Abstract
Background and Aims
Xylem vessels containing gases (embolized) must be refilled with water if they are to resume transport of water through the plant, so refilling is of great importance for the maintenance of water balance in plants. However, the refilling process is poorly understood because of inadequate examination methods. Simultaneous measurements of plant anatomy and vessel refilling are essential to elucidate the mechanisms involved. In the present work, a new technique based on phase-contrast X-ray imaging is presented that visualizes, in vivo and in real time, both xylem anatomy and refilling of embolized vessels.
Methods
With the synchrotron X-ray micro-imaging technique, the refilling of xylem vessels of leaves and a stem of Phyllostachys bambusoides with water is demonstrated under different conditions. The technique employs phase contrast imaging of X-ray beams, which are transformed into visible light and are photographed by a charge coupled device camera. X-ray images were captured consecutively at every 0·5 s with an exposure time of 10 ms.
Key Results
The interface (meniscus) between the water and gas phases in refilling the xylem vessels is displayed. During refilling, the rising menisci in embolized vessels showed repetitive flow, i.e. they temporarily stopped at the end walls of the vessel elements while gas bubbles were removed. The meniscus then passed through the end wall at a faster rate than the speed of flow in the main vessels. In the light, the speed of refilling in a specific vessel was slower than that in the dark, but this rate increased again after repeated periods in darkness.
Conclusions
Real-time, non-destructive X-ray micro-imaging is an important, useful and novel technique to study the relationship between xylem structure and the refilling of embolized vessels in intact plants. It provides new insight into understanding the mechanisms of water transport and the refilling of embolized vessels, which are not understood well.
Key words: Micro-imaging, Phyllostachys bambusoides, water refilling, X-ray, xylem vessel
INTRODUCTION
In plants, the long-distance transport of sap plays a crucial role in the exchange of nutrients and signal messengers between different plant organs (Köckenberger et al., 1997). An intricate network of xylem vessels facilitates the transport of sap within the plant but the precise mechanisms involved are unclear. To understand how sap flows within and between xylem vessels, it is necessary to monitor the flow in situ. Most conventional observation methods, however, are unable directly to visualize this flow of sap in the xylem vessels of intact plants.
Lee et al. (1987) used magnetic resonance imaging (MRI) to measure the velocity of thermally stratified, unsteady water flow by means of phase-encoding methods. MRI was also used recently to study sap flow in xylem (Köckenberger et al., 1997) as well as to monitor the occurrence of cavitation (embolism) and the subsequent embolism-repair process (Wagner et al., 2000; Holbrook et al., 2001). Although MRI is able to distinguish between individual xylem vessels, the limited spatial resolution of 10–100 µm makes accurate visualization of sap transport through such vessels difficult. In addition, the limited temporal resolution of a few hundred microseconds also hampers observation of the dynamics of the refilling process. Moreover, the utility of MRI is limited if the sample contains components which are influenced by a magnetic field (e.g. ferromagnetic materials such as iron).
In contrast to MRI, synchrotron X-ray micro-imaging as used in the present study has superior spatial resolution of 1 to approx. 2 µm. In addition, it is also possible to record X-ray images in real time. This transmission-type imaging technique shows the inner structure of opaque objects without the need for injecting chemical tracers or for preparing thin samples. The potential of synchrotron X-ray imaging was recently demonstrated with the visualization of tracheal respiration in insects (Westneat et al., 2003). X-ray micro-tomography has also been used to investigate the three-dimensional structure of living organisms (Jenneson et al., 2003). Other measurement techniques such as confocal laser scanning microscopy (CLSM), optical coherence microscopy (OCM) and electron tomography (ET) have greater resolution than synchrotron X-ray micro-imaging (Stuppy et al., 2003). However, compared with the requirements for X-ray micro-imaging and MRI, the sample specimens for CLSM, OCM and ET must be very thin and slightly transparent, which substantially restricts their application (Stuppy et al., 2003).
The main aim here was to assess the use of the advanced synchrotron X-ray micro-imaging technique in a study of the mechanism by which sap (water) refills embolized xylem vessels. Xylem refilling under a high rate of transpiration, in which xylem sap is still under tension, has been reported but has not been explained (Steudle, 2001). It is generally assumed that radial water transport from surrounding cells is responsible for refilling the embolized vessels, and a number of different driving forces have been proposed such as tissue pressure produced by a high osmotic concentration in the cells, a pressure gradient produced by the low osmotic concentration in neighbouring cells or a pressure gradient produced by the high concentration of sap in the refilling vessel (Canny, 1998; Tyree et al., 1999; Salleo et al., 2004). It is essential to explain how water in the refilling vessel is not lost to the surroundings, which have lower water potential than the refilling vessels. Holbrook and Zwieniecki (1999) and Zwieniecki and Holbrook (2000) suggested a hydraulic isolation of refilling vessels based on the geometry and wall chemistry of the bordered pits in the vessel wall. There is a numerical model based on this idea (Vesala et al., 2003). Involvement of cellular metabolism in refilling was also proposed (Salleo et al., 2004; Lovisolo and Schubert, 2006). However, most of these proposed models suffer from flaws and thus new experimental evidence and a new model are needed (Tyree et al., 1999; Steudle, 2001). Simultaneous measurements of xylem vessel anatomy, and of water refilling, together with changes in the gas bubbles within them, may provide more understanding of the processes and help to elucidate the mechanism of water refilling. The experimental technique applied here to the problem of refilling of embolized xylem vessels will provide high-resolution, real-time data that will be useful for testing hypotheses and developing new models of xylem function.
