Abstract
Background and Aims
Plant growth responses to the rare earth elements lanthanum (La) and cerium (Ce) have been reported, but little is known about the effects of these two elements on plant mineral nutrition.
Methods
Corn (Zea mays ‘Hycorn 82’) and mungbean (Vigna radiata ‘Berken’) were grown in continuous flowing nutrient solutions containing 0, 0·2, 1·0 and 5·0 µm La or Ce. At harvest plants were divided into roots and shoots, dried, weighed and analysed for macro- and micronutrients, as well as for La and Ce.
Key Results
La and Ce did not increase the growth of corn or mungbean. The dry weight of corn shoots was decreased by 32 % in the presence of 5·0 µm Ce; the other La and Ce concentrations had no effect. La and Ce concentrations of 0·9 and 5·0 µm decreased the shoot dry weight of mungbean by 75 or 95 %, the two elements having closely similar effects. Decreases in the uptake of Ca, Na, Zn and Mn by corn were observed with increases in solution La and Ce. For mungbean, the uptake rates of all measured elements decreased with increases in solution La and Ce. The concentrations of La and Ce in the roots of both species were higher than in the shoots and increased strongly with increasing concentrations of La or Ce in solution. The La and Ce concentrations in mungbean shoots were always higher than in corn shoots.
Conclusions
La and Ce did not enhance the growth of corn or mungbean, but decreased the growth, root function and consequently the nutritional status of mungbean at concentrations >0·2 µm in solution. It is concluded that if La or Ce have positive effects on corn and mungbean growth, they can only occur at solution concentrations below 0·2 µm.
Key words: Cerium, corn, Zea mays, lanthanum, mineral nutrition, mungbean, Vigna radiata, plant growth, rare earth elements
INTRODUCTION
The periodic table currently contains 118 elements (Karol et al., 2003). Seventeen of these elements have been classified as essential for all higher plants, with other elements such as Na, Ni and Si being beneficial to some plants under certain conditions (Asher, 1991; Epstein and Bloom, 2005). However, many other elements from the periodic table can also be found in plant tissues. Consequently, some of these elements could potentially in the future be added to the list of elements beneficial to some plants, if experimental evidence can be presented which subsequently is supported and verified (Asher, 1991). There have been many claims for the beneficial effects of various elements on plants over the years (Asher, 1991).
The rare earth elements (REEs) are a collection of 16 elements, namely lanthanum (La), cerium (Ce), the remaining 13 lanthanides, together with scandium and yttrium. China contains the largest mineral deposits of REEs in the world (Drew et al., 1990; Yang and Woolley, 2006). Reports from China on the use of these elements in agriculture have increased the interest of plant biologists and agriculturists in the REEs (Asher et al., 1990; Brown et al., 1990; Asher, 1991; Hu et al., 2004; Tyler, 2004; von Tucher and Schmidhalter, 2005). Chinese reports suggest increased plant growth, yield and quality of a range of crops, pasture and horticultural species. However, critical evaluation of these claims has been difficult owing to ambiguities and lack of clarity in language translation, and the lack of details relating to experimental design, methodologies or statistical treatment of the data (Asher et al., 1990). The absence of such essential information precludes the scrutiny required to analyse critically many of the Chinese claims. In addition, to date we have been unable to find scientific papers in peer-reviewed journals in western countries that have shown similar effects of REEs on plants. The effects of foliar application of La and Ce were initially examined (Diatloff et al., 1999). The recommended rates of a commercially available REE fertilizer obtained from China, which contains a mixture of REE nitrates, were applied to mungbean and corn. No beneficial effects on the growth and yield of mungbean and corn were observed. A series of carefully conducted solution culture experiments were subsequently initiated to examine the effects of La and Ce on the growth and mineral nutrition of corn (Zea mays) and mungbean (Vigna radiata) (Diatloff et al., 1995a, b, c).
When La and Ce were maintained in a soluble form in nutrient solutions comparable in composition with that of soil solutions, concentrations of La and Ce of 1–16 µm were found to be toxic to the root elongation of corn and mungbean (Diatloff et al., 1995a). It was concluded that if La and Ce do have positive effects on plant growth, these effects are likely to be manifested at very low solution La or Ce concentrations, probably below 1 µm. When grown in continuously flowing nutrient solution containing low concentrations of La or Ce, the dry weight of corn roots was significantly increased by increasing the concentrations of La or Ce in the nutrient solutions from about 0·007 µm to approx. 0·6 µm (Diatloff et al., 1995b, c). However, effects on the dry weight of shoots and of whole plants were not significant. In the same experiments, the dry weights of mungbean shoots and roots were depressed by La concentrations ≥0·4 µm or Ce concentrations ≥0·2 µm. In both corn and mungbean, shoot tissue phosphorus (P) concentrations were high under the conditions of these experiments (≥0·7 %), but not necessarily in the toxic range. In addition, in the Ce experiment, increasing Ce concentrations depressed shoot manganese (Mn) concentrations in mungbean to very low levels (≤5 mg kg−1) and resulted in the development of Mn-deficiency symptoms. Hence it was not possible to separate the direct effects of Ce on mungbean growth from the indirect effects associated with Mn deficiency.
The present paper describes an experiment in which responses of corn and mungbean to La and Ce were studied further in flowing solution culture at a lower P concentration and a higher Mn concentration than had been employed in previous experiments.
MATERIALS AND METHODS
Plant culture
The experiment was conducted in a controlled environment room with a 14-h light period at 600 µmol photons m–2 s−1, a constant temperature of 25 °C, 70 % relative humidity and 0·03 % CO2. Plants were grown in continuously flowing solution culture units (FSCUs; Diatloff et al., 1995b, c), filled with a dilute, low-P nutrient solution containing (μm): N (as nitrate), 1000; Ca, 1000; S, 825; K, 255; Mg, 200; P, 1; Na, 34; Cl, 25; Fe, 9; B, 3; Mn, 0·3; Zn, 0·05; Cu, 0·05; Mo, 0·02 and Co 0·02. This basal nutrient solution was similar to that used in Diatloff et al. (1995b, c), except that the P concentration was reduced from 5 to 1 µm and the Mn concentration was increased from 0·1 to 0·3 µm. These modifications were designed to reduce the above-adequate concentrations of P in corn and mungbean shoots and to overcome the Mn deficiency symptoms observed in Diatloff et al. (1995b, c). The macronutrient stock solutions used to prepare the basal nutrient solution were purified with Chelex® 100 resin (Rayment and Andrews, 1972). The solution P was maintained at 1 ± 0·2 µm with a slow, continuous injection of concentrated KH2PO4 solution into each FSCU via a peristaltic pump. Solutions were analysed daily for P by the method of Motomizu et al. (1983) and the rate of P addition was adjusted according to plant demand. Solution pH was maintained at 4·5 ± 0·05 with re-distilled 0·2 m HNO3 to prevent the precipitation of added La and Ce with phosphate (Diatloff et al., 1995a).
Appropriate volumes of 2 mm La or Ce nitrate stock solutions were added to each FSCU to impose treatments of approx. 0·2, 1·0 or 5·0 µm La or Ce. In addition, there was a common control treatment without any added La or Ce, making a total of seven FSCUs. The test solutions were analysed for La or Ce the day before transplanting of seedlings, and subsequently every 2 or 3 d, using inductively coupled plasma mass spectrometery (ICP-MS) with indium (In) as the internal standard. The control treatment contained La and Ce concentrations below the detection limit of 0·007 µm. As the La and Ce concentrations in solution remained constant, no additional La or Ce was supplied during the plant growing period. The mean concentrations (±s.e.) measured in the other treatments were 0·18 ± 0·01, 0·91 ± 0·04 and 5·09 ± 0·12 µm La, and 0·19 ± 0·02, 0·86 ± 0·02 and 5·02 ± 0·09 µm Ce.
