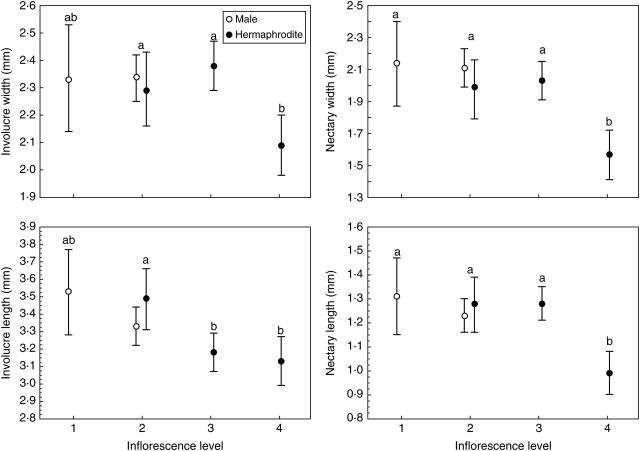
Cyathium size
The size of the involucre and the nectaries of the cyathia were significantly different between the levels of the inflorescence (Table 3, Fig. 3). The cyathia of the three first levels of the inflorescence were the same size (except the involucre length at level 3), and those at the last level were smaller (Fig. 3). The size of the involucre was similar in both sexes, but there was a significant difference in the sizes of the nectaries (Table 3). However, these differences are attributable to the smaller size of the hermaphrodite cyathia at the last level (Fig. 3). When male and hermaphrodite cyathia were compared at level 2 of the inflorescence (the only level that can produce both types of cyathia), no significant differences in size were detected (Wilk's lambda for involucre size = 0·94, F2,39 = 1·35, P = 0·27; Wilk's lambda for nectary size = 0·89, F2,39 = 2·43, P = 0·10; Fig. 3).
Table 3.
Results from MANOVAs comparing the size of involucre and nectaries from male and hermaphrodite cyathia of different inflorescence levels in the La Camilla population of E. nicaeensis
| Involucre |
Nectary |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate test |
Univariate test for variable |
Multivariate test |
Univariate test for variable |
|||||||||
| Factor | Wilk's lambda | F | d.f. | P | Width | Length | Wilk's lambda | F | d.f. | P | Width | Length |
| Level | 0·72 | 5·44 | 6, 182 | **** | *** | ** | 0·70 | 6·05 | 6, 182 | **** | **** | **** |
| Sexuality | 0·96 | 2·13 | 2, 93 | ns | ns | ns | 0·89 | 5·63 | 2, 93 | ** | ** | ns |
Interaction between both factors was not recorded because male and hermaphrodite cyathia do not occur at each level of the inflorescence (see Fig. 1). The levels of significance were corrected with a sequential Bonferroni test. **** P < 0·0001; *** P < 0·001; ** P < 0·01; ns, not significant.
Fig. 3.
Size of the involucre and the nectaries of male and hermaphrodite cyathia of E. nicaeensis in successive levels of inflorescence. Values are means ±95 % confidence intervals. Different letters indicate significant differences in size between inflorescence levels with Bonferroni-corrected P level (0·05/4 = 0·013). Note that both types of cyathia of level 2 were combined for the analysis.
Nectar secretion
Most of the male cyathia and hermaphrodite cyathia in the male phase produced nectar: 83 % (n = 792) and 96 % (n = 277), respectively. However, only 9 % (n = 368) of the hermaphrodite cyathia in the female phase produced nectar. This proportion was significantly different from that of the male cyathia (χ2 = 195·45, d.f. = 1, P < 0·00001) and that of the hermaphrodite cyathia in the male phase (χ2 = 201·12, d.f. = 1, P < 0·00001). In the same inflorescence, all cyathia either produced or did not produce nectar, and this was true for the three types of cyathia.