In this study, a synchrotron X-ray micro-imaging technique was employed to visualize the entry of water into embolized vessels in real time and in vivo. The effects of plant anatomy and illumination on the refilling were demonstrated in cut leaves and a stem segment without leaves of bamboo (Phyllostachys bambusoides).
MATERIALS AND METHODS
Plant material
Phyllostachys bambusoides Sieb. et. Zucc. (bamboo) shoots that contained leaves and stems were excised during the daytime. The cut ends were placed in tap water for 24 h to hydrate the shoots fully. At the beginning of the experiments, leaves were cut from the hydrated shoots to allow air to enter the cut end. Leaf blades were 8–12 cm long. A stem segment 6 cm in length was prepared cutting from the hydrated shoot, and the leaves attached in the stem were removed.
X-ray micro-imaging method
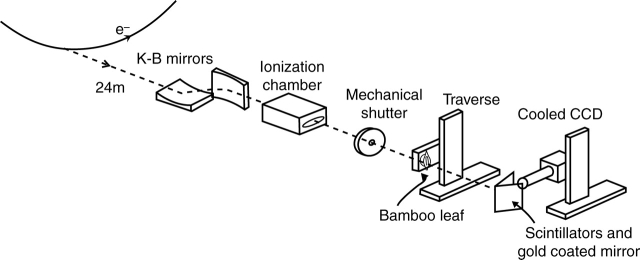
The principle of the synchrotron X-ray micro-imaging technique is based on the phase contrast imaging of X-ray beams. The X-ray beam is phase-shifted at interfaces between two substances with different absorption, refraction and diffraction characteristics: the phase shift can be detected and presented as images (Hwu et al., 1999). The synchrotron X-ray micro-imaging system (Fig. 1) consists of mirrors, an ionization chamber, a mechanical shutter and a charged coupled device (CCD) camera. Lee and Kim (2003) have described a technique applied to the quantitative measurement of the velocity field of a liquid flow inside an opaque tube. A hard X-ray beam from the 1B2 beamline at Pohang Light Source (Pohang, Korea) was used as the illuminating source. This beam was focused on the sample using a K-B mirror. The X-ray images of nanometre-scale resolution were transformed into visible light by a thin CdWO4 scintillator crystal. The distance between the sample specimen and the scintillator crystal was approx. 30 cm. The phase contrast X-ray images were then recorded using a cooled digital CCD camera with a resolution of 1024 × 1280 pixels. Real-time X-ray images, with a field of view of 0·686 × 0·858 mm, were obtained with a 10× objective lens.
Fig. 1.
Schematic diagram of the synchrotron X-ray micro-imaging system.
A mechanical shutter with an exposure time of 10 ms was mounted in front of the test sample to prevent unnecessary exposure to high-intensity X-ray radiation. Overexposure leads to the generation of vapour bubbles within the liquid or living organs, and may result in cavitation within the xylem. The mechanical shutter and the CCD camera were synchronized using a delay generator to open the shutter only when the X-ray images were recorded. These X-ray images were captured every 0·5 s with 10-ms exposure of the X-ray beam. Thus, the test sample was exposed to the X-ray beam for just 20 ms every second. The test sample was placed in a cabinet located in an air-conditioned hall in which temperature and humidity were nearly constant.
Dehydration–rehydration
The leaves were cut from the hydrated shoot in air (see above). The cut leaf was placed vertically on a manipulation plate. After dehydrating it for about 8 min in the dark, the cut end of the specimen was immersed in water to rehydrate. During the rehydration, the xylem vessels were observed using the X-ray micro-imaging technique. After 3 min of rehydration in the dark, the specimen was dehydrated again by removing the water supply from the cut end. Water around the cut end was drained through a tube, while the specimen was fixed and kept in the same position. Dehydration and rehydration were repeated several times on the same specimen. Each cycle lasted about 11 min: dehydration took about 8 min, and the following rehydration about 3 min. For the stem segment without leaves, the same dehydration and rehydration procedure was applied. On a leaf sample, once a relatively constant refilling rate was observed after several dehydration–rehydration cycles, the light intensity was increased, in steps, from darkness (0 µmol m−2 s−1) to 30, 60 and 140 µmol m−2 s−1 during rehydration. Finally, refilling on the leaf sample was repeated in darkness. Dehydrations of the test sample were made in darkness.
Tracking of water-rise kinetics
The water-rise kinetics in the xylem were investigated by tracking the water-front in the consecutively captured X-ray images. We detected the position of each meniscus from the digital images by reading the pixel number. The position of the meniscus was determined every second. From these data, the water-rise kinetics in the xylem vessel were determined. It was possible to obtain up to 15 X-ray images per second with the camera used.
RESULTS
X-ray micro-imaging
The typical internal structure of embolized xylem vessels of a bamboo leaf, obtained by the synchrotron X-ray micro-imaging technique, is shown in Fig. 2, together with the meniscus rising inside the embolized vessel during rehydration. Here the end wall is situated between two adjacent vessel elements.
Fig. 2.
