Abstract
Background and Aims
Along snowmelt gradients, the canopies of temperate alpine meadows differ strongly in their structural and biochemical properties. Here, a study is made of the effects of these canopy dissimilarities combined with the snow-induced changes in length of growing season on seasonal gross primary production (GPP).
Methods
Leaf area index (LAI) and community-aggregated values of leaf angle and leaf nitrogen content were estimated for seven alpine plant canopies distributed along a marked snowmelt gradient, and these were used as input variables in a sun–shade canopy bulk-photosynthesis model. The model was validated for plant communities of early and late snowmelt sites by measuring the instantaneous CO2 fluxes with a canopy closed-chamber technique. A sensitivity analysis was conducted to estimate the relative impact of canopy properties and environmental factors on the daily and seasonal GPP.
Key Results
Carbon uptake was primarily related to the LAI and total canopy nitrogen content, but not to the leaf angle. For a given level of photosynthetically active radiation, CO2 assimilation was higher under overcast conditions. Sensitivity analysis revealed that increase of the length of the growing season had a higher effect on the seasonal GPP than a similar increase of any other factor. It was also found that the observed greater nitrogen content and larger LAI of canopies in late-snowmelt sites largely compensated for the negative impact of the reduced growing season.
Conclusions
The results emphasize the primary importance of snow-induced changes in length of growing season on carbon uptake in alpine temperate meadows. It was also demonstrated how using leaf-trait values of the dominants is a useful approach for modelling ecosystem carbon-cycle-related processes, particularly when continuous measurements of CO2 fluxes are technically difficult. The study thus represents an important step in addressing the challenge of using a plant functional-trait approach for biogeochemical modelling.
Key words: Alpine meadows, gross primary production, plant functional traits, snowmelt gradient, sun–shade model
INTRODUCTION
The carbon budget of cold, snow-covered ecosystems is of particular interest because they are known to sequester a large amount of organic carbon in their soils and to be particularly sensitive to global warming (Hobbie et al., 2000). Much attention has been drawn to the carbon balance of arctic tundra (Vourlitis et al., 2000; Grant et al., 2003; Campbell et al., 2005; Euskirchen et al., 2006), but carbon balance estimations for temperate alpine tundra and meadows are relatively uncommon (Cernusca, 1989; Diemer, 1994). In cold ecosystems, snow determines the length of the season, which is a main driver of carbon exchange between land and atmosphere (Arora and Boer, 2005; Churkina et al., 2005). Recent climatic studies have highlighted the impact of rising temperatures on snow-cover depth and duration at high elevations (Keller et al., 2005), but their consequences for the carbon budget remain poorly understood (Brooks et al., 1997; Monson et al., 2006).
In situ continuous recording of CO2 fluxes remains difficult in high-elevation terrains (but see Cernusca et al., 1998; Li et al., 2005; Hammerle et al., 2007). The alpine landscape generally exhibits very fine-scale changes in vegetation cover, a feature that would preclude the designation of turbulent fluxes to a particular ecosystem. For the same reason, remote-sensing-based estimates of gross primary production (GPP) in alpine landscapes also suffer from insufficient spatial resolution (Turner et al., 2004). This makes spatial and temporal scaling-up a major challenge. A growing body of literature suggests using plant functional traits in ecosystem modelling in order to scale-up ecosystem processes on a mechanistic basis (Diaz and Cabido, 2001; Lavorel and Garnier, 2002). Although this approach has recently been used in modelling primary productivity and litter decomposition (Quetier et al., 2007), to our knowledge, this promising avenue has seldom been explored to model seasonal variations in GPP of herbaceous ecosystems.
According to the ‘biomass ratio hypothesis’ (Grime, 1998), ecosystem properties and function (i.e. carbon and nitrogen cycles) should be related to the trait values of the dominant contributors to the plant biomass. Several key ecosystem processes, such as decomposition rate or productivity, may be predicted from the traits of the dominant species (Chapin et al., 1996; Cornelissen et al., 1999; Epstein et al., 2001). A main challenge is to mechanistically link ecosystem processes to a set of key plant functional traits that could easily be measured in the field (Diaz and Cabido, 2001). In this respect, it has been proposed that the use of quantitative traits would be more powerful than a broad categorization into discrete plant functional groups (Garnier et al., 2004). The mean of trait values weighted by the relative abundance of each species – i.e. a community-aggregated value (Violle et al., 2007) – provides a route to scale-up from organ to community level and offers a linkage between comparative plant ecology and ecosystem modelling. The goal of this paper is to develop such a modelling approach to estimate the seasonal GPP of temperate alpine meadows. Gross primary production is a key variable of the carbon balance as it quantifies the amount of autotrophic carbon available for growth, reserve and respiratory demands at the ecosystem level.
In alpine ecosystems, the landscape-scale distribution of snow (which is tightly related to the mesotopography) is a main driver of ecosystem structure and functioning. Through its effect on the length of the growing season, snow provides a complex ecological gradient affecting the seasonal course of temperature, light, wind exposure, soil water content and nitrogen availability (Jones et al., 2000). It has long been known that arctic and temperate alpine plant communities exhibit high species turnover along snowmelt gradients (e.g. Komarkova and Webber, 1978; Kudo and Ito, 1992; Onipchenko and Blinnikov, 1994; Theurillat et al., 1994). More recent studies have indicated that consistent shifts in plant functional diversity occur from early to late-snowmelt sites (Kudo et al., 1999; Choler, 2005). A greater leaf nitrogen concentration (Nmass), a higher specific leaf area (SLA) and a predominance of horizontal leaves (i.e. trait values generally associated with a high capacity for resource acquisition) are common features of species from snowy sites (Choler, 2005). Conversely, species from early melting sites are characterized by upright and thick leaves and low SLA, i.e. trait values generally associated with nutrient-conservation strategies (Wright et al., 2004).
Many studies have investigated the relationships among several canopy properties – leaf area index (LAI), nitrogen content, canopy architecture – and their effect on carbon uptake in different light conditions (Anten et al., 1995; Hikosaka and Hirose, 1997; Anten, 2005). But, to our knowledge, there has been no attempt to examine these relationships for multispecies assemblages distributed along gradients of length of growing season. In this study, we addressed the following questions. (1) What are the differences in the community-aggregated values of leaf functional traits along the snow-cover gradient? (2) What is the relative effect of these canopy functional properties and environmental factors (light and temperature regime, length of growing season) on the seasonal GPP? (3) To what extent do plant communities from late-snowmelt sites overcome the negative impact of a reduced growing season on GPP?
This study was based on previous work investigating changes in plant functional traits at the species level along snowmelt gradients in the alpine zone of the south-western Alps (Choler, 2005). For this work, a sun–shade canopy bulk-photosynthesis model was implemented to simulate the light interception and the GPP at the ecosystem level. The model was validated with instantaneous measurements of CO2 fluxes using a closed-chamber technique. Finally, a sensitivity analysis was conducted in order to determine the relative effects of climatic factors and canopy functional properties on the seasonal GPP.
MATERIALS AND METHODS
Study site and plant trait measurements
The research site was located in the south-western Alps (France) between the Lautaret and Galibier Pass (45°7′N, 6°5′E). The 2-ha site is a slightly inclined depression located between 2700 and 2780 m a.s.l. It exhibits a typical mosaic of alpine meadow species, ranging from Kobresia myosuroides-dominated plant communities in the early snowmelt sites to Carex foetida- and Alchemilla pentaphyllea-dominated plant communities in the late-snowmelt sites. For this work, the seven plant communities that are the most abundant in the studied area were selected. The communities are designated here according to the mean date of snowmelt in Julian days (e.g. C130, C140, C150, etc). Leaf angle, specific leaf area and leaf nitrogen concentration [expressed on a mass (Nmass) or area (Narea) basis] were used in this study because of their known effect on carbon-cycle-related processes. Further details of the site and trait measurements are given in Choler (2005).
