Abstract
Background and Aims
Plants growing in altered gravity conditions encounter changes in vascular development and cell wall deposition. The aim of this study was to investigate xylem anatomy and arrangement of cellulose microfibrils in vessel walls of different organs of soybean seedlings grown in Space.
Methods
Seeds germinated and seedlings grew for 5 d in Space during the Foton-M2 mission. The environmental conditions, other than gravity, of the ground control repeated those experienced in orbit. The seedlings developed in space were compared with those of the control test on the basis of numerous anatomical and ultrastructural parameters such as number of veins, size and shape of vessel lumens, thickness of cell walls and deposition of cellulose microfibrils.
Key Results
Observations made with light, fluorescence and transmission electron microscopy, together with the quantification of the structural features through digital image analysis, showed that the alterations due to microgravity do not occur at the same level in the various organs of soybean seedlings. The modifications induced by microgravity or by the indirect effect of space-flight conditions, became conspicuous only in developing vessels at the ultrastructural level. The results suggested that the orientation of microfibrils and their assembly in developing vessels are perturbed by microgravity at the beginning of wall deposition, while they are still able to orient and arrange in thicker and ordered structures at later stages of secondary wall deposition.
Conclusions
The process of proper cell-wall building, although not prevented, is perturbed in Space at the early stage of development. This would explain the almost unaltered anatomy of mature structures, accompanied by a slower growth observed in seedlings grown in Space than on Earth.
Key words: Cell wall, cellulose microfibrils, Glycine max, microgravity, soybean seedlings, space, xylem anatomy
INTRODUCTION
The evolution of plants and their spreading in the terrestrial environment have been accompanied by a progressive increase in complexity of the composition and structure of cell walls. The latter perform major functions in the development and survival of land plants because they support the aerial organs under 1-g conditions in much the same way as bones and muscles support the animal body (Taylor et al., 1992; Hoson, 1998). Development of a tough cell wall for supporting an upright stand against gravity leads to restricting the free growth of tissues and organs, thus affecting growth and morphogenesis (Hoson et al., 2003).
Changes in the gravitational stimulus are responsible for alterations in the chemical and mechanical properties of cell walls. Hypergravity (gravity conditions higher than 1 g, created in the laboratory by using centrifuges) conditions are reported to affect the development of xylem cells due to enhancement of the deposition of cell wall polysaccharides and lignin, the development of thicker and more rigid cell walls, and a reduction in the extensibility of secondary walls (Kasahara et al., 1995; Hoson et al., 1996; Nakabayashi et al., 2006; Tamaoki et al., 2006). On the other hand, simulated or real microgravity are considered responsible for changes in the amount and organization of cellulose and lignin, modifications in the levels and composition of cell wall polysaccharides and other events leading to the alteration of cell wall thickness and extensibility (Cowles et al., 1984; Halstead and Dutcher, 1987; Nedukha, 1996; Levine et al., 2001; Hoson et al., 2002, 2003).
Few experiments have been performed to understand the possible changes encountered by cell walls developing under real microgravity (10−6 g) in Space. Indeed, opportunities for space experiments are limited and most of the knowledge currently available is based on the results of tests conducted in simulated microgravity or on the interpretation of the data obtained in hypergravity experiments, accepting that results follow the opposite trend under microgravity conditions (Hoson et al., 1996). Moreover, often protoplasts were used as models (Iversen et al., 1992; Rasmussen et al., 1992, 1994a, b; Skagen and Iversen, 1999), partly to overcome the technical constraints imposed by flight experiments, namely the restricted availability of room, time and crew-time for plant cultivation. Cell cultures were also useful to investigate the relationships between cortical microtubules and cellulose microfibrils during the synthesis of cell walls (Baskin, 2001; Roberts et al., 2004). The orientation of cortical microtubules was proved to change following the alteration of the gravitational field, thus causing modifications in the arrangement of cellulose microfibrils (Skagen and Iversen, 1999). The few investigations conducted to visualize cortical microtubules or cellulose microfibrils in planta during flight experiments have often produced conflicting observations. For example, Sytnik et al. (cited in Halstead and Dutcher, 1987) claimed the randomization of microfibrils in relation to the longitudinal axis of cells developed in microgravity conditions. On the other hand, the deposition of ordered cellulose microfibrils seemed to be not perturbed by microgravity in primary walls of wheat (Levine et al., 2001).
The conflicting results obtained in different experiments performed in microgravity may be explained by the fact that metabolic and structural responses of plants to microgravity are affected by numerous factors such as the species, cultivar, age and physiological state of the plant, and may differ in various organs or regions of the same organ (Levine et al., 2001; Nakabayashi et al., 2006). Moreover, in the experiments performed in space, results can be influenced by other variables such as (a) the rapid changes in the gravitational conditions on the way back to Earth, when fixation of samples is not carried out while in microgravity, or (b) the type of fixation itself, when different protocols and hardware are used (Levine et al., 2001; Hoson et al., 2002).
This paper reports the results of a space-flight experiment to describe, for the first time, the features of developing xylem cells in different organs of soybean seedlings grown in a microgravity environment. The anatomical features of xylem are quantified and the ultrastructure of cell walls is investigated, focusing on the assembly of cellulose microfibrils.
MATERIALS AND METHODS
Flight and 1-g control experiment
Thirty-two soybean seeds [Glycine max (L.) Merr. ‘Adzuchi’, 2004 seed lot] were screened for size, weight, uniformity and lack of defects. They were sterilized and used to supply the automatic growth support systems used during the flight experiment on board of Foton-M2 capsule, launched by a Soyuz rocket (mission period: 31 May 2005 to 16 June 2005), and in the 1-g control test (De Micco et al., 2006c). The flight hardware was inserted into a portable incubator (temperature was set at 22 °C) and transferred to the launch base at the Baikonur cosmodrome (Kazakhstan). On day 1 (0930 h, time zero), the flight hardware was switched on and was installed in the Foton-M2 capsule. Foton-M2 took off on day 4 at 1800 h and returned to Earth after orbiting for 16 d. Switching on the hardware primed the countdown to start all the planned operations, namely the recording of temperature and humidity data, injection of water for seed germination and injection of the chemical fixative (formaldehyde 40 % : glacial acetic acid : ethanol 50 % in the volume ratio of 5 : 5 : 90) to stop seedling growth. More specifically, water injection started 8 d (11 520 min) after switching on the hardware and was completed 17 min later. This operation primed seed germination and seedling growth that was blocked after 5 d (at 18 720 min) by the injection of the chemical fixative. Seedlings were allowed to grow in the dark, at a temperature range of 18–20 °C. After the flight, identical experiments in terms of hardware, materials and procedures were performed in normal gravity conditions (1-g control) at the laboratories of the University of Naples. Since seedlings developed in Space were, in average, smaller in size than those grown in 1 g (De Micco et al., 2006b), anatomical and ultrastructural analyses were performed on seedlings showing the same morphology and size. In this way, the different anatomical features can be considered as due to the effect of Space factors and not biased by the comparison of different developmental stages. The seedlings were dissected to isolate cotyledons, the hypocotyl hook and hypocotyl zone corresponding to the maximum diameter. The sub-samples obtained were subjected to preparation for light-, epi-fluorescence microscopy and transmission electron microscopy, setting the protocols to have the same time of fixation and embedding procedure for seedlings developed in Space and in 1 g on Earth.
