Abstract
Background and Aims
Recent studies have shown that small structures on plant surfaces serve ecological functions such as resistance against herbivores. The morphology, distribution, chemical composition and changes during shoot and leaf development of such small structures were examined on Paulownia tomentosa.
Methods
The morphology and distribution of the structures were studied under light microscopy, and their chemical composition was analysed using thin-layer chromatography and high-performance liquid chromatography. To further investigate the function of these structures, several simple field experiments and observations were also conducted.
Key Results
Three types of small structures on P. tomentosa were investigated: bowl-shaped organs, glandular hairs and dendritic trichomes. The bowl-shaped organs were densely aggregated on the leaves near flower buds and were determined to be extrafloral nectarines (EFNs) that secrete sugar and attract ants. Nectar production of these organs was increased by artificial damage to the leaves, suggesting an anti-herbivore function through symbiosis with ants. Glandular hairs were found on the surfaces of young and/or reproductive organs. Glandular hairs on leaves, stems and flowers secreted mucilage containing glycerides and trapped small insects. Secretions from glandular hairs on flowers and immature fruits contained flavonoids, which may provide protection against some herbivores. Yellow dendritic trichomes on the adaxial side of leaves also contained flavonoids identical to those secreted by the glandular hairs on fruits and flowers. Three special types of leaves, which differed from the standard leaves in shape, size and identity of small structures, developed near young shoot tips or young flower buds. The density of small structures on these leaf types was higher than on standard leaves, suggesting that these leaf types may be specialized to protect young leaves or reproductive organs. Changes in the small structures during leaf development suggested that leaves of P. tomentosa are primarily protected by glandular hairs and dendritic trichomes at young stages and by the EFNs at mature stages.
Conclusions
The results indicate that P. tomentosa protects young and/or reproductive organs from herbivores through the distribution and allocation of small structures, the nature of which depends on the developmental stage of leaves and shoots.
Key words: Anti-herbivore defence, dendritic trichome, extrafloral nectary, flavonoids, glandular hair, glycerides, indirect defence, leaf development, morphology, optimal defence hypothesis, Paulownia tomentosa, shoot development
INTRODUCTION
Small structures on the plant surface (epidermal appendages), such as various types of trichomes, extrafloral nectaries (EFNs) and food bodies (small, nutrient-rich structures that can easily be removed by foraging ants), serve various ecological functions, particularly in resistance against herbivores. For example, hook-like trichomes of Passiflora adenopoda provide defence against heliconiine butterfly larvae, a major class of Passiflora herbivores, by injuring the body surfaces of the larvae (Gilbert, 1971). In addition, glandular trichomes on the stems and leaves of tomato (Solanum lycopersicum) impede the rate of movement of caterpillars on plants (Wilkens et al., 1996). The stinging hairs of nettles (Urtica dioica) deter mammalian herbivores, such as rabbits and sheep (Pollard and Briggs, 1984). Several studies of various species have demonstrated that EFNs and food bodies play important roles in plant–ant mutualisms wherein plants provide food for the ants, which in turn provide protection from herbivores (for reviews see Bentley, 1977; O'Dowd, 1982; Beattie, 1985; Koptur, 1992; Heil and McKey, 2003). For example, in Catalpa speciosa (Bignoniaceae) bearing EFNs visited by ants, significantly fewer herbivore larvae were removed from ant-excluded branches, which also experienced significantly more leaf herbivory (Stephenson, 1982). Plants of the neotropical genus Cecropia (Moraceae) provide food bodies (Müllerian bodies) as resources for Azteca ants living in the Cecropia; in turn, ants attack herbivores and remove vines growing on the trunks of the plants (Janzen, 1969; Eugene, 1986). Some structures may also serve protective functions against ultraviolet (UV) radiation. For example, non-glandular trichomes on the leaves of Cydonia oblonga and Eriobotrya japonica contain UV-B-absorbing compounds. The removal of these trichomes results in UV-B radiation damage, including a reduction of the photochemical efficiency of photosystem II and epidermal browning (Karabourniotis et al., 1995).
In most cases, the functions of these structures have not yet been determined. In addition, most studies have focused on a particular structure, even though a single species often has several types of small structures. The comprehensive examination of the small structures on a single species is important to fully understand the functions of these structures. A plant may gain ecological benefits, such as resistance against herbivores, by basing the distribution and allocation of these structures on its developmental state and environmental conditions.
This study reports a thorough investigation of the characteristics and functions of three types of small structures found on Paulownia tomentosa (Scrophulariaceae), a deciduous tree native to eastern Asia and distributed throughout China, Korea and Japan. This tree grows to 10–25 m tall and 40–50 cm in trunk diameter at breast height (DBH). The chemical composition, distribution and allocation of these small structures on the plant were analysed, as well as changes in the structures with leaf and/or shoot development. Field observations and simple field experiments were also conducted to investigate the ecological function of the small structures. Based on the results, we discuss how P. tomentosa organizes these structures to fulfill its ecological requirements during leaf and/or shoot development.
MATERIALS AND METHODS
Experimental trees
The surface structures of the leaves from six mature Paulownia tomentosa were observed and collected for chemical analysis. Five of these trees (Nos. 1–5) grew on the campus of the Tokyo Institute of Technology (35°36′N, 139°41′E), and the sixth tree (No. 6) was located on the grounds of the Kawasaki Mizonokuchi Post Office (35°36′N, 139°36′E). The sizes of these trees (Nos. 1–6) at the time of the study were 12, 10, 12, 12, 9 and 8 m in height and 25, 20, 20, 25, 20 and 20 cm in DBH, respectively.
For the chemical analysis of glandular hairs on the leaf, leaves of bud flushes were collected from the stump of a mature tree (No. 7) felled at the base in the previous year. The size of the bud flush at the time of the study was 1·5 m in height and 2 cm in DBH.
For the field experiment examining the response of the bowl-shaped organ (see Results for description) to artificial leaf damage, leaves of bud flushes from tree stumps (Nos. 8–11) were used and also tree No. 1. The sizes of these bud flushes during the study period were 1·5, 1·7, 1·5, 2·2 and 12 m in height and 2·5, 3·0, 2·0, 3·5 and 25 cm in DBH, respectively.
Stages of leaf development
The developmental stages of leaves were defined as follows, based on the longitudinal length of the leaves (‘AL’ represents the average length of fully developed leaves of each individual tree determined from the previous year's growth; AL ranged from 20·8 to 30·6 cm, and the mean AL of the six experimental trees was 24·7 cm): Stage 1, leaves fully unfolded with longitudinal length <20 % of AL; Stage 2, longitudinal length between 20 % and 80 % of AL; and Stage 3, longitudinal length >80 % of AL.
Leaf samples were harvested between April and August (1997 to 1999, and 2005) to examine changes in the leaf-surface structures during leaf development. Stage 1 of the ‘first leaf’ (see Results, ‘Changes in leaf type during shoot development’) was defined as the leaf with a longitudinal length <50 % of AL, because this type of leaf grows to approximately 40 % of AL prior to unfolding.
The sizes of ‘flower-bud leaves’ (see Results, ‘Changes in leaf type during shoot development’) differed greatly depending on the position of leaves in sub-branches with flower buds. Therefore, the AL of flower-bud leaves was defined as the AL of fully expanded leaves at the same position.
Density and total number of structures per leaf
To estimate the density and total number of each structure on leaves, 8–65 leaves were sampled from five trees (Nos. 1–4, 6) for analyses. Leaf area was measured by scanning an image of the leaf and analysing it using NIH-image software. The total numbers of each structure on a leaf or in a small area (1·0 mm2) on the leaf surface were counted under light microscopy. Densities were calculated by dividing the total number of each structure by the observed leaf area. See Results for descriptions of structures referred to in the following sections.
Chemical analysis of structures
Constituents of the secretions of the bowl-shaped organ
Secretions from the bowl-shaped organ were collected in capillary pipettes from one tree (No. 1) from May to August 1997 and from five trees (Nos. 1–5) from May to August 2004. All samples were stored at –80 °C until just prior to analyses. In 1997, secretions were analysed using the Benedict test. In 2004, pipettes were dipped in methanol to extract surface components, and the extract was analysed using thin-layer chromatography [TLC; Merck pre-coated TLC plates, Silicagel 60F-254 (0·1 mm thickness), solvent system: isopropanol–water 7 : 1] with a silver nitrate–sodium hydroxide reagent (Jork, 1990) and glucose and sucrose as reference samples.
Constituents of the secretions of glandular hairs on leaf, stem and flower surfaces
Secretions from the glandular hairs on the surface of fresh leaves from four adult trees (Nos. 1–4) were collected by removing secretions with glass fibre filters (ADTEC, GA200, 47 mm, cut into 1-cm2 pieces) from May to August 2005. The filters were dipped in methanol for 5 min. The extract was analysed using TLC (solvent system: chloroform–methanol 10 : 1) with a 5 % ethanolic phosphomolybdic acid reagent).
