Abstract
Background
The cells of growing plant organs secrete an extracellular fibrous composite (the primary wall) that allows the turgid protoplasts to expand irreversibly via wall-yielding events, which are regulated by processes within the cytoplasm. The role of the epidermis in the control of stem elongation is described with special reference to the outer epidermal wall (OEW), which forms a ‘tensile skin’.
Novel Facts
The OEW is much thicker and less extensible than the walls of the inner tissues. Moreover, in the OEW the amount of cellulose per unit wall mass is considerably greater than in the inner tissues. Ultrastructural studies have shown that the expanding OEW is composed of a highly ordered internal and a diffuse outer half, with helicoidally organized cellulose microfibrils in the inner (load-bearing) region of this tension-stressed organ wall. The structural and mechanical backbone of the wall consists of helicoids, i.e. layers of parallel, inextensible cellulose microfibrils. These ‘plywood laminates’ contain crystalline ‘cables’ orientated in all directions with respect to the axis of elongation (isotropic material). Cessation of cell elongation is accompanied by a loss of order, i.e. the OEW is a dynamic structure. Helicoidally arranged extracellular polymers have also been found in certain bacteria, algae, fungi and animals. In the insect cuticle crystalline cutin nanofibrils form characteristic ‘OEW-like’ herringbone patterns.
Conclusions
Theoretical considerations, in vitro studies and computer simulations suggest that extracellular biological helicoids form by directed self-assembly of the crystalline biopolymers. This spontaneous generation of complex design ‘without an intelligent designer’ evolved independently in the protective ‘skin’ of plants, animals and many other organisms.
Keywords: Cellulose, cell elongation, epidermis, growth, helicoidal wall
INTRODUCTION
Land plants (embryophytes) continue to grow as long as the multicellular system is alive. This developmental strategy of the sessile, green (photoautotrophic) organisms, which form the kingdom Plantae, is known as indeterminate growth. In contrast to plants, most animals are motile: they move through their environment in search of food. Typically, animals cease to grow after the organism has reached a defined, species-specific size (determinate growth).
The contrasting survival strategies of animals versus plants are paralleled by the behaviour of the ‘building blocks’ of these multicellular macro-organisms. Animal physiology is to some extent related to cell movement (circulation of blood cells, migration of cells during embryonic development, etc.). In contrast, the cells of the plant body are immobile, fixed ‘boxes’ that are encased by walls. Plant cells contain photosynthetically active organelles (chloroplasts), which evolved from once free-living cyanobacteria. In the light, chloroplasts fix carbon dioxide and hence provide a surplus of sugars that form the polysaccharides of the extracellular walls which surround the protoplasts within the plant body (Kutschera and Niklas, 2004, 2005).
Much progress in photosynthesis research over past decades is documented in the current literature. However, the structure and function of the growing plant cell wall is still far from clear (Fry, 2004; Somerville, 2006). The aim of this Briefing is to review one aspect of cell wall research that has recently gathered momentum owing to the publication of a major research paper (Savaldi-Goldstein et al., 2007): the architecture and physiological role of the sturdy outer epidermal wall (OEW). In addition, striking similarities at the ultrastructural level of extracellular matrices are shown here to exist between plants and animals. This little-known example for convergent evolution of a complex biological structure is discussed.
THE BOX CONSTRUCTION OF PLANTS: CELL WALLS AND CELLULOSE
In plant tissues, the walls of adjacent cells are glued together by a middle lamella so that sliding between the cell layers within the organism is prevented. However, considerable intercellular spaces remain between the individual cells, thus facilitating gas exchange (Fig. 1C). Owing to the turgor pressure of the vacuolar contents of the encased protoplasts, the walls of well-watered plants are under considerable stress; this gives rise to a hydrostatic skeleton similar to that of earthworms. However, plant cells grow by turgor-driven cell enlargement, a mode of volume increase that usually does not occur in animals.
Fig. 1.
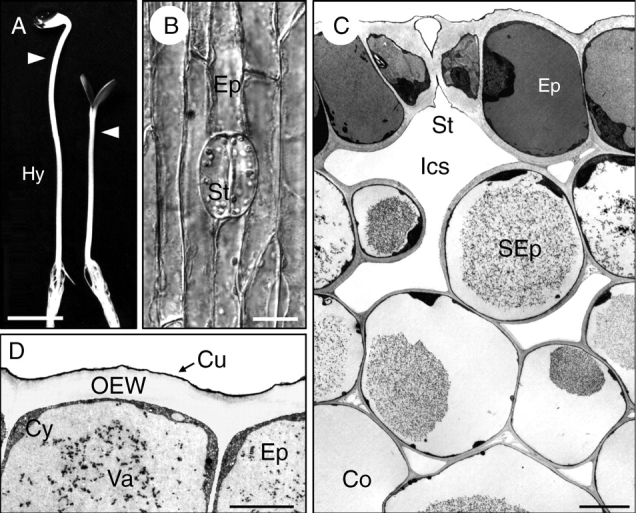
Axial plant organs and the growth-limiting outer cell layers. (A) Photograph of sunflower (Helianthus annuus) seedlings that were either grown for 4 d in darkness (left) or for 3 d in darkness and 1 d in continuous white light (irradiated plant, right). (B) Light micrograph of the epidermis in the sub-apical region of the hypocotyl (arrowheads in A) of an irradiated seedling. (C, D) Electron micrographs of transverse sections through the peripheral four cell layers in the sub-apical region of a 4-d-old irradiated seedling (C) and the epidermis (D). Co, cortex; Cu, cuticle; Cy, cytoplasm; Ep, epidermis; Hy, hypocotyl; Ics, intracellular space; OEW, outer epidermal wall; SEp, sub-epidermis; St, stoma; Va, vacuole. Scale bars: (A) 1 cm, (B) 20 µm, (C) 10 µm, (D) 2 µm.
