Abstract
Background and Aims
The hypothesis was tested that pectin content and methylation degree participate in regulation of cell wall mechanical properties and in this way may affect tissue growth and freezing resistance over the course of plant cold acclimation and de-acclimation.
Methods
Experiments were carried on the leaves of two double-haploid lines of winter oil-seed rape (Brassica napus subsp. oleifera), differing in winter survival and resistance to blackleg fungus (Leptosphaeria maculans).
Key Results
Plant acclimation in the cold (2 °C) brought about retardation of leaf expansion, concomitant with development of freezing resistance. These effects were associated with the increases in leaf tensile stiffness, cell wall and pectin contents, pectin methylesterase (EC 3·1·1·11) activity and the low-methylated pectin content, independently of the genotype studied. However, the cold-induced modifications in the cell wall properties were more pronounced in the leaves of the more pathogen-resistant genotype. De-acclimation promoted leaf expansion and reversed most of the cold-induced effects, with the exception of pectin methylesterase activity.
Conclusions
The results show that the temperature-dependent modifications in pectin content and their methyl esterification degree correlate with changes in tensile strength of a leaf tissue, and in this way affect leaf expansion ability and its resistance to freezing and to fungus pathogens.
Key words: Brassica napus subsp, oleifera, cell wall, cold acclimation, de-acclimation, freezing, growth, leaf stiffness, pathogen, pectins, pectin methylesterase
INTRODUCTION
Prolonged growth of non-endodormant chilling resistant plants in the cold (>0 °C) is associated with an adjustment of cellular metabolism to low temperature conditions and results in both retardation of growth and in increased resistance of cells to extracellular freezing (Kacperska, 1989). This phenomenon is known as plant cold acclimation or plant cold-hardening. Cold-acclimated plants may resume growth and lose resistance to extracellular freezing if exposed to the growth-promoting conditions (warmer environmental temperature, longer photoperiods), i.e. they may undergo de-acclimation (Kalberer et al., 2006).
During extracellular freezing, the difference in chemical potential of water between the extracellular ice and the intracellular solutions results in migration of cellular water to the extracellular ice and leads to dehydration of cells and concentration of intracellular solutes (Rajashekar and Burke, 1996). The extent of cell dehydration increases in parallel with a gradual reduction in temperature. Freezing-induced cell dehydration results in the collapse of the cell wall around the shrinking protoplasm and, depending on the properties of cell components, may lead to different degrees of frost injuries (Levitt, 1980).
Aside from other factors (such as membrane and protoplast properties), the resistance of plants to extracellular freezing depends on the mechanical properties of cell walls (Tao et al., 1983; Bartolo et al., 1987; Murai and Yoshida, 1998), which may modify the kinetics and the degree of freezing-induced dehydration, as well as the temperature of ice nucleation (Rajashekar and Burke, 1996; Rajashekar and Lafta, 1996).
It has been accepted that mechanical properties of cell walls are determined by the amount and structure of wall polysaccharides (Taiz, 1984; Carpita and Gibeaut, 1993). In the leaves of cold-acclimated winter oil-seed rape plants (Brassica napus subsp. oleifera), increased cell wall thickness and considerable alterations in cell wall ultrastructure (Stefanowska et al. 1999) and composition were observed (Kubacka-Zębalska and Kacperska, 1999). The most remarkable feature of the cold-modified cell walls was increased content of non-covalently bound pectins. These modifications seem to be the reason for inhibition of leaf area expansion in the cold and the promotion of leaf growth in thickness (Maciejewska and Kacperska, 1987). However, no experimental evidence of the relationship between the cold-induced changes in leaf cell wall properties and leaf resistance to extracellular freezing has been given so far. Nor is information concerning modifications of cell wall properties in de-acclimated leaves available, although promotion of tissue elongation growth during de-acclimation of oil-seed rape plants has been observed (Rapacz et al., 2001).
In contrast to the cold effects, a short, sublethal, freezing treatment, aside from other effects, increased leaf capacity for surface area expansion (Kacperska and Kulesza, 1987), decreased cell wall thickness (Stefanowska et al., 1999), and reduced the content of non-covalently bound pectins in leaf cell walls (Kubacka-Zębalska and Kacperska, 1999).
Pectins are a highly heterogeneous group of polymers composed of homogalacturonans and rhamnogalactouronans. They are proposed to function in the control of cell wall porosity, in cell adhesion, in plant defense responces (Jarvis, 1984; Baron-Epel et al., 1988; Hahn et al., 1989; Vorwerk et al., 2004) and in tissue ability to supercool intracellular water during freezing (Ashworth and Abeles, 1984). It is known that the degree of the pectin methyl-esterification affects the cell wall extension capability and cell growth (Parre and Geitmann, 2005), since de-esterification permits Ca2+ ions to cross-link the acidic pectins to form a semi-rigid pectate gel (Jarvis, 1984; Carpita and Gibeaut, 1993).
Availability of free carboxyl groups for calcium binding depends on the activity of pectin methylesterase (PME, EC 3·1·1·11) in cell walls, which catalyses the hydrolysis of the methyl ester of homogalactouronan, the back-bone of pectin, to release acidic pectins and methanol (Micheli, 2001). The activity substantially influences physiological and physical properties of the cell wall matrix (McQueen-Mason, 1997). Depending on the pH of a medium and the cell wall properties, the enzymes can act randomly, promoting the action of pH-dependent cell wall hydrolases (i.e. endopolygalacturonases) and contributing to cell wall loosening, or it can act linearly, giving rise to blocks of free carboxyl groups that interact with bivalent cations (Ca2+) and thus rigidifying the cell wall (Micheli, 2001; Ezaki et al., 2005). Involvement of PME in numerous plant growth and defence reactions has been recently reported by Pelloux et al. (2007).
Based on the above observations, the present work was aimed at verification of the hypothesis that in cold-acclimated and de-acclimated leaves, differences in cell growth capability and resistance to extracellular freezing may be connected with modifications in the pectin content and their methyl-esterification degree, which, in turn, affects cell wall mechanical properties and cell expansion capability. Since a transient non-lethal freezing reversed the cold-acclimation effects on oil-seed rape leaf cell expansion ability and the cell wall structure and pectin content, the treatment could be used as a tool to verify the above hypothesis. In these studies, two haploid lines of winter oil-seed rape plants, differing in their over-wintering capability and resistance to blackleg fungus [Leptosphaeria maculans (Desm.) Ces. et de Not.], were used. It is known that the ability of non-endodormant plants to survive winter depends not only on their resistance to freezing temperatures but is also associated with plant resistance to pathogen attacks (McKersie and Leshem, 1994). The role of cell wall polysaccharide composition in disease resistance has been recently pointed out (Vorwerk et al., 2004). Interestingly, it has been proposed that the amount of highly methylated pectins and the isoform pattern of the PME activity could serve as potential markers for resistance of potato stems to blackleg disease (Marty et al., 1997). Therefore, the question was raised, whether the genotype-dependent differences in resistance to the pathogen could correlate with differences in cold acclimation- or de-acclimation-induced modifications in leaf extension growth, cell wall properties and pectin status.
