Abstract
Background and Aims
Leaves expand during a given period of time until they reach their final size and form, which is called determinate growth. Duration of leaf expansion is stable when expressed in thermal-time and in the absence of stress, and consequently it is often proposed that it is controlled by a robust programme at the plant scale. The usual hypothesis is that growth cessation occurs when cell expansion becomes limited by an irreversible tightening of cell wall, and that leaf size is fixed once cell expansion ceases. The objective of this paper was to test whether leaf expansion could be restored by rewatering plants after a long soil water-deficit period.
Methods
Four experiments were performed on two different species (Arabidopsis thaliana and Helianthus annuus) in which the area of leaves that had apparently reached their final size was measured upon reversal of water stresses of different intensities and durations.
Key Results
Re-growth of leaves that had apparently reached their final size occurred in both species, and its magnitude depended only on the time elapsed from growth cessation to rewatering. Leaf area increased up to 186% in A. thaliana and up to 88% in H. annuus after rewatering, with respect to the leaves of plants that remained under water deficit. Re-growth was accounted for by cell expansion. Increase in leaf area represented actual growth and not only a reversible change due to increased turgor.
Conclusions
After the leaf has ceased to grow, leaf cells retain their ability to expand for several days before leaf size becomes fixed. A response window was identified in both species, during which the extent of leaf area recovery decreased with time after the ‘initial’ leaf growth cessation. These results suggest that re-growth after rewatering of leaves having apparently attained their final size could be a generalized phenomenon, at least in dicotyledonous plants.
Key words: Arabidopsis thaliana, sunflower (Helianthus annuus), leaf expansion, leaf growth cessation, water deficit, rewatering
INTRODUCTION
With only a few exceptions (e.g. the Guarea genus; Steingraeber and Fisher, 1986), leaves expand during a given period of time until they reach their final size and form. This is called determinate growth and it is considered a critical feature that distinguishes leaves from shoots (Tsukaya, 1995) and roots (Fitter, 2002). In pea, sunflower or sorghum, duration of leaf expansion differs between successive leaves of the plant, but each of these durations are constant over a large number of experiments, excluding stressing environmental conditions, when they are expressed in thermal time (Turc and Lecoeur, 1997; Granier and Tardieu, 1998b; Lafarge and Tardieu, 2002; Dosio et al., 2003). In Arabidopsis thaliana, duration of expansion is similar for all leaf positions and stable when expressed in thermal time without any stress (Chenu et al., 2005). As a consequence, it is often proposed that duration of leaf expansion is controlled by a robust programme at the scale of the plant, which mainly depends on temperature and which is predictable when expressed in thermal time in non-stressing conditions.
Many studies on the plasticity of leaf expansion in response to abiotic stresses have shown the duration of leaf expansion to be consistently affected by stress. It was increased in response to a reduction in incident light, both in sunflower (Rawson and Dunstone, 1986; Granier and Tardieu, 1999a) and in A. thaliana (Chenu et al., 2005; Cookson and Granier, 2006). In response to soil water deficit, duration of leaf expansion has been shown to either increase (Takami et al., 1981, Rawson and Turner, 1982, Aguirrezábal et al., 2006) or remain unaffected (Karamanos et al., 1982, Granier and Tardieu, 1999b), depending on water deficit intensity and plant species.
At the cellular level, changes in duration of expansion by soil water deficit or shading are systematically accompanied by a decrease in epidermal cell number, whereas the epidermal cell area could be increased or decreased depending on the position and the intensity of the stressing treatment during leaf development (Aguirrezábal et al., 2006; Cookson and Granier, 2006; Granier et al., 2006b).
Knowledge of the mechanisms underlying leaf growth cessation is still scarce (in sunflower, Granier and Tardieu, 1998a; in Festuca, MacAdam and Nelson, 2002), but it is usually hypothesized that cell expansion becomes limited by an irreversible tightening of cell walls (e.g. Kutschera, 1996). Dimerization of ferulic acids could play a role in the control of some mechanical properties of the cell wall (Locher et al., 1994). Such bonds result from the activity of cell wall peroxidases (Fry, 1986; Lin and Kao, 2001). Cell wall peroxidase activity increases at the end of leaf expansion in Festuca (MacAdam et al., 1992) and is considered to be a likely candidate involved in growth cessation. Under this hypothesis, leaf size should be irreversibly fixed once cell expansion ceases.
The objective of this paper was to test whether leaf expansion could be restored by rewatering plants after a long soil water deficit period. Four experiments were performed in two different species (Arabidopsis thaliana and Helianthus annuus) in which leaves that had apparently reached their final size resumed growth upon modification of the environmental conditions (reversal of water stress). These experiments show that after the leaf has ceased to grow, leaf cells retain their ability to expand for several days before leaf size becomes fixed.
MATERIALS AND METHODS
Arabidopsis thaliana experiment (expt 1)
Two hundred plants of Arabidopsis thaliana (L.) Heynh ecotype Col-0 were grown in a growth chamber. Seeds were sown in cylindrical pots (9 cm high and 4·5 cm diameter) filled with a mixture (1:1, v/v) of a loamy soil and organic compost. Soil water content was determined before planting to estimate the amount of dry soil and water in each pot. Subsequent changes in pot weight were attributed to changes in soil water status after correction for plant weight (as described in Granier et al., 2006a). This allowed the calculation and adjustment of soil water content to be precisely defined. This adjustment was made manually. The substrate was previously characterized by a water-release curve relating plant predawn water potential to soil water content (Granier et al., 2006a).
The water-deficit treatment started at 13 d after emergence (Table 1 and Fig. 1A) when water was withheld until a soil water content of 0·22 g H2O g−1 dry soil (soil water potential = −0·55 MPa) was obtained. Leaf 1 was not fully expanded at the start of the water-deficit treatment (Table 1). A batch of 70 control plants was kept under well-watered conditions (soil water content = 0·5 g H2O g−1 dry soil, soil water potential = –0·3Mpa; Fig. 1A). At 31 d after emergence, when plants in the water-deficit treatment had 12 fully expanded leaves (Table 1), a batch of 115 of these was rewatered to a soil water potential similar to control plants (=0·5 g H2O g−1 dry soil, –0·3 MPa, Fig. 1A). The experiment ended when leaf 15 reached its final leaf area.