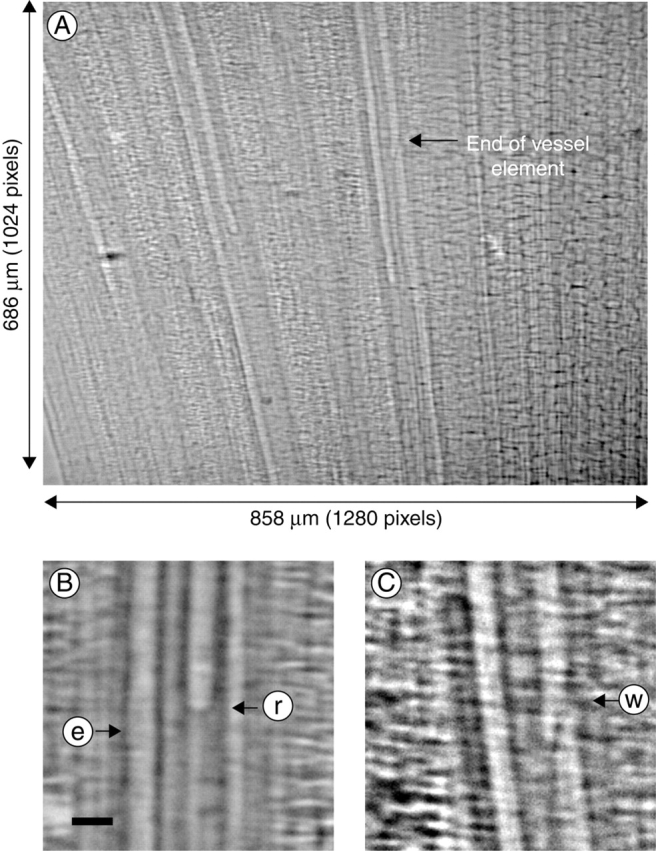
A typical X-ray image of the xylem vessels within a bamboo leaf cut in air, allowed to dry briefly and rehydrated. Image A is an X-ray image of the leaf, while images B and C are micrographic sections showing the meniscus (between water and air) in a xylem vessel and the end wall of the xylem vessel element. In B, arrows e and r show an empty and refilling xylem vessel, respectively. In C, the end wall between two adjacent vessel elements is shown by arrow w. Scale bars = 20 µm.
Dehydration
When a leaf was cut from a shoot in air, the severed vessels were drained. Drainage stopped when the meniscus reached the end wall of the vessel. Following complete hydration of the leaf, which required about 40 min, all xylem vessels were filled with water. When the leaf was allowed to dehydrate in air, water within the vessels was retained while the cut end was wet. However, after a short period, as water evaporated from the leaf and water on the cut end moved into the open vessels or evaporated into the atmosphere (data not shown), severed vessels were drained. The stem segment without attached leaves showed similar results after dehydration.
Pattern of refilling in cavitated vessels
Rising menisci were observed in two adjacent xylem vessels after water was supplied to a dehydrated leaf (Fig. 3). Interestingly, the rate of water movement in the left conduit slowed and stopped temporarily when the meniscus reached the end of the vessel element (V1). This is in contrast to the meniscus in the adjacent conduit, which continued to rise at the same rate (V2).
Fig. 3.
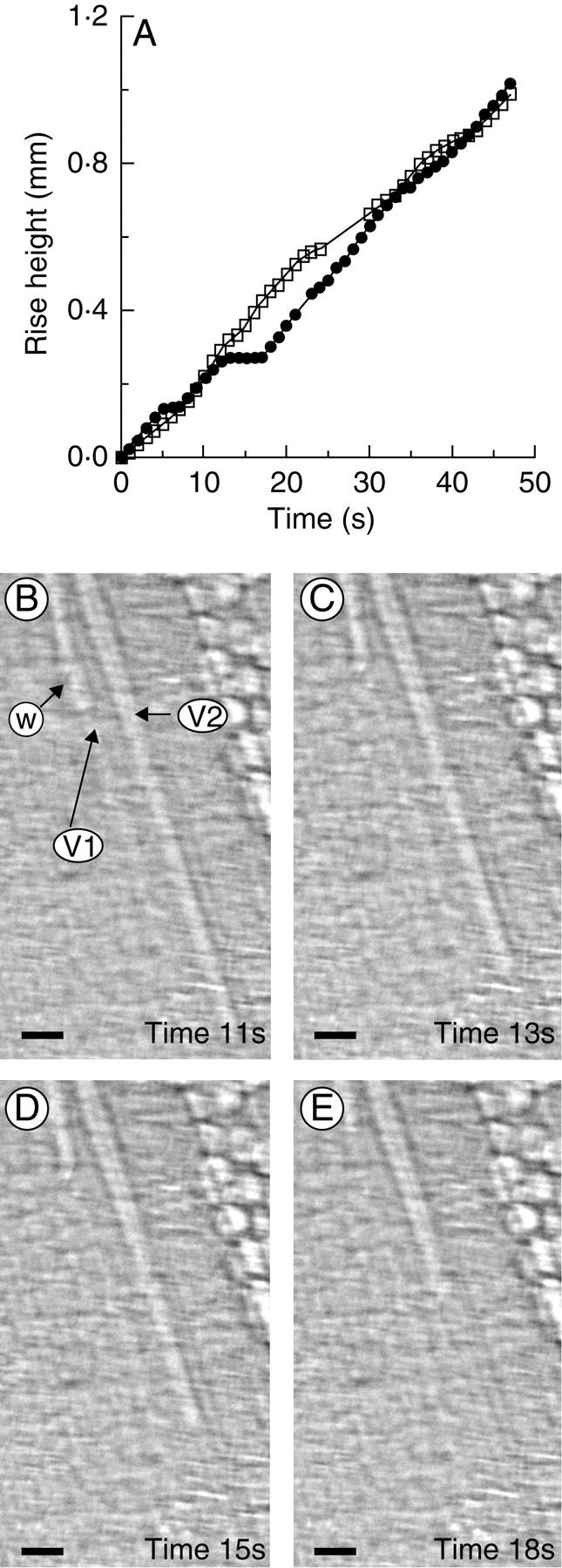
Measurement of the position of the meniscus in a vessel of bamboo leaf with time, showing that the end wall stopped the movement of the water-front. (A) The water-rise kinetics in two neighbouring xylem vessels were plotted from analysis of consecutive X-ray images. Closed circles and open squares represent vessel 1 (V1) and vessel 2 (V2), respectively. In the X-ray images B–E, V1 and V2 exhibited similar moving speeds of flow initially (B). After 13 s, the meniscus in V1 stopped moving when it reached the end wall of the vessel element (arrow w), while the meniscus in V2 continued to rise (C). After 15 s, the water moved only slightly in the right-hand portion of V1, while in the left, the meniscus remained unchanged, enabling the empty volume below the end wall of the vessel element to be filled (D). After 18 s, the meniscus began to move again in V1 (E). Scale bars = 20 µm. Time and water movement are relative to the start of the experiment. Observation was under normal room light of 5 µmol m−2 s−1. A supplementary video is available upon request.