MATERIALS AND METHODS
Plant material and growth conditions
Experiments were performed on winter oil-seed rape, Brassica napus L. subsp. oleifera (Delile) Sinskaya (note that the taxonomy of this species is controversial, and it has also been referred to as Brassica napus L. var. oleifera L.). Seedlings of two double haploid lines (DH-1 and DH-2, provided by Resistance Breeding Laboratory, Plant Breeding and Acclimation Institute, Poznań, Poland) were germinated and grown in pots containing a mixture of sand and peat (in proportion 1 : 1, v/v) for 5 weeks at 12 °C (day and night) under a 12 h photoperiod, i.e. under prehardening conditions (Rapacz et al., 2001). The level of photosynthetically active radiation at the top of the plants was approx. 250 µmol m−2 s−1 PPFD (Philips, Poland). Then, the prehardened plants were subjected to acclimation in cold (2 ± 1 °C, day and night) for 3 weeks (cold-acclimated plants); the light conditions were not changed. On the 20th day of cold acclimation, some of the cold-acclimated plants were subjected to a transient sublethal freezing treatment (–5 °C for 6 h in darkness) in a Dual Program Illuminated Incubator 818 (Precision Scientific, USA). After the treatment, the frost-treated plants were transferred back to the cold chamber (+2 °C) for 1 d. On the 22nd day of the experiment, cold-acclimated plants were subjected to the 7 d de-acclimation at 12 °C (day and night), under the same light conditions as described for prehardened plants (de-acclimated plants, DA). Such conditions allow the effective de-acclimation of winter oil-seed plants (Rapacz, 2002).
All analyses were performed on laminas of the 4th leaves (from the plant bottom), sampled on different days of plant cold-acclimation and de-acclimation. The terms ‘leaf’ and ‘lamina’ in the text are used as synonyms.
Determination of growth
The leaves were scanned with the Epson Perfection 4870 PHOTO scanner and the areas of their laminas were measured using WinFOLIA Pro computer program (Regent Instruments, Canada). On each sampling day, ten leaves were used to determine lamina growth and the relative growth rate was calculated.
Determination of freezing tolerance
The frost half killing temperature (LT50) was determined according to Kacperska and Kulesza (1987). Leaf discs (15 mm diameter) were frozen in cryostats at desired temperatures for 2 h (cooling rate 0·2 °C min −1) in ten replicates. The leaf samples were seeded with ice at −1 °C to provoke extracellular ice formation. After freezing, leaf samples were thawed (at a rate of 0·5 °C min−1) and allowed to recover from reversible injury for 20 h at +2 °C in darkness. Then, leakage of intracellular electrolytes from the discs was determined with a conductometer N 572 (MERA ELWRO, Poland) and taken as a measure of freezing injury. The index of injury was calculated according to Flint et al. (1967) and plotted as a function of temperature to estimate the LT50.
Determination of leaf tensile stiffness
Determination of leaf tensile stiffness was chosen to estimate potential changes in the mechanical properties of leaf cell walls during plant cold acclimation and de-acclimation. Providing that alive tissues are maintained at full turgor, changes in their tensile stiffness reflect modifications in cell wall mechanical properties which may be caused by genetic or chemical manipulations of cell wall composition or intermolecular links (Ryden et al., 2003).
Strips (5 mm wide and 20 mm long) were cut off the mid-part of the 4th leaf lamina, from the region located between the 1st and 2nd main lateral midribs below the tip. Before mechanical testing, they were kept in 0·05 M HEPES buffer, pH 7·4 for at least 20 min to regain full turgidity. Then, their thickness was measured at three various locations in the lamina cross-section, at an accuracy of 0·01 mm, using a dial micrometer (model MDAa10; Predom-Termet, Poland). The average thickness, multiplied by the width of the specimen surface, provided the initial cross-sectional area of the tested lamina strip.
Tensile tests of lamina strips were performed at room temperature, using the computer-controlled Instron system (model 4452, High Wycombe, UK) and a load cell of 10 N. The leaf strips were fixed at a gauge length of 10·0 mm between special grips with shallow face serrations to protect a specimen against slippage. Then, they were subjected to extension at a rate of 20 mm min−1 up to failure. The relationship σ(ε) between stress (σ) and strain (ε), was recorded, where the stress was the load per unit of initial area of the specimen cross-section, and the strain was the ratio of the extension to the initial gauge length. The lamina tensile stiffness was calculated using Merlin software, as the maximum slope of the stress–strain curve to the strain axis, which corresponds to the effective Young's modulus, E = σ/ε (Niklas, 1990) at the initial phase of stretching. It is expressed in MPa. Data presented in the figures are the average of at least 20 measurements.
Cell wall preparation
Cell walls from leaf laminas were prepared using a modified method of Wu et al. (1998). Fresh leaf tissues were homogenized in 0·05 M HEPES buffer, pH 6·5, containing a mixture of protease inhibitors (PMSF, aprotinin, bestatin, pepstatin A and leupeptine, 1 mm each), filtered through a miracloth and washed several times with cold water. After air drying, dry matter of crude cell wall preparations was determined by weighing.
Protein determinations
Cell wall proteins were extracted from crude cell wall preparations with 0·05 m HEPES buffer containing 1 m NaCl and a mixture of protease inhibitors (PMSF, aprotinin, bestatin, pepstatin A and leupeptine, 1 mm each), under gentle agitation at 4 °C for 2 h. Protein concentration in the extracts was determined by the method of Bradford (1976), using bovine serum albumin (Sigma, Germany) as a standard.
Protein extracts from cell walls were used to determine PME activity.
Determination of PME activity
Determination of the enzymatic activity was performed according to Richard et al. (1994). Reaction mixtures contained 0·5 % (w/w) highly methylated citrus pectins (Sigma, Germany), 0·2 m NaCl and 0·015 (w/v) Methyl Red (Sigma, Germany) as a pH indicator. Protein sample (50 µL) was added to 950 µL of reaction mixture in a microcuvette. Changes in colour from yellow to red (due to pH lowering during pectin de-esterification) were measured spectrophotometrically at 525 nm (Shimadzu, Japan) for 3 min at 25 °C. A calibration curve was obtained by adding 1–200 nEq H+ to 1 mL of reaction mixture and the enzyme activity was calculated on the basis of cell wall protein. The enzymatic activity was expressed in PME units [one unit was defined as one nanoequivalent of protons (nEH+), released by milligrams of cell wall proteins during 1 min], and was also recalculated per gram of cell wall matter.
In preliminary experiments, the PME activity was measured at three different pH values: 5·0, 6·8 and 8·5 PME activities determined at acidic or alkaline pH were rather low (about 20 % of that observed at pH 6·8) and were not significantly affected by the cold-acclimation or de-acclimation conditions (data not shown). Therefore, only the enzymatic activities determined at neutral pH are reported in the present paper.