The ‘box construction’ of the plant body has yet another consequence. It has precluded the phylogenetic development of an ‘animal-like’ contractile circulatory system: water is transported in fixed tubes of the xylem via the transpiration stream, and sugars (sucrose etc.) are translocated osmotically within the sieve-tubes of the phloem.
Most studies on the composition and ultrastructure of the cell walls have been carried out with seedlings of dicotyledonous plants (angiosperms). Figure 1A shows two 4-d-old seedlings of sunflower (Helianthus annuus) that were either raised in darkness (rapidly growing, etiolated) or irradiated with white light (green, de-etiolated). Light treatment not only has considerable consequences for the developmental strategy of the juvenile plant (scoto- versus photomorphogenesis in dark- and light-grown plants, respectively), but also affects the thickness and mechanical properties of the cell walls.
By well-established fractionation techniques (Carpita and Gibeaut, 1993; Fry, 2004) it was shown that the isolated, dry walls of growing sunflower hypocotyls are composed of approx. 25 % pectic substances, approx. 40 % hemicelluloses and approx. 25 % cellulose. In addition, glycoproteins and phenolic substances can be extracted from cell walls. These compounds usually constitute less than 10 % of the wall mass (Kutschera, 1990). Hence, the expanding (primary) wall of growing stems is a composite structure that displays characteristic yielding properties. Under the force of turgor pressure (approx. 0·5 MPa in the sub-apical region of the hypocotyls shown in Fig. 1A) the walls are maintained under considerable stress. As a result, wall extensibility (plasticity) determines the rate of cell elongation and hence of organ growth (Niklas, 1992; Kutschera, 2000).
Experiments with plant cell cultures that almost completely lacked a cellulose–hemicellulose (xyloglucan) network led to the conclusion that cellulose microfibrils (approx. 15 % of the volume of the wall) contribute at least 70 % of the mechanical strength to the primary wall (Kutschera, 2001). Cellulose microfibrils (Fig. 2A) are composed of about 36 hydrogen-bonded chains that each contain 500–14 000 β‐1,4-linked glucose units (Somerville, 2006). These insoluble cable-like structures, which have a stiffness of about 130 GPa (Vincent and Wegst, 2004), are laid down by apposition on the inner surface of the growing wall, whereas pectins, hemicelluloses and glycoproteins (i.e. the matrix components) are secreted via the Golgi apparatus into the network of microfibrils. Hence, the primary wall can be regarded as a pliable (plastic/elastic) fibrous composite like a flexible fibreglass, with stiff cellulose microfibrils that are embedded into a less stiff matrix of polysaccharides and glycoproteins (Neville, 1985; Niklas, 1992, 2004; Carpita and Gibeaut, 1993; Fry, 2004).
Fig. 2.
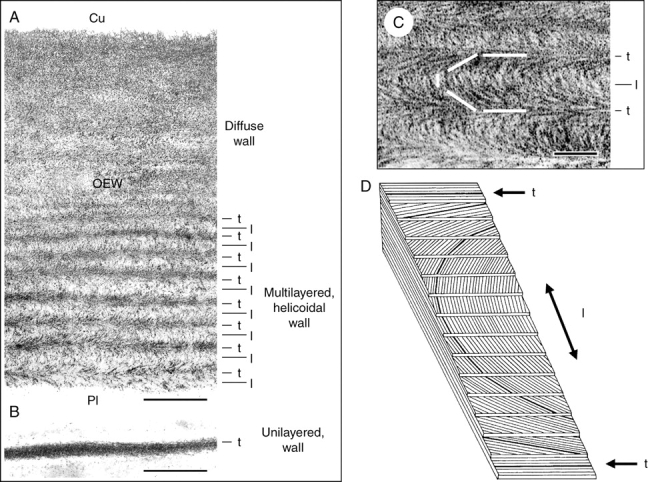
Cellulose architecture of outer and internal walls of a 4-d-old sunflower seedling that was grown in darkness and white light (see Fig. 1A). Transmission electron micrographs of oblique transverse sections through the outer epidermal wall (A, C) and a wall in the pith (B). The cellulose microfibrils were stained with periodic acid–thiocarbohydrazide silver protein (PATAg) as described by Hodick and Kutschera (1992). A model of a helicoid (D) shows parallel cellulose microfibril layers that are packed with a constant rotation (adapted from Mulder and Emons, 2001). l, longitudinally orientated; t, transversely orientated cellulose layers with respect to the axis of cell elongation; Cu, position of the cuticle; OEW, outer epidermal wall; Pl, position of the plasma membrane. Scale bars = 0·5 µm (A, B), 0·1 µm (C).