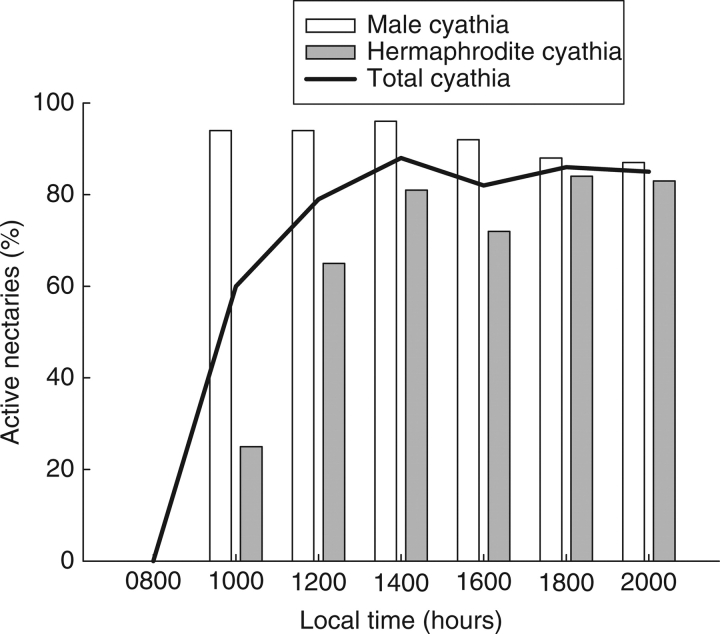
The nectar secretion throughout the day, measured as the proportion of active nectaries, began before 1000 h, increased quickly until the middle of the day, and remained high during the afternoon (Fig. 4). The proportions of active nectaries in the male cyathia and the hermaphrodite cyathia in the male phase were significantly different (χ2 = 118·95, d.f. = 5, P < 0·0001). In the overall census, the proportion of active nectaries was higher in the male cyathia, especially during the morning, when the differences were more noticeable (Fig. 4).
Fig. 4.
Daily nectar secretion rate measured as percentage of active nectaries in male cyathia, hermaphrodite cyathia in the male phase and total cyathia.
The volume of nectar that accumulated in 24 h in the male cyathia was highly variable, ranging between 0·4 and 8·3 µL, with a mean of 2·7 ± 0·29 µL. The hermaphrodite cyathia in the male phase produced a smaller volume, between 0·3 and 6·0 µL, with a mean of 1·9 ± 0·17 µL, but the difference between both types of cyathia was not significant because of the great variability within and between plants (Table 4). In contrast, the mean concentration of nectar in the male cyathia was smaller than that in the hermaphrodite cyathia in the male phase (mean ± s.e. = 7·4 ± 0·46 °Brix versus 13·4 ± 1·27 °Brix, respectively), but again these differences were not significant because of the great variability within and between plants (Table 4). As a consequence, the amount of sugar produced was highly variable in both male (32–634 µg; mean ± s.e. = 188 ± 21·4 µg) and hermaphrodite cyathia in the male phase (28–438 µg; mean ± s.e. = 181 ± 14·0 µg), and their means were not significantly different (Table 4). Hermaphrodite cyathia in the male phase produced similar amounts of sugar (98–389 µg; mean ± s.e. = 208 ± 54·8 µg) to those of the hermaphrodite cyathia in the female phase (U = 81, n1 = 6, n2 = 39, P = 0·54) and those of the male cyathia (U = 103, n1 = 6, n2 = 46, P = 0·70).
Table 4.
Results of nested ANOVAs comparing volume, concentration and sugar weight of nectar between male and hermaphrodite cyathia in the male phase from the level 2 of the inflorescence
| Volume |
Concentration (°Brix) |
Sugar weight |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | S.S. | F | P | S.S. | F | P | S.S. | F | P | |
| Sex | 1 | 8·20 | 1·28 | 0·2934 | 361·68 | 1·06 | 0·3376 | 0·004 | 0·01 | 0·9450 |
| Plant (sex) | 7 | 46·97 | 3·66 | 0·0018 | 2549·26 | 24·54 | < 0·0001 | 5·043 | 2·00 | 0·0656 |
| Error | 76 | 139·21 | 1127·64 | 27·346 | ||||||
P-values in bold are significant at Bonferroni-corrected P level (0·05/3 = 0·017).