Light and epi-fluorescence microscopy
Samples (3 sub-samples × 5 seedlings × 2 treatments) were dehydrated in an ethanol series up to 90 % and embedded in JB4 acrylic resin (Polysciences Europe, Eppelheim, Germany). Semi-thin cross-sections (1–3 µm thick) of the middle part of the dissected sub-samples were obtained by using a rotative microtome and collected on glass slides. Several sections per sample were stained with 0·5 % Toluidine Blue (Feder and O'Brien, 1968), mounted with Canadian Balsam and observed under a transmitted light microscope (BX 60, Olympus, Hamburg, Germany,).
The unstained sections were observed under an epi-fluorescence microscope (BX60, Olympus) set to detect the UV induced fluorescence of lignin (excitation 330–385 nm, emission 400–420 nm) (Fukuzawa, 1992).
Quantification of anatomical parameters
Microphotographs were obtained by means of a digital camera (CAMEDIA C4040, Olympus). Images were analysed with Plant Meter-Root (Aronne and Eduardo, 2001) and AnalySIS 3·2 (Olympus), two software programs devised to quantify anatomical features.
The anatomy of the vascular system was studied in cotyledons, the hypocotyl and hypocotyl hook by counting the number of principal veins (Fig. 1A and B) or xylem poles (Fig. 1C–F), and number of vessels per vein/pole. In each section, 15 vessels were selected among those where lignin was completely deposited and the thickness of cell walls was measured avoiding cell corners. Moreover, the area and shape of vessel lumen were quantified; cell shape was defined by the following indices (definitions are based on the AnalySIS manual): aspect ratio – the maximum ratio of width and height of a rectangle circumscribing the cell; convexity – the fraction of the cell's area and the area of its convex; elongation – the lack of roundness of the cell (the more elongated the cell, the higher the value of the index); shape factor – this provides information about the ‘roundness’ of the cell by considering the deviations of the two-dimensional cell outline from the standard image of a circle (for a spherical, turgid cell, whose boundaries are straight and smooth, the shape factor assumes the maximum value of 1); sphericity – this describes the ‘roundness’ of the cell by using central moments (for a spherical cell it is 1, for all other cell shapes it is smaller than 1; sphericity is meant as circularity that is a more appropriate two-dimensional term).
Fig. 1.
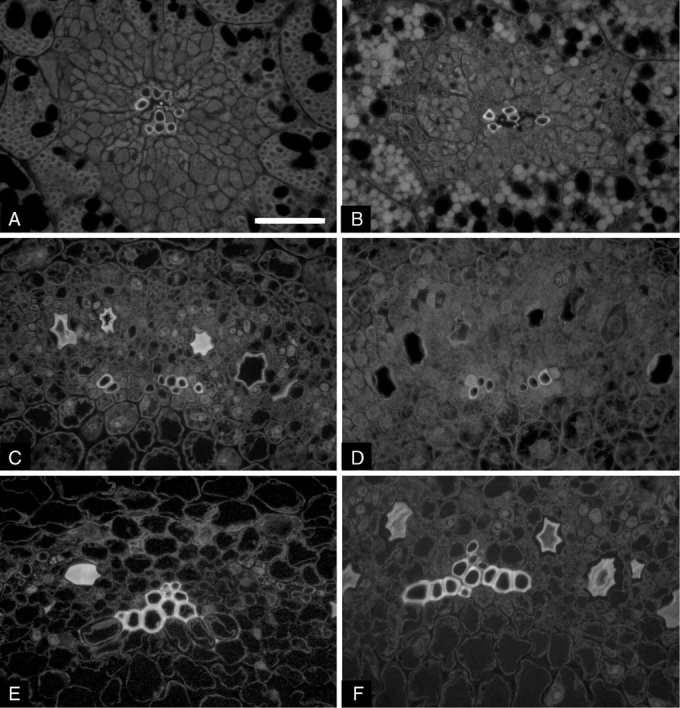
Microphotographs of cross-sections of soybean seedlings grown in 1 g on Earth (A, C, E) and in microgravity (B, D, F): cotyledons (A, B), hypocotyl hook (C, D) and hypocotyl (E, F). Vessel walls appear fluorescent because of the presence of lignin. Single principal veins are surrounded by parenchyma cells in cotyledons (A, B). Single poles of xylem in the stele of hypocotyl zones are shown (C, D, E, F). Scale bar = 50 µm.
Transmission electron microscopy
Sub-samples of cotyledons and the hypocotyl were dehydrated in an ethanol series up to 100 %. The samples were then transferred to solutions of ethanol/LR White acrylic resin, hard grade (Ted Pella, Inc., Redding, CA, USA), 3/1, 1/1, 1/3 (v/v). Finally, the samples were infiltrated with 100 % LR White and embedding in fresh LR White resin was performed in gelatine capsules with polymerization for 24 h at 50 °C. Ultra-thin sections (50 nm) were cut using an ultramicrotome (Leica UC6, Leica Microsystems, Wetzlar, Germany) and collected with plastic rings (Marinozzi, 1964). Sections floating on plastic rings were subjected to the periodic acid–thiocarbohydrazide–silver-proteinate method in order to mark the polysaccharide moiety of the cell walls, following the procedure by Thiéry (1967) modified by Ruel et al. (1981) to highlight cellulose microfibrils. In brief, the sections were floated on a 5 % solution of periodic acid in water for 90 min, the reaction with thiocarbohydrazide was carried out for 24 h and silver proteinate was applied for 30 min in the dark. Finally, the sections were transferred to copper grids coated with carbon–collodion (collodion 2 % in amyl acetate) and examined with a Philips CM 200-Cryo-TEM at an accelerating voltage of 80 kV. Photographs of the cell walls of developing vessels in both cotyledon and hypocotyl were taken on electron microscope films (Kodak 4489, Rochester, NY, USA).
