Abstract
Background and Aims
Cold acclimation modifies the balance of the energy absorbed and metabolized in the dark processes of photosynthesis, which may affect the expression of cold-regulated (COR) genes. At the same time, a gradual acclimation to the relatively high light conditions is observed, thereby minimizing the potential for photo-oxidative damage. As a result, the resistance to photoinhibition in the cold has often been identified as a trait closely related to freezing tolerance. Using four barley genotypes that differentially express both traits, the effect of cold acclimation on freezing tolerance and high-light tolerance was studied together with the expression of COR14b, one of the best-characterized barley COR genes.
Methods
Plants were cold acclimated for 2 weeks at 2 °C. Freezing tolerance was studied by means of electrolyte leakage. Changes in photosynthetic apparatus and high-light tolerance were monitored by means of chlorophyll fluorescence. Accumulation of COR14b and some proteins important in photosynthetic acclimation to cold were studied with western analysis. COR14b transcript accumulation during cold acclimation was assessed with real-time PCR.
Key Results
Cold acclimation increased both freezing tolerance and high-light tolerance, especially when plants were treated with high light after non-lethal freezing. In all plants, cold acclimation triggered the increase in photosynthetic capacity during high-light treatment. In two plants that were characterized by higher high-light tolerance but lower freezing tolerance, higher accumulation of COR14b transcript and protein was observed after 7 d and 14 d of cold acclimation, while a higher transient induction of COR14b expression was observed in freezing-tolerant plants during the first day of cold acclimation. High-light tolerant plants were also characterized with a higher level of PsbS accumulation and more efficient dissipation of excess light energy.
Conclusions
Accumulation of COR14b in barley seems to be important for resistance to combined freezing and high-light tolerance, but not for freezing tolerance per se.
Key words: Chlorophyll a fluorescence, cold acclimation, COR14b, non-photochemical energy quenching, freezing tolerance, gene expression, Hordeum vulgare, photoinhibition, photosynthetic acclimation to cold, photosynthetic capacity
INTRODUCTION
Temperature changes that trigger cold acclimation may be analysed by studying the photosynthetic apparatus (Ensminger et al., 2006). Changes of temperature and light intensity modify the balance of energy absorbed during the light phase of photosynthesis and the energy metabolized in dark processes. These changes influence the relative state of photosystem II (PSII) oxidation (Huner et al., 1998). Such changes can modify the balance between energy utilization in the processes of growth and cold acclimation (Rapacz, 1998a, b), as well as modifying expression of some genes directly involved in cold acclimation (Ndong et al., 2001).
Among the cold-regulated genes, COR14b is one of the best-characterized in cereals (Crosatti et al., 2003). This gene is differentially expressed in freezing-sensitive and freezing-tolerant plants under both laboratory and field conditions (Stanca et al., 1996; Giorni et al., 1999; Crosatti et al., 2003). Accumulation of this protein is evidence of a direct interaction between the cold-regulated (COR) gene expression originating from a cold-induced signalling pathway and the redox state of the chloroplast (Ensminger et al., 2006). The redox state of chloroplast plastoquinone promotes the accumulation of COR14b protein in the light, while the level of COR14 mRNA depends on cold treatment only (Dal Bosco et al., 2003). COR14b protein is accumulated in the stromatal component of the chloroplast (Crosatti et al., 1999).
On the other hand, the relative excess of light energy can lead to increased production of damaging reactive oxygen species (Müller et al., 2001). In such circumstances, regulation of the balance between absorption and utilization of light energy is necessary, thereby minimizing the potential for photo-oxidative damage. To regulate and protect photosynthesis against this stress event, so-called low-temperature photoinhibition – a gradual acclimation to the conditions in which light-energy absorption exceeds the capacity for light utilization – has been observed during low-temperature growth (Huner et al., 1993). Two general strategies of photosynthetic apparatus acclimation to relative excess of light can be observed in higher plants during cold hardening (Huner et al., 1993). The first strategy is that of increasing light-energy consumption in photochemical processes by increasing photosynthetic capacity. This strategy is observed mainly in over-wintering herbaceous plants, which must accumulate high amounts of photoassimilates before and during winter. The second strategy relies on an intensification of protective non-photochemical mechanisms that harmlessly dissipate excess excitation energy as heat, which is more typical of evergreen trees and shrubs. However, it is hard to assume that non-photochemical mechanisms, which are the most important in equilibration of the absorption and utilization of light energy (Müller et al., 2001) are not involved, partially at least, in any case of photosynthetic acclimation to cold. In herbaceous plants the development of a non-photochemical mechanism has been observed, depending on genotype (Rapacz et al. 2004; Humphreys et al., 2007) as well as environmental factors (Adams et al., 2002).
Changes in the relative redox state of PSII affect both increasing freezing tolerance and acclimation of photosynthetic apparatus to avoid low-temperature photoinhibition. Therefore, the resistance to photoinhibition in cold has often been identified as a trait closely related to cold tolerance (Huner et al., 1998; Pocock et al., 2001; Rapacz et al., 2004) and plants that were not able to increase the resistance to photoinhibition were also not able to increase freezing tolerance during cold acclimation (Rapacz et al., 2007). However, data obtained previously has indicated that the approximate correlation between winter hardiness (or freezing tolerance) and the ability of phosynthetic acclimation to cold was about 0·7, which means that, in the case of some plants, freezing tolerance and the tolerance to cold-induced photoinhibition are, at least partially, independent (Rapacz et al., 2004). The aim of the present experiment was to characterize freezing tolerance and photosynthetic acclimation to cold in barley plants that differentially expressed both traits. Most attention was paid to the possible connections between COR14b expression, freezing tolerance and photosynthetic acclimation to cold.
MATERIALS AND METHODS
Plant material
The experiments were performed on four winter barley Hordeum vulgare L. varieties and advanced breeding lines of generations F6–F8 (‘Carola’ and lines POA4006, POA4044 and POA4784) supplied by Dr W. Mikulski (Szelejewo Plant Breeding, Poland) and R. Madejewski (Piast Plant Breeding, Poland), selected from about 60 breeding lines during three winters. These selected two- (POA4006 and POA4044) and six-rowed genotypes represented a high spectra of variation in freezing tolerance (as measured under field-laboratory and laboratory conditions), de-acclimation ability and also changes in photosynthetic apparatus observed during cold acclimation, which could suggest opposing abilities to adjust to cold and photosynthetic acclimation to cold (Rapacz et al., 2008).
Plant growth and cold acclimation
Seeds of the plants studied were sown in 20-cm pots containing a sand : peat mixture (1 : 1, v/v) and placed in an air-conditioned greenhouse (+21/ +17 °C, day/night, with a natural photoperiod of about 12/12 h). After 7 d, plants were moved to a growth chamber [1 week at +20 °C, photoperiod 10/14 h, 250 µmol m−2 s−1 photosynthetic photon flux density (PPFD); Philips AGRO sodium light source] and then cold acclimated (2 weeks at +2 °C, with the same PPFD and photoperiod). The experiment was carried out twice, in autumn 2006 and spring 2007. High-light treatment studies were repeated twice on plants growing in the spring, sown in two series at 1-week intervals. In this case, plants of particular genotypes were sown on different days to maintain the same length of cold acclimation period. Real-time PCR studies were done for the first time during the main experiment in the spring and were repeated in June 2007. All data presented in this paper are means from two independent experiments.