At the root end of the hypocotyl (Fig. 1A) the cells have reached their final size. These non-growing cells deposit a lignified secondary wall, which provides additional mechanical strength due to a cellulose content of up to 80 % per dry mass. As wood contains considerable amounts of cellulose, this polysaccharide is arguably the most abundant biopolymer of the biosphere. It should be noted that some heterotrophic bacteria (e.g. Acetobacter xylinum, Agrobacterium tumefaciens), slime moulds and one group of animals (the urochordates, e.g. ascidians such as Cionia intestinalis) have the ability to biosynthesize cellulose (Matthysse et al., 2004). However, on a biomass basis, plants, cyanobacteria and protists (algae) are the ‘deposits’ of almost all the cellulose on Earth.
Despite of its importance at a global scale, the exact mode of cellulose biosynthesis in plants has not yet been elucidated. Although the basic biosynthetic event consists of the simple polymerization of glucose units from a substrate such as UDP-glucose to form the homopolymer β‐1,4-d-glucan (i.e. cellulose; the glucan chains further associate into microfibrils), the biochemical and genetic regulators of this process are still unclear (Somerville, 2006; Joshi and Mansfield, 2007). Moreover, in wounded cells the β‐1,3-glucan callose is made (Scherp et al., 2001). The interactions between cellulose synthase and callose synthase are complex and remain a matter of debate.
It should be noted that the relationship between cortical microtubules and the orientation of cellulose microfibrils is another problem of ongoing debate that is beyond the scope of this article (Kutschera and Bette, 1998; Emons et al., 2007).
THE EPIDERMAL CELL LAYER AND THE REGULATION OF GROWTH
Although developing seedlings (Fig. 1A) consist of three organs (root, hypocotyl, cotyledons) that display co-ordinated, light-dependent growth patterns, physiologists have largely focused on the plant axis (hypocotyl) in their efforts to elucidate the mechanism of cell enlargement. Axial organs (stems, coleoptiles) grow almost exclusively in length owing to the anisotropic properties of the cell walls. Numerous studies have shown that the dermal tissue system, which forms an outer protective covering analogous to the skin of animals, is composed of thick-walled cells that display specific mechanical properties (relatively low plastic extensibility etc.), whereas the walls of the internal tissues (cortex, vascular bundles and pith) are extensible and spontaneously elongate upon isolation. As a result of these differences in the mechanical properties of outer and inner walls that are ‘glued’ together, growing stems are characterized by tissue tension (for historical reviews of this topic, see Niklas, 1992; Peters and Tomos, 1996; Kutschera, 1992, 2001; Kutschera and Niklas, 2007). As the ‘tension-stressed skin’ of the hypocotyl (essentially the epidermis) is stretched by the inner core of turgid cells that are maintained in a state of relative compression, the term ‘tissue stresses’ is more appropriate (tension and compression are stresses, i.e. forces per unit cross-sectional area). In hypocotyls and coleoptiles, the OEW (Fig. 1D) is the structure that bears most of the stress exerted by the expanding internal tissues; accordingly, this ‘organ wall’, which contains up to half the total wall material of the hypocotyl (Kutschera, 1992, 2001; Derbyshire et al., 2007), represents a growth-limiting sheath of the multicellular system (Kutschera, 1992, 2001, 2006; Niklas and Paolillo, 1997, 1998; Passioura and Boyer, 2003; Kutschera and Niklas, 2007; Derbyshire et al., 2007).
This well-supported ‘epidermal growth control theory’ of axis elongation has recently been challenged by Savaldi-Goldstein et al. (2007), who argued that the epidermal cell layer both promotes and restricts stem growth by providing an unspecified signal to the internal tissues. Their conclusion is based on experiments with brassinosteroid (BR) mutants of Arabidopsis thaliana. In the absence of BRs, A. thaliana plants are dwarfs, which is to a large extent attributable to defects in cell elongation. Savaldi-Goldstein et al. (2007) documented that the expression of a BR-biosynthetic enzyme (or a BR receptor) in the epidermis, but not in the internal tissues, of BR-minus mutants can overcome their dwarf phenotype. Based on these and other findings they concluded that the epidermis may have a dual role in the regulation of organ growth.
Kutschera and Niklas (2007) have shown that the classical ‘tensile skin’ theory of stem elongation is supported by a solid body of experimental evidence. Moreover, they presented novel data that further document that the epidermis represents the growth-controlling tissue and that in plants grown in relatively dry air the thickened cuticle may play a major role in restricting the rate of organ expansion. In accordance with Savaldi-Goldstein et al. (2007), it was concluded that hormonal signals may be exchanged between the epidermal and internal tissues that co-ordinate the growth process of the multicellular system. This ‘tissue interaction model’ of stem elongation postulates that as-yet unknown ‘messages’ from the growth-limiting peripheral cell(s) to the ground tissue exist (Kutschera and Niklas, 2007). However, a role of the epidermis in actively driving shoot growth is not supported by empirical evidence.
