Abstract
Background and Aims
Previous studies have suggested that velamen characteristics are useful as taxonomic markers in Orchidaceae. Members of tribe Cranichideae have been assigned to two velamen types constructed based on combinations of characters such as the presence of secondary cell-wall thickenings and pores. However, such characters have not been analysed on an individual basis in explicit cladistic analyses.
Methods
The micromorphology of roots of 26 species of Cranichideae was examined through scanning electron microscopy and light microscopy, scoring the variation and distribution of four characters: number of velamen cell layers, velamen cell-wall thickenings, presence and type of tilosomes, and supraendodermal spaces. The last three characters were analysed cladistically in combination with DNA sequence data of plastid trnK/matK and nuclear ribosomal internal transcribed spacer (ITS) regions and optimized on the resulting phylogenetic tree.
Key Results
Thickenings of velamen cell walls group Prescottiinae with Spiranthinae, whereas tilosomes, documented here for the first time in Cranichideae, provide an unambiguous synapomorphy for subtribe Spiranthinae. Supraendodermal spaces occur mostly in species dwelling in seasonally dry habitats and appear to have evolved three times.
Conclusions
Three of the four structural characters assessed are phylogenetically informative, marking monophyletic groups recovered in the combined molecular–morphological analysis. This study highlights the need for conducting character-based structural studies to overcome analytical shortcomings of the typological approach.
Key words: Cranichideae, Cranichidinae, ITS, Orchidaceae, phylogeny, Prescottiinae, trnK/matK, root anatomy, Spiranthinae, tilosomes, velamen
INTRODUCTION
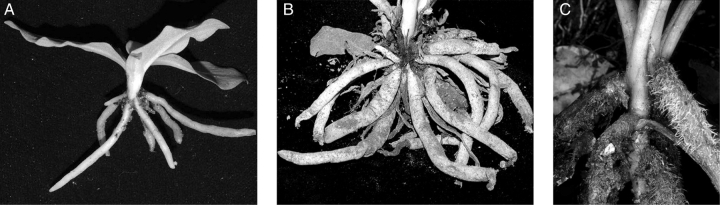
Tribe Cranichideae encompasses about 90 genera and 1600 species of mainly geophytic orchids found in all continents except Antarctica, but over 95 % of such diversity is confined to tropical and subtropical regions (Dressler, 1993; Chase et al., 2003; Pridgeon et al., 2003). Plants of most species in this group have fleshy roots, which are either fasciculate or produced from the nodes of a creeping or subterranean rhizome. In taxa with fasciculate roots, such as most members of subtribes Cranichidinae, Prescottiinae and Spiranthinae, the soft, non-articulate leaves are typically arranged in a basal rosette and are usually drought-deciduous, lasting only a few months after which they wither with all other aerial parts. The roots function as perennating organs that sustain the new shoot during the next growth season (Fig. 1A–C; Salazar, 2003; Hágsater et al., 2005). Members of Manniellinae and some Prescottiinae that live in permanently moist or wet tropical forests and cloud forests form a rosette that persists for several growth seasons and the base of the rosette is held a few centimetres above the ground by thick stilt-like roots produced on an upright rhizome (Fig. 1C). Subtribe Goodyerinae has rhizomatous, decumbent stems crowned by a rosette of leaves, and the leaves often persist for several years.
Fig. 1.
Roots of representative members of tribe Cranichideae. (A) Cylindrical, thin roots in Ponthieva schaffneri. (B) Fusiform roots in Dichromanthus sp. (C) Stilt-like roots in Manniella gustavi. The roots are approximately 3 mm (A), 10 mm (B) and 6 mm in diameter (C) at their maximum thickness.
Many workers have studied structural and functional aspects of orchid roots (reviewed in Pridgeon, 1987). Recent focus has been on the search for characters useful for ascertaining systematic and evolutionary relationships in the family, and various studies have included representatives of Cranichideae. For instance, Pridgeon et al. (1983) surveyed the structure and distribution of tilosomes (excrescences from the innermost periclinal cell wall of velamen cells adjacent to the passage cells of the exodermis), finding that these thickenings are more common in epiphytic, mostly Neotropical orchids and describing several structural types. They reported the absence of tilosomes in the eight representatives of Cranichideae examined, as found in later studies (Porembski and Barthlott, 1988; Stern et al., 1993b).
Porembski and Barthlott (1988) conducted a comparative survey of velamen micromorphology across Orchidaceae, distinguishing 12 velamen types on the basis of characters such as the number of cell layers, stratification in epi- and endovelamen, presence/absence and other features of cell-wall thickenings and pores, tilosomes, thickening of exodermal cell walls and tracheoid idioblasts in the cortex. They studied 19 species belonging to 16 genera currently included in Cranichideae, including five species of Goodyerinae, four of Cranichidinae s.l. [i.e. including two genera later transferred by Dressler (1990, 1993) to Prescottiinae] and eight species of Spiranthinae. Porembski and Barthlott (1988) found a simple rhizodermis in three of the five representatives of Goodyerinae examined, but in the other two, Ludisia discolor and Platylepis heteromorpha, they recorded a one-layered velamen of the Calanthe type (defined as a one- to four-layered velamen without helical thickenings but with relatively small pores on the cell walls). All members of Spiranthinae exhibited a velamen of the Spiranthes type (usually one- or two-layered, with rather fine helical thickenings and small pores in the cell walls), with Sauroglossum elatum having a six-layered velamen. Representatives of Cranichidinae, by contrast, showed variation in velamen characteristics: Ponthieva schaffneri (as Cranichis) and Ponthieva petiolata had velamen of the Calanthe type whereas Aa palacea (as Altensteinia) and Prescottia colorans had velamen of the Spiranthes type. Dressler (1990, 1993) segregated several genera included previously in Cranichidinae, including Aa, Altensteinia and Prescottia (plus a few others) into a new subtribe, Prescottiinae, distinguishing it from Cranichidinae by possessing a velamen of the Spiranthes type, in addition to several floral features. Dressler (1993) hypothesized a sister-group relationship between Prescottiinae and Spiranthinae because of their shared possession of retrorse nectariferous lobules at the base of the labellum and velamen of the Spiranthes type.
Stern et al. (1993a) reported a specialized type of amyloplast, termed by them ‘spiranthosomes’, in the root cortex of many species of Cranichideae, which might represent a synapomorphy for the tribe (Salazar et al., 2003). Stern et al. (1993b) also carried out a study of the vegetative anatomy of subfamily Spiranthoideae, in which Cranichideae were included at that time, concluding that Spiranthoideae sensu Dressler (1993) were a polyphyletic assemblage. According to their data, tribes Tropidieae and Diceratosteleae were more closely related to ‘lower’ members of subfamily Epidendroideae (Palmorchis) than to tribe Cranichideae. In turn, the members of Cranichideae studied by Stern et al. (1993b) appeared to be more closely related to Diuris (tribe Diurideae, subfamily Orchidoideae). Stern et al. (1993b) also found evidence suggesting that Cryptostylis, placed by Dressler (1981) in Cranichideae, belonged with Diuris. Their findings have subsequently been largely corroborated by several molecular phylogenetic studies (e.g. Kores et al., 1997, 2000, 2001; Cameron et al., 1999; Freudenstein et al., 2000; Chase et al., 2001, 2003).