DISCUSSION
The pleiochasia of Euphorbia nicaeensis developed different numbers of branching levels in the two populations studied and, as a result, the numbers of cyathia per inflorescence also differed between them. In the Aracena population, the inflorescences were more branched and carried a greater number of cyathia, probably the result of the shady habitat, as has been reported in French populations of E. nicaeensis (Al-Samman et al., 2001).
All E. nicaeensis plants produced both male and hermaphrodite cyathia, and were thus functionally andromonoecious. Moreover, every plant showed a very well-defined pattern of distribution of both types of cyathia in its inflorescences. Male cyathia were produced predominantly in the first and second levels, whereas the cyathia were almost exclusively hermaphrodite in the subsequent levels. A similar pattern of cyathia distribution was observed in two other populations of E. nicaeensis in the Iberian Peninsula (Narbona et al., 2002), and thus it seems an established character in this species. In fact, the production of male cyathia in the first levels of the inflorescence seems to be a common feature in perennial species of Euphorbia (Eichberger, 2001; Narbona et al., 2002). However, in the perennial E. boetica, in which the pleiochasia develop up to eight levels, male cyathia are also found in the latest inflorescence levels (Narbona et al., 2005).
In the hermaphrodite cyathia of E. nicaeensis, dichogamy is totally effective in avoiding autogamy because there is no overlap between the female and male phases of each cyathium. Moreover, all hermaphrodite cyathia of an inflorescence level show perfect synchrony, exhibiting the same sexual phase, and there is no overlap in the flowering between levels (Narbona, 2002). All these features, marked protogyny, intra-level synchrony, and inter-level asynchrony, should be effective in avoiding geitonogamy in E. nicaeensis. Brunet and Charlesworth (1995) presented an evolutionary stable strategy analysis of the resource allocation in hermaphroditic plants and suggested that, in plants with more or less marked protogyny and sequential blooming, early flowers should be expected to allocate more resources to male function because the ratio of potential mates to ovule competitors is lowest in these flowers. This evolutionary strategy could operate in the markedly protogynous E. nicaeensis, causing its lower cyathia to specialize as male, without female flowers.
A true floral dimorphism in the primary sexual characteristics does not exist in E. nicaeensis, because male and hermaphrodite cyathia at the same inflorescence level produce similar amounts of pollen. When males and hermaphrodites at different inflorescence levels (2 and 3, respectively) were compared, the number of viable pollen grains was higher in the males. These facts suggest that the position of the cyathia in the inflorescence, but not their sex, determines the observed differences in viable pollen production. The results for E. nicaeensis do not support the hypothesis that staminate flowers of andromonoecious species invest more in male function than do perfect flowers (Anderson and Symon, 1989; Spalik and Woodell, 1994).
Euphorbia nicaeensis only shows a true but slight floral dimorphism in some of its secondary sexual characteristics associated with nectar secretion. In terms of cyathium size, only a slight dimorphism in the nectary width was observed, which may have been apparent because the cyathia of the last level of the inflorescence (which contains hermaphrodites exclusively) were markedly smaller than those of the other levels. In fact, no differences in the sizes of cyathia were detected when males and hermaphrodites were compared at level 2, the only level at which both types of cyathia occur, although in different plants. A decrease in flower size in the last levels of the inflorescence has been reported in the andromonoecious E. boetica and Anthriscus sylvestris (Spalik and Woodell, 1994; Narbona et al., 2005). In E. nicaeensis, this can be attributed to the effect of the inflorescence architecture or to the phenotypic plasticity, which is related to the availability of resources (Diggle and Miller, 2004). However, the present study did not allow discrimination between these two factors (see Diggle, 1997; Ashman and Hitchens, 2000). With regard to nectar secretion, male and hermaphrodite cyathia of the same inflorescence level produced similar amounts of sugar in 24 h. However, male cyathia produced nectar throughout their whole lifespans, whereas hermaphrodites produced it exclusively during their male phase, which constitutes a true dimorphism in their secretion patterns. A true sexual dimorphism was also identified which affected the nectar secretion rate throughout the day, in that the nectary activity of the male cyathia started earlier in the day than did that of the hermaphrodites. A similar dimorphism in nectar secretion patterns has been reported in another spurge species, E. boetica (Narbona et al., 2005).
