Abstract
Background and Aims
Many notorious alien invasive plants are clonal, but little is known about some roles and aspects of clonal integration. Here, the hypothesis is tested that clonal integration affects growth, photosynthetic efficiency, biomass allocation and competitive ability of the exotic invasive weed Alternanthera philoxeroides (Amaranthaceae).
Methods
The apical parts of Alternanthera were grown either with or without the lawn grass Schedonorus phoenix (tall fescue) and their stolon connections to the basal parts grown without competitors were either severed or left intact.
Key Results
Competition greatly reduced the maximum quantum yield of photosystem II (Fv/Fm) and growth (biomass, number of ramets and leaves, total stolon length and total leaf area) of the apical Alternanthera, but not the biomass of S. phoenix. Stolon connections significantly increased Fv/Fm and growth of Alternanthera. However, such effects on growth were smaller with than without competition and stolon connections did not alter the relative neighbour effect of Alternanthera. Stolon connections increased Alternanthera's biomass allocation to roots without competition, but decreased it with competition.
Conclusions
Clonal integration contributed little to Alternanthera's competitive ability, but was very important for Alternanthera to explore open space. The results suggest that the invasiveness of Alternanthera may be closely related to clonal integration.
Key words: Alien species, alligator weed, Alternanthera philoxeroides, chlorophyll fluorescence, clonal invasive plants, competition, physiological integration, Schedonorus phoenix
INTRODUCTION
Connected ramets of clonal plants can share water, carbohydrates and nutrients through clonal integration (Alpert and Mooney, 1986; Marshall, 1990; Stuefer et al., 1994, 1996; Alpert, 1996, 1999; Wijesinghe and Hutchings, 1997). Many studies have shown that clonal integration facilitates establishment of newly produced ramets, improves survival, growth and/or reproduction of adult ramets in stressful environments, and helps genets to occupy open space (Hartnett and Bazzaz, 1985; Hester et al., 1994; Brewer and Bertness, 1996; Jónsdóttir and Watson, 1997; Yu et al., 2002, 2004; Lötscher, 2006). These positive effects of clonal integration may provide clonal plants with a competitive advantage over non-clonal plants or clonal plants with little integration. On the other hand, integration-mediated increases in performance of clonal plants may reduce the growth and reproduction of their competitors. As a result, clonal integration may influence species co-existence, community structure and ecosystem functioning (Oborny and Podani, 1995; Pyšek, 1997; Wilsey, 2002).
In past decades, plant invasion has become a great threat to global ecosystems (Mack et al., 2000; Pimental et al., 2000; Mitchell and Power, 2003). Many notorious alien invasive plants are clonal (Liu et al., 2006). Some have vigorous clonal growth that enables them to invade local communities and to displace native plant species (Crawley, 1986; Blossey and Kamil, 1996; Liu et al., 2006). For example, the alien invasive rhizomatous herb Solidago gigantea and the stoloniferous herb Alternanthera philoxeroides (alligator weed) form very dense stands, which exclude almost all other species (Julien and Bourne, 1988; Jakobs et al., 2004). Some studies have suggested that the competitive ability, and thus the invasiveness, of alien clonal plants may be closely related to clonal traits such as clonal integration (Pyšek, 1997; Reichard, 1997; Maurer and Zedler, 2002; Pyšek et al., 2003; Liu et al., 2006). To date, however, very few studies have addressed how clonal integration affects growth of alien invasive clonal plants when competing with native species.
Several studies have addressed how clonal integration affects growth of clonal plants when they compete with neighbours (Hartnett and Bazzaz, 1985; Schmid and Bazzaz, 1987; Hartnett, 1993; Price and Hutchings, 1996; Pennings and Callaway, 2000; Peltzer, 2002; Březina et al., 2006), but the results disagree. Clonal integration was not shown to affect growth of Glechoma hederacea (Price and Hutchings, 1996), Panicum virgatum (Hartnett, 1993), Populus tremuloides (Peltzer, 2002) or Calamagrostis epigejos (Březina et al., 2006). Hartnett and Bazzaz (1985), however, found that the growth response of a Solidago canadensis ramet to a given neighbour species varied with the neighbour species encountered by its interconnected ramets, suggesting that clonal integration played an important role. Pennings and Callaway (2000) also found that clonal integration significantly increased growth of several salt marsh plants when they competed with neighbours for below-ground resources and tended to increase growth when they competed with neighbours for both above- and below-ground resources. However, none of these studies had investigated whether clonal integration reduced the growth of the neighbour plants (i.e. competitors).
Effects of clonal integration on photosynthetic efficiency of plants have not been widely studied (but see Hartnett and Bazzaz, 1983; Roiloa and Retuerto, 2005, 2006a, b). Photosynthetic efficiency can be estimated by measuring chlorophyll fluorescence (Schreiber et al., 1998), and the maximum quantum yield of photosystem II (Fv/Fm) derived from the parameters of chlorophyll fluorescence is a sensitive indicator of plant photosynthetic performance (Björkman and Demmig, 1987; Johnson et al., 1993; Roiloa and Retuerto, 2005, 2006a,b). Fv/Fm usually decreases when plants are exposed to environmental stress (Björkman and Demmig, 1987; Johnson et al., 1993; Roiloa and Retuerto, 2005, 2006a, b). Because clonal integration may alleviate the competition-mediated stress on ramets, it may greatly reduce the negative effects of competition on Fv/Fm. However, no study has been conducted to test this hypothesis.