The phylogenetic relationships in tribe Cranichideae were assessed by Salazar et al. (2003) by using sequence and indel data from plastid and nuclear DNA. In that work, Prescottiinae were recovered as a grade in which subtribes Cranichidinae and Spiranthinae were nested, with the members of Prescottiinae forming two clades: Prescottia and a mostly high-Andean group of genera including Aa, Gomphichis, Porphyrostachys and Stenoptera. Thus, the molecular study of Salazar et al. (2003) supported neither monophyly of subtribe Prescottiinae nor its sister relationship to Spiranthinae, as suggested by Dressler (1990, 1993).
In the present study the value of several root anatomical characters for inferring the phylogenetic relationships among Cranichidinae, Prescottiinae and Spiranthinae is assessed. This was achieved by examining individual characters rather than the so-called ‘velamen types’ (Porembski and Barthlott, 1988) constructed as groups of various characters that often occur in different combinations among the taxa. Herein, several of the attributes considered by Porembski and Barthlott (1988) in defining their velamen types were studied, namely the number of velamen layers, presence/absence of pores and thickenings of the secondary wall of velamen cells, and scalariform thickenings of exodermal cell walls. In addition, two attributes not considered in previous anatomical studies of the roots of Cranichideae were surveyed: intercellular spaces in the cortex and the structure and distribution of tilosomes. Neither of these two features has been previously reported in Cranichideae (Pridgeon et al., 1983; Porembski and Barthlott, 1988; Stern et al., 1993b) and both are reported here for the first time. To explore possible evolutionary paths for such characters and their taxonomic value, they were optimized on a phylogenetic tree derived from a cladistic analysis of molecular data and the structural characters themselves, gathering both structural data and DNA sequences for the same species. The molecular information used consists of DNA sequence data from two compartments of the plant genome: plastid trnK/matK region (including the gene matK and the 3′ portion of the trnK intron; Johnson and Soltis, 1994; Kelchner, 2002) and the internal transcribed spacer (ITS) region of the nuclear ribosomal DNA, including ITS1, the 5·8S gene and ITS2 (Baldwin et al., 1995). The utility of both these regions for reconstructing phylogenetic relationships has been demonstrated in Cranichideae (Salazar et al., 2003) as well as many other groups of orchids (e.g. Kores et al., 2000, 2001; Gravendeel et al., 2001, 2004; Pridgeon et al., 2001; Whitten et al., 2000; Williams et al., 2001; van den Berg et al., 2005).
MATERIALS AND METHODS
Study species
Twenty-seven exemplars of 26 species and 19 genera belonging to subtribes Cranichidinae, Goodyerinae, Manniellinae, Prescottiinae and Spiranthinae sensu Dressler (1993) were studied. As we were specifically interested in root character evolution in subtribes Cranichidinae, Prescottiinae and Spiranthinae, representatives of Manniellinae and Goodyerinae were used as outgroups on the basis of previous phylogenetic studies (Cameron et al., 1999; Kores et al., 2001; Salazar et al., 2003). A list of the taxa studied with voucher information and GenBank accession numbers is given in Table 1.
Table 1.
Taxa studied, voucher information and GenBank accession numbers of DNA sequences
| GenBank accession no. |
|||||
|---|---|---|---|---|---|
| Taxon | Voucher | Anatomy | DNA | matK | ITS |
| Cranichidinae | |||||
| Cranichis cochleata Dressler | Salazar 6547, MEXU. | + | + | AM900817 | AM419782 |
| Cranichis revoluta Hamer & Garay | Soto 10097, AMO. | + | + | AM900818 | AM419783 |
| Ponthieva ephippium Rchb.f. | Salazar 6440, MEXU. | + | + | AM900824 | AM419789 |
| Ponthieva formosa Schltr. | Salazar 6250, EXU;Salazar 6539, MEXU | + | + | AM900828 | AM419793 |
| Ponthieva tuerckheimii Schltr. | Salazar 6512, MEXU. | + | + | AM900829 | AM419794 |
| Pterichis habenarioides (F.Lehm. & Kraenzl.) Schltr. | Davidse 24767, MEXU.; Aldana 2, MEXU. | + | + | AJ543937 | AJ539509 |
| Goodyerinae | |||||
| Goodyera brachyceras (A.Rich & Galeotti) Garay & G.A.Romero | Hernández 2005, MEXU.; Salazar 6144, MEXU. | + | + | AM902104 | AM778169 |
| Ludisia discolor (Ker Gawl.) A.Rich. | Salazar s.n., MEXU (spirit) | + | + | AJ543911 | AJ539483 |
| Manniellinae | |||||
| Manniella gustavi Rchb.f. | Salazar 6505, MEXU. (photograph); Etuge 4515 R, YA. | + | + | AJ543944 | AJ539517 |
| Prescottiinae | |||||
| Aa colombiana Schltr. | Aldana 012, MEXU. | + | + | AM900802 | AM419766 |
| Altensteinia fimbriata Kunth | Salazar 6789, MEXU. | + | + | AM900801 | AM419765 |
| Prescottia tubulosa (Lindl.) L.O.Williams | Salazar 6888, MEXU.; Salazar 6054, MEXU. | + | + | AJ543938 | AJ539510 |
| Prescottia stachyodes (Sw.) Lindl. | Soto 6515, MEXU (photograph); Salazar 6092, MEXU | + | + | AM900808 | AM419773 |
| Pseudocranichis thysanochila (B.L.Rob. & Greenm.) Garay | Salazar 6887, MEXU.; Tenorio 17900, MEXU. | + | + | AM900810 | AM419775 |
| Spiranthinae | |||||
| Aulosepalum pyramidale (Lindl.) M.A.Dix & M.W.Dix | Figueroa 045, MEXU.; Salazar 6061, MEXU. | + | + | AM884240 | AM778174 |
| Cyclopogon aff. comosus (Rchb.f.) Burns-Bal. & E.W.Greenw. | Soto 94034, MEXU.; Salazar 6115, MEXU | + | + | AM902107 | AM778172 |
| Dichromanthus cinnabarinus (La Llave & Lex.) Garay | Figueroa 043, MEXU.; Linares 4469, MEXU. | + | + | AJ543914 | AJ539486 |
| Dichromanthus cinnabarinus (La Llave & Lex.) Garay subsp. galeottianus Soto Arenas & Salazar | Salazar et al 6895 MEXU. | + | + | AM902110 | AM778176 |
| Dichromanthus michuacanus (La Llave & Lex.) Salazar & Soto Arenas | Salazar 6583, MEXU.; Salazar 6047, MEXU. | + | + | AM902111 | AM778177 |
| Mesadenus lucayanus (Britton) Schltr. | Salazar 6714, MEXU.; Salazar 6043, MEXU. | + | + | AJ543916 | AJ539488 |