Seeds of corn (Zea mays ‘Hycorn 82’) and mungbean (Vigna radiata ‘Berken’) were surface sterilized for 15 min with 1 % w/v calcium hypochlorite, then thoroughly rinsed with deionized water. Next, they were imbibed for 3 h in a continuously aerated 200 µm CaSO4 solution, then wrapped in paper towels moistened with a similar solution. When the roots were 30–40 mm long, the seedlings were transplanted into the FSCUs, giving ten single-plant replicates of each species in each unit. Also at transplanting, 20 seedlings of each species were harvested for chemical analysis. These plants were divided into roots and shoots, fresh weights were recorded, and these were then oven dried for 72 h at 80 °C, weighed and stored. All samples were finely ground and thoroughly mixed.
Harvest
Corn plants were grown for 14 d and mungbean for 18 d. At harvest, each plant was divided into roots and shoots. Fresh weights were recorded, and dry weights were measured after oven drying for 72 h at 80 °C. Prior to elemental analysis, shoots from the ten corn plants per treatment were bulked into five samples per treatment to provide sufficient material for analysis, and the corresponding roots were bulked into two samples per treatment. Shoots of mungbean in the control, 0·2 and 0·9 µm La and Ce treatments were bulked into five samples and the shoots in the 5 µm La and Ce treatments were bulked into two samples. Roots of mungbean were bulked into two samples.
Plant tissue analysis
Subsamples of shoots and roots (500–1000 mg) were weighed into 50-mL digestion tubes, digested for 3 h in re-distilled HNO3 and made up to 25 mL using MilliQ® water. The samples were then analysed for La, Ce, K, Mg, Ca, Na, B, Fe, Mn, Zn and Cu by ICP-MS using In as the internal standard. Separate sub-samples were analysed also for N and P (Kjeldahl digest: autoanalyser), and S (nitric perchloric digest: turbidimetric analysis) (Johnson et al., 1985). After these analyses, sufficient plant material remained to allow the measurement of chloride in the corn roots and shoots using an automated colorimetric method based on the thiocyanate–chloride complex after hot water extraction (Johnson et al., 1985).
Calculation of mean nutrient uptake rates
Approximate mean nutrient uptake (IM) rates by corn and mungbean were calculated using the formula (Williams, 1948):
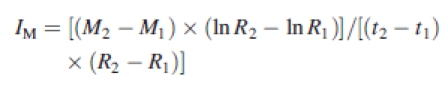 |
where R1 and R2 are the root fresh weights, and M1 and M2 are the nutrient amounts in the total plant at harvests taken at times t1 (transplanting) and t2 (final harvest).
Statistical analyses
Analyses of variance were performed using Genstat 4·0.
RESULTS
Plant growth
The dry weight of corn shoots was unaffected by increasing solution La concentrations from 0 to 5 µm and was unaffected also by solution Ce concentrations up to 0·9 µm (Fig. 1A). However, increasing the Ce concentration to 5·0 µm significantly reduced the shoot dry weight by 32 %. Although not apparent from Fig. 1A, the dry weight of corn roots was unaffected by solution La or Ce concentrations up to approx. 0·9 µm, but significantly decreased by 22 % in the presence of 5 µm La and by 33 % at 5 µm Ce.
Fig. 1.
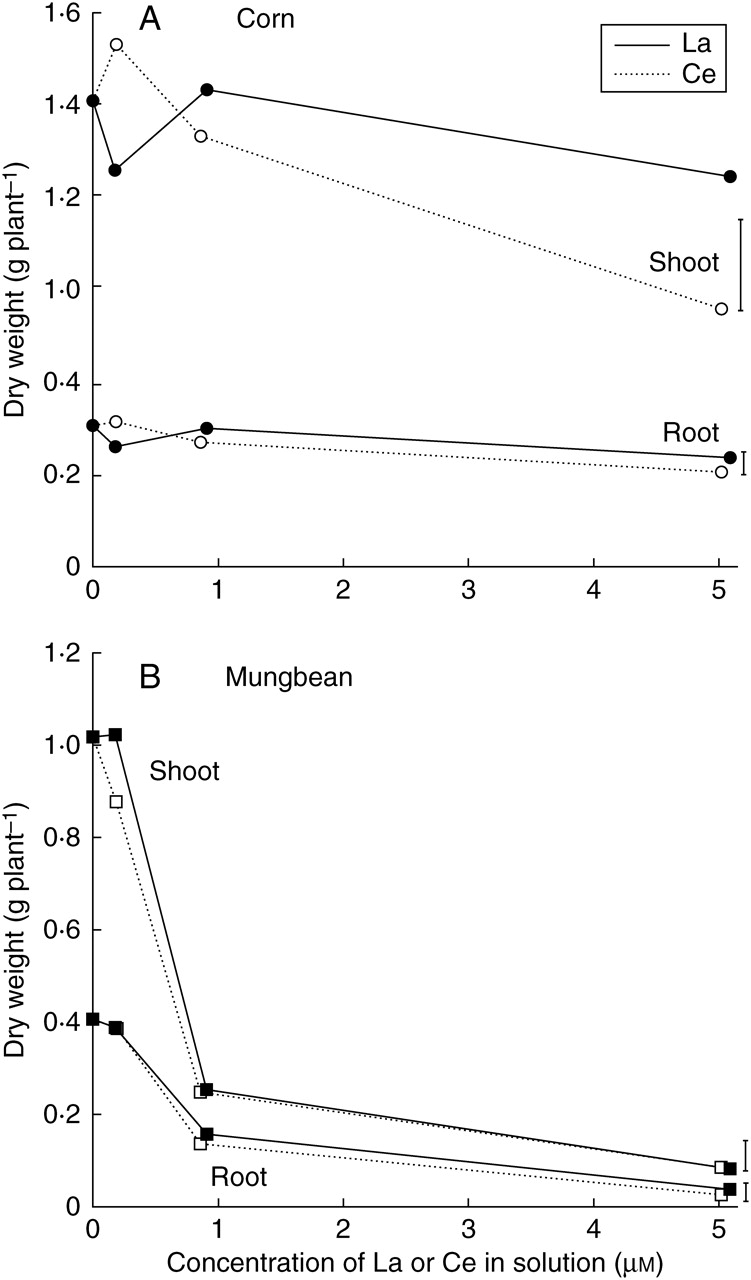
Effects of solution La (closed symbols, solid line) or Ce (open symbols, broken line) concentrations on the shoot and root dry weight of (A) corn and (B) mungbean. Vertical bars indicate least significant difference (5 %) for the REE × concentration interaction (interaction was not significant for mungbean roots).
The dry weight of mungbean shoots was unaffected by 0·2 µm La, but 0·2 µm Ce significantly reduced the dry weight by 14 % (Fig. 1B). Increasing the La or Ce concentrations to approximately 0·9 or 5 µm decreased the shoot dry weight of mungbean by 75 or 92 %, the two elements having similar effects. The root dry weight of mungbean was unaffected by approximately 0·2 µm La or Ce; however, concentrations of approx. 0·9 or 5 µm of these elements reduced the dry weight by 64 or 92 %, again the two elements having similar effects.