The nectar secretion pattern can affect pollinator movements between flowers (Johnson and Hubbell, 1975; Kendall and Smith, 1975), producing a directionality in their movements (Willson and Price, 1979; Waddington, 2001). In E. nicaeensis, the differential pattern of nectar secretion between male and hermaphrodite cyathia during the day could cause pollinators to visit the male cyathia first, and then the hermaphrodites. However, most hermaphrodite cyathia in the female phase do not produce nectar, and fruiting is high in E. nicaeensis (Narbona, 2002), indicating successful pollination. Thus, it seems that most visitors to E. nicaeensis cannot discriminate between nectariferous and nectarless cyathia.
The fact that most hermaphrodite cyathia in the female phase are nectarless has been observed in successive years in La Camilla, and in other populations (E. Narbona, pers. obs.). Thus, most hermaphrodite cyathia in the female phase offer no rewards to pollinators, and this suggests that pollination in E. nicaeensis is, to a great extent, based on deception (Baker, 1976; Wilson and Ågren, 1989). This mode of pollination has been found in several monoecious and dioecious species (e.g. Wilson and Ågren, 1989; Costich and Meagher, 2001), including the Euphorbiaceae monoecious species Cnidoscolus urens (Bawa et al., 1982). Because pollen is also an important reward for many floral visitors, andromonoecy only permits a deceit pollination system if the hermaphrodite flowers are dichogamous. However, all the floral visitors observed in the La Camilla and Aracena populations of E. nicaeensis sought nectar exclusively, and these populations were not pollen limited (Narbona, 2002). Therefore, as suggested for other species (Wilson and Ågren, 1989; Le Corff et al., 1998), pollination by deceit may be a strategy of E. nicaeensis to reduce the cost of nectar production, while simultaneously maintaining the attraction of pollinators and female fitness.
The above results show clearly a relationship between floral sexual dimorphism and inflorescence development in Euphorbia nicaeensis. A true sexual dimorphism was only found in some characteristics related to the nectar secretion patterns and this would support a functional hypothesis in which sexual dimorphism could have evolved to maximize pollination success and allocation of resources (Costich and Meagher, 2001; Miller and Venable, 2003). However, most differences between the male and hermaphrodite cyathia of E. nicaeensis (viable pollen production and cyathium size) were the result of positional effects. These differences based on inflorescence position could be contemplated as a result of developmental constraints (Diggle, 1997, 2003), but alternatively the fact that the male and hermaphrodite cyathia of E. nicaeensis are produced at different positions along the inflorescence could be considered as an evolutionary mechanism to promote floral dimorphism in relation to gender expression (Meagher, 2007).
ACKNOWLEDGEMENTS
This work was supported by grant of the Programa de Ayuda a los Grupos de Investigación (Junta de Andalucía) and by a grant to P. L. Ortiz from the Spanish Ministerio de Educación y Ciencia (CGL2005– 03731).