| Mesadenus polyanthus (Rchb.f.) Schltr. | Salazar 6370, MEXU. | + | + | AM902109 | AM778175 |
| Microthelys constricta (Szlach.) Szlach. | Hernández s.n., MEXU.; Soto s.n., MEXU. | + | + | AM902108 | AM778173 |
| Pelexia sp. | Salazar 6421, MEXU. | + | + | AM902105 | AM778170 |
| Sacoila lanceolata (Aubl.) Garay | Salazar 6718, MEXU.; Da Silva 874, MG. | + | + | AJ543933 | AJ539504 |
| Sarcoglottis schaffneri (Rchb.f.) Ames. | Figueroa 044, MEXU.; Salazar 6060, MEXU. | + | + | AM902106 | AM778171 |
| Schiedeella llaveana (Lindl.) Schltr. | Salazar 6508, MEXU.; Salazar 6105, MEXU. | + | + | AJ543915 | AJ539487 |
Light microscopy
Roots were collected from living plants directly in the field or from plants kept in cultivation under greenhouse conditions, with the exception of Aa colombiana and Pterichis habenarioides for which roots were obtained from herbarium specimens (Table 1). Root fragments taken 1–4 cm above the root tip were fixed in FAA (5 % formalin, 5 % acetic acid, 50 % ethanol; Sass, 1958) or 70 % ethanol for at least 24 h and stored in 50 % ethanol until further processing. Transverse sections (50 µm thick) were cut on a hand microtome (Reichert Jung, AG Heidelberg, Germany). Sections were stained in an aqueous mix of 0·5 % (w/v) methylene blue in 0·5 % (w/v) borax and 0·5 % (w/v) azure II (Ruzin, 1999). Stained sections were mounted in glycerine jelly. Observations were made with an Axiostar Plus photomicroscope (Carl Zeiss, Göttingen, Germany). Photomicrographs were taken with a Sony CyberShot digital camera (Japan).
Scanning electron microscopy (SEM)
Cross- and paradermal root sections (2 mm thick) were fixed for 24 h in 4 % (v/v) glutaraldehyde in Sorensen's phosphate buffer, pH 7·2 (Ruzin, 1999). After two 1-h washes in phosphate buffer, the samples were dehydrated in an ethanol series, critical-point dried, coated with gold, and examined using a scanning electron microscope (Hitachi S-2460 N, Tokyo, Japan) operating at 15 kV. Micrographs were taken with a camera (Pentax Z10, Japan) using 35-mm Kodak 100 TMAX film and the negatives were subsequently digitized using a scanner (Nikon Super Coolscan 5000, Tokyo, Japan).
DNA extraction, amplification and sequencing
We followed standard molecular procedures, including extraction of genomic DNA from fresh or silica-dried plant tissue using a 2× cetyltrimethylammonium bromide (CTAB) protocol based on Doyle and Doyle (1987) and polymerase chain reaction (PCR) using commercial kits (PCR Master Mix, Advanced Biotechnologies Ltd, Epsom, Surrey, UK or Taq PCRCore Kit, Qiagen, Crawley, West Sussex, UK), following the manufacturers’ protocols. PCR products were purified with QIAquick silica columns (Qiagen) and used in cycle sequencing reactions with the ABI Prism Big Dye® Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq® DNA polymerase, versions 3 or 3·1 (Applied Biosystems Inc., Warrington, Cheshire, UK). The products of cycle sequencing were cleaned by precipitation with ethanol (for a detailed description of the molecular protocols see Salazar et al., 2003). Both DNA strands were sequenced and the chromatograms were assembled and edited with the program Sequencher versions 3·1 or 4·1 (Gene Codes Corp., Ann Arbor, MI, USA) and aligned visually.
Phylogenetic analyses
A maximum-parsimony analysis was conducted with the computer program PAUP* version 4·02b for Macintosh (Swofford, 2002). In a previous study (Salazar et al., 2003), the trnK/matK and ITS regions were shown to recover similar patterns of relationships in Cranichideae when analysed individually, and both resolution and internal support (e.g. as evaluated by non-parametric bootstrapping; Felsenstein, 1985) were found to increase when the two regions are analysed in combination. Therefore, we analysed both DNA regions together with the structural characters that varied in a potentially informative way (excluding autapomorphies). This ‘total evidence’ approach allows the interaction of structural and molecular characters in the analysis, maximizes the explanatory power of the data (Kluge, 1989; Eernise and Kluge, 1993; Nixon and Carpenter, 1996) and imposes a stringent test on our hypothesis of primary homology through character congruence (see de Pinna, 1991). However, the effect of removing the structural characters on the resulting phylogenetic hypotheses was also assessed.
The cladistic analysis consisted of a heuristic search with 1000 replicates of random addition of sequences for the starting trees and tree-bisection-reconnection (TBR) branch swapping, saving all most-parsimonious trees (MPTs). All characters were treated as unordered and equally weighted. Clade support was evaluated by 300 bootstrap replicates, each of these with 20 random sequence additions and TBR branch swapping, saving up to 20 MPTs per replicate to reduce the time spent in swapping on large islands of trees (Maddison, 1991). Changes in the structural characters of interest were analysed examining their optimization on the MPT(s) using the program MacClade version 4 (Maddison and Maddison, 2001) using the ‘accelerated transformation’ (ACCTRAN) option, which maximizes the interpretation of postulated hypotheses of primary homology as valid in the absence of evidence to the contrary (de Pinna, 1991).
RESULTS
Root structural characters
Root morphology is relatively homogeneous in the taxa studied, and the observations confirm those of Stern et al. (1993b), except as noted below. As mentioned earlier, attention was focused on particular attributes of the velamen (number of cell layers, presence/absence and characteristics of pores, thickening of the cell walls, and presence and type of tilosomes), the exodermis (distribution of scalariform thickenings on cell walls) and intercellular spaces in the cortex. A detailed description of the root anatomy of the species studied will be published elsewhere (C. Figueroa, unpubl. res.). A list of species with their anatomical characters studied in this work is given in Table 2.
Table 2.
