Abstract
Background and Aims
Seed physiological dormancy (PD) limits the use and conservation of some of Queensland's (Qld) native forb species. It was hypothesised that optimum dormancy-alleviating treatments would reflect environmental conditions that seeds experience in situ, and this premise was tested for PD seeds of four species native to south-west Qld.
Methods
High temperatures and increased rainfall during summer are characteristic of this semi-arid tropical environment. Ex situ treatments were designed to mimic conditions that seeds dispersed in spring experience during the summer months before germinating in cooler autumn temperatures. Seeds received between 4 and 20 weeks of a dry after-ripening (DAR), warm stratification or dry/wet cycling treatment (DAR interspersed with short periods of warm stratification), in darkness, before being transferred to germination test conditions. In addition, natural dormancy alleviation of one of the Goodeniaceae species was investigated in situ.
Key Results
Dry/wet cycling resulted in higher levels of germination of Actinobole uliginosum (Asteraceae), Goodenia cycloptera and Velleia glabrata (Goodeniaceae) when compared with constant DAR or stratification, while Goodenia fascicularis (Goodeniaceae) responded better to short durations of warm stratification. Long durations of DAR partially alleviated PD of A. uliginosum; however, stratification induced and maintained dormancy of this species. Modifications to the dry/wet cycling treatment and germination test conditions based on data collected in situ enabled germination of G. cycloptera and V. glabrata to be further improved.
Conclusions
Treatments designed using temperature, relative humidity and rainfall data for the period between natural seed dispersal and germination can successfully alleviate PD. Differences between the four species in conditions that resulted in maximum germination indicate that, in addition to responding to broad-scale climate patterns, species may be adapted to particular microsites and/or seasonal conditions.
Key words: Seed dormancy, germination, dry after-ripening, dry/wet cycling, south-west Queensland, Australia, semi-arid tropical, Actinobole uliginosum, Asteraceae, Goodenia fascicularis, Goodenia cycloptera, Velleia glabrata, Goodeniaceae
INTRODUCTION
Seeds of many Australian native plants exhibit physiological dormancy (PD) and there remain species in a range of families, including Goodeniaceae and Asteraceae, with unknown dormancy-alleviation requirements (Merritt et al., 2007). It is often possible to bypass or terminate PD and stimulate germination through the application of chemical agents such as gibberellins (Cohn et al., 1989; Foley, 1992). However ‘germination inducing factors’ are not believed to affect dormancy status (Vleeshouwers et al., 1995), can result in seedling abnormalities, and reveal little about why PD exists and how it is overcome in the natural environment. In contrast, recent intuitive approaches have attempted to alleviate PD by removing the block(s) to germination. This involves mimicking the environment into which dormant seeds are dispersed (Baskin and Baskin, 2004) and is therefore likely to initiate germination and development of healthy seedlings. Temperature is the primary factor regulating seasonal changes in dormancy status, with modification by seed moisture content (Benech-Arnold et al., 2000; Vleeshouwers and Bouwmeester, 2001). Treatments that involve controlling these factors include dry after-ripening (DAR), stratification and dry/wet cycling.
South-west Queensland's (Qld) natural climate is classified as semi-arid tropical. Average climatic data collected between 1971 and 2000 by the Australian Bureau of Meteorology indicate that summer months are hot (approx. 22–35 °C) and receive more rain than any other time of the year (up to 50 mm per month). During winter months temperatures are lower (approx. 7–20 °C), and rainfall is at its lowest (approx. 22 mm per month). Seeds of some Asteraceae and Goodeniaceae species native to south-west Qld are naturally dispersed in spring and exhibit PD upon dispersal. With the aid of germination stimulants, a proportion of these seeds will germinate at temperatures reminiscent of autumn months (15–20 °C; Hoyle et al., 2008). It is reasonable to hypothesize that seeds dispersed in spring have evolved dormancy mechanisms to prevent immediate germination during superficially favourable pre-summer temperatures. Instead, cooler autumn temperatures initiate germination only after PD has been alleviated over summer. Postponing germination until after summer would allow seedling establishment, growth and reproduction to take place during the cooler winter months. Indeed, many Asteraceae and Goodeniaceae are known to flower throughout the winter in south-eastern Qld (Stanley and Ross, 2002). Based on dispersal, germination and flowering phenology, we hypothesized that mimicking summer conditions during the post-dispersal/pre-germination period may provide insights into the natural dormancy alleviation of Qld's forbs and how PD might be overcome ex situ.
Many plant families worldwide have responded to DAR as a means of alleviating seed PD (Baskin and Baskin, 2001), including several families native to Australia. For example, dormancy of Actinotus leucocephalus (Apiaceae) seeds was alleviated by dry storage at alternating 20/50 °C or constant 37 °C, temperatures that simulated summer soil conditions for the Mediterranean-type climate region of south-west Australia (Baker et al., 2005). Although rainfall in south-west Qld is greatest in summer, rain events are sporadic (an average of only 3·3 days of ≥1 mm of rain per summer month) and are followed by extended dry periods. Therefore seeds dispersed in spring are likely to experience significant dry periods between heavy showers, suggesting that DAR may be involved in dormancy alleviation. Dry after-ripening has also been reported to alleviate PD in several Australian Asteraceae, although differences exist between species. For example, high temperature storage (25 and 38 °C) for 8 months increased germination of Schoenia filifolia subsp. subulifolia, but germination of Craspedia sp. remained low after storage of >16 months at the same temperature (Peishi et al., 1999). However, after-ripening during storage in ambient laboratory conditions for >12 months (e.g. Turner et al., 2006a) is not reminiscent of what seeds might experience in natural field conditions.