Measurement of microfibrils
The electron microscope films were scanned with an HP scanjet 7400c scanner. Given the magnification of microscope films and the resolution of digital images, pixel size ranged between 0·658 and 1·316 nm, thus allowing measurement at accuracy of no less than nanometre level. (Lower values of pixel size corresponded to the photos taken at the highest magnification and used for the detection of the thinnest structures.) The digital images were subjected to digital image analysis with the Plant Meter–Root software. The thickness of microfibrils and of the structures formed after the progressive assembly of adjacent microfibrils (lamellae) was measured repeating three times the measurement of each structure in three nearby measurements. Micrographs of three to five replicates were analysed per treatment, measuring at least 15 microfibrils and lamellae in each developing vessel. In the Results, mean values are rounded up to 10−2 but this should not give the misleading impression that accuracy of measurement was higher than nanometre level. To be more conservative, the size of single microfibrils might have an indicative value, while data on the other classes of lamellae are more consistent.
Statistical analysis of data
All results were subjected to statistical analysis (ANOVA) using the SPSS statistical package (SPSS Inc., Chicago, IL, USA). Data on convexity, shape factor and sphericity were transformed through arcsine function before statistical analysis.
RESULTS
Light and epi-fluorescence microscopy
As a preliminary observation, it was verified that primary xylem in cotyledons and hypocotyl in soybean seedlings is formed during germination starting from provascular bundles. Primary xylem in soybean seedlings occurred as numerous veins interspersed in the surrounding parenchyma in the cotyledons (Fig. 1A, B) and as poles of developing vessels in the central cylinder of the hypocotyl hook and hypocotyl (Fig. 1C–F). In the seedlings developed in 1-g conditions, the median sections of each cotyledon were characterized by the presence of 16·2 ± 0·86 (mean value ± s.e.) bundles, each having 9·20 ± 0·75 vessels. Similarly, seedlings grown in Space showed 15·2 ± 0·71 veins made of 10·82 ± 0·51 cellular elements.
The hypocotyl hook and hypocotyl presented eight principal veins coupled to form four opposite poles (Fig. 1C, D); the latter are gradually joined throughout the hypocotyl towards the root (Fig. 1E, F). In both the hypocotyl hook and hypocotyl, the mean number of vessels per vein was not significantly different between seedlings developed in Space and on Earth: values ranged between 7·12 ± 0·30 and 8·31 ± 0·28.
Both in cotyledons and hypocotyl zones, most of the vessels had cell walls with secondary thickenings that appeared fluorescent due to lignification (Fig. 1); in some elements, UV-induced fluorescence of lignin appeared evident only at cell corners.
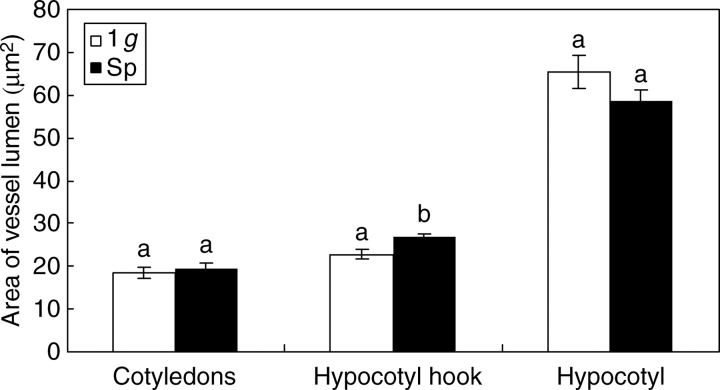
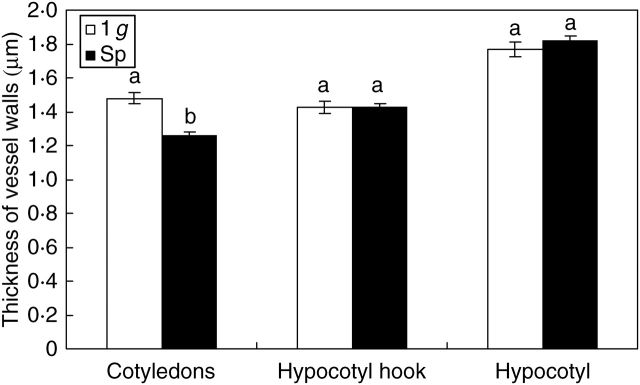
Vessel lumen of the hypocotyl hook grew significantly larger (P < 0·05) in Space than on Earth, while no significant differences were found in the other regions of the seedlings (Fig. 2). The shape of vessel lumen, measured as the ratio between opposite diameters (aspect ratio), enlargement of the cell in a preferential direction (elongation) or lack of sphericity, underwent no significant changes in any of the organs analysed during the development of seedlings in microgravity (Table 1). However, the analysis of other indices, namely convexity and shape factor, evidenced significantly lower values in cotyledons of seedlings grown in Space, suggesting the occurrence of less straight and less smooth inner boundaries of the cell wall (Table 1) compared with seedlings developed in 1 g. Regarding vessel walls, a significant difference was revealed only by vessels of cotyledons whose cell walls were significantly thicker in seedlings developed in 1-g conditions than in Space (Fig. 3).
Fig. 2.
Comparison of seedlings developed in normal gravity conditions (1 g) and in microgravity (Sp) on the basis of vessel lumen size. Mean values and standard errors are shown. Different letters correspond to significantly different values after using LSD and Student–Newman–Keuls coefficients for multiple comparison tests (P < 0·05).
Table 1.
Mean values of indices describing the effect of space-flight conditions on the shape of vessel lumen in the various organs of soybean seedlings developed in microgravity (Sp) and in normal gravity conditions (1 g)
| Cotyledons | Hypocotyl hook | Hypocotyl | ||
|---|---|---|---|---|
| Aspect Ratio | Sp | 1·359a | 1·450a | 1·369a |
| 1 g | 1·335a | 1·431a | 1·361a | |
| Convexity | Sp | 0·952a | 0·958a | 0·968a |
| 1 g | 0·960b | 0·961a | 0·966a | |
| Elongation | Sp | 1·384a | 1·479a | 1·389a |
| 1 g | 1·355a | 1·466a | 1·376a | |
| Shape factor | Sp | 0·882a | 0·885a | 0·905a |
| 1 g | 0·905b | 0·899a | 0·895a | |
| Sphericity | Sp | 0·575a | 0·518a | 0·584a |
| 1 g | 0·591a | 0·530a | 0·582a |
Different letters correspond to significantly different values after using LSD and Student–Newman–Keuls coefficients for multiple comparison tests (P < 0·05).