Measurements of chlorophyll fluorescence parameters before and during cold acclimation
All PSII chlorophyll a fluorescence measurements were made at room temperature with a FMS2 fluorometer (Hansatech, Kings Lynn, UK) on the middle section of the youngest, fully expanded leaves. Before measurements of minimum and maximum fluorescence of dark-adapted leaves (F0 and Fm, respectively), leaves were dark adapted for at least 15 min in leaf-clips. Values of steady-state fluorescence of light-adapted leaves (Fs) and maximum fluorescence of light-adapted leaves (F′m) were recorded sequentially when Fs became stable after re-exposure to actinic light (250 µmol m−2 s−1). In the case of Fm and F′m measurements, the saturating pulse intensity was about 10 000 µmol m−2 s−1. Next, minimal fluorescence of light-adapted leaves (F′0) was measured on removal of the actinic light but with the addition of far-red light to ensure rapid opening of PSII reaction centres. Photochemical quenching coefficient (qP) was calculated according to Schreiber et al. (1994): qP = (F′m – Fs)/(F′m – F′0). Non-photochemical quenching (NPQ) was calculated as: NPQ = (Fm – F′m)/F′m (Bilger and Bjorkman, 1991). Measurements were made in five replications of each experimental series, 1 d before cold acclimation started (day 0) and on the 7th and 14th days of cold acclimation.
Estimation of frost resistance
The youngest (but fully expanded) leaves were cut from plants (ten for each freezing temperature: −3, −6, −9, −12 and −15 °C), divided into 2-cm-long segments, and placed on ice (5 cm3 of frozen deionized water) in conductivity vessels. Then vessels were put into a programmed freezer with the temperature of 0 ± 0·5 °C. A freezing–thawing cycle was performed in darkness, separately for each freezing temperature. The temperature was decreased at a rate of 2 °C h−1. Freezing temperature was kept for 90 min and then the temperature was increased up to 0 °C at a rate of approx. 3 °C h−1. Measurements of plasma membrane injuries were done as described in Rapacz (1999) and the index of injuries (% EL) was calculated according to Flint et al. (1967). The temperature causing a 50 % electrolyte leakage (TEL50) was estimated from a linear regression fitted to the central part of the sigmoid relationship between the freezing temperature and electrolyte leakage, using at least three temperatures.
Responses of chlorophyll fluorescence in hardened and non-hardened plants exposed to high light
Plant response to photoinhibitory treatment was studied 1 d before cold acclimation started (non-acclimated plants) and on 14th day of cold acclimation (cold-acclimated plants). Chlorophyll fluorescence was measured using a pulse-amplitude-modulated fluorometer FMS2 (Hansatech) with saturating light flashes of 10 000 µmol m−2 s−1. Measurements were done in an Oxylab leaf chamber (Hansatech) cooled with a water bath at +5 °C. The temperature was controlled by an internal sensor (Hansatech). The initial measurements (F0, Fm) were made in dark-adapted (20 min) middle fragments of the youngest fully expanded leaves detached immediately before measurements were taken. Samples were subsequently exposed for 20 min to high light of 1200 µmol m−2 s−1, which was measured close to the leaf with an internal sensor (Hansatech), and provided by an LS2H lamp (Hansatech), supplied with a halogen light source, Decostar 51S 50W (Osram, Germany). After the exposure, F′m and F′0 were measured as described above and the next leaves were darkened. After 10 min of dark relaxation, maximum fluorescence (Fm10) was recorded and, after a subsequent 20 min of relaxation, minimum chlorophyll fluorescence after high-light treatment (F0HL) was measured. On the basis of theses measurements, qP and the kinetics of relaxation of non-photochemical fluorescence quenching were calculated. The fast-relaxing (within the first 10 min of dark relaxation after light treatment) component of fluorescence quenching (qE) was assigned to the energy-dependent mechanism connected with photoprotection and the slowly relaxing (after 10 min of dark relaxation) component of fluorescence quenching (qI) was assigned to a mix of photoprotection and photodamages (Müller et al., 2001). Quenching parameters of the Stern–Volmer type were calculated according to Thiele et al. (1997) as follows:
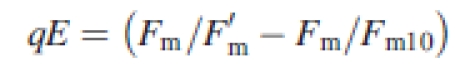 |
and
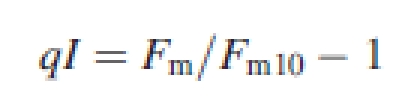 |
To distinguish between photoprotective mechanisms and photoinhibition inside the qI component (F0 – F0HL)/F0 was proposed as the indicator of photoinhibitory damages. According to Gilmore et al. (1996), F0 is decreased in direct proportion to Fm by the photoprotective quenching, whereas photoinhibition normally increases the F0 level while decreasing Fm.
All measurements were done with eight replications (four for each experimental series).
The effect of non-lethal freezing on the response to high light
After 14 d of cold acclimation, plants were subjected during the night to non-lethal freezing (−6 °C) for 4 h. Freezing and de-frosting rates were about 1·5 °C h−1. After defrosting, plants were transferred back to the conditions of cold acclimation and reaction to the high-light treatment was measured as described above. Measurements were made on six replicates (three for each experimental series).
Protein accumulation
The western experiment was repeated twice with three replications (leaves) in each experiment. Samples were always collected at the same time of the day (between the 6th and 7th hour of the day), a day before cold acclimation and on the 1st, 7th and 14th day of hardening. Basic characteristics of the proteins and antibodies used in the experiment are presented in Table 1. Frozen leaves (one for each sample – the youngest and fully expanded) were ground to a fine powder in liquid nitrogen. Total leaf proteins were precipitated with 10 % (w/v) trichloro-acetic acid and 0·07 % (v/v) β-mercaptoethanol dissolved in acetone (5 mL for 1 g of the leaf). Proteins were separated by SDS–PAGE using the buffer system of Laemmli (1970). Exactly 5 mg of lyophilized protein powder were dissolved in 280 µL of loading buffer (4 % w/v SDS, 12 % w/v glycerol, 2 % v/v β-mercaptoethanol, 0·01 % w/v bromophenol blue in 50 mm Tris, pH 6·8), boiled for 2 min and centrifuged (7 min, 12000 g). Thirty microlitres of supernatant were loaded into a 12 % polyacrylamide gel with a 5 % stacking gel. Together with samples, molecular weight markers (Sigma) were loaded into the gel. Electrophoresis was run in Mini-Protean III apparatus (Bio-Rad, Hercules, CA, USA) as follows: 195 V, 45 min in the case of higher-molecular-weight proteins [Rubisco large subunit (RbcL) and sucrose phosphate synthase (SPS) together with high-molecular-weight range SigmaMarker] and 165 V, 55 min in the case of COR14b, PsbA and PsbS with low-molecular-weight range SigmaMarkers. In addition, a control sample from 14-d cold-acclimated ‘Carola’ plants was loaded on every gel. This enabled an indirect comparison of protein accumulation from different blots (different times of cold acclimation). In the cases in which observed accumulation of target protein in control samples was clearly different between membranes, such membranes were discarded.
Table 1.