Seeds in their natural environment of south-west Qld are likely to experience moist (imbibed) periods of several days duration following heavy summer rainfall. In temperate environments, cold stratification has been found to be the main factor governing changes in dormancy status of imbibed seeds (Benech-Arnold et al., 2000). Cold stratification pre-treatments have increased seed germination of some species from sub-alpine, Mediterranean-type and temperate climates within Australia (Beardsall and Mullet, 1984; Curtis, 1996; Moncur et al., 1997). However in Qld's semi-arid tropical climate, seeds that lie dormant over summer are more likely to require a warm stratification treatment to alleviate dormancy. Stratification at warm temperatures (33/18 °C or 26/13 °C) for several weeks was successful in alleviating dormancy for the majority of Acanthocarpus preissii (Dasypogonaceae) seeds, enabling germination at cooler temperatures (18/7 °C; Turner et al., 2006b). It was concluded that A. preissii seeds required treatments simulating temperature and soil moisture conditions representative of early to mid-autumn in south-west Western Australia (WA), prior to germinating in winter.
A small number of studies have combined DAR and warm, wet stratification to simulate periodic rainfall. Lolium rigidum (Poaceae) seeds from WA exposed to one or more hydration events during a 12-week DAR period were less dormant than seeds that remained dry throughout, with hydration events earlier in the DAR period promoting greatest dormancy alleviation (Gallagher et al., 2004). Weekly wet/dry cycling of Actinotus leucocephalus (Apiaceae) seeds stored at 37 °C increased the rate of dormancy alleviation when compared with seeds stored air-dry at the same temperature (Baker et al., 2005). Indeed, Merritt et al. (2007) found that fluctuations in seed moisture content, including seeds of a Goodeniaceae species, is a natural phenomenon experienced by water-permeable seeds during periods of sporadic rainfall in WA's Mediterranean-type climate regions during autumn and early winter.
Consequently, we hypothesized that the most effective treatment for the alleviation of PD of species native to south-west Qld would be warm, dry storage (DAR) interspersed with periods of warm, wet stratification. A DAR treatment interspersed with 2-d periods of warm stratification at 4 week intervals, as well as a continuous DAR and warm stratification treatment, were applied to PD seeds of three Goodeniaceae and one Asteraceae species. For one of the study species, Velleia glabrata, these ex situ treatments were complemented by comparison with dormancy loss over the summer months for seeds buried in the natural environment.
MATERIALS AND METHODS
Seed collection and viability testing
Seeds of the four study species [Goodenia cycloptera, G. fascicularis, Velleia glabrata (Goodeniaceae) and Actinobole uliginosum (Asteraceae)] were collected in the spring of 2004 or 2005 (see Table 1 for seed lot and site details). At least 10 000 seeds were collected from >50 individual plants per species. Only mature seeds at the point of natural dispersal were collected, i.e. seed capsules were dehiscing and seeds were brown in colour and dispersing naturally. In the laboratory, seeds were processed (non-seed material was removed by hand, aspirator or rubbing seed capsules through a sieve using a rubber bung), and stored at 15 °C and 15–20 % relative humidity (RH). Prior to storage, three replicates of 20 seeds per species were tested for viability using the tetrazolium chloride (TZ) staining technique (ISTA, 2003). Seeds were initially hydrated on water agar for 24 h at room temperature before being nicked (away from the embryo axis) and placed in TZ solution at 30 °C and darkness for 24 h. Seeds were then cut in half and examined. Only uniformly stained red/dark pink embryos were considered ‘viable’.
Table 1.
Study species, global positioning system (GPS) co-ordinates of their collection sites and date of collection
| Family | Species | GPS collection co-ordinates | Date collected |
|---|---|---|---|
| Asteraceae | Actinobole uliginosum (A. Gray) Eichler | 8°06′8·7″S, 145°76′39·2″E | 22 Oct 2004 |
| Goodeniaceae | Goodenia fascicularis F. Muell & Tate | 28°06′10·7″S, 145°81′88·6″E | 22 Oct 2004 |
| Goodeniaceae | Goodenia cycloptera R. Br. | 27°57′48·5″S, 148°17′27·3″E | 27 Oct 2005 |
| Goodeniaceae | Velleia glabrata Carolin | 28°00′26·3″S, 146°02′23·5″E | 27 Oct 2005 |
Design of treatments and germination test conditions based on climate data
Annual weather data collected in Cunnamulla, central to all experimental seed origins, between 1971 and 2000 is summarized in Table 2 (Bureau of Meteorology, 2007). Summer air temperature, RH and rainfall averages were used to design the ex situ dry after-ripening (DAR), stratification and dry/wet cycling (D/W cycling) treatments. All treatments were applied to seeds of all species at 34/20 °C with a 12/12 h thermoperiod, in darkness. In each case, five replicates of 20 seeds (G. fascicularis, V. glabrata and A. uliginosum) or three replicates of 10 seeds (G. cycloptera) were used and completely randomized. Seeds in the DAR treatment were placed in an air-tight, thermo-stable, plastic box over a lithium chloride (LiCl) solution that created a daily mean RH of 40·0 ± 0·3 %, with a daily mean maximum of 45·0 ± 0·8 % and a daily mean minimum of 34·0 ± 3·6 % RH. Seeds receiving a stratification treatment were placed on agar and became fully imbibed at approx. 100 % RH. Seeds receiving the D/W cycling treatment were periodically moved between the two aforementioned treatments, remaining in stratification (wet) for 48 h and DAR (dry) for 4 weeks, per cycle. In and beyond phase II of water uptake, seeds are sufficiently hydrated to begin certain preparative steps towards germination (Meyer et al., 2000). Therefore, during the D/W cycling treatment, imbibed seeds were moved from wet to dry conditions under a green ‘safelight’. Due to the mucilage surrounding all imbibed seeds, wet seeds were dried on baking paper, in darkness, for 12 h at 20 °C, approx. 47 % RH before being returned to the DAR treatment. Control seeds remained at 15 °C, 15–20 % RH.