Table 1.
Description of water-deficit treatments imposed: soil water content and potential, dates of start of water deficit and rewatering, and number of fully expanded leaves at these moments
| Days after emergence |
Fully expanded leaves |
|||||||
|---|---|---|---|---|---|---|---|---|
| Expt | Species | Treatment | Soil water content (g g−1) | Soil water potential (Mpa) | Start of water deficit | Rewatering | Start of water deficit | Rewatering |
| 1 | A. thaliana | Early, long water deficit | 0·22 | −0·55 | 13 | 31 | 0 | 12 |
| 2 | H. annuus | Early, long water deficit | 0·20 | −0·65 | 18 | 49 | 1 | 9 |
| 3 | H. annuus | Early, long water deficit | 0·20 | −0·65 | 16 | 49 | 2 | 8 |
| Early, short water deficit | 0·20 | −0·65 | 16 | 30 | 2 | 4 | ||
| Late water deficit | 0·20 | −0·65 | 29 | 49 | 4 | 8 | ||
| 4 | H. annuus | Early, long water deficit | 0·20 | −0·65 | 17 | 56 | 2 | 8 |
| Early, short water deficit | 0·20 | −0·65 | 17 | 28 | 2 | 4 | ||
| Late water deficit | 0·20 | −0·65 | 27 | 56 | 4 | 8 | ||
| Severe water deficit | 0·18 | −1·31 | 17 | 56 | 2 | 8 | ||
Control plants of A. thaliana were kept at a soil water content of 0·50 g g−1 (soil water potential = −0·3 MPa), and control plants of H. annuus at a soil water content = 0·30 g g−1 (soil water potential = −0·04 MPa).
Fig. 1.
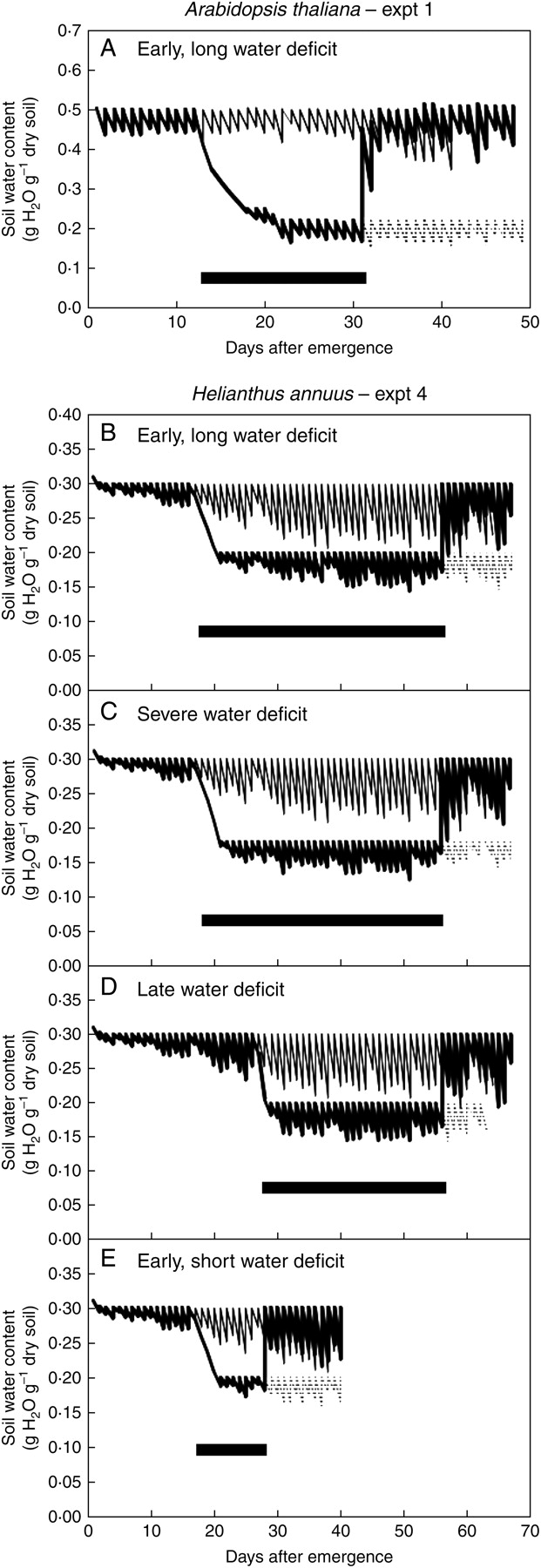
Changes with time in soil water content: (A) data from expt 1 (A. thaliana) and (B–E) expt 4 (H. annuus). Plants were initially grown in well-watered conditions (soil water content adjusted daily to 0·5 g H2O g−1 dry soil in A. thaliana and 0·3 g H2O g−1 dry soil in H. annuus), followed by a water-deficit treatment (soil water content adjusted daily to 0·22, 0·20 or 0·18 g H2O g−1 dry soil, depending on species and treatment), and then followed by rewatering to initial well-watered conditions (thick continuous line). Control plants were kept under well-watered conditions throughout the experiment (thin continuous line). In each water-deficit treatment, a batch of plants was kept under water deficit conditions after the moment of rewatering (dotted line). The thick horizontal bar indicates the length of each water-deficit treatment.