For each trait, a community-aggregated value (Ti) was calculated as follows:
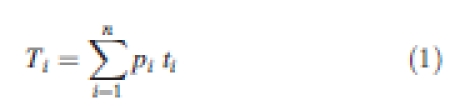 |
1 |
where pi is the relative cover of species i in the sampling unit (SU), n is the number of species accounting for 80 % of the cover in the SU, and ti is the trait value of species i. Although the total coverage of the n species could slightly exceed 80 %, we did not correct the community-aggregated value. In practical terms, the change in Ti value because of this discrepancy is of very limited magnitude (Cornelissen et al., 2003).
For each plant community, the peak standing biomass was harvested at the end of July in square plots of 50 × 50 cm. To calculate the leaf area index (LAI), the whole projected surface of green biomass for three harvests per community was measured using a leaf area meter (WinDIAS, Delta-T Devices Ltd). Separation between green and dead phytoelements was hard to achieve in early snowmelt sites, and hence LAI might have been slightly over-estimated for these communities. Material was then dried at 85 °C for 48 h and weighed.
Climate
Hourly soil temperatures were recorded using Hobo probes (Onset Computer Corporation, Bourne, MA) buried at 5-cm depth over the period 1999–2005. During snow-covered periods, soil temperatures were close to 0 °C (usually between –1 and 1 °C) throughout the day and did not exhibit circadian variations, a recording consistent with a continuous snow cover of at least 1 m depth. The length of the growing season was calculated as the number of snow-free days with a mean soil temperature above 0 °C.
During the summer season, air temperature, relative humidity and photosynthetically active radiation (PAR) were recorded hourly by an automatic weather station [Campbell Scientific (Canada) Corp.] located in the Jardin Alpin du Lautaret (2100 m a.s.l.), 5 km from the study site. The diffuse and direct components of PAR were calculated according to Spitters (1986). Daily integrated values of incoming solar radiation were obtained from a longer time series (1999–2004) at Briançon climatic station (1300 m a.s.l.), located at 30 km from the site and maintained by Météo France.
The Clear Sky Model (CSM) developed in the framework of the European Solar Radiation Atlas (Rigollier et al., 1999) was used to model the incoming global irradiance on a horizontal plane under a cloudless sky. In the CSM, the direct (or beam) radiation (I0b) and the diffuse radiation (I0d) are simulated separately. Details of the CSM can be found in Rigollier et al. (1999). Solar geometry was implemented using equations written by L. Wald and O. Bauer (École des mines de Paris, Centre d'Energétique, Groupe de Télédétection, February, 1997). The model was implemented with no mask effect due to the relief. The long-term recordings of daily irradiance in Briançon were used to parameterize the monthly mean atmospheric turbidity in the area.
Canopy bulk-photosynthesis model and simulation of seasonal GPP
We developed a modified version of the sun–shade model of De Pury and Farquhar (1997) to estimate the canopy-intercepted radiation and CO2 fixation. For light interception, the model splits the canopy into a sunlit and a shaded fraction, which experience distinct light regimes. Shaded leaves receive scattered light and diffuse sky irradiance while sunlit leaves receive direct-beam irradiance in addition to this. CO2 fixation is based on the von Caemmerer and Farquhar biochemical model of C3 leaf CO2 assimilation (Farquhar et al., 1980; von Caemmerer and Farquhar, 1981). Below, we only detail the main changes made to the original sun–shade model. The main constants, parameters and equations of the model are given in the Supplementary Information, available online.
We used the ellipsoidal distribution model (Campbell, 1990) to describe the leaf-inclination distribution (or leaf eccentricity) in the canopy. For simplicity, the canopy is considered as spatially homogeneous, i.e. with no clumped leaves in either the vertical or horizontal dimensions. The model assumes that the leaf-angle distribution is similar to the distribution of area on the surface of an ellipsoid. The model requires only one parameter (ε), which is the ratio of the axes of the ellipsoid. The leaf-azimuth-angle distribution is assumed to be uniform; as such, the leaf-inclination probability density function g will solely depend on zl, the zenith angle of the leaf normal (see Supplementary Information, Table S2, eqn 1, available online). We estimated the parameter ε from community-aggregated values of leaf angle (Table S2, eqn 2). The use of the ellipsoidal distribution model modifies the calculations of the canopy extinction coefficient for direct radiation (kb) as well as the canopy reflection coefficient (Table S2, eqns 4–6).
The Rubisco capacity is linearly related to the leaf nitrogen content. The mean slope of the Narea–Vc,max relationship (the parameter sN) was derived from ACi response curves of six alpine species (see Supplementary Information online) and from published data for 13 other species (Wohlfahrt et al., 1998). The minimum leaf nitrogen content, Nmin, corresponds to the x-intercept of the Narea–Vc,max linear relationship. Much attention has been devoted to the vertical distribution of nitrogen in models of canopy photosynthesis (Friend, 2001). Under non-uniform distribution of nitrogen within the canopy, Vc,max is also a function of canopy depth (Table S2, eqn 12). Following Schieving et al. (1992), we used generalized circle equations to model, with a single parameter pN, different curvatures in the profile of the nitrogen distribution within the canopy while keeping the total amount of nitrogen, Ntot, constant (Table S2, eqn 13). If pN = 1, Narea decreases linearly with canopy depth, while a uniform distribution is achieved when pN tends towards infinity. Our preliminary measurements did not support non-uniform nitrogen distribution in early and late-snowmelt alpine meadows. Nevertheless, we maintained this model parameterization in order to compare the potential impact of nitrogen distribution with other factors.
The photosynthesis model was combined with a model of stomatal conductance using the empirical approach developed by Ball et al. (1987; Table S2, eqns 24–28, online). Lack of data for accurate parameterization required that several simplifications had to be made. First, we used the relative humidity of ambient air as a surrogate for the leaf surface water vapour pressure. Secondly, boundary layer conductance to H2O was set constant. Due to the interdependence of leaf net photosynthesis and stomatal conductance, we used an iteration method to calculate Ci (Harley and Tenhunen, 1991). Parameters for the stomatal conductance sub-model were taken from the work of Wohlfahrt et al. (1998).
- Seasonal GPP was estimated by running the canopy bulk-photosynthesis model from 10 May to 20 August. This 100-d period was divided into ten sub-periods of 10 d. For each sub-period, we calculated on an hourly basis the daily time course of air temperature, light and relative humidity for sunny, cloudy and intermediate days. Each sub-period comprised six sunny days, two cloudy days and two intermediate days. These relative contributions were derived from an analysis of the long-term recordings of daily irradiance in Briançon (see ‘Climate’, above). The seasonal evolution of LAI was described as a linear function of cumulative degree days (DD) after snowmelt, as follows:
where LAImax is the measured LAI at the time of peak standing biomass (topt). This parameterization allows the capture of the faster initial development of canopies from late-snowmelt sites.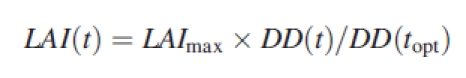
To assess the relative importance of canopy functional properties and climatic factors on carbon uptake, we conducted sensitivity analyses by systematically varying one factor, i.e. a model parameter or a variable, while keeping the other factors constant and calculating the relative effect on the seasonal GPP.
Model validation by canopy gas-exchange measurements
A closed system was used to measure the CO2 exchange of alpine meadow monoliths taken from an early and a late-snowmelt site (communities C140 and C180, respectively). Early snowmelt monoliths were sampled in the vicinity of the site of where plant trait measurements were taken. But for practical reasons, late-snowmelt monoliths were sampled near the Agnel Pass at 2760 m a.s.l., located in the Queyras mountain range. For each community, five monoliths of 20-cm depth and 50 × 25 cm area were excavated during autumn 2005, and brought to the Lautaret Alpine field station (2100 m a.s.l.) where they remained until the following summer for measurements. Species' identity and relative cover in the monoliths were assessed before the measurements and no changes were found in the floristic composition compared with the field. This indicated that the monoliths recovered well from excavation.