Pectin content determination
Pectin isolation was performed as described by Kubacka-Zębalska and Kacperska (1999). The crude cell wall preparations were subjected to 90 % (v/v) DMSO treatment for 24 h at 20 °C to remove starch. Samples were air-dried and weighed. Cell wall aliquots (200 mg) were extracted with a 20-mL mixture of 50 mm CDTA and 50 mm Na-acetate, pH 6·5, for 6 h and then with 50 mm CDTA for 2 h at room temperature. The combined extracts were centrifuged (12 000 g, 15 min) and concentrated by evaporation under reduced pressure. The concentrate was dialysed for 72 h against deionized water (three changes, 4 °C), evaporated to dryness under reduced pressure and weighed. Pectin content was expressed in grams per 100 g of cell wall preparations. In preliminary experiments it has been checked that the method allows for extraction of all the pectins, differing in their solubility in calcium/magnesium chelating or diluted alkaline solutions (Kubacka-Zębalska and Kacperska, 1999).
Determination of degree of pectin methylesterification in muro
The degree of pectin methylation in vivo was determined according to Willats et al. (2000), using the monoclonal antibodies JIM5 and JIM7 (PlantProbes, Leeds, UK). The epitopes recognized by JIM5 or JIM7 have been shown to be regions of de-metylesterified or methylesterified homogalacturonans, respectively (Clausen et al., 2003). Hand-cut sections of fresh leaves were placed immediately in the fixative consisting of 0·5 % glutaraldehyde with 4 % paraformaldehyde in Na-phosphate buffer, pH 7·2. Following 2 h of fixation, sections were blocked in TTBS buffer with 2 % of non-fat milk (2 % M/TTBS) for 1 h and then incubated for 12 h at 4 °C in primary antibody diluted in the same buffer. JIM5 and JIM7 were used as 5-fold dilutions of hybridoma supernatants. Sections were washed in TTBS prior to incubation for 1 h in secondary antibody, conjugated with Alexa 488 fluorochrome (Molecular Probes, Leeds, UK). Sections were mounted in anti-fade agent Vectashield (Vector, USA), examined under a fluorescent microscope and photographed (Leica DMI 6000).
Statistics
All experiments were repeated at least three times. In each experiment, three independent samples were collected on each sampling day, unless indicated otherwise. The presented values are the means of at least nine determinations ± standard deviations. Correlation coefficients were calculated using STATISTICA 7·0 PL software (Statsoft, USA) at 0·05 and 0·01 probability levels.
RESULTS
Growth characteristics
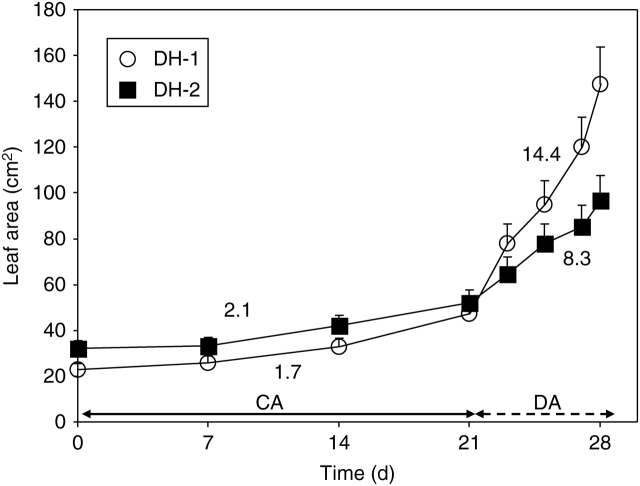
During plant acclimation in the cold, a slow expansion of leaf areas was noted (Fig. 1), the growth rates for the DH-1 and DH-2 leaves being similar.
Fig. 1.
Leaf growth during plant cold-acclimation (CA, first 21 d) and de-acclimation (DA, next 7 d) conditions (see Methods, ‘Plant material and growth conditions’). Numbers given in the figure show the relative growth rate values for the lines studied, DH-1 and DH-2. Error bars are ± s.d.
During de-acclimation, the genotype-dependent differences in leaf growth rates were observed (Fig. 1). In the DH-1 line, a substantial promotion of lamina expansion was already noted after 2 d of de-acclimation, and 6 d later expansion of the leaf area reached a level almost 4-fold higher than that noted in cold-acclimated leaves. In the DH-2 line, the rate of leaf expansion was much lower and at the end of the experiment, the leaf area reached a 30 % lower level than that in the DH-1 plants.
Freezing resistance
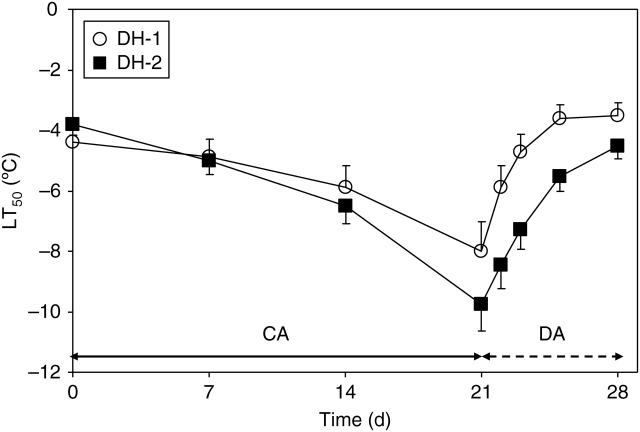
Three-week acclimation of oil-seed rape plants in the cold (+2 °C) resulted in a gradual increase in the resistance of leaves to extracellular freezing, as indicated by changes in LT50 (Fig. 2). The resistance increased more rapidly in the DH-2 line and reached –9 °C and at the end of the acclimation period. At that time, the resistance of the DH-1 line was lower by about 2K.
Fig. 2.
Changes in freezing resistance of leaves of DH-1 and DH-2 lines over the course of plant cold acclimation (CA) and de-acclimation (DA). Error bars are ± s.d.
During plant de-acclimation at 12 °C, a rapid decrease in freezing resistance was observed in the leaves of both genotypes studied. In the DH-1 leaves, freezing resistance returned to the level observed in non-acclimated plants after 4 d. In more frost-resistant DH-2 leaves, such a level of freezing resistance was observed as late as after 7 d.
Leaf tensile stiffness
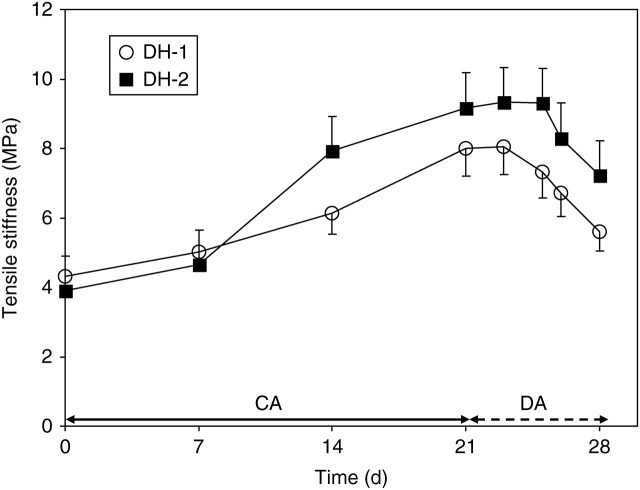
Tensile stiffness (effective Young's modulus) of fully hydrated leaves increased during a 3-week acclimation in the cold (Fig. 3). The increase was more pronounced in the DH-2 than in the DH-1 leaves. After 2 weeks, the stiffness of DH-2 leaves reached an approximately 20 % higher level than that in the DH-1 plants.