Characteristics of detected proteins and antibodies used in the experiment, together with the dilutions applied
| Protein | Mol. weight (kD) | Primary antibody | Secondary antibody (alkaline-phosphatase conjugated) |
|---|---|---|---|
| COR14b | 14·4 | Rabbit anti-COR14b raised against a synthetic polypeptide, AgriSera, Vännäs, Sweden | Goat anti-rabbit IgG (whole molecule), Sigma1 : 50 000 |
| 1 : 20 000 | |||
| SPS (sucrose phosphate synthase) | 130 | Chicken anti-SPS, AgriSera | Rabbit anti-chicken IgY (IgG) (whole molecule), Sigma 1 : 50 000 |
| 1 : 40 000 | |||
| RbcL (RuBisCO large subunit) | 58 | Chicken anti-RbcL (global antibody), AgriSera | |
| 1 : 20 000 | |||
| PsbS | 22 | Chicken anti-PsbS (global antibody), AgriSera | |
| 1 : 40 000 | |||
| PsbA (D1) | 32 | Chicken anti-PsbA (global antibody), AgriSera | |
| 1 : 20 000 |
Polypeptides from freshly electrophoresed gels were electrophoretically transferred (Semi-Dry Electrophoretic Transfer Cell, Bio-Rad) to ImmunBlot PVDF-membranes (0·2 µm pore size; Bio-Rad) by applying changing voltage (10 V, 15 min; 13 V, 15 min; 15 V, 15 min) and non-limiting current in transfer buffer (Tris-glycine, pH 9·0) as described by Towbin et al. (1979) and Kurien and Scofield (2003). The quality of the transfer and the position of the desired protein bands were checked by stacking the gel with 0·2 % (w/v) Coomassie brilliant blue R-250 (Sigma) and the membrane with ATX Ponceau S red staining solution (Sigma). Both gels and membranes were photographed with fixed settings (EOS 300D, Canon). After overnight blocking of the membrane with 2 % (w/v) fat-free milk powder in TBS and washing in TBS, the membrane was incubated with a primary antibody (Table 1) for 1 h. After the samples had been washed with TBS, polypeptide–primary antibody complexes were incubated for 1 h with a secondary antibody conjugated with alkaline phosphatase (Table 1) and washed in TBS and AP-buffer. The complexes were visualized using SIGMAFAST BCIP/NBT alkaline phosphatase activity detection kit. After staining, membranes were photographed with fixed settings (EOS 300D, Canon).
The COR14b antibody used in the experiments showed some cross-reaction with the second cold-induced band (of higher molecular weight), which has been reported before by Crosatti et al. (1999) for a different antibody, but, as was suggested there, only the lower molecular weight band was taken into consideration.
Analysis of COR14b transcript accumulation during the first day of cold acclimation
Plants were transferred at time 0 from darkness (+20 °C) to the light (250 µmol m−2 s−1 PPFD). For cold treatment, plants were transferred from +20 °C to +2 °C. The experiment was repeated twice. Each time, two samples were collected from each genotype.
Samples (approx. 0·04 g from the middle part of the youngest, fully expanded leaf) were collected for both control and cold-acclimated plants just before the transfer, after 3 h and 6 h of the first day of cold acclimation, as well as in the 6th hour of the 7th and the 14th days of cold acclimation (150th and 318th hours of cold acclimation, respectively) and immediately frozen in liquid nitrogen. To analyse the expression of the COR14b gene by real-time RT PCR (Higuchi et al., 1993), total RNA was isolated using RNeasy Plant Mini Kit (Qiagen) and than subjected to reverse transcription reaction integrated with removal of genomic DNA contamination (QuantiTect Reverse Transcription Kit, Qiagen). The final concentration and quality of cDNA was determined spectrophotometricaly (Ultrospec 2100 Pro, supplied with ultramicrovolume cell; Amersham Biosciences, Buckinghamshire, UK).
PCR amplification was performed using a 7500 real-time PCR System (Applied Biosystems, Foster City, CA, USA) in 96-well plates using TaqMan MGB probes to detect dsDNA synthesis (Livak et al., 1995). Each PCR reaction was done in triplicate, in 25 µL volume containing 900 nm of each primer, 250 nm of TaqMan MBG probe, 2·5 µL of cDNA (corresponding to approx. 38 ng), 12·5 µL of TaqMan Universal PCR Master Mix and 2·5 µL of RNase-free water. Reactions were run using default cycling parameters (initial denaturation at 95 °C for 10 min followed by 40 cycles of incubation at 95 °C for 15 s, and 60 °C for 1 min). In every cDNA expression of both COR14b and actin genes, a reference housekeeping gene (An et al., 1996) was studied separately using: for COR14b, specific forward primer (AGACCCAGATCGATGGCTTCT), specific reverse primer (GCACGGCCTGGGAAGAG) and specific probe (6-FAM-TCGGAGGAGGCGCG-MGB); and for actin, specific forward primer (TCCTCTGTGGAGAAGAGCT ACGA), specific reverse primer (TCAGCCCCAATGGTGATCA) and specific probe (6-FAM-TGCCCGATGGGCAG-MGB). Primers and probes were designed using Primer Express 3·0 software (Applied Biosystems) on the basis of Hordeum vulgare sequences published in the NCBI database [public domain information on the US National Library of Medicine web pages (http://www.ncbi.nlm.nih.gov); accession numbers: AJ512944, COR14b; AY145451, actin] and supplied by Applied Biosystems. Data were analysed using 7500 real-time PCR Sequence Detection Software v1·3 (Applied Biosystems,). CT values were determined as the numbers of amplification cycles needed to reach a fixed threshold in the exponential growth region of the amplification curve (representing fluorescence emission intensity due to production of a particular amplicon).
Expression of the COR14b gene in cold relative to the control was calculated with the 2−ΔΔCT method as described by Livak and Schmittgen (2001).
RESULTS
Resistance to freezing and photoinhibition
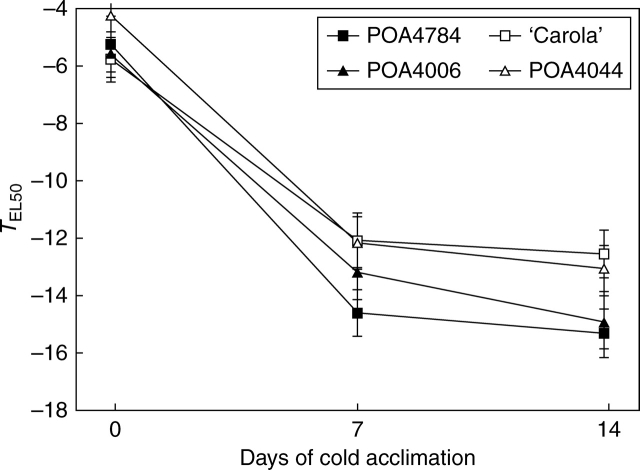
Genotypes of winter barley showed different freezing tolerance (Fig. 1). The temperature in which 50 % of electrolyte leakage from leaf tissue was observed decreased clearly during cold acclimation in every genotype and this decrease was statistically significant only during the first week of acclimation. During the second week, freezing tolerance increased only slightly and, on the basis of the measurements taken at this time, two groups of genotypes could be identified according to their freezing tolerance: POA4044 and ‘Carola’ were less freezing tolerant than POA4784 and POA4006, by about 2·5 °C on average. The results obtained confirmed previous data obtained during 3 years of field-laboratory studies (Rapacz et al., 2008).
Fig. 1.
Changes in freezing tolerance of winter barley genotypes during cold acclimation (2 °C, photoperiod 10/14 h, PAR = 250 µmol m−2 s−1). TEL50 means the temperature at which 50 % of total electrolytes were released from leaf tissues. Values of TEL50 and coefficient intervals for P = 0·05 were calculated on the basis of freezing tests made in two separate experiments at five freezing temperatures: −3, −6, −9, −12 and −15 °C; in total 20 replicates for each freezing temperature.