For CO2 flux measurements, a 48-L Perspex chamber was placed over each monolith and sealed around the base by seating it in a trough of water. Within the chamber, a fan (Radio Spare) provided air mixing above the canopy. The chamber was connected to a portable IRGA (EGM-4, PPSystems, Hitchin, UK) measuring the CO2 concentration every minute. The rate of change of CO2 in the chamber was determined by averaging the measurements under steady-state conditions, which typically began after 1 min. The measurement periods were generally brief, not exceeding 3 min in order to minimize chamber effects. We simultaneously recorded photosynthetically active radiation (PAR), air and soil temperature, and relative humidity (RH) within the chamber. The rate of change in chamber CO2 concentration was converted into a CO2 exchange rate per ground area. Net CO2 fluxes under light conditions (Fn) were collected on both cloudy and sunny days in mid-July, 2006. Dark respiration rates (Rd) were measured before and after the light treatment by placing a dark cover over the chamber. There was no indication that respiration increased after the light treatment. Instantaneous rates of gross CO2 uptake (GPP) were calculated as GPP = Fn + Rd. LAI and Ntot were measured on three 5 × 5 cm square plots per monolith. The mean LAI of early snowmelt monoliths was 1·3, and the mean Ntot was 208 mmol N m−2. The mean LAI of late-snowmelt monoliths was 2·2, and the mean Ntot was 280 mmol N m−2.
Model performance was evaluated quantitatively by calculating the square of Pearson's correlation coefficient (r2), and qualitatively by the root-mean-square error (RMSE) and the mean absolute error (MAE), summarizing the mean differences between observed and predicted values (Willmott and Matsuura, 2005).
Numerical simulations, statistical analyses and graphics were performed with the R software environment (R Development Core Team, 2006). The source code is available upon request.
RESULTS
Climatic conditions along the snowmelt gradient
The yearly course of soil temperature for the early (C140) and the late (C180) snowmelt sites is depicted on Fig. 1. The delayed onset of growing season, by nearly 40 d, is constant throughout the 7 years of recordings (Fig. 1A). This difference accounts for a loss of around 280 °Cd at the snowy site at the time of peak standing biomass, which is 30 % fewer degree-days than at the early snowmelt site (Fig. 1B). By comparison, inter-annual variability in cumulative degree-days in the snowy sites is about an order of magnitude below that of the early snowmelt sites (Fig. 1B).
Fig. 1.
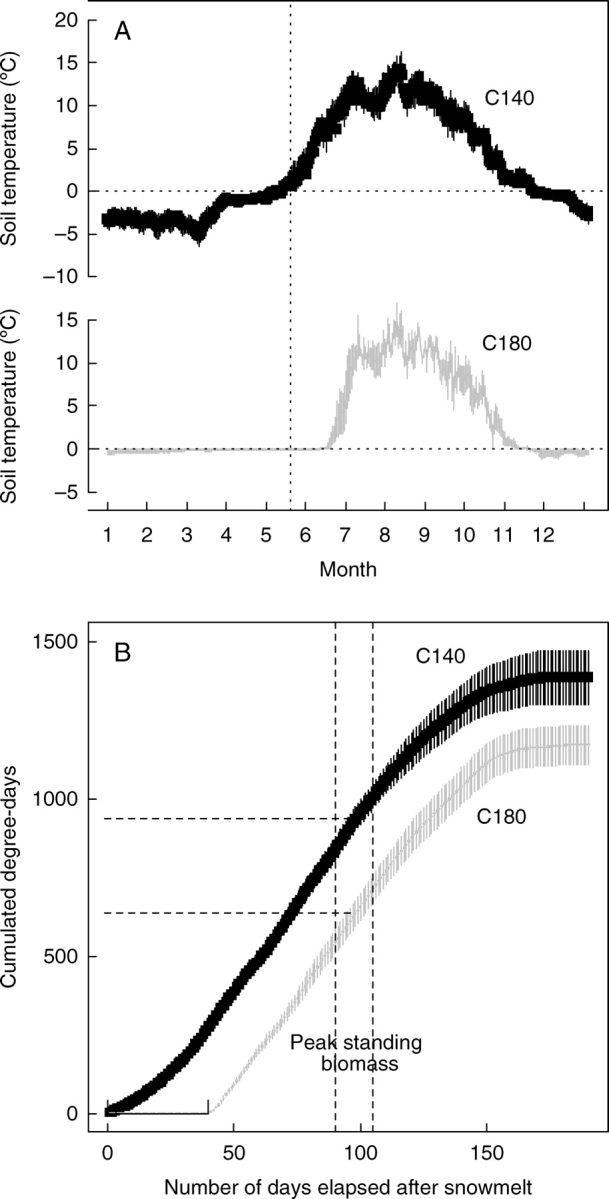
(A) Time course of daily mean (± s.e.) soil temperature at 5 cm below ground and (B) cumulative degree-days in early and late-snowmelt sites in the C140 and C180 communities (see Table 1). Data are averaged over the period 1999–2005 and were recorded at two or three different sites depending on the year.
The annual cycle of incident solar radiation measured during cloudless days is fitted well by the clear sky model (Fig. 2A). Around 60 % of days during the growing season received more than 70 % clear-sky radiation, hereafter referred to as ‘sunny days’. Thus, cloudless weather conditions predominate in this area of the south-western Alps. The effect of cloudiness accounts for a 10 % reduction in total radiation received by the early snowmelt sites compared to the clear sky model (Fig. 2B). Later snowmelt in C180 is responsible for a 40 % reduction in the cumulative incoming solar radiation over the growing season when compared with early snowmelt sites, i.e. from 0·22 MJ cm−2 to 0·13 MJ cm−2 (Fig. 2B). Inter-annual variations are small compared with those attributable to the timing of snowmelt (Fig. 2B). The daily time course of climatic data for the three types of day is given in the Supplementary Information (Fig. S1, available online).
Fig. 2.
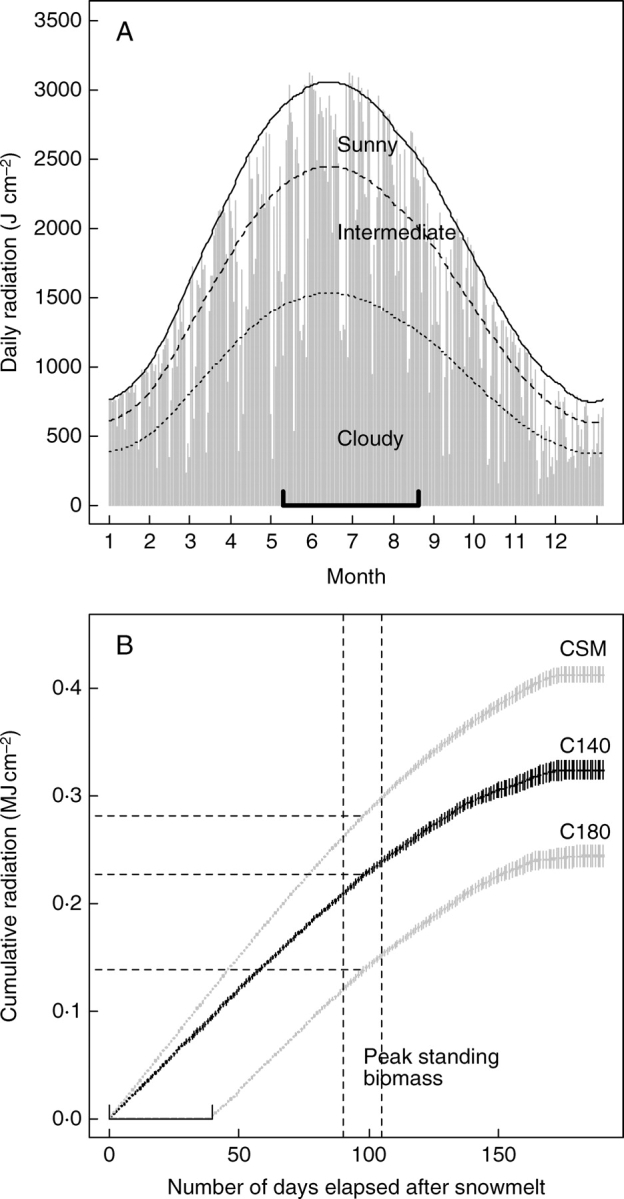
(A) Time course of daily integrated incoming radiation in 2002 and comparison with a clear sky model (solid line). Broken lines indicate reductions of 20 % and 50 % of the clear sky amount of radiation, i.e. the threshold values chosen for intermediate and cloudy days. The horizontal segment indicates the growing season period. (B) Cumulative radiation during the growing season in the C140 and C180 communities. The clear sky model is shown for comparison. Means (± s.e.) were calculated over the period 1999–2004. Data were recorded at Briançon (1300 m a.s.l.), 30 km from the study site.