Many studies have investigated effects of clonal integration on biomass allocation of clonal plants (Salzman and Parker, 1985; Friedman and Alpert, 1991; Evans and Whitney, 1992; Birch and Hutchings, 1994; Stuefer et al., 1994, 1996; Wijesinghe and Hutchings, 1997; Yu et al., 2002; Roiloa et al., 2007). In heterogeneous conditions consisting of a mixture of rich and poor resource patches, clonal integration can modify biomass allocation so that relatively more biomass is allocated to the organs (roots or leaves) to acquire more abundant resources, a phenomenon called ‘division of labour’ (Stuefer et al., 1996; Alpert and Stuefer, 1997; Hutchings and Wijesinghe, 1997). This is in contrast with the pattern of non-clonal plants or clonal plants grown in homogeneous conditions (Birch and Hutchings, 1994; Stuefer et al., 1996; Alpert and Stuefer, 1997; Hutchings and Wijesinghe, 1997). However, very few studies have been conducted to address how clonal integration affects biomass allocation of plants when they were grown with competitors, especially for exotic invasive clonal plants.
Alternanthera philoxeroides (hereafter referred to by the genus name only) is a stoloniferous alien invasive weed of the Amaranthaceae and originates from the Parana River region of South America (Gunasekera and Bonila, 2001). It is amphibious, growing both in riparian and in terrestrial habitats (Julien and Stanley, 1999). In terrestrial situations Alternanthera can displace native species (Julien and Bourne, 1988) and block irrigation and drainage systems of cropland; in riparian systems it can cover the entire water surface, reducing oxygen exchange, preventing flow and potentially increasing flood damage (Ma and Wang, 2005). In China, Alternanthera has invaded different ecosystems, including cropland, lawn, garden, marshes and lakes, and has caused great economic and environmental problems (Wang and Wang, 1988; Ma and Wang, 2005). It is one of the world's worst invasive weeds and one of the 16 worst alien invasive weeds in China (Julien et al., 1995; Holm et al., 1997; Ma and Wang, 2005).
To examine the effect of clonal integration on growth, photosynthetic performance, biomass allocation and thus competitive ability of exotic invasive clonal plants, we grew clonal fragments of Alternanthera in containers with two sections. The apical (with the apical parts of the Alternanthera clonal fragments) were grown either with or without a common lawn grass, Schedonorus phoenix (tall fescue), whereas the basal sections (with basal parts of the clonal fragments) were grown without competitors. Stolons connecting the two parts were either severed or left intact to test the effect of clonal integration. Specifically, we test the following hypotheses. (1) Stolon connections (clonal integration) will significantly improve growth and competitive ability of Alternanthera. (2) Clonal integration will buffer the decrease in Fv/Fm of Alternanthera grown with competitors. (3) Stolon connections will change biomass allocation of Alternanthera grown with competitors. According to the theory of labour division (Alpert and Stuefer, 1997; Hutchings and Wijesinghe, 1997), we predict that stolon connection will increase biomass allocation to leaves if the below-ground competition (for nutrients and/or water) is more severe and will increase that to roots if the above-ground competition (for light) is more severe. (4) Severing stolons of Alternanthera will increase growth of S. phoenix.
MATERIALS AND METHODS
Experimental design
The experiment used a factorial design involving competition (with or without) and stolon connection (intact or severed). The plants of Alternanthera philoxeroides (Mart.) Griseb. used in this experiment were 28 similar-sized clonal fragments (12·74 ± 0·29 cm in length; mean ± s.e.), each consisting of a stolon with six ramets. Each clonal fragment was classified into two parts, one termed as the ‘basal part’ consisting of four relatively old ramets (relatively proximal to their mother ramets) and the other as the ‘apical part’ consisting of two relatively young ramets (relatively distal to the mother ramets) and a stolon apex. Because previous studies have shown that all Alternanthera plants in China are genetically identical (Xu et al., 2003; Wang et al., 2005), the clonal fragments used in this experiment were derived only from a single clone collected from cropland in Yunnan Province, China.
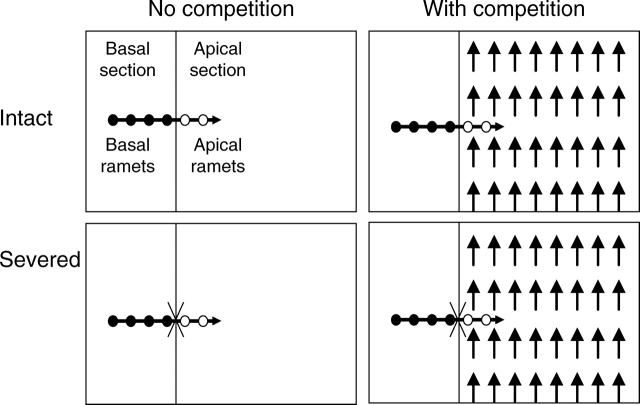
The experimental containers were 35 plastic trays (50 × 37 × 15 cm; length × width × height), each having two separated sections (Fig. 1)–the basal section (16·7 cm long) and the apical section (33·3 cm long). Nutrients, water and roots in the two sections did not interfere with each other. The trays were filled with a mixture of sand and peat at a volume ratio of 4 : 1 and with 0·8 g L−1 of slow-release fertilizer (Osmocote 301, Scotts, USA) having an elemental ratio of 15 N: 11P: 13K: 2 Mg. On 25 November 2005, the apical sections of 21 trays were planted with the lawn grass Schedonorus phoenix (Scop.) Holub. (tall fescue, previously named Festuca arundinacea Schreb.), sown at a seed density of 30 g m−2 (approx. 12 228 ± 309·97 seeds m−2; mean ± s.e., n = 3). The remaining 14 trays were kept with the apical sections unsown.
Fig. 1.
Schematic representation of the experimental design. The clonal fragments of Alternanthera, each consisting of four basal ramets (filled circles) and two apical ramets (open circles) with a stolon apex (horizontal arrow), were grown either with (left) or without (right) competitors (Schedonorus phoenix, vertical arrows) and with stolon connections between basal and apical ramets either intact (top) or severed (bottom).