Fig. 3.
Changes in stiffness of leaf lamina of DH-1 and DH-2 lines over the course of plant acclimation (CA) and de-acclimation (DA). Error bars are ± s.d.
During de-acclimation, the leaf stiffness markedly decreased (Fig. 3). In the DH-2 leaves, the decrease was delayed for 2 d as compared with the DH-1 ones.
Cell wall and pectin contents
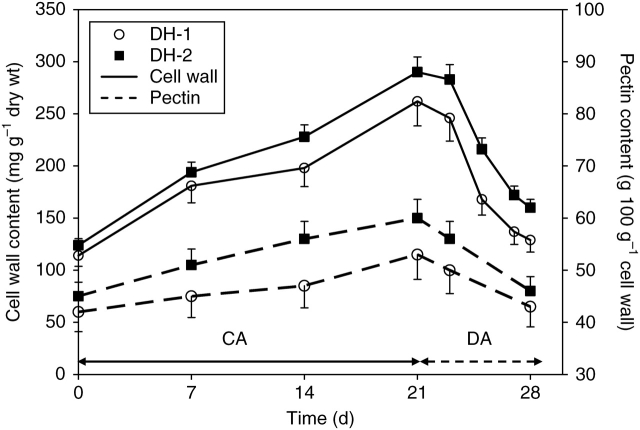
Acclimation of plants in the cold resulted in a marked, 2-fold increase of cell wall content in the leaves of both DH lines (Fig. 4). The effect was less pronounced in the DH-1 leaves. Pectin content in cell walls increased during plant acclimation in the cold, especially in the DH-2 leaves (Fig. 4). At the end of cold acclimation, the content of pectins in DH-2 cell walls was higher by about 20 % in comparison to the DH-1 samples.
Fig. 4.
Changes in cell wall and pectin contents in leaves of DH-1 and DH-2 lines during plant cold-acclimation (CA) and de-acclimation (DA). Pectin content is shown as a percentage of cell wall amount. Error bars are ± s.d.
During plant de-acclimation, cell wall and pectin contents decreased to the original levels in both studied lines.
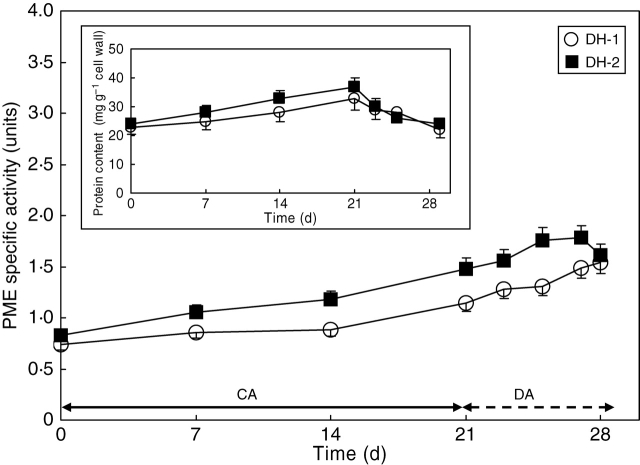
PME activity
The enzyme activity increased substantially during cold acclimation, especially in DH-2 leaves. After 3 weeks of plant growth in the cold, the level of the enzyme activity increased by about 90 % and 70 % in DH-2 and DH-1 leaves, respectively.
During the 6 d of plant de-acclimation, a further increase of the PME-specific activity was observed in the DH-1 line (Fig. 5). In the DH-2 genotype, the enzyme activity started to decrease after 4 d of plant de-acclimation.
Fig. 5.
Changes in PME activity of lines DH-1 and DH-2 over the course of plant cold-acclimation (CA) and de-acclimation (DA). Insert: changes in the cell wall-bound protein content. Error bars are ± s.d.
The total activity of PME (calculated per unit of cell wall dry matter) showed a similar pattern of modifications in the cold-acclimated and de-acclimated leaves of the genotypes studied (data not shown).
Degree of pectin esterification
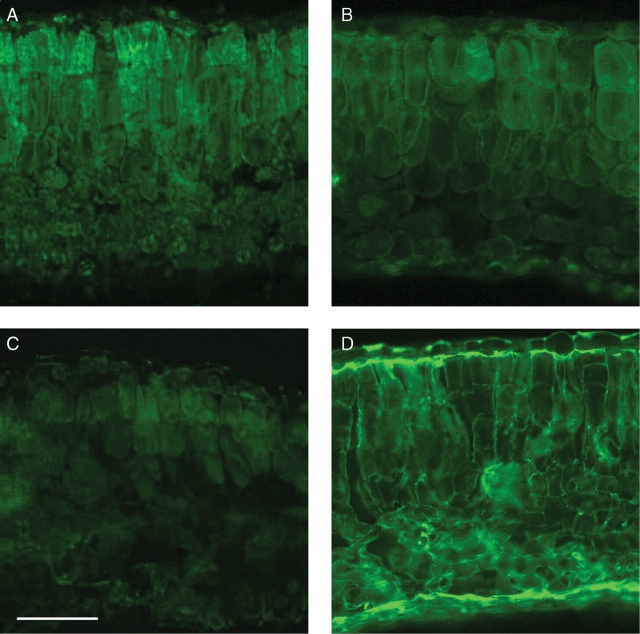
Immunochemical studies showed that the level of methyl-esterification of pectins, as indicated by green fluorescence of the JIM7-treated leaf sections, was relatively high in the DH-2 leaves not subjected to cold-acclimation (Fig. 6A). In the respective leaves incubated with JIM5 (the antibody detecting de-esterified pectins), fluorescence was weak and localized preferentially in walls of palisade cells (Fig. 6C). After 3 weeks of plant acclimation in the cold, the reverse situation was observed: the JIM-7-dependent fluorescence markedly decreased (Fig. 6B), whereas that of JIM5 increased markedly (Fig. 6D). A similar pattern of the cold acclimation- and de-acclimation-induced modifications in the JIM7- and JIM 5-dependent fluorescence was observed in the DH-1 leaves (data not shown). All of these observations indicate that a degree of pectin de-methylation increased markedly in the cold-acclimated leaves. Interestingly, the JIM5-dependent fluorescence was located preferentially in the inner cell walls of upper and lower epidermis (Fig. 6D).
Fig. 6.