In discussions on the role of the epidermis the functional significance of the stomata (Fig. 1B), which consist of two guard-cells that surround a pore, is usually ignored. Transverse sections through the epidermal cell layer reveal that the guard-cells are characterized by considerably thickened outer walls (Fig. 1C). The question of how the rate of elongation of the bean-shaped guard-cells, which are symplastically isolated, is co-ordinated with that of the ‘normal’ cylindrical epidermal cells remains unanswered. In the following discussion of the thickness and cellulose architecture of the OEW, only the ‘average epidermal cells’ (Fig. 1B, D) are addressed, which represent more than 90 % of the ‘building blocks’ of this dermal tissue system.
WALL BIOSYNTHESIS AND CELL ELONGATION
In numerous books and articles ‘the plant cell wall’ is described at length. Most of the authors implicitly assume that embryophytes are characterized by a more or less structurally uniform extracellular polymeric ‘sacculus’, which can be depicted by one model (for a recent example, see Cosgrove, 2005, and references cited therein). The results summarized here document that this view is not correct.
In the model plant Helianthus annuus, the OEW of dark-grown (etiolated) versus light-grown seedlings (Fig. 1A) was analysed in detail, with a focus on the sub-apical region of the hypocotyls (Kutschera, 1990; Hodick and Kutschera, 1992). In a study based on a series of standard transmission electron micrographs, it was shown that during the growth of the etiolated sunflower hypocotyl the OEW does not maintain its thickness: in 2-d-old hypocotyls (length approx. 5 mm), wall thickness was 1·79 ± 0·01 µm, whereas in 6-d-old hypocotyls an average value of 0·90 ± 0·01 µm was measured (mean ± s.e.m., n = 24). Hence, considerable wall thinning occurs during the development of the sunflower stem in darkness (about –50 %). A comparison between 4-d-old etiolated and irradiated (de-etiolated) seedlings (Fig. 1A, B) revealed that the thickness of the OEW was considerably enhanced as a result of light-treatment: values of 1·14 ± 0·01 µm (darkness) and 2·18 ± 0·02 µm (irradiated) were measured (mean ± s.e.m., n = 24).
A detailed quantitative analysis of the thicknesses of the outer and internal cell walls in 4-d-old de-etiolated (green) seedlings (Fig. 1A) revealed that the OEW is the thickest wall of the organ (about 2 µm; Fig. 2A). The walls of the sub-epidermis are considerably thinner and in the cortex of the hypocotyl an average wall thickness of 0·2 µm was measured. The walls of the pith cells, which are shown in Fig. 2B, had an average thickness of about 0·1 µm. Hence, in light-grown sunflower hypocotyls, the OEW is approximately 20 times thicker than the walls in the centre of the organ. These results are in accordance with corresponding values measure on other axial plant organs (Kutschera, 1992; Niklas and Paollilo, 1997; Refregier et al., 2004; Derbyshire et al., 2007).
The two-fold thinning of the OEW in etiolated sunflower seedlings between 2 and 6 d after sowing is accompanied by an approx. 6·5-fold increase in the average length of the epidermal cells (approx. 40 vs. approx. 260 µm in 2- and 6-d-old seedlings, respectively) (my unpubl. data). These results demonstrate that considerable amounts of wall polymers must have been added to the expanding walls during stem elongation. However, the relationship between wall synthesis and growth is still unclear (Proseus and Boyer, 2006). Experiments with sunflower seedlings have shown that the net deposition of all three classes of polysaccharides (cellulose, hemicellulose, pectins) is independent of the rate of elongation in both the epidermal and the internal walls (Carrington and Firn, 1985; Kutschera, 1990; Edelmann and Kutschera, 1993; Kutschera and Heiderich, 2002). A detailed study with dark-grown seedlings of A. thaliana yielded similar results. Cellulose deposition and net wall biosynthesis occur at rates that are not related to cell elongation (Refregier et al., 2004; Derbyshire et al., 2007). However, these data do not preclude the possibility that subtle, growth-related changes may occur, such as the secretion and incorporation of expansion-limiting glycoproteins (Kutschera and Edelmann, 2005; Proseus and Boyer, 2006).
The studies summarized above led to the conclusion that the amount of cellulose per cell is much larger in the epidermis than in the inner tissues. This thick epidermal ‘cellulose sheath’ is discussed in the next section.
CELLULOSE ARCHITECTURE OF THE CELL WALLS
In conventionally stained ultrathin cross-sections, the thickened peripheral wall(s) of sunflower hypocotyls display no fine structural pattern (Figs 1C, D). However, after extraction of pectic substances (which are rich in calcium ions; Fry, 2004) from excised 2-mm stem segments, ultrathin sections can be obtained that are amenable to a specific staining technique for cellulose (Hodick and Kutschera, 1992). Figure 2A–C show transmission electron micrographs of extracted/stained sections that were cut oblique to the cross-sectional plane by about 45°. In the thick OEW, a striation or polylamellation is apparent that is not visible in the thin walls of the pith. In the inner half of the OEW, the arced patterns typical of helicoidal cell walls become apparent. This specific wall architecture was postulated two decades ago (see Neville, 1985). According to Neville, a helicoid is defined as a composite structure in which parallel cellulose microfibril monolayers are packed with a stepwise rotation with respect to their neighbours. This ‘rotated ply structure’ is depicted schematically in Fig. 2D, indicating a rotation during deposition of the cellulose monolayers. The positions of longitudinally and transversely orientated layers of microfibrils are indicated by arrows in this figure. In the thickened walls of the sub-epidermal cells of Helianthus hypocotyls, helicoidal wall structures were also detected. However, in the cortex and pith very thin monolayered walls occur with cellulose microfibrils orientated primarily transversely (Fig. 2B).