Light in the growth-chamber was provided by a bank of cool-white fluorescent tubes and sodium lamps. Day length was maintained at 10 h (Table 2). Photosynthetic photon flux density (PPFD) was measured continuously at plant level, using a PPFD sensor (LI-190SB, LI COR, Lincoln, NV, USA). Air temperature and relative humidity were measured every 20 s (HMP35A Vaisala Oy, Helsinki, Finland). All measurements of temperature, PPFD and relative humidity were averaged and stored every 600 s in a datalogger (Campbell Scientific, LTD-CR10 Wiring Panel, Shepshed, Leics, UK). Mean air vapour pressure difference (VPD) was calculated during the light period. Mean micrometeorological conditions during the experiment are presented in Table 1. Thermal time was calculated using a base temperature of 3 ° C (Granier et al., 2002).
Table 2.
Growth conditions during the four experiments
| Expt | Species | Day length (h) | Mean air temperature (° C) | Daily incident light (mol m–2 d–1) | VPD (kPa) |
|---|---|---|---|---|---|
| 1 | A. thaliana | 10·0 | 21·0 | 9·0 | 0·80 |
| 2 | H. annuus | 9·4 | 22·0 | 29·0 | 0·90 |
| 3 | H. annuus | 11·0 | 21·0 | 18·0 | 1·00 |
| 4 | H. annuus | 8·0 | 19·0 | 28·0 | 0·75 |
The effect of rewatering on leaf expansion was investigated in Arabidopsis thaliana grown in a growth chamber and Helianthus annuus grown in a greenhouse (expts 2, 3 and 4) For each experiment, mean micrometeorological conditions from sowing to end of rosette leaf development (expt 1) or from sowing to end of expansion of leaf 12 (expts 2, 3 and 4) are presented.
Digital pictures of the plants were taken manually two or three times a week. The area of leaves 6, 8, 10 and 12 was measured on these pictures using an image-analysis software. A leaf was considered as fully expanded when three consecutive measurements with equal or decreasing leaf area were obtained, considering the first as the moment of leaf growth cessation.
At the end of the experiment, transparent replication films of the adaxial epidermis of leaf 12 were obtained after evaporation of a varnish spread on the upper surface of the leaf. Films were placed under a microscope (Leica, Leitz DM RB, Wetzlar, Germany) coupled to an image analyser. The area of 25 epidermal cells (excluding guard cells and trichomes) was measured at four different places on the leaf: near the base, near the tip and on each side of the mid-vein of the leaf.
Helianthus annuus experiments (expts 2, 3 and 4)
One hundred and fifty plants of H. annuus cv. HAR2 were grown in a heated greenhouse in three different experiments. Seeds were sown in cylindrical pots (35 cm high and 10 cm diameter) filled with soil (Typic Argiudoll, horizon A). Soil water content measurements and adjustments were similar to expt 1. The water release curve of the soil in the pots had been previously characterized (Pereyra-Irujo et al., 2007).
Sunflower experiments included one to four water-deficit treatments (Table 1), consisting of two different imposition dates (when plants had one or two expanded leaves – ‘early’; or when plants had four expanded leaves – ‘late’), two different rewatering dates (when plants had four expanded leaves – ‘short water deficit’; or when plants had eight or nine expanded leaves – ‘long water deficit’), and two soil water potential levels (mild: soil water content = 0·20 g H2O g−1, soil water potential = –0·65 MPa, or severe: soil water content = 0·18 g H2O g−1, soil water potential = –1·31 MPa). In early water-deficit treatments, water deficit was imposed by using previously sown maize plants as described in Pereyra-Irujo et al. (2007). In late water-deficit treatments, no maize plants were used since leaf area and rooting depth of sunflower plants were enough to reach the defined soil water content rapidly. In all treatments, only half of the plants were rewatered, keeping the rest under water deficit. In all cases, a batch of plants was kept as a control without water deficit throughout the experiment (soil water content = 0·3 g H2O g−1, soil water potential = –0·04 MPa; Fig. 1B–E). The change in time of soil water content in the four water-deficit treatments imposed in expt 4, as compared with the controls, is shown in Fig. 1(B–E).
PPFD, air temperature and relative humidity were measured continuously at plant level. Measurements were made by using, respectively, a PPFD sensor, a thermistor and a humidity sensor, and stored every 15 min in a datalogger (Cavadevices, Buenos Aires, Argentina). Sensors were placed in a ventilated shelter. All measurements were averaged daily. Mean air VPD was calculated from air humidity and temperature during the light period. Mean micrometeorological conditions during the experiment are presented in Table 2. Thermal time was calculated using a base temperature of 4·8° C (Granier and Tardieu, 1998b).
Width and length of leaves 4, 5, 6, 7 and 8 were non-destructively measured every 2 or 3 d. Leaf area was calculated with a highly significant relationship (R2 > 0·99, n = 1755, P < 0·0001) obtained from actual leaf area data from different leaves of control and water-stressed plants of 18 sunflower genotypes (G. Pereyra-Irujo and L. Aguirrezábal, unpubl. res.). This equation was checked against destructive measurements of leaf area at the end of the experiment. As in expt 1, a leaf was considered as fully expanded when three consecutive measurements with equal or decreasing leaf area were obtained, considering the first as the moment of leaf growth cessation.
At the end of the experiment, transparent replication films of the adaxial epidermis of leaf 8 were obtained as described for expt 1. Cell areas were measured as described for expt 1, except that 50 cells of three leaf zones (base, middle and tip) were measured in this case.
Statistical analysis
Leaves considered were those that were fully expanded at the moment of rewatering. The effect of treatments on the final leaf area of each leaf was assessed by analysis of variance (0·05 significance level). Treatment means were compared by least significant difference tests (0·05 significance level). The effect of water deficit on leaf area was quantified as: (final leaf area water deficit – final leaf area control)/final leaf area control. The increase in leaf area after rewatering was quantified as: (final leaf area rewatered – final leaf area water deficit)/final leaf area water-deficit.
RESULTS
In both species, water-deficit treatments significantly reduced leaf growth. In all water-deficit treatments, leaves continued to grow at reduced rates, and the duration of leaf expansion was maintained or even increased (Fig. 2). Upon rewatering, leaves that were still growing increased their expansion rates, and some of the leaves that had already ceased to grow resumed expansion (Fig. 2). In expt 1, leaf 12 of A. thaliana plants subjected to a water deficit expanded for 5 d (88° Cd) after rewatering, increasing leaf area by 186 %. In expt 3, leaf 8 of H. annuus plants grown under mild water deficit resumed expansion for 6 d (71° Cd) upon rewatering, increasing leaf area by 27 %.