For nuclear magnetic resonance (NMR) analysis of the secretions from glandular hairs of adult trees, the secretions on the surface of fresh leaves (500 g) were collected from the bud flush (No. 7) on 28 April 2006 and were dissolved into diethyl ether (600 mL) by briefly dipping the leaves in the solvent, and the solution was then concentrated to dryness (700 mg). TLC analysis indicated that the extract included the same compounds isolated from the secretions of glandular hairs on the leaf surfaces of adult trees. Then, the extract was separated by column chromatography (CC) on neutral silica gel (63–210 µm, 25 g). The column was eluted successively with a gradient of n-hexane–AcOEt (6 : 1–1 : 2) and four fractions (Fr. 1–4) were collected. Fraction 1 was subjected to CC using flash silica gel (40–100 µm, 2 g) and the column was eluted with n-hexane–AcOEt (8 : 1–3 : 1) to give compounds 1a–c and 2 (see Fig. 4A). Fraction 2 was subjected to CC using flash silica gel (40–100 µm, 5 g) and the column was eluted with n-hexane–AcOEt (3 : 1–1 : 1) to give compound 3 (see Fig. 4A). Fraction 3 was composed of compounds 4a and 4b (see Fig. 4A) and Fr. 5 gave compounds 5a–c (see Fig. 4A). 1H- and 13C-NMR spectra of these fractions were recorded on a Bruker DRX500 spectrometer (500 MHz for 1H and 125 MHz for 13C) in CDCl3 or CDCl3/CD3OD (9 : 1) solution (for details see Asai et al., 2008).
Fig. 4.
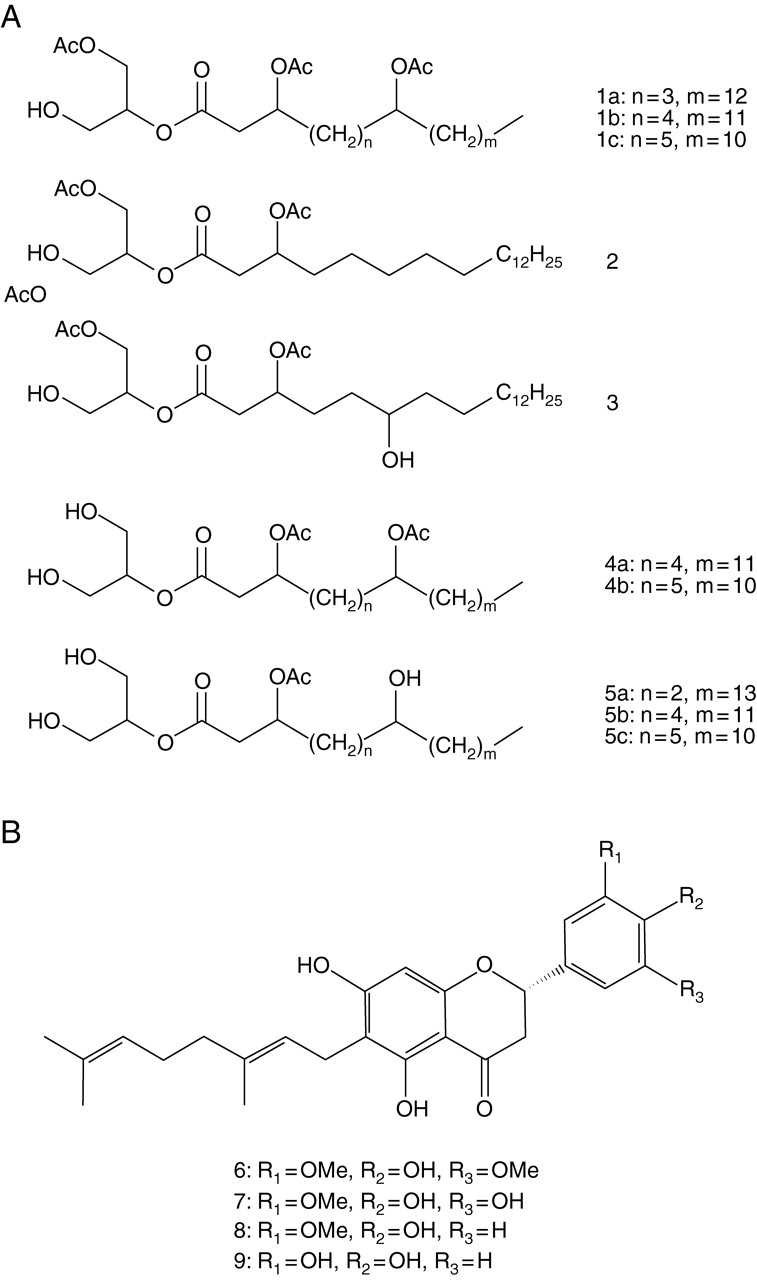
Constituents of the small structures of P. tomentosa. (A) Constituents of the secretions of glandular hairs on the leaf surface. (B) Constituents of yellow dendritic trichomes on the leaf surface.
Flowers were collected from one adult tree (No. 1) in May 2006 and stored at –80 °C until immediately prior to analysis. Secretions from the glandular hairs on the flower surface were collected by briefly washing the surface with methanol. These methanol extracts were analysed using TLC (solvent system: chloroform–methanol 10 : 1) with a 5 % ethanolic phosphomolybdic acid reagent.
Constituents of the secretions of glandular hairs on the surface of immature fruit
The viscous material on the surfaces of 260 fresh, immature fruits of P. tomentosa was collected from tree No. 5 on 14 October 2005 and was dissolved in methanol (300 mL) by briefly dipping the fruits in the solvent. The methanol solution was concentrated to dryness (5 g). The extract was chromatographed over silica gel with hexane–EtOAc–MeOH as eluents followed by a combination of chromatographies over silica gel, Sephadex LH-20, and/or reversed-phase HPLC (flow rate: 1·0 mL min−1, solvent: 10 or 25 % aqueous MeOH, UV detection at 282 nm). The methanol extract was analysed by NMR spectroscopy (for details see Asai et al., 2008).
Constituents of dendritic trichomes on the leaf surface
From May to August 2005, the yellow or white dendritic trichomes on the surfaces of leaves from four trees (Nos. 1–4) and the brown dendritic trichomes on the surfaces of flower buds from one tree (No. 1) were removed using tweezers. Trichomes were dipped in methanol to extract components. The extracts of yellow dendritic trichomes on adaxial leaf surfaces were analysed by direct comparison to reference samples isolated from the secretions of glandular hairs on the fruit surface [TLC and high-performance liquid chromatography (HPLC) analysis with a Shimadzu LC-6A apparatus equipped with a UV detector using a reversed-phase column (Inertsil ODS-3, 15 cm × 4·6 mm i.d.) under isocratic solvent condition]. The methanol extract of the white dendritic trichomes on the abaxial leaf surface and the brown dendritic trichomes on the flower bud surface were analysed by direct comparison to reference samples isolated from the secretions of glandular hairs on the fruit surface as well as the methanol extract of the yellow dendritic trichomes on the adaxial leaf surface (TLC and HPLC analysis).
Observations of arthropods feeding on the secretions of the bowl-shaped organ
From 7 May to 5 November 1997, the number of each type of arthropod that fed on the secretions of the bowl-shaped organs was recorded once a week by visual observation for seven branches of an adult tree (No. 1). The total number of observations was 27.
Observations of the time spent by ants on leaves
The time that ants spent on leaves was recorded on one branch of an adult tree (No. 1) by visual observation. The amount of time spent by ants on mature leaves with secretions and young leaves without secretions was recorded once a week for 10 min from April to May 1997, for a total of five observations.
Changes in the amount and rate of secretion of the bowl-shaped organ due to artificial leaf damage
On 27–28 August and 3–4 September 2007 (4 d of no rain), changes in the amount and rate of secretion from the bowl-shaped organs of leaves were compared after application of two different treatments (see below). Before treatments were applied, all animals (i.e. possible nectar consumers) on the leaves were carefully eliminated after checking the rate and amount of secretion. The first treatment involved artificially damaging leaves by puncturing them 200 times using injector needles (Terumo Needle; 19 G × 1·5, 1·1 mm in diameter). The damage corresponded to a loss of approximately 0·74 % of the leaf area (mean of 30 leaves, s.d. = 0·56) evenly spread over the leaf blade. After the treatment was applied, the branch was covered with gauze bags (mesh size 0·5 mm) to protect the nectaries from nectar consumers. Control leaves were not damaged and were covered with gauze bags after checking the amount and rate of secretion.
Five plants (Nos. 1, 8–11) were used in these experiments. For the first study in August, leaves on one branch of each of four plants (Nos. 8–11) were used. For the second investigation in September, leaves on one branch of each of four plants (Nos. 8–11) and those on eight branches of tree No. 1 were used. The amount secreted by the bowl-shaped organs on four fully expanded ‘standard’ leaves (mean leaf area = 383 cm2, n = 30 leaves, s.d. = 230) near the tip of each branch was measured.
The amount and rate of secretion of the bowl-shaped organs on the surface of the adaxial side of leaves were measured using a field microscope (50×). The secretion amount was quantified by rating the size of the secretion droplet as follows: Level 0, no secretion (score = 0); Level 1, diameter of the secretion droplet was <50 % of the depth of the bowl-shaped organ (score = 1); Level 2, diameter of the secretion droplet was >50 % of the depth of the bowl-shaped organ (score = 2); Level 3, diameter of the secretion droplet exceeded the depth of the bowl-shaped organ (score = 3; see Fig. 1A).
Fig. 1.

Small structures on leaves of Paulownia tomentosa. (A) Bowl-shaped organ (EFN). (B) Section of a bowl-shaped organ (0·01 mm) stained with light green and safranin. (C) Glandular hairs. (D) Yellow dendritic trichomes. (E) White dendritic trichomes. Scale bars = 100 µm.