Root anatomical characters of representatives of tribe Cranichideae included in the cladistic analysis
| Velamen cell wall |
||||||
|---|---|---|---|---|---|---|
| Taxon | Velamen layers | Tilosomes* | Continuous | Pores | Thickenings | Supraendodermal spaces† |
| Subtribe Cranichidinae | ||||||
| Cranichis cochleata | 1 | – | – | + | – | – |
| Cranichis revoluta | 1 | – | – | + | – | – |
| Ponthieva ephippium | 1 | – | – | + | – | – |
| Ponthieva formosa | 1 | – | – | + | – | – |
| Ponthieva tuerckheimii | 1 | – | – | + | – | – |
| Pterichis habenarioides | 1 | – | – | + | – | – |
| Subtribe Goodyerinae | ||||||
| Goodyera brachyceras | 1 | – | + | – | – | – |
| Ludisia discolor | 1 | – | + | + | – | – |
| Subtribe Manniellinae | ||||||
| Manniella gustavi | 1 | – | + | + | – | – |
| Subtribe Prescottiinae | ||||||
| Aa colombiana | 1 | – | – | + | + | S |
| Altensteinia fimbriata | 1 | – | – | + | + | – |
| Prescottia tubulosa | 1 | L | – | + | + | – |
| Prescottia stachyodes | 3 | B | – | + | + | – |
| Pseudocranichis thysanochila | 1 | – | + | + | – | S |
| Subtribe Spiranthinae | ||||||
| Aulosepalum pyramidale | 1 | L | – | + | + | S |
| Cyclopogon aff. comosus | 1 | L | – | + | + | – |
| Dichromanthus cinnabarinus subsp. cinnabarinus | 1 | L | – | + | + | S |
| Dichromanthus cinnabarinus subsp. galeottianus | 1 | L | – | + | + | S |
| Dichromanthus michuacanus | 1 | L | – | + | + | S |
| Mesadenus lucayanus | 1 | L | – | + | + | S |
| Mesadenus polyanthus | 1 | L | – | + | + | S |
| Microthelys constricta | 1 | L | – | + | + | S |
| Pelexia sp. | 1 | L | – | + | + | – |
| Sacoila lanceolata | 1 | L | – | + | + | E |
| Sarcoglottis schaffneri | 1 | L | – | + | + | – |
| Schiedeella llaveana | 1 | L | – | + | + | S |
* L, tilosomes of the lamellate type; and B, of the baculate type (after Pridgeon et al., 1983).
† S, short supraendodermal spaces; E, elongated supraendodermal spaces (see text).
Number of velamen layers
The species examined in the present study have a uniseriate velamen, with the sole exception of Prescottia stachyodes, in which the velamen consists of three cell layers. In cross-section, velamen cells are isodiametric in members of Cranichidinae and Manniellinae (Fig. 2A, B), radially compressed in Goodyera and Ludisia (Goodyerinae), and radially elongated in Spiranthinae and Prescottiinae (Fig. 2D, E). One-celled root hairs are present in all the species studied and are particularly abundant in Manniella gustavi (Fig. 1C). The three-layered velamen is autapomorphic for Prescottia stachyodes and was not included in the cladistic analysis.
Fig. 2.
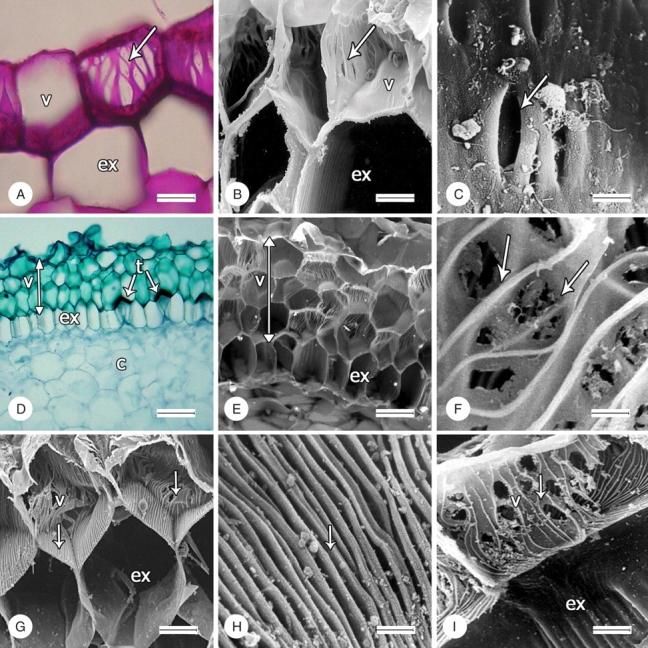
Structural characters of velamen (indicated by arrows) in representatives of tribe Cranichideae. (A–H) Transverse sections, (I) longitudinal section; (A, D), light micrographs, all the others scanning electron micrographs. (A) Pores in cell walls of Manniella gustavi; scale bar = 40 µm. (B, C) Pores in cell walls of Ponthieva ephippium; scale bars = 20 and 5 µm, respectively. (D, E) Three-layered velamen of Prescottia stachyodes; scale bars = 100 and 40 µm, respectively. (F) Anastomosing cell-wall thickenings of P. stachyodes; scale bar = 5 µm. (G) Thickenings in cell walls of Sarcoglottis schaffneri; scale bar = 40 µm. (H) Thickenings in tangential wall (adjacent to the exodermis) of S. schaffneri; scale bar = 5 µm. (I) Anastomosed thickenings in radial cell walls in velamen of S. schaffneri; scale bar = 20 µm. v, velamen; ex, exodermis; c, cortex; t, tilosome.
Pores and thickenings of secondary cell walls of velamen
Velamen of Goodyera brachyceras has continuous cell walls. Manniella gustavi is polymorphic for these characters, showing both a continuous cell wall and pores in different cells (Fig. 2A). In Ludisia discolor, all the species of Cranichidinae and Pseudocranichis thysanochila (Prescottiinae) velamen cell walls lack distinct thickenings but have pores of various sizes, which are usually elongated in parallel to the radius of the root. The velamen cell walls of Spiranthinae and Prescottiinae (except Pseudocranichis) have conspicuous thickenings, which vary in arrangement and orientation on different portions of the wall (Fig. 2D–I). The innermost tangential cell wall (i.e. that adjacent to the exodermis) has straight, densely arranged, parallel thickenings aligned with the main axis of the root (Fig. 2G, H). Radial walls have irregularly spaced thickenings orientated transversely to the main axis of the root and often curved and anastomosed (Fig. 2G–I). In all cases, the velamen radial cell walls have pores of various sizes on the thin wall areas between the thickenings (Fig. 2I). As Goodyera brachyceras is the only species lacking any sort of pores on the walls, that character was not used in the cladistic analysis. Cell-wall thickenings were coded as a binary character (presence/absence).
Exodermal thickenings
In all the species the exodermis is uniseriate, and all have scalariform thickenings on the radial walls at maturity (Fig. 2E, I).