Fig. 3.
Comparison of seedlings developed in normal gravity conditions (1 g) and in microgravity (Sp) on the basis of wall thickness of vessels. Mean values and standard errors are shown. Different letters correspond to significantly different values after using LSD and Student–Newman–Keuls coefficients for multiple comparison tests (P < 0·05).
Transmission electron microscopy
The ground control seedlings showed vessel walls with a clearly well-organized structure both in cotyledons and hypocotyl. Single cellulose microfibrils were laid parallel to each other and were arranged in progressively thicker bundles (lamellae) from the deposition of middle lamellae and primary wall up to the complete formation of the secondary wall (Fig. 4A, C, E, G). The ordered arrangement of cellulose fibrils in vessel walls was still recognizable in all the vessels where the assembly of secondary wall and lignification was completed (Fig. 4G). The ultrastructure of primary vessel walls of seedlings grown in microgravity appeared disorganized in 100 % developing vessels, with no differences between cotyledons and hypocotyl. More specifically, the lack of orientation of these microfibrils was evident in wide areas of all developing vessel walls where single cellulose fibrils were not laid parallel to each other but were spread without any preferential direction (Fig. 4B, D, F). Although microfibrils were preferentially separated from each other, in some regions they were arranged into lamellae whose boundaries were not so conspicuous as in the 1-g control; consequently they were less easily detectable. The disorganization was also visualized in the form of a separation of the microfibrils from cellulose bundles appearing more compact. The lack of orientation endured by these fibrils in microgravity caused their spreading in a wider area between two adjacent cells compared with cellular elements developing in 1-g conditions.
Fig. 4.
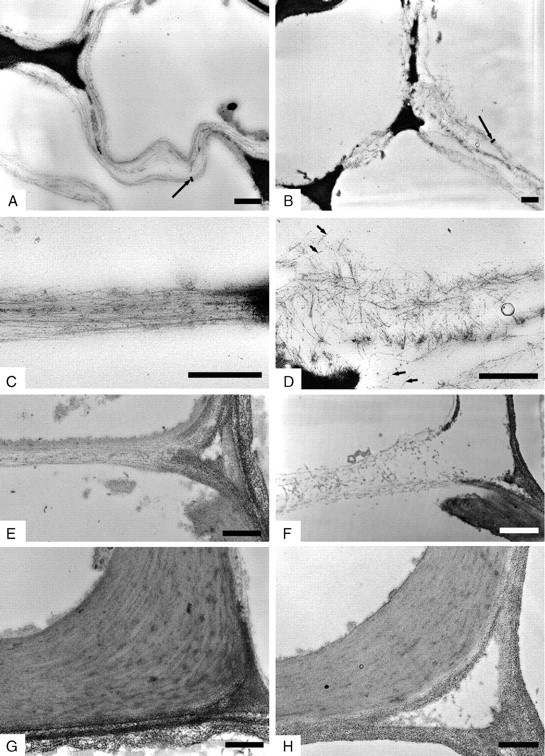
Transmission electron microphotographs of thin sections of developing vessel walls of soybean seedlings grown in 1 g on Earth (A, C, E, G) and in microgravity (B, D, F, H). Periodic acid–thiocarbohydrazide–silver-proteinate staining. Cellulose microfibrils lie parallel to each other and are arranged in lamellae in cotyledons (A, C) and hypocotyl (E) of seedlings grown in 1 g. Microfibrils are scattered and are not easily arranged in thicker bundles in cotyledons (B, D) and hypocotyl (F) of seedlings developed in microgravity. Vessel walls with complete deposition of secondary wall show ordered arrangement in both 1 g (G) and microgravity (H). Long arrows = lamellae; short arrows = microfibrils. Scale bars = 0·5 µm.
A more ordered arrangement of the cell wall was visualized in those vessel walls where the deposition of secondary wall and lignification was completed (Fig. 4H).
Measurement of cellulose microfibrils and bundles
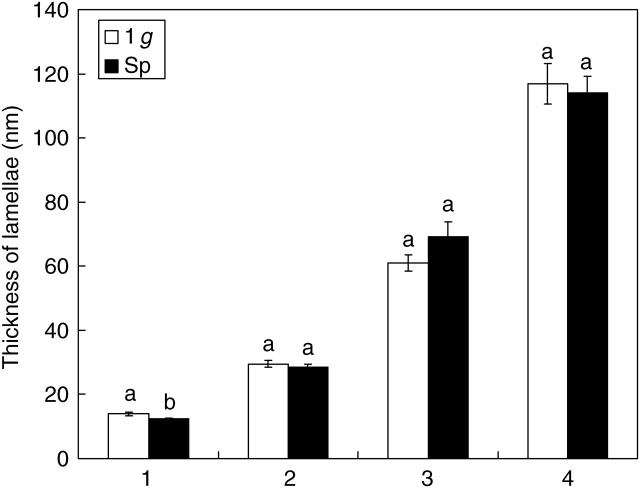
Cellulose microfibrils of primary vessel walls were 6·82 ± 0·17 nm (mean value ± s.e.) thick in seedlings developed in 1-g conditions and 6·37 ± 0·13 nm in microgravity. This difference is statistically significant; however, it is close to the limit imposed by measurement accuracy. Two or more microfibrils were arranged in progressively thicker bundles (lamellae), showing a similar trend of size increment in both Space and 1-g seedlings (Fig. 5; the numbers from 1 to 4 indicate the classes of progressively thicker lamellae deriving from the arrangement between microfibrils). The thickness of lamellae was not significantly different between Space and 1-g seedlings in all classes considered but the first. The mean multiplying factor that marks the passage from the thinner structures up to thicker bundles was 2·035 in 1-g conditions and 2·080 in microgravity. Variability in the microfibril arrangement was estimated by the coefficient of variation that was 0·042 in the control versus 0·167 in Space. This suggests that the assemblage modality is more stable in 1-g than in microgravity.
Fig. 5.