The secretion amount of the leaf was calculated as the sum of the scores of all bowl-shaped organs on the leaf divided by the total number of structures (for normalization). The secretion amount of the branch was calculated as the sum of the secretion amounts of the four leaves on the branch. The secretion rate was estimated as the percentage of bowl-shaped organs with secretion droplets (levels 1–3). The secretion rate of the branch was calculated as the average of the secretion rates of the four leaves on the branch. After the initial measurements, the amount and rate of secretion of the same leaves were measured again after 24 h.
Leaf-slice specimens for microscopy
Leaves were cut to approximately 5 mm2, dipped in 10 % formalin (in H2O) for ≥1 h, dipped consecutively in 70, 90, 100 and 100 % ethanol for 30 min each, placed in 1:1 xylene : paraffin (54 °C, 30 min), sliced into 0·01-mm pieces, and then stained with 1 % safranine (in 50 % ethanol) and 1 % light-green (in 100 % ethanol).
Statistical analyses
Differences in the densities of bowl-shaped organs, glandular hairs and dendritic trichomes among the four leaf types, changes in the density and secretion rate of bowl-shaped organs, densities of glandular hairs and dendritic trichomes during leaf development, and changes in the amount and rate of secretion of the bowl-shaped organs due to artificial damage were analysed statistically using one-way analysis of variance (ANOVA).
RESULTS
Morphology, distribution and chemical constituents of the structures found on the surface of P. tomentosa
The following three types of structures were found on the surface of P. tomentosa, and are referred to here as bowl-shaped organs, glandular hairs and dendritic trichomes.
Bowl-shaped organ
The bowl-shaped organ (Fig. 1A) is a structure shaped like a small bowl attached at its base to the plant surface. This structure was afound to be approx. 0·2–0·3 mm in diameter and was distributed on the leaf, stem, and calyx of the flower and fruit. The organ was distributed on both sides of leaves, particularly at the base of the leaf and near the main vein. This structure was also found near the base of the leaf stalk on the stem and in the cleavage position of the flower and fruit calyx (Table 1, Fig. 2A). The cross-section of this structure when stained with light-green and safranine (Fig. 1B) indicated that the main part of this organ and its basement membrane consist of many cells with vacuole-like structures stained by safranine.
Table 1.
Distribution of small structures on P. tomentosa
| Leaf |
Flower and fruit |
|||||
|---|---|---|---|---|---|---|
| Structure | Immature | Mature | Stem (immature) | Surface | Calyx | Flower bud |
| Bowl-shaped organ (EFN) | * | * | * | |||
| Glandular hair | ** | ** | ** | |||
| Yellow dendritic trichome | * | * | ||||
| White dendritic trichome | ** | * | ||||
| Brown dendritic trichome | ** | ** | ||||
* Structure present; ** density of structure especially high.
Fig. 2.
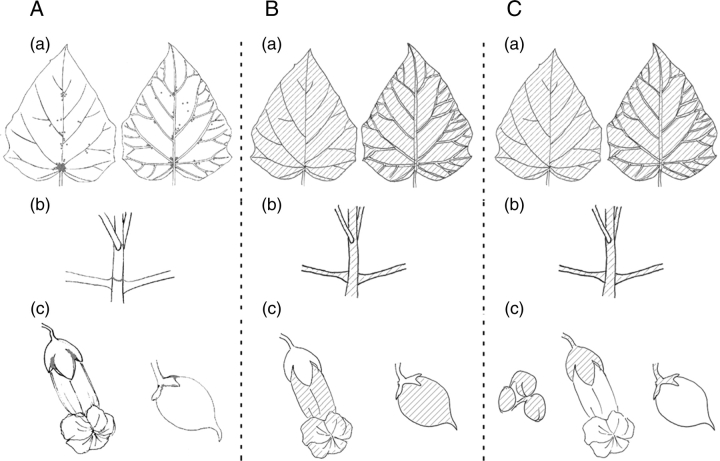
Distribution of three types of small structures on the surface of P. tomentosa. (A) Bowl-shaped organs (EFNs; dots) were found on (a) leaf (left: adaxial side; right: abaxial side), (b) stem, and (c) calyx. (B) Glandular hairs (shaded area) were found on (a) leaf (left: adaxial side; right: abaxial side in young trees and some adult trees), (b) petiole and stem in young trees and immature parts of adult trees, and (c) on flowers and fruits. (C) Dendritic trichomes (shaded area) were found on (a) leaf in mature trees (left: adaxial side; right: abaxial side), (b) on petiole and stem in mature trees, and (c) on calyx of flower buds, flowers and fruits.
The bowl-shaped organs produced liquid from the hollow side of the structure (Fig. 1A), and the Benedict test indicated that the secretions contained sugar. Further TLC analysis and comparison with standards determined that the sugar was a monomeric reducing sugar. Secretions were minimal in young leaves and became more prolific with leaf growth. In very mature leaves, the organ gradually became brown in colour, exhibited a decrease in the rate of secretion, and dropped off the leaf.
Various insects, spiders and mites were observed feeding on the secretions of the bowl-shaped organ; however, ants were the most frequently observed animals feeding (85 % of the observed animals, Table 2). The length of stay by ants was compared between mature leaves with secretions and young leaves without secretions, and it was found that ants stayed on mature leaves for significantly longer periods of time than on young leaves (F1,9 = 6·71, P = 0·03). These results indicate that the bowl-shaped organ is probably an extrafloral nectary (EFN) that attracts ants.
Table 2.
Arthropods observed feeding on the secretion of the bowl-shaped organ (EFN)
| Group of arthropod | Observed number |
|---|---|
| Ants* | 65 |
| Aphids | 3 |
| Leaf beetles | 3 |
| Spider mites | 2 |
| Ladybirds | 2 |
| Ant-mimicking spiders | 1 |
* The ants observed included Formica japonica, Camponotus japonicus and Lasius sakagamii.
The number of arthropods was recorded approximately once a week from 7 May to 5 November 1997 (27 weeks) for seven branches in an adult tree. Total number of the arthropods observed feeding = 76.
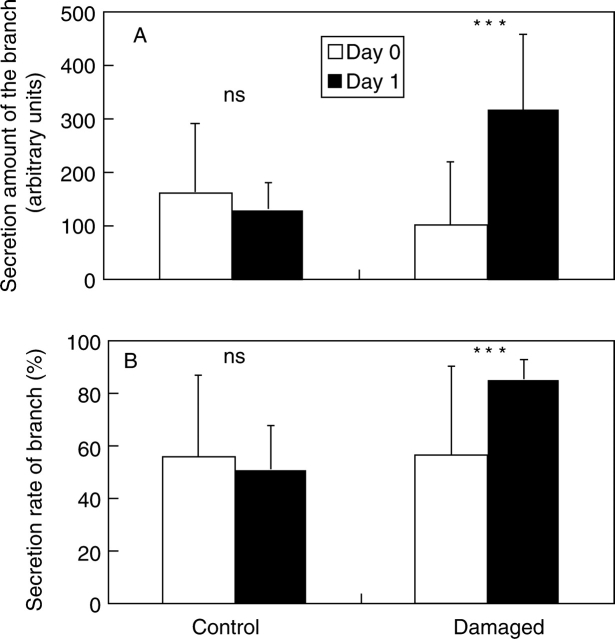
Artificial damage to leaves significantly increased the secretions of the bowl-shaped organ. The amount of secretion in branches with artificially damaged leaves significantly increased to 335 % of the level before treatment within 1 d (F1,14 = 17·5, P < 0·001). In contrast, the amount of secretion of control branches did not change significantly and instead exhibited a slight, non-significant decrease (F1,14 = 0·24, P > 0·5; Fig. 3A). The rate of secretion of branches with artificially damaged leaves also significantly increased (F1,14 = 21·1, P < 0·001), whereas control branches showed a slight, although non-significant, decrease in secretion rate (F1,14 = 0·079, P > 0·5; Fig. 3B). Even within the same tree (No. 1), the amount and rate of secretion of branches with artificially damaged leaves also significantly increased to levels 205 % and 32 % higher, respectively, than before treatment (amount: F1,6 = 8·68, P < 0·05; rate: F1,6 = 20·6, P < 0·05). In contrast, control branches exhibited a slight, but non-significant, decrease in the amount and rate of secretion of the bowl-shaped organs (amount: F1,6 = 1·00, P > 0·5; rate: F1,6 = 0·62, P > 0·5). These results suggest that the leaf-damage treatment minimally affected the secretions of remote branches on the same plant.
Fig. 3.
Changes in the amount and rate of secretion of the bowl-shaped organ (EFN) due to artificial damage to the leaf. (A) Changes in the amount of the secretion (n = 8 branches for each treatment); and (B) changes in the rate of secretion (n = 8 branches for each treatment). See text for details of how values were determined. Values are means ± s.d. Significant differences between Day 0 and Day 1: *** P < 0·001; ns, not significant.
Glandular hairs
Glandular hairs are transparent and hollow tubular structures on the plant surface that were found to secrete mucous liquid from the tip (Fig. 1C). The length of these structures ranged from 0·1 to 0·7 mm. Glandular hairs were distributed on the leaf surface, stem, flower petals and fruits (Table 1, Fig. 2B). Glandular hairs on the leaf were distributed only on the adaxial side in adult trees, with the exception of the ‘first leaves’ (see ‘Changes in leaf type and small structures during shoot development’ below). In contrast, the hairs were distributed on both sides of the leaf in young trees. The density of glandular hairs on the stem was higher in young trees than in old ones. The length of hairs was longer on flower petals (∼0·7 mm) and shorter on fruits (∼0·1 mm) compared with those on the leaf or stem (0·2–0·4 mm). The amount of secretions from the tip appeared greatest in glandular hairs on fruits.