Tilosomes
This study provides the first evidence for the presence of tilosomes in Cranichideae (see Pridgeon et al., 1983; Pridgeon, 1987; Porembski and Barthlott, 1988; Stern et al., 1993b). Tilosomes are present in Prescottia tubulosa and Prescottia stachyodes (Prescottiinae) and in all the species of Spiranthinae examined here. In Spiranthinae and Prescottia tubulosa tilosomes are formed by densely packed parallel ridges and furrows (Fig. 3A–C), corresponding to the lamellate type of Pridgeon et al. (1983). However, Prescottia stachyodes is unique in having masses of free-standing rods perpendicular to the innermost tangential wall of the velamen cell (Fig. 3D–F), matching the baculate type of Pridgeon et al. (1983). Tilosomes are absent in species of Goodyerinae, Cranichidinae and Manniellinae examined in this work. This feature was coded as a three-state unordered character (0 = tilosomes absent; 1 = lamellate tilosomes; 2 = baculate tilosomes).
Fig. 3.
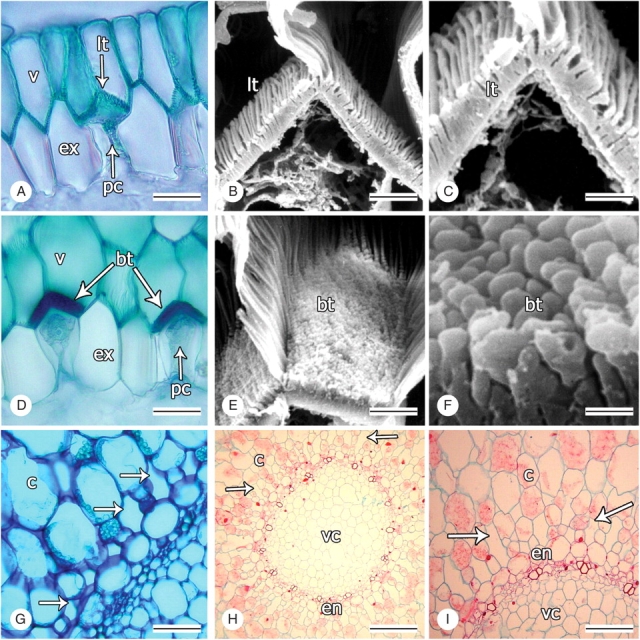
Tilosomes and supraendodermal spaces in representatives of tribe Cranichideae. Transections. (A, D, G–I) Light micrographs, all others scanning electron micrographs. (A) Velamen, tilosome and passage cell of exodermis of Mesadenus polyanthus; scale bar = 40 µm. (B, C) Lamellate tilosome of Prescottia tubulosa; scale bars = 10 µm, 5 µm. (D) Velamen, tilosome and passage cells of exodermis of Prescottia stachyodes; scale bar = 40 µm. (E, F) Baculate tilosome of P. stachyodes; scale bars = 10 and 1 µm, respectively. (G) Short supraendodermal spaces of Mesadenus polyanthus (arrows); scale bar = 100 µm. (H, I) Elongated supraendodermal spaces of Sacoila lanceolata (arrows); scale bar = 100 and 50 µm, respectively. v, velamen; ex, exodermis; pc, passage cells; lt, lamellate tilosome; bt, baculate tilosome; c, cortex; en, endodermis; vc, vascular cylinder.
Supraendodermal spaces
In most Spiranthinae, with the exception of Cyclopogon, Pelexia and Sarcoglottis, small intercellular spaces roughly the size of one of the surrounding cortical cells occur immediately outside the endodermis and are thus referred to here as supraendodermal spaces (Fig. 3G). Similar spaces were also found in two distantly related species of Prescottiinae, namely Aa colombiana and Pseudocranichis thysanochila. In Sacoila lanceolata (Spiranthinae), supraendodermal spaces are radially elongated and of a length comparable with that of three or four cells of the adjacent cortical cells (Fig. 3H, I). The supraendodermal spaces were scored as a three-state, unordered character (0 = absent; 1 = short; 2 = elongated).
Phylogenetic analysis
The combined matrix comprised 2663 characters, of which 531 were potentially parsimony-informative. The heuristic search recovered a single tree with a length of 1671 steps, consistency index (CI) of 0·66 and retention index (RI) of 0·72 (Fig. 4). Manniella gustavi (Manniellinae) is strongly supported [bootstrap value (BP) = 100] as sister to a clade that includes all the species studied of Cranichidinae, Prescottiinae and Spiranthinae. Cranichidinae sensu Dressler (1993) is paraphyletic because Pseudocranichis thysanochila is embedded in Prescottia. Excluding Pseudocranichis, Cranichidinae is monophyletic with strong support (BP = 100; Fig. 4). Pterichis habenarioides is sister to the rest, which in turn form two well-supported clades: Cranichis (BP = 99) and Ponthieva (BP = 100).
Fig. 4.
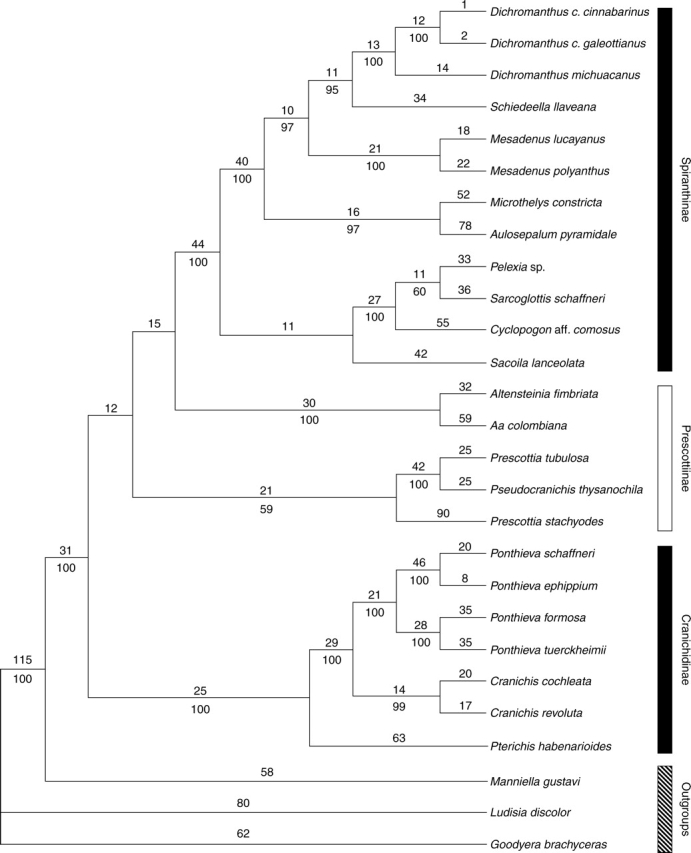
Single most-parsimonious cladogram found by the heuristic search of combined molecular and structural data (L = 1671 steps, CI = 0·66, RI = 0·72). Numbers above branches are branch lengths; numbers below branches are bootstrap values.