These results, which are in accordance with studies on the fine structure of the epidermal walls in other growing organs (Roland et al., 1982; Neville, 1985, 1993; Levy, 1992; Niklas and Paollilo, 1997; Emons and Mulder, 2000; Mulder and Emons, 2001; Suslov and Verbelen, 2006) led to the following conclusions. (1) The OEW (approx. 2 µm thick) is the sturdiest wall of the hypocotyl; this ‘organ sheath’ differs in thickness depending on the age and growing conditions of the juvenile plant (darkness vs. light). (2) There is a gradient in wall thickness across the diameter of the hypocotyl, with the thinnest walls in the pith. (3) The thick OEW is a polylamellated, helicoidal structure with cellulose microfibrils orientated more or less longitudinally with respect to the axis of elongation, whereas the extensible walls of the inner tissues (notably the pith) are unilayered with transversely orientated microfibrils packed together into very thin layers (thickness approx. 0·1 µm).
Hence, the notion of a more or less uniform ‘plant cell wall’ (Cosgrove, 2005) is an oversimplification of reality. We have to distinguish between thick, multilayered peripheral and much thinner, unilayered internal walls. In sunflower seedlings that were raised at approx. 100 % relative humidity (closed plastic boxes) the OEW is covered by a thin waxy cuticle (Fig. 1D), which is absent in the extracted/stained wall preparation because of the chemical treatments of the samples (Fig. 2A). The structure and function of this waxy outer ‘skin’, which can reach a considerable thickness in plant organs that grow in air of 50 % relative humidity (i.e. natural conditions), has been discussed by Kutschera and Niklas (2007).
According to Neville (1985), helicoids are ‘plywood laminates’, in each component layer of which the cellulose microfibrils lie in parallel, in the plane of the layer. As consecutive layers are set at a small angle, the ‘grain’ changes direction so that a structure resembling the steps of a spiral staircase becomes apparent (Fig. 2C, D). As mentioned above, these arced patterns in oblique sections of the OEW are restricted to the ‘young’, load-bearing inner half of the wall (multilayered, helicoidal region). In the ‘older’ outer part of the OEW no clear lamellation is apparent, i.e. a diffuse or non-ordered cellulose architecture can be deduced from the electron micrographs (Fig. 2A).
In a classical study, Roland et al. (1982) analysed the growth gradient in hypocotyls of etiolated mung bean (Vigna radiata) seedlings. They observed that, in the OEW, thickness and cellulose order (helicoids in the inner half) reached a maximum during the exponential phase of cell elongation. Cessation of growth was accompanied by wall thinning and a loss of order (diffuse elongated walls without helicoids). These results are in accordance with the representative electron micrograph shown in Fig. 2A, i.e. the growing OEW is a structure that dissipates from ordered (inner) to disordered (outer) regions. It follows that during cell growth an active realigning process occurs that creates the diffuse structure within the non-growing part of the peripheral wall.
HELICOIDAL STRUCTURES IN ALL FIVE KINGDOMS OF LIFE
The results summarized above document that helicoidal structures occur in the OEW of the growing peripheral cells in stems and coleoptiles. Such cell walls, in which layers of parallel cellulose microfibrils are assembled in such a way as to give a characteristic herringbone pattern in oblique sections (Fig. 2C, D), were also found in the supporting tissues of various plants, such as collenchyma, sclerenchyma fibres and conifer tracheids (Neville, 1985). Hence, this specific cellulose microfibril architecture is widely distributed in the kingdom Plantae.
In green algae, such as the giant unicellular stonewort Nitella translucens (one of the nearest living relatives of the embryophytes; Scherp et al., 2001), helicoidal walls were observed and analysed in detail (Levy, 1992). According to Neville (1993), helicoidal extracellular structures have also been documented in other algae (kingdom Protoctista), some bacteria (Xanthomonas) and cyanobacteria (Rivularia) (kingdom Monera), spore walls of some primitive Zygomycetes (kingdom Fungi), and animals.
It has already been mentioned that urochordates (e.g. ascidians), ubiquitous marine invertebrates, have evolved the ability to biosynthesize cellulose (Matthysse et al., 2004). In these sessile animals, cellulose is a product of the epidermis and is incorporated into the outer coat. According to Neville (1993), the tunica of at least one species (Halocynthia papillosa) displays a helicoidal cellulose architecture, analogous to that of the OEW in plants (Fig. 2). In many other groups of invertebrate animals, such as nematodes, leeches (Erpobdella octoculata) and insects (the silk moth Hyalophora cecropia), helicoidal extracellular structures were found in eggshells (Neville, 1993).