LITERATURE CITED
- Al-Samman N, Martin A, Puech S. Inflorescence architecture variability and its possible relationship to environment or age in a Mediterranean species, Euphorbia nicaeensis All. (Euphorbiaceae) Biological Journal of the Linnean Society. 2001;136:99–105. [Google Scholar]
- Anderson GJ, Symon DE. Functional dioecy and andromonoecy in Solanum. Evolution. 1989;43:204–219. doi: 10.1111/j.1558-5646.1989.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Arroyo MTK. Breeding systems and pollination biology in Leguminosae. In: Polhill RM, Raven PH, editors. Advances in legume systematics. London: Royal Botanic Garden, Kew; 1981. pp. 723–769. [Google Scholar]
- Ashman TL, Hitchens MS. Dissecting the causes of variation in intra-inflorescence allocation in sexually polymorphic species, Fragaria virginiana (Rosaceae) American Journal of Botany. 2000;87:197–204. [PubMed] [Google Scholar]
- Assouad MW, Dommee B, Lumaret R, Valdeyron G. Reproductive capacities in the sexual forms of the gynodioecious species Thymus vulgaris L. Botanical Journal of the Linnean Society. 1978;77:29–39. [Google Scholar]
- Baker HG. ‘Mistake’ pollination as a reproductive system with special reference to the Caricaceae. In: Burley J, Styles BT, editors. Tropical trees. New York, NY: Academic Press; 1976. pp. 161–169. [Google Scholar]
- Bar-Shalom D, Mattson O. Mode of hydration, an important factor in the germination of trinucleate pollen grains. Botanisk Tidsskrift. 1977;71:245–251. [Google Scholar]
- Bawa KS, Webb CJ, Tuttle AF. The adaptative significance of monoecism in Cnidoscolus urens (L.) Arthur (Euphorbiaceae) Botanical Journal of the Linnean Society. 1982;85:213–223. [Google Scholar]
- Bell G. On the function of flowers. Proccedings of the Royal Society London B. 1985;224:223–265. [Google Scholar]
- Benedí C, Molero J, Simón J, Vicens J. Euphorbia. In: Castroviejo S, Aedo C, Benedí C, Laínz MR, Muñoz-Garmendia F, Nieto-Feliner G, Paiva J, editors. Vol. 7. Madrid: Real Jardín Botánico de Madrid-CSIC; 1997. pp. 210–285. Flora Iberica. [Google Scholar]
- Bertin RI. The evolution and maintenance of andromonoecy. Evolutionary Theory. 1982;6:25–32. [Google Scholar]
- Brunet J, Charlesworth D. Floral sex allocation in sequentially blooming plants. Evolution. 1995;49:70–79. doi: 10.1111/j.1558-5646.1995.tb05959.x. [DOI] [PubMed] [Google Scholar]
- Buide ML. Intra-inflorescence variation in floral traits and reproductive success of the hermaphrodite Silene acutifolia. Annals of Botany. 2004;94:441–448. doi: 10.1093/aob/mch164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly BA, Anderson GJ. Functional significance of the androecium in staminate and hermaphroditic flowers of Solanum carolinense (Solanaceae) Plant Systematcs and Evolution. 2003;240:235–243. [Google Scholar]
- Costich DE, Meagher TR. Impacts of floral gender and whole-plant gender on floral evolution in Ecballium elaterium. Biological Journal of the Linnean Society. 2001;74:475–487. [Google Scholar]
- Cruden RW, Hermann SM. Studying nectar? Some observation on the art. In: Bentley B, Elias T, editors. The biology of nectaries. New York, NY: Columbia University Press; 1983. pp. 223–241. [Google Scholar]
- Cuevas J, Polito V. The role of staminate flowers in the breeding system of Olea europaea (Oleaceae): an andromonoecious, wind-pollinated taxon. Annals of Botany. 2004;93:547–553. doi: 10.1093/aob/mch079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RW, Quinn GP. Comparisons of treatments after an analysis of variance in ecology. Ecological Monographs. 1989;59:433–463. [Google Scholar]
- Delph LF. Flower size dimorphism in plants with unisexual flowers. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman and Hall; 1996. pp. 217–237. [Google Scholar]
- Delph LF, Galloway LF, Stanton L. Sexual dimorphisms in flower size. American Naturalist. 1996;148:299–320. [Google Scholar]