Cranichidinae is sister to a clade consisting of two successively diverging prescottioid clades, i.e. Prescottia/Pseudocranichis (BP = 59) and Aa–Altensteinia (BP = 100), and Spiranthinae. The Aa–Altensteinia clade is sister, with no support, to a monophyletic Spiranthinae (BP = 100). Within the latter, Sacoila lanceolata is sister, without bootstrap support, to a strongly supported group comprising Cyclopogon, Pelexia and Sarcoglottis. This group of four genera is in turn sister to a clade encompassing Aulosepalum, Microthelys, Mesadenus, Schiedeella and Dichromanthus. Internal relationships of these groups are all strongly supported.
When the three structural characters were removed from the matrix, the heuristic search found three shortest trees, one of which is topologically identical with the single shortest tree recovered by the combined analysis. The other two trees differ in the position of the Prescottia/Pseudocranichis and the Aa–Altensteinia clades relative to Cranichidinae and Spiranthinae, but in the strict consensus those four clades formed a polytomy (not shown). These results agree with the previous molecular analyses by Salazar et al. (2003), which recover essentially the same four main clades (Pseudocranichis was not included in that study), but the relationships among them lack support. The analysis conducted here, which included structural as well as molecular data, likewise failed to provide support for the relationships for those four main clades, but we consider it as our best estimate of the phylogenetic relationships in the group and a reasonable background in which to analyse the evolution of the structural characters.
DISCUSSION
Our observation of a three-layered velamen in Prescottia stachyodes agrees with previous studies by Pridgeon (1987) and Stern et al. (1993b), who reported three and two or three velamen cell layers for the same species, respectively. There seems to be substantial variation in this character within the genus: Stern et al. (1993b) also reported four or five cell layers in the velamen of Prescottia plantaginea (as P. plantaginifolia), but only one layer has been recorded for Prescottia tubulosa (this study) and Prescottia colorans (Porembski and Barthlott, 1988). However, information is only available for this feature for four of the 24 or so species known in the genus (Ackerman, 2003), which precludes drawing firm conclusions about its systematic significance.
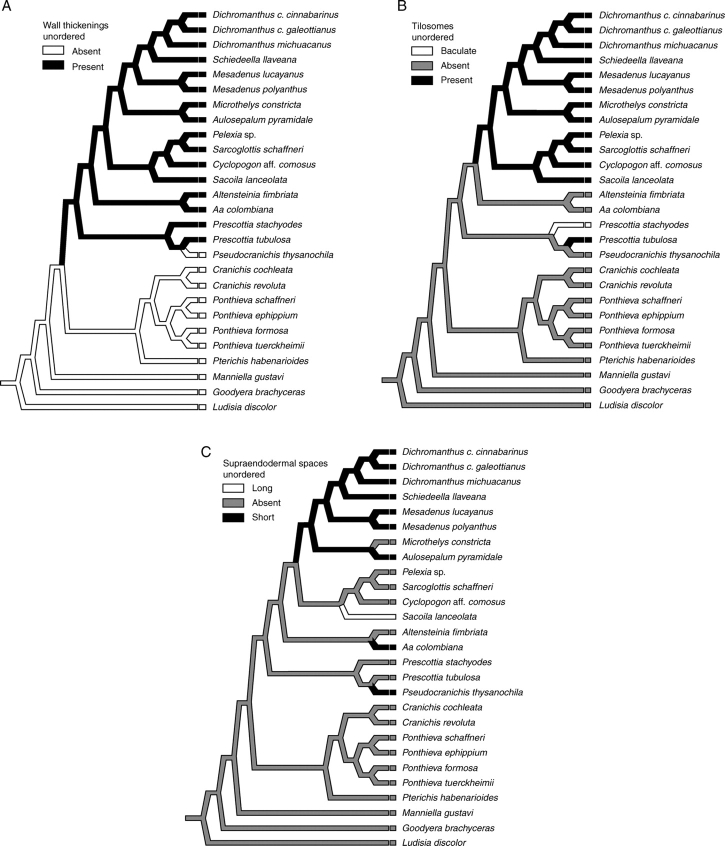
Absence of pores and wall thickenings is a distinctive feature of Goodyera brachyceras. Pores, but not thickenings, occur in the velamen of Ludisia discolor, Manniella gustavi, Pseudocranichis thysanochila and all the representatives of Cranichidinae. All the other species analysed in the present study have both pores and conspicuous cell-wall thickenings. Thus, neither a continuous cell wall nor the presence/absence of pores seems to be useful in establishing relationships among the species sampled herein. Nevertheless, the presence of cell-wall thickenings is synapomorphic for the clade that includes the two Prescottiinae clades and Spiranthinae, with the absence of thickenings in Pseudocranichis being most parsimoniously interpreted as a secondary loss (Fig. 5A). Our results agree with the proposal of Dressler (1990, 1993) that Prescottiinae might be the closest relative of Spiranthinae. However, this analysis failed to provide support for monophyly of Prescottiinae, as did our previous assessment of phylogenetic relationships in Cranichideae based on analysis of a larger number of taxa and characters (Salazar et al., 2003). The absence of thickenings in Cranichidinae distinguished them from Prescottiinae but is symplesiomorphic for the sample of Cranichideae examined and does not represent a uniquely derived feature of that subtribe.
Fig. 5.
ACCTRAN optimization of root characters on the cladogram in Fig. 4. (A) Cell-wall thickenings, (B) tilosomes, (C) supraendodermal spaces.
Previous surveys of root anatomy that focused either on the spiranthoid orchids (Stern et al., 1993b) or on Orchidaceae as a whole (Pridgeon et al., 1983; Pridgeon, 1987; Porembski and Barthlott, 1988) have reported the absence of tilosomes in Cranichideae, as interpreted today (Dressler, 1993; Chase et al., 2003; Salazar et al., 2003). Porembski and Barthlott (1988) and Stern et al. (1993b) found tilosomes in Cryptostylis, at that time considered a member of Cranichideae (following Dressler, 1981), but recent convincing evidence has accumulated that places Cryptostylis among Diurideae (e.g. Stern et al., 1993a, b; Kores et al., 1997, 2000, 2001; Cameron et al., 1999). Tilosomes were observed in all the representatives of Spiranthinae and the two species of Prescottia examined herein. Lack of tilosomes is interpreted here as the plesiomorphic condition, whereas lamellate tilosomes arose independently in Spiranthinae and in Prescottia tubulosa (Fig. 5B). Baculate tilosomes represent an autapomorphy of Prescottia stachyodes. A different interpretation would result if tilosomes had been coded as present/absent, independently of their structural characteristics. Under this scenario, a single origin in the last common ancestor of Prescottiinae and Spiranthinae could be postulated, with independent losses in Pseudocranichis and Aa–Altensteinia. Nevertheless, given the marked structural differences between the lamellate and baculate tilosomes and the lack of detailed information on their developmental pathways (Pridgeon, 1987), these two types of tilosome are best seen as different conditions evolving in parallel.