On 20 December, 28 clonal fragments of Alternanthera were positioned in 28 trays (14 with and 14 without the grass competitor). The remaining seven trays with grasses were used as a control for grass growth without competition. Each clonal fragment was arranged such that the four ramets of the basal part were placed within the basal section of a tray and the two ramets and the apex of the apical part were placed within the apical section of the same tray (see Fig. 1). At this time, the S. phoenix sward was 15·12 ± 0·27 cm (mean ± s.e.) high and at a density of 1·18 ± 0·07 plants cm−2. The stolon of the apical ramets was anchored to the soil surface to facilitate rooting. On 10 January 2006, when the first three basal nodes of the apical ramets of all clonal fragments had rooted, the stolon connections between the apical and the basal parts were severed in 14 trays, whilst the other 14 trays were kept untreated (Fig. 1). The experiment was ended on 12 March 2006. During the experiment, new stolons and ramets derived from the basal (or apical) part of a clonal fragment were prevented from growing into the basal (or apical) section of the tray.
Both material preparation and experiment were conducted in a heated glasshouse (18–25 °C) at the Institute of Botany, Chinese Academy of Sciences in Beijing, China. During the experiment, artificial light was provided to ensure a lighting period of 12 h. All plants were watered two or three times a week, and sprayed twice during the experiment with fungicide against fungal infection. To avoid the effects of possible environmental patchiness within the glasshouse, the trays were systematically repositioned in the glasshouse every 2 weeks so that each tray experienced all possible conditions. To simulate mowing in a lawn ecosystem, all S. phoenix plants were clipped to 3 cm high on 12 January and 12 February 2006.
Measurements
Between 1930 h and 2030 h on 8 March 2006, after a dark adaptation of at least 1·5 h sufficient for the photosystem II (PSII) reaction centres to open, the minimal (F0) and the maximum fluorescence yield (Fm) were measured for a fully developed, healthy leaf on the second-youngest Alternanthera ramet in each apical part using a portable chlorophyll fluorometer (PAM-2100, Walz, Effeltrich, Germany) with the saturation pulse method (Schreiber et al., 1998). The maximum quantum yield of PSII (Fv/Fm) was calculated as (Fm – F0)/Fm (Björkman and Demmig, 1987; Johnson et al., 1993; Maxwell and Johnson, 2000; Roiloa and Retuerto, 2006a, b).
At harvest, the number of ramets, leaves and stolons were counted, and stolon length and total leaf area of Alternanthera were measured for the apical section of each tray. Then Alternanthera in the apical sections were harvested and separated into leaves, stolons and roots and their biomass was determined after drying at 70 °C for 48 h. Above-ground parts of S. phoenix plants in the apical section of each tray were also harvested and their root biomass was obtained based on the dry mass measured in five systematically sampled soil cores (4·5 cm in diameter). The biomass of S. phoenix clipped on the two dates (see above) was also measured.
Data analysis
Two-way analysis of variance (ANOVA) was used to test the effect of stolon connection (clonal integration) and competition on Fv/Fm of Alternanthera in the apical sections (Sokal and Rohlf, 1981). Two-way multivariate analysis of variance (MANOVA) was employed to investigate the global effects of stolon connection and competition on growth and percentage biomass allocation of Alternanthera, and corresponding univariate analyses were also conducted. One-way ANOVA followed by Student–Newman–Keuls (SNK) tests were used to assess whether biomass of S. phoenix in the apical sections differed among the three treatments (no competition; with competition with severed stolon connection of Alternanthera; with competition with intact stolon connection).
The index of the relative neighbour effect (RNE) was calculated to measure the competitive intensity of S. phoenix on Alternanthera (Kikvidze et al., 2006). The RNE of Alternanthera was calculated as (C–A)/max(C, A), where A is the average biomass of Alternanthera across replicates without competition, C is biomass of Alternanthera with competition, and max(C, A) is the larger value between A and C. Under conditions of competition (not facilitation), the RNE values range between −1 and 0; the greater the RNE values are, the smaller the neighbour's competition effect is. A t-test was conducted to investigate whether stolon connection affected the RNE of Alternanthera. A significantly larger RNE with than without stolon connection would indicate that clonal integration improves Alternanthera's competitive ability. The RNE of S. phoenix was not considered because a significant competition effect was not observed (see Results). The main stolon tip of one Alternanthera clonal fragment was damaged during the experiment, so data relevant to this plant were excluded from all analyses. The software package SPSS 13·0 (SPSS, Chicago, IL, USA) was used for all analyses.
RESULTS
Photosynthetic performance of Alternanthera
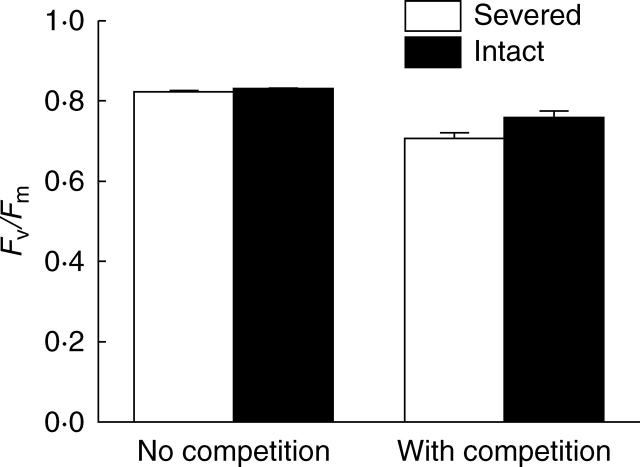
Both competition with the lawn grass, S. phoenix, and severing the stolon markedly reduced the value of Fv/Fm of Alternanthera in the apical sections (competition effect: F1,23 = 72·8, P < 0·001; stolon connection effect: F1,23 = 7·6, P = 0·011; Fig. 2). However, the effect of stolon connection tended to be larger when Alternanthera was grown with rather than without competition (interaction effect: F1,23 = 3·8, P = 0·058; Fig. 2).