Arrangement of cellulose microfibrils into thicker bundles in vessel walls of soybean seedlings. Size of lamellae resulting from the progressive arrangement of microfibrils in seedlings developed in normal gravity conditions (1 g) and in microgravity (Sp). The numbers from 1 to 4 label the lamellae of increasing thickness starting from the thinnest detectable. Mean values and standard errors are shown. Different letters correspond to significantly different values after using LSD and Student–Newman–Keuls coefficients for multiple comparison tests (P < 0·05).
DISCUSSION
It is well known that xylem is differentiated from meristematic cells: primary xylem is produced by procambial cells derived from apical meristems, and secondary xylem originates from the activity of the vascular cambium during secondary growth. After being generated by a meristematic cell, a xylem cell undergoes a process of growth and differentiation encountering radial and longitudinal elongation, thickening and lignification of walls. Within the xylem, the degree of cell wall thickening as well as the pattern and chemical nature of thickenings vary according to the function of the cell and are the result of the influence of numerous factors. Changes in gravitational conditions, whether microgravity or hypergravity, can affect the morphology, mechanical and chemical properties of xylem cells, thus causing modifications to plant growth and development (Hoson et al., 1996). Moreover, such modifications can involve various levels of the plant organization and are not always revealed concurrently at structural, ultrastructural, chemical or molecular level. The analysed soybean seedlings grown in microgravity seemed to form a normal xylem pattern propagating in the cotyledons and throughout the axis of developing organs. The quantification of anatomical features and ultrastructural observations showed that the formation of xylem cells is somewhat perturbed in Space samples. More specifically, the ultrastructural organization of primary cell walls was affected by microgravity or the indirect action of space-flight conditions only in developing vessels.
Being predetermined in the seed, the number of veins did not change during the development of soybean seedlings in Space. The mechanisms regulating the pattern of vascular bundles in cotyledons and leaves are poorly understood and they can be controlled at hormonal level, as evidenced in arabidopsis seedlings (Sieburth, 1999). The number of xylem cells formed within a vein can be modified by altered gravity conditions as demonstrated by Nakamura et al. (1999) who found that stems of Prunus jamasakura growing on a 3D-clinostat formed a smaller amount of secondary xylem with fewer fibres and a higher incidence of vessels. Hypergravity is also a factor affecting the formation of xylem cells; indeed, the development of a higher number of metaxylem elements with larger lumen size in the immature inflorescence stem of Arabidopsis thaliana is promoted by hypergravity, while the formation of protoxylem cells is not disturbed (Nakabayashi et al., 2006). This is in agreement with the present findings that the number of xylem cells in cotyledon veins and in vascular bundles of the hypocotyl in soybean seedlings does not undergo significant changes in altered gravity conditions despite showing a tendency to increase. Previous experiments on the clinostat also showed that soybean seedlings developing in simulated microgravity had a tendency to form more xylem cells with larger lumen compared with those growing in 1-g conditions (De Micco et al., 2006a). The increased dimensions of vessel lumen were confirmed only in the hypocotyl hook in the case of soybean seedlings developed in Space. The formation of larger vessel lumens in Space than 1-g seedlings could be related to the altered deposition of cellulose microfibrils in microgravity. Indeed, alignment of cellulose microfibrils is considered responsible for mechanical anisotropy of cell walls, thus influencing growth phenomena (Suslov and Verbelen, 2006). However, significant differences in other organs were not found where tendencies to both increase and decrease in vessel lumen size were found. No strict relationship has been established yet between levels of gravity and cell size in various tissues in planta because either increase or decrease of size is reported after exposure to altered gravity, but the results are not always significant (Darbelley et al., 1989; Moore, 1990; Nakamura et al., 1999; Aronne et al., 2003). Moreover, the response can be different in various regions of the same organ (Nakabayashi et al., 2006).
Several experiments have been also conducted using protoplasts as model systems to examine the effect of microgravity and hypergravity on the morphological development of cells during the formation of cell walls (Wymer et al., 1996; Skagen and Iversen, 1999; Baskin, 2001; Roberts et al., 2004). The size of protoplasts is reported to increase after exposure to simulated or real microgravity (Rasmussen et al., 1994a,b; Iversen et al., 1999; Skagen and Iversen, 1999). It is believed that the increased volume of protoplasts is an indirect result of the inadequate quantity or disturbed organization of cortical microtubules causing the formation of less-developed or abnormal cell walls (Skagen and Iversen, 1999), suggesting lowered mechanical resistance.
There is evidence to support the prominent thinning of the cell walls in plants growing in real microgravity or under microgravity conditions simulated by water submergence (Masuda et al., 1994a,b; Hoson et al., 2003). On the other hand, plants appear to form thick and tough cell walls when exposed to hypergravity (Hoson et al., 1996; Nakabayashi et al., 2006).
Following these experiments, a positive trend is delineated with the cell wall becoming thicker according to the increase in gravity levels. The finding that vessel walls of soybean cotyledons are thinner in microgravity than in 1-g conditions is in agreement with this trend, although it is in contrast with results obtained in simulated microgravity (De Micco et al., 2006a). Moreover, this relationship between gravity and wall thickness was only observed in the case of cotyledons, but was not significant in hypocotyl hook and hypocotyl.
In soybean cotyledons, the thinning of cell walls is associated with the presence of less-definite internal boundaries as supported by the slightly, but still significant, lower values of convexity and shape factor occurring in microgravity. This is consistent with the ultrastructural observations which provided evidence for perturbations in the arrangement of cellulose microfibrils in primary cell walls. Indeed, these fibrils tended to lose their orientation and lay preferentially separated from each other in primary vessel walls of seedlings developed in microgravity, barely assembling into orderly thicker structures. However, in those regions where microfibrils were arranged into thicker structures, the size of these lamellae was the same as in 1-g conditions, albeit showing higher variability. It can be speculated that the disorganization of cellulose microfibrils is a transient phenomenon in vessel walls of soybean seedlings occurring in the early stages of the assembly of primary cell wall, because a more ordered arrangement of the fibrils can be visualized in vessel walls with advanced state of thickening, maturation and lignification. The observed alterations in the orientation and aggregation of cellulose microfibrils in the seedlings developed in Space reveal that wall assembly has been perturbed. It is difficult to say whether these ultrastructural alterations are directly or indirectly caused by microgravity and space-flight conditions. If they are not directly caused by microgravity, they could result from partial extraction of matrix polymers during the chemical fixation following the space experiment, such as pectin material removal. In any case, the difference with the control (which was fixed and embedded following exactly the same procedure as the Space samples) shows that the assembly of cellulose microfibrils did not occur in a normal way in Space samples. These defects concern primary walls and they cannot be seen after secondarization of the wall. The presence of normally completed lignified xylem vessels, together with the occurrence of normal walls in other cell-types, would explain why the morphological distortion is not conspicuous. A similar situation has been reported in genetically engineered A. thaliana plants down-regulated in some matrix components (Goujon et al., 2003).