Fig. 2.
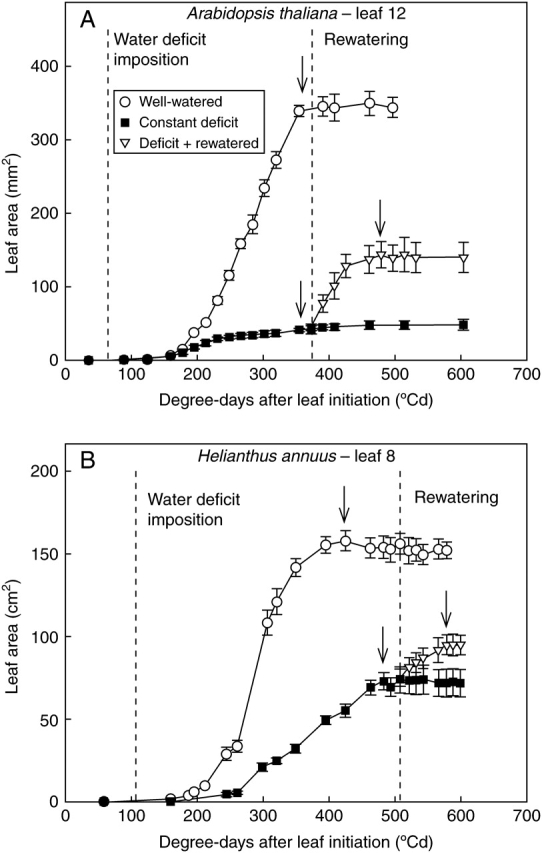
Response of leaf area to water deficit and rewatering: growth of leaf 12 of Arabidopsis thaliana (A) or leaf 8 of Helianthus annuus (B), under well-watered conditions, constant water deficit, or a water deficit followed by a rewatering after the studied leaf had ceased to expand. Leaf area is plotted against growing degree days after the initiation of each leaf. Arrows indicate the date when the leaf was considered fully expanded in each treatment. Note the difference in area units between (A) and (B). Vertical bars represent ± s.e. (n = 6).
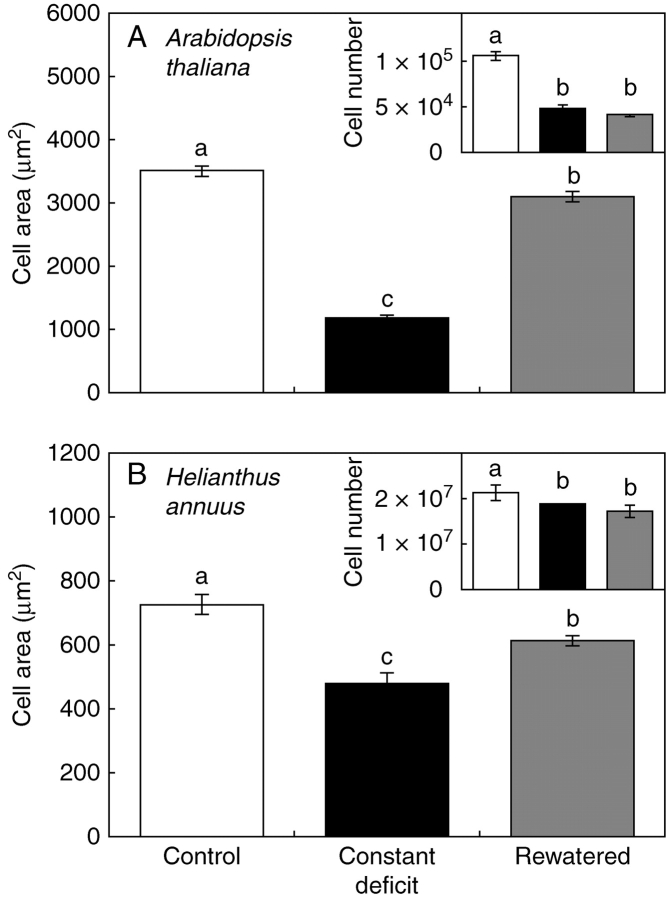
After rewatering, the increase in area of leaves having apparently attained their final size (Fig. 2) was paralleled by an increase in the area of epidermal cells, in both species (Fig. 3). Rewatering did not affect epidermal cell number with respect to plants that remained under water deficit, neither in A. thaliana nor in H. annuus (insets in Fig. 3A, B). In H. annuus, all the rewatered plants showed a re-growth of some of the leaves which had ceased to expand, regardless of the duration (Fig. 4A vs. C), the intensity (Fig. 4A vs. B) or the moment of imposition (Fig. 4C vs. D) of the water-deficit treatment. In A. thaliana, all the measured leaves which had ceased to expand at the moment of rewatering, drastically increased their area (Fig. 5, top). In the case of leaf 6, re-growth occurred despite plants were rewatered 11 d after cessation of expansion. In H. annuus, only the leaves that had recently ceased to expand (<5 d: leaves 7 and 8 in expt 3) responded significantly to rewatering (Fig. 5, bottom).
Fig. 3.
Response of epidermal cell area and cell number to water deficit and rewatering: final average cell area of leaf 12 of Arabidopsis thaliana (A) or leaf 8 of Helianthus annuus (B); under well-watered conditions (white); constant water deficit (black); or a water deficit followed by a rewatering after the studied leaf had ceased to expand (grey). Insets: final cell number per leaf calculated from final leaf area (Fig. 2) and cell area data. Data represent the mean value of measurements in three leaf zones of at least four plants. Vertical bars represent ± s.e. and different letters indicate significant differences between means (P < 0·05).
Fig. 4.