- Diggle PK. Developmental plasticity, genetic variation, and the evolution of andromonoecy in Solanum hirtum (Solanaceae) American Journal of Botany. 1993;80:967–973. [Google Scholar]
- Diggle PK. Architectural effects and the interpretation of patterns of fruit and seed development. Annual Review of Ecology and Systematics. 1995;26:531–552. [Google Scholar]
- Diggle PK. Ontogenetic contingency and floral morphology: the effects of architecture and resource limitation. International Journal of Plant Sciences. 1997;158:S99–S107. [Google Scholar]
- Diggle PK. Architectural effects on floral form and functions: a review. In: Stuessy TF, Mayer V, Horandl E, editors. Deep morphology: towards a renaissance of morphology in plant systematics. Koenigstein: Koeltz Scientific Books; 2003. pp. 63–80. [Google Scholar]
- Diggle PK, Miller JS. Architectural effects mimic floral sexual dimorphism in Solanum (Solanaceae) American Journal of Botany. 2004;91:2030–2040. doi: 10.3732/ajb.91.12.2030. [DOI] [PubMed] [Google Scholar]
- Eckhart VM. The effects of floral display on pollinator visitation vary among populations of Phacelia linearis (Hydrophyllaceae) American Journal of Botany. 1991;79:792–800. [Google Scholar]
- Ehrenfeld J. Reproductive biology of three species of Euphorbia subgenus chamaesyce (Euphorbiaceae) American Journal of Botany. 1976;63:406–413. [Google Scholar]
- Eichberger C. biology synecology, plant sociology and sociocultural position of a Mediterranean species. Stuttgart: Science Publishers; 2001. The tree spurge Euphorbia dendroides L. Dissertationes Botanicae 344. [Google Scholar]
- Elle E, Meagher TR. Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). II. Paternity and functional gender. American Naturalist. 2000;156:622–636. doi: 10.1086/316997. [DOI] [PubMed] [Google Scholar]
- Emms SK. Andromonoecy in Zigadenus paniculatus (Liliaceae): spatial and temporal patterns of sex allocation. American Journal of Botany. 1993;80:914–923. [Google Scholar]
- Gibbs PE, Lewis GP, Lughadha EN. Fruit-set induced changes in the sex of flowers in Caesalpinia calycina (Leguminosae) Plant Biology. 1999;1:665–669. [Google Scholar]
- Huan S-Q. Flower dimorphism and the maintenance of andromonoecy in Sagittaria guyanensis ssp. lappula (Alismataceae) New Phytologist. 2003;157:357–364. doi: 10.1046/j.1469-8137.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- Johnson LK, Hubbell SP. Contrasting foraging strategies and coexistence of two bee species on a single resource. Ecology. 1975;56:1398–1406. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Niwot, CO: University Press of Colorado; 1993. [Google Scholar]
- Kendall DA, Smith BD. The foraging behaviour of honeybees on ornamental Malus spp. used as pollinizers in apple orchards. Journal of Applied Ecology. 1975;12:465–471. [Google Scholar]
- Koul P, Sharma N, Koul AK. Pollination biology of Apiaceae. Current Science. 1993;50:219–222. [Google Scholar]
- Le Corff J, Ågren J, Schemske DW. Floral display, pollinator discrimination, and female reproductive success in two monoecious Begonia species. Ecology. 1998;79:1610–1619. [Google Scholar]
- Lovett Doust J. Floral sex ratios in andromonoecious Umbelliferae. New Phytologist. 1980;85:265–273. [Google Scholar]
- Meagher TR. Linking the evolution of gender variation to floral development. Annals of Botany. 2007;100:165–176. doi: 10.1093/aob/mcm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Diggle PK. Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): the roles of phenotypic plasticity and architecture. American Journal of Botany. 2003;90:707–715. doi: 10.3732/ajb.90.5.707. [DOI] [PubMed] [Google Scholar]
- Miller JS, Venable DL. Floral morphometrics and the evolution of sexual dimorphism in Lycium (Solanaceae) Evolution. 2003;57:74–86. doi: 10.1111/j.0014-3820.2003.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Narbona E. Seville, Spain: University of Seville; 2002. Estrategias reproductivas de dos especies perennes de Euphorbia. PhD Thesis. [Google Scholar]