Changes in photosynthetic apparatus during cold acclimation were studied by means of chlorophyll fluorescence parameters (Table 2). A progressive decrease in apparent (maximum) quantum yield of PSII (Fv/Fm, where Fv = Fm – F0) together with an increase in minimum fluorescence (F0) was observed in every plant except ‘Carola’, which may suggest photodamage occurred during growth in cold (2 °C) and relatively high (250 µmol m−2 s−1) light intensity. Between 0 and the 14th day of cold acclimation a decrease in both Fv/Fm and F0 was observed in ‘Carola’ (by 6 % and 4 %, respectively), while the photosynthetic apparatus of POA4784 and POA4006 can be classified as the most cold sensitive (19 % decrease in Fv/Fm and 17 % increase in F0 for POA4784 and 13 % and 32 % for POA4006). Changes observed in POA4044 were rather small (8 % decrease in Fv/Fm and 12 % increase in F0). On the 14th day of cold acclimation, genotypes were characterized with diverse Fv/Fm values (in descending order: ‘Carola’, POA4044, POA4006 and POA4784).
Table 2.
Changes in chlorophyll fluorescence parameters of four winter barley genotypes during cold acclimation at +2 °C
| Day of cold acclimation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
7 |
14 |
||||||||||
| Genotype | F0 | Fv/Fm | qP | NPQ | F0 | Fv/Fm | qP | NPQ | F0 | Fv/Fm | qP | NPQ |
| ‘Carola’ | 253 c | 0·822 a | 0·842 a | 0·852 e | 248 c | 0·803 a | 0·780 b | 0·883 de | 242 cd | 0·775 b | 0·694 c | 0·930 cd |
| POA4006 | 273 bc | 0·801 a | 0·736 b | 0·971 bc | 318 b | 0·752 bc | 0·686 c | 1·015 b | 360 a | 0·694 d | 0·619 d | 1·093 a |
| POA4044 | 291 b | 0·805 a | 0·780 b | 0·864 e | 310 b | 0·777 b | 0·738 b | 0·958 bcd | 325 b | 0·743 c | 0·680 c | 1·107 a |
| POA4784 | 235 d | 0·817 a | 0·876 a | 0·853 e | 257 c | 0·757 bc | 0·785 b | 0·910 cde | 276 bc | 0·659 e | 0·548 e | 0·992 bc |
Means of ten replications from two independent experiments.
Values of the same parameter with the same letter did not differ significantly at P = 0·05 (Duncan's multiple range test).
A decrease in the photochemical chlorophyll fluorescence quenching coefficient (qP) during cold acclimation was observed in every genotype and the highest decrease (by 37 %) was seen in POA4784. The coefficient of non-photochemical chlorophyll fluorescence quenching (NPQ) increased during cold acclimation in every plant and the highest increase was observed in POA4044 (by 28 %). The changes described are in fact a sum of two effects: cold-induced photodamage and photosynthetic acclimation to cold. A more detailed fluorescence study was performed under conditions of controlled high-light treatment. Short-term exposure of plants to very high light intensity in cold (20 min of 1200 µmol m−2 s−1 at 5 °C) gave an opportunity to indicate both the different sensitivities to photoinhibition of the genotypes studied as well as the presence of mechanisms of the photosynthetic acclimation triggered by 14 d of cold hardening (Table 3). Cold-acclimated plants showed higher values of qP during high-light treatment compared with non-acclimated plants. The highest values of qP, indicating the higher photosynthetic capacity of cold-acclimated plants were noticed in every plant, and the highest values were observed in the most freezing-tolerant POA4006 and POA4784.
Table 3.
The response of winter barley plants non-acclimated, cold-acclimated (14 d at 2 °C) and cold-acclimated + subjected to non-lethal freezing temperatures (–5 °C during the night) to the high-light treatment (20 min of 1200 µmol m−2 s−1 at 5 °C)
| Plants |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-acclimated |
Cold acclimated |
Cold acclimated and frozen |
||||||||||
| Genotype | qP | qE | qI | (F0 – F0HL)/F0 | qP | qE | qI | (F0 – F0HL)/F0 | qP | qE | qI | (F0 – F0HL)/F0 |
| ‘Carola’ | 0·079c | 1·832g | 0·261b | 0·104ab | 0·103b | 2·010de | 0·196c | 0·025e | 0·103b | 2·011de | 0·195c | 0·030de |
| POA4006 | 0·081c | 2·064cd | 0·198c | 0·045cde | 0·136a | 2·209a | 0·199c | 0·046cde | 0·135a | 2·136b | 0·238b | 0·059cd |
| POA4044 | 0·078c | 1·937ef | 0·283a | 0·072bc | 0·105b | 2·093bc | 0·199c | 0·065c | 0·102b | 2·081bc | 0·200c | 0·069c |
| POA4784 | 0·074c | 1·870fg | 0·273ab | 0·098b | 0·131a | 1·971e | 0·268b | 0·101b | 0·131a | 1·911f | 0·296a | 0·129a |
Photochemical quenching of chlorophyll fluorescence (qP), components of non-photochemical quenching–fast-relaxating qE and slow relaxating qI, as well as the change in F0 observed during high-light treatment: (F0–F0HL)/F0 was measured in two independent experiments, total in eight or six (frozen plants) replications.
Values of the same parameter with the same letter did not differ significantly at P = 0·05 (Duncan's multiple range test).
An alternative mechanism of excess light utilization depends on excess light energy dissipation and is evident in higher values of the fast-relaxating component of NPQ, qE. Before cold acclimation the highest value of qE was measured in POA4006. During cold acclimation, qE increased in every genotype and the observed increase was lowest in POA4784 (5 %), resulting in the lowest values of qE observed in this genotype after cold acclimation and the highest in ‘Carola’ and POA4044 (by 10 % and 8 %, respectively). Although the increase in the slow-relaxating component of NPQ, qI, may sometimes be an effect of some acclimatory mechanisms, together with the increase in F0 observed during high-light treatment, it can be evidence of a photoinhibitory event (Müller et al., 2001). Before cold acclimation, POA4006 was less susceptible to photoinhibition, while after cold acclimation POA4784 suffered most from high light, having the highest qI and increasing F0 by 10 % during high-light treatment. When cold-acclimated plants were subjected to non-lethal freezing (−5 °C) during the night before high-light treatment, susceptibility to photoinhibition and qE was strongly influenced, while qP remained almost the same (Table 3). The ability to dissipate energy, as indicated by qE, decreased in POA4784 and POA4006 and probably was responsible for the increase in photoinhibitory damage observed in these two genotypes (qI increased after freezing by 10 % and by 19 % in POA4784 and POA4006, respectively), but the increase in F0 was statistically significant only in POA4784. In the case of ‘Carola’ and POA4044 both parameters remained unaffected.