The lack of orientation of cellulose microfibrils in cell walls of soybean seedlings developed in microgravity conditions is in agreement with the observations made by other authors who reported the occurrence of randomized cellulose microfibrils on the longitudinal axis of cells, also bacteria, developed in altered gravity conditions (Sytnik et al. cited in Halstead and Dutcher, 1987; Brown et al., 1992). Levine et al. (2001) also reported the occurrence of perturbations during the development of secondary walls in wheat grown in the milligravity (10−3–10−4) environment of a space-shuttle mission. More specifically, they found indications of the thinning of secondary walls and a reduced deposition of cellulose, although their experiment did not reveal perturbations in the organization of cellulose microfibrils. It has to be noted that these authors examined cells from completely developed tissues. According to the broadened view of the microtubule/microfibril paradigm, which states that cortical microtubules provide positional information for the nascent cellulose microfibrils and that the latter supply biophysical information back to the microtubules, the excessively low density of nascent cellulose causes the failure of the ordering principles during the assemblage of cell walls and consequently generates the random deposition of microfibrils (Giddings and Staehelin, 1991; Wymer et al., 1996; Fisher and Cyr, 1998; Baskin, 2001; Roberts et al., 2004). In cell walls of soybean seedlings developing in space, the lack of orientation of the fibrillar structures was accompanied by their spreading on a wider area between two adjacent cells and consequently determined a lower density of cellulose microfibrils which, in turn, probably constrained the functioning of the ordering principles.
Experiments using protoplasts as models supported the hypothesis that, while hypergravity triggers an increased organization of cortical microtubules, low-gravity conditions not only reduce the ability of the cell to organize the cortical microtubules into parallel arrays but also reduce their quantity, thus lowering the capability to regenerate an adequate cell wall (Skagen and Iversen, 1999; Soga et al., 2006 ). The scattering of microfibrils in cell walls developing in Space could also be due to the lack of crystallization of glucan chains through H–H bonding during cellulose formation (Brown et al., 1992; Nedukha, 1996).
The formation of the cell wall is a complex phenomenon which involves not only the correct formation and assembly of cellulose microfibrils, but also their interaction with other compounds, such as hemicelluloses, pectins and lignin, as visualized through immunological probes (Esau, 1977; Ruel et al., 1994; Joseleau and Ruel, 1997; Ruel, 2003). Altered gravity conditions affect the chemical composition of cell walls, changing the proportion between different macromolecules. Experiments conducted in microgravity conditions provide evidence for the reduction in cellulose and matrix polysaccharides production, the alteration of the ratio of the high molecular mass polysaccharides in the hemicellulose fraction, and changes in the lignin content and composition (Cowles et al., 1984, 1988; Nedukha, 1996; Levine et al., 2001; Genot et al., 2003; Hoson et al., 2002, 2003; Soga et al., 2002). Alteration of the degree of crystallization of cellulose as well as the different interactions with the other cell wall components could be hypothesized to explain the lower estimation of the size of microfibrils and some classes of lamellae in soybean seedlings grown in Space. The interaction between cellulose and other compounds of polysaccharidic nature is also the reason, besides the techniques used, deemed responsible for the difference in apparent size of microfibrils measured by various authors (Kerr and Goring, 1975; Ruel et al., 1978; Ruel and Barnoud, 1981; McCann et al., 1990; Donaldson and Singh, 1998; Davies and Harris, 2003).
In conclusion, the overall analysis suggests that, although cellulose microfibrils show randomized orientation at the beginning of the primary vessel wall deposition in soybean seedlings developing in microgravity, they seem to be able to orient parallel to each other and arrange in progressively thicker bundles at a later stage, at least during the deposition of secondary wall layers. Moreover, the perturbations observed at the beginning of primary wall formation in vessels could furnish a reason to explain the almost unaltered anatomy accompanied by a slower growth observed in seedlings developed in Space than in 1 g on Earth. Although to confirm the present results and interpretations, a space experiment with in-flight 1-g control would be consequential, it can be hypothesized that the process of proper cell-wall building is not prevented but somewhat slowed under Space conditions.
ACKNOWLEDGEMENTS
We acknowledge funding by the European Space Agency (ESA) through the ISS Education Fund. Financial support was also provided by the Palynological Analyses Centre (CeAP) of University of Naples Federico II, Microglass Heim s.r.l., Natural Technologies Italia–N.T.I. s.r.l. and Olympus Italia s.r.l. The authors are grateful to Michele Scala (University of Naples Federico II, Naples, Italy), Raimondo Fortezza, Dario Castagnolo and Salvatore Sorrentino (MARS Center, Naples, Italy), Pasquale Eduardo, Marco Colandrea and Massimo Nicolazzo (Informatica Service, Naples, Italy) for their advice, cooperation and technical support.