Plant functional traits along the snowmelt gradient
Graminoids, erect forbs and large leafy rosettes are the main plant life forms accounting for up to 80 % of the vegetation cover in the study area (Table 1). Early snowmelt sites exhibit a discontinuous vegetation cover and a high proportion of bunch graminoids (e.g. Kobresia myosuroides), whereas tiny erect forbs and stoloniferous graminoids are predominant at the snowy sites. The snowmelt gradient was also characterized by a marked shift in community-aggregated values of leaf traits, with planophilous canopies with a high Nmass and high SLA at the snowy sites, and erectophilous canopies with low SLA and high Narea at the early snowmelt sites (Fig. 3).
Table 1.
Features of the seven alpine meadows distributed along the snowmelt gradient. The measurements were made at the time of peak standing biomass at the end of July 2004. The leaf eccentricity was calculated with an ellipsoidal leaf-distribution model (see Material and Methods)
| Mean snowmelt (Julian day) |
|||||||
|---|---|---|---|---|---|---|---|
| 130 | 140 | 150 | 160 | 170 | 180 | 190 | |
| n | 7 | 9 | 6 | 4 | 9 | 4 | 8 |
| Percentage cover of plant life form | |||||||
| Bunch graminoids | 59 ± 1·6 | 33 ±1·7 | 18 ± 0·9 | <1 | <1 | <1 | <1 |
| Other graminoids | 15 ± 1·4 | 12 ± 0·8 | 13 ± 0·5 | 28 ± 2·5 | 20 ± 1·1 | 39 ± 4·3 | 48 ± 4·8 |
| Erect forbs | 14 ± 0·6 | 30 ± 1·3 | 33 ± 1·0 | 36 ± 1·6 | 43 ± 2·1 | 52 ± 3·6 | 52 ± 4·8 |
| Large leafy rosette | 3 ± 0·3 | 17 ± 1·1 | 17 ± 0·9 | 22 ± 3·3 | 17 ± 2·3 | 4 ± 0·5 | <1 |
| Leaf eccentricity | 0·5 | 1·5 | 1·6 | 2·7 | 2·3 | 2·5 | 3·1 |
| Leaf area index* | 0·8 ± 0·09 | 1·5 ± 0·06 | 2·5 ± 0·35 | 1·2 ± 0·10 | 1·12 ± 0·01 | 2·0 ± 0·09 | 2·4 ± 0·18 |
| Ntot (mmol m−2) | 133 ± 4·0 | 218 ± 10·3 | 356 ± 10·2 | 148 ± 6·1 | 159 ± 5·9 | 257 ± 7·4 | 275 ± 16·7 |
| Above-ground phytomass (g m−2) | 435 ± 154 | 486 ± 65 | 443 ± 89 | 352 ± 77 | 402 ± 45 | 398 ± 67 | 370 ± 36 |
Means ± s.e. are shown.
*n = 3 for LAI.
Fig. 3.
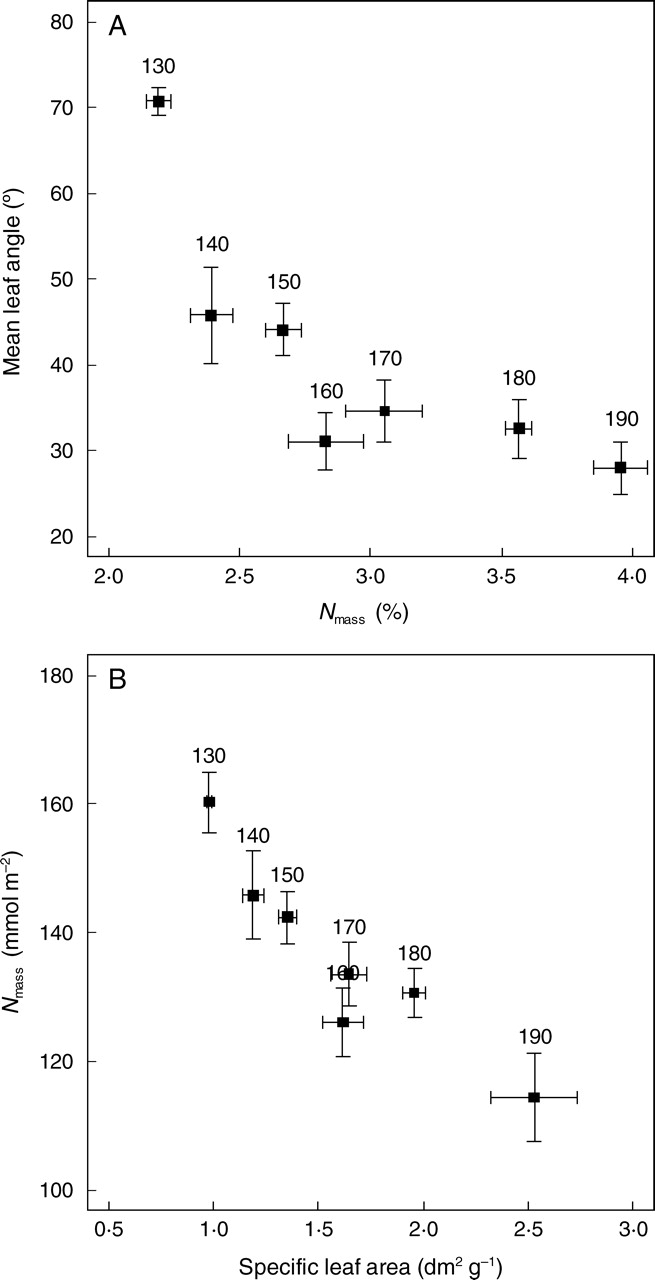
Relationships between community-aggregated values of (A) leaf nitrogen concentration on a mass basis (Nmass) and mean leaf angle, and (B) leaf nitrogen content on an area basis (Narea) and specific leaf area (SLA). Means (± s.e.) were calculated from 4–9 different plots (see Table 1). The numbers above the squares indicate the mean snowmelt date for each community in Julian days.
Canopies of late-snowmelt sites exhibited larger LAI and greater Ntot (Table 1). It should be stressed that the range of variation for LAI (from less than 1·0 at the early snowmelt sites to around 2·5 at the mid-part of the gradient) largely exceeded that of Narea. Therefore, the total nitrogen pool in the canopy, Ntot = LAI × Narea is primarily determined by LAI, and to a lesser extent by Narea.
Daily and seasonal gross primary production
Modelled values of instantaneous gross CO2 uptake corresponded well with measurements under cloudless and overcast conditions, although there was a slight over-estimation for early snowmelt canopies (Fig. 4). Increased carbon fixation under conditions of high diffuse radiation was particularly noteworthy for canopies with high LAI (Fig. 4A). At a PAR of 1000 µmol m−2 s−1, the CO2 gross uptake of late snowmelt monoliths was 1·5 times higher under overcast conditions compared to sunny conditions (Fig. 4A), even though ambient air temperature was around 5 °C lower (Supplementary Information, Fig. S1).
Fig. 4.