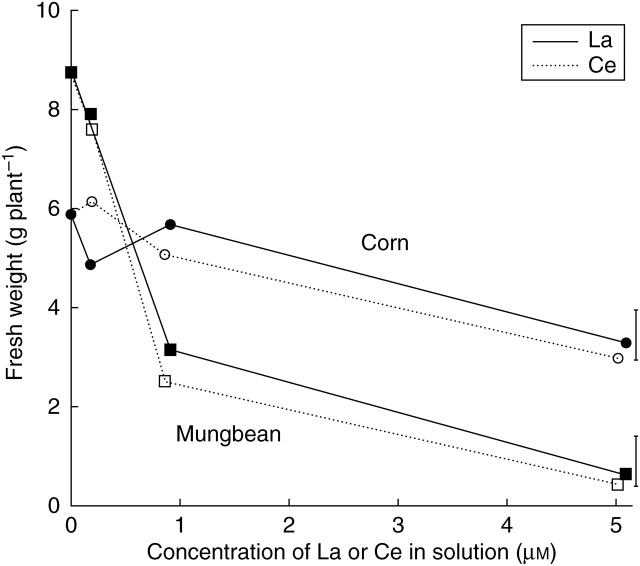
The effects of La or Ce on root fresh weights (Fig. 2), which were used in the calculation of mean nutrient uptake rates, were closely similar to those described for the root dry weights (Fig. 1). The root fresh weight of corn was unaffected by solution La or Ce concentrations up to approx. 0·9 µm, but significantly decreased by 44 % in the presence of 5 µm La, and by 49 % at 5 µm Ce (Fig. 2A). The addition of La or Ce at approx. 0·2, 0·9 or 5 µm significantly decreased the root fresh weight of mungbean by 11, 68 and 94 % respectively, the two elements having similar effects.
Fig. 2.
Effects of solution La (closed symbols, solid line) or Ce (open symbols, broken line) concentrations on the fresh weight of corn and mungbean roots. Vertical bars indicate least significant difference (5 %) for the REE × concentration interaction (the interaction was not significant for mungbean).
Concentrations of La and Ce in roots and shoots
In the zero REE treatment, the shoots of corn contained 0·12 mg kg−1 La and 0·25 mg kg−1 Ce, and the corresponding roots contained 0·52 mg kg−1 La and 0·84 mg kg−1 Ce. For mungbean, the concentrations were 0·17 mg kg−1 La and 0·46 mg kg−1 Ce in the shoots, and 0·73 mg kg−1 La and 0·90 mg kg−1 Ce in the roots.
Where REEs were added to the nutrient solution, the roots of corn and mungbean contained La or Ce concentrations 25–200 times higher than the corresponding shoots (Fig. 3). In addition, at equivalent concentrations of La or Ce in solution, mungbean roots and with one exception mungbean shoots, contained higher La or Ce concentrations than corn roots or shoots. These results are similar to those previously reported (Diatloff et al., 1995b, c), demonstrating that corn accumulated less La and Ce than mungbean.
Fig. 3.
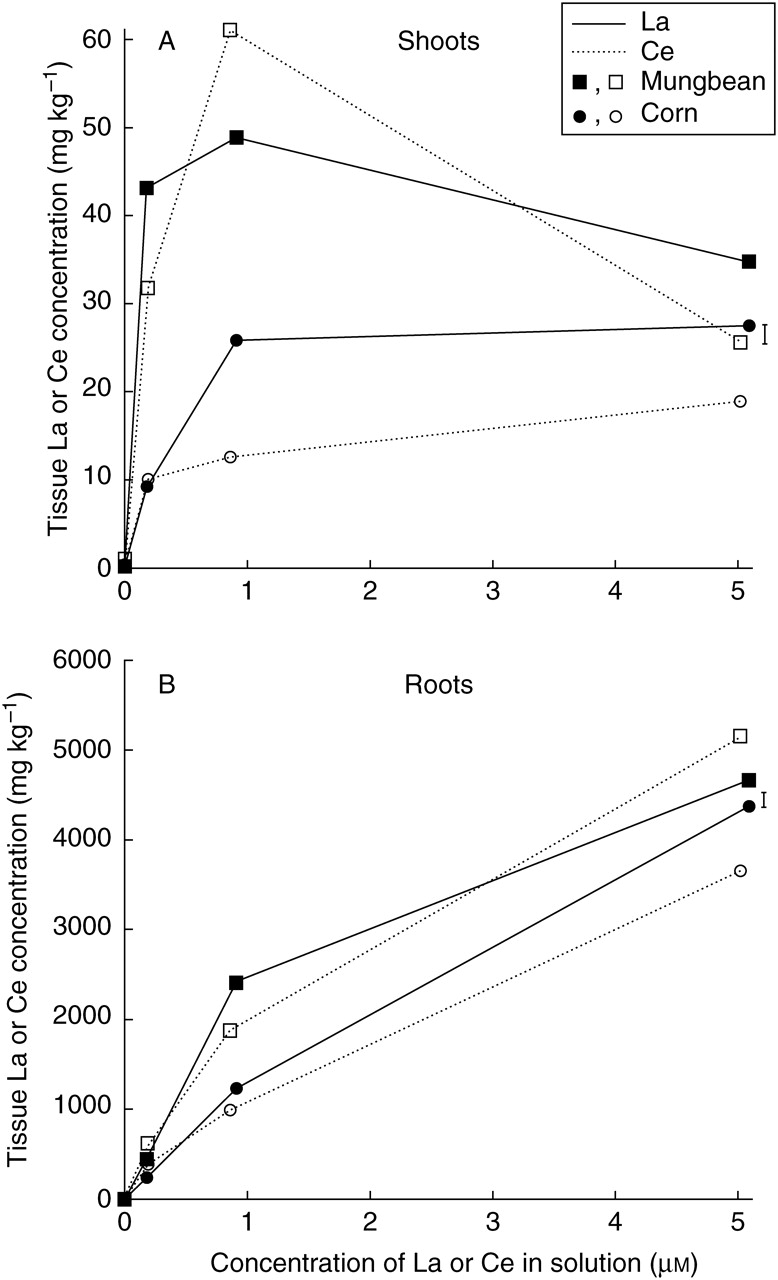
Effects of solution La or Ce concentrations on the concentrations of La (solid line) and Ce (broken line) in the (A) shoots and (B) roots of corn (circles) and mungbean (squares). Vertical bars indicate least significant difference (5 %) for the plant species × REE × concentration interaction.
The concentrations of La and Ce in the roots of both species increased strongly with increasing concentrations of La or Ce in solution over the entire range studied (Fig. 3B). The concentrations of La and Ce in the shoots of both plant species followed a different pattern (Fig. 3A). The concentrations of La and Ce in the shoots of mungbean increased to a maximum of 49 mg kg−1 La or 62 mg kg−1 Ce at 0·9 µm La or Ce, but then significantly declined to 35 mg kg−1 La or 26 mg kg−1 Ce in the presence of 5 µm La or Ce. These shoot concentrations in the highest solution treatments were significantly lower than those at 0·2 µm. At 0·2 µm Ce, corn shoots accumulated 10 mg kg−1 Ce and increasing the Ce concentration in solution to 5 µm significantly increased the shoot Ce concentration to 19 mg kg−1 (Fig. 3A). Similarly, corn accumulated 9 mg kg−1 La in the presence of 0·2 µm La. However, both at 0·9 and at 5 µm La corn shoots contained 26–28 mg kg−1, significantly higher than shoot Ce concentrations in the corresponding Ce treatments.
The concentrations of La and Ce in corn and mungbean shoots did not increase strongly over the entire range of solution concentrations studied (as seen in roots), despite a 25-fold increase in the concentrations of La or Ce in solution and a five- to 25-fold increase in their concentrations in the roots. Again these results are similar to those reported previously (Diatloff et al., 1995b, c), confirming that both plant species are able to restrict the uptake of La and Ce into the shoots.
Concentrations of nutrients in shoots and roots
Tables 1–4 present the concentrations of nutrients in the whole shoots and roots of corn and mungbean. For each nutrient, seven values are shown corresponding to the La and Ce treatments, along with the least-significant difference (LSD; 5 %) for this REE × concentration interaction. Below this line are shown three values in parentheses for the means of the La and Ce treatments, along with the LSD (5 %) for this REE concentration effect. The three columns at the right-hand side of each table indicate the concentration means for each REE, and the LSD (5 %) values for this REE effect.
Table 1.
Effect of solution La or Ce concentrations on the nutrient concentrations in the whole shoots of corn
| La or Ce concentration (μm) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0·2 |
0·9 |
5 |
Mean |
||||||||
| Nutrient | La | Ce | La | Ce | La | Ce | LSD (5 %)* | Adequate range† | La | Ce | LSD (5%)‡ | |
| Per cent | ||||||||||||
| N | 5·05 | 4·87 | 5·02 | 4·85 | 4·69 | 4·40 | 3·96 | 0·17 | 3·5–5·0 | 4·71 | 4·56 | 0·10 |
| P | 0·47 | 0·46 | 0·46 | 0·48 | 0·42 | 0·41 | 0·40 | 0·02 | 0·3–0·5 | 0·45 | 0·43 | 0·01 |
| K | 5·79 | 5·56 | 5·81 | 5·58 | 5·44 | 5·18 | 4·79 | n.s. | 2·5–4·0 | 5·44 | 5·35 | n.s. |
| S | 0·23 | 0·21 | 0·23 | 0·23 | 0·25 | 0·32 | 0·33 | n.s. | 0·2–0·3 | 0·25 | 0·27 | 0·01 |
| Ca | 0·81 | 0·74 | 0·70 | 0·70 | 0·42 | 0·48 | 0·33 | 0·08 | 0·3–0·7 | 0·64 | 0·48 | 0·05 |
| Mg | 0·20 | 0·19 | 0·18 | 0·20 | 0·22 | 0·22 | 0·27 | 0·01 | 0·15–0·45 | 0·20 | 0·22 | 0·01 |
| Cl | 0·98 | 0·95 | 1·03 | 0·95 | 1·08 | 0·93 | 1·04 | n.s. | Toxic >2·0 | 0·94 | 1·05 | 0·05 |
| mg kg−1 | ||||||||||||
| Na | 467 | 478 | 245 | 353 | 142 | 321 | 110 | n.s. | – | 384 | 166 | 51 |
| Cu | 21 | 21 | 14 | 17 | 22 | 15 | 21 | 3 | 5–20 | 17 | 19 | n.s. |
| Zn | 141 | 112 | 101 | 84 | 120 | 88 | 80 | 14 | 20–60 | 94 | 100 | n.s. |
| Mn | 100 | 113 | 73 | 71 | 92 | 64 | 60 | 9 | 25–300 | 83 | 75 | 5 |
| Fe | 159 | 129 | 152 | 164 | 162 | 129 | 135 | n.s. | 50–250 | 141 | 149 | n.s. |
| B | 11 | 12 | 12 | 13 | 24 | 13 | 30 | 8 | 5–25 | 12 | 22 | 5 |
* Least significant difference (5 %) for REE × concentration interaction
‡ Least significant difference (5 %) for REE effect.
n.s. = not significant at P = 0·05.
Table 4.
Effect of solution La or Ce concentrations on the nutrient concentrations in mungbean roots
| La or Ce concentration (μm) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0·2 |
0·9 |
5 |
Mean |
|||||||
| Nutrient | La | Ce | La | Ce | La | Ce | LSD (5 %)* | La | Ce | LSD (5 %)† | |
| Per cent | |||||||||||
| N | 5·75 | 5·70 | 5·48 | 4·78 | 4·86 | 3·84 | 3·40 | 0·17 | 4·77 | 4·58 | 0·10 |
| P | 0·60 | 0·63 | 0·60 | 0·80 | 0·65 | 0·55 | 0·50 | 0·03 | 0·66 | 0·59 | 0·02 |
| K | 8·27 | 7·98 | 7·47 | 7·22 | 6·79 | 3·57 | 2·69 | n.s. | 6·25 | 5·65 | 0·15 |
| S | 0·62 | 0·55 | 0·54 | 0·56 | 0·49 | 0·29 | 0·18 | n.s. | 0·47 | 0·40 | n.s. |
| Ca | 0·31 | 0·30 | 0·31 | 0·14 | 0·18 | 0·14 | 0·10 | 0·03 | 0·19 | 0·19 | n.s. |
| Mg | 0·32 | 0·40 | 0·38 | 0·20 | 0·29 | 0·13 | 0·09 | 0·03 | 0·25 | 0·23 | n.s. |
| mg kg−1 | |||||||||||
| Na | 314 | 314 | 316 | 540 | 574 | 750 | 1061 | 42 | 535 | 650 | 24 |
| Cu | 27 | 18 | 20 | 30 | 40 | 31 | 35 | n.s. | 27 | 32 | n.s. |
| Zn | 289 | 199 | 342 | 137 | 138 | 209 | 120 | 48 | 182 | 200 | n.s. |
| Mn | 113 | 142 | 157 | 45 | 55 | 29 | 42 | n.s. | 72 | 85 | n.s. |
| Fe | 358 | 362 | 322 | 708 | 582 | 619 | 868 | 36 | 563 | 591 | 21 |
| B | 40 | 45 | 30 | 57 | 59 | 40 | 77 | 19 | 47 | 55 | n.s. |
* LSD (5 %) for REE × concentration interaction.
† LSD (5 %) for REE effect.
n.s. = not significant at P = 0·05.
Corn
In corn, the concentrations in the whole shoots of N, P, S, Ca, Mg, Cl, Cu, Mn, Fe and B in all La and Ce treatments (Table 1) were generally within the adequate ranges suggested for healthy field-grown corn at a similar growth stage (Reuter and Robinson, 1986). Decreasing the concentration of P in solution from 5 µm (Diatloff et al., 1995b, c) to 1 µm (present experiment) successfully reduced the high P concentrations of 0·83–0·98 % in the shoots of control corn plants reported by Diatloff et al. (1995b, c) to 0·47 %, which is within the adequate range (Table 1). However, the concentrations of K and Zn, were somewhat above the adequate range in all treatments, but the highest Zn concentration (141 mg kg−1) was well below the critical value of 460 mg kg−1 reported by Boawn and Rasmussen (1971).
Increasing the concentrations of La or Ce in solution from 0 to 5 µm significantly decreased the concentrations of N, P, K, Ca, Na, Zn, Mn and Fe in the shoots of corn (Table 1). At 5 µm, there were decreases of 41 % (La) and 59 % (Ce) in the Ca concentrations, and an approximately 38 % decrease in the Mn concentrations as compared with control plants. The concentration of Zn in the shoots of corn decreased approximately 40 % in the 5 µm treatments relative to control plants. The concentration of Cl in the shoots of corn was unaffected by increasing concentrations of La or Ce in solution. La or Ce at 5 µm increased the concentration of S approximately 40 % compared with controls. The concentration of Mg in the shoots of corn increased significantly by 10 % (La) and 35 % (Ce) in the presence of 5 µm La or Ce. There was a 2·7-fold increase in the concentration of B in the shoots of corn plants grown in the presence of 5 µm Ce.
Ce was more detrimental to the concentrations of N, P, Ca, Na and Mn in corn shoots than La (Table 1). La and Ce had similar effects on the concentrations of K, Cu, Zn and Fe, but Ce increased the concentrations of S, Cl, Mg and B more than did La.
In corn roots, the concentrations of P increased 15 and 47 % relative to controls in the presence of 0·9 and 5 µm La or Ce, respectively (Table 2). The concentrations of the remaining nutrients in corn roots were either unaffected (K, S, Cl, Na, Cu, B) or decreased (N, Ca, Mg, Zn, Mn, Fe) over the range of La or Ce concentrations in solution. The Ca concentrations in roots decreased with increasing concentration of La or Ce in solution (also observed in corn shoots), with the Ca concentration in the 5 µm La or Ce treatment being 30 % of that measured in the control plants. Similarly, the concentrations of Mg in the roots of plants in the 5 µm La or Ce treatments were 60 % of those in the control plants, a trend opposite to that noted in the corresponding shoots.
Table 2.
Effect of solution La or Ce concentrations on the nutrient concentrations in corn roots
| La or Ce concentration (μm) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0·2 |
0·9 |
5 |
Mean |
|||||||
| Nutrient | La | Ce | La | Ce | La | Ce | LSD (5 %)* | La | Ce | LSD (5 %)† | |
| Per cent | |||||||||||
| N | 4·44 | 4·71 | 4·87 | 4·66 | 4·50 | 4·08 | 3·92 | n.s. | 4·48 | 4·43 | n.s. |
| P | 0·34 | 0·37 | 0·38 | 0·45 | 0·36 | 0·52 | 0·47 | n.s. | 0·45 | 0·40 | 0·04 |
| K | 4·56 | 4·77 | 5·02 | 5·44 | 5·33 | 4·98 | 4·79 | n.s. | 5·06 | 5·04 | n.s. |
| S | 1·04 | 0·89 | 0·71 | 0·89 | 0·91 | 1·14 | 0·96 | n.s. | 0·97 | 0·86 | 0·10 |
| Ca | 1·19 | 0·96 | 0·99 | 0·55 | 0·69 | 0·37 | 0·42 | n.s. | 0·63 | 0·70 | n.s. |
| Mg | 0·34 | 0·37 | 0·36 | 0·32 | 0·28 | 0·23 | 0·20 | n.s. | 0·31 | 0·28 | 0·02 |
| Cl | 0·33 | 0·39 | 0·34 | 0·36 | 0·38 | 0·37 | 0·44 | 0·06 | 0·37 | 0·39 | n.s. |
| Na | 0·12 | 0·12 | 0·14 | 0·12 | 0·10 | 0·13 | 0·12 | n.s. | 0·12 | 0·12 | n.s. |
| mg kg−1 | |||||||||||
| Cu | 53 | 42 | 35 | 83 | 72 | 43 | 77 | n.s. | 56 | 61 | n.s. |
| Zn | 257 | 183 | 355 | 200 | 123 | 153 | 95 | 38 | 179 | 191 | n.s. |
| Mn | 120 | 142 | 147 | 86 | 54 | 76 | 82 | 20 | 102 | 94 | n.s. |
| Fe | 300 | 271 | 292 | 390 | 270 | 268 | 224 | 61 | 310 | 262 | 35 |
| B | 15 | 16 | 10 | 12 | 14 | 10 | 12 | 4 | 13 | 12 | n.s. |
* LSD (5 %) for REE × concentration interaction.
† LSD (5 %) for REE effect.
n.s. = not significant at P = 0·05.
La and Ce had similar effects on the root concentrations of N, K, Ca, Cl, Na, Cu, Zn, Mn and B, but Ce was more detrimental than La to the concentrations of P, S, Mg and Fe.
Mungbean
The shoots of mungbean plants in the control treatment contained adequate concentrations of all nutrients when compared with the limited number of documented adequate ranges for mungbean and other tropical grain legumes (Table 3). Increasing the concentration of Mn in solution from 0·1 µm (Diatloff et al., 1995b, c) to 0·3 µm (present experiment) successfully increased the concentration of Mn in the shoots of control mungbean plants from 44 mg kg−1 (Diatloff et al., 1995b, c) to 80 mg kg−1 (Table 3). However, at ≥0·9 µm La or Ce, Mn concentrations were again marginal or deficient. As with corn, reducing the P concentration in solution from 5 to 1 µm successfully reduced the high P concentration of 0·97 % in mungbean shoots in the zero Ce treatment (Diatloff et al., 1995b, c) to 0·5 %, which is within adequate levels (Table 3).
Table 3.
Effect of solution La or Ce concentrations on the nutrient concentrations in whole shoots of mungbean.
| La or Ce concentration (μm) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0·2 |
0·9 |
5 |
Mean |
||||||||
| Nutrient | La | Ce | La | Ce | La | Ce | LSD (5 %)* | Adequate range† | La | Ce | LSD (5 %)‡ | |
| Per cent | ||||||||||||
| N | 5·69 | 5·70 | 5·60 | 5·31 | 5·01 | 2·10 | 1·82 | n.s. | 3·6A | 4·81 | 4·62 | 0·18 |
| P | 0·50 | 0·53 | 0·52 | 0·84 | 0·62 | 0·24 | 0·19 | 0·05 | 0·18–0·56A | 0·54 | 0·46 | 0·03 |
| K | 5·45 | 5·28 | 5·49 | 5·16 | 5·01 | 1·43 | 0·85 | n.s. | 4·9–6·6B | 4·39 | 4·35 | n.s. |
| S | 0·28 | 0·28 | 0·27 | 0·34 | 0·30 | 0·17 | 0·19 | 0·03 | – | 0·27 | 0·25 | 0·02 |
| Ca | 2·88 | 3·36 | 2·77 | 1·83 | 1·68 | 0·41 | 0·22 | n.s. | 0·72–1·0B | 2·36 | 1·94 | 0·21 |
| Mg | 0·38 | 0·44 | 0·45 | 0·38 | 0·36 | 0·14 | 0·12 | n.s. | 0·17–0·31B | 0·36 | 0·36 | n.s. |
| mg kg−1 | ||||||||||||
| Na | 85 | 73 | 71 | 118 | 129 | 89 | 97 | n.s. | <200C | 87 | 81 | n.s. |
| Cu | 20 | 10 | 9 | 10 | 9 | 11 | 7 | n.s. | 6–15C | 10 | 9 | n.s. |
| Zn | 61 | 52 | 65 | 68 | 68 | 37 | 19 | n.s. | >28A | 52 | 55 | n.s. |
| Mn | 80 | 60 | 53 | 15 | 17 | 8 | 5 | n.s. | 54–300C | 38 | 35 | n.s. |
| Fe | 583 | 486 | 408 | 349 | 472 | 194 | 54 | 95 | >100B | 391 | 362 | n.s. |
| B | 42 | 41 | 40 | 43 | 36 | 35 | 18 | n.s. | >20A | 40 | 34 | 6 |
* LSD (5 %) for REE × concentration interaction.
† Reuter and Robinson (1986); A mungbean; B cowpea; C soybean.
‡ LSD (5 %) for REE effect.
n.s. = not significant at P = 0·05.
At 0·2 µm La or Ce the concentrations of all nutrients were again within the adequate range (Table 3). At 0·9 µm La or Ce, most nutrients were within the adequate range, although P was above adequate and Mn was deficient. At 5 µm La or Ce, Mn and N were in the deficient range, and P was marginal for mungbean, and K, Mg would have been marginal for soybean and Ca deficient for soybean, no corresponding values being available in the literature for mungbean.
In the presence of 0·2 µm La or Ce, there was a significant decrease in the shoot concentrations of Cu (50 %), Mn (29 %) and Fe (17 %, La; 30 %, Ce) compared with the control plants. The concentrations of all other nutrients were generally unaffected by 0·2 µm La or Ce, although 0·2 µm Ce increased Mg concentrations and 0·2 µm La increased Ca concentrations relative to the controls. Increasing the concentrations of La and Ce to 0·9 µm significantly decreased the concentrations of N, K, Ca, Mg, Mn and Fe. The concentrations of Na were unaffected by La or Ce concentrations in solution. The concentrations of Mn in the shoots of mungbean decreased 11-fold in the 5 µm treatments relative to controls. The concentration of Ca in the shoots of mungbean decreased seven-fold (La) and 13-fold (Ce) in the 5 µm treatments compared with controls. Ce was more detrimental than La to the concentrations of N, P, S, Ca and B, the two elements having similar effects on all the remaining nutrients.
In mungbean roots (Table 4), the concentrations of Na and Fe increased with increasing concentrations of La or Ce in solution, in contrast to the shoots (Table 3). The concentrations of the remaining nutrients in mungbean roots were either generally unaffected (Cu, B) or decreased (N, P, K, S, Ca, Mg, Zn, Mn) by increasing concentrations of La or Ce in solution.
Nutrient uptake rates
For corn, increasing the concentrations of La or Ce in solution from 0 to 5 µm increased the uptake rates of Cl, S, Mg and B (Fig. 4 B, D, F). An increased rate of S uptake was observed only in the 5 µm La and Ce treatments, there being a 65 % (La) and 34 % (Ce) increase relative to the control (Fig. 4D). Similarly, increased Mg uptake was observed only in the highest La and Ce treatments, with a 36 % (La) and 29 % (Ce) increase in the 5 µm treatments compared with control plants (Fig. 4D). The uptake of S and Mg corresponded to increased nutrient concentrations in the shoots and not the roots (Tables 1 and 2). Boron uptake rate by corn increased 39 % and 1·6-fold in the presence of 5 µm La and Ce, respectively, compared with controls (Fig. 4F).
Fig. 4.
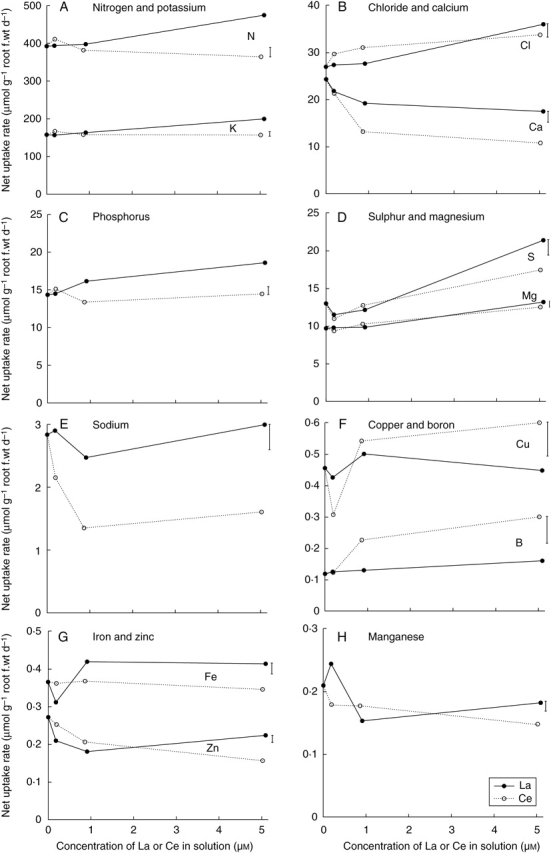
Effects of La (closed symbols, solid line) or Ce (open symbols, broken line) concentrations in solution on the net nutrient uptake rates of corn. Vertical bars indicate least significant difference (5 %) for the REE × concentration interaction. The interaction was significant for all nutrients except Mg, for which only the concentration effect was significant.
Decreases in the uptake rate of Ca, Na, Zn and Mn by corn were observed with increased concentrations of La or Ce in solution (Figs. 4B, E, G, H). Ce decreased the uptake rate of Na by corn to a greater degree than La (Fig. 4E). Similarly, solution Ce concentrations ≥0·9 µm decreased Ca uptake rate by over 50 %, with only a 20 % reduction observed at equivalent La concentrations (Fig. 4B).
For mungbean, the uptake rates of all nutrients decreased with increasing concentrations of La or Ce in solution >0·2 µm (Fig. 5). There was a 50 % decrease in the Cu uptake rate at approx. 0·2 µm La or Ce, but this rate was unaffected by higher concentrations of La or Ce in solution (Fig. 5G). The largest decrease in nutrient uptake rate in the presence of 0·2 µm La or Ce was observed with Mn, which decreased over 80 % relative to controls (Fig. 5H). The uptake rate of all nutrients was severely depressed in the presence of 5 µm La or Ce in solution.
Fig. 5.
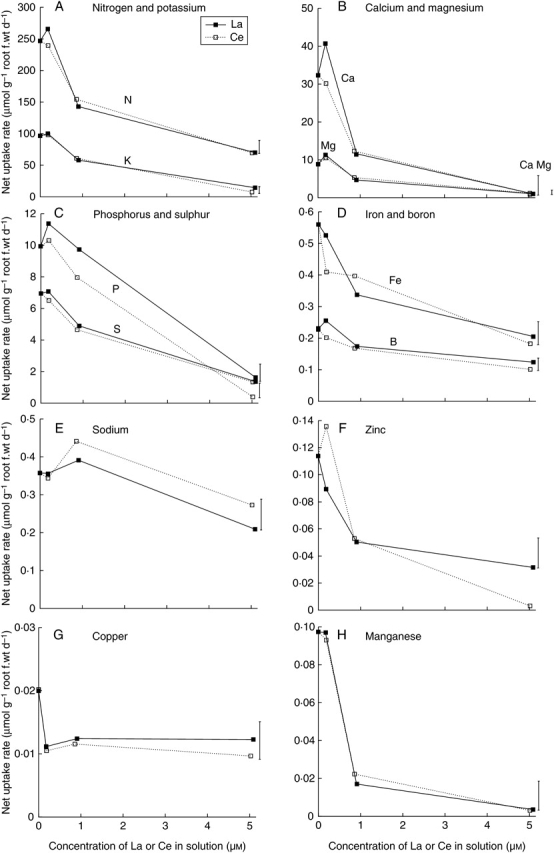
Effects of La (closed symbols, solid line) or Ce (open symbols, broken line) concentrations in solution on the net nutrient uptake rates of mungbean. Vertical bars indicate least significant difference (5 %) for the REE × concentration interaction. The interaction was significant for N, Ca, Fe, Zn, Cu and Na, but not for P, K, Mg, B or Mn.
DISCUSSION
Plant growth
Corn has previously been shown to be more tolerant than mungbean of La or Ce concentrations in solution ranging from 0 to 1·4 µm (Diatloff et al., 1995b, c). Results of the present experiment have confirmed this differential sensitivity of corn and mungbean to these two REEs. Concentrations of La or Ce in solution up to approx. 0·9 µm did not significantly affect corn dry weight, whereas mungbean dry weight was depressed by over 70 % at La or Ce concentrations >0·2 µm (Fig. 1). Chemical analysis of plant tissues from the present experiment (Fig. 3) and previous experiments (Diatloff et al., 1995b, c) demonstrate that the shoots and roots of corn accumulated less La and Ce than mungbean, which may have contributed to the greater tolerance of corn than of mungbean to both elements. Reduced accumulation of metals is one strategy commonly employed by plants to tolerate aluminium (Al) and heavy metals (Cumming and Taylor, 1990; Taylor, 1991).
Small beneficial effects of Ce and, to a lesser extent, La were observed on the root growth of corn in flowing solution culture (Diatloff et al., 1995b, c). No such beneficial effects of La or Ce were observed on corn root dry weight or fresh weight in the present experiment (Figs 1A and 2), where higher Mn (0·3 µm) and lower P concentrations (1 µm) were added to the nutrient solution than in our previous experiments (Diatloff et al., 1995b, c). This suggests that the concentrations of these nutrients in solution may influence the effects of La and Ce on corn root growth. Further investigations into the beneficial effects would require attention to the interaction between solution concentrations of Mn, P and H.
Separate experiments by Diatloff et al. (1995b, c) indicated Ce may be more toxic to the growth of mungbean than La, but interpretation was difficult owing to Mn deficiency in the presence of Ce but not of La. In the present experiment, in which higher concentrations of Mn in solution were provided, no significant differences were observed between the effects of La or Ce on mungbean dry matter production in the 0·9 and 5 µm treatments (Fig. 1B). Therefore, the concentration of Mn in solution may also influence the effects of La and Ce on the growth of mungbean. The influence of Mn on the response of mungbean to La and Ce is discussed further below.
The present experiment allowed a direct comparison of the effects of La and Ce on plant growth. La and Ce have very similar chemical properties with respect to cation charge, ionic radii, and stability of organic and inorganic complexes (Evans, 1990). The similarity in the chemical properties of La and Ce was reflected in the closeness of their effects on plant growth in the present experiment (Figs 1 and 2).
Corn root dry weight was decreased at 5 µm La or Ce and 5 µm Ce decreased the shoot dry weight (Fig. 1A). These decreases in plant growth were unlikely to be due to nutritional effects, as the concentrations of all nutrients were within the adequate range for all REE treatments (Table 1). It is most probable that decreased corn growth was due to direct effects of La or Ce rather than indirect effects such as decreased nutrient uptake.
Effects of La and Ce on the nutrition of corn
The effects of La and Ce on the nutrition of each plant species is discussed separately due to the contrasting growth responses of the plant species to increasing concentrations of La or Ce in solution. For corn, the dry weight of shoots and roots was not significantly affected by La or Ce concentrations up to approx. 0·9 µm. This enables the effects of La and Ce on the nutrition of corn to be examined in the absence of gross growth effects.
Calcium
For corn, the nutrient most severely affected by the treatments was Ca. Calcium concentrations in corn shoots and roots (Table 1) and the uptake rate of Ca by corn plants (Fig. 4B) significantly decreased with increasing concentrations of La or Ce in the nutrient solution. In this dilute solution, soluble La and Ce are predicted to occur as REE3+ and REESO4+ complexes (Diatloff et al., 1993). These positively charged species would be expected to displace Ca from the cell walls of roots, and this is supported by chemical analyses showing a decrease in Ca concentrations in corn roots with increasing concentrations of La or Ce in solution (Table 1). Once adsorbed onto cell walls, REEs repel Ca from the apoplast (Clarkson and Sanderson, 1969). Consequently, there will be a reduced reservoir of Ca that can be transported radially either by apoplastic or by symplastic flow to the xylem. The REEs are also known to reduce the influx of Ca into root cells by blocking Ca channels in the roots of maize (Marshall et al., 1994) and wheat (Huang et al., 1994). The decreased Ca nutrition of corn in the presence of La and Ce is consistent with their known antagonism of Ca. Results in this study show Ce to be a more potent inhibitor of net Ca uptake by corn than La (Fig. 4B).
Other cations
Apart from Ca, the effects of REEs on the nutrition of plants are very poorly documented (Brown et al., 1990; Hu et al., 2004). In contrast to Ca, the uptake of all other cations by corn was much less affected by increasing concentrations of La or Ce. For example, uptake of Mg was unaffected by ≥0·9 µm La or Ce, but slightly increased by the 5 µm treatments (Fig. 4D). This effect is in contrast to that of Al, which generally decreases the uptake of both Ca and Mg (Rengel, 1992; Marschner, 1995).
The uptake of K was also relatively unaffected, although 5 µm La resulted in a slight increase (Fig. 4A). No consistent picture about La effects on K uptake by plants exists, with both increased and decreased K uptake reported, and at solution concentrations from 25 to 1000 µm La (Brown et al., 1990; Hue et al., 2004). These differences in K uptake are mostly caused by different experimental protocols. Although K and Na have similar ionic radii, their uptake rates by corn were differently affected by La and Ce. Ce was more detrimental than La to the rate of uptake and shoot concentrations of Na in corn, but Na concentrations in the roots were unaffected by La or Ce (Table 1; Fig. 4e). This indicates that the translocation of Na from the roots to the shoots is disturbed more by La than by Ce, and presumably the uptake of Na by corn roots is unaffected by La or Ce.
The uptake of Fe by corn was relatively unaffected by La or Ce (Fig. 4G), suggesting that the mechanism of phytosiderophore-mediated Fe uptake attributed to corn (von Wiren et al., 1995) may not be adversely influenced by La or Ce. Similarly, the shoot and root concentrations of Cu and the uptake of Cu were unaffected by La or Ce (Tables 1 and 2; Fig. 4F). However, tissue concentrations of Mn and Zn and their uptake rates decreased with increasing concentrations of La or Ce (Tables 1 and 2; Fig. 4G, H).
Despite the decrease in the uptake of Ca, Zn and Mn, there did not appear to be any increase in the uptake of any other of the cations or a corresponding decrease in anion uptake that potentially could counteract the charge imbalance due to decreased cation uptake. This charge imbalance may have been accommodated by decreased organic acid synthesis within the plant.
Anions
Increasing the concentration of La or Ce in solution up to 5 µm decreased the concentration of P in corn shoots (Table 1), but increased the P concentration in the roots (Table 2). Leonard et al. (1975) also noted that short-term exposure of corn roots to La resulted in increased 32P uptake; however, this phenomenon was concluded to be due to the non-metabolic accumulation of an La–P complex in the root apoplast. It is most probable that the increased P concentrations in corn roots were the result of phosphate precipitation of La or Ce in the apoplast (Clarkson and Sanderson, 1969). In contrast, La and Ce at >0·2 µm increased the S concentration in corn shoots, but not in the roots (Tables 1 and 2) for which at present there is no explanation. The Cl concentrations in root and shoots of corn were unaffected by La or Ce (Tables 1 and 2).
No consistent pattern of effects of La and Ce on either divalent or monovalent cations or anions were observed to suggest any general effect on any of these groupings of nutrients. This would suggest interactions of La or Ce with each individual nutrient rather than any effect on global factors affecting corn nutrition.
Effects of La and Ce on the nutrition of mungbean
The dry and fresh weight of mungbean roots was severely depressed by concentrations of La or Ce in solution ≥0·9 µm (Figs 1B and 2). Similarly, root elongation studies also showed La and Ce to be potent inhibitors of mungbean root growth (Diatloff et al., 1995a). The present study shows that in addition to the reduced root growth, the function of these roots was impaired by La and Ce. The shoot concentrations and uptake rates of all nutrients decreased over the range of La and Ce concentrations studied (Table 2; Fig. 5), suggesting a general reduction in root function.
Mungbean dry matter production was reduced by 64–75 % at 0·9 µm La or Ce, the solution concentration at which adequate concentrations of all nutrients (except perhaps Mn) were measured in mungbean shoots (Figs 1 and 2; Table 2). Initially, this would seem to suggest that the growth of mungbean was depressed directly by La and Ce in advance of reduced growth due to impaired nutrient uptake. However, the pattern of decreased mungbean growth (Figs 1 and 2) was closely similar to that of decreased Mn uptake (Fig. 5H). In addition, the decrease in Mn uptake with increased concentrations of La or Ce in solution was larger than for the decrease in uptake of any other nutrients (Fig. 5), and the Mn concentrations in mungbean shoots were probably deficient at 0·9 µm La or Ce, and deficient at 5 µm La or Ce (Table 2). Together these results indicate an association of decreased mungbean growth with reduced Mn uptake. Despite the increased Mn concentration in solution (Diatloff et al., 1995b, c), separation of direct La and Ce toxicity on mungbean from reduced Mn uptake was again not possible. To observe the direct effects of La and Ce in the absence of Mn deficiency, still higher concentrations of Mn in solution would be required. Recalculation of the results from Ashwath (1990) suggest that mungbean (‘Berken’) tolerated concentrations of Mn in solution up to 3 µm, and concentrations in the shoots of up to 600 mg kg−1 without any decrease in total dry weight resulting. However, these results were obtained from flowing solution culture experiments conducted at pH 5·5 (Ashwath, 1990), and it is known that at pH 4·5 (pH used in the present experiment) Mn uptake is three times lower than at pH 5·5 (Maas et al., 1968). Although little has been documented on the tolerance of mungbean to low concentrations of Mn, mungbean is known to be sensitive to excess levels of Mn (Smith et al., 1983; Ashwath, 1990).
CONCLUSIONS
Lanthanum and cerium did not enhance the growth of corn or mungbean, but decreased the growth, root function and consequently the nutritional status of mungbean at concentrations >0·2 µm in solution. While La and Ce had similar effects on plant growth, Ce was more detrimental than La to the uptake of Ca and Na by corn. Corn accumulated significantly lower concentrations of La and Ce than mungbean, which may contribute to its higher tolerance to both elements. We conclude that if La or Ce have positive effects on corn and mungbean growth, they can only occur at solution concentrations below 0·2 µm.
ACKNOWLEDGEMENTS
This paper is dedicated to Emeritus Professor Colin J. Asher and Dr Frank W. Smith for their contributions to the teaching and science of plant nutrition.
LITERATURE CITED
- Asher CJ. Beneficial elements, functional nutrients and possible new essential elements. In: Mortvedt JJ, Cox FR, Shuman LM, Welch RM, editors. Micronutrients in agriculture. 2nd edn. Madison, WI: SSSA; 1991. pp. 703–723. [Google Scholar]
- Asher CJ, Reghenzani JR, Robards KH, Tribe DE. Rare earths in Chinese agriculture. The Australian Academy of Technological Sciences and Engineering; 1990. Report of an Australian Mission which visited China from 31st March – 15th April 1990. [Google Scholar]
- Ashwath DC. Effects of manganese toxicity on growth and nodulation of tropical grain legumes. The University of Queensland, Department of Agriculture; 1990. MSc thesis. [Google Scholar]
- Boawn LC, Rasmussen PE. Crop responses to excessive Zn fertilization of a1kaline soils. Agronomy Journal. 1971;63:874–876. [Google Scholar]
- Brown PH, Rathjen AH, Graham RD, Tribe DE. Rare earth elements in biological systems. In: Gschneider KA Jr, Eyring L, editors. Handbook on the physics and chemistry of rare earths. vol. 13. Amsterdam: North-Holland Publishing Company; 1990. pp. 423–452. [Google Scholar]
- Clarkson DT, Sanderson J. The uptake of a polyvalent cation and its distribution in root apices of Allium cepa, tracer and autoradiographic studies. Planta. 1969;89:136–154. doi: 10.1007/BF00386981. [DOI] [PubMed] [Google Scholar]
- Cumming JR, Taylor GJ. Mechanisms of metal tolerance in plants: adaption for exclusion of metal ions from the cytoplasm. In: Alseher G, Cumming JR, editors. Stress responses in plants: Adaption and acclimation mechanisms. New York: John Wiley and Sons; 1990. pp. 329–356. [Google Scholar]
- Diatloff E, Asher CJ, Smith FW. Use of GEOCHEM-PC to predict rare earth element (REE) species in nutrient solutions. Plant and Soil. 1993;156:251–254. [Google Scholar]
- Diatloff E, Smith FW, Asher CJ. Rare-earth elements and plant growth. 1. Effects of lanthanum and cerium on root elongation of corn and mungbean. Journal of Plant Nutrition. 1995;a 18:1963–1976. [Google Scholar]
- Diatloff E, Smith FW, Asher CJ. Rare-earth elements and plant growth. 2. Responses of corn and mungbean to low concentrations of lanthanum in dilute, continuously flowing nutrient solutions. Journal of Plant Nutrition. 1995;b 18:1977–1989. [Google Scholar]
- Diatloff E, Smith FW, Asher CJ. Rare-earth elements and plant growth. 3. Responses of corn and mungbean to low concentrations of cerium in dilute, continuously flowing nutrient solutions. Journal of Plant Nutrition. 1995;c 18:1991–2003. [Google Scholar]
- Diatloff E, Asher CJ, Smith FW. Foliar application of rare earth elements to maize and mungbean. Australian Journal of Experimental Agriculture. 1999;39:189–194. [Google Scholar]
- Drew LJ, Qingrun M, Weijun S. The Bayan Obo iron rare-earth niobium deposits, Inner-Mongolia, China. Lithos. 1990;26:43–65. [Google Scholar]
- Epstein E, Bloom AJ. Mineral nutrition of plants: principles and perspectives. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Evans CH. Biochemistry of the lanthanides. New York: Plenum Press; 1990. [Google Scholar]
- Hu ZY, Richter H, Sparovek G, Schnug E. Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: a review. Journal of Plant Nutrition. 2004;27:183–220. [Google Scholar]
- Huang JWW, Grunes DL, Kochian LV. Voltage-dependent Ca2+ influx into right-side-out plasma-membrane vesicles isolated from wheat roots – characterization of a putative Ca2+ channel. Proceedings of the National Academy of Sciences of the United States of America; 1994. pp. 3473–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Simons JG, Hansen RW, Daniel RA. Chemical procedures for the analysis of plant material: multi-element, oils sugars and gum. Lucia, Brisbane: CSIRO Division of Tropical Agronomy; 1985. Tropical Agronomy Technical Memorandum No. 40. St. [Google Scholar]
- Karol PJ, Nakahara H, Petley BW, Vogt E. On the claims for discovery of elements 110, 111, 112, 114, 116, and 118 – (IUPAC Technical Report) Pure and Applied Chemistry. 2003;75:1601–1611. [Google Scholar]
- Leonard RT, Nagahashi G, Thomson WW. Effect of lanthanum on ion absorption in corn roots. Plant Physiology. 1975;55:542–546. doi: 10.1104/pp.55.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas EV, Moore DP, Mason BJ. Manganese absorption by excised barley roots. Plant Physiology. 1968;43:527–530. doi: 10.1104/pp.43.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition in higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Marshall J, Corzo A, Leigh RA, Sanders D. Membrane potential-dependent calcium-transport in right-side-out plasma-membrane vesicles from Zea mays L. roots. The Plant Journal. 1994;5:683–694. [Google Scholar]
- Motomizu S, Wakimoto T, Toei K. Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. Analyst. 1983;108:361–367. doi: 10.1016/0039-9140(84)80269-6. [DOI] [PubMed] [Google Scholar]
- Rayment G, Andrews CS. Biological and chemical comparisons of methods for removing copper from macronutrient solutions for growth investigations. Plant and Soil. 1972;36:547–559. [Google Scholar]
- Rengel Z. Role of calcium in aluminium toxicity. New Phytologist. 1992;121:499–513. doi: 10.1046/j.1469-8137.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- Reuter DJ, Robinson JB. Plant analysis: an interpretation manual. Sydney: Inkata Press; 1986. [Google Scholar]
- Smith FW, Imrie BC, Pieters WHJ. Foliar symptoms of nutrient disorders in mungbean (Vigna radiata). Collingwood, Victoria, Australia: CSIRO Publishing; 1983. Technical Paper No. 24. CSIRO Australia Division of Tropical Crops and Pastures. [Google Scholar]
- Taylor GJ. Current views of the aluminium stress response; the physiological basis of tolerance. Current Topics in Plant Biochemistry and Physiology. 1991;10:57–93. [Google Scholar]
- von Tucher S, Schmidhalter U. Lanthanum uptake from soil and nutrient solution and its effects on plant growth. Journal of Plant Nutrition and Soil Science-Zeitschrift fur Pflanzenernahrung und Bodenkunde. 2005;168:574–580. [Google Scholar]
- Tyler G. Rare earth elements in soil and plant systems – A review. Plant and Soil. 2004;267:191–206. [Google Scholar]
- Yang ZM, Woolley A. Carbonatites in China: a review. Journal of Asian Earth Sciences. 2006;27:559–575. [Google Scholar]
- Williams RF. The effects of phosphorus supply on the rate of intake of phosphorus and nitrogen and upon certain aspects of phosphorus metabolism in graminaceous plants. Australian Journal of Science Research. 1948;1:333–361. (Series B) [Google Scholar]
- von Wiren N, Marschner H, Romheld V. Uptake kinetics of iron-phytosiderophores in two maize genotypes differing in iron efficiency. Physiologia Plantarum. 1995;93:611–616. [Google Scholar]