Table 2.
Summary of temperature, relative humidity and rainfall data (mean ± s.e.) collected by the Bureau of Meteorology in Cunnamulla, south-west Queensland between 1971 and 2000
| Season | Months | Max. air temp (°C) | Min. air temp (°C) | % RH, 0900 h | % RH, 1500 h | No. of rainy days ≥1 mm | Rainfall (mm) |
|---|---|---|---|---|---|---|---|
| Spring | Sep, Oct, Nov | 29 ± 2 | 15 ± 2 | 39 ± 1 | 25 ± 1 | 2·8 ± 0·3 | 24 ± 3 |
| Summer | Dec, Jan, Feb | 35·4 ± 0·3 | 21·5 ± 0·4 | 42 ± 4 | 28 ± 2 | 3·3 ± 0·1 | 45 ± 3 |
| Autumn | Mar, Apr, May | 28 ± 3 | 14 ± 3 | 54 ± 4 | 36 ± 3 | 2·5 ± 0·3 | 33 ± 4 |
| Winter | Jun, Jul, Aug | 20 ± 1 | 7.0 ± 0.4 | 63 ± 5 | 39 ± 4 | 2.4 ± 0.3 | 22 ± 3 |
Germination test conditions (12/12 h light/dark photoperiod and 25/15 °C thermoperiod) were designed using mean autumn air temperatures for south-west Qld (Table 2). Fortnightly or monthly for 20 weeks, five replicates of 20 seeds of each species (or five replicates of 10 seeds for G. cycloptera), were removed from the DAR and stratification treatment conditions (and exhumed from the field in the case of V. glabrata – see below), and their dormancy status assessed in the germination tests. Seeds in the D/W cycling treatment were sown in germination tests both before and after every stratification event, to investigate whether the effects of the wet component persisted after seeds were dried. Seeds were sown into 9-cm diameter plastic Petri dishes containing 1 % agar–water medium and placed into sealed transparent plastic bags to prevent desiccation. Bags were placed into germination incubators in which light was provided by fluorescent tubes (approx. 50 µmol m−2 s−1). Germination, defined as visible radicle emergence by at least 1 mm, was counted every 7 d, germination tests ran for 63 d and any ungerminated seeds were cut-tested and examined for viability. A firm, fresh endosperm and white embryo were considered viable.
Velleia glabrata seed burial and retrieval
A proportion of the V. glabrata collection were processed in situ immediately after collection. Seeds were separated from seed capsules and chaff by hand, and replicates of 20 seeds placed into secure 10 × 10 cm mosquito-net bags, which were tagged and labelled. Bags were buried 1·5 cm below the soil surface, approx. 15 km north of Cunnamulla towards Charleville (28°01′37·9″S, 145°44′54·3″E). A fence was built around and over the burial site using iron star posts and wire mesh to protect seeds from large predators. Five replicates of 20 seeds were exhumed every 2 weeks for the next 20 weeks and sown directly into ex situ germination tests (see above). Data probes logged percentage soil moisture (TinytagPlus; Hastings Data Loggers, NSW, Australia) and temperature (Datahog 2; Skye Instruments, Powys, UK), approx. 1·5 cm below the soil surface for the duration of the experiment.
Modifications to the dry/wet cycling treatment and germination test conditions
In a second set of experiments, conducted 18 months after the first, attempts were made to improve dormancy alleviation and germination of V. glabrata and G. cycloptera seeds. Velleia glabrata seeds were sown in the original D/W cycling treatment as described above, but each cycle was shortened to 12 d of DAR and 2 d of stratification so that 4·5 cycles took a total of 10 weeks rather than 20 weeks. Treatment conditions were modified based on soil temperature data collected in the field during the seed burial experiment. Velleia glabrata seeds were placed in a D/W cycling treatment in which the DAR component was held at a higher temperature of constant 40 °C. This was the mean maximum soil temperature in the field during the period in which buried V. glabrata seeds lost dormancy. The 2-d stratification component remained at 20/34 °C. Finally, germination test conditions were modified based on the premise that, in situ, the majority of seeds may not be ready to germinate until winter months (i.e. cooler temperatures). Post treatment, seeds were sown at a 12/12 h 25/15 °C thermoperiod and constant 20 °C, 12/12 h photoperiod. Due to limited seed numbers, G. cycloptera seeds were subjected to only one modified scenario; a D/W cycling treatment of increased number of cycles and warmer DAR temperature, and a cooler germination temperature, as described for V. glabrata.