Fig. 2.
The maximum quantum yield of photosystem II (Fv/Fm, ± 1 s.e.) of Alternanthera in the apical sections, grown either with or without competitors and with stolon connections either severed or kept intact.
Growth and competitive intensity of Alternanthera
Overall, stolon connection, competition and the interaction had significant effects on growth of Alternanthera in the apical sections (Table 1A). Competition greatly reduced biomass, number of ramets and leaves, total stolon length and total leaf area of Alternanthera (Table 1B, Figs 3A and 4). Severing stolons also significantly reduced these growth traits (Table 1B, Figs. 3A and 4), but such effects were, or tended to be, smaller when Alternanthera was grown with, rather than without, competition (Table 1B, Figs. 3A and 4). The relative neighbour effect of Alternanthera was −0·91 ± 0·02 (mean ± s.e.) when the stolons were kept intact and −0·92 ± 0·01 when they were severed; stolon connections had no effect (t11 = 0·14, P = 0·89).
Table 1.
Summary of MANOVA for effects of stolon connection, competition and their interaction on growth measures of Alternanthera in the apical sections (A) Multivariate test statistics and exact F-statistics
| Effect | Wilk's Lambda | F | d.f. | P |
|---|---|---|---|---|
| Stolon connection | 0·371 | 6·46 | 5,19 | <0·001 |
| Competition | 0·043 | 84·01 | 5,19 | <0·001 |
| SC × C | 0·207 | 14·52 | 5,19 | <0·001 |
| (B) Univariate test statistics | |||||||
|---|---|---|---|---|---|---|---|
| Stolon connection |
Competition |
SC × C |
|||||
| Trait | d.f. | F | P | F | P | F | P |
| Total biomass | 1, 23 | 21·91 | <0·001 | 175·56 | <0·001 | 15·24 | <0·001 |
| Total leaf area | 1, 23 | 11·78 | 0·002 | 131·42 | <0·001 | 3·97 | 0·058 |
| Total stolon length | 1, 23 | 8·81 | 0·007 | 132·01 | <0·001 | 4·89 | 0·037 |
| Number of leaves | 1, 23 | 6·51 | 0·018 | 182·30 | <0·001 | 3·50 | 0·074 |
| Number of ramets | 1, 23 | 6·45 | 0·018 | 174·66 | <0·001 | 4·52 | 0·045 |
Fig. 3.
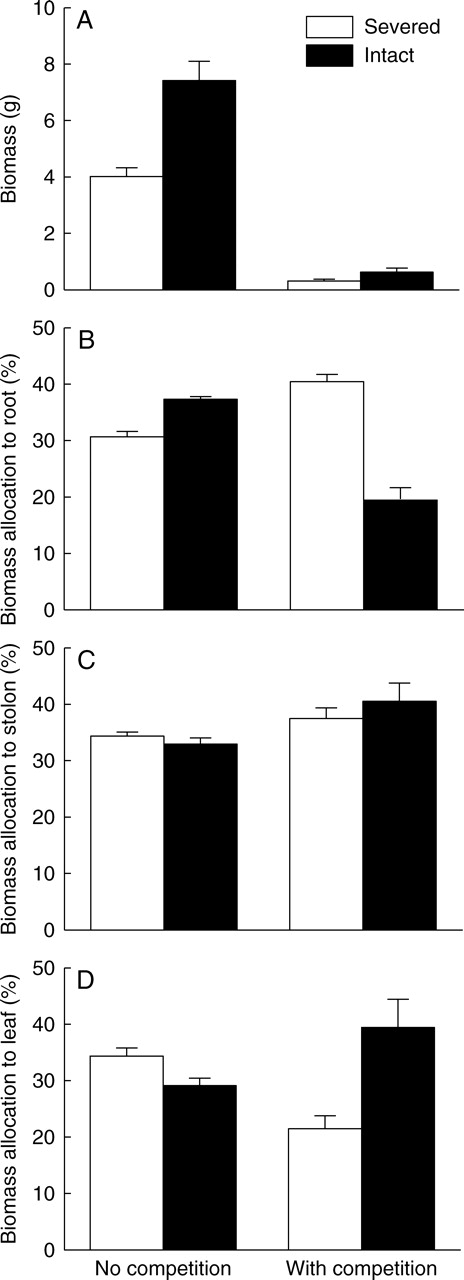
(A) Biomass and (B, C, D) percentage biomass allocation (±1 s.e.) of Alternanthera in the apical sections, grown either with or without competitors and with stolon connections either severed or kept intact.
Fig. 4.
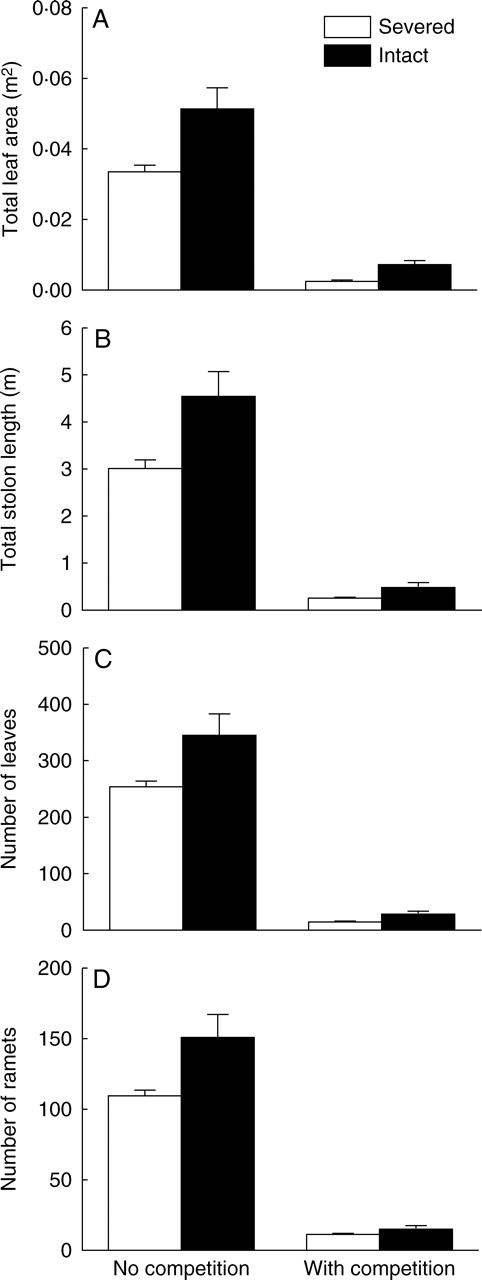
(A) Total leaf area, (B) total stolon length, (C) number of leaves, and (D) number of ramets (±1 s.e.) of Alternanthera in the apical sections, grown either with or without competitors and with stolon connections either severed or kept intact.