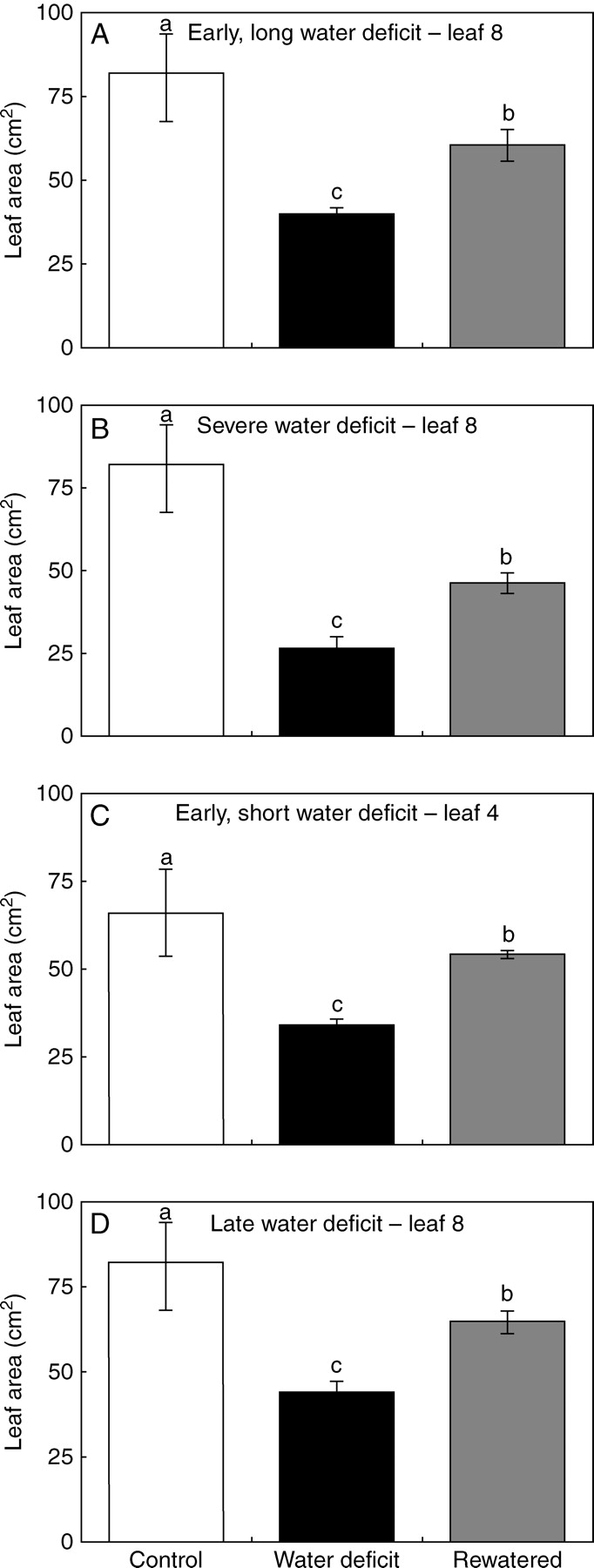
Response of leaf area to rewatering after water deficits of different duration, intensity, or applied at different moments: final area of leaves of Helianthus annuus, under well-watered conditions (white), constant water deficits of different duration, intensity, or applied at different moments (black), and the same water deficits followed by a rewatering after the studied leaf had ceased to expand (grey). Water deficits in (A) and (B) are long water deficits (34 d) of different intensity (–0·65 and –1·31 MPa, respectively). Water deficits in (C) and (D) are shorter (11 and 26 d), mild (–0·65 MPa) water deficits, applied at 17 and 28 d after emergence. Vertical bars represent ± s.e. (n = 6) and different letters indicate significant differences between means (P < 0·05)
Fig. 5.
Response of leaf area to water deficit and rewatering in different leaves of a plant: final area of leaves 6, 8, 10 and 12 of Arabidopsis thaliana or leaves 5, 6, 7 and 8 of Helianthus annuus, under well-watered conditions (white), constant water deficit (black), or a water deficit followed by a rewatering after the studied leaves had ceased to expand (grey). Vertical bars represent ± s.e. (n = 6) and different letters indicate significant differences between means (P < 0·05)
The decrease in final leaf area under water deficit was greater in younger leaves. Among leaves which had reached their final size, re-growth was also greater in the younger ones. No clear relationship was found, however, between both effects (P > 0·08). The effect of rewatering in leaves having apparently attained their final size was inversely related to the time elapsed between the end of leaf expansion and the moment of rewatering, which was lower in younger leaves (Fig. 6). In sunflower, this relationship accounted for the effect of rewatering (R2 = 0·79; P < 0·001), regardless of the moment of imposition of water deficit, its duration, its intensity or the moment of rewatering. This relationship determines a time window during which a leaf which has ceased to expand under stress is able to resume growth upon rewatering. In sunflower, this response window was found to be of approx. 60 ° Cd (approx. 4 d), during which the response decreases from 80 % to nearly zero (Fig. 6B). In A. thaliana, this window appears to be longer than 200 ° Cd (approx. 11 d), and the magnitude of the response is about twice that of sunflower (Fig. 6A).
Fig. 6.