JIM7 (A and B) and JIM5 (C and D) -dependent immunofluorescence in non-acclimated (A and C) and cold-acclimated (B and D) leaves. Scale bar = 100 µm.
Leaf tensile stiffness, cell wall and pectin contents, and PME activity in plants subjected to a transient frost
The treatment resulted in a 20–30 % decrease in leaf tensile stiffness as well as in cell wall and pectin contents in the leaves of both lines studied (Table 1). The freezing-induced alterations were transient and disappeared 3 d later, after transferring the plants back to the cold acclimation conditions (data not shown). The specific activity of PME was practically not affected by the treatment (Table 1).
Table 1.
Effects of a transient frost on leaf tensile stiffness, cell wall and pectin contents, and PME activity
| Trait | DH-1 line | DH-2 line |
|---|---|---|
| Stiffness (MPa) | 6·23 (78%) | 6·34 (69%) |
| Cell wall content (mg g −1 dry wt) | 209 (79%) | 221 (76%) |
| Pectin content (mg g −1 cell wall) | 400 (75%) | 420 (70%) |
| PME activity (nEH+ mg−1 protein min−1) | 1·2 (97%) | 1·5 (91%) |
The numbers in brackets indicate freezing-induced changes as a percentage of the respective values obtained for cold-acclimated leaves, which were taken as 100 %.
DISCUSSION
Effects of cold acclimation and transient freezing treatment
The experiments performed show that growing plants in the cold has brought about a marked retardation of leaf lamina expansion, with the rate of expansion being similar in both the genotypes studied (Fig. 1). This was followed by the development of leaf resistance to extracellular freezing (Fig. 2). The hardening process was more effective in the DH-2 than in the DH-1 genotype. The observation that inhibition of leaf area expansion precedes development of freezing tolerance in cold-acclimated plants is in line with other findings made for oil-seed rape (e.g. Kacperska and Szaniawski, 1993). The observations collectively indicate that cell growth and development of tolerance to extracellular freezing are under the control of different cold-induced mechanisms.
Development of leaf tolerance to extracellular freezing in cold-acclimated winter oil-seed rape plants (Fig. 1) was found to be associated with modifications in leaf tensile stiffness (Fig. 3). Statistical analysis showed that the correlation between resistance of leaves to extracellular freezing and leaf stiffness was highly significant in both genotypes examined (Table 2).
Table 2.
Correlations between the studied leaf characteristics and lamina stiffness
| Traits | Treatment | DH1 line | DH2 line |
|---|---|---|---|
| Freezing tolerance and lamina stiffness | CA | ** | ** |
| DA | n.s. | n.s. | |
| Cell wall content and lamina stiffness | CA | * | * |
| DA | n.s. | n.s. | |
| Pectin content and lamina stiffness | CA | ** | * |
| DA | n.s. | n.s. | |
| PME activity and lamina stiffness | CA | * | * |
| DA | n.s. | n.s. |
Correlation coefficients were calculated using STATISTICA 7·0 PL software (Statsoft, USA).
CA and DA, cold-acclimated and de-acclimated leaves, respectively; DH1 and DH2 lines, less or more pathogen-resistant genotypes, respectively.
**, significant at P = 0·01; s, significant at P = 0·05; n.s., non-significant at P = 0·05.
In the studies concerning the role of cell wall mechanical properties in the control of freezing-induced phenomena, rigid leaves of broadleaf evergreen species, pith and xylem ray parenchyma cells of cold hardy, able to supercool, grape twigs (Rajashekar and Burke, 1982, 1996) and xylem ray parenchyma cells in softwood species (Fujikawa et al., 1999) have been used. In herbaceous leaves, which were the object of the present studies, primary cell walls are held in tension created by turgor pressure and cell wall rigidity plays a less important role in coping with mechanical stresses (Jarvis and McCann, 2000). As far as is known, the data presented in the current work show for the first time that tensile stiffness of such leaves could be modified by cold treatment. Extracellular freezing in these leaves results in the dehydration-driven lateral collapse of the epidermal and mesophyll cells that become flattened (Pearce, 1988). It seems, therefore, that the cold-dependent increase in leaf tensile stiffness observed in the current work, may reflect the presence of a mechanism that protects cells against cell volume collapse (Pearce, 1988), cell wall deformation (Fujikawa et al., 1999) and cytorrhysis (Yamada et al., 2002), which are all brought about by freezing-induced cell dehydration. Development of negative pressures (tensions) in leaf cells of herbaceous plants can also be predicted (Rajashekar and Lafta, 1996), but this supposition needs experimental verification.
The present experiments show that increases in leaf tensile stiffness, observed in cold acclimated winter oil-seed rape leaves, were associated with increased contents of the CDTA-soluble pectins in cell walls (Fig. 4), with the correlation coefficient between these two leaf characteristics being significant for both the genotypes studied (Table 2). This observation is in a full accordance with our previous findings indicating that the increased cell wall content in these leaves was associated with higher levels of pectins (Kubacka-Zębalska and Kacperska, 1999). Changes in cell wall and pectin contents were previously found to be associated with a marked increase in the wall thickness in epidermis cells and with a promotion of palisade and parenchyma cell expansion in the plane perpendicular to the leaf surface (Stefanowska et al., 1999). These modifications may play an important role in withstanding both tensile and compressive stresses, brought in the plane of the wall by rain, snow loads, and winds during the late autumn and winter (Żebrowski, 2003). Therefore we want to suggest, that the cold-dependent modifications in cell wall structure, associated with increased pectin content, not only protect cells against freezing-induced damage, but also reflect the plant acclimation strategy, ascribed so far for the secondary, lignified tissues in plant organs exposed to the mechanical stresses evoked by environmental factors (Jarvis and McCann, 2000).
Results of the present experiments showed that the activity of pectin methyl-esterase increased in cell walls of cold-acclimated leaves (Fig. 5), with the increase being much lower in the DH-1 genotype. The latter observation may point to a different genotype-dependent sensitivity of the enzyme or its isoforms to low temperature, as shown for the PMEs from chicory roots (Thonar et al., 2006). It is interesting that the PME activity in chicory roots was generally repressed in tissues sampled at the end of a growing season when the air temperature dropped below 5 °C. On the other hand, a high promotion of the PME gene expression was observed in cold-treated arabidopsis pollen (Lee and Lee, 2003). Obviously, searching for the genotype- or tissue-dependent mechanisms responsible for the temperature control of PME activity requires further studies that were not the object of the experiments presented here.