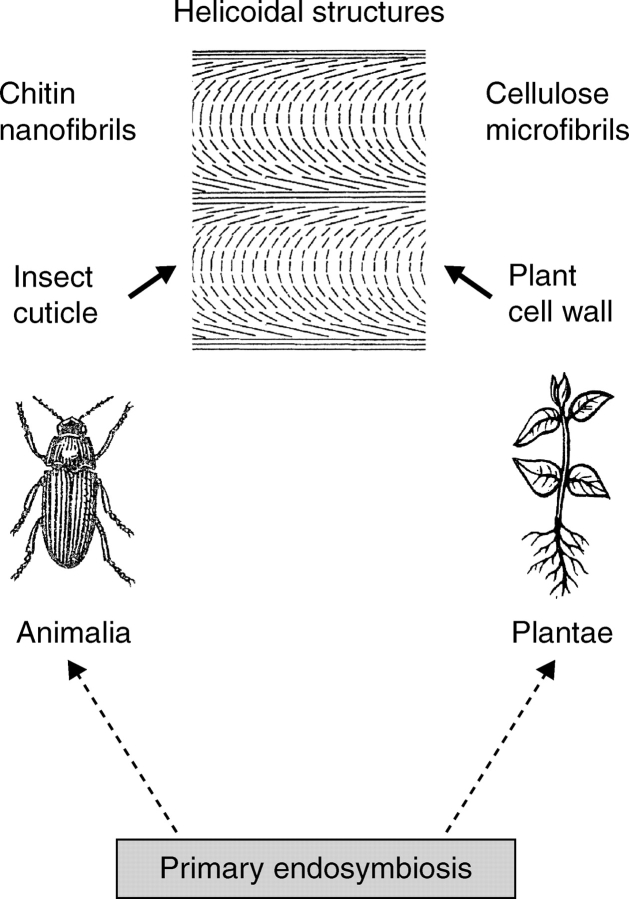
The most striking example in the kingdom Animalia is the insect cuticle, a fibrous composite secreted by a single layer of epidermal cells that covers the entire surface of the invertebrate. This multi-layered lightweight exoskeleton of the Insecta can be thin (1 µm in the hindgut of the Ephemeroptera) or relatively thick (>200 µm in the elytra of large Coleoptera). The insect cuticle is a natural biocomposite that consists of arrangements of crystalline chitin nanofibrils that are embedded into an aqueous matrix of proteins, polyphenols and lipids (Vincent and Wegst, 2004). Numerous studies have shown that the cuticle of insects is a helicoidal structure analogous to that of the OEW in plants, with chitin nanofibres arranged in typical ‘plywood laminates’ (Neville, 1985, 1993; Chen et al., 2006). This striking example for convergent evolution (Fig. 3) is discussed below.
Fig. 3.
Convergent evolution of helicoidal structures in animals and plants (kingdoms Animalia and Plantae, respectively). Chitin nanofibrils in the exoskeleton (cuticle) of insects and cellulose microfibrils in the epidermal wall(s) of plants display the same kind of architecture (design), which is largely the result of directed molecular self-assembly. Primary serial endosymbiosis was a key event in the history of life that gave rise to the evolution of multicellular organisms.
CONCLUSIONS
Although the developmental strategies of sessile plants are very different from those of higher (motile) animals, in both types of organism a peripheral ‘skin-system’ has evolved that protects the living organism from attack by pathogens. Therefore, it is not surprising that the OEW forms a mechanically sturdy yet flexible sheath that covers the above-ground phytosphere like a pliable coat. Recent studies have corroborated the concept that the tension-stressed OEW is the growth-limiting structure, wherein wall-loosening (and concomitant wall-tightening) processes occur that determine the rate of turgor-dependent organ elongation (Kutschera, 2001, 2003, 2006; Schopfer, 2006; Kutschera and Niklas, 2007).
In this article the specific helicoidal cellulose architecture of the growing OEW is described, with special reference to the loss of order (dissipation) in the outermost (i.e. oldest) wall layers (Fig. 2A). The isotropic mechanical properties of the helicoidal OEW prevents longitudinal rupture of the mechanically stressed, expanding peripheral organ wall, which contains layers of cellulose microfibrils in all directions with respect to the axis of the hypocotyl (Fig. 2D). It should be pointed out that the biochemical processes responsible for the co-ordinated yielding of the OEW are still a matter of debate with no consensus among researchers as to the nature of the ‘wall-loosening factor’ (Kutschera, 2001, 2003, 2006; Fry, 2004; Cosgrove, 2005; Schopfer, 2006). The results summarized here demonstrate that these elusive ‘wall-weakening events’ occur independently of the deposition of the helicoidal cellulose network, which is assembled at a constant rate in both the rapidly and the slowly growing OEW of sunflower (Carrington and Firn, 1985; Kutschera, 1990; Hodick and Kutschera, 1992) and Arabidopsis hypocotyls (Refregier et al., 2004; Derbyshire et al., 2007).