In field observations, small insects, primarily aphids, were often trapped by the sticky mucous secretions of the glandular hairs. On average, six aphids per leaf (n = 11, s.d. = 6·34, range = 0–23) were trapped by the glandular hairs (11 branches of three young trees were examined during all 11 field observations from April to June, 1998).
Analyses (TLC, HPLC and NMR) of the secretions of glandular hairs on the leaves of both bud flushes and adult trees as well as the flowers of adult trees indicated that the secretions contained five types of glycerides (Fig. 4A, 1–5). In addition, TLC and NMR analyses of the secretions of hairs on immature fruits and flowers showed that secretions contained nine new (see Asai et al., 2008, for details) and six known geranylated flavonoids: 3′-O-methyldiplacone, (Fig. 4B, 8; Wollenweber et al., 1989; Phillips et al., 1996; Phommart et al., 2005); diplacone (Fig. 4B, 9; Phillips et al., 1996; Phommart et al., 2005; Kumazawa et al., 2004); diplacol 3′-O-methyl ether (Phillips et al., 1996), diplacol (Phillips et al., 1996; Lee et al., 2001); tanariflavanone (Phommart et al., 2005); and 3′,4′,5,7-pentahydroxy-6-[7-hydroxy-7-methyl-2(E)-octenyl] flavanone (Kumazawa et al., 2007)]. 6-Geranyl-4′,5,7-trihydroxy-3′,5′-dimethoxyflavanone (Fig. 4B, 6) and the flavonoids 6-geranyl-4′,5,5′,7-tetrahydroxy-3′-methoxyflavanone (Fig. 4B, 7), 3′-O-methyldiplacone (only in fruit; Fig. 4B, 8) and diplacone (only in fruit; Fig. 4B, 9) were the primary constituents. The total amount of flavonoids per unit area on the fruit surface was approximately 900 µg cm−2.
Fig. 5.
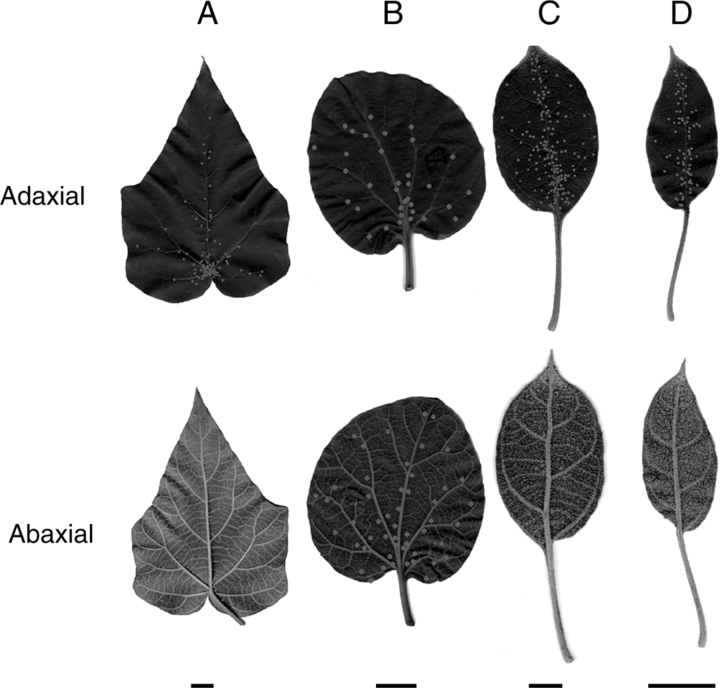
Four leaf types of P. tomentosa. (A) Standard leaf; (B) first leaf; (C) small leaf; and (D) flower-bud leaf. Dots indicate positions of bowl-shaped organs (EFNs). Average number of EFNs per leaf for (A–D) was 129, 49, 233 and 73 on adaxial side and 71, 76, 11 and 4 on abaxial side, respectively. Scale bars = 20 mm.
Fig. 8.
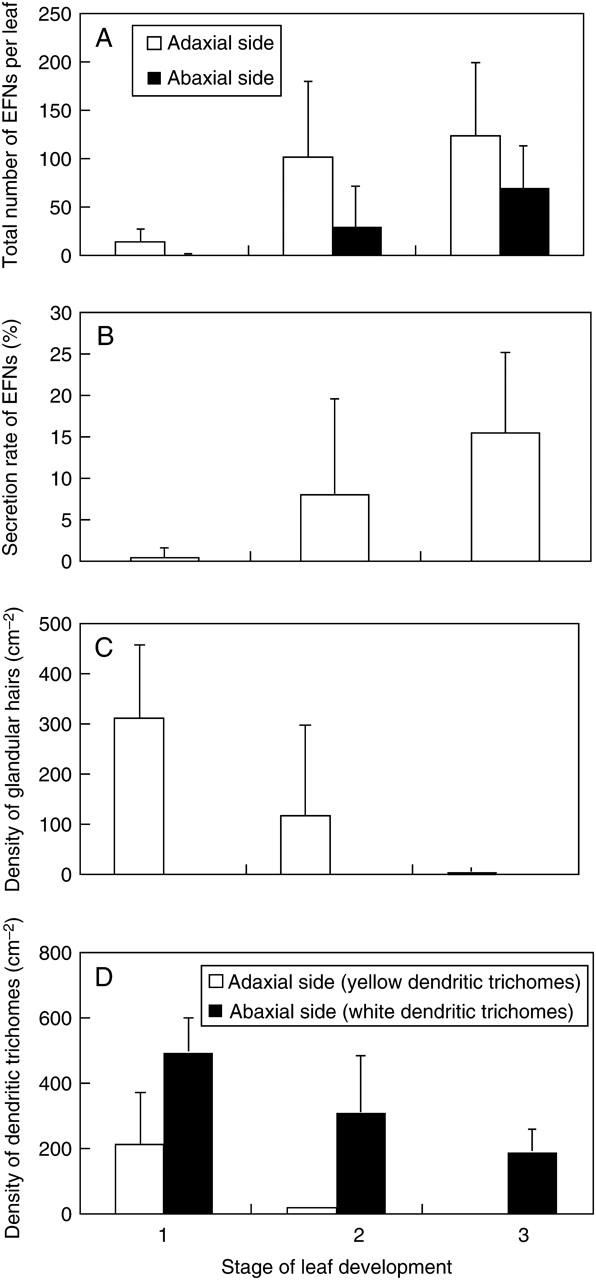
Change in small structures on standard leaves during leaf development in P. tomentosa. (A) Change in the total number of bowl-shaped organs (EFNs; adaxial side: n = 12 for stage 1, n = 30 for stage 2, and n = 27 for stage 3; P < 0·001; abaxial side: n = 10 for stage 1, n = 26 for stage 2, and n = 22 for stage 3; P < 0·001). (B) Change in the secretion rate of bowl-shaped organs (see text for calculation of values; n = 12 for stage 1, n = 30 for stage 2, and n = 13 for stage 3; P < 0·05). (C) Change in the density of glandular hairs (adaxial side: n = 11 for stage 1, n = 33 for stage 2 and n = 65 for stage 3; P < 0·001; abaxial side: n = 13 for stage 1, n = 33 for stage 2, n = 65 for stage 3; P > 0·05). (D) Change in the density of dendritic trichomes (adaxial side, yellow dendritic trichomes: n = 11 for stage 1, n = 33 for stage 2 and n = 65 for stage 3; P < 0·001; abaxial side, white dendritic trichomes: n = 13 for stage 1, n = 28 for stage 2, n = 34 for stage 3; P < 0·001). Error bars represent ± s.d.
Fig. 6.
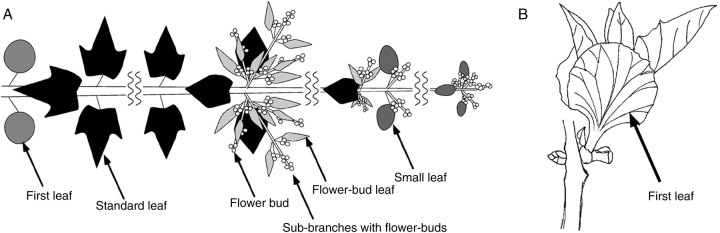
Distribution of four leaf types of P. tomentosa. (A) Distribution of four leaf types in a shoot. (B) A pair of the first leaves develop that cover young leaves at the tip of the shoot from both sides.
Fig. 7.