Higher activity of the enzyme in the cold-grown leaves was reflected by a lower level of methyl-esterified pectins, concomitant with a higher level of de-esterified pectins in the cold-acclimated DH-2 leaves (Fig. 6). Low-methylated pectins contribute to the overall rigidity of the cell wall (Fenwick et al., 1997). The degree of methyl-esterification was found to play the key role in epidermal cell elongation in pea internodes (Fujino and Itoh, 1998) and in pollen tube growth (Parre and Geitman, 2005). Promotion of PME activity in transgenic tobacco plants was shown to induce dwarfism in tobacco plants (Hasunuma et al., 2004) and one of the pectin methylesterase isoforms appeared to be involved in stiffening of cell walls in flax (Al-Qsous et al., 2004). Therefore, it is proposed that increased activity of PME in the cold-acclimated leaves has led to the increased cell wall rigidity, reflected by the increased leaf stiffness. This, in turn, allowed the increase in resistance of leaf cells to dehydration brought about by extracellular freezing and was the reason for the inhibition of leaf lamina expansion growth. The calcium-binding ability of de-esterified pectins may also allow a reduction of pore sizes, which has been observed in the walls of cold-acclimated cells (Rajashekar and Lafta, 1996). No direct evidence of the role of calcium pectate gel in reduction in cell wall pore sizes has been provided so far, but the suggestion is supported by the observation that the pore sizes in non-graminaceous plant cell walls were rapidly decreased by borate ester cross-linking of pectic polysaccharides (Fleischer et al., 1999). Reduced pore sizes are known to contribute to the formation of an effective barrier against the propagation of extracellular ice in cell walls and to the avoidance of intracellular freezing, as shown for such freezing-resistant plant tissues as the leaves of orchard grass, Arabidopsis thaliana and the cortical tissues of mulberry (Yamada et al., 2002). Modification of the wall porosity may also facilitate supercooling since water in small pores freezes at lower temperature than bulk water (Ashworth and Abeles, 1984). Pore sizes appeared to be an important element in the phenomenon of deep supercooling in the xylem parenchyma of peach (Wisniewski et al., 1991; Jones et al., 2000). A higher level of un-esterified pectins was observed in the cell walls of supercooling peach buds, whereas in the cell walls of non-supercooling buds, more methylesterified pectins were found (Wisniewski and Davis, 1995). Increased supercooling ability has also been observed in the cold-acclimated oil-seed rape leaves (Kulesza et al., 1986).
Exposure of the cold-acclimated leaves to a transient freezing brought about approx. 20–30 % decreases in the leaf tensile stiffness and cell wall and pectin contents (Table 1). These observations are in line with previous findings indicating that a transient freezing destabilizes the cell wall structure of the cold-acclimated winter oil-seed rape leaves (Kubacka-Zębalska and Kacperska, 1999; Stefanowska et al. 1999). The freezing-induced decrease of non-covalently bound pectins in cold-acclimated oil-seed rape leaves was associated with the decrease of amorphous structure in the inner layers of epidermal cell walls (Stefanowska et al., 1999). The decrease of the amorphous matrix may allow wall relaxation in the load-bearing region (Carpita and Gibeaut, 1993) and results in a reduction in turgor pressure and water potential, thereby enabling cells to take up water and to expand physically (Cosgrove, 1997), as actually observed in previous studies (Kacperska and Kulesza, 1987). Therefore, the effects of the freezing treatment on cell wall and pectin contents in cold-acclimated leaves provide an additional argument for the supposition that alterations in the cell wall pectin status may be an important element in the control of cell expansion growth.
De-acclimation effects
During plant de-acclimation at 12 °C, the remarkable increase in the leaf expansion capability, paralleled by the decrease in leaf resistance to freezing, was observed, especially in the DH-1 leaves (compare respective data in Figs 1 and 2). This observation is in line with the conviction that there is an inverse relationship between growth capability and freezing resistance in de-acclimated plant organs (Kalberer et al., 2006). It has been suggested that initiation of elongation growth during de-acclimation of oil-seed rape plants triggers loss of frost resistance (Rapacz et al., 2001).
The present observations indicate that the de-acclimation related events are under the control of mechanisms different from those involved in the cold-acclimation process. This suggestion is based on the following observations: (a) de-acclimation-induced modifications, such as resumption of leaf growth (Fig. 1), decrease in leaf freezing resistance (Fig. 2), reduction in leaf stiffness (Fig. 3) and reduced amounts of cell wall and pectin contents (Fig. 4), occurred in a much shorter time than those observed during plant acclimation in the cold (in 7 d or in 21 d, respectively); (b) much higher leaf expansion rates in the DH-1 plants (Fig. 1) were not associated with more rapid losses in their resistance to freezing (Fig. 2); (c) no correlation has been found among modifications in leaf stiffness, cell wall and pectin contents in de-acclimated leaves (Table 2); (d) a decrease in leaf stiffness was not associated with the decreased PME activity (Fig. 5 and Table 2).
Different kinetics of acclimation and de-acclimation processes in plants are commonly observed and thought to be related to divergent energy requirements for structural and functional adjustments of plants grown at low or warm ambient temperatures (Kalberer et al., 2006). Acclimation involves changes in structure and function, necessitating large amounts of energy (Browse and Lange, 2004). De-acclimation, however, seems to be a relatively less energy-intensive process and may be fuelled by the catabolism of metabolites accumulated during cold acclimation (Kalberer et al., 2006). In agreement with this suggestion, a substantial decrease in protein content in the cell walls of de-acclimated leaves was observed (Figs 4 and 5, insert). It is interesting that similar effects were induced by a short transient freezing (Table 1). In the prefrozen cold-acclimated winter oil-seed rape leaves, the promotion of the polysaccharide-degrading enzyme was also observed (Sawicka and Kacperska, 1995). It seems that products of cell wall degradation may be used to produce energy and synthesize other cell components during rapid tissue growth during plant de-acclimatiion (Fig. 1) or during cell recovery from freezing stress.
Results of the present experiments show that an increase in the environmental temperature did not affect the specific activity of PME in the de-acclimated leaves, despite a rapid and marked decrease in the total cell wall-bound protein content in these leaves (Fig. 5). This may indicate that either the turnover of the enzyme is relatively insensitive to a change in environmental temperature, or that a preferential synthesis of certain PME isoforms of different sensitivity to ambient temperature took place during plant de-acclimation. The solution to this problem requires further study.
The decrease in leaf stiffness and cell wall and pectin contents, observed after 2 d or 4 d of de-acclimation of DH-1 or DH-2 plants, respectively, was associated with the promotion of leaf expansion (Fig. 1). At that time, however, the PME activity in the cell walls continued to increase or was maintained at a high level (Fig. 5). This may mean that either the mode of action of the enzyme or its isoforms has been changed by decreased pH of the cell walls (Micheli, 2001; Pelloux et al., 2007) in response to an increased environmental temperature (Brauer et al., 1991) or some other mechanisms responsible for wall-loosening were put into operation (Cosgrove, 1999). In fact, a high PME activity may prepare molecules for further degradation by combined activities of polygalactouronases, pectin esterases and pectate lyases and, in this way, the enzyme may contribute to cell wall loosening and promotion of cell enlargement (Lee and Lee, 2003).