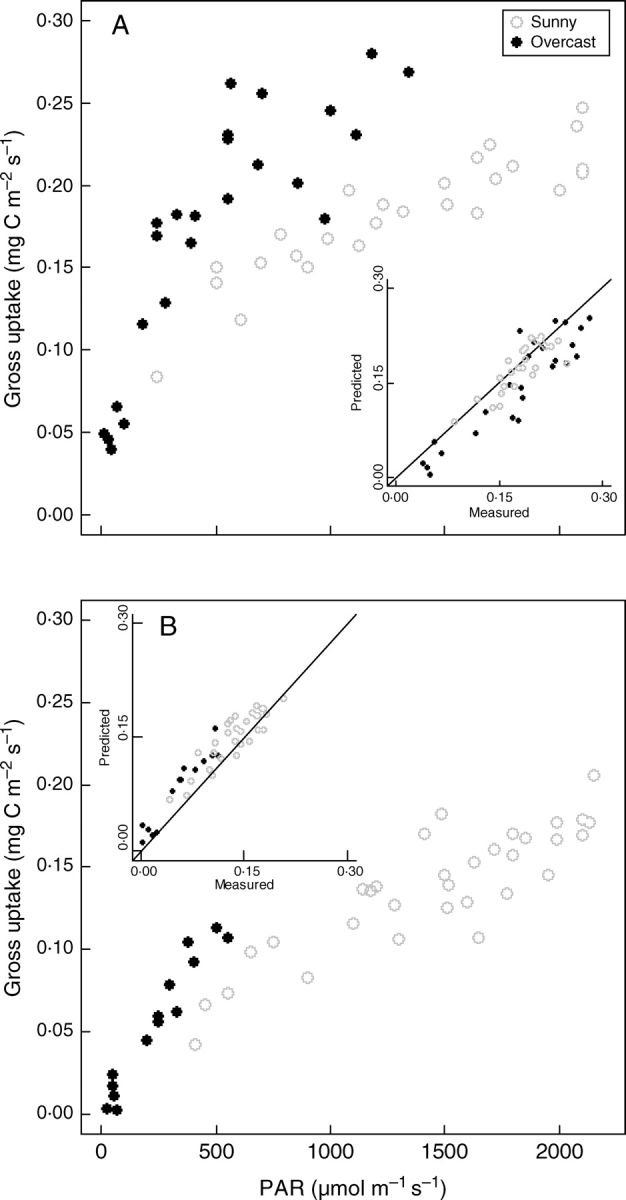
Canopy gross CO2 uptake for sunny and overcast conditions in mid-July 2006. Closed chamber measurements were performed (A) on monoliths dominated by Carex foetida sampled at a late-snowmelt site (C180), and (B) on monoliths dominated by Kobresia myosuroides sampled at an early snowmelt site (C140). The relationships between measured and predicted values are shown in the two insets. Evaluation of the model performance is as follows: (A) n = 50, r2 = 0·89, RMSE = 0·033, MAE = 0·026; (B) n = 46, r2 = 0·95, RMSE = 0·025, MAE = 0·021.
For sunny and overcast conditions, the daily GPP values for the seven canopies were linearly related to the LAI (Fig. 5). This may be explained by the strong relationship between LAI and Ntot (see above and Table 1). For a given amount of Ntot, we simulated the daily carbon uptake for a range of LAI (Fig. 5). For low LAI values, the daily GPP strongly increases with LAI, indicating that light capture is the main limiting factor of carbon uptake. Then, the slight decrease of GPP with LAI may be explained by a decrease in Nmass due to a ‘dilution’ effect. The modelled GPP under cloudy skies was lowered by around 10 % compared with cloudless skies, for example 6·5 against 7·3 g C m−2 d−1 for C180 canopies (Fig. 4). We found a negligible effect of leaf eccentricity (see Table 1) on this trade-off between light capture – driven by LAI – and CO2 fixation – driven by Nmass (data not shown). Finally, it should be noted that for a given Ntot, alpine canopies operate at a higher LAI than the optimal LAI (Fig. 4).
Fig. 5.
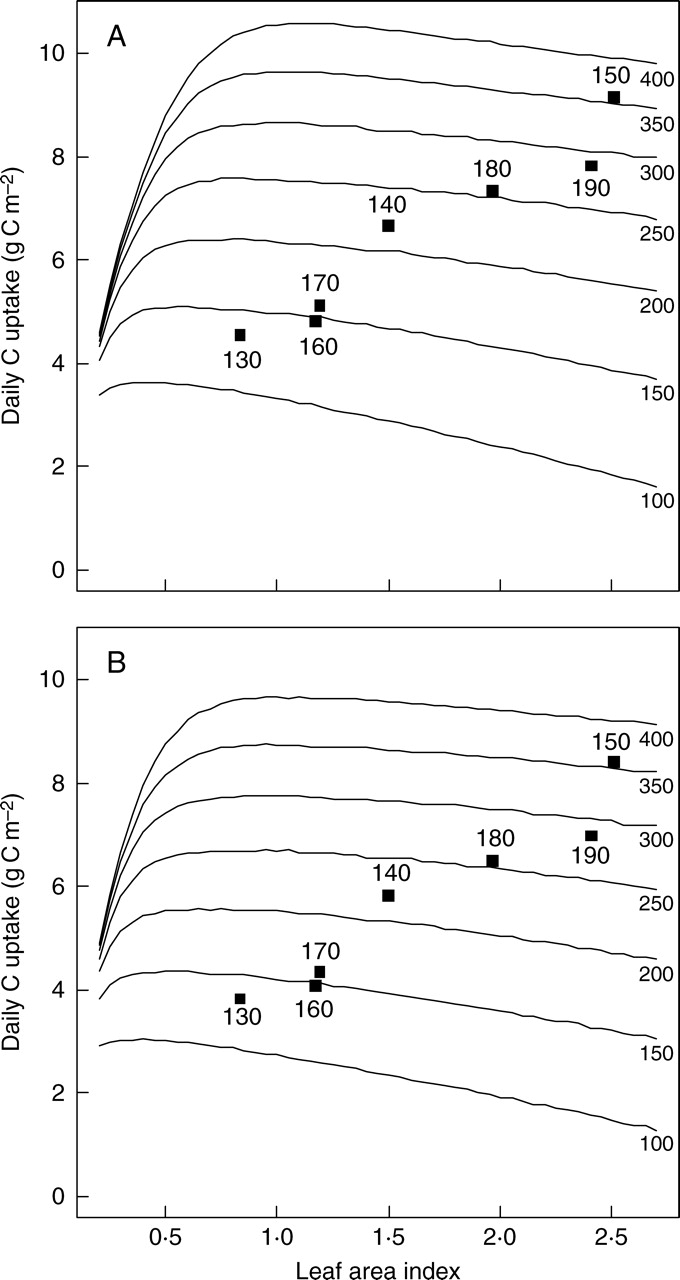
Daily estimates of GPP (in g C m−2 ground d−1) for (A) sunny and (B) overcast conditions in mid-July as a function of LAI. Simulations were run for a range of canopy nitrogen contents, Ntot (in mmol N m−2 ground), represented by the lines. The black squares correspond to the GPP estimates obtained with the LAI–Ntot combinations of the seven alpine plant communities studied. Numbers above the squares as in Fig. 3.
A sensitivity analysis of the seasonal GPP model was conducted by estimating the effect of a 10 % increase in a factor while keeping all other characteristics constant (Fig. 6A). The simulations were run for a 100-d period (see Material and Methods). A 10 % increase in the length of growing season (corresponding to a shift of 10 d for the snowmelt) had the greatest impact on the seasonal GPP (Fig. 6A). By comparison, a shift to clear sky conditions throughout the season had a weaker effect. Similarly, increased temperature during the whole season did not compensate for 10 d lost in the growing season. LAI and two nitrogen-related factors, Nmass and sN, had a noticeable impact on GPP, but still lower than that of the length of growing season (Fig. 6A). The shift from a uniform to a linearly decreasing nitrogen distribution (pN = 1) also had a weaker effect on GPP, roughly similar to the effect of a temperature or an atmospheric CO2 increase. Finally, a 10 % change in relative humidity, leaf angle, or physiological parameters related to stomatal conductance changed the GPP by less than 1 %.
Fig. 6.