Accumulation of COR14b and selected proteins from photosynthetic apparatus
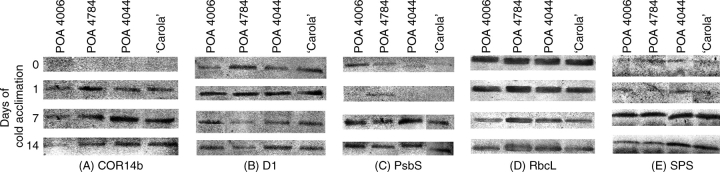
Analysis of western blots showed some differences in protein accumulation between genotypes (Fig. 2). COR14b was below detection levels before cold acclimation (Fig. 2A). At the end of the first day of cold acclimation, the highest amount of protein was recorded in freezing-tolerant and susceptible-to-photoinhibition POA4784, medium amounts were recorded in freezing-susceptible and tolerant-to-photoinhibition POA4044 and ‘Carola’, and the lowest amount was in POA4006. On the 7th day of cold acclimation, the picture was quite different – tolerant-to-photoinhibition POA4044 and ‘Carola’ accumulated more COR14b than susceptible-to-photoinhibition POA4784 and, especially, POA4006. On the 14th day the differences between genotypes were very similar but the accumulation level was lower.
Fig. 2.
Western blots of changes in the accumulation of (A) COR14b, (B) D1, (C) PsbS, (D) RbcL and (E) SPS proteins during cold acclimation (2 °C, photoperiod 10/14 h, PAR = 250 µmol m−2 s−1) of winter barley genotypes. Representative scans were chosen from six replicated bands (three in each independent experiment).
The accumulation of D1, the core protein of PSII, was similar in all genotypes before cold acclimation and after the first day of cold acclimation (Fig. 2B). A transient increase of D1 accumulation was visible during the first day of cold acclimation. During the following days the level of accumulation was lower in POA4784 than in other plants.
On days 0 and 14, PsbS protein had accumulated in amounts proportional to the qE values presented above (Fig. 2C and Table 3). Prior to cold acclimation, the highest accumulation of PsbS was observed in POA4006, which was also less sensitive to high-light treatment at that time. An induction of PsbS synthesis was observed on the 7th day of hardening and, during the 2nd week of cold acclimation, PsbS accumulation probably slightly decreased. In both terms, POA4784, which showed the lowest qE and was less susceptible to photoinhibition after cold acclimation, accumulated less PsbS than remaining genotypes.
Differences in the accumulation of RbcL during cold acclimation can be attributed neither to freezing- nor to photoinhibition-tolerance of plants (Fig. 2D). The highest accumulation during the first day of cold acclimation was observed in POA4784. In the following days in cold the accumulation level decreased.
On the 7th day of cold acclimation, a clear increase in SPS protein accumulation was observed (Fig. 2E). The accumulation seemed to decrease again on the 14th day, when the lowest accumulation was observed in freezing-tolerant genotypes POA4006 and POA4784.
COR14b transcript accumulation during the first day of cold acclimation
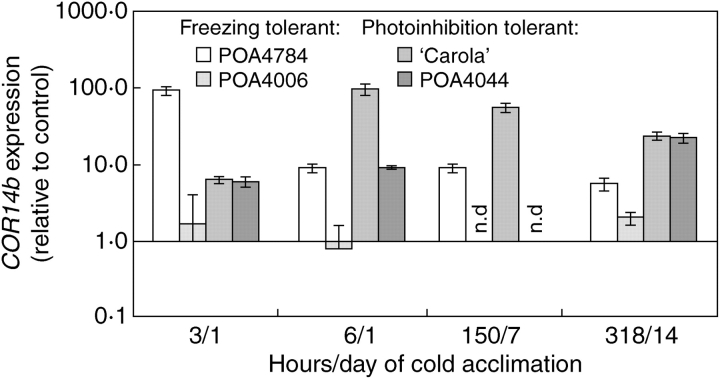
Real-time RT-PCR measurements showed that COR14b transcript levels were already affected by low temperature after 3 h (Fig. 3). At this time, differences in transcript accumulation between plants were very similar to those observed in COR14b protein accumulation 3–4 h later. The highest COR14b expression was evident for POA4784, a medium level was seen for POA4044 and ‘Carola’, whereas induction of transcript accumulation in POA4006 was within the range of measurement error. By the 6th hour of low-temperature treatment, a decrease in relative expression level was observed in freezing-tolerant POA4784 and POA4006, while in ‘Carola’ and POA4044, which are insensitive to low-temperature photoinhibition and susceptible to freezing, a further increase in transcript accumulation was observed. COR14b transcript accumulation was also higher in ‘Carola’ and POA4044 than in plants sensitive to photoinhibition after 7 d and 14 d of cold acclimation (6th hour of the day). Although data for POA4006 and POA4044 after 7 d of cold acclimation are missing, in the remaining plants the decrease in the transcript level was observed between the 7th and the 14th day of cold acclimation, which was also visible in the case of protein accumulation (Fig. 2A).
Fig. 3.
COR14b transcript accumulation during cold acclimation at +2 °C, relative to non-acclimated plants. Both cold-acclimated and non-acclimated plants were transferred from +20 °C at time 0 from darkness to the light (250 µmol m−2 s−1). Means and errors for four leaves (cDNAs) from two independent series (12 real-time PCR reactions in total). n.d., No data.
DISCUSSION
The results presented here indicate that the genotypes studied showed different degrees of resistance to freezing and low-temperature photoinhibition. POA4006 and particularly POA4784 were freezing tolerant but susceptible to photoinhibition, while ‘Carola’ and POA4044 were freezing susceptible but tolerant to photoinhibition. Differences in sensitivity to photoinhibition were more visible when non-lethal freezing treatment preceeded high-light treatment. POA4784 showed a similar reaction in previous experiments, having a very high survival rate after controlled freezing of plants cold-acclimated in the field (a 3-year experiment) and low freezing tolerance measured as the decrease in Fv/Fm after freezing (Rapacz et al., 2008).
All genotypes studied in the present paper showed an increasing photosynthetic capacity during cold acclimation. This increase was connected with freezing tolerance rather than with resistance against photoinhibition in cold, which was in turn connected mainly with the increased ability to dissipate energy during high-light treatment. Previous studies conducted in other Poaceae (wheat and rye) and Brassicaceae showed that cold acclimation in these plants was accompanied by increased photosynthetic capacity (Huner et al., 1998; Pocock et al., 2001; Savitch et al., 2002). However, studies on the npq1 mutant of Arabidopsis thaliana revealed that the xanthophyll cycle and the associated NPQ seem to be relevant to the protection of photosynthesis against sudden increases in light intensity during cold treatment (Havaux and Kloppstech, 2001).
PSII protein, PsbS, belonging to the LHC protein superfamily but different in structure and pigment-binding characteristics (Funk et al., 1995) is essential for qE (Li et al., 2000). In the present experiment, accumulation of PsbS protein was associated with higher qE values both before and after 14 d of cold acclimation. Savitch et al. (2002) observed similar changes in pine (Pinus contorta) but not in winter wheat, indicating differences in excess light utilization strategies during cold acclimation between evergreen and herbaceous plants. In the present paper, changes in qE and PsbS accumulation were responsible for the different sensitivity to high-light treatment observed between genotypes. Three reasons are possible. (1) Savitch et al. (2002) studied wheat plants cold acclimated for a longer time (68 d). After such a long cold treatment, mechanisms different from those connected with PsbS and the xantophyll cycle may be involved in photoprotection (Havaux and Kloppstech, 2001). In addition, in the present experiment some decrease in PsbS accumulation was probably visible between the 7th and the 14th days of cold acclimation. (2) The authors did not distinguish between qE and qI. (3) In that experiment plants were not affected by a sudden increase in high-light treatment when changes in qE are most important for photoprotection (Havaux and Kloppstech, 2001).