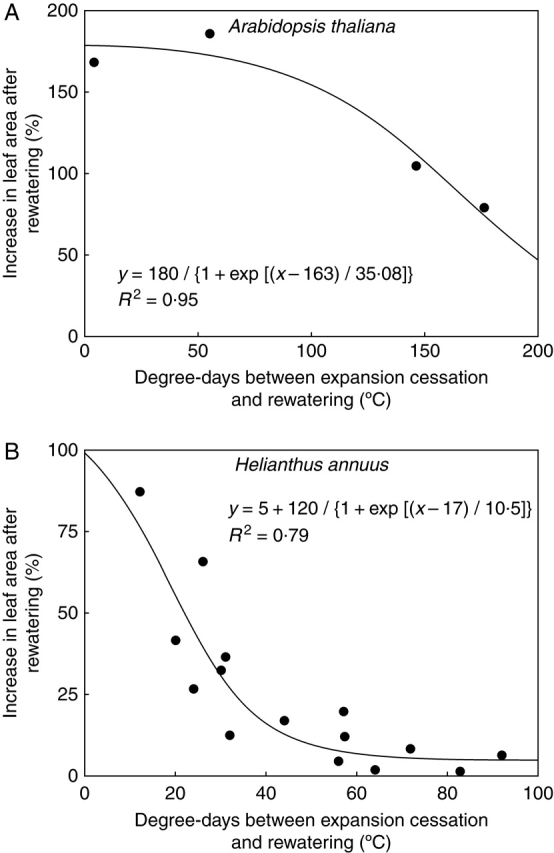
Response window of leaf area to rewatering: percentage increase in final leaf area of the rewatered treatments, relative to the final leaf area in the corresponding water-deficit treatment, in Arabidopsis thaliana (A) or Helianthus annuus (B). Data are plotted against the moment of rewatering, relative to the moment of cessation of leaf expansion in the water-deficit treatment. Data include all the experiments and treatments described.
DISCUSSION
The effect of rewatering on plants under water deficit has received a lot of attention as plants are often exposed to variations in soil water availability. Most of previous works mainly focused on the changes in different plant processes after rewatering, such as photosynthesis (Galmés et al., 2007), photoassimilate partitioning (Dai et al., 2007), and plant water relations (Secchi et al., 2007). The effect of rewatering on leaf expansion has been investigated on growing leaves of plants subjected to short water deficit periods (Singh et al., 2000; Alves and Setter, 2004). This paper focuses on re-growth after rewatering of leaves which have apparently attained their final size, a phenomenon that has not received attention in the literature. The main result is that this process could be significant in the recovery of plant leaf area after a water-deficit treatment. As an example, in expt 1, A. thaliana leaves of rewatered plants recovered to an average of 60 % of the area of well-watered plants whereas plants that remained under water deficit reached only 28 % of the area of the controls (calculated from Fig. 5).
Re-growth after rewatering of leaves having apparently attained their final size occurred both in the model species A. thaliana and the oil crop H. annuus. Both species are relatively genetically unrelated (Dominguez et al., 2003). Despite the wide range of conditions previous to rewatering explored in H. annuus, no clear effect of timing, duration or intensity of water deficit was observed. The magnitude of re-growth depended only on the time elapsed from the cessation of growth to rewatering. These results suggest that re-growth after rewatering of leaves having apparently attained their final size could be a generalized phenomenon, at least in dicotyledonous species, and a quite unspecific response of plants to variations in water availability.
The present results support the idea that the increase in area of leaves which had previously ceased to expand represents a new growth and not a reversible change due to increased turgor after rewatering. (a) Re-growth of leaves having attained their apparent final area occurred during several days depending on the species and the time elapsed from cessation of leaf expansion. The duration of re-growth ranged between 87 and 144 ° Cd for leaves 6–12 of A. thaliana, and between 12 and 71 ° Cd for leaves 6–8 of H. annuus. (b) Leaf area increased up to 186% in A. thaliana and up to 88 % in H. annuus after rewatering, with respect to the leaves of plants that remained under water deficit. In H. annuus plants, leaves which had ceased to expand >50 ° Cd before rewatering showed only a slight increase in area upon rewatering (5 % on average, Fig. 6B); this age-independent increase is presumably due to increased turgor. (c) the phenomenon was observed under water deficit levels previously characterized as moderate (Aguirrezabal et al., 2006; Granier et al., 2006a) for the ecotype Columbia of A. thaliana. Collectively, these results question the commonly accepted idea that leaf size should be irreversibly fixed once cell expansion ceases.
In both species, cell division in the leaf epidermis ceases well before the end of leaf expansion, when the leaf has attained approx. 50 % of its final area (Pyke et al., 1991; Granier and Tardieu, 1998a). During the phase with cell division in the sunflower leaf, epidermal cell area is lower than 200 µm2, whereas at the end of expansion all cells fall outside this range (Granier and Tardieu, 1998a), suggesting that no cells remain in the epidermis with a potential to re-enter the division cycle. As expected, in the present experiments cell proliferation was not detected upon rewatering in leaves that had previously ceased to expand. In both species, cell expansion accounted for growth of leaves having previously ceased to expand. A developmental gradient within the leaf determines that cells closer to the tip cease to expand before those closer to the base, both in A. thaliana (Pyke et al., 1991) and in H. annuus (Granier and Tardieu, 1998a), making it likely that re-growth be larger in basal than apical cells.
Biochemical and molecular mechanisms underlying re-growth of cells having ceased to expand are unknown. Cell expansion depends on cell turgor and rheological properties of cell wall (Lockhart, 1965), and both could theoretically be points of regulation of the re-growth process. It is currently considered that cell turgor is the driving force of cell expansion but it does not play a key role in the control of growth (e.g. Spollen and Sharp, 1991), the constitution of the cell wall and the activity of cell wall enzymes being the most likely points of regulation of this process. For instance, expansins are proteins involved in non-reversible cell wall extension (Catalá et al., 2000; Li et al., 2002; Belfield et al., 2005). Cell wall peroxidases could play a key role in cell wall tightening as their activity drastically increases after the end of leaf expansion in several species (MacAdam et al., 1992; Thompson et al., 1998). Consistently with the duration of the response window in H. annuus, a delay of 2–4 d between leaf growth cessation and the completion of cell wall deposition was found in two Festuca genotypes (MacAdam and Grabber, 2002). In the present experiments, the response of leaf area to rewatering depended on the time elapsed from the end of leaf expansion to the moment of rewatering and not on the magnitude of the change in soil water potential, making it unlikely that cell turgor alone regulates this response. Insufficient cell turgor for cell wall displacement could, however, be responsible for the ‘initial’ growth cessation. Processes linked to cell wall tightening seem to be delayed under water deficit, and the end of the response window for growth resumption could therefore represent the release from this condition. While more research is needed to clarify the biophysical and biochemical mechanisms underlying the re-growth after cessation of leaf expansion, studying this phenomenon could probably be useful to elucidate the control of leaf growth cessation.