LITERATURE CITED
- Aronne G, Eduardo P. ‘ROOT’: a computer system to measure plant anatomical features automatically. In: Mazzoleni S, Colin CJ, editors. ModMED: Modelling Mediterranean Ecosystem Dynamics, Final Report ModMED III Project. 2001. pp. 125–128. EU-DGXII Environment (IV) Framework, ENV 4-ct97-0680. [Google Scholar]
- Aronne G, De Micco V, Ariaudo P, De Pascale S. The effect of uni-axial clinostat rotation on germination and root anatomy of Phaseolus vulgaris L. Plant Biosystems. 2003;137:155–162. [Google Scholar]
- Baskin TI. On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma. 2001;215:150–171. doi: 10.1007/BF01280311. [DOI] [PubMed] [Google Scholar]
- Brown RM, Jr, Kudlicka KK, Cousins SK, Nagy R. Gravity effects on cellulose assembly. American Journal of Botany. 1992;79:1247–1258. [PubMed] [Google Scholar]
- Cowles JR, Scheld HW, Lemay R, Peterson C. Growth and lignification in seedlings exposed to eight days of microgravity. Annals of Botany. 1984;54:33–48. doi: 10.1093/oxfordjournals.aob.a086865. [DOI] [PubMed] [Google Scholar]
- Cowles JR, Lemay R, Jahns G. Microgravity effects on plant growth and lignification. Astrophysical Letters and Communications. 1988;127:223–228. [PubMed] [Google Scholar]
- Darbelley N, Driss-Ecole D, Perbal G. Elongation and mitotic activity of cortical cells in lentil roots grown in microgravity. Plant Physiology and Biochemistry. 1989;27:341–347. [Google Scholar]
- Davies LM, Harris PJ. Atomic force microscopy of microfibrils in primary cell walls. Planta. 2003;217:283–289. doi: 10.1007/s00425-003-0979-6. [DOI] [PubMed] [Google Scholar]
- De Micco V, Aronne G, De Pascale S. Effect of simulated microgravity on seedling development and vascular differentiation of soy. Acta Astronautica. 2006;a 58:139–148. [Google Scholar]
- De Micco V, Aronne G, Scala M. Biometric anatomy of seedlings developed onboard of Foton M2 in an automatic system supporting growth. Proceedings of the 57th International Astronautical Congress, IAC-06-A1·5·07; Bremen: IAF Secretariat and ZARM; 2006. pp. 1–11. [Google Scholar]
- De Micco V, Aronne G, Scala M, Castagnolo D, Fortezza R. Growth-support system for seedling development onboard of unmanned spacecrafts. Space Technology. 2006;c 25(3–4):1–8. [Google Scholar]
- Donaldson LA, Singh AP. Bridge-like structures between cellulose microfibrils in radiata pine (Pinus radiata D. Don) kraft pulp and holocellulose. Holzforschung. 1998;52:449–454. [Google Scholar]
- Esau K. Anatomy of seed plants. 2nd edn. New York: John Wiley & Sons; 1977. [Google Scholar]
- Feder N, O'Brien TP. Plant microtechnique: some principles and new methods. American Journal of Botany. 1968;55:123–142. [Google Scholar]
- Fisher DD, Cyr RJ. Extending the microtubule/microfibril paradigm. Plant Physiology. 1998;116:1043–1051. doi: 10.1104/pp.116.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa K. Ultraviolet microscopy. In: Lin SY, Dence CW, editors. Methods in lignin chemistry. Berlin: Springer-Verlag; 1992. pp. 110–131. [Google Scholar]
- Genot P, Cabané M, Banvoy J, Ladouce N, Dauphin A, Pollet B, et al. The effects of simulated microgravity on the lignification of young Eucaliptus globulus. ELGRA News. 2003;23:118. [Google Scholar]
- Giddings TH, Jr, Staehelin LA. Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW, editor. The cytoskeletal basis of plant growth and form. London: Academic Press; 1991. pp. 85–99. [Google Scholar]
- Goujon T, Ferret V, Mila I, Pollet B, Ruel K, Burlat V, et al. Down-regulation of AtCCR1 gene in Arabidopsis thaliana: effects on phenotype, lignins and cell wall degradability. Planta. 2003;217:218–228. doi: 10.1007/s00425-003-0987-6. [DOI] [PubMed] [Google Scholar]
- Halstead TW, Dutcher FR. Plants in space. Annual Review of Plant Physiology. 1987;38:317–345. doi: 10.1146/annurev.pp.38.060187.001533. [DOI] [PubMed] [Google Scholar]
- Hoson T. Apoplast as the site of response to environmental signals. Journal of Plant Research. 1998;111:167–177. doi: 10.1007/BF02507163. [DOI] [PubMed] [Google Scholar]
- Hoson T, Nishitani K, Miyamoto K, Ueda J, Kamisaka S, Yamamoto R, et al. Effects of hypergravity on growth and cell wall properties of cress hypocotyls. Journal of Experimental Botany. 1996;47:513–517. doi: 10.1093/jxb/47.4.513. [DOI] [PubMed] [Google Scholar]
- Hoson T, Soga K, Mori R, Saiki M, Nakamura Y, Wakabayashi K, et al. Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant Cell Physiology. 2002;43:1067–1071. doi: 10.1093/pcp/pcf126. [DOI] [PubMed] [Google Scholar]
- Hoson T, Soga K, Wakabayashi K, Kamisaka S, Tanimoto E. Growth and cell wall changes in rice roots during spaceflight. Plant and Soil. 2003;255:19–26. doi: 10.1023/a:1026105431505. [DOI] [PubMed] [Google Scholar]
- Iversen T-H, Rasmussen O, Gmünder F, Baggerud C, Kordyum EL, Lozovaya VV, et al. The effect of microgravity on the development of plant protoplasts flown on Biokosmos 9. Advances in Space Research. 1992;12:123–131. doi: 10.1016/0273-1177(92)90274-2. [DOI] [PubMed] [Google Scholar]
- Iversen T-H, Johnsson A, Skagen EB, Ødegaard E, Beisvåg T, Chinga G, et al. Effect of a microgravity environment and influences of variations in gravity on the regeneration of rape seed plant protoplasts flown on the S/MM-03 mission. ESA SP. 1999;1222:1–11. [Google Scholar]
- Joseleau J-P, Ruel K. Study of lignification by non invasive techniques in growing maize internodes – an investigation by Fourier transform infrared, cross-polarisation-magic angle spinning 13C-nuclear magnetic resonance spectroscopy and immunocytochemical transmission electron microscopy. Plant Physiology. 1997;114:1123–1133. doi: 10.1104/pp.114.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara H, Shiwa M, Takeuchi Y, Yamada M. Effects of hypergravity on the elongation growth in radish and cucumber hypocotyls. Journal of Plant Research. 1995;108:59–64. doi: 10.1007/BF02344306. [DOI] [PubMed] [Google Scholar]
- Kerr AJ, Goring DAI. The ultrastructural arrangement of the wood cell wall. Cellulose Chemistry and Technology. 1975;9:563–573. [Google Scholar]