Pridgeon et al. (1983) and Pridgeon (1987) reported absence of tilosomes in Sarcoglottis acaulis (as Spiranthes acaulis) and Spiranthes odorata (as Spiranthes cernua var. odorata). The general occurrence of tilosomes in Spiranthinae documented here suggests the need to re-examine those species to confirm whether tilosomes of the lamellate type may have been overlooked or if there is variation for this feature in the subtribe. Furthermore, much more work under controlled conditions needs to be conducted on the role of the environment (substrate, water relations, mycorrhizas, etc.) on development of tilosomes. At least some of the previous work that did not report tilosomes in Cranichideae appears to have been based on plants that had been in cultivation for some time, most likely with ample water supply and without mycorrhizas. It would be interesting to compare the process of development of tilosomes in an orchid population in the wild and in plants from the same population removed and cultivated for extended periods. It might also be instructive to sample meristemmed, flasked plants from the same population for tilosomes. This would contribute to an understanding of the influence of the environment (e.g. drought) on expression of tilosomes.
Pridgeon (1987) proposed that presence and type of tilosomes are most useful as taxonomic markers at the generic and subtribal levels. The present results seem to corroborate Pridgeon's assertion as presence of lamellate tilosomes in our analysis is unambiguously reconstructed as a synapomorphy of subtribe Spiranthinae, which is notable as this group otherwise lacks diagnostic morphological characters (Salazar, 2003; Salazar et al., 2003). Traditionally Spiranthinae are distinguished from other Cranichideae mostly by the combination of fasciculate roots, resupinate flowers and a lip with distinct nectar glands and adherent to the sides of the column, but one or more of these features may be absent in particular genera.
The most-parsimonious reconstruction of supraendodermal spaces on the phylogenetic tree (Fig. 5C) points to three independent origins for short supraendodermal spaces: once each in Pseudocranichis, Aa and the most recent common ancestor of a clade of Spiranthinae that includes Aulosepalum through Dichromanthus, with a secondary loss in Microthelys. Elongated supraendodermal spaces are autapomorphic for Sacoila. We are not sure of the nature of such spaces. A possibility is that they represent secretory channels formed by periclinal divisions of young endodermal cells, as in some Asteraceae (Guttenberg, 1968, cited in Esau, 1987). A copious secretion of mucilage occurs when the roots of various Spiranthinae are cut transversely (C. Figueroa and G. A. Salazar, unpubl. res.). Mucilage, consisting of polysaccharides, may aid in water storage and is particularly abundant in plants adapted to arid environments (Esau, 1987). Most of the species we studied that show supraendodermal spaces occur in seasonally dry habitats, such as xerophilous scrub, Pinus–Quercus forest, tropical deciduous forest (most Spiranthinae and Pseudocranichis thysanochila), or high Andean paramos (Aa colombiana). On the other hand, Sacoila lanceolata, which possesses elongated supraendodermal spaces, is one of the more widely distributed orchids in the Neotropics and usually thrives in marginal or disturbed habitats, including roadsides, pastures, abandoned crop fields and burnt savannahs (Salazar, 2003). Further study is required to determine whether such cortical spaces are filled with mucilage or represent something else.
CONCLUSIONS
This study documents the presence of tilosomes in the velamen of various representatives of Cranichideae, in contrast to previous surveys that reported their absence (Pridgeon et al., 1983; Pridgeon, 1987; Porembski and Barthlott, 1988; Stern et al., 1993b). Tilosome distribution is phylogenetically informative, and the lamellate type is supported in our analysis as a synapomorphy grouping all the species of Spiranthinae we sampled. The presence of velamen cell-wall thickenings supports a close relationship between Spiranthinae and Prescottiinae, as suggested by Dressler (1990, 1993). Presence of supraendodermal spaces in the cortex showed more variation, but they are more common among species of Spiranthinae and Prescottiinae that live in seasonally dry habitats. However, the number of genera and species that have been available for study is small compared with the diversity of the group and further research is required to verify the generality of our observations and gain insight into the ecological and functional roles of those structures.
Our results agree in general with the observations of Porembski and Barthlott (1988) regarding the structure and distribution of velamen attributes in Cranichideae, which served as the basis for the segregation of Prescottiinae from Cranichidinae by Dressler (1990, 1993). The construction of ‘types’ of velamen by Porembski and Barthlott (1988) contributed to our understanding of orchid velamen by summarizing information on its various attributes. Nevertheless, as such types represent assemblages of different characters, not all of which are necessarily present in all the taxa assigned to them, they are of limited use in studies focused on testing hypotheses about phylogenetic relationships and character evolution explicitly (i.e. by their inclusion in a cladistic analysis). Such studies require instead the generation of data matrices in which each character is clearly defined and coded, which can be easily updated and reanalysed as required.
ACKNOWLEDGEMENTS
We thank Miguel A. Soto for providing material of several taxa, Berenit Mendoza and Miguel Vega for their technical aid with SEM and light microscopy, Lidia I. Cabrera and Laura Márquez Valdelamar for their assistance with DNA sequencing, and Mike Fay, Alec Pridgeon and an anonymous reviewer for insightful comments and suggestions on the manuscript.