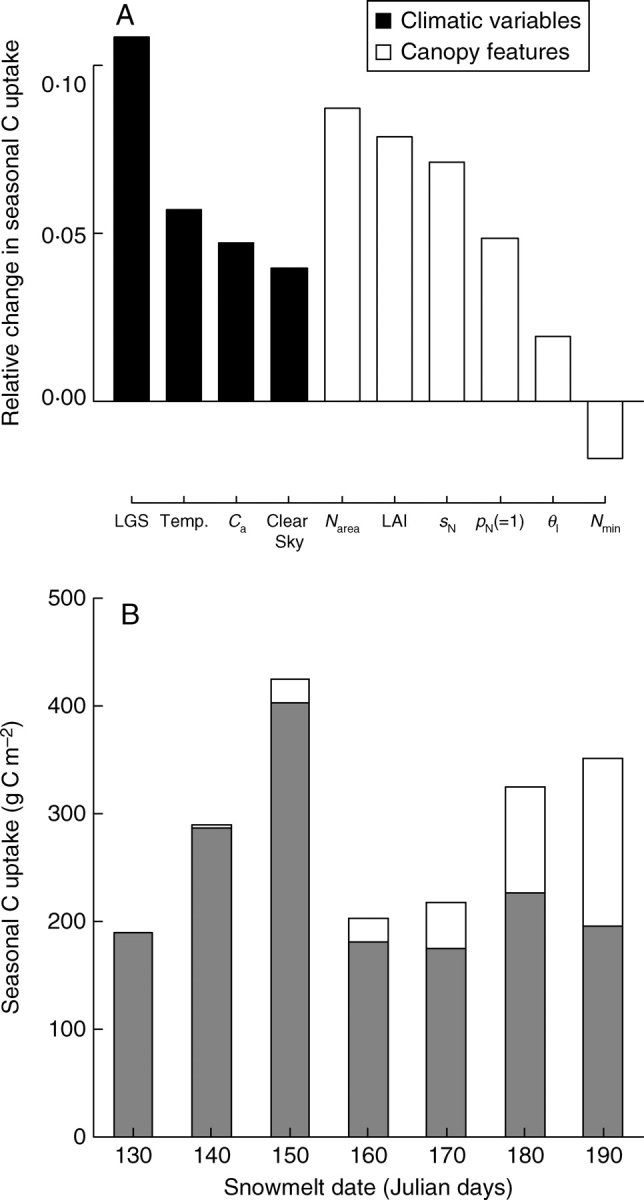
(A) Sensitivity analysis of the seasonal GPP model. The relative change in GPP following a 10 % increase in a given factor was calculated as (GPPnew – GPP)/GPP where GPPnew is the GPP obtained after changing the factor. Factors for which the relative effect on GPP did not exceed 1 % are not shown. Climatic variables and canopy features are distinguished. LGS is for a 10-day increase of the length of growing season. Ca, ambient CO2 partial pressure; pN, parameter for nitrogen distribution (see Material and Methods); sN, slope of the linear relation between Narea and Vc,max; Nmin, minimum leaf nitrogen content; θl, curvature of the leaf response of electron transport rate to irradiance. (B) Seasonal estimates of GPP (in g C m−2) for the seven alpine communities studied. For each bar, the grey part accounts for the carbon uptake during the observed length of the growing season, and the white part accounts for the missing carbon uptake due to the delayed snowmelt. Numbers indicate the mean snowmelt date of each community in Julian days.
The integrated values of GPP over the growing season were around 200 g C m−2, except for the plant communities C140 and C150 (Fig. 6B). GPP simulations were also performed without the snow-induced shortening of the growing season. For the late-snowmelt sites, the model predicted a severe reduction in the carbon uptake because of the delayed snowmelt. However, the results indicated that the functional properties of these canopies (greater Ntot and LAI) largely compensated for this negative impact of a reduced growing season.
DISCUSSION
Our study emphasizes the interplay between short-term and long-term effects of snow-cover duration on the seasonal carbon uptake of alpine canopies. Short-term effects are driven by the direct influence of snow cover on the seasonal light and temperature regimes, whereas long-term effects correspond to the ecological sorting of species and plant traits along snow-cover gradients. A main result is that the snow-induced reduction in the carbon uptake period is counterbalanced by increased efficiency of carbon gain, which is made possible by the particular leaf trait combination of exploitative strategists that occur at the late-snowmelt sites. To our knowledge, this is the first attempt to determine the complex impact of snow on the seasonal GPP of temperate alpine canopies.
Instantaneous CO2 flux measurements under different light conditions are predicted well by our canopy photosynthesis model. We are thus confident that the use of community-aggregated values of the chosen leaf traits is relevant to simulate the carbon uptake at the community level, as hypothesized in previous conceptual work (Diaz and Cabido, 2001). We found that the assimilation rate of the whole canopy (expressed per unit ground area) was of the same magnitude as the leaf assimilation rate (expressed per unit leaf area), which is consistent with other studies (Grabherr and Cernusca, 1977; Diemer, 1994; Tappeiner and Cernusca, 1998). Compared with the most widely investigated temperate alpine community, i.e. Carex curvula-dominated alpine meadow (Diemer and Körner, 1998), the measured and modelled carbon uptake are higher for canopies of late-snowmelt sites and lower for early snowmelt sites.
Scaling-up from detailed plant physiological studies to ecosystem or regional scales requires taking into account variations in vegetation composition and community structure. Approaches based on a priori classification of plants into functional types have shown some limitations (Naeem and Wright, 2003; Reich et al., 2004). Our study shows how integrating the trait-based approaches of comparative plant ecology with canopy-functioning models addresses this difficulty. However, recent findings suggest that functional diversity, i.e. the distribution and range of trait values in a given plant community, might be another key driver of ecosystem functioning (Naeem and Wright, 2003; Reich et al., 2004). Clearly, a further challenge in ecosystem modeling would be to explicitly incorporate functional diversity effects into biogeochemical models.
There are several possible shortcomings when simulating the GPP over the growing season. For example, we considered seasonal variations in incoming radiation and degree-days, excluding the potential impact of changes in soil water content on canopy assimilation. Our continuous measurements of gravimetric soil water content, at 5 cm below ground, did not show significant differences between early and late-snowmelt sites, and only a slight decrease through the growing season (F. Baptist, unpubl. res.). The soils of the study site are deep and the average rainfall between mid-May and mid-August for the three years, 2004–2006, was around 300 mm. These features should ensure enough water is available to plants in the conditions of low evaporative demand normal for high-elevation meadows (Körner, 1999). Moreover, we did not consider all the potential changes in plant functioning over the growing season. For example, the nitrogen content of late-snowmelt canopies is particularly high immediately after their release from snow cover, but then decreases slightly (F. Baptist, unpubl. res.). This may cause an over-estimation of the seasonal GPP at late-snowmelt sites. These limitations call for further refinements of the model and, as such, absolute values of the seasonal GPP estimates should be considered with caution. However, these limitations should not distort the conclusions about the relative impact of growing season length vs. canopy functional properties on carbon uptake along the snowmelt gradient.
In our simulations, leaf geometrical properties did not exert a strong influence on GPP. Canopy photosynthesis models, based on the Monsi–Saeki theory, also show that the assimilation rate of canopies with a LAI under 2·5 is largely independent of the extinction coefficient, K (Hirose, 2005). However, our model neglects other potential effects of leaf inclination, for example the effect on night-time frost and possible low-temperature photoinhibition (Germino and Smith, 2001). Presently, we do not have enough empirical data to assess the relative importance of such mechanisms at the canopy level and over the growing season.
The results suggest that the alpine canopies do not operate at their optimal LAI (Fig. 5). It is clear that stand-level properties are not exclusively dependent upon maximizing carbon uptake and that individuals are the units under selection, not canopies (Hirose, 2005). Some theoretical approaches have also highlighted that the optimal LAI is not evolutionary stable if one takes into account competition among individuals (Anten and Hirose, 2001). Perhaps more interestingly, the comparison of canopy functioning along the snowmelt gradient might call for a more detailed investigation of the optimal LAI–N relationships for different lengths of growing season.
As reported in other studies (De Pury and Farquhar, 1997; Gu et al., 2002), the sun–shade model allows a more realistic treatment of the difference in the canopy photosynthetic response to direct and diffuse radiation. Increased carbon uptake under diffuse radiation has already been reported (Roderick et al., 2001). The deep-shaded leaf fraction within vegetation canopies is strongly reduced on cloudy days compared to cloudless days as a result of the increased diffuse fraction of incoming radiation. Furthermore, at noon there is a lower probability that canopy photosynthetic saturation will occur under overcast conditions compared to sunny conditions (Gu et al., 2002).