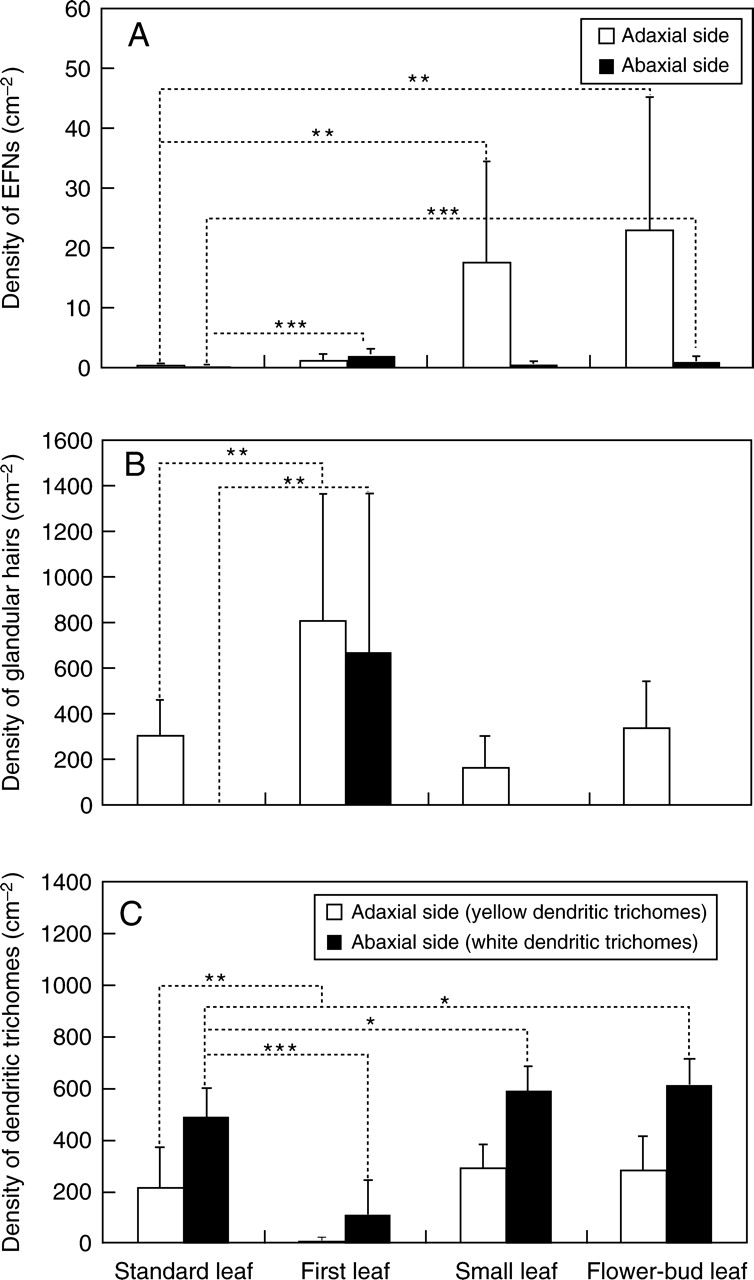
Density of small structures in each leaf type. (A) Density of bowl-shaped organs (EFNs) in each leaf type (mature leaves, stage 3) on adaxial side (n = 9 for first leaf, n = 13 for standard leaf, n = 11 for small leaf, and n = 21 for flower-bud leaf; P < 0·001) and abaxial side (n = 9 for first leaf, n = 13 for standard leaf, n = 11 for small leaf, and n = 20 for flower-bud leaf, P < 0·001). (B) Density of glandular hairs in each leaf type (young leaves, stage 1) on adaxial side (n = 8 for first leaf, n = 11 for standard leaf, n = 10 for small leaf, and n = 5 for flower-bud leaf; P < 0·001) and abaxial side (n = 8 for first leaf, n = 13 for standard leaf, n = 10 for small leaf, and n = 6 for flower-bud leaf; P < 0·001). (C) Density of dendritic trichomes in each leaf type (young leaves, stage 1) on adaxial side (yellow dendritic trichomes, n = 8 for first leaf, n = 11 for standard leaf, n = 10 for small leaf, and n = 6 for flower-bud leaf; P < 0·001) and abaxial side (white dendritic trichomes, n = 8 for first leaf, n = 13 for standard leaf, n = 10 for small leaf, and n = 6 for flower-bud leaf; P < 0·001). Error bars represent ± s.d. Asterisks indicate significant differences compared with standard leaf, as indicated: * P < 0·05; ** P < 0·01; *** P < 0·001
Dendritic trichomes
These structures are hair-like and shaped like a small tree with a main stem and many branches with spine-like tips; the diameter of the area covered by these branches was approx. 0·3 mm. The dendritic trichomes were distributed on both sides of the leaf surface as well as the surfaces of the calyx and flower buds (Table 1, Fig. 2C). Dendritic trichomes on the adaxial side of the leaf were tinted yellow (Fig. 1D), whereas those on the abaxial side were transparent (Fig. 1E). Trichomes on the surfaces of the calyx and flower buds were brown and appeared empty and desiccated. These trichomes are referred to here as yellow, white and brown dendritic trichomes, respectively.
The TLC analysis indicated that the yellow dendritic trichomes on the adaxial side of leaves contained three flavonoids identical to those found in the glandular hairs on the surfaces of immature fruits. These compounds were identified as 3′-O-methyldiplacone (Fig. 4B, 8), 6-geranyl-4′,5,5′,7-tetrahydroxy-3′-methoxyflavanone (Fig. 4B, 7), and diplacone (Fig. 4B, 9) by direct comparison (TLC and HPLC analyses) to the reference samples isolated from the secretions of glandular hairs on the surfaces of immature fruits. The total amount of flavonoids per unit area on the surface of young leaves was approximately 0·63 µg cm−2. TLC analysis also indicated that the white dendritic trichomes on the abaxial side of leaves and the brown dendritic trichomes on flower buds did not contain any significant amounts of organic substances.
During field observations, 12 branches of four young trees were examined during each of 17 visits from April to May 1998. During this period, two aphids were observed trapped by brown dendritic trichomes on the calyx surface.
Changes in leaf type during shoot development
Leaves of various shapes and sizes and with differing small structures developed during the different growth stages of the shoot. Leaves were classified into the following four types according to their morphology: ‘first leaf’ (Fig. 5B), ‘standard leaf’ (Fig. 5A), ‘small leaf’ (Fig. 5C), and ‘flower-bud leaf’ (Fig. 5D). The first leaves were round and approximately 9 cm in diameter. These leaves emerged first during the earliest stage of shoot development (Fig. 6A), and a pair of these leaves developed to cover young leaves at the tip of the shoot from both sides (Fig. 6B). Standard leaves were heart-shaped and approx. 24 cm in longitudinal diameter. This type was the largest and most abundant leaf type. Small leaves were oval and approximately 10 cm in longitudinal diameter; these leaves emerged near the tip of the shoot. Flower-bud leaves were small, oval and approx. 5 cm in longitudinal diameter; this type of leaf emerged on sub-branches with flower buds that branched off from the main shoot.
The distribution and densities of the small structures also differed among the leaf types. The densities of bowl-shaped organs (EFNs) on the first, small and flower-bud leaves were significantly higher than on the standard leaf (adaxial side: F3,50 = 7·6, P < 0·001; abaxial side: F3,49 = 14·9, P < 0·001; Fig. 7A). In particular, the densities of EFNs were 38 and 49 times higher, respectively, on small and flower-bud leaves compared to the adaxial side of the standard leaf (small leaf: F1,22 = 13·7, P < 0·005; flower-bud leaf: F1,32 = 13·0, P < 0·005). The EFNs were also significantly more dense on first leaves relative to the abaxial side of the standard leaves (F1,20 = 45·6, P < 0·001).
The density of glandular hairs on first leaves was significantly higher than on the other leaf types (adaxial side: F3,30 = 7·46, P < 0·001; abaxial side: F3,33 = 9·08, P < 0·001; Fig. 7B). The first leaves had glandular hairs on both sides of the leaf, whereas the other leaf types only had hairs on the adaxial side.
The densities of yellow (adaxial side) and white (abaxial side) dendritic trichomes also varied among the four leaf types (yellow dendritic trichome: F3,31 = 10·39, P < 0·001; white dendritic trichome: F3,33 = 38·12, P < 0·001; Fig. 7C). The densities of white dendritic trichomes on small and flower-bud leaves were significantly higher relative to the other leaf types (small leaf: F1,21 = 5·01, P < 0·05; flower-bud leaf: F1,17 = 5·73, P < 0·05). In general, the density of dendritic trichomes was very small on first leaves.
Changes in small structures on leaves during leaf development
The total number of bowl-shaped organs (EFNs) on standard leaves increased during leaf development (adaxial side: F2,66 = 10·1, P < 0·001; abaxial side: F2,55 = 13·4, P < 0·001; Fig. 8A). These results indicated that EFNs were formed continuously during leaf development. The secretion rate of EFNs on standard leaves also significantly increased with leaf growth (F2,52 = 7·6, P < 0·01; Fig. 8B), suggesting that these organs are most active on mature leaves.
In contrast, the densities of glandular hairs and dendritic trichomes on standard leaves were highest during stage 1 (young leaves) and decreased with leaf growth (Fig. 8C, D). Most glandular hairs and yellow dendritic trichomes dropped off the leaves before stage 3 (mature leaves), suggesting that these structures primarily function in young leaves.
The density of white dendritic trichomes on the abaxial side of standard leaves was highest during stage 1 and decreased with leaf development; however, many of these structures remained even at stage 3 (Fig. 8D). These results suggest that white dendritic trichomes primarily function in young leaves but also maintain activity in mature leaves.