Statistical analysis
Statistical analysis was carried out on arcsine-transformed germination data (arcsine of the square root of proportions; Zar, 1984). Control data was excluded from analysis, to satisfy the ANOVA assumption of equal variances. ANOVA of means (General Linear Model, GLM) was carried out to assess the effect of treatments on germination of each species and to obtain common standard deviation values per species for use in t-tests. The GLM was also used to test the effect of duration per treatment. Treatment means were compared to a mean control germination value using a 1-sample t-test, and compared to one another using a 2-sample t-test for summarized data. All statistical analysis was carried out in Minitab 15 (Minitab Inc., Chicago).
RESULTS
Actinobole uliginosum
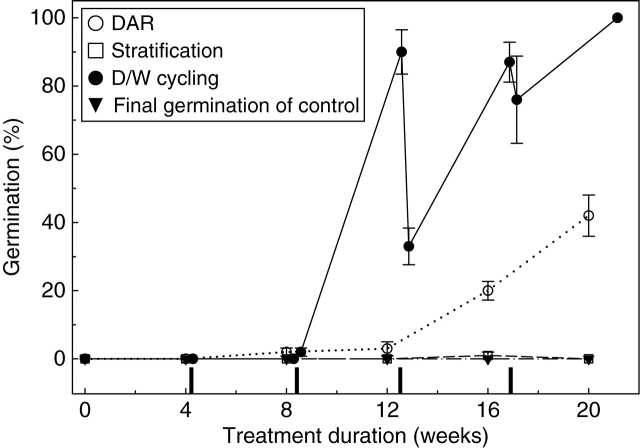
There was no germination of A. uliginosum seeds that were simply removed from storage (15 °C, 15–20 % RH) and sown at 15/25 °C (control), despite TZ staining assessment indicating 93 % viability. Nor did seeds respond to the warm stratification treatment when compared with the control (one-sample t-test: t = –0·41, n = 5, P = 0·700). Seeds gradually responded (GLM: F = 34·53, d.f. = 4, P ≤ 0·001) to DAR at 20/34 °C and 40 % RH to achieve a final germination of 42 % after 20 weeks; a clear improvement over the control treatment (one-sample t-test: t = 6·54, n = 5, P = 0·003; Fig. 1). Dormancy was completely alleviated by the D/W cycling treatment (one-sample t-test: t = 8·54, n = 5, P = 0·001), which was more effective than DAR (two-sample t-test: t = –4·10, d.f. = 8, P = 0·003). Each cycle consisted of one ‘dry’ period (4 weeks of DAR) followed by one ‘wet’ period (48 h of stratification). Two cycles were needed before seeds would germinate at 25/15 °C, gradually increasing to 100 % germination after 4·5 cycles (Fig. 1). During the third cycle, as seeds became able to germinate, the wet phase partially reversed dormancy alleviation (two-sample t-test: t = 5·54, d.f. = 8, P = 0·001; Fig. 1).
Fig. 1.
Final germination (mean ± s.e.) of Actinobole uliginosum seeds at 15/25 °C, 12/12 h light/dark after various durations of DAR, stratification and D/W cycling at 20/34 °C in darkness. Control seeds (in storage at 15 °C/15 % RH) are also shown. Markers on the x-axis indicate the 48-h wet phases of the D/W cycling treatment.
Goodenia fascicularis
Only 5·3 % of G. fascicularis seeds germinated in the control treatment despite TZ staining suggesting 98 % viability, and there was no response of seeds to the DAR treatment (one-sample t-test: t = 1·02, n = 5, P = 0·366). Seeds responded increasingly (GLM: F = 2·77, d.f. = 7, P = 0·023) to the D/W cycling treatment, achieving 35 % germination after 4·5 cycles; a significant improvement on the final germination of the control (one-sample t-test: t = 3·71, n = 5, P = 0·021; Fig. 2A). However, approx. 58 % of G. fascicularis seeds germinated after receiving warm stratification, an improvement upon the control (one-sample t-test: t = 11·19, n = 5, P ≤ 0·001), and the D/W cycling treatment (two-sample t-test: t = –5·29, d.f. = 8, P = 0·007), regardless of whether treatment duration was 4 or 20 weeks (GLM: F = 0·34, d.f. = 4, P = 0·847; Fig. 2A).
Fig. 2.