The results support the view that the traits of exploitative strategists (especially the high leaf Nmass, the rapid growth of photosynthetic organs) should permit the constraint of a shortened carbon-uptake period to be overcome. However, both conservative and exploitative strategies are known to be adaptive under severe limitation of the carbon uptake period (Kikuzawa and Kudo, 1995). Obviously, the exploitative strategy is strongly dependent upon sufficient soil nutrient availability. The late-snowmelt sites that were investigated benefit from a pulse of inorganic nitrogen at the time of snowmelt, as compared to early snowmelt sites (F. Baptist, unpubl. res.; see Supplementary Information online). These results are also consistent with other comparisons along snowmelt gradients (Bowman et al., 1993; Fisk et al., 1998; Jaeger et al., 1999). It is therefore likely that in the late-snowmelt sites that were studied, this nutrient pulse at the onset of growing season allowed a rapid expansion of photosynthetic tissues, which ensured efficient light capture and carbon fixation.
Conclusions
For cold ecosystems, the carbon-uptake period is primarily determined by the snow-cover duration. Here, we have demonstrated that the snow-induced changes in the length of growing season had the highest impact on the seasonal GPP. Our study is among the first ones to integrate the functional trait approach with instantaneous measurements and integrated estimates of ecosystem functioning. This approach is particularly promising because continuous recordings of CO2 fluxes have been shown to be technically difficult in alpine environments with strong bioclimatic gradients.
Climatic change in will affect snow regimes in temperate mountains (Keller et al., 2005; Euskirchen et al., 2006). Increased temperature and reduced snow precipitation may be responsible for earlier snowmelt. But the phenological responses of alpine species to a lengthening growing season are still hard to predict (Starr et al., 2000; Wipf et al., 2006; Bjork and Molau, 2007). One might predict a small impact on carbon uptake if plant communities are dominated by periodic species, i.e. species with a fixed, genetically controlled growing period (Sorenson, 1941). Conversely, the short-term consequences for ecosystem productivity would be stronger if aperiodic species, i.e. species able to extend their vegetative growth, are dominatant. Future studies should address these issues for a better estimate of seasonal GPP in alpine meadows in response to global climate change.
SUPPLEMENTARY INFORMATION
Supplementary material is available online at http://aob.oxfordjournals.org/ and consists of the following figures and tables. Figure S1: the daily course of temperature, relative humidity and photosynthetically active radiation for sunny, intermediate and cloudy days in May–August. Figure S2: Relationship between the maximum rate of carboxylation (Vc,max) and the leaf nitrogen content per unit area (Narea). Figure S3: daily uptake of nitrate and ammonium by a resin bag inserted at 5 cm below ground in the C140, C160 and C180 communities. Table S1: list of constants, parameters and lumped variables used in the model. Table S2: list of the main equations of the model.
ACKNOWLEDGEMENTS
The authors would like to acknowledge S. Aubert, K. Charra-Vascou, C. Flahault and G. Girard, who assisted in the field and the laboratory. We are indebted to M. Robson and F. Quétier for discussions and comments on an earlier version of this manuscript. Logistical support was provided by the Station Alpine J. Fourier of Grenoble. This work was supported by the University of Grenoble and by the Centre National de la Recherche Scientifique (CNRS). F. Baptist received a Ph.D. grant from the French Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche.
LITERATURE CITED
- Anten NPR. Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Annals of Botany. 2005;95:495–506. doi: 10.1093/aob/mci048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten NPR, Hirose T. Limitations on photosynthesis of competing individuals in stands and the consequences for canopy structure. Oecologia. 2001;129:186–196. doi: 10.1007/s004420100718. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Schieving F, Werger MJA. Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono- and dicotyledonous species. Oecologia. 1995;101:504–513. doi: 10.1007/BF00329431. [DOI] [PubMed] [Google Scholar]
- Arora VK, Boer GJ. A parameterization of leaf phenology for the terrestrial ecosystem component of climate models. Global Change Biology. 2005;11:39–59. [Google Scholar]
- Ball JT, Woodrow IE, Berry JA. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggens J., editor. Progress in photosynthesis research. The Netherlands: Martinus Nijhoff Publishers; 1987. [Google Scholar]
- Bjork RG, Molau U. Ecology of alpine snowbeds and the impact of global change. Arctic Antarctic and Alpine Research. 2007;39:34–43. [Google Scholar]
- Bowman WD, Theodose TA, Schardt JC, Conant RT. Constraints of nutrient avaibility on primary production in two alpine communities. Ecology. 1993;74:2085–2097. [Google Scholar]
- Brooks PD, Schmidt SK, Williams MW. Winter production of CO2 and N2O from alpine tundra: environmental controls and relationship to inter-system C and N fluxes. Oecologia. 1997;110:403–413. doi: 10.1007/PL00008814. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relatoinships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Campbell GS. Derivation of an angle density function for canopies with ellipsoidal leaf angle distributions. Agricultural and Forest Meteorology. 1990;49:173–176. [Google Scholar]
- Campbell JL, Mitchell MJ, Groffman PM, Christenson LM, Hardy JP. Winter in northeastern North America: a critical period for ecological processes. Frontiers in Ecology and the Environment. 2005;3:314–322. [Google Scholar]
- Cernusca A. Struktur und Funktion von Graslandökosystemen im Nationalpark Hohe tauern. Innsbruck: Österreich Akademische Wiss and Wagner; 1989. Veröffenentlichungen des Österreich MaB-Hochgebirgsprogramms Hohe Tauern. [Google Scholar]
- Cernusca A, Bahn M, Chemini C, Graber W, Siegwolf R, Tappeiner U, Tenhunen J. ECOMONT: a combined approach of field measurements and process-based modelling for assessing effects of land-use changes in mountain landscapes. Ecological Modelling. 1998;113:167–178. [Google Scholar]
- Chapin FS, Bret-Harte MS, Hobbie SE, Zhong H. Plant functional types as predictors of transcient responses of arctic vegetation to global change. Journal of Vegetation Science. 1996;7:693–702. [Google Scholar]
- Choler P. Consistent shifts in Alpine plant traits along a mesotopographical gradient. Arctic Antarctic and Alpine Research. 2005;37:444–453. [Google Scholar]
- Churkina G, Schimel D, Braswell BH, Xiao XM. Spatial analysis of growing season length control over net ecosystem exchange. Global Change Biology. 2005;11:1777–1787. [Google Scholar]
- Cornelissen JHC, Perez Harguindeguy N, Diaz S, Grime JP, Marzano B, Cabido M, Vendramini F, Cerabolini B. Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytologist. 1999;143:191–200. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- De Pury DGG, Farquhar GD. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant, Cell and Environment. 1997;20:537–557. [Google Scholar]
- Diaz S, Cabido M. Vive la difference: plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution. 2001;16:646–655. [Google Scholar]
- Diemer M, Körner C. Transient enhancement of carbon uptake in an alpine grassland ecosystem under elevated CO2. Arctic and Alpine Research. 1998;30:381–387. [Google Scholar]
- Diemer MW. Mid-season gas exchange of an alpine grassland under elevated CO2. Oecologia. 1994;98:429–435. doi: 10.1007/BF00324233. [DOI] [PubMed] [Google Scholar]
- Epstein HE, Chapin FS, Walker MD, Starfield AM. Analyzing the functional type concept in arctic plants using a dynamic vegetation model. Oikos. 2001;95:239–252. [Google Scholar]
- Euskirchen ES, McGuire AD, Kicklighter DW, Zhuang Q, Clein JS, Dargaville RJ, et al. Importance of recent shifts in soil thermal dynamics on growing season length, productivity, and carbon sequestration in terrestrial high-latitude ecosystems. Global Change Biology. 2006;12:731–750. [Google Scholar]