Biomass allocation of Alternanthera
Stolon connection, competition and the interaction significantly affected biomass allocation of Alternanthera in the apical sections (Table 2A). Severing stolons increased biomass allocation to the leaves and decreased that to the roots when Alternanthera was grown without competition, whereas it decreased biomass allocation to the leaves and increased that to the roots when Alternanthera was grown with competition (Table 2B; Fig. 3B, D). Biomass allocation to the stolons was not affected by stolon connection, but was significantly larger when the apical ramets were grown with rather than without competition (Table 2B; Fig. 3C).
Table 2.
Summary of MANOVA for effects of stolon connection, competition and their interaction on percentage biomass allocation of Alternanthera in the apical sections (A) Multivariate test statistics and exact F-statistics
| Effect | Wilk's Lambda | F | d.f. | P |
|---|---|---|---|---|
| Stolon connection | 0·410 | 15·81 | 2, 22 | <0·001 |
| Competition | 0·575 | 8·13 | 2, 22 | 0·002 |
| SC × C | 0·178 | 50·72 | 2, 22 | <0·001 |
| (B) Univariate test statistics | |||||||
|---|---|---|---|---|---|---|---|
| Stolon Connection |
Competition |
SC × C |
|||||
| Trait | d.f. | F | P | F | P | F | P |
| Biomass allocation to leaves (%) | 1, 23 | 26·61 | <0·001 | 1·10 | 0·305 | 88·68 | <0·001 |
| Biomass allocation to stolons (%) | 1, 23 | 0·20 | 0·660 | 8·39 | 0·008 | 1·48 | 0·235 |
| Biomass allocation to roots (%) | 1, 23 | 7·12 | 0·014 | 2·27 | 0·146 | 26·49 | <0·001 |
Growth of S. phoenix
Biomass of S. phoenix in the apical sections grown without interspecific competition (no Alternanthera) was 63·6 ± 1·6 g (mean ± s.e.), not significantly different from that when grown with Alternanthera with intact (66·4 ± 3·0 g) or severed (68·7 ± 1·4 g) stolon connections (F2,17 = 1·05, P = 0·37).
DISCUSSION
Integration effects on photosynthetic efficiency of Alternanthera
For several species in favourable conditions, the normal range for Fv/Fm values is between 0·75 to 0·85 (Butler and Kitajima, 1975; Björkman and Demmig, 1987; Demmig and Björkman, 1987). Without competition, Fv/Fm values of both connected and severed ramets of Alternanthera in the apical parts are within the normal range for healthy plants (Fig. 2). Growing with S. phoenix decreased Fv/Fm of Alternanthera to the degree that was outside this range (Fig. 2), suggesting that competition imposed severe stress on Alternanthera (Maxwell and Johnson, 2000). However, the decrease in Fv/Fm of Alternanthera was greatly alleviated by stolon connections, and clonal integration allowed the ramets with competition to maintain their Fv/Fm values within the normal range (Fig. 2). The results, therefore, support our hypothesis and suggest that clonal integration markedly reduced the stress imposed by competition and significantly increased the photosynthetic activity of Alternanthera. Roiloa and Retuerto (2006a) also found that clonal integration significantly buffered the decrease in Fv/Fm of the offspring ramets of Fragaria vesca grown in soils contaminated by heavy metals. As a result, clonal integration improved growth of Alternanthera when it was grown with competitors. However, repeated measurements at different time points are required for a full understanding of the integration effects on Fv/Fm.
Integration effects on growth and competitive ability of Alternanthera
Without competition, stolon connections markedly increased growth of Alternanthera in the apical sections. This is probably because the established adult ramets of Alternanthera in the basal sections supported the growth of the interconnected young, apical ramets and also facilitated the production of new meristems, probably by acropetal (i.e. from older to younger ramets) translocation of carbohydrates through clonal integration. A positive effect of clonal integration on the survival and/or growth of newly produced ramets has also been reported in several other clonal species, including Solidago canadensis (Hartnett and Bazzaz, 1983), Holcus lanatus (Bullock et al., 1994), Psammochloa villosa (Dong, 1999; Dong and Alaten, 1999), Hedysarum laeve (Zhang et al., 2002) and Calamagrostis epigejos (Březina et al., 2006). The results suggest that clonal integration is very important for Alternanthera to explore open space and thus increase its invasiveness in natural habitats.