- Levine LH, Heyenga AG, Levine HG, Choi J-W, Davin LB, Krikorian AD, et al. Cell-wall architecture and lignin composition of wheat developed in a microgravity environment. Phytochemistry. 2001;57:835–846. doi: 10.1016/s0031-9422(01)00148-0. [DOI] [PubMed] [Google Scholar]
- McCann MC, Wells B, Roberts K. Direct visualization of cross-links in the primary plant cell wall. Journal of Cell Science. 1990;96:323–334. [Google Scholar]
- Maninozzi V. Cytochimie ultrastructurale du nucléole-RNA et protéines intranucléolaires. Journal of Ultrastructural Research. 1964;10:433. doi: 10.1016/s0022-5320(64)80021-6. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Kamisaka S, Yamamoto R, Hoson T, Nishitani K. Plant responses to simulated microgravity. Advances in Space Biology and Medicine. 1994;a 4:111–126. doi: 10.1016/s1569-2574(08)60137-9. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Kamisaka S, Yamamoto R, Hoson T, Nishitani K. Changes in the rheological properties of the cell wall of plant seedlings under simulated microgravity conditions. Biorheology. 1994;b 31:171–177. doi: 10.3233/bir-1994-31205. [DOI] [PubMed] [Google Scholar]
- Moore R. How effectively does a clinostat mimic the ultrastructural effects of microgravity on plant cells? Annals of Botany. 1990;65:213–216. doi: 10.1093/oxfordjournals.aob.a087926. [DOI] [PubMed] [Google Scholar]
- Nakabayashi I, Karahara I, Tamaoki D, Masuda K, Wakasugi T, Yamada K, et al. Hypergravity stimulus enhances primary xylem development and decreases mechanical properties of secondary cell walls in inflorescence stems of Arabidopsis thaliana. Annals of Botany. 2006;97:1083–1090. doi: 10.1093/aob/mcl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Sassa N, Kuroiwa E, Negishi Y, Hashimoto A, Yamashita M, et al. Growth of Prunus tree stems under simulated microgravity conditions. Advances in Space Research. 1999;23:2017–2020. doi: 10.1016/s0273-1177(99)00343-9. [DOI] [PubMed] [Google Scholar]
- Nedukha EM. Possible mechanisms of plant cell wall changes at microgravity. Advances in Space Research. 1996;17(6–7):37–45. doi: 10.1016/0273-1177(95)00610-q. [DOI] [PubMed] [Google Scholar]
- Rasmussen O, Klimchuk DA, Kordyum EL, Danevich LA, Tarnavskaya EB, Lozovaya MG, et al. The effect of exposure to microgravity on the development and structural organisation of plant protoplasts flown on Biokosmos 9. Physiologia Plantarum. 1992;84:162–170. [PubMed] [Google Scholar]
- Rasmussen O, Bondar RL, Baggerud C, Iversen T-H. Development of plant protoplasts during the IML-1 mission. Advances in Space Research. 1994;a 14:189–196. doi: 10.1016/0273-1177(94)90403-0. [DOI] [PubMed] [Google Scholar]
- Rasmussen O, Baggerud C, Larssen HC, Evjen K, Iversen T-H. The effect of 8 days of microgravity on regeneration of intact plants from protoplasts. Physiologia Plantarum. 1994;b 92:404–411. [Google Scholar]
- Roberts AW, Frost AO, Roberts EM, Haigler CH. Roles of microtubules and cellulose microfibril assembly in the localization of secondary-cell-wall deposition in developing tracheary elements. Protoplasma. 2004;224:217–229. doi: 10.1007/s00709-004-0064-4. [DOI] [PubMed] [Google Scholar]
- Ruel K. Immunochemical probes for microscopy study of the plant cell walls. In: Mendez-Vilas A, editor. Science, technology and education of microscopy: an overview. Vol. 2. Spain: FORMATEX; 2003. pp. 445–454. (edn A.), Series No. 1. [Google Scholar]
- Ruel K, Barnoud F. Supramolecular aspects of wood constituents as seen by electron microscopic investigations. ISWPC Stockholm. 1981;1:11–15. [Google Scholar]
- Ruel K, Barnoud F, Goring DAI. Lamellation in the S2 layer of softwood tracheids as demonstrated by scanning transmission electron microscopy. Wood Science and Technology. 1978;12:287–291. [Google Scholar]
- Ruel K, Barnoud F, Eriksson KE. Micromorphological and ultrastructural aspects of spruce wood degradation by wild-type Sporotrichum pulverulentum and its cellulase-less mutant cel 44. Holzforschung. 1981;35:157–171. [Google Scholar]
- Ruel K, Faix O, Joseleau J-P. New immunogold probes for studying the distribution of the different lignin types during plant cell wall biogenesis. Journal of Trace and Microprobe Technique. 1994;12:2247–2265. [Google Scholar]
- Sieburth LE. Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiology. 1999;121:1179–1190. doi: 10.1104/pp.121.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagen EB, Iversen T-H. Simulated weightlessness and hyper-g results in opposite effects on the regeneration of the cortical microtubule array in protoplasts from Brassica napus hypocotyls. Physiologia Plantarum. 1999;106:318–325. doi: 10.1034/j.1399-3054.1999.106309.x. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta. 2002;215:1040–1046. doi: 10.1007/s00425-002-0838-x. [DOI] [PubMed] [Google Scholar]
- Soga K, Wakabayashi K, Kamisaka S, Hoson T. Hypergravity induces reorientation of cortical microtubules and modifies growth anisotropy in azuki bean epicotyls. Planta. 2006;224:1485–1494. doi: 10.1007/s00425-006-0319-8. [DOI] [PubMed] [Google Scholar]
- Suslov D, Verbelen J-P. Cellulose orientation determines mechanical anisotropy in onion epidermis cell walls. Journal of Experimental Botany. 2006;57:2183–2192. doi: 10.1093/jxb/erj177. [DOI] [PubMed] [Google Scholar]
- Tamaoki D, Karahara I, Schreiber L, Wakasugi T, Yamada K, Kamisaka S. Effects of hypergravity conditions on elongation growth and lignin formation in the inflorescence stem of Arabidopsis thaliana. Journal of Plant Research. 2006;119:79–84. doi: 10.1007/s10265-005-0243-1. [DOI] [PubMed] [Google Scholar]
- Taylor JG, Owen TP, Jr, Koonce LT, Haigler CH. Dispersed lignin in tracheary elements treated with cellulose synthesis inhibitors provides evidence that molecules of secondary cell wall mediate wall patterning. The Plant Journal. 1992;2:959–970. [Google Scholar]
- Thiéry JP. Mise en evidence des polysaccharides sur coupes fines en microscopie éléctronique. Journal of Microscopy. 1967;6:987–1018. [Google Scholar]
- Wymer CL, Wymer SA, Cosgrove DJ, Cyr RJ. Plant cell growth responds to external forces and the response requires intact microtubules. Plant Physiology. 1996;110:425–430. doi: 10.1104/pp.110.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]