When the meniscus passed through the end wall of the vessel element after slowing and stopping, the rate of movement of the meniscus exceeded the normal refilling speed of water transport, both in the stem segment (Fig. 4A) and in the leaves (Fig. 4B; see also Figs 6 and 7 below). The distance between end walls, where menisci halted, was about 100–500 µm and this short distance suggests that the end walls probably contain perforation plates (see Discussion). The moving speed of the meniscus after halting increased in proportion to the duration for which movement of the meniscus stopped at the end wall of the vessel element (Fig. 4). For example, in Fig. 4A, flow halted for 4 and 14 s, after which the meniscus moved at 40 and 230 µm s−1, respectively.
Fig. 4.
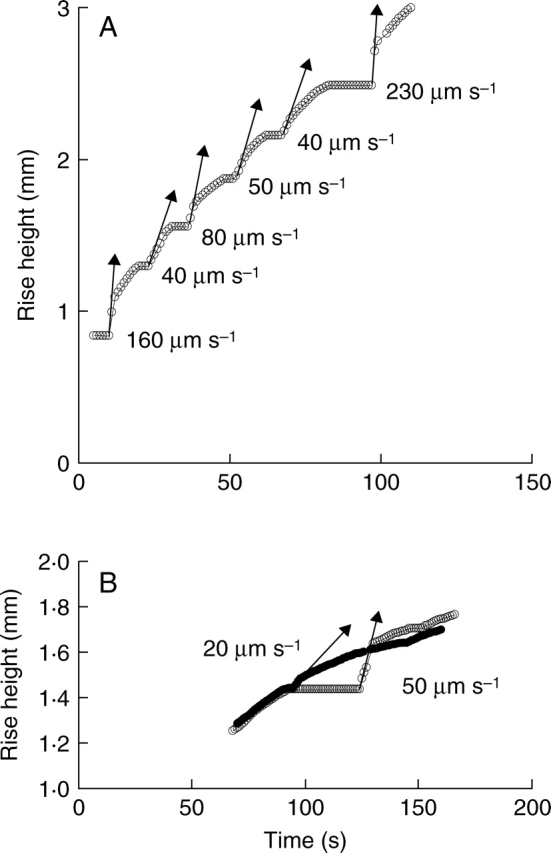
(A) The kinetics of movement of the meniscus in xylem vessels of a stem are similar to those in a leaf. Water movement stopped at the end wall of a vessel element: when the meniscus passed the end wall, water movement was faster (arrows) than that of water during normal refilling of a vessel element. (B) During repeated refilling in the same vessel of a bamboo leaf, the speed of the rising meniscus at the same position relative to the end wall varied depending on the period for which the meniscus had stopped. Here, time 0 indicates the start of water supply after dehydration, and water-rise height is the position of the refilling meniscus relative to the cut end of a leaf or base of the stem. Observation was made under room light of 5 µmol m−2 s−1.
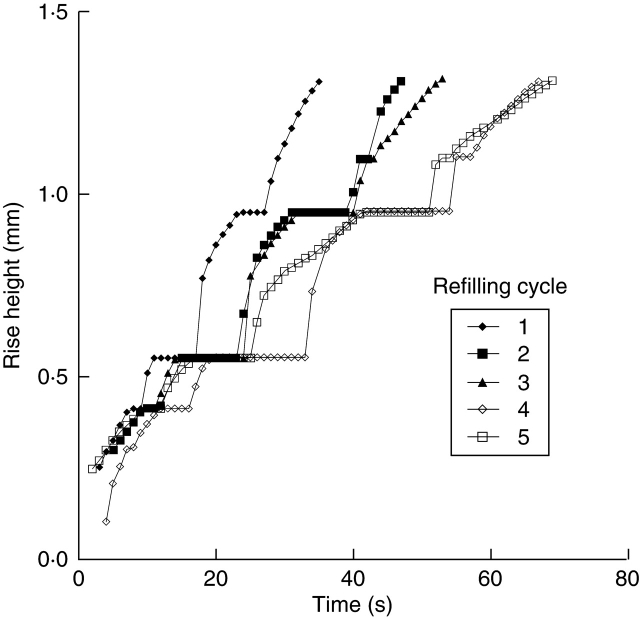
Fig. 6.
Variation in the kinetics of movement of the meniscus during repeated refilling cycles of a xylem vessel in a bamboo leaf. Numbers 1–5 indicate the order of refilling cycles. Experiments were repeated on a specific conduit of a leaf in the dark (0 µmol m−2 s−1). Here, time 0 indicates the start of water supply after dehydration, and water-rise height refers to the position of the meniscus from the cut end of the leaf.