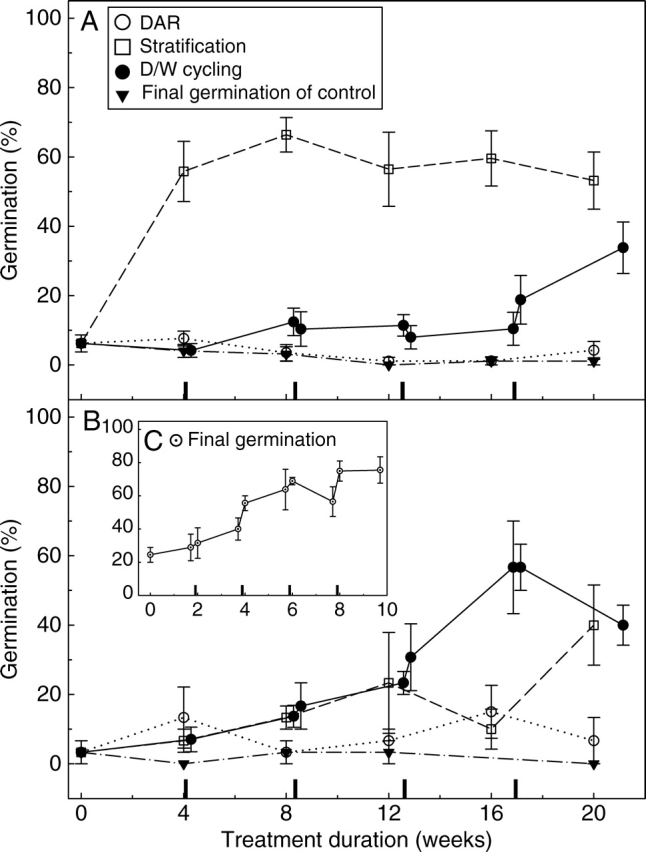
Final germination (mean ± s.e.) of (A) Goodenia fascicularis and (B) G. cycloptera seeds at 15/25 °C, 12/12 h light/dark after various durations of DAR, stratification and D/W cycling at 20/34 °C in darkness. Control seeds (in storage at 15 °C/15 % RH) are also shown. (C) Modified D/W cycling of G. cycloptera at 20 °C, 12/12 h light/dark after various shorter durations of a modified D/W cycling treatment (40 °C DAR component). Markers on all x-axis indicate the 48-h wet phases of the D/W cycling treatment.
Goodenia cycloptera
Only 3·7 % of G. cycloptera seeds in the control treatment germinated despite 88 % viability. Twenty weeks of warm stratification elicited 39 % germination (one-sample t-test: t = 4·50, n = 5, P = 0·011; Fig. 2B), but the treatment that resulted in the greatest dormancy release was D/W cycling (one-sample t-test: t = 6·74, n = 5, P = 0·003), with between 31 and 57 % of seeds responding after three cycles (Fig. 2B). After 20 weeks of application, germination was greater after D/W cycling when compared with DAR (two-sample t-test: t = –3·13, d.f. = 8, P = 0·014), but not stratification (two-sample t-test: t = −1·59, d.f. = 8, P = 0·151). Modifications to the D/W cycling treatment based on soil temperature data (Fig. 3B) (a constant 40 °C DAR component instead of 20/34 °C, 12/12 h thermoperiod), and modifications to the germination tests (constant 20 °C instead of 25/15 °C, 12/12 h thermoperiod), achieved 76 % final germination, an increase of 36 % when compared with the original D/W cycling treatment (two-sample t test: t = 3·46, d.f. = 4, P = 0·026; Fig. 2C).
Fig. 3.
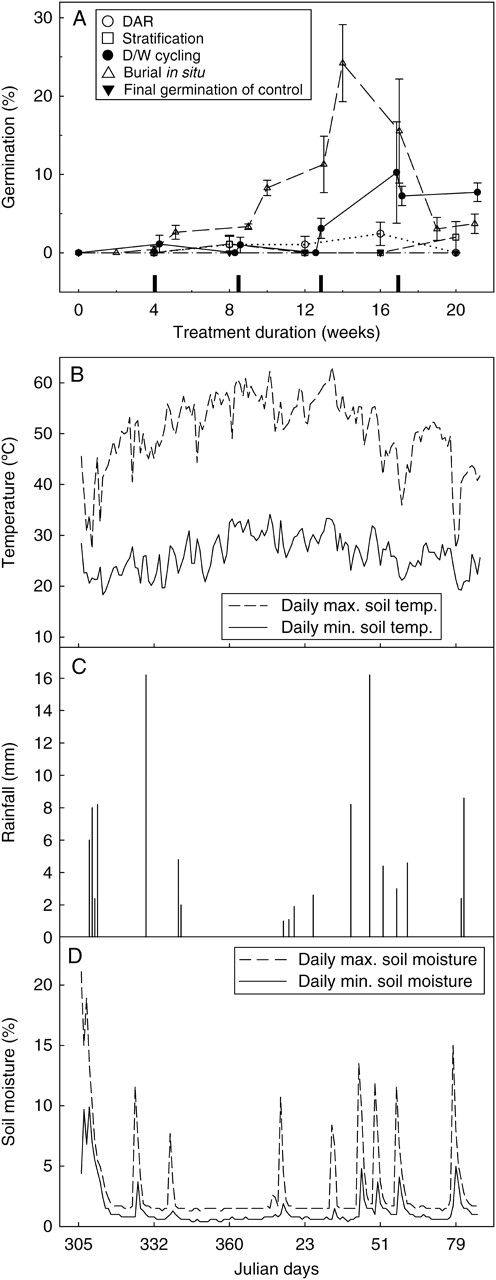
(A) Final germination (mean ± s.e.) of Velleia glabrata seeds at 15/25 °C, 12/12 h light/dark after various durations of DAR, stratification and D/W cycling at 20/34 °C in darkness, and burial 1·5 cm below soil surface in situ. Control seeds (in storage at 15 °C/15 % RH) are also shown. Markers on the x-axis indicate the 48-h wet phases of the D/W cycling treatment. (B) Daily maximum and minimum soil temperatures 1·5 cm below soil surface, (C) daily rainfall, and (D) daily maximum and minimum soil moisture content (% mineral) at seed burial site. Julian days are from November 2005 to March 2006, inclusive.