A response window of leaf area to rewatering was identified in both species. Determining a response window could be useful to incorporate the effect of a recovery in plant water conditions into developmental models of whole-plant leaf area (e.g. Dosio et al., 2003). The results presented here show that cessation of leaf expansion in plants subjected to water deficit is a process which can be reversed by an increase in water availability. This attribute of leaf cells allows for the duration of the expansion of the leaf to increase drastically if the plant goes through a drying/rewatering cycle. Thus, the duration of leaf expansion under well-watered conditions or under constant water deficit was lower than the maximum (‘potential’) duration of growth. This result clearly shows the limitations of the conventional curvilinear models to describe the growth of leaves under non-constant water conditions. Moreover, while the study of rate and duration of leaf expansion under constant homogeneous water deficit was useful to identify clearly the response of different genotypes to water conditions (e.g. Aguirrezábal et al, 2006), results presented here show that extrapolation of these results would be of little value in predicting leaf growth of plants growing under fluctuating water availability.
ACKNOWLEDGMENTS
We thank J. J. Thioux, Ph. Hamard and L. Méndez for their help with plant growth measurements, and soil water content adjustment during the experiments. We also thank the useful comments of the anonymous reviewers. L.A.N.A. is a member of CONICET. L.L. and G.P.-I. hold scholarships from CONICET. Arabidopsis data were obtained during a post-doctoral stay of L.A.N.A. in France (INRA-LEPSE). Sunflower data are part of a thesis submitted by L.L. in partial fulfilment for the requirements for a doctorate. This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (PAV 00137 and PICTO ASAGIR 13168), GABI-GENOPLANTE (AF 2001 094), Institut National de la Recherche Agronomique, Département Environnement et Agronomie (support to L.A.N.A.'s stay in France) and Instituto Nacional de Tecnología Agropecuaria (PNCER 1336).
LITERATURE CITED
- Aguirrezábal L, Bouchier-Combaud S, Radiejwosky A, Dauzat M, Cookson SJ, Granier C. Plasticity to soil water deficit in Arabidopsis thaliana dissections of leaf development into underlying growth dynamic and cellular variables reveals invisible phenotypes. Plant, Cell and Environment. 2006;29:2216–2227. doi: 10.1111/j.1365-3040.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- Alves AAC, Setter TL. Response of cassava leaf area expansion to water deficit: cell proliferation, cell expansion and delayed development. Annals of Botany. 2004;94:605–613. doi: 10.1093/aob/mch179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfield EJ, Ruperti B, Roberts JA., McQueen-Mason S. Changes in expansin activity and gene expression during ethylene-promoted leaflet abscission in Sambucus nigra. Journal of Experimental Botany. 2005;56:817–823. doi: 10.1093/jxb/eri076. [DOI] [PubMed] [Google Scholar]
- Catalá C, Rose JKC, Bennett AB. Auxin-regulated genes encoding cell wall-modifying proteins are expressed during early tomato fruit growth. Plant Physiology. 2000;122:527–534. doi: 10.1104/pp.122.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu K, Franck N, Dauzat J, Barczi JF, Rey H, Lecoeur J. Integrated responses of rosette organogenesis, morphogenesis and architecture to reduced incident light in Arabidopsis thaliana results in higher efficiency of light interception. Functional Plant Biology. 2005;32:1123–1134. doi: 10.1071/FP05091. [DOI] [PubMed] [Google Scholar]
- Cookson SJ, Granier C. A dynamic analysis of the shade-induced plasticity in Arabidopsis thaliana rosette leaf development reveals new components of the shade adaptative response. Annals of Botany. 2006;97:443–452. doi: 10.1093/aob/mcj047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai ZW, Wang LJ, Zhao JY, Fan PG, Li SH. Effect and after-effect of water stress on the distribution of newly-fixed 14C-photoassimilate in micropropagated apple plants. Environmental and Experimental Botany. 2007;60:484–494. [Google Scholar]
- Dominguez I, Graziano E, Gebhardt C, Barakat A, Berry S, Arús P, Delseny M, Barnes Plant genome archaeology: evidence for conserved ancestral chromosome segments in dicotyledonous plant species. Plant Biotechnology Journal. 2003;1:91–99. doi: 10.1046/j.1467-7652.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- Dosio GAA, Rey H, Lecoeur J, Izquierdo NG, Aguirrezábal LAN, Tardieu F, Turc O. A whole-plant analysis of the dynamics of expansion of individual leaves of two sunflower hybrids. Journal of Experimental Botany. 2003;54:2541–2552. doi: 10.1093/jxb/erg279. [DOI] [PubMed] [Google Scholar]
- Fitter A. Characteristics and functions of root systems. In: Waisel Y, Eshel A, Kafkafi U, Dekker M, editors. Plant roots: the hidden half. 2nd edn. New York, NY: Marcel Dekker; 2002. [Google Scholar]
- Fry SC. Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annual Review of Plant Physiology. 1986;37:165–186. [Google Scholar]
- Galmés J, Medrano H, Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytologist. 2007;175:81–93. doi: 10.1111/j.1469-8137.2007.02087.x. [DOI] [PubMed] [Google Scholar]
- Granier C, Tardieu F. Spatial and temporal analyses of expansion and cell cycle in sunflower leaves: a common pattern of development for all zones of a leaf and different leaves of a plant. Plant Physiology. 1998;a 116:991–1001. doi: 10.1104/pp.116.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Tardieu F. Is thermal time adequate for expressing the effects of temperature on sunflower leaf development? Plant, Cell and Environment. 1998;b 21:695–703. [Google Scholar]