When the apical parts of Alternanthera were grown with the common lawn grass S. phoenix, its biomass decreased sharply to 8·4–8·7 % of those without competition and ramet and stolon production to 6·3–14·2 %, suggesting that interspecific competition in the present experiment imposed very strong stress on Alternanthera. Unexpectedly, although stolon connection increased growth of Alternanthera with competition, such beneficial effects of clonal integration were much smaller as compared with those without competition. In addition, stolon connection did not affect the relative neighbour effect, suggesting that clonal integration did not affect the competitive ability of Alternanthera (Callaway et al., 2002; Kikvidze et al., 2006). In a south-eastern USA salt marsh, Pennings and Callaway (2000) also found that clonal integration was of little importance for the plants to compete with neighbours for both above- and below-ground resources. Similarly, clonal integration has not been found to influence the competitive ability of several other clonal plants (Schmid and Bazzaz, 1987; Hartnett, 1993; Price and Hutchings, 1996; Peltzer, 2002; Březina et al., 2006). Therefore, clonal integration may be of most importance for clonal plants to explore open, stressful habitats, to better use resource patches and to flourish in low-productivity habitats (Jónsdóttir and Watson, 1997; Pennings and Callaway, 2000; Peltzer, 2002), but may contribute little to their competitive ability under severe stress (Pennings and Callaway, 2000; Peltzer, 2002).
Integration effects on biomass allocation of Alternanthera
Clonal integration significantly modified biomass allocation of Alternanthera, agreeing with previous findings on many other clonal plants (Salzman and Parker, 1985; Friedman and Alpert, 1991; Evans and Whitney, 1992; Birch and Hutchings, 1994; Stuefer et al., 1994, 1996; Wijesinghe and Hutchings, 1997; Roiloa et al., 2007). Without competition, clonal integration increased Alternanthera's biomass allocation to roots at the expense of that to leaves. This is most likely because without competition soil resources (i.e. nutrients and water) were relatively more limiting for the quick spreading of Alternanthera ramets and stolons in the apical sections. For the connected apical ramets, the required carbohydrates could be transported efficiently from the basal ramets so that relatively more biomass could be allocated to roots in order to increase the growth of the whole ramet system in the apical parts. With competition, however, clonal integration significantly increased biomass allocation to leaves and decreased that to roots. This may be because, under severe competition, allocating more biomass to leaves allowed the connected apical Alternanthera ramets to be more easily placed above the canopy of the dense swards of S. phoenix, thus allowing them to harvest the relatively more abundant light resources at that level, whereas relatively scarce soil resources (due to the abundant roots of S. phoenix) could be compensated for by transport from the basal ramets grown without competition. This explanation was supported by the observation that stolons of Alternanthera plants could turn upright when they were grown with the dense swards. Biomass allocation of Alternanthera under competition agrees with the theory of labour division in clonal plants (Alpert and Stuefer, 1997; Hutchings and Wijesinghe, 1997). Thus, effects of clonal integration on the biomass allocation pattern may potentially improve the uptake of resources and enhance the invasiveness of Alternanthera.
Integration effects on the growth of S. phoenix
Unexpectedly, biomass of S. phoenix was not affected by the presence of Alternanthera, suggesting that competition treatments used in the experiment did not impose severe stress on S. phoenix. In addition, clonal integration did not suppress the growth of S. phoenix, in contrast to our prediction. This is probably because in the present experiment the density of S. phoenix was too high to allow an efficient invasion by Alternanthera apical parts (Brown and Fridley, 2003). This view was supported by the fact that with competition, biomass of Alternanthera in the apical sections was reduced to less than 10 % (8·4–8·7 %) as compared with that recorded without competition. Under such severe competition, even though clonal integration contributed significantly to the growth of Alternanthera in the apical sections, the negative effects of severe competition were still substantial due to the relatively short experimental duration (9 weeks). Thus, roles of clonal integration may be more important if the experiment had lasted longer.
CONCLUSIONS
When Alternanthera was grown with dense swards of S. phoenix, clonal integration contributed little to its competitive ability, even though clonal integration greatly benefited its photochemical activity. However, clonal integration was very important for Alternanthera to explore open space. We hypothesize that the effects of clonal integration on the competitive ability of Alternanthera and S. phoenix would be more pronounced if the density of S. phoenix was reduced and/or the duration of the experiment was prolonged. The results suggest that the invasiveness of Alternanthera may be closely related to clonal integration. To allow a generalization and robust extrapolation, more studies are needed.
ACKNOWLEDGEMENTS
We thank two anonymous reviewers for comments on an early version of the manuscript, H. Xu for help with the chlorophyll fluorescence measurements, and L.-L. Zhang, J. Du, H. Yu, Y.-H. Wang, R.-Q. Li, S.-Q. Gao and Q.-G. Cui for assistance with plant harvest. This research was supported by the National Key Basic Research Program of China (2007CB106802) and NSFC (30500070, 30770357).