Velleia glabrata
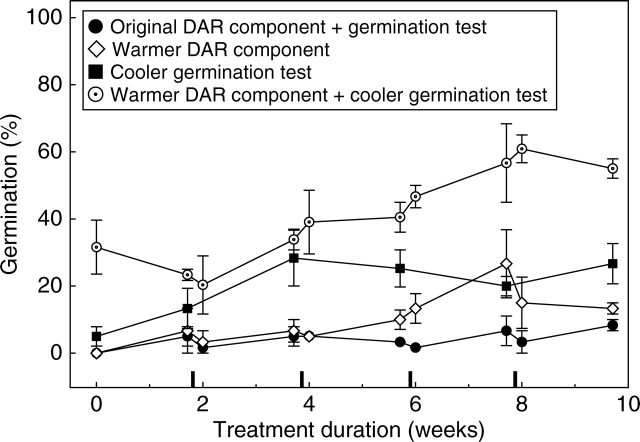
Velleia glabrata seeds exhibited 90 % viability according to TZ staining patterns, but none of the initial ex situ treatments resulted in >10 % germination (Fig. 3A). No germination of seeds buried at the collection site was recorded in situ. However, seeds exhumed periodically over the summer months and sown in ex situ germination tests germinated following 14 weeks of burial to achieve 24 % germination (one-sample t-test: t = 3·75, n = 5, P = 0·021), before dropping again to 4 % germination after 21 weeks (Fig. 3A). Field temperature and rainfall data indicated how the D/W cycling treatment might be improved. Mean maximum daily temperatures 1·5 cm below the soil surface (50 ± 6 °C) reached approx. 22 °C higher than air temperatures; minimum soil temperatures were also higher in situ (26 ± 3 °C; Fig. 3B) and rainfall frequency in the field was greater than that included in the original D/W cycling treatment (Fig. 3C). Therefore the D/W cycling treatment and subsequent germination test were repeated with cycles applied at a higher frequency (four cycles in 10 weeks compared with two in 10 weeks). In addition, the treatment and the germination test conditions were modified as described for G. cycloptera. Velleia glabrata seeds did not respond to the increased frequency of cycles (GLM: F = 0·000, d.f. = 1, P = 0·969; Fig. 4). Nor did they respond significantly to a warmer treatment temperature (GLM: F = 5·39, d.f. = 1, P = 0·146). However, seeds did respond to a cooler germination-test temperature post treatment (GLM: F = 207·4, d.f. = 1, P = 0·005), and a combination of a warmer treatment and cooler germination conditions led to a maximum germination of 60 % (GLM: F = 261, d.f. = 1, P = 0·004; Fig. 4), an increase of almost 50 % germination compared with the original treatment and germination test result.
Fig. 4.
Final germination (mean ± s.e.) of Velleia glabrata seeds in 12/12 h light/dark after various modifications to the D/W cycling treatment: original DAR (20/34 °C) and germination test (25/15 °C), a warmer (40 °C) DAR, a cooler (20 °C) germination temperature and a combination of the warmer DAR and cooler germination test. Markers on the x-axis indicate the 48-h wet phases of the D/W cycling treatment.
DISCUSSION
Attempting to mimic environmental conditions that seeds experience during hot, wet summers followed by germination at autumn temperatures was successful in alleviating, partially or fully, PD of all four species investigated. The D/W cycling treatment most closely mimicked natural conditions that seeds experienced in the field, post dispersal and before germination (Fig. 3B–D). Indeed, all four species responded to D/W cycling, and maximum ex situ germination of A. uliginosum, G. cycloptera and V. glabrata was achieved after longer durations of this treatment. Physiological dormancy of A. uliginosum was completely alleviated by the D/W cycling; after a total of 12 weeks of DAR interspersed with two ‘rain events’, the majority of A. uliginosum seeds were non-dormant, and after 4·5 cycles all the seeds germinated at 25/15 °C (Fig. 1). Similarly, dormancy of G. cyloptera and V. glabrata was alleviated to the greatest extent by D/W cycling (Figs 2B and 3A), results that, with subsequent modifications to the treatment and germination test conditions, increased even further (Figs 2C and 4).
Wetting and drying cycles have not been used in dormancy release trials to any great extent but clearly show promise for species exposed to sporadic rainfall events in their natural post-dispersal environment. Dry/wet cycling increased germination of dormant annual ryegrass (Lolium rigidum, Poaceae) and Actinotus leucocephalus (Apiaceae) seeds (Gallagher et al., 2004; Baker et al., 2005), both from a Mediterranean-type climate. In the present study we have shown that D/W cycling can alleviate PD of an Asteraceae and Goodeniaceae species from a semi-arid tropical climate. While DAR offers a simple method of alleviating dormancy ex situ, and is commonly used to alleviate PD of Australian Asteraceae (Peishi et al., 1999; Schutz et al., 2002), it is not characteristic of the natural environment in south-west Qld, where seeds are unlikely to experience continuous warm, dry periods of longer than 6 weeks during summer. According to field data collected during the in situ burial trial, soil moisture content peaked on at least nine separate occasions in the field (Fig. 3D), confirming that shallow-buried seeds would experience cycles of hydration and dehydration and that the D/W cycling treatment is more ecologically representative. Seed hydration events in the soil may be analogous to seed-priming techniques used to promote more rapid or uniform germination of non- or low-dormant seed lots (Taylor et al., 1998; Gallagher et al., 2004). Priming may stimulate embryo cell elongation, loosen endosperm and/or testa and activate germination enzymes (McDonald, 1999).