Neville (1993) argued that fibrous (helicoidal) composites may have evolved convergently in members of all five kingdoms of life. This hypothesis has been corroborated by detailed studies on the ultrastructure of insect cuticles, which display helicoidal chitin nanofibre patterns analogous to those in the OEW of growing plants (Fig. 3; Vincent and Wengst, 2004; Chen et al., 2006).
Which processes are responsible for the highly ordered architecture (i.e. the three-dimensional design) of the OEW and the insect cuticle? Neville (1985, 1993), Jarvis (1992), Satiat-Jeunemaître (1992) and others have provided evidence indicating that biological helicoids form in muro by directed self-assembly via a liquid crystalline stage. These biochemical processes can be simulated in vitro and by computer modelling. Extracellular structures serving supporting function are composed of large, water-insoluble, inextensible molecules that self-assemble into helicoids in order to achieve a low state of free energy (Neville, 1993). It should be noted that molecular self-assembly of helicoidal structures in extracellular matrices is a striking example of the occurrence of complex design in biological systems without an ‘intelligent designer’ (Kutschera and Niklas, 2004). However, more experimental work is required to support further this concept of the generation of ordered structures from disordered cable-like biopolymers in the extracellular compartments of living organisms.
ACKNOWLEDGEMENTS
I thank Dr Z.-Y. Wang for helpful comments on the manuscript. The financial support provided by the Alexander von Humboldt-Stiftung (Bonn, Germany) is gratefully acknowledged.
LITERATURE CITED
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Carrington CMS, Firn RD. Polysaccharide synthesis and turnover in the cell walls of growing and non-growing cells of gravistimulated sunflower hypocotyls. Journal of Plant Physiology. 1985;118:41–48. doi: 10.1016/S0176-1617(85)80164-4. [DOI] [PubMed] [Google Scholar]
- Chen B, Peng X, Cai C, Niu H, Wu X. Helicoidal microstructure of Scarabaei cuticle and biomimetic research. Materials Science and Engineering A. 2006;423:237–242. [Google Scholar]
- Cosgrove DJ. Growth of the plant cell wall. Nature Reviews Molecular Cell Biology. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Derbyshire P, Findlay K, McCann MC, Roberts K. Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. Journal of Experimental Botany. 2007;58:2079–2089. doi: 10.1093/jxb/erm074. [DOI] [PubMed] [Google Scholar]
- Edelmann HG, Kutschera U. Tissue pressure and cell-wall metabolism in auxin-mediated growth of sunflower hypocotyls. Journal of Plant Physiology. 1993;142:467–473. [Google Scholar]
- Emons AMC, Mulder BM. How the deposition of cellulose microfibrils builds cell wall architecture. Trends in Plant Science. 2000;5:35–40. doi: 10.1016/s1360-1385(99)01507-1. [DOI] [PubMed] [Google Scholar]
- Emons AMC, Höfte H, Mulder BM. Microtubules and cellulose microfibrils: how intimate is their relationship? Trends in Plant Science. 2007;12:279–281. doi: 10.1016/j.tplants.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Fry SC. Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist. 2004;161:641–675. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- Hodick D, Kutschera U. Light-induced inhibition of elongation growth in sunflower hypocotyls. Biophysical and ultrastructural investigations. Protoplasma. 1992;168:7–13. [Google Scholar]
- Jarvis MC. Self-assembly of plant cell walls. Plant, Cell and Environment. 1992;15:1–5. [Google Scholar]
- Joshi CP, Mansfield SD. The cellulose paradox–simple molecule, complex biosynthesis. Current Opinion in Plant Biology. 2007;10:220–226. doi: 10.1016/j.pbi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Kutschera U. Cell-wall synthesis and elongation growth in hypocotyls of Helianthus annuus L. Planta. 1990;181:316–323. doi: 10.1007/BF00195882. [DOI] [PubMed] [Google Scholar]
- Kutschera U. The role of the epidermis in the control of elongation growth in stems and coleoptiles. Botanica Acta. 1992;105:246–252. [Google Scholar]
- Kutschera U. Cell expansion in plant development. Revista Brasileira de Fisiologia Vegetal. 2000;12:65–95. [Google Scholar]
- Kutschera U. Stem elongation and cell wall proteins in flowering plants. Plant Biology. 2001;3:466–480. [Google Scholar]
- Kutschera U. Auxin-induced cell elongation in grass coleoptiles: a phytohormone in action. Current Topics in Plant Biology. 2003;4:27–46. [Google Scholar]
- Kutschera U. Acid growth and plant development. Science. 2006;311:952–953. doi: 10.1126/science.311.5763.952b. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Bette A. In growing epidermal cells of rye coleoptiles microtubules are associated with the nuclei. Journal of Plant Physiology. 1998;152:463–467. [Google Scholar]
- Kutschera U, Edelmann HG. Osmiophilic nanoparticles in epidermal cells of grass coleoptiles: implications for growth and gravitropism. Recent Research Developments in Plant Science. 2005;3:1–14. [Google Scholar]