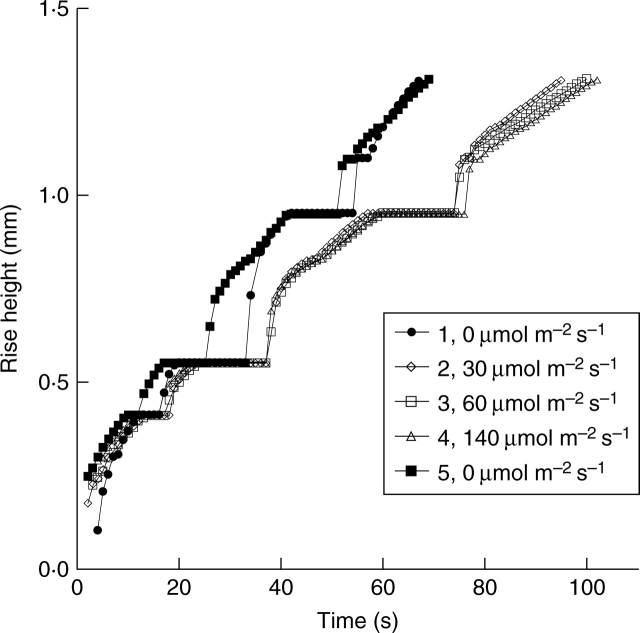
Fig. 7.
The effect of light intensity on the kinetics of water movement on the same vessel in the leaf used in Fig. 6, which experienced repeated refilling cycles in the dark and had achieved a constant rate of movement of the meniscus. Light intensities of 0, 30, 60 and 140 µmol m−2 s−1 were applied during each rehydration cycle. The light intensity was reduced again to 0 µmol m−2 s−1 in the final cycle of refilling. Numbers 1–5 indicate the order of the refilling cycles.
The gas bubble in the sap of the refilling vessel moved with the meniscus without decreasing its size (Fig. 5A). However, it became smaller and finally disappeared within 11 s once the meniscus stopped at the end wall of the vessel element (Fig. 5C, D).
Fig. 5.
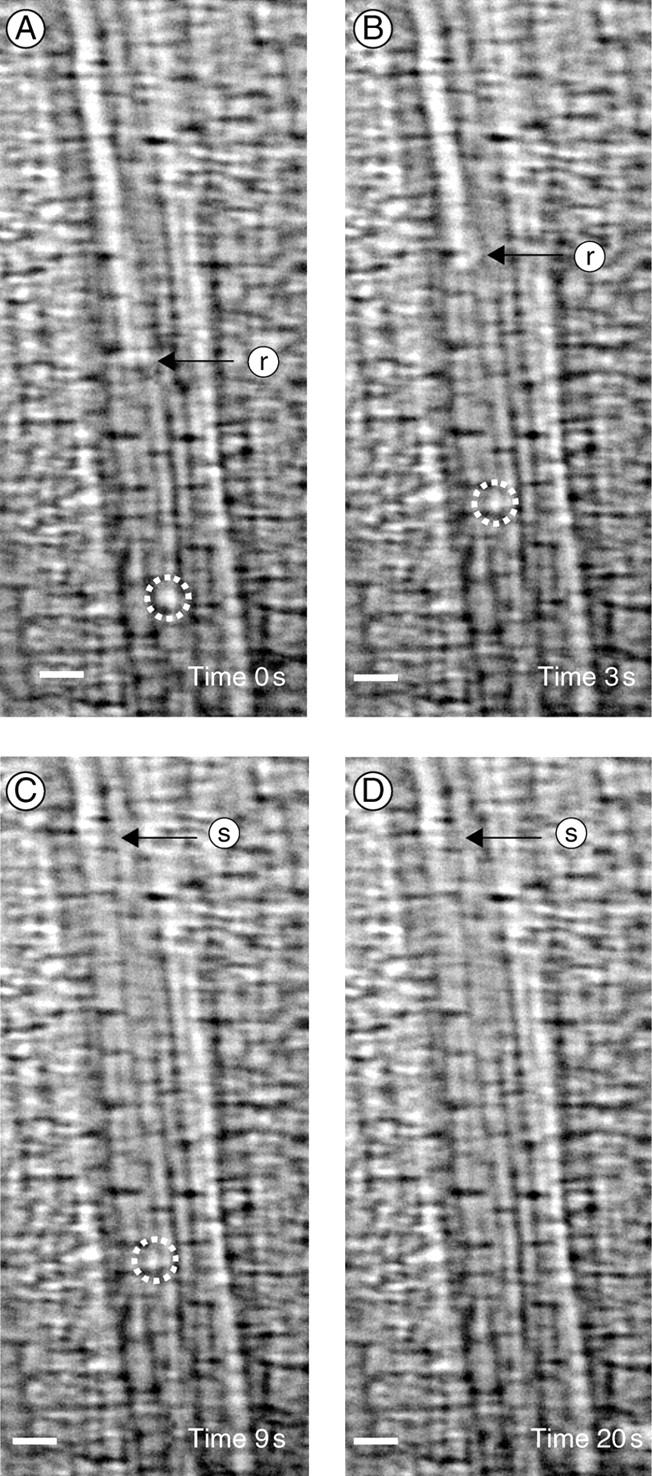
The removal of gas bubbles in xylem vessels of a bamboo leaf. A gas bubble (dashed circle) moved with the water without decreasing in size as the meniscus moved through the xylem vessel from the start of observation (A, time = 0) to t = 3 s. At t = 3 s, the gas bubble stuck to the xylem vessel wall, while the meniscus continued to rise (B). The bubble changed little in size until the meniscus stopped at the end wall of the vessel element at t = 9 s (C). The gas bubble became smaller while the meniscus was not moving. Finally, the gas bubble was removed after 20 s (D). The arrows show the position of the meniscus: r, rising front; s, static front. Scale bars = 20 µm. Observation under normal room light. A supplementary video is available upon request.