One of the first sites of photoinhibitory damage in thylakoids is the D1 polypeptide of the PSII reaction centre. The damaged D1 protein is degraded, and the recovery of PSII activity needs de novo synthesis of D1 (Aro et al., 1993). Studies by Schäfer et al. (1993) showed that the actual level of D1 reflected the balance between D1 degradation and resynthesis. In addition, the changes in D1 accumulation did not depend on a pronounced increase of the slowly reversible non-photochemical quenching qI. Cleland et al. (1990) also suggested that the loss of photochemical activity during photoinhibition can be uncoupled from D1 degradation. Savitch et al. (2002) showed a decrease in D1 accumulation 68 d after wheat was transferred to cold. The sudden increase in PsbA (D1-coding gene) transcript accumulation was observed in freezing- and photoinhibition-tolerant Festuca pratensis, which may suggest that the resistance to photoinhibition during the first days of cold treatment may be connected with activation of D1 resynthesis (Canter et al., 2000). Comprehensive results were obtained in the present paper: D1 accumulation increased initially and then gradually decreased.
Freezing-tolerant genotypes of winter barley studied in the present experiment showed increasing capacity for photochemical quenching of excess light after cold acclimation, which suggests changes occurring in photosynthetic carbon metabolism. Changes in the accumulation of RbcL, ribulose-1,5-carboxylase-oxygenase, a main enzyme of the Calvin cycle, were not connected with either freezing tolerance or the tolerance against photoinhibition of the genotypes studied. A transient increase in RbcL accumulation was observed in POA4784 during the first day of cold acclimation. An increase in many Calvin cycle enzymes, including Rubisco activity, during cold acclimation has been observed repeatedly (Hurry et al., 1994, 1995a, b). After long-term cold treatment the increase in Rubisco activity was higher in freezing-tolerant plants (Huner et al., 1993; Hurry et al., 1995b), which was attributed to increasing specific activity rather than increased accumulation.
In comparison to Rubisco, accumulation of SPS protein was associated with freezing tolerance of the genotypes of barley studied. After clear induction of SPS accumulation observed after 7 d of cold acclimation, a further decrease was observed and this decrease was higher in freezing-tolerant plants. This observation is in agreement with the results obtained by Bascunan-Godoy et al. (2006) for Colobanthus quitensis, an Antarctic plant with high freezing tolerance. Some increase in SPS accumulation was observed there after 7 d of cold treatment while, after 21 d, SPS protein levels returned to those of the control. It is probable that in freezing-tolerant genotypes an increase in SPS-specific activity, which has been reported many times for prolonged cold acclimation of freezing-tolerant plants (Hurry et al., 1994; Savitch et al., 2000; Bascunan-Godoy et al., 2006), can compensate for a lower level of the protein.
The most interesting question that should be asked on the basis of the results presented here is about the role of COR14b protein during cold acclimation. The present results indicate that accumulation of both COR14b transcript and protein after 1 and 2 weeks of cold acclimation is connected to the differences in photoinhibition tolerance, but not with freezing tolerance per se. In previous reports, observed accumulation of COR14b protein was higher in winter cultivars, while some variations for this character were detected in less-freezing-tolerant spring cultivars (Stanca et al., 1996; Giorni et al., 1999; Crosatti et al., 2003). As in the case of the relationship between COR14b accumulation and freezing tolerance, the relationship between freezing tolerance and the tolerance of low-temperature-induced photoinhibition has been reported as a particular discriminant between spring- and winter-type plants, with spring plants, in general, being deficient in freezing and photoinhibition tolerance (Huner et al., 1993). In addition, winter plants lose the ability to avoid photoinhibition after completing vernalization, or when the intense elongation growth starts (Rapacz, 2002; Gusta, 2006), and COR14b accumulation decreases in winter barley at later developmental stages (Crosatti et al., 2008).
Although many advanced molecular studies have been performed on COR14b, little is certain about the function of the final protein produced. COR14b is a member of a small family of COR genes widespread in cereals and including wheat WCS19 (Chauvin et al., 1993), WCOR14 (Tsvetanov et al., 2000) and WCOR15 (Takumi et al., 2003), all of which encode chloroplast-targeted COR proteins analogous to the Arabidopsis protein COR15a (Lin and Thomashow, 1992; Thomashow, 1994). In addition, COR14b protein is targeted to chloroplast stroma (Crosatti et al., 1999). The main function of COR15a protein in Arabidopsis thaliana seems to be in vivo stabilization of thylakoid membranes against freezing-induced damage, which was demonstrated in transgenic plants overexpressing this gene (Artus et al., 1996). On the other hand, in the same paper some protective effects on isolated protoplasts were also observed, but this did not significantly affect the LT50 values of the protoplast survival curves. Over-expression of COR15a increased the freezing tolerance of protoplasts frozen at temperatures between −4 °C and −8 C (the protective effect on thylakoids was the highest over the same range), and decreased survival over the range of −2 °C to −4 °C. According to Uemura et al. (1995), the predominant form of membrane injury during freezing between −2 °C to −4 °C is expansion-induced lysis, while over the range of −4 °C to −8 °C membrane damage resulted from freeze-induced lamellar-to-hexagonal II phase transitions, which were observed both in the plasma membrane and various endomembranes, mainly in the chloroplast envelope. Thus, it seems possible that constitutive expression of COR15a might decrease the incidence of freeze-induced formation of hexagonal II phase lipids, which should be associated with increasing membrane fluidity (Uemura et al., 1995). High membrane fluidity seems to be crucial not only for membrane protection from low-temperature stress but also for resistance to photoinhibition (Vijayan and Browse, 2002). Although an indirect photoprotective effect of COR14b via modification of thylakoid membrane properties seems to be possible, the exact role of COR14b in photoprotection needs to be verified in future studies.
The decrease in COR14b transcript and protein accumulation observed in the present experiment after longer period of cold acclimation confirms the previous reports of Stanca et al. (1996) and Giorni et al. (1999). This effect may be connected with the fact that qE quenching of excess light energy in herbaceous plants is more important during the first few days after a low-temperature shift, and afterwards different protective mechanisms are developed (Havaux and Kloppstech, 2001; Müller et al., 2001). One of the possible mechanisms of the down-regulation of COR14b may be higher photosynthetic capacity, which is fully developed after longer low-temperature growth (Huner et al., 1993).
The results presented here showed that high levels of COR14b accumulation and tolerance to photoinhibition may not be necessary during the development of freezing tolerance. However, the differences between photoinhibition-susceptible and -tolerant genotypes were recorded under a very high-light treatment that triggers photoinhibition on a scale that usually does not apply under field conditions. Cold acclimation is also a very complex trait with a number of genes involved and the lowered expression of just one of them (with the exception of some regulatory genes) cannot easily decrease freezing tolerance (Fowler et al., 1999).
The induction of COR14b expression may be assumed to be the symptom of co-ordinated gene expression regulated by the CBF family of transcription factors in the most important and best-recognized gene expression regulation network acting in cold acclimation (Chinnusamy et al., 2006). COR14b contains a single CRT motif controlling the LT-responsiveness of the gene (Dal Bosco et al., 2003). CBFs bind CRT elements and barley HvCBF products bind the COR14b CRT motif, implicating a direct interaction between the HvCBFs and COR14b gene regulation (Skinner et al., 2005, 2006). During the first 3 h of cold acclimation, the highest (but transient) increase in COR14b expression was observed in the most freezing-tolerant genotype POA4784, but not in the second-most freezing-tolerant POA4006. The barley CBF3 gene is transiently up-regulated within 15 min of exposure to cold (Choi et al., 2002), thus an increase in COR14b expression, followed by down-regulation, could occur in POA4006 before the 3rd hour of cold acclimation. This hypothesis can be additionally supported by the observation that at the 6th hour of cold acclimation the accumulation of COR14b protein in POA4006 was at the level as in POA4784 and ‘Carola’, while the transcript level was lower both at that time and at the 3rd hour after transfer to cold; thus protein must have been accumulated earlier.