On first, small and flower-bud leaves, many glandular hairs and yellow dendritic trichomes remained even at stage 3, whereas nearly all of these structures were lost in mature standard leaves (Fig. 8D). The mean density of glandular hairs on the adaxial and abaxial sides of mature first leaves was 72·3 cm−2 (s.d. = 70·9) and 31·9 cm−2 (s.d. = 31·9), respectively, which represented 9 % and 5 % of the density on young leaves (stage 1). The densities of these structures on the adaxial side of mature small and flower-bud leaves were 58·5 cm−2 (s.d. = 128·6) and 19·5 cm−2 (s.d. = 24·4), respectively, which was 35 % and 6 % of the density on young leaves. The mean densities of yellow dendritic trichomes on mature small and flower-bud leaves was 75·4 cm−2 (s.d. = 107·9) and 44·0 cm−2 (s.d. = 54·8), respectively, which was 26 % and 16 % of the density on young leaves. Given the large increase in leaf area during leaf development (at least 1500 %), these results strongly suggest that most of the glandular hairs and yellow dendritic trichomes from stage 1 remained until stage 3 and/or that the formation of these structures continued during the development of these leaf types.
Changes in leaf type and small structures during shoot development
Shoot development in P. tomentosa can be divided into six growth stages, comprising 2 years in total. The leaf types and structures observed in each stage were as follows (Fig. 6A). (1) New-shoot formation stage (April to May of the first year): new shoots begin to grow from an auxiliary bud on the previous year's shoot. Young organs at the shoot tip are covered by a pair of first leaves (Fig. 6B) with dense glandular hairs and bowl-shaped organs (EFNs). (2) Photosynthetic stage (May to August of the first year): standard leaves, the largest and most abundant type of leaf, develop. Standard leaves develop many glandular hairs and yellow dendritic trichomes during the young stage and many EFNs during the mature stage. (3) Anthogenesis stage (August to September of the first year): sub-branches with flower buds are formed near the tip of the main shoot. Small leaves and flower-bud leaves dense with bowl-shaped organs and dendritic trichomes develop around the flower buds. (4) Overwintering stage (October of the first year to April of the second year): the shoot loses its leaves and only flower buds remain on the shoot. Flower buds are densely covered with hard, brown dendritic trichomes. (5) Blooming stage (April to May of the second year): the trees come into bloom. Flowers are dense with glandular hairs on the petals and with brown dendritic trichomes and EFNs on the calyx. (6) Fruiting stage (May to September of the second year): fruits are formed and mature. Immature fruits have dense glandular hairs on the surface, and the calyx is dense with brown dendritic trichomes and EFNs. Glandular hairs on the fruit contain many more flavonoids per unit area than those on the leaf.
DISCUSSION
Function of the small structures on P. tomentosa
Bowl-shaped organ
The bowl-shaped organs secreted sugar and attracted ants (Table 2), indicating that these structures are EFNs. The morphology of these organs is similar to that of ‘scale-like nectaries’ reported from the floral bracts of Thunbergia grandiflora (Acanthaceae), the petiole and calyx of Clerodendron species (Verbenaceae), and the petioles, leaves, flowers and some fruits of most genera of Bignoniacae (Elias, 1983). EFNs are found in a wide variety of plant taxa, i.e. in at least 93 species of plants (Koptur, 1992), especially among angiosperms, and these EFNs generally secrete sucrose, glucose and fructose (Bentley, 1977). Ants attracted by EFNs provide protection against herbivores (Bentley, 1977; Laine and Niemelä, 1980; Messina, 1981; Skinner and Whittaker, 1981; Koptur, 1992; Heil et al., 2001; Sobrinho et al., 2002; Heil and McKey, 2003). For example, Catalpa speciosa (Bignoniaceae) has extrafloral nectaries on its leaves and is visited by ants that remove the eggs and young larvae of its principal herbivore. Furthermore, ant-excluded branches of C. speciosa had significantly fewer herbivore larvae removed, experienced significantly more leaf herbivory, and produced significantly fewer mature fruits than control branches (Stephenson, 1982).
The secretions of bowl-shaped organs (EFNs) increased due to artificial leaf damage that mimicked damage by herbivores, suggesting that these structures provide protection against herbivores. EFN secretions by the Asian pioneer tree Macaranga tanarius (Euphorbiaceae) were induced by both artificial and natural herbivore damage (Heil et al., 2001). Subsequent to these increased EFN secretions of M. tanarius, the number of EFN-visiting insects increased and the number of herbivores decreased (Heil et al., 2001).
EFNs of P. tomentosa (i.e. bowl-shaped organs) were densely distributed around reproductive organs such as flowers, flower buds and fruits. For example, during flower-bud formation, small leaves and flower-bud leaves with high densities of EFNs emerged near the flower buds. This pattern also suggests that these organs provide protection against herbivores in P. tomentosa, because the protection of reproductive organs from herbivores is particularly crucial to plant fitness. The protection of reproductive organs by ants is also especially important and contributes greatly to plant reproductive success (Sobrinho et al., 2002). Messina (1981) reported that during chrysomelid beetle outbreaks in meadow goldenrods (Solidago spp.) in central New York, only Solidago stems bearing Formica ants for most of the season produced flowers and seeds, suggesting that protection by ants was critical to the reproductive success of the plant. Formica japonica, the ant species most frequently found feeding on the secretions of the EFNs, is one of the most aggressive ants in Japan. Therefore, EFNs of P. tomentosa may contribute to plant reproductive success.
Glandular hairs
The observation that many aphids were trapped by glandular hairs suggests that this organ serves to obstruct the activity of herbivores on plants. Glandular hairs on the leaf of wild potato (Solanum berthaultii) also produced sticky and/or toxic exudates that could trap arthropods, leading to their death via starvation or other causes (Kennedy, 2003). In addition, glandular trichomes on the stems and leaves of tomato (Solanum lycopersicum) impeded the rate of movement of caterpillars (Wilkens et al., 1996). In mountain birch (Betula pubescens ssp. czerepanovii), the growth rate and pupal mass of autumnal moth (Epirrita autumnata) larvae, an insect herbivore, were negatively correlated with the density of glandular trichomes, suggesting that these structures play a role in herbivore resistance (Valkama et al., 2005). Thus, the glandular hairs of P. tomentosa may also provide protection against herbivores. Glandular hairs were primarily distributed on young and reproductive organs, further indicating that these structures serve to protect the most vulnerable and important parts of the plant.
Glycerides were the most abundant compounds isolated from the secretions of glandular hairs on the leaves and flowers of P. tomentosa (Fig. 4A). These compounds were sticky but non-toxic to several insects (Fujimoto et al., unpubl. res.). Oncidinol, a glyceride structurally related to the glycerides of the glandular hairs, was isolated from the floral oil of Ornithophora radicans and may act as a reward for pollinating bees (Reis et al., 2003), also suggesting that the glycerides in the glandular hairs of P. tomentosa are not toxic. Therefore, the glandular hairs on the leaves and flowers may only physically obstruct herbivores.
In contrast, the secretions of the glandular hairs on the surface of immature fruit were also rich in flavonoids. The most abundant flavonoids isolated from the secretions of these glandular hairs were diplacone (Fig. 4B, 9) and 3′-O-methyldiplacone (Fig. 4B, 8). Previous studies have also isolated diplacone and 3′-O-methyldiplacone from an ethanolic extract of P. tomentosa fruit (Smejkal et al., 2007a, b), and diplacone has been isolated from an ethanolic extract of the flower (Du et al., 2004), although the secretions of the glandular hairs were not analysed separately. Diplacone is a type of ortho-dihydroxyflavonoid, which may provide protection against some herbivores. Elliger et al. (1980) demonstrated that ortho-dihydroxyflavonoids inhibit the growth of lepidopteran larvae (Heliothis zea) when incorporated into an artificial diet. Diplacone is also the most abundant flavonoid in the resin on leaves of Diplacus aurantiacus (a synonym of Mimulus aurantiacus Curtis; Rhoades, 1977; Lincoln, 1980). This resin reduces the fitness of Euphydryas chalcedona (Lepidoptera, Nymphalidae), a primary herbivore of D. aurantiacus (Lincoln, 1985; Lincoln and Walla, 1986). Simmonds (2003) found that diplacone may be effective for protection against some herbivores, even though this substance did not provide D. aurantiacus with resistance against its primary lepidopteran herbivore, E. chalcedona (Hare, 2002a, b), probably because the larvae have adapted to the compound (Simmonds, 2003). Therefore, diplacone in the secretions of the glandular hairs on the surface of P. tomentosa fruit may be effective for protection against herbivores. Glandular hairs on young reproductive organs contained rich flavonoids with concentrations over 1000 times higher than on the surfaces of young leaves, also suggesting that these chemicals play a role in protecting important organs against herbivores.
The substance 3′-O-methyldiplacone is also a major flavonoid in the resin of D. aurantiacus (Lincoln and Walla, 1986), which may help reduce water loss and provide some protection from UV light as well as from herbivory. Lincoln and Walla (1986) hypothesized that methoxylated flavonoids such as 3′-O-methyldiplacone are more effective in protecting plants from water loss because of their reduced polarity. Secretions of the glandular hairs on surfaces of P. tomentosa fruit may also be effective against UV radiation and water loss, because the fruit surface was often covered by a thin layer of the secretions.
Dendritic trichomes
The observation that some aphids were trapped by the brown dendritic trichomes suggests that these structures are effective in physically obstructing the activity of herbivores on the plants. Wilkens et al. (1996) suggested that trichomes, even those without glandular exudates, also play a role in impeding insect herbivores on tomatoes (Solanum lycopersicum). On tomato stems with non-glandular trichomes, the amount of time that caterpillars spent searching for food decreased and the amount of time spent removing impediments to movement increased relative to artificially de-haired stems (Wilkens et al., 1996). Because brown dendritic trichomes are harder and denser than other types of dendritic trichomes, these structures on the surfaces of reproductive organs such as flower buds and the calyx of flowers and fruits appear most effective in obstructing the activity of herbivores. However, white and yellow trichomes may also impede herbivore activity, because these structures have spine-like tips very similar to those of brown dendritic trichomes.