- Kutschera U, Heiderich A. Sucrose metabolism and cellulose biosynthesis in sunflower hypocotyls. Physiologia Plantarum. 2002;114:372–379. doi: 10.1034/j.1399-3054.2002.1140306.x. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Niklas KJ. The modern theory of biological evolution: an expanded synthesis. Naturwissenschaften. 2004;91:255–276. doi: 10.1007/s00114-004-0515-y. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Niklas KJ. Endosymbiosis, cell evolution, and speciation. Theory in Biosciences. 2005;124:1–24. doi: 10.1016/j.thbio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Niklas KJ. The epidermal-growth-control theory of stem elongation: an old and a new perspective. Journal of Plant Physiology. 2007;164:1395–1409. doi: 10.1016/j.jplph.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Levy S. Two separate zones of helicoidally orientated microfibrils are present in the walls of Nitella internodes during growth. Protoplasma. 1992;163:145–155. [Google Scholar]
- Matthysse AG, Deschet K, Williams M, Marry M, White AR, Smith WC. A functional cellulose synthase from ascidian epidermis. Proceedings of the National Academy of Sciences USA. 2004;101:986–991. doi: 10.1073/pnas.0303623101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder BM, Emons AMC. A dynamical model for plant cell wall architecture formation. Journal of Mathematical Biology. 2001;42:261–294. doi: 10.1007/s002850000063. [DOI] [PubMed] [Google Scholar]
- Neville AC. Molecular and mechanical aspects of helicoid development in plant cell walls. BioEssays. 1985;3:4–8. [Google Scholar]
- Neville AC. Biology of Fibrous Composites. Development Beyond the Cell Membrane. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Niklas KJ. Plant Biomechanics. An Engineering Approach to Plant Form and Function. Chicago and London: The University of Chicago Press; 1992. [Google Scholar]
- Niklas KJ. The walls that bind the tree of life. BioScience. 2004;54:831–841. [Google Scholar]
- Niklas KJ, Paolillo DJ. The role of the epidermis as a stiffening agent in Tulipa (Liliaceae) stems. American Journal of Botany. 1997;84:735–744. [PubMed] [Google Scholar]
- Niklas KJ, Paolillo DJ. Preferential states of longitudinal tension in the outer tissues of Taraxacum officinale (Asteraceae) peduncles. American Journal of Botany. 1998;85:1068–1081. [PubMed] [Google Scholar]
- Passioura JB, Boyer JS. Tissue stresses and resistance to water flow conspire to uncouple the water potential of the epidermis from that of the xylem in elongating plant stems. Functional Plant Biology. 2003;30:325–334. doi: 10.1071/FP02202. [DOI] [PubMed] [Google Scholar]
- Peters WS, Tomos AD. The history of issue tension. Annals of Botany. 1996;77:657–665. doi: 10.1006/anbo.1996.0082. [DOI] [PubMed] [Google Scholar]
- Proseus TE, Boyer JS. Periplasm turgor pressure controls wall deposition and assembly in growing Chara coralline cells. Annals of Botany. 2006;98:93–105. doi: 10.1093/aob/mcl098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refregier G, Pelletier S, Jaillard D, Höfte H. Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis. Plant Physiology. 2004;135:959–968. doi: 10.1104/pp.104.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J-C, Reis D, Mosiniak M, Vian B. Cell wall texture along the growth gradient of the mung bean hypocotyl: ordered assembly and dissipative processes. Journal of Cell Science. 1982;56:303–318. [Google Scholar]
- Satiat-Jeunemaître B. Spatial and temporal regulations in helicoidal extracellular matrices: comparison between plant and animal systems. Tissue Cell. 1992;24:315–334. doi: 10.1016/0040-8166(92)90049-d. [DOI] [PubMed] [Google Scholar]
- Savaldi-Goldstein S, Peto C, Chory J. The epidermis both drives and restricts plant shoot growth. Nature. 2007;446:199–202. doi: 10.1038/nature05618. [DOI] [PubMed] [Google Scholar]
- Scherp P, Grotha R, Kutschera U. Occurrence and phylogenetic significance of cytokinesis-related callose in green algae, bryophytes, ferns and seed plants. Plant Cell Reports. 2001;20:143–149. doi: 10.1007/s002990000301. [DOI] [PubMed] [Google Scholar]
- Schopfer P. Biomechanics of plant growth. American Journal of Botany. 2006;93:1415–1425. doi: 10.3732/ajb.93.10.1415. [DOI] [PubMed] [Google Scholar]
- Somerville C. Cellulose synthesis in higher plants. Annual Review of Cell and Developmental Biology. 2006;22:57–78. doi: 10.1146/annurev.cellbio.22.022206.160206. [DOI] [PubMed] [Google Scholar]
- Suslov D, Verbelen J-P. Cellulose orientation determines mechanical anisotropy in onion epidermis cell walls. Journal of Experimental Botany. 2006;57:2187–2192. doi: 10.1093/jxb/erj177. [DOI] [PubMed] [Google Scholar]
- Vincent JFV, Wegst UGK. Design and mechanical properties of insect cuticle. Arthropod Structure and Development. 2004;33:187–199. doi: 10.1016/j.asd.2004.05.006. [DOI] [PubMed] [Google Scholar]