Finally, it can be concluded that increasing expression of COR14b may, on the one hand, lead to increasing light-energy dissipation via the qE component of NPQ and, on the other hand, may be a symptom of a more ‘productive’ gene expression network that runs during cold acclimation. As a result, the increase in energy dissipation during cold acclimation may, in average genotypes and conditions, reflect in fact the cold-acclimation ability of plants, as was observed by Rapacz et al. (2004) in the Lolium–Festuca complex. Moreover, the increased ability to dissipate excess light observed in freezing-tolerant Lolium–Festuca hybrids as an effect of gene transfer from the more-freezing-tolerant Festuca to Lolium (Humphreys et al., 2007) may indicate the transfer of some components of mechanisms controlling gene expression during cold acclimation and not the exigency of NPQ mechanism for the development of freezing tolerance.
ACKNOWLEDGEMENTS
Funding was provided by the Polish Ministry of Science and Higher Education (2 P06A 002 28).
LITERATURE CITED
- Adams WW, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V. Photosynthesis and photoprotection in overwintering plants. Plant Biology. 2002;4:545–557. [Google Scholar]
- An Y-Q, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB. Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. The Plant Journal. 1996;10:107–121. doi: 10.1046/j.1365-313x.1996.10010107.x. [DOI] [PubMed] [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochimica et Biophysica Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Artus NN, Uemura M, Steponkus PL, Gilmour SJ, Lin C, Thomashow MF. Constitutive expression of the cold-regulated Arabidopsis thaliana COR15a gene affects both chloroplast and protoplast freezing tolerance. Proceedings of the National Academy of Sciences of the USA. 1996;93:13404–13409. doi: 10.1073/pnas.93.23.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascunan-Godoy L, Uribe E, Zuniga-Feest A, Corcuera LJ, Bravo LA. Low temperature regulates sucrose-phosphate synthase activity in Colobanthus quitensis (Kunth) Bartl. by decreasing its sensitivity to Pi and increased activation by glucose-6-phosphate. Polar Biology. 2006;29:1011–1017. [Google Scholar]
- Bilger W, Björkman O. Temperature-dependence of violaxanthin deepoxidation and nonphotochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta. 1991;184:226–234. doi: 10.1007/BF00197951. [DOI] [PubMed] [Google Scholar]
- Canter PH, Bettany AJE, Donnison I, Timms E, Humphreys MW, Jones RN. Expressed sequence tags (ESTs) during cold-acclimation in Festuca pratensis include a homologue of the chloroplast gene PsbA. Journal of Experimental Botany. 2000;51:72. [Google Scholar]
- Chauvin LP, Houde M, Sarhan F. A leaf-specific gene stimulated by light during wheat acclimation to low temperature. Plant Molecular Biology. 1993;23:255–265. doi: 10.1007/BF00029002. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu J-K. Gene regulation during cold acclimation in plants. Physiologia Plantarum. 2006;126:52–61. [Google Scholar]
- Choi D-W, Rodriguez EM, Close TJ. Barley Cbf3 gene identification, expression pattern, and map location. Plant Physiology. 2002;129:1781–1787. doi: 10.1104/pp.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland RE, Ramage RT, Critchley C. Photoinhibition causes loss of photochemical activity without degradation of D1 protein. Australian Journal of Plant Physiology. 1990;17:641–651. [Google Scholar]
- Crosatti C, Delaureto PP, Bassi R, Cattivelli L. The interaction between cold and light controls the expression of the cold-regulated barley gene cor14b and the accumulation of the corresponding protein. Plant Physiology. 1999;119:671–680. doi: 10.1104/pp.119.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosatti C, Mare C, Mazzucotelli E, Selioni S, Barilli S, Bassi R, et al. Genetic analysis of the expression of the cold-regulated gene cor14b: a way toward the identification of components of the cold response signal transduction in Triticeae. Canadian Journal of Botany. 2003;81:1162–1167. [Google Scholar]
- Crosatti C, Pagani D, Cattivelli L, Stanca AM, Rizza F. Effects of growth stage and hardening conditions on the association between frost resistance and the expression of the cold induced protein COR14b in barley. Environmental and Experimental Botany. 2008;62:93–100. [Google Scholar]
- Dal Bosco C, Busconi M, Govoni C, Baldi P, Stanca AM, Crosatti C, et al. cor gene expression in barley mutants affected in chloroplast development and photosynthetic electron transport. Plant Physiology. 2003;131:793–802. doi: 10.1104/pp.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger I, Busch F, Huner NPA. Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiologia Plantarum. 2006;126:28–44. [Google Scholar]
- Flint HJ, Boyce BR, Brattie DJ. Index of injury, a useful expression of freezing injuries to plant tissues as determined by the electric method. Canadian Journal of Plant Science. 1967;47:229–239. [Google Scholar]
- Fowler DB, Limin AE, Ritchie JT. Low temperature tolerance in cereals: model and genetic interpretation. Crop Science. 1999;39:626–633. [Google Scholar]
- Funk C, Schröder WP, Napiwotzki A, Tjus SE, Renger G, Andersson B. The PSII-S protein of higher plants: a new type of pigment-binding protein. Biochemistry. 1995;34:11133–11141. doi: 10.1021/bi00035a019. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Hazlett TL, Debrunner PG, Govindjee Comparative time-resolved photosystem II chlorophyll a fluorescence analyses reveal distinctive differences between photoinhibitory reaction center damage in young canopy and xanthophyll cycle-dependent energy dissipation. Photochemistry and Photobiology. 1996;64:552–563. doi: 10.1111/j.1751-1097.1996.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Giorni E, Crosatti C, Baldi P, Grossi M, Marè C, Stanca AM, et al. Cold-regulated gene expression during winter in frost tolerant and frost susceptible barley cultivars grown under field conditions. Euphytica. 1999;106:149–157. [Google Scholar]
- Gusta LV. Plant and Microbe Adaptation to Cold. Italy: Salsomaggiore Terme; 2006. Cold acclimation, de-acclimation and re-acclimation of spring canola, winter canola and winter wheat: the role of vernalization, low-temperature associated proteins and carbohydrates. May 16–20 2006. [Google Scholar]
- Havaux M, Kloppstech K. The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta. 2001;213:953–966. doi: 10.1007/s004250100572. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR: real time monitoring of DNA amplification reactions. Biotechnology. 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- Humphreys MW, Gąsior D, Leśniewska-Bocianowska A, Zwierzykowski Z, Rapacz M. Androgenesis as a means of dissecting complex genetic and physiological controls: selecting useful gene combinations for breeding freezing tolerant grasses. Euphytica. 2007;158:337–345. [Google Scholar]
- Huner NPA, Öquist G, Hurry VM, Krol M, Falk S, Griffith M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynthetic Research. 1993;37:19–39. doi: 10.1007/BF02185436. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Öquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends in Plant Science. 1998;3:224–230. [Google Scholar]