LITERATURE CITED
- Alpert P. Nutrient sharing in natural clonal fragments of Fragaria chiloensis. Journal of Ecology. 1996;84:395–406. [Google Scholar]
- Alpert P. Clonal integration in Fragaria chiloensis differs between populations: ramets from grassland are selfish. Oecologia. 1999;120:69–76. doi: 10.1007/s004420050834. [DOI] [PubMed] [Google Scholar]
- Alpert P, Mooney HA. Resource sharing among ramets in the clonal herb. Fragaria chiloensis. Oecologia. 1986;70:227–233. doi: 10.1007/BF00379244. [DOI] [PubMed] [Google Scholar]
- Alpert P, Stuefer JF. Division of labour in clonal plants. In: de Kroon H, van Groenendal J, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishing; 1997. pp. 137–154. [Google Scholar]
- Birch CPD, Hutchings MJ. Exploitation of patchlly distributed soil resources by the clonal herb Glechoma hederacea. Journal of Ecology. 1994;82:653–664. [Google Scholar]
- Björkman O, Demmig B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- Blossey B, Kamil J. What determines the increased competitive ability of non-indigenous plants? In. In: Moran VC, Hoffmann JH, editors. Proceedings of the IX international symposium on biological control of weeds; Stellenbosch: University of Cape Town Press; 1996. pp. 3–9. [Google Scholar]
- Brewer JS, Bertness MD. Disturbance and intraspecific variation in the clonal morphology of salt marsh perennials. Oikos. 1996;77:107–116. [Google Scholar]
- Březina S, Koubek T, Münzbergová Z, Herben T. Ecological benefits of integration of Calamagrostis epigejos ramets under field conditions. Flora. 2006;201:461–467. [Google Scholar]
- Brown RL, Fridley JD. Control of plant species diversity and community invasibility by species immigration: seed richness versus seed density. Oikos. 2003;102:15–24. [Google Scholar]
- Bullock JM, Mortimer AM, Begon M. Physiological integration among tillers of Holcus lanatus: age-dependence and responses to clipping and competition. New Phytologist. 1994;128:737–747. [Google Scholar]
- Butler WL, Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochimica et Biophysica Acta. 1975;376:116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Callaway RM, Rrooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- Crawley MJ. The population biology of invaders. Philosophical Transactions of the Royal Society of London, Series B. 1986;314:711–731. [Google Scholar]
- Demmig B, Björkman O. Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta. 1987;171:171–184. doi: 10.1007/BF00391092. [DOI] [PubMed] [Google Scholar]
- Dong M. Effects of severing rhizome on clonal growth in rhizomatous grass species Psammochloa villosa and Leymus secalinus. Acta Botanica Sinica. 1999;41:194–198. [Google Scholar]
- Dong M, Alaten B. Clonal plasticity in response to rhizome severing and heterogeneous resource supply in the rhizomatous grass Psammochloa villosa in an Inner Mongolian dune, China. Plant Ecology. 1999;141:53–58. [Google Scholar]
- Evans JP, Whitney S. Clonal integration across a salt gradient by a nonhalophyte, Hydrocotyle bonariensis (Apiaceae) American Journal of Botany. 1992;79:1344–1347. [Google Scholar]
- Friedman D, Alpert P. Reciprocal transport between ramets increases growth of Fragaria chiloensis when light and nitrogen occurred in separate patches but only if patches are rich. Oecologia. 1991;86:76–80. doi: 10.1007/BF00317392. [DOI] [PubMed] [Google Scholar]
- Gunasekera L, Bonila J. Alligator weed: tasty vegetable in Australian backyards? Journal of Aquatic Plant Management. 2001;39:17–20. [Google Scholar]
- Hartnett DC. Regulation of clonal growth and dynamics of Panicum virgatum (Poaceae) in tallgrass prairie: effects of neighbour removal and nutrient addition. American Journal of Botany. 1993;80:1114–1120. [Google Scholar]
- Hartnett DC, Bazzaz FA. Physiological integration among intraclonal ramets in Solidago canadensis. Ecology. 1983;64:779–788. [Google Scholar]
- Hartnett DC, Bazzaz FA. The integration of neighbourhood effects by clonal genets of Solidago canadensis. Journal of Ecology. 1985;73:415–428. [Google Scholar]
- Hester MW, Mckee KL, Burdick DM, Kock MS, Flynn KM, Patterson S, Mendelssohn IA. Clonal integration in Spartina patens across a nitrogen and salinity gradient. Canadian Journal of Botany. 1994;72:767–770. [Google Scholar]
- Holm L, Doll J, Holm E, Pancho J, Herberger J. World weeds: natural histories and distribution. New York: John Wiley & Sons, Inc; 1997. [Google Scholar]
- Hutchings MJ, Wijesinghe DK. Patchy habitats, division of labour and growth dividends in clonal plants. Trends in Ecology and Evolution. 1997;12:390–394. doi: 10.1016/s0169-5347(97)87382-x. [DOI] [PubMed] [Google Scholar]
- Jakobs G, Weber E, Edwards PJ. Introduced plants of the invasive Solidago gigantea (Asteraceae) are larger and grow denser than conspecifics in the native range. Diversity and Distribution. 2004;10:11–19. [Google Scholar]
- Johnson GN, Scholes JD, Young AJ, Horton P. The dissipation of excess excitation energy in British plant species. Plant, Cell and Environment. 1993;16:673–679. [Google Scholar]
- Jónsdóttir IS, Watson MA. Extensive physiological integration: an adaptive trait in resource-poor environments? In. In: de Kroon H, van Groenendal J, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishing; 1997. pp. 109–136. [Google Scholar]
- Julien MH, Bourne AS. Alligator weed is spreading in Australia. Plant Protection Quarterly. 1988;3:91–96. [Google Scholar]
- Julien MH, Stanley JN. The management of alligator weed, a challenge for the new millennium. In: Blackmore P, editor. Proceedings of the 10th Biennial Noxious Weeds Conference, New South Wales Department of Agriculture, Ballina, Australia; July 20–22; 1999. pp. 2–13. Practical weed management: protecting agriculture and the environment. [Google Scholar]
- Julien MH, Skarratt B, Maywald GF. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. Journal of Aquatic Plant Management. 1995;33:55–60. [Google Scholar]
- Kikvidze Z, Khetsuriani L, Kikodze D, Callaway RM. Seasonal shifts in competition and facilitation in subalpine plant communities of the central Caucasus. Journal of Vegetation Science. 2006;17:77–82. [Google Scholar]