LITERATURE CITED
- Ackerman JD. In: Genera Orchidacearum. Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Vol. 3. Oxford: Oxford University Press; 2003. pp. 47–50. Prescottia. [Google Scholar]
- Baldwin BG, Sanderson MJ, Porter JM, Wojciechowski MF, Campbell CS, Donoghue MJ. The ITS region of nuclear ribosomal DNA: a valuable source of evidence on angiosperm phylogeny. Annals of the Missouri Botanical Garden. 1995;82:247–277. [Google Scholar]
- van den Berg C, Goldman DH, Freudenstein JV, Pridgeon AM, Cameron KM, Chase MW. An overview of the phylogenetic relationships within Epidendroideae inferred from multiple DNA regions and recircumscription of Epidendreae and Arethuseae (Orchidaceae) American Journal of Botany. 2005;92:613–624. doi: 10.3732/ajb.92.4.613. [DOI] [PubMed] [Google Scholar]
- Cameron KM, Chase MW, Whitten WM, Kores PJ, Jarrell DC, Albert VA, et al. A phylogenetic analysis of the Orchidaceae: evidence from rbcL nucleotide sequences. American Journal of Botany. 1999;86:208–224. [PubMed] [Google Scholar]
- Chase MW, Kurzweil H, Linder P, Cribb P. Phylogenetics of Orchidoideae. In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera Orchidacearum, 2. Oxford: Oxford University Press; 2001. pp. 1–5. [Google Scholar]
- Chase MW, Cameron KM, Barrett RL, Freudenstein JV. DNA data and Orchidaceae systematics: a new phylogenetic classification. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Borneo: Natural History Publications; 2003. pp. 69–89. [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Phytochemical Bulletin, Botanical Society of America. 1987;19:11–15. [Google Scholar]
- Dressler RL. The orchids: natural history and classification. Cambridge, MA: Harvard University Press; 1981. [Google Scholar]
- Dressler RL. The Spiranthoideae: grade or subfamily? Lindleyana. 1990;5:110–116. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Portland: Dioscorides Press; 1993. [Google Scholar]
- Eernise DJ, Kluge AG. Taxonomic congruence versus total evidence, and amniote phylogeny inferred from fossils, molecules, and morphology. Molecular Biology and Evolution. 1993;10:1170–1195. doi: 10.1093/oxfordjournals.molbev.a040071. [DOI] [PubMed] [Google Scholar]
- Esau K. Anatomía de las Plantas con Semilla. Buenos Aires: Editorial Hemisferio Sur; 1987. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Freudenstein JV, Senyo DM, Chase MW. Mitochondrial DNA and relationships in the Orchidaceae. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Collingwood: CSIRO; 2000. pp. 421–429. [Google Scholar]
- Gravendeel B, Chase MW, de Vogel EF, Roos MC, Mes THM, Bachmann K. Molecular phylogeny of Coelogyne (Epidendroideae; Orchidaceae) based on plastid RFLPs, matK and nuclear ribosomal ITS sequences: evidence for polyphyly. American Journal of Botany. 2001;88:1915–1927. [PubMed] [Google Scholar]
- Gravendeel B, Eurlings MCM, van den Berg C, Cribb PJ. Phylogeny of Pleione (Orchidaceae) and parentage analysis of its wild hybrids based on plastid and nuclear ribosomal ITS sequences and morphological data. Systematic Botany. 2004;29:50–63. [Google Scholar]
- Guttenberg HV. Der primäre Bau der Angiospermenwurzel. Handbuch der Pflanzenanatomie Band VIII, Teil 5. Berlin, Stuttgart: Gebr. Borntraeger; 1968. [Google Scholar]
- Hágsater E, Soto MA, Salazar GA, Jiménez R, López MA, Dressler RL. Orchids of Mexico. Mexico City: Instituto Chinoín; 2005. [Google Scholar]
- Johnson LA, Soltis DE. matK DNA sequences and phylogenetic reconstruction in Saxifragaceae s.str. Systematic Botany. 1994;19:143–156. [Google Scholar]
- Kelchner SA. Group II introns as phylogenetic tools: structure, function and evolutionary constraints. American Journal of Botany. 2002;89:1651–1669. doi: 10.3732/ajb.89.10.1651. [DOI] [PubMed] [Google Scholar]
- Kluge AG. A concern for evidence and and a phylogenetic hypothesis of relationships among Epicrates (Boidae, Serpentes) Systematic Zoology. 1989;38:7–25. [Google Scholar]
- Kores PJ, Cameron KM, Molvray M, Chase MW. The phylogenetic relationships of Orchidoideae and Spiranthoideae (Orchidaceae) as inferred from rbcL plastid sequences. Lindleyana. 1997;12:1–11. [Google Scholar]
- Kores PJ, Weston PH, Molvray M, Chase MW. Phylogenetic relationships within the Diurideae (Orchidaceae): inferences from plastid matK DNA sequences. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Collingwood: CSIRO; 2000. pp. 449–456. [Google Scholar]
- Kores PJ, Molvray M, Weston PH, Hopper SD, Brown AP, Cameron KM, Chase MW. A phylogenetic analysis of Diurideae (Orchidaceae) based on plastid DNA sequence data. American Journal of Botany. 2001;88:1903–1914. [PubMed] [Google Scholar]
- Maddison RD. The discovery and importance of multiple islands of most-parsimonious trees. Systematic Zoology. 1991;40:315–328. [Google Scholar]
- Maddison RD, Maddison WP. MacClade 4, version 4·02. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Nixon KC, Carpenter JM. On simultaneous analysis. Cladistics. 1996;12:221–241. doi: 10.1111/j.1096-0031.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- de Pinna MCC. Concepts and tests of homology in the cladistic paradigm. Cladistics. 1991;7:367–394. [Google Scholar]
- Porembski S, Barthlott W. Velamen radicum micromorphology and classification of Orchidaceae. Nordic Journal of Botany. 1988;8:117–137. [Google Scholar]
- Pridgeon AM. The velamen and exodermis of orchid roots. In: Arditti J, editor. Orchid biology, reviews and perspectives, IV. Ithaca, NY: Cornell University Press; 1987. pp. 139–192. [Google Scholar]
- Pridgeon AM, Stern WL, Benzing DH. Tilosomes in roots of Orchidaceae: morphology and systematic occurrence. American Journal of Botany. 1983;70:1365–1377. [Google Scholar]
- Pridgeon AM, Solano R, Chase MW. Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. American Journal of Botany. 2001;88:2286–2308. [PubMed] [Google Scholar]
- Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN. Genera Orchidacearum, 3: Orchidoideae part 2, Vanilloideae. Oxford: Oxford University Press; 2003. [Google Scholar]
- Ruzin ES. Plant microtechnique and microscopy. Oxford: Oxford University Press; 1999. [Google Scholar]
- Salazar GA. Spiranthinae. In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN, editors. Genera Orchidacearum, 3. Oxford: Oxford University Press; 2003. pp. 164–278. [Google Scholar]
- Salazar GA, Chase MW, Arenas MS, Ingrouille M. Phylogenetics of Cranichideae with emphasis on Spiranthinae (Orchidaceae, Orchidoideae): evidence from plastid and nuclear DNA sequences. American Journal of Botany. 2003;90:777–795. doi: 10.3732/ajb.90.5.777. [DOI] [PubMed] [Google Scholar]
- Sass JE. Botanical microtechnique. Ames: Iowa State College Press; 1958. [Google Scholar]
- Stern WL, Aldrich HC, McDowell LM, Morris MW, Pridgeon AM. Amyloplasts from cortical root cells of Spiranthoideae (Orchidaceae) Protoplasm. 1993;a 172:49–55. [Google Scholar]
- Stern WL, Morris MW, Judd WS, Pridgeon AM, Dressler RL. Comparative vegetative anatomy and systematics of Spiranthoideae (Orchidaceae) Botanical Journal of the Linnean Society. 1993;b 113:161–197. [Google Scholar]
- Swofford DL. Phylogenetic analysis using parsimony (* and other methods), version 4·02b. Sunderland, MA: Sinauer Associates; 2002. PAUP. [Google Scholar]
- Whitten WM, Williams NH, Chase MW. Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis on Stanhopeinae: combined molecular evidence. American Journal of Botany 87: 2000:1842–1856. [PubMed] [Google Scholar]
- Williams NH, Chase MW, Fulcher T, Whitten WM. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscription of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus. Lindleyana. 2001;16:113–139. [Google Scholar]