- Hurry VM, Malmberg G, Gardestrom P, Öquist G. Effects of a short-term shift to low temperature and of long-term cold hardening on photosynthesis and ribulose-1,5-bisphosphate carboxylase/oxygenase and sucrose phosphate synthase activity in leaves of winter rye (Secale cereale L.) Plant Physiology. 1994;106:983–990. doi: 10.1104/pp.106.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurry VM, Keerberg O, Parnik T, Gardestrom P, Öquist G. Cold-hardening results in increased activity of enzymes involved in carbon metabolism in leaves of winter rye (Secale cereale L.) Planta. 1995;a 195:554–562. [Google Scholar]
- Hurry VM, Strand A, Tobiaeson M, Gardestrom P, Öquist G. Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiology. 1995;b 109:697–706. doi: 10.1104/pp.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurien BT, Scofield RH. Protein blotting: a review. Journal of Immunological Methods. 2003;274:1–15. doi: 10.1016/s0022-1759(02)00523-9. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403:391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- Lin C, Thomashow MF. DNA sequence analysis of a complementary DNA for cold-regulated Arabidopsis cor15 and characterization of the COR15 polypeptide. Plant Physiology. 1992;115:171–180. doi: 10.1104/pp.99.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Flood SJ, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods and Applications. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK. Non-photochemical quenching: a response to excess light energy. Plant Physiology. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndong C, Danyluk J, Huner NPA, Sarhan F. Survey of gene expression in winter rye during changes in growth temperature, irradiance or excitation pressure. Plant Molecular Biology. 2001;45:691–703. doi: 10.1023/a:1010684719225. [DOI] [PubMed] [Google Scholar]
- Pocock TH, Hurry V, Savitch LV, Huner NPA. Susceptibility to low-temperature photoinhibition and the acquisition of freezing tolerance in winter and spring wheat: the role of growth temperature and irradiance. Physiologia Plantarum. 2001;113:499–506. [Google Scholar]
- Rapacz M. The after-effects of temperature and irradiance during early growth of winter oilseed rape (Brassica napus L. var oleifera cv. Gorczanski) seedlings on the progress of their cold acclimation. Acta Physiologiae Plantarum. 1998;a 20 [Google Scholar]
- Rapacz M. The effects of day and night temperatures during early growth of winter oilseed rape (Brassica napus L. var oleifera cv. Gorczanski) seedlings on their morphology and cold acclimation responses. Acta Physiologiae Plantarum. 1998;b 20:67–72. [Google Scholar]
- Rapacz M. Frost resistance and cold acclimation abilities of spring-type oilseed rape. Plant Science. 1999;147:55–64. [Google Scholar]
- Rapacz M. Regulation of frost resistance during cold deacclimation and reacclimation in oilseed rape: a possible role of PSII redox state. Physiologia Plantarum. 2002;115:236–243. doi: 10.1034/j.1399-3054.2002.1150209.x. [DOI] [PubMed] [Google Scholar]
- Rapacz M, Gąsior D, Zwierzykowski Z, Leśniewska-Bocianowska A, Humphreys MW, Gay AP. Changes in cold tolerance and the mechanisms of acclimatation of photosystem II to cold hardening generated by anther culture of Festuca pratensis × Lolium multiflorum cultivars. New Phytologist. 2004;161:105–114. [Google Scholar]
- Rapacz M, Gąsior D, Kościelniak J, Kosmala A, Zwierzykowski Z, Humphreys MW. The role of the photosynthetic apparatus in cold acclimation of Lolium multiflorum: characteristics of novel genotypes low-sensitive to PSII over-reduction. Acta Physiologiae Plantarum. 2007;29:309–316. [Google Scholar]
- Rapacz M, Tyrka M, Kaczmarek W, Gut M, Wolanin B, Mikulski W. Photosynthetic acclimation to cold as a potential physiological marker of winter barley freezing tolerance assessed under variable winter environment. Journal of Agronomy and Crop Science. 2008 (in press) [Google Scholar]
- Savitch LV, Harney T, Huner NPA. Sucrose metabolism in spring and winter wheat in response to high irradiance, cold stress and cold acclimation. Physiologia Plantarum. 2000;108:270–278. [Google Scholar]
- Savitch LV, Leonardos ED, Krol M, Jansson S, Grodzinski B, Huner NPA, et al. Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant, Cell and Environment. 2002;25:761–771. [Google Scholar]
- Schäfer C, Vogg G, Schmid V. Evidence for loss of D1 protein during photoinhibition of Chenopodium rubrum L. culture cells. Planta. 1993;189:433–439. doi: 10.1007/BF00194442. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Bilger W, Neubauer C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schultze E-D, Caldwell MM, editors. Ecophysiology of photosynthesis. Berlin: Springer; 1994. pp. 49–70. [Google Scholar]
- Skinner JS, von Zitzewitz J, Szűcs P, Marquez-Cedillo L, Filichkin T, Amundsen K, et al. Structural, functional, and phylogenetic characterization of a large CBF gene family in barley. Plant Molecular Biology. 2005;59:533–551. doi: 10.1007/s11103-005-2498-2. [DOI] [PubMed] [Google Scholar]
- Skinner JS, Szűcs P, von Zitzewitz J, Marquez-Cedillo L, Filichkin T, Stockinger EJ, et al. Mapping of barley homologs to genes that regulate low temperature tolerance in Arabidopsis. Theoretical and Applied Genetics. 2006;112:832–842. doi: 10.1007/s00122-005-0185-y. [DOI] [PubMed] [Google Scholar]
- Stanca AM, Crosatti C, Grossi M, Lacerenza NG, Rizza F, Cattivelli L. Molecular adaptation of barley to cold and drought conditions. Euphytica. 1996;92:215–219. [Google Scholar]
- Takumi S, Koike A, Nakata M, Kume S, Ohno R, Nakamura C. Cold-specific and light-stimulated expression of a wheat (Triticum aestivum L.) Journal of Experimental Botany. 2003;54:2265–2274. doi: 10.1093/jxb/erg247. [DOI] [PubMed] [Google Scholar]
- Thiele A, Winter K, Krause GH. Low inactivation of D1 protein of photosystem II leaves of Anacardium excelsum under high-light stress. Journal of Plant Physiology. 1997;151:286–292. [Google Scholar]
- Thomashow MF. Arabidopsis thaliana as a model for studying mechanisms of plant cold tolerance. In: Meyerowitz E, Somerville C, editors. Arabidopsis. New York, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 807–834. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and applications. Proceedings of the National Academy of Sciences of the USA. 1979;76:4350. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanov S, Ohno R, Tsuda K, Takumi S, Mori N, Atanassov A, et al. A cold-responsive wheat (Triticum aestivum L.) gene wcor14 identified in a winter-hardy cultivar ‘Mironovska 808. Genes and Genetic Systems. 2000;75:49–57. doi: 10.1266/ggs.75.49. [DOI] [PubMed] [Google Scholar]
- Uemura M, Joseph RA, Steponkus PL. Cold acclimation of Arabidopsis thaliana: effect on plasma membrane lipid composition and freeze-induced lesions. Plant Physiology. 1995;109:15–30. doi: 10.1104/pp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P, Browse J. Photoinhibition in mutants of Arabidopsis deficient in thylakoid unsaturation. Plant Physiology. 2002;129:876–885. doi: 10.1104/pp.004341. [DOI] [PMC free article] [PubMed] [Google Scholar]