Effects of repeated dehydration–rehydration cycles on refilling
In general, the refilling ability of xylem vessels gradually decreased as the number of dehydration–rehydration cycles increased. Figure 6 shows the repeated water-rise kinetics of a xylem vessel when dehydration and rehydration were repeated on the same sample in the absence of light. In each case, the kinetics of water movement were similar, with the meniscus stopping for 2–13 s at the end wall of the vessel element. The speed of flow restarting to pass through it was then much faster than that of normal refilling. However, the time taken for a meniscus in a vessel to reach 1·3 mm from the cut end of the leaf, ts, increased by 35 s as the number of cycles was increased from one to four. After the fourth refilling cycle, ts was nearly constant at 70 s.
Effect of light on refilling
In general, refilling was faster in the dark than in the light. On the same vessel used for Fig. 6, which showed a saturated ts in the absence of light, the dehydration–rehydration cycles were repeated while increasing the light intensity during rehydration. Dehydration was done in the dark all the time. As seen in Fig. 7, at light intensities of 30, 60 and 140 µmol m−2 s−1, ts was longer than that of the previous experiment in the dark. The water-rise kinetics appeared to be similar regardless of the light intensity. When refilling was repeated in the dark for the same vessel previously monitored under bright illumination, ts returned to the previous low value obtained in the dark, indicating that the rate of refilling was reversible. For the same vessel shown in Fig. 7, three sets of dark–light–dark (light at 30 µmol m−2s−1) cycles were repeated; values of ts averaged over the cycle were 70 ± 4 s in the dark and 90 ± 4 s in the light (30 µmol m−2 s−1 (mean ± s.d., n = 3–4).
DISCUSSION
This study illustrates that the X-ray micro-imaging technique is useful for visualizing the internal structure of individual xylem vessels in leaves and stems, the position of menisci as the vessels refill with water, and the transport of gas bubbles. From the X-ray images recorded in real time, it is possible to obtain the water-rise kinetics and their relationship with xylem structure. This advanced measurement technique facilitates the investigation of water refilling in intact plants, both for leaves which are relatively thin and for thick stems. Although three-dimensional structures of the xylem vessels of a stem were converted into two-dimensional X-ray images, it was possible to recognize individual vessels.
Conventionally, to investigate cavitation and refilling of xylem vessels, the percentage loss of hydraulic conductance has been evaluated (Tyree et al., 1999; Salleo et al., 2004). However, this method has limitations in examining the role of plant anatomy. Cryoscanning electron microscopy could provide images of high spatial resolution, but it is not suited for non-invasive measurements in real time. In addition, it has been criticized because freezing tissues may disturb the natural situation (Tyree et al., 2003). MRI would be comparable with the X-ray micro-imaging method as it could visualize refilling non-invasively. However, the X-ray micro-imaging technique has advantages over MRI in terms of better temporal and spatial resolution.
X-ray micro-imaging of water refilling
To check the feasibility and usefulness of the X-ray micro-imaging technique in the study of embolism repair, simple model experiments were conducted. Bamboo leaves and a stem segment were cut in air and dehydrated. Water was supplied to their cut ends, resulting in rehydration. According to the conventional model attributed to Scholander, it is assumed that when transpiring stems or leaves are cut, the xylem vessels are severed and the water in them is removed and replaced by air (Tyree and Cochard, 2003). When the meniscus reaches the ends of the vessels and with further dehydration, air can be trapped at the bordered pits located at the vessel ends, and move into uncut vessels, allowing further cavitation to propagate into the uncut vessels. When leaves and stems are rehydrated by placing the cut ends in water, air is partly or completely removed to allow vessels to fill and the tissues to rehydrate. However, when these air blockages are not sufficiently removed, leaves become wilted. Visualization using the X-ray micro-image method shows that this model is valid. Xylem vessels cut in air are shown to be filled with gas. Then, when the cut ends of the leaves and stems were put in water, the vessels refilled with water and the gas phase was removed.
The simple model experiments undertaken in this study clearly allow the movement of water in xylem vessels to be visualized and rates of movement to be quantified. The experimental conditions may be different from those experienced by intact plants in the field. However, the X-ray imaging technique also can be utilized to visualize sap flow in the xylem vessels of intact plants, and will be of interest to those attempting to understand how plants recover from cavitation, which is encountered frequently. Because repair of embolism under tension in xylem vessels and at low water potential has not been fully understood, several hypotheses have been discussed to explain the mechanism (Steudle, 2001). The X-ray imaging technique employed in this study may be used systematically, under defined conditions, to gain more accurate data that is not available from any other techniques, which will be valuable for establishing the mechanism involved.
Implications of water-front stoppage during refilling
During refilling of a vessel, water-rise must be driven by a capillary force opposing the gravitational field. However, the temporary halt to water-rise at the end of the vessel element, as shown in Figs 3 and 4, suggests that there should be another driving force. Water in the refilled vessel element seems to be pressurized when the water-front has stopped moving because the meniscus moved at an increased speed when it began to rise again (Fig. 4). This pressurization of water in the refilled vessel element during the period when the movement of the meniscus ceases may help to dissolve the gas bubbles in the water within the xylem, as observed in Fig. 5.
The fluid dynamics of water transport through the end wall of a vessel element appear to be similar to flow in a pressurized syringe with a safety valve. Up to a certain threshold pressure, the internal pressure increases with movement of the plunger. When the internal pressure increases sufficiently to activate the safety valve, it opens and water is ejected from the syringe. If the plunger is pushed continuously with constant force, the velocity of the flow upon its release from the valve increases in proportion to the pressure in the syringe and thus to the duration of the period for which pressure is applied and how long the valve takes to open.