Cell wall modifications and plant resistance to the pathogen
In the two winter oil-seed rape genotypes studied, which differ in their resistance to blackleg fungus, a lower resistance of plants to the pathogen was reflected by lower responsiveness of leaves to the cold in terms of leaf tolerance to extracellular freezing (Fig. 2), leaf stiffness (Fig. 3), cell wall and pectin contents (Fig. 4), and the cold-dependent modifications in PME activity (Fig. 5). These observations indicate that tissue resistance to a pathogen may rely on the effectiveness of low temperature in induction of chemical and structural changes in the leaf cell wall properties, especially in modifications of pectin content and composition.
In summary, the present experiments show that pectins are involved in the modification of mechanical properties of leaf cell walls during plant cold-acclimation and de-acclimation of oil-seed rape plants of different resistance to fungi pathogens. In this way, they play an important role in the control of expansion growth and development of frost tolerance of the leaves. A high activity of pectin methyl esterase in the leaves seems to result in two different effects, dependent on the cold-acclimation status of plants. In cold-acclimated leaves, the enzyme contributes to the increased rigidity of cell walls, reflected by the increased leaf stiffness, resulting in the limited expansion ability of leaf laminas. In de-acclimated leaves, the enzyme may prepare pectin molecules for degradation by other enzymes, contributing to cell wall loosening and promotion of growth. It seems that further studies on the mechanisms controlling PME activity may add important information to the knowledge of the molecular bases of cell growth and resistance to abiotic stresses.
ACKNOWLEDGEMENTS
This work was financially supported by grant No. 2 P06A 018 26 from the Ministry of Science and Higher Education of Poland. The authors are very grateful to Jaroslaw Korczyński (Laboratory of Confocal Microscopy, Nencki Institute of Experimental Biology) and Agnieszka Abratowska for their valuable help with the immunolocalization of pectin. We also thank Mrs Barbara Dobrska and Urszula Daciuk for their skillful assistance.
LITERATURE CITED
- Al-Qsous S, Carpentier E, Klein-Eude D, Burel C, Mareck A, Dauchel H, et al. Idetification and isolation of a pectin methylesterase isoform that could be involved in flax cell wall stiffening. Planta. 2004;219:369–378. doi: 10.1007/s00425-004-1246-1. [DOI] [PubMed] [Google Scholar]
- Ashworth EN, Abeles FB. Freezing behaviour of water in small pores and the possible role in the freezing of plant tissues. Plant Physiology. 1984;76:201–204. doi: 10.1104/pp.76.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Epel O, Gharyal PK, Schindler M. Pectins as mediators of wall porosity in soybean cells. Planta. 1988;175:389–395. doi: 10.1007/BF00396345. [DOI] [PubMed] [Google Scholar]
- Bartolo ME, Wallner SJ, Ketchum RE. Comparison of freezing tolerance in cultured plant cells and their respective protoplasts. Cryobiology. 1987;24:53–57. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:1151–1154. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brauer D, Loper M, Schubert C, Tu SI. Effects of temperature on the coupled activities of the vanadate-sensitive proton pump from maize root microsomes. Plant Physiology. 1991;96:1114–1117. doi: 10.1104/pp.96.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Lange BM. Counting the cost of a cold-blooded life: metabolomics of cold acclimation. Proceedings of the National Academy of Sciences of the USA; 2004. pp. 14996–14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Clausen MH, Willats WGT, Knox JP. Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydrate Research. 2003;338:1797–1800. doi: 10.1016/s0008-6215(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Relaxation in a high-stress environment: the molecular bases of extensible cell wall and cell enlargement. The Plant Cell. 1997;9:1031–1041. doi: 10.1105/tpc.9.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Annual Review of Plant Physiology Plant Molecular Biology. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- Ezaki N, Kido N, Takahashi K, Katou K. The role of wall Ca2+ in the regulation of wall extensibility during the acid-induced extension of soybean hypocotyl cell walls. Plant and Cell Physiology. 2005;46:1831–1838. doi: 10.1093/pcp/pci199. [DOI] [PubMed] [Google Scholar]
- Fenwick KM, Jarvis MC, Apperley DC. Estimation of polymer rigidity in cell walls of growing and nongrowing celery collenchyma by solid-state nuclear magnetic resonance in vivo. Plant Physiology. 1997;115:587–592. doi: 10.1104/pp.115.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R. The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiology. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint HL, Boyce BR, Brattie DJ. Index of injury – a useful expression of freezing injury to plant tissues as determined by the electrolytic method. Canadian Journal of Plant Science. 1967;47:229–239. [Google Scholar]
- Fujikawa S, Jitsuyama Y, Kuroda K. Determination of the role of cold acclimation-induced diverse changes in plant cells from the viewpoint of avoidance of freezing injury. Journal of Plant Research. 1999;112:237–244. [Google Scholar]
- Fujino T, Itoh T. Changes in pectin structure during epidermal cell elongation in pea (Pisum sativum) and its implications for cell wall architecture. Plant and Cell Physiology. 1998;39:1315–1323. [Google Scholar]
- Hahn MG, Bucheli P, Cervone F, Doares SH, O'Neill RA, Darvill A, Albersheim P. Roles of cell wall constituents in plant–pathogen interactions. In: Kosuge T, Nester EW, editors. Plant–microbe interactions: molecular and genetic perspectives. Vol. 3. New York, NY: McGraw-Hill; 1989. pp. 131–181. [Google Scholar]
- Hasunuma T, Fukusaki E, Kobayashi A. Expression of fungal pectin methylesterase in transgenic tobacco leads to alteration in cell wall metabolism and a dwarf phenotype. Journal of Biotechnology. 2004;111:241–251. doi: 10.1016/j.jbiotec.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Jarvis MC. Structure and properties of pectin gels in plant cell walls. Plant, Cell and Environment. 1984;7:153–164. [Google Scholar]
- Jarvis MC, McCann C. Macromolecular biophysics of the plant cell wall: concepts and methodology. Plant Physiology and Biochemistry. 2000;38:1–13. [Google Scholar]
- Jones KS, MacKersie BD, Paroschy J. Prevention of ice propagation by permeability barriers in bud axes of Vitis vinifera. Canadian Journal of Botany. 2000;78:3–9. [Google Scholar]
- Kacperska A. Metabolic consequences of low temperature stress in chilling-insensitive plants. In: Li PH, editor. Low temperature stress physiology in crops. Boca Raton, FL: CRC Press; 1989. pp. 27–40. [Google Scholar]
- Kacperska A, Kulesza L. Frost resistance of winter rape leaves as related to changes in water potential and growth capability. Physiologia Plantarum. 1987;71:483–488. [Google Scholar]
- Kacperska A, Szaniawski RK. Frost resistance and water status of winter rape leaves as affected by differential shoot:root temperature. Physiologia Plantarum. 1993;89:775–782. [Google Scholar]