Yellow dendritic trichomes on the adaxial sides of leaves contained flavonoids, particularly diplacone (Fig. 4B, 9) and 3′-O-methyldiplacone (Fig. 4B, 8), that were also found in the secretions of glandular hairs on immature fruit. Therefore, these structures may also be chemically (as well as physically) effective in protecting plants against herbivores. In contrast, white and brown dendritic trichomes contained minimal chemical components relative to the yellow dendritic trichomes, suggesting that the primary function of these trichomes is physical protection against herbivores.
Flavonoids in yellow dendritic trichomes may also protect young leaves from UV-B radiation. Non-glandular trichomes of a variety of species contain UV-B-absorbing compounds such as flavonoids (Karabourniotis et al., 1992; Skaltsa et al., 1994). Furthermore, exposure of artificially de-haired young leaves to UV-B radiation resulted in a reduction of the photochemical efficiency of photosystem II and epidermal browning in Olea europaea, Cydonia oblonga, Eriobotrya japonica (Karabourniotis et al., 1995) and Quercus ilex (Skaltsa et al., 1994). In O. europaea, the density of trichomes on the leaf and UV-B-absorbing compounds of the leaf hairs were positively correlated with the midday UV-B instant irradiance, suggesting protective functions of the trichomes against UV-B (Liakoura et al., 1997). The concentration of flavonoids in dendritic trichomes on the adaxial side of leaves was much higher than on the abaxial side. These results suggest that flavonoids of yellow dendritic trichomes may protect against UV-B as well as against herbivory, because the adaxial side of leaves is more susceptible to UV radiation than the abaxial side.
Functions of different leaf types
The densities of the small structures, especially those of EFNs, on first, small and flower-bud leaves were higher relative to standard leaves (Fig. 7). In addition, the distribution of these leaf types around young and/or important parts of the plant (Fig. 6) suggests that these leaf types are specialized to protect vulnerable and important organs from herbivory.
The characteristics of first leaves indicate a specialization to protect young leaves at the shoot tip during the earliest stage of shoot development. For example, in first leaves, the densities of the EFNs and glandular hairs were higher than those of standard leaves (Fig. 7A, B). In addition, only the first leaves had dense glandular hairs on the abaxial side and these hairs remained on mature leaves, whereas most were lost before the mature stage in standard leaves (Fig. 8C). The dense amounts of EFNs and glandular hairs on the abaxial sides of first leaves may be an adaptation to protect the shoot tip, because the abaxial sides of first leaves face outward when a pair of first leaves develops to cover the young shoot tip from both sides (Fig. 6B).
Small and flower-bud leaves had higher densities of EFNs and dendritic trichomes compared with standard leaves (Fig. 7A, C). In particular, the density of EFNs on the adaxial side of small and flower-bud leaves was approx. 37 and 48 times higher, respectively, than that of standard leaves. Additionally, in small and flower-bud leaves, yellow dendritic trichomes on the adaxial side remained on mature leaves (stage 3), whereas most of the trichomes were lost before the mature stage in standard leaves (Fig. 8D). These two types of leaves develop around flower buds (i.e. important reproductive organs) during the anthogenesis stage (August to September), suggesting that small and flower-bud leaves are specialized to protect flower buds.
Standard leaves were the largest and most abundant leaf type, developed during summer (May to August), and appeared to be specialized for photosynthesis. The rapid decrease of glandular hairs and yellow dendritic trichomes on the adaxial side of this leaf type during leaf growth may enable the leaf surface to receive more light for photosynthesis during the mature stage.
Changes in small structures on the leaf during leaf development
In many plant species, trichome density is very high in young leaves but decreases rapidly with leaf expansion (Maffei et al., 1989; Werker et al., 1993; Pérez-Estrada et al., 2000). Karabourniotis et al. (1995) showed that trichome density as well as the relative concentration of UV-B-absorbing phenolics in hairs declined considerably with leaf age, especially on the adaxial side of leaves in Olea europaea, Cydonia oblonga and Eriobotrya japonica. Karabourniotis et al. (1995) suggested that the dense trichomes often covering young leaves may, in addition to other functions, transiently protect the underlying cells against UV-B radiation damage during the time period required for the maturation of internal avoidance and/or repairing mechanisms. This function may also help to explain the observed drastic decrease in the density of yellow dendritic trichomes with flavonoids on the adaxial side of mature standard leaves (Fig. 8D), if we assume that this trichome also functions to protect young leaves from UV-B damage. However, the observed decrease in glandular hairs and white dendritic trichomes during leaf development cannot be explained by the UV-B protection hypothesis, because these structures contained no UV-B-absorbing compounds.
The results strongly indicated that glandular hairs and dendritic trichomes as well as the EFNs exhibit some anti-herbivory functions. Therefore, changes in these structures on standard leaves during leaf development suggest that these particular leaves of P. tomentosa are primarily protected against herbivory by glandular hairs and dendritic trichomes during the young-leaf stage, but are protected by EFNs during the mature-leaf stage. The fact that glandular hairs and dendritic trichomes primarily function during younger leaf stages when EFNs are not yet active (Fig. 8C, D) suggests that these structures compensated for the lack of anti-herbivory protection by the EFNs. Many plant species may use trichomes during the young-leaf stage to obstruct the activity of herbivores, because young, growing leaves cannot mechanically be tough due to the constraints of cell expansion and cell-wall development. Furthermore, young leaves still do not have enough photosynthetic product to produce many protective chemicals and/or nectar for symbiosis with ants.
Changes in leaf type and small structures during shoot development
The observed changes in leaf type and the three small structures during shoot development indicated that these structures were preferentially distributed spatially and temporally to weak and/or important organs, such as young leaves, flower buds, flowers and fruits, that particularly required protection against herbivores. Defensive compounds such as alkaloids are predicted to be concentrated in the most valuable and vulnerable parts of a plant if the supply of such compounds is limited (‘the optimal defence hypothesis’, McKey, 1974; Heil and McKey, 2003). EFNs of P. tomentosa, which may provide protection for the plant, were concentrated in small leaves and flower-bud leaves that develop near flower buds when these valuable organs were developing and vulnerable. EFNs as well as glandular hairs were also concentrated on first leaves covering young leaves at the shoot tips. According to the optimal defence hypothesis, young leaves need to be well defended, because damage to them may cause a larger decline in future photosynthesis than damage to mature leaves (McKey, 1974; Riipi et al., 2002); thus, our results support the optimal defence hypothesis. Assuming that the optimal defence hypothesis functions within our system, the fact that glandular hairs and brown dendritic trichomes were concentrated in flowers and fruits, yellow and white dendritic trichomes were concentrated on special leaves near flower buds, and flavonoids such as diplacone were concentrated in young fruits and special leaves near flower buds, all suggest that these structures and chemicals also serve protective functions against herbivores.
In conclusion, our results strongly suggest that three types of small structures found on P. tomentosa are anti-herbivore structures. The results also indicate that the plant protects young and reproductive organs against herbivores through a distribution and allocation of these structures that is commensurate with the developmental stage of leaves and shoots.