- Narbona E, Ortiz PL, Arista M. Functional andromonoecy in Euphorbia (Euphorbiaceae) Annals of Botany. 2002;89:571–577. doi: 10.1093/aob/mcf099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbona E, Ortíz PL, Arista M. Dichogamy and sexual dimorphism in floral traits in the andromonoecious Euphorbia boetica. Annals of Botany. 2005;95:779–787. doi: 10.1093/aob/mci077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz PL, Arista M, Oliveira PE, Talavera S. Pattern of flowers and fruit production in Stryphnodendron adstringes, an adromonoecious legume tree of Central Brazil. Plant Biology. 2003;5:592–599. [Google Scholar]
- Plack A. Sexual dimorphism in Labiatae. Nature. 1957;180:1218–1219. [Google Scholar]
- Podolsky RD. Evolution of a flower dimorphism: how effective is pollen dispersal by ‘male’ flowers? Ecology. 1993;74:2255–2260. [Google Scholar]
- Prenner G, Rudall PJ. Comparative ontogeny of the cyathium in Euphorbia (Euphorbiaceae) and its allies: exploring the organ–flower–inflorescence boundary. American Journal of Botany. 2007;94:1612–1629. doi: 10.3732/ajb.94.10.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primack RB, Lloyd DG. Andromonoecy in the New Zealand montane shrub manuka, Leptospermun scoparium (Myrtaceae) American Journal of Botany. 1980;67:9–13. [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Schlessman MA, Graceffa LM. Protogyny, pollination, and sex expression of andromonecious Pseudocymopterus montanus (Apiaceae, Apioideae) International Journal of Plant Sciences. 2002;163:409–417. [Google Scholar]
- Spalik K. On evolution of andromonoecy and ‘overproduction’ of flowers: a resource allocation model. Biological Journal of Linnean Society. 1991;42:325–336. [Google Scholar]
- Spalik K, Woodell RJ. Regulation of pollen production in Anthriscus sylvestris, an andromonoecious species. International Journal of Plant Sciences. 1994;155:750–754. [Google Scholar]
- StatSoft. STATISTICA for Windows. Tulsa, OK: StatSoft; 2001. Computer program manual. [Google Scholar]
- Traveset A. Reproductive ecology of Cneorun tricoccom L. (Cneoraceae) in the Balearic Islands. Botanical Journal of the Linnean Society. 1995;117:221–232. [Google Scholar]
- Uno GE. Comparative reproductive biology of hermaphrodite and male-sterile Iris douglasiana Herb. (Iridaceae) American Journal of Botany. 1982;69:818–823. [Google Scholar]
- Waddington KD. Subjective evaluation and choice behaviour by nectar- and pollen-collecting bees. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination. Cambridge: Cambridge University Press; 2001. pp. 41–60. [Google Scholar]
- Weberling F. Morphology of flowers and inflorescences. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Weiss D, Halevy AH. Stamens and gibberellin in the regulation of corolla pigmentation and growth in Petunia hybrida. Planta. 1989;179:89–96. doi: 10.1007/BF00395775. [DOI] [PubMed] [Google Scholar]
- Whalen MD, Costich DE. Andromonoecy in Solanum. In: D'Arcy WG, editor. Solanaceae: biology and systematics. New York, NY: Columbia University Press; 1986. pp. 284–302. [Google Scholar]
- Willson MF, Ågren J. Differential floral rewards and pollination by deceit in unisexual flowers. Oikos. 1989;55:23–29. [Google Scholar]
- Willson MF, Price PW. The evolution of inflorescence size in Asclepias (Asclepiadaceae) Evolution. 1979;31:495–511. doi: 10.1111/j.1558-5646.1977.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Yampolsky C. The occurrence and inheritance of sex intergradation in plants. American Journal of Botany. 1920;7:21–38. [Google Scholar]
- Yampolsky C, Yampolsky H. Distribution of sex forms in the phanerogamic flora. Bibliotheca Genetica. 1922;3:1–62. [Google Scholar]
- Zar JH. Biostatistical analysis. Upper Saddle River, NJ: Prentice-Hall; 1999. [Google Scholar]