- Farquhar GD, Von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:341–347. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Fisk MC, Schmidt SK, Seastedt TR. Topographic patterns of above- and belowground production and nitrogen cycling in Alpine tundra. Ecology. 1998;79:2253–2266. [Google Scholar]
- Friend AD. Modelling canopy CO2 fluxes: are ‘big-leaf’ simplifications justified? Global Ecology and Biogeography. 2001;10:603–619. [Google Scholar]
- Garnier E, Cortez J, Billes G, Navas ML, Roumet C, Debussche M, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Germino MJ, Smith WK. Relative importance of microhabitat, plant form and photosynthetic physiology to carbon gain in two alpine herbs. Functional Ecology. 2001;15:243–251. [Google Scholar]
- Grabherr G, Cernusca A. Influence of radiation, wind, and temperature on the CO2 gas exchange of the Alpine dwarf shrub community Loiseleurietum cetrarietosum. Photosynthetica. 1997;11:22–28. [Google Scholar]
- Grant RF, Oechel WC, Ping CL. Modelling carbon balances of coastal arctic tundra under changing climate. Global Change Biology. 2003;9:16–36. [Google Scholar]
- Grime JP. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. Journal of Ecology. 1998;86:902–910. [Google Scholar]
- Gu LH, Baldocchi D, Verma SB, Black TA, Vesala T, Falge EM, Dowty PR. Advantages of diffuse radiation for terrestrial ecosystem productivity. Journal of Geophysical Research Atmospheres. 2002;107 D6, 4050, doi:10.1029/2001JD001242. [Google Scholar]
- Hammerle A, Haslwanter A, Schmitt M, Bahn M, Tappeiner U, Cernusca A, Wohlfahrt G. Eddy covariance measurements of carbon dioxide, latent and sensible energy fluxes above a meadow on a mountain slope. Boundary Layer Meteorology. 2007;122:397–416. doi: 10.1007/s10546-006-9109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Tenhunen JD. Modeling the photosynthetic response of C3 leaves to envionmental factors. In: Boote KJ, Loomis RS, editors. Modelling crop photosynthesis – from biochemistry to canopy. Madison: Crop Science Society of America; 1991. pp. 17–39. [Google Scholar]
- Hikosaka K, Hirose T. Leaf angle as a strategy for light competition: optimal and evolutionarily stable light-extinction coefficient within a leaf canopy. Ecoscience. 1997;4:501–507. [Google Scholar]
- Hirose T. Development of the Monsi–Saeki theory on canopy structure and function. Annals of Botany. 2005;95:483–494. doi: 10.1093/aob/mci047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie SE, Schimel JP, Trumbore SE, Randerson JR. Controls over carbon storage and turnover in high-latitude soils. Global Change Biology. 2000;6(Suppl. 1):196–210. doi: 10.1046/j.1365-2486.2000.06021.x. [DOI] [PubMed] [Google Scholar]
- Jaeger CH, Monson RK, Fisk MC, Schmidt SK. Seasonal partitioning of nitrogen by plants and soil microorganisms in an alpine ecosystem. Ecology. 1999;80:1883–1891. [Google Scholar]
- Jones HG, Pomeroy JW, Walker DA, Hoham RW. Snow ecology: an interdisciplinary examination of snow-covered ecosystems. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Keller F, Goyette S, Beniston M. Sensitivity analysis of snow cover to climate change scenarios and their impact on plant habitats in alpine terrain. Climatic Change. 2005;72:299–319. [Google Scholar]
- Kikuzawa K, Kudo G. Effects on the length of the snow-free period on leaf longevity in alpine shrubs: a cost–benefit model. Oikos. 1995;73:98–104. [Google Scholar]
- Komarkova V, Webber PJ. An alpine vegetation map of Niwot Ridge, Colorado. Arctic and Alpine Research. 1978;1:1–29. [Google Scholar]
- Körner C. Alpine plant life. Berlin: Springer Verlag; 1999. [Google Scholar]
- Kudo G, Ito K. Plant distribution in relation to the length of the growing season in a snow-bed in the Taisetsu mountains, northern Japan. Vegetatio. 1992;98:319–328. [Google Scholar]
- Kudo G, Nordenhall U, Molau U. Effects of snowmelt timing on leaf traits, leaf production, and shoot growth of alpine plants: comparisons along a snowmelt gradient in northern Sweden. Ecoscience. 1999;6:439–450. [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. [Google Scholar]
- Li SG, Asanuma J, Eugster W, Kotani A, Liu JJ, Urano T, et al. Net ecosystem carbon dioxide exchange over grazed steppe in central Mongolia. Global Change Biology. 2005;11:1941–1955. [Google Scholar]
- Monson RK, Lipson DL, Burns SP, Turnipseed AA, Delany AC, Williams MW, Schmidt SK. Winter forest soil respiration controlled by climate and microbial community composition. Nature. 2006;439:711–714. doi: 10.1038/nature04555. [DOI] [PubMed] [Google Scholar]
- Naeem S, Wright JP. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecology Letters. 2003;6:567–579. [Google Scholar]
- Onipchenko VG, Blinnikov MS. Experimental investigation of alpine plant communities in the Northwestern Caucasus. Veröffentlichungen des Geobotanischen Institutes der ETH, Stiftung Rübel, Zürich. 1994;115:91–98. [Google Scholar]
- Quetier F, Thebault A, Lavorel S. Plant traits in a state and transition framework as markers of ecosystem response to land-use change. Ecological Monographs. 2007;77:33–52. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Reich PB, Tilman D, Naeem S, Ellsworth DS, Knops J, Craine J, Wedin D, Trost J. Species and functional group diversity independently influence biomass accumulation and its response to CO2 and N. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10101–10106. doi: 10.1073/pnas.0306602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigollier C, Bauer O, Wald L. On the clear sky model of the 4th European Solar Radiation Atlas with respect to the Heliosat method. Solar Energy. 1999;68:33–48. [Google Scholar]
- Roderick ML, Farquhar GD, Berry SL, Noble IR. On the direct effect of clouds and atmospheric particles on the productivity and structure of vegetation. Oecologia. 2001;129:21–30. doi: 10.1007/s004420100760. [DOI] [PubMed] [Google Scholar]
- Schieving F, Werger MJA, Hirose T. Canopy structure, nitrogen distribution and whole canopy photosynthesic carbon gain in growing and flowering stands of tall herbs. Vegetatio. 1992;102:173–181. [Google Scholar]
- Sorenson T. Temperature relations and phenology of north-east Greenland flowering plants. Meddelelser. 1941;125:1–305. [Google Scholar]
- Spitters CJT. Separating the diffuse and direct component of global radiation and its implications for modelling canopy photosynthesis. Part II. Calculation of canopy photosynthesis. Agricultural and Forest Meteorology. 1986;38:231–242. [Google Scholar]
- Starr G, Oberbauer SF, Pop EW. Effects of lengthened growing season and soil warming on the phenology and physiology of Polygonum bistorta. Global Change Biology. 2000;6:357–369. [Google Scholar]
- Tappeiner U, Cernusca A. Model simulation of spatial distribution of photosynthesis in structurally differing plant communities in the Central Caucasus. Ecological Modelling. 1998;113:201–223. [Google Scholar]
- Theurillat J-P, Aeschimann D, Küpfer P, Spichiger R. The higher vegetation units of the alps. Colloques Phytosociologiques. 1994;23:189–239. [Google Scholar]
- Turner DP, Ollinger SV, Kimball JS. Integrating remote sensing and ecosystem process models for landscape- to regional-scale analysis of the carbon cycle. BioScience. 2004;54:573–584. [Google Scholar]
- Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. Let the concept of trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- Vourlitis GL, Harazono Y, Oechel WC, Yoshimoto M, Mano M. Spatial and temporal variations in hectare-scale net CO2 flux, respiration and gross primary production of Arctic tundra ecosystems. Functional Ecology. 2000;14:203–214. [Google Scholar]
- Willmott CJ, Matsuura K. Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Climate Research. 2005;30:79–82. [Google Scholar]
- Wipf S, Rixen C, Mulder CPH. Advanced snowmelt causes shift towards positive neighbour interactions in a subarctic tundra community. Global Change Biology. 2006;12:1496–1506. [Google Scholar]
- Wohlfahrt G, Bahn M, Horak I, Tappeiner U, Cernusca A. A nitrogen sensitive model of leaf carbon dioxide and water vapour gas exchange: application to 13 key species from differently managed mountain grassland ecosystems. Ecological Modelling. 1998;113:179–199. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]