The germination response of both A. uliginosum and G. fascicularis to all three ex situ treatments reveals an interesting and contrasting interaction between DAR and stratification when applied cyclically. The short bursts of stratification allowed the DAR component, which only alleviated PD of A. uliginosum partially when applied alone, to become more effective at alleviating PD of this species (Fig. 1). Similar work with annual ryegrass seeds also concluded that short periods of hydration during after-ripening not only increased dormancy alleviation but also changed the DAR process (Gallagher et al., 2004). In contrast, although stratification was the optimal treatment for G. fasicularis, D/W cycling achieved greater dormancy alleviation of this species than would be expected simply from an accumulation of the 2-d stratification component (8 d in total), suggesting that the DAR phases caused seeds to become increasingly responsive to stratification.
In the case of A. uliginosum, the stratification component of the D/W cycling, as well as enabling the DAR to be more effective, also appeared to induce PD. For example, the proportion of seeds that were able to germinate after 2·5 cycles appeared to exhibit dormancy again after 3·0 cycles (i.e. after re-hydration; Fig. 1). Fluctuations in soil moisture content (and thus seed moisture content) were also found to cause fluctuations in dormancy status of Polygonum aviculare (Polygonaceae) seeds (Batlla and Benech-Arnold, 2006). As in the present study, these effects were more pronounced during the earlier stages of dormancy alleviation. Our results suggest that stratification, when applied continuously, did not merely prevent PD alleviation of A. uliginosum but actively maintained PD (Fig. 1). In the field, this kind of response to intermittent rain events may act to stagger germination over time and increase seedling survival rates.
Related species responded very differently to the treatments investigated, despite the fact that seeds were collected at the same time, from the same location. For example, 4 weeks of continuous warm stratification alleviated PD of G. fascicularis (Fig. 2A), whereas 10 to 20 weeks of D/W cycling alleviated PD of G. cycloptera (Fig. 2B, C). In the field, long periods of stratification are unlikely, due to the sporadic and short-lived nature of rain events, which may explain the rapid response of G. fascicularis to short durations of stratification. Longer durations failed to increase dormancy alleviation beyond approx. 60 % germination, a limited response that may have been determined by the maternal environment during seed development (Steadman et al., 2004), the treatment itself or the germination test conditions. Thus, G. fascicularis may occur preferentially in wet microsites, e.g. cracks in the earth in which puddles form. In contrast, G. cycloptera gradually responded to D/W cycling, DAR and stratification, (Fig. 2B) and therefore may have evolved to lose dormancy in a variety of different environmental scenarios since this species is known to flower (and presumably set seed) throughout the year (Stanley and Ross, 2002).
Ex situ germination tests suggested that PD of V. glabrata was partially alleviated in the field (Fig. 3A). However beyond 14 weeks burial, germination began to decrease, despite continued seed viability. This decrease in germination coincided with a drop in daily maximum soil temperatures (Fig. 3B) and a number of successive rain events (Fig. 3C, D), suggesting that buried V. glabrata seeds experienced cold stratification at the onset of autumn that may have induced PD. These seeds may be capable of dormancy cycling if primary dormancy is not sufficiently alleviated during summer, or if conditions for germination in autumn/winter are not optimal.
Both G. cycloptera and V. glabrata responded positively to a combination of a warmer treatment and cooler germination test (Figs 2C and 4, respectively); temperatures that mimicked more closely what V. glabrata seeds experienced in situ. Results also suggest that treatments of longer than 20 weeks duration are likely to be even more effective. However, despite the fact that all four species responded positively to an increase in the number of D/W cycles applied, an increased frequency of cycles was found to be of no importance to V. glabrata seeds (Fig 4). In the field, PD may be maintained during the early summer rains, regardless of rainfall frequency, to prevent all seeds from germinating after any particular rainfall event and to ensure that germination occurs more towards the end of summer.
In conclusion, forb species from semi-arid tropical Queensland appear to exhibit physiological dormancy that, to varying degrees, is lost during a summer season of natural wetting and drying cycles, conditions that can be mimicked ex situ to achieve germination of otherwise difficult-to-germinate species. Subtle differences between species in their responses to relative proportions of wet versus dry periods might be expected to contribute towards determining which forbs dominate the landscape each year.
ACKNOWLEDGEMENTS
We are grateful to all those who helped to collect seed and manage field and laboratory experiments, particularly Jitka Kochanek, Chris O'Donnell, David Bowen, Rowena Long and Pedro Kingman (University of Qld) and Matthew Wolnicki (Cunnamulla). Financial support to G. Hoyle from the Australian Mining Industry and the Millennium Seed Bank Project, The Royal Botanic Gardens, Kew, UK is gratefully acknowledged.