- Liu J, Dong M, Miao S, Li Z, Song M, Wang R. Invasive alien plants in China: role of clonality and geographical origin. Biological Invasions. 2006;8:1461–1470. [Google Scholar]
- Lötscher M. Resource allocation in clonal plants. Progress in Botany. 2006;67:537–561. [Google Scholar]
- Ma R, Wang R. Invasive mechanism and biological control of alligator weed, Alternanthera philoxeroides (Amaranthaceae), in China. Chinese Journal of Applied and Environmental Biology. 2005;11:246–250. [Google Scholar]
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications. 2000;10:689–710. [Google Scholar]
- Marshall C. Source–sink relations of interconnected ramets. In: de Kroon H, van Groenendal J, editors. Clonal growth in plants: regulation and function. The Hague: SPB Academic Press; 1990. pp. 23–41. [Google Scholar]
- Maurer DA, Zedler JB. Differential invasion of a wetland grass explained by tests of nutrients and light availability on establishment and clonal growth. Oecologia. 2002;131:279–288. doi: 10.1007/s00442-002-0886-8. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature. 2003;42:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- Oborny B, Podani J. Clonality in plant communities. Uppsala: Opulus Press; 1995. [Google Scholar]
- Peltzer DA. Does clonal integration improve competitive ability? A test using aspen (Populus tremuloides [Salicaceae]) invasion into prairie. American Journal of Botany. 2002;89:494–499. doi: 10.3732/ajb.89.3.494. [DOI] [PubMed] [Google Scholar]
- Pennings SC, Callaway RM. The advantages of clonal integration under different ecological conditions: a community-wide test. Ecology. 2000;81:709–716. [Google Scholar]
- Pimental D, Lach L, Zuniga R, Morrison D. Environmental and economic costs of nonindigenous species in the United States. BioScience. 2000;50:53–65. [Google Scholar]
- Price EAC, Hutchings MJ. The effects of competition on growth and form in Glechoma hederacea. Oikos. 1996;75:279–290. [Google Scholar]
- Pyšek P. Clonality and plant invasion: can a trait make a difference? In. In: de Kroon H, van Groenendal J, editors. The ecology and evolution of clonal plants. Leiden: Backhuys Publishers; 1997. pp. 405–427. [Google Scholar]
- Pyšek P, Brock JH, Bímová K, Mandák B, Jarošík V, Koukolíková I, Pergl J, Štěpánek J. Vegetative regeneration in invasive Reynoutria (Polygonaceae) taxa: the determinant of invasibility at the genotype level. American Journal of Botany. 2003;90:1487–1495. doi: 10.3732/ajb.90.10.1487. [DOI] [PubMed] [Google Scholar]
- Reichard S. Invasive woody plant species in North America. Seattle: University of Washington Press; 1997. [Google Scholar]
- Roiloa SR, Retuerto R. Presence of developing ramets of Fragaria vesca L. increases photochemical efficiency in parent ramets. International Journal of Plant Sciences. 2005;166:795–803. [Google Scholar]
- Roiloa SR, Retuerto R. Physiological integration ameliorates effects of serpentine soils in the clonal herb Fragaria vesca. Physiologia Plantarum. 2006;a 128:662–676. [Google Scholar]
- Roiloa SR, Retuerto R. Small-scale heterogeneity in soil quality influences photosynthetic efficiency and habitat selection in a clonal plant. Annals of Botany. 2006;b 98:1043–1052. doi: 10.1093/aob/mcl185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiloa SR, Alpert P, Tharayil N, Hancock G, Bhowmik PC. Greater capacity for division of labour in clones of Fragaria chiloensis from patchier habitats. Journal of Ecology. 2007;95:397–405. [Google Scholar]
- Salzman AG, Parker MA. Neighbours ameliorate local salinity stress for a rhizomatous plant in a heterogeneous environment. Oecologia. 1985;65:273–277. doi: 10.1007/BF00379229. [DOI] [PubMed] [Google Scholar]
- Schmid B, Bazzaz FA. Clonal integration and population structure in perennials: effects of severing rhizome connections. Ecology. 1987;68:2016–2022. doi: 10.2307/1939892. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Bilger W, Hormann H, Neubauer C. Chlorophyll fluorescence as a diagnostic tool: basics and some aspects of practical relevance. In: Raghavendra AS, editor. Photosynthesis: a comprehensive treatise. Cambridge: Cambridge University Press; 1998. pp. 320–336. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 2nd edn. San Francisco: Freeman; 1981. [Google Scholar]
- Stuefer JF, During HJ, de Kroon H. High benefits of clonal integration in two stoloniferous species, in response to heterogeneous light environments. Journal of Ecology. 1994;82:511–518. [Google Scholar]
- Stuefer JF, de Kroon H, During HJ. Exploitation of environmental heterogeneity by spatial division of labor in a clonal plant. Functional Ecology. 1996;10:328–334. [Google Scholar]
- Wang B, Li W, Wang J. Genetic diversity of Alternanthera philoxeroides in China. Aquatic Botany. 2005;81:277–283. [Google Scholar]
- Wang R, Wang Y. The discussion on feasibility of biological control and occurrence of Alternanthera philoxeroides in south China. Journal of Weed Science. 1988;3:36–40. [Google Scholar]
- Wilsey B. Clonal plants in a spatially heterogeneous environment: effects of integration on Serengeti grassland response to defoliation and urine-hits from grazing mammals. Plant Ecology. 2002;159:15–22. [Google Scholar]
- Wijesinghe DA, Hutchings MJ. The effects of spatial scale of environmental heterogeneity on the growth of a clonal plant: an experimental study with Glechoma hederacea. Journal of Ecology. 1997;85:17–28. [Google Scholar]
- Xu C, Zhang W, Fu C, Lu B. Genetic diversity of alligator weed in China by RAPD analysis. Biodiversity and Conservation. 2003;12:637–645. [Google Scholar]
- Yu F, Chen Y, Dong M. Clonal integration enhances survival and performance of Potentilla anserina, suffering from partial sand burial on Ordos plateau, China. Evolutionary Ecology. 2002;15:303–318. [Google Scholar]
- Yu F, Dong M, Krüsi B. Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytologist. 2004;162:697–704. doi: 10.1111/j.1469-8137.2004.01073.x. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang C, Dong M. The significance of rhizome connection of semi-shrub Hedysarum laeve in an Inner Mongolian dune, China. Acta Oecologica. 2002;23:109–114. [Google Scholar]