- Kalberer SR, Wisniewski M, Arora R. Deacclimation and reacclimation of cold-hardy plants: current understanding and emerging concepts. Plant Science. 2006;171:3–16. [Google Scholar]
- Kubacka-Zębalska M, Kacperska A. Low temperature-induced modifications of cell wall content and polysaccharide composition in leaves of winter oilseed rape (Brassica napus L. var. oleifera L.) Plant Science. 1999;148:59–67. [Google Scholar]
- Kulesza L, Pukacki P, Kacperska A. Ice formation and frost killing temperatures as related to cold acclimation of winter rape plants. Acta Physiologiae Plantarum. 1986;8:185–193. [Google Scholar]
- Lee JY, Lee DH. Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiology. 2003;132:517–529. doi: 10.1104/pp.103.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J. Responses of plants to environmental stresses. Vol. I.Chilling, freezing, and high temperature stresses. New York, NY: Academic Press; 1980. [Google Scholar]
- McKersie BD, Leshem YY. Stress and stress coping in cultivated plants. Dordrecht: Kluwer Academic; 1994. pp. 104–131. [Google Scholar]
- McQueen-Mason S. Plant cell walls and the control of growth. Biochemical Society Transitions. 1997;25:204–214. doi: 10.1042/bst0250204. [DOI] [PubMed] [Google Scholar]
- Maciejewska U, Kacperska A. Changes in the level of oxidized and reduced pyridine nucleotides during cold acclimation of winter rape plants. Physiologia Plantarum. 1987;69:687–691. [Google Scholar]
- Marty P, Jouan B, Bertheau Y, Vian B, Goldberg R. Charge density in stem cell walls of Solanum tuberosum genotypes and susceptibility to blackleg. Phytochemistry. 1997;44:1435–1441. [Google Scholar]
- Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends in Plant Science. 2001;6:414–416. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- Murai M, Yoshida S. Evidence for the cell wall involvement in temporal changes in freezing tolerance of Jerusalem artichoke (Helianthus tuberosus L.) tubers during cold acclimation. Plant and Cell Physiology. 1998;39:97–105. doi: 10.1093/oxfordjournals.pcp.a029295. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. The mechanical significance of clasping leaf sheaths in grasses: evidence from two cultivars of Avena sativa. Annals of Botany. 1990;65:505–512. [Google Scholar]
- Parre E, Geitmann A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta. 2005;220:582–592. doi: 10.1007/s00425-004-1368-5. [DOI] [PubMed] [Google Scholar]
- Pearce RS. Extracellular ice formation and cell shape in frost-stressed cereal leaves: a low-temperature scanning-electron-microscopy study. Planta. 1988;175:313–324. doi: 10.1007/BF00396336. [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rusterucci C, Mellerowicz EJ. New insight into pectin methylesterase structure and function. Trends in Plant Science. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Rajashekar CB, Burke MJ. Liquid water during slow freezing based on cell water relations and limited experimental testing. In: Li PH, Sakai A, editors. Plant cold hardiness and freezing stress. Vol. 2. New York, NY: Academic Press; 1982. pp. 211–220. [Google Scholar]
- Rajashekar CB, Burke MJ. Freezing characteristics of rigid plant tissues. Plant Physiology. 1996;111:597–603. doi: 10.1104/pp.111.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekar CB, Lafta A. Cell-wall changes and cell tension in response to cold acclimation and exogenous abscisic acid in leaves and cell cultures. Plant Physiology. 1996;111:605–612. doi: 10.1104/pp.111.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapacz M. Cold-deacclimation of oilseed rape (Brassica napus var. oleifera) in response to fluctuating temperatures and photoperiod. Annals of Botany. 2002;89:543–549. doi: 10.1093/aob/mcf090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapacz M, Tokarz K, Janowiak F. The initiation of elongation growth during long-term low- temperature stay of spring-type oilseed rape may trigger loss of frost resistance and changes in photosynthetic apparatus. Plant Science. 2001;161:221–230. doi: 10.1016/s0168-9452(00)00341-1. [DOI] [PubMed] [Google Scholar]
- Richard L, Qin LX, Gadal P, Goldberg R. Molecular cloning and characterization of a putative pectin methylesterase cDNA in Arabidopsis thaliana (L.) FEBS Letters. 1994;355:135–139. doi: 10.1016/0014-5793(94)01187-7. [DOI] [PubMed] [Google Scholar]
- Ryden P, Sugimoto-Shirasu K, Smith AC, Findlay K, Reiter WD, McCann MC. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiology. 2003;132:1–8. doi: 10.1104/pp.103.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicka T, Kacperska A. Soluble and cell wall-associated β-galactosidases from cold-grown winter rape (Brassica napus L. var. oleifera L.) leaves. Journal of Plant Physiology. 1995;145:357–362. [Google Scholar]
- Stefanowska M, Kuraś M, Kubacka-Zębalska M, Kacperska A. Low temperature affects pattern of leaf growth and structure of cell walls in winter oilseed rape (Brassica napus L., var. oleifera) Annals of Botany. 1999;84:313–319. [Google Scholar]
- Taiz L. Plant cell expansion: regulation of cell wall mechanical properties. Annual Review in Plant Physiology. 1984;35:585–657. [Google Scholar]
- Tao D, Li PH, Carter JV. Role of cell wall in freezing tolerance of cultured potato cells and their protoplasts. Physiologia Plantarum. 1983;58:527–532. [Google Scholar]
- Thonar C, Liners F, Van Cutsem P. Polymorphism and modulation of cell wall esterase enzyme activities in the chicory root during the growing season. Journal of Experimental Botany. 2006;57:81–89. doi: 10.1093/jxb/erj006. [DOI] [PubMed] [Google Scholar]
- Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends in Plant Science. 2004;9:203–209. doi: 10.1016/j.tplants.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Limberg G, Buchholt HC, van Alebeek G-J, Benen J, Christensen TMIE, et al. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides and enzymatic degradation. Carbohydrate Research. 2000;327:309–320. doi: 10.1016/s0008-6215(00)00039-2. [DOI] [PubMed] [Google Scholar]
- Wisniewski M, Davis G. Immunogold localization of pectins and glycoproteins in tissues of peach with reference to deep supercooling. Trees – Structure and Function. 1995;9:253–260. [Google Scholar]
- Wisniewski M, Davis G, Schaffer K. Mediation of deep supercooling of peach and dogwood by enzymatic modifications in cell-wall structure. Planta. 1991;184:254–260. doi: 10.1007/BF00197955. [DOI] [PubMed] [Google Scholar]
- Wu Y, Sharp RE, Durachko DM, Cosgrove DJ. Growth maintenance of the maize primary root at low water potentials involves increases in cell-wall extension properties, expansin activity, and wall susceptibility to expansins. Plant Physiology. 1998;111:765–772. doi: 10.1104/pp.111.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Kuroda K, Jitsuyama Y, Takezawa D, Arakawa K, Fujikawa S. Roles of the plasma membrane and the cell wall in the responses of plant cells to freezing. Planta. 2002;215:770–778. doi: 10.1007/s00425-002-0814-5. [DOI] [PubMed] [Google Scholar]
- Żebrowski J. Pandalai SG, editor. Biomechanical design of non-woody plant organs. Recent research development in plant biology. Reseach Signpost. 2003;Vol. 3:155–188. [Google Scholar]