LITERATURE CITED
- Baker KS, Steadman KJ, Plummer JA, Merritt DJ, Dixon KW. The changing window of conditions that promote germination of two fire ephemerals, Actinotus leucocephalus (Apiaceae) and Tersonia cyathiflora (Gyrostemonaceae) Annals of Botany. 2005;96:1225–1236. doi: 10.1093/aob/mci274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin C, Baskin J. Seeds: ecology, biogeography and evolution of dormancy and germination. London: Academic Press; 2001. [Google Scholar]
- Baskin CC, Baskin JM. Determining dormancy-breaking and germination requirements from the fewest seeds. In: Guerrant E, Havens K, Maunder M, editors. Ex situ plant conservation: supporting species survival in the wild. Covelo, CA: Island Press; 2004. pp. 162–179. [Google Scholar]
- Batlla D, Benech-Arnold RL. The role of fluctuations in soil water content on the regulation of dormancy changes in buried seeds of Polygonum aviculare L. Seed Science Research. 2006;16:47–59. [Google Scholar]
- Beardsall D, Mullett J. Seed germination of Eucalyptus pauciflora Sieb. ex Spreng. from low and high altitude populations in Victoria. Australian Journal of Botany. 1984;32:475–480. [Google Scholar]
- Benech-Arnold RL, Sanchez RA, Forcella F, Kruk BC, Ghersa CM. Environmental control of dormancy in weed seed banks in soil. Field Crops Research. 2000;67:105–122. [Google Scholar]
- Bureau of Meteorology. Climate statistics for Australian locations. 2007. http://www.bom.gov.au/climate/averages/tables/cw_. 044026.shtml Accessed May 2007.
- Cohn MA, Jones KL, Chiles LA, Church DF. Seed dormancy in Red Rice VII. Plant Physiology. 1989;89:879–882. doi: 10.1104/pp.89.3.879. Structure-activity studies of germination stimulants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis NP. Germination and seedling survival studies of Xanthorrhoea autralis in the Warby Range State Park, North-eastern Victoria, Australia. Australian Journal of Botany. 1996;44:635–647. [Google Scholar]
- Foley ME. Effect of soluble sugars and gibberellic-acid in breaking dormancy of excised Wild Oat (Avena fatua) embryos. Weed Science. 1992;40:208–214. [Google Scholar]
- Gallagher RS, Steadman KJ, Crawford AD. Alleviation of dormancy in annual ryegrass (Lolium rigidum) seeds by hydration and after-ripening. Weed Science. 2004;52:968–975. [Google Scholar]
- Hoyle GL, Steadman KJ, Daws MI, Adkins SW. Physiological dormancy in forbs native to south-west Queensland: diagnosis and classification. South African Journal of Botany. 2008 In press. doi: 10·1016/j.sajb.2007·11·005. [Google Scholar]
- ISTA (International Seed Testing Association) ISTA working sheets on tetrazolium testing. Vols I and II. Bassersdorf, Switzerland: ISTA; 2003. [Google Scholar]
- McDonald MB. Seed deterioration: physiology, repair and assessment. Seed Science and Technology. 1999;27:177–237. [Google Scholar]
- Merritt DJ, Turner SR, Clarke S, Dixon KW. Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany. 2007;55:336–344. [Google Scholar]
- Meyer SE, Debaene-Gill SB, Allen PS. Using hydrothermal time concepts to model seed germination response to temperature, dormancy loss and priming effects in Elymus elymoides. Seed Science Research. 2000;10:213–223. [Google Scholar]
- Moncur MW, Boland DJ, Harbard JL. Aspects of the floral biology of Allocasuarina verticillata (Casuarinaceae) Australian Journal of Botany. 1997;45:857–869. [Google Scholar]
- Peishi Z, Plummer J, Turner D, Choengsaat D, Bell D. Low- and high-temperature storage effects on viability and germinability of seeds of three Australian Asteraceae. Australian Journal of Botany. 1999;47:265–275. [Google Scholar]
- Schutz W, Milberg P, Lamont BB. Seed dormancy, after-ripening and light requirements of four annual Asteraceae in South-western Australia. Annals of Botany. 2002;90:707–714. doi: 10.1093/aob/mcf250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman KJ, Ellery AJ, Chapman R, Moore A, Turner NC. Maturation temperature and rainfall influence seed dormancy characteristics of annual ryegrass (Lolium rigidum) Australian Journal of Agricultural Research. 2004;55:1047–1057. [Google Scholar]
- Stanley TD, Ross EM. Flora of south-eastern Queensland. vol. 2. Brisbane: Queensland Department of Primary Industries; 2002. [Google Scholar]
- Taylor A, Allen P, Bennet M, Bradford K, Burris J, Misra M. Seed enhancements. Seed Science Research. 1998;8:245–256. [Google Scholar]
- Turner SR, Merritt DJ, Baskin JM, Baskin CC, Dixon KW. Combinational dormancy in seeds of the Western Australian endemic species Diplopeltis huegelii (Sapindaceae) Australian Journal of Botany. 2006;a 54:565–570. [Google Scholar]
- Turner SR, Merritt DJ, Ridley EC, Commander LE, Baskin JM, Baskin CC, Dixon KW. Ecophysiology of seed dormancy in the Australian endemic species Acanthocarpus preissii (Dasypogonaceae) Annals of Botany. 2006;b 98:1137–1144. doi: 10.1093/aob/mcl203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ. A simulation model for seasonal changes in dormancy and germination of weed seeds. Seed Science Research. 2001;11:77–92. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. Redefining seed dormancy: an attempt to integrate physiology and ecology. The Journal of Ecology. 1995;83:1031–1037. [Google Scholar]
- Zar JH. Biostatistical analysis. 2nd edn. Englewood Cliffs, NJ: Prentice Hall; 1984. [Google Scholar]