Similarly, we can hypothesize that the water inside the vessel elements is pressurized when the meniscus stops moving at the end wall of the vessel element. The pressure in the refilled vessel element increases up to a threshold value at which the surface force formed by the gas/water interface in a pit chamber or in a perforation plate balances the hydrostatic pressure within the refilled vessel element. Radial water transport may play an important role in building up the pressure when the meniscus has stopped at the end wall of a vessel element. If there were no radial water transport, capillary force would be the only force available to refill a vessel because, as revealed in the present study, leaf or stem segments were tested without root pressure. It is unlikely that the surface force increased whenever the water-front passed through the end wall of the vessel elements, although the wettability of the wall surface may not be homogeneous in the vessels. Also, when water passed through the end wall of the vessel element without stopping, rapid downstream movement of the meniscus did not occur (data not shown). From this, we speculate that water may have been transported to a refilling xylem vessel from surrounding tissues such as adjacent living cells and/or conducting vessels. When the movement of the meniscus has stopped, the increasing hydrostatic pressure is balanced by a surface force, which may be changed due to the variation of the contact angle between the meniscus and the cell wall. The surface force acts in the opposite direction to water-rise, when the meniscus halts at the end wall. Recently, the role of living cells in removal of embolism has been emphasized, showing that cellular metabolic processes were involved in refilling (Salleo et al., 2004; Lovisolo and Schubert, 2006). However, the mechanism of refilling xylem vessels is not yet clear (Holbrook and Zwieniecki, 1999; Tyree et al., 1999; Steudle, 2001; Vesala et al., 2003).
The meniscus may stop at the bordered pits at the end of the vessel or at perforation plates between the xylem elements. The spatial resolution of the X-ray imaging technique may not be adequate to distinguish between a perforation plate and bordered pits. In most cases, perforation plates were most likely based on the short distance between the positions where the meniscus halted. However, some vessel ends containing bordered pits could possibly be present.
Another reasonable interpretation for the fast movement of the meniscus after stopping is dissolution of the gas above the meniscus in the liquid as it advances. When the meniscus is stopped at the end wall, the gas pressure may decrease due to air dissolving in the liquid; this may create a suction force, which allows the static meniscus to advance faster. In this case, the rate of advance would be limited by how fast the trapped gas can be dissolved. The excised leaf segments, which contain vessels cut at both ends, could be used to estimate the relative contribution of these two processes, namely radial water transport and dissolution of gas in refilling vessels. If only the latter were the case, there should be no faster movement of the meniscus after its halt at the end wall of the vessel element. Schneider et al. (2000) proposed a quantitative model that describes the capillary rise of water in leaky vessels, in which no compression of air was assumed during rise of the meniscus. However, it is not clear if their assumption would be valid when the model is applied to the present case. In order to validate existing models or to develop a new model, it would be necessary to make systematic measurements of parameters under defined conditions using the X-ray imaging technique.
Effect of repeated dehydration–rehydration cycles on refilling
Repetition of the dehydration–rehydration cycle affected the refilling process in embolized vessels, when the same xylem vessel was used to determine the water-rise kinetics. Repetition appears to reduce the ability of vessels to refill. As there was no root pressure, water availability in neighbouring xylem vessels and living cells may affect water transport to refilling vessels from surrounding tissues: on repetition, dehydration of neighbouring cells may occur or the number of cavitated xylem vessels may increase. After the fourth cycle, the rate of refilling was almost constant, suggesting that water fluxes in the dehydrated leaf may have stabilized.
Effect of light on refilling
The experiments to determine the effect of light on water refilling were carried out for the same xylem vessel of a leaf, under standard conditions in an enclosure where temperature and humidity were nearly constant, through several cycles of dehydration and rehydration until the time taken for the meniscus to ascend a height of 1·3 mm from the cut surface had become nearly constant. Although continued illumination of the high-intensity X-ray beam increases the temperature of the sample up to a maximum of 3 °C, the water-rise velocity may not be changed, largely due to just minor changes to the water characteristics such as viscosity, density and surface tension. Water-rise kinetics in the presence of light were apparently different from those in the absence of light. However, they were similar irrespective of the light intensity. This suggests that a small temperature change did not affect noticeably the water-rise kinetics in a refilling xylem.
Repeatability of the X-ray micro- imaging experiments
In the present study, 20 leaves and one stem were tested. One of the main objectives was to show the feasibility of using the X-ray imaging technique to study refilling of xylem vessels and removal of embolism: it has been applied successfully to visualize opaque flows inside non-living objects and blood flow in opaque tubes (Lee and G. B. Kim, 2003, 2005; Lee and S. Kim, 2005; Kim and Lee, 2006). With appropriate precautions, the X-ray imaging system has shown good repeatability. A few hours are needed in setting up the camera and optics suited to this kind of flow imaging experiment.
Higher spatial resolution of X-ray imaging
While the leaves and a stem segment of bamboo were used in this study as test samples, the X-ray micro-imaging technique can be directly applied to a whole intact plant. Further improvements to the spatial resolution down to the nanometre scale are underway by developing special X-ray optics.
To summarize, the synchrotron X-ray imaging technique revealed the internal structure of xylem vessels in bamboo leaves and stem, and the movement of the meniscus in vessels refilling with water in real time, non-invasively, in vivo. This provides dynamic, experimental data on water transport not previously obtainable. The technique is a promising method to explore water movement into embolized vessels in plants.