ACKNOWLEDGEMENTS
We thank all the members of our laboratories in the Tokyo Institute of Technology, especially Dr Noriyuki Hara, for their kind help and support of our studies. We also thank the staff of Kawasaki Mizonokuchi Post Office who kindly permitted us to study the tree at their site. This research was partly supported by COE Program (A10 and K6) of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
LITERATURE CITED
- Asai T, Hara N, Kobayashi S, Kohshima S, Fujimoto Y. Geranylated flavanones from the secretion on the surface of the immature fruits of Paulownia tomentosa. Phytochemistry. 2008;69:1234–1241. doi: 10.1016/j.phytochem.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Beattie AJ. The evolutionary ecology of ant–plant mutualisms. Cambridge, UK: Cambridge University Press; 1985. [Google Scholar]
- Bentley BL. Extrafloral nectaries and protection by pugnacious bodyguards. Annual Review of Ecology and Systematics. 1977;8:407–427. [Google Scholar]
- Du X, Shi YP, Li ZG, Li Y. Isolation and structural elucidation of flavones from flower of Paulownia tomentosa. Chinese Traditional and Herbal Drugs. 2004;35:245–247. [Google Scholar]
- Elias TS. Extrafloral nectaries: their structure and distribution. In: Bentley BL, Elias TS, editors. The biology of nectaries. Irvington, NY: Columbia University Press; 1983. pp. 174–203. [Google Scholar]
- Elliger CA, Chan BC, Waiss AC. Flavonoids as larval growth inhibitors. Naturwissenschaften. 1980;67:358–360. [Google Scholar]
- Eugene WS. Azteca protection of Cecropia: ant occupation benefits juvenile trees. Oecologia. 1986;70:379. doi: 10.1007/BF00379500. [DOI] [PubMed] [Google Scholar]
- Gilbert LE. Butterfly–plant coevolution: has Passiflora adenopoda won the selectional race with heliconiine butterflies? Science. 1971;172:585–586. doi: 10.1126/science.172.3983.585. [DOI] [PubMed] [Google Scholar]
- Hare JD. Geographic and genetic variation in the leaf surface resin components of Mimulus aurantiacus from southern California. Biochemical Systematics and Ecology. 2002;a 30:281–296. [Google Scholar]
- Hare JD. Seasonal variation in the leaf resin components of Mimulus aurantiacus. Biochemical Systematics and Ecology. 2002;b 30:709–720. [Google Scholar]
- Heil M, McKey D. Protective ant–plant interactions as model systems in ecological and evolutionary research. Annual Review of Ecology, Evolution, and Systematics. 2003;34:425–553. [Google Scholar]
- Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE. Extrafloral nectar production of the ant-associated plant. Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid; Proceedings of the National Academy of Sciences of the USA; 2001. pp. 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen DH. Allelopathy by myrmecophytes: the ant Azteca as an allelopathic agent of Cecropia. Ecology. 1969;50:147–153. [Google Scholar]
- Jork H. Thin-layer chromatography: reagents and detection methods. V. 1a. Physical and chemical detection methods: fundamentals, reagents I. New York, NY: VCH Publishers; 1990. pp. 408–410. [Google Scholar]
- Karabourniotis G, Papadopoulos K, Papamarkou M, Manetas Y. Ultraviolet-B radiation absorbing capacity of leaf hairs. Physiologia Plantarum. 1992;86:414–418. [Google Scholar]
- Karabourniotis G, Kotsabassidis D, Manetas Y. Trichome density and its protective potential against ultraviolet-B radiation damage during leaf development. Canadian Journal of Botany. 1995;73:376–383. [Google Scholar]
- Kennedy GG. Tomato, pests, parasitoids, and predators: tritrophic interactions involving the genus Lycopersicon. Annual Review of Entomology. 2003;48:51–72. doi: 10.1146/annurev.ento.48.091801.112733. [DOI] [PubMed] [Google Scholar]
- Koptur S. Extrafloral nectary-mediated interactions between insects and plants. In: Bernays EA., editor. Insect–plant interactions. IV. Boca Raton, FL: CRC Press; 1992. pp. 81–129. [Google Scholar]
- Kumazawa S, Goto H, Hamasaka T, Fukumoto S, Fujimoto T, Nakayama T. A new prenylated flavonoid from propolis collected in Okinawa, Japan. Bioscience, biotechnology, and biochemistry. 2004;68:260–262. doi: 10.1271/bbb.68.260. [DOI] [PubMed] [Google Scholar]
- Kumazawa S, Ueda R, Hamasaka T, Fukumoto S, Fujimoto T, Nakayama T. Antioxidant prenylated flavonoids from propolis collected in Okinawa, Japan. Journal of Agricultural and Food Chemistry. 2007;55:7722–7725. doi: 10.1021/jf071187h. [DOI] [PubMed] [Google Scholar]
- Laine KJ, Niemelä P. The influence of ants on the survival of mountain birches during an Oporinia autumnata (Lep., Geometridae) outbreak. Oecologia. 1980;47:39–42. doi: 10.1007/BF00541773. [DOI] [PubMed] [Google Scholar]
- Lee D, Bhat KPL, Fong HHS, Farnsworth NR, Pezzuto JM, Kinghorn AD. Aromatase inhibitors from Broussonetia papyrifera. Journal of Natural Products. 2001;64:1286–1293. doi: 10.1021/np010288l. [DOI] [PubMed] [Google Scholar]
- Liakoura V, Stefanou M, Manetas Y, Cholevas C, Karabourniotis G. Trichome density and its UV-B protective potential are affected by shading and leaf position on the canopy. Environmental and Experimental Botany. 1997;38:223–229. [Google Scholar]
- Lincoln DE. Leaf resin flavonoids of Diplacus aurantiacus. Biochemical Systematics and Ecology. 1980;8:397–400. [Google Scholar]
- Lincoln DE. Host-plant protein and phenolic resin effects on larval growth and survival of a butterfly. Journal of Chemical Ecology. 1985;11:1459–1467. doi: 10.1007/BF01012192. [DOI] [PubMed] [Google Scholar]
- Lincoln DE, Walla MD. Flavonoids from Diplacus aurantiacus leaf resin. Biochemical Systematics and Ecology. 1986;14:195–198. [Google Scholar]
- Maffei M, Chialva F, Sacco T. Glandular trichomes and essential oils in developing peppermint leaves. New Phytologist. 1989;111:707–716. doi: 10.1111/j.1469-8137.1989.tb02366.x. [DOI] [PubMed] [Google Scholar]
- McKey D. Adaptive patterns in alkaloid physiology. The American Naturalist. 1974;108:305–320. [Google Scholar]
- Messina FJ. Plant protection as a consequence of an ant–membracid mutualism: interactions on goldenrod (Solidago Sp.) Ecology. 1981;62:1433–1440. [Google Scholar]
- O'Dowd D. J. Pearl bodies as ant food: an ecological role for some leaf emergences of tropical plants. Biotropica. 1982;14:40–49. [Google Scholar]
- Pérez-Estrada LB, Canto-Santan Z, Oyama K. Variation in leaf trichomes of Wigandia urens: environmental factors and physiological consequence. Tree Physiology. 2000;20:629–632. doi: 10.1093/treephys/20.9.629. [DOI] [PubMed] [Google Scholar]
- Phillips WR, Baj NJ, Gunatilaka AAL, Kingston DGI. C-geranyl compounds from Mimulus clevelandii. Journal of Natural Products. 1996;59:495–497. doi: 10.1021/np960240l. [DOI] [PubMed] [Google Scholar]
- Phommart S, Sutthivaiyakit P, Chimnoi N, Ruchirawat S, Sutthivaiyakit S. Constituents of the leaves of Macaranga tanarius. Journal of Natural Products. 2005;68:927–930. doi: 10.1021/np0500272. [DOI] [PubMed] [Google Scholar]
- Pollard AJ, Briggs D. Genecological studies of Urtica dioica L. III. Stinging hairs and plant–herbivore interactions. New Phytologist. 1984;97:507–522. [Google Scholar]
- Reis MG, de Faria AD, Amaral MCE, Marsaioli AJ. Oncidinol – a novel diacylglycerol from Ornithophora radicans Barb. Rodr. (Orchidaceae) floral oil. Tetrahedron Letters. 2003;44:8519–8523. [Google Scholar]
- Riipi M, Ossipov V, Lempa K, Haukioja E, Koricheva J, Ossipova S, Pihlaja K. Seasonal changes in birch leaf chemistry: are there trade-offs between leaf growth and accumulation of phenolics? Oecologia. 2002;130:380–390. doi: 10.1007/s00442-001-0826-z. [DOI] [PubMed] [Google Scholar]
- Rhoades D. Integrated antiherbivore, antidesiccant and ultraviolet screening properties of creosotebush resin. Biochemical Systematics and Ecology. 1977;50:281–290. [Google Scholar]
- Simmonds MSJ. Flavonoid–insect interactions: recent advances in our knowledge. Phytochemistry. 2003;64:21–30. doi: 10.1016/s0031-9422(03)00293-0. [DOI] [PubMed] [Google Scholar]
- Skaltsa H, Verykokidou E, Harvala C, Karabourniotis G, Manetas Y. UV-B protective potential and flavonoid content of leaf hairs of Quercus ilex. Phytochemistry. 1994;37:987–990. [Google Scholar]
- Skinner GJ, Whittaker JB. An experimental investigation of inter-relationships between the wood-ant (Formica rufa) and some tree-canopy herbivores. The Journal of Animal Ecology. 1981;50:313–326. [Google Scholar]
- Smejkal K, Holubova P, Zima A, Muselik J, Dvorska M. Antiradical activity of Paulownia tomentosa (Scrophulariaceae) extracts. Molecules. 2007;a 12:1210–1219. doi: 10.3390/12061210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkal K, Grycová L, Marek R, Lemière F, Jankovská D, Forejtníková H, Vanco J, Suchý V. C-geranyl compounds from Paulownia tomentosa fruits. Journal of natural products. 2007;b 70:1244–1248. doi: 10.1021/np070063w. [DOI] [PubMed] [Google Scholar]
- Sobrinho TS, Schoereder JH, Rodrigues LL, Collevatti RG. Ant visitation (Hymenoptera: Formicidae) to extrafloral nectaries increases seed set and seed viability in the tropical weed Triumfetta semitriloba. Sociobiology. 2002;39:353–372. [Google Scholar]
- Stephenson AG. The role of the extrafloral nectaries of Catalpa Speciosa in limiting herbivory and increasing fruit production. Ecology. 1982;63:663–669. [Google Scholar]
- Valkama E, Koricheva J, Salminen J, Helander M, Saloniemi I, Saikkonen K, Pihlaja K. Leaf surface traits: overlooked determinants of birch resistance to herbivores and foliar micro-fungi? Trees: Structure and Function. 2005;19:191–197. [Google Scholar]
- Werker E, Putievsky E, Ravid U, Dudai N, Katzir I. Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae) Annals of Botany. 1993;71:43–50. [Google Scholar]
- Wilkens RT, Shea GO, Halbreich S, Stamp NE. Resource availability and the trichome defenses of tomato plants. Oecologia. 1996;106:181–191. doi: 10.1007/BF00328597. [DOI] [PubMed] [Google Scholar]
- Wollenweber E, Schober I, Schilling G, Giner FJA, Roitman JN. A geranyl alpha-pyrone from the leaf resin of Diplacus aurantiacus. Phytochemistry. 1989;28:3493–3496. [Google Scholar]