- Granier C, Tardieu F. Leaf expansion and cell division are affected by reducing absorbed light before but not after the decline in cell division rate in the sunflower leaf. Plant, Cell and Environment. 1999;a 22:1365–1376. [Google Scholar]
- Granier C, Tardieu F. Water deficit and spatial pattern of leaf development: variability in responses can be simulated using a simple model of leaf development. Plant Physiology. 1999;b 119:609–620. doi: 10.1104/pp.119.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Massonnet C, Turc O, Muller B, Chenu K, Tardieu F. Individual leaf development in Arabidopsis thaliana: a stable thermal-time-based programme. Annals of Botany. 2002;89:595–604. doi: 10.1093/aob/mcf085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, et al. PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytologist. 2006;a 169:623–635. doi: 10.1111/j.1469-8137.2005.01609.x. [DOI] [PubMed] [Google Scholar]
- Granier C, Cookson SJ, Tardieu F, Muller B. Cell cycle and environmental stresses. In: Inzé D, editor. Cell cycle control and plant development. Annual Plant Reviews. b Vol. 32. Oxford: Blackwell Publishing; 2006. pp. 335–355. [Google Scholar]
- Karamanos A, Elston J, Wadsworth R. Water stress and leaf growth of field beans (Vicia faba L.) in the field: water potentials and laminar expansion. Annals of Botany. 1982;49:815–826. [Google Scholar]
- Kutschera U. Cessation of cell elongation in rye coleoptiles is accompanied by a loss of cell-wall plasticity. Journal of Experimental Botany. 1996;47:1387–1394. [Google Scholar]
- Lafarge T, Tardieu F. A model co-ordinating the elongation of all leaves of a sorghum cultivar was applied to both Mediterranean and Sahelian conditions. Journal of Experimental Botany. 2002;53:715–725. doi: 10.1093/jexbot/53.369.715. [DOI] [PubMed] [Google Scholar]
- Li Y, Darley CP, Ongaro V, Fleming A, Schipper O, Baldauf SL, et al. Plant expansins are a complex multigene family with an ancient evolutionary origin. Plant Physiology. 2002;128:854–864. doi: 10.1104/pp.010658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Kao CH. Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Science. 2001;160:323–329. doi: 10.1016/s0168-9452(00)00396-4. [DOI] [PubMed] [Google Scholar]
- Locher R, Martin HV, Grison R, Pilet PE. Cell wall-bound trans- and cis-ferulic acids in growing maize roots. Physiologia Plantarum. 1994;90:734–738. [Google Scholar]
- Lockhart JA. An analysis of irreversible plant cell elongation. Journal of Theoretical Biology. 1965;8:264–275. doi: 10.1016/0022-5193(65)90077-9. [DOI] [PubMed] [Google Scholar]
- MacAdam JW, Grabber JH. Relationship of growth cessation with the formation of diferulate cross-links and p-coumaroylated lignins in tall fescue leaf blades. Planta. 2002;215:785–793. doi: 10.1007/s00425-002-0812-7. [DOI] [PubMed] [Google Scholar]
- MacAdam JW, Nelson C. Secondary cell wall deposition causes radial growth of fibre cells in the maduration zone of elongation tall fescue leaf blades. Annals of Botany. 2002;89:89–96. doi: 10.1093/aob/mcf010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAdam JW, Nelson CJ, Sharp RE. Peroxidase activity in the leaf elongation zone of tall fescue: I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiology. 1992;99:872–878. doi: 10.1104/pp.99.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra-Irujo GA, Velázquez L, Granier C, Aguirrezábal LAN. A method for drought tolerance screening in sunflower. Plant Breeding. 2007:445–448. [Google Scholar]
- Pyke KA, Marrison JL, Leech AM. Temporal and spatial development of the cells of the expanding first leaf of Arabidopsis thaliana (L.) Heynh. Journal of Experimental Botany. 1991;42:1407–1416. [Google Scholar]
- Rawson H, Dunstone R. Simple relationships describing the responses of leaf growth to temperature and radiation in sunflower. Australian Journal of Plant Physiology. 1986;13:321–327. [Google Scholar]
- Rawson H, Turner N. Recovery from water stress in five sunflower (Helianthus annuus L.) cultivars. II. The development of leaf area. Australian Journal of Plant Physiology. 1982;9:449–460. [Google Scholar]
- Secchi F, Lovisolo C, Uehlein N, Kaldenhoff R, Schubert A. Isolation and functional characterization of three aquaporins from olive (Olea europaea L.) Planta. 2007;225:381–392. doi: 10.1007/s00425-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Singh DK, Sale PWG, Pallaghy CK, Singh V. Role of proline and leaf expansion rate in the recovery of stressed white clover leaves with increased phosphorus concentration. New Phytologist. 2000;146:261–269. doi: 10.1046/j.1469-8137.2000.00643.x. [DOI] [PubMed] [Google Scholar]
- Spollen WG, Sharp RE. Spatial distribution of turgor and root growth at low water potentials. Plant Physiology. 1991;96:438–443. doi: 10.1104/pp.96.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingraeber DA, Fisher JB. Indeterminate growth of leaves in Guarea (Meliaceae): a twig analogue. American Journal of Botany. 1986;73:852–862. [Google Scholar]
- Takami S, Turner N, Rawson H. Leaf expansion of four sunflower (Helianthus annuus L.) cultivars in relation to water deficits. I. Patterns during plant development. Plant, Cell and Environment. 1981;4:399–407. [Google Scholar]
- Thompson DS, Davies WJ, Ho LC. Regulation of tomato fruit growth by epidermal cell wall enzymes. Plant, Cell and Environment. 1998;21:589–599. [Google Scholar]
- Tsukaya H. Developmental genetics of leaf morphogenesis in dicotyledonous plants. Journal of Plant Research. 1995;108:407–416. [Google Scholar]
- Turc O, Lecoeur J. Leaf primordium initiation and expanded leaf production are co-ordinated through similar response to air temperature in pea (Pisum sativum L.) Annals of Botany. 1997;80:265–273. [Google Scholar]