ACKNOWLEDGEMENTS
This work was supported by the National Research Laboratory Program of the Korea Science and Engineering Foundation. Experiments at Pohang Light Source (PLS, Pohang, Korea) were supported in part by the Ministry of Science and Technology of Korea and POSTECH. We thank Bu-Geun Paik, Seok Kim, Guk-Bae Kim and Chang-Sub Yeo for experimental help, and Hwa-Shik Youn and Soo-Yeun Baik who operated the 1B2 beam line at PLS. Thanks also to Mel Tyree (University of Alberta, Canada) for suggesting the gas dissolution model.
LITERATURE CITED
- Canny MJ. Application of the compensating pressure theory of water transport. American Journal of Botany. 1998;85:897–909. [PubMed] [Google Scholar]
- Holbrook NM, Zwieniecki MA. Embolism repair and xylem tension: do we need a miracle? Plant Physiology. 1999;120:7–10. doi: 10.1104/pp.120.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook NM, Ahrens ET, Burns MJ, Zwieniecki MA. In vivo observation of cavitation and embolism repair using magnetic resonance imaging. Plant Physiology. 2001;126:27–31. doi: 10.1104/pp.126.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu Y, Hsieh HH, Lu MJ, Tsai WL, Lin HM, Goh WC, et al. Coherence-enhanced synchrotron radiology: refraction versus diffraction mechanisms. Journal of Applied Physics. 1999;86:4613–4618. [Google Scholar]
- Jenneson PM, Gilboy WB, Morton EJ, Gregory PJ. An x-ray micro-tomography system optimized for the low-dose study of living organisms. Applied Radiation and Isotopes. 2003;58:177–181. doi: 10.1016/s0969-8043(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Kim GB, Lee SJ. X-ray PIV measurements of blood flows without tracer particles. Experiments in Fluids. 2006;41:195–200. [Google Scholar]
- Köckenberger W, Pope JM, Xia Y, Jeffrey KR, Komor E, Callaghan PT. A non-invasive measurement of phloem and xylem water flow in castor bean seedlings by nuclear magnetic resonance micro-imaging. Planta. 1997;201:53–63. [Google Scholar]
- Lee SJ, Kim GB. X-ray particle image velocimetry for measuring quantitative flow information inside opaque objects. Journal of Applied Physics. 2003;94:3620–3623. [Google Scholar]
- Lee SJ, Kim GB. Synchrotron micro-imaging technique for measuring the velocity field of real blood flows. Journal of Applied Physics. 2005;97:064701. [Google Scholar]
- Lee SJ, Kim S. Simultaneous micro-imaging of size and velocity of micro-bubbles moving in an opaque tube. Experiments in Fluids. 2005;39:492–497. [Google Scholar]
- Lee SJ, Chung MK, Mun CW, Cho ZH. Experimental study of thermally stratified unsteady flow by NMR-CT. Experiments in Fluids. 1987;5:273–281. [Google Scholar]
- Lovisolo C, Schubert A. Mercury hinders recovery of shoot hydraulic conductivity during grapevine rehydration: evidence from a whole-plant approach. New Phytologist. 2006;172:469–478. doi: 10.1111/j.1469-8137.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, Trifilò P, Nardini A. New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant, Cell and Environment. 2004;27:1065–1076. [Google Scholar]
- Schneider H, Wistuba N, Wagner HJ, Thürmer F, Zimmermann U. Water rise kinetics in refilling xylem after desiccation in a resurrection plant. New Phytologist. 2000;148:221–238. doi: 10.1046/j.1469-8137.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- Steudle E. The cohesion-tension mechanism and the acquisition of water by plant roots. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:847–875. doi: 10.1146/annurev.arplant.52.1.847. [DOI] [PubMed] [Google Scholar]
- Stuppy WH, Maisano JA, Colbert MW, Rudall PJ, Rowe TB. Three-dimensional analysis of plant structure using high-resolution x-ray computed tomography. Trends in Plant Science. 2003;8:2–6. doi: 10.1016/s1360-1385(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Cochard H. Vessel contents of leaves after excision: a test of the Scholander assumption. Journal of Experimental Botany. 2003;54:2133–2139. doi: 10.1093/jxb/erg237. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Salleo S, Nardini A, Lo Gullo MA, Mosca R. Refilling of embolized vessels in young stems of Laurel. Do we need a new paradigm? Plant Physiology. 1999;120:11–21. doi: 10.1104/pp.120.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Cochard H, Cruiziat P. The water-filled versus air-filled status of vessels cut open in air: the ‘Scholander assumption’ revisited. Plant, Cell and Environment. 2003;26:613–621. [Google Scholar]
- Vesala T, Hölttä T, Perämäki M, Nikinmaa E. Refilling of hydraulically isolated embolized xylem vessel: model calculations. Annals of Botany. 2003;91:419–428. doi: 10.1093/aob/mcg022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner HJ, Schneider H, Mimietz S, Wistuba N, Rokkita M, Krohne G, et al. Xylem conduits of a resurrection plant contain a unique lipid lining and refill following a distinct pattern after desiccation. New Phytologist. 2000;148:239–255. doi: 10.1046/j.1469-8137.2000.00755.x. [DOI] [PubMed] [Google Scholar]
- Westneat MW, Betz O, Blob RW, Fezzaa K, Cooper WJ, Lee WK. Tracheal respiration in insects visualized with synchrotron X-ray imaging. Science. 2003;299:558–560. doi: 10.1126/science.1078008. [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Holbrook NM. Bordered pit structure and vessel wall surface properties. Plant Physiology. 2000;123:1015–1020. doi: 10.1104/pp.123.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]