Abstract
Background and Aims
Variation in mating patterns may be particularly evident in colonizing species because they commonly experience wide variation in plant density. Here, the role of density for the mating system of Ambrosia artemisiifolia (common ragweed), a wind-pollinated annual colonizing species previously reported as self-compatible, is explored.
Methods
The effect of population density on the proportion of self- and cross-fertilized seeds was examined using allozyme markers and experimental arrays conducted over two seasons in the field. Also the reproductive success of isolated plants located in diverse habitats was measured. The potential occurrence of a physiological mechanism preventing self-fertilization, i.e. self-incompatibility, following controlled self- and cross-pollinations in the glasshouse was examined.
Key Results
Outcrossing rates estimated using allozyme markers were uniformly high, regardless of the spacing between plants. However, when single plants were isolated from congeners they set few seeds. Observations of pollen-tube growth and seed set following controlled pollinations demonstrated that plants of A. artemisiifolia possess a strong self-incompatibility mechanism, contrary to earlier reports and assumptions.
Conclusions
The maintenance of high outcrossing rates in colonizing populations of A. artemisiifolia is likely to be facilitated by the prodigious production of wind-borne pollen, high seed production and extended seed dormancy.
Key words: Self-incompatibility, outcrossing rate, density dependence, colonization, wind-pollination, Ambrosia artemisiifolia (ragweed), Asteraceae
INTRODUCTION
The immobility of plants exerts an important influence on mating which is commonly ‘context dependent’, varying with local ecological and demographic conditions and the availability of compatible mating partners. Density-dependent and frequency-dependent influences on rates of self- and cross-fertilization have now been widely reported (e.g. Farris and Mitton, 1984; Kohn and Barrett, 1994; Cheptou et al., 2002; Kalisz et al., 2004; Eppley and Pannell, 2007). For species that grow in ephemeral habitats, and particularly those that are annual, demographic factors and mate availability can vary greatly among neighbourhoods and between years. This unpredictability can have important reproductive consequences with respect to mating and fertility and may explain the common occurrence of self-compatibility in many annual colonizing species (Baker, 1955; Mulligan and Findlay, 1970; Price and Jain, 1981; Pannell and Barrett, 1998; Cheptou, 2004; although for exceptions, see Abbott and Forbes, 1993; Sun and Ritland, 1998; Cheptou et al., 2002).
Demographic factors, such as the local density of plants, are well known to influence mating and fertility. Both animal- and wind-pollinated species often show a positive correlation between plant density and outcrossing rate (e.g. Vaquero et al., 1989; Murawski and Hamrick, 1991; Motten and Antonovics, 1992; van Treuren et al., 1993; but see Karron et al., 1995; Herlihy and Eckert, 2004; Brunet and Sweet, 2006). For animal-pollinated plants this relationship can arise because of density-dependent changes in pollinator behaviour (reviewed in Antonovics and Levin, 1980). In contrast, because wind-pollinated plants do not rely on visits by pollinators, demographic effects on outcrossing are a feature of the interaction with local microclimatic conditions and the plants themselves. For example, Eppley and Pannell (2007) recently demonstrated that in Mercurialis annua, a wind-pollinated androdioecious annual, selfing rates were context-dependent depending on the local density and relative frequencies of hermaphrodites and males. In general, context-dependent mating arises from either differences in the amount of pollen captured (pollen quantity) or the type of pollen captured (pollen quality).
Here, the effect of plant density on mating in Ambrosia artemisiifolia (common ragweed) was investigated. This wind-pollinated monoecious annual is common in eastern North America as a weed of arable crops but has been introduced to Europe, Asia and Australia where it has become invasive (Bassett and Crompton, 1975; Bass et al., 2000; Chauvel et al., 2006). There have been no studies of the mating system of A. artemisiifolia but most workers, probably because of its annual weedy habit, have assumed the species is self-compatible and capable of selfing (e.g. Genton et al., 2005). Indeed, Jones (1936) conducted genetic studies of sexual variation in A. artemisiifolia and reported all monoecious forms to be self-fertile. Similarly, Bassett and Crompton (1975) stated that A. artemisiifolia produces viable seed through both self- and cross-fertilization and McKone and Tonkyn (1986) and Lundholm and Aarssen (1994) both report that the species is self-fertile. The present studies were therefore motivated on the assumption that, like many annual colonizers, A. artemisiifolia was self-compatible.
The present study had three objectives concerning the reproductive biology of A. artemisiifolia. First, using allozyme markers, the influence of plant density on mating patterns in field experiments conducted over 2 years was investigated. Secondly, the reproductive success of isolated A. artemisiifolia plants in the field was examined to assess to what extent low mate availability might reduce seed fertility. Finally, because the results from these two experiments suggested that A. artemisiifolia does not have the capacity for significant self-fertilization, the compatibility status of plants was investigated using controlled self- and cross-pollinations in the glasshouse.
METHODS AND MATERIALS
Study system
Ambrosia artemisiifolia (Asteraceae) grows abundantly in disturbed habitats on a variety of soil types, including cultivated fields, roadsides, waste places and gardens. It is a successful pioneer in early successional ecosystems (Bazzaz, 1974). In Ontario, Canada, where the present studies were conducted, plants flower in August–September with female flowers produced in the axils of bracts or upper leaves and male flowers in terminal racemes (Payne, 1963). There is no vegetative propagation, so that all reproduction occurs through seed (Bassett and Crompton, 1975).
Density arrays
During summer 2005, 750 ragweed seedlings were transplanted from ten different sites at the Koffler Scientific Reserve (KSR) at Jokers Hill in Southern Ontario (44°03′N, 79°29′W). Seedlings that were uniform in size with between six and eight leaves were selected. The seedlings were then grown in a common environment in a glasshouse at the University of Toronto in 4-inch pots until developing buds were visible. In early August, just before flowers opened, the plants were transported back to KSR and they were planted in three freshly tilled fields each separated by approx. 500 m. Plants were randomly assigned to one of three density arrays (plants 30 cm, 90 cm, 150 cm apart). Each array was represented twice in each field for a total of six replicates per density array. In each density treatment, a total of 37 plants was used, with 18 focal plants with neighbours on all sides. Plants were positioned in hexagonal arrays so that all inter-plant distances were equal. In 2006 the experiments were repeated using the same general protocols but with two modifications. First, density arrays of 90 cm, 450 cm and 900 cm were used, and only one array per field. Secondly, plants were germinated from seed and the arrays set up 3 weeks earlier in the season so that flowering in the experiment did not overlap with flowering of local ragweed populations. Both of these modifications were undertaken in an effort to reduce the potential influence of background levels of pollen on outcrossing rates.
For the 2005 experiment, stigmas were collected to assess stigmatic pollen loads from one of the three density treatments in each field once a week for 4 weeks. At the end of the season, 20 seeds were collected from each maternal plant to assess mating patterns in each array. Seeds were collected at two time intervals 2 weeks apart, to span the range of maturation (and stigma exposure) times. Stigmatic pollen loads were analysed with repeated-measures, general linear models (Neter et al., 1996: mixed procedure of SAS, release 9·1, SAS Inst. Inc., 2002). The dependent variable was log-transformed to assure normally distributed residuals.
Allozyme markers and gel electrophoresis were used to determine the outcrossing rate of each density array (2005: 18 arrays, six of each density treatment; 2006: six arrays, two of each density treatment). For each density treatment, 20 seeds from each of the 18 focal plants were used to estimate outcrossing rates for that array. Variation at four isozyme loci was resolved using horizontal starch gel electrophoresis. Seeds from each maternal plant were separately ground in three drops of 0·1 M Tris–HCl extraction buffer (Soltis et al., 1983), and the extract absorbed onto 3-mm chromatography paper wicks and placed directly onto 11–12 % starch gels. Using a lithium-borate buffer system (pH 8·3), four variable loci were resolved from three enzyme systems: alcohol dehydrogenase (Adh), glutamate oxaloacetate transaminase (Got) and phosphoglucomutase (Pgm). Gels were stained for enzyme activity following recipes in Wendel and Weeden (1991). Four alleles were detected at an Adh locus, three alleles at Got-1, four alleles at Got-2 and three alleles at Pgm.
Genotypes were inferred based on segregation patterns characteristic of either dimeric or monomeric codominant enzymes. Single and multi-locus outcrossing rates (t and tm, respectively), and pollen (p) and ovule (u) allele frequencies were jointly estimated using the program MLTR (vers. 0·9; Ritland, 1986). This program uses maximum-likelihood to infer the genotypes of the maternal parents, allele frequencies in the pollen pool, and the proportion of progeny that are the result of outcrossing versus selfing. Standard errors of the estimates were obtained from the standard deviation of 1000 bootstrap values, using the seed family as the unit of re-sampling. Within each field, separate outcrossing rates were estimated for the density arrays (2005: six arrays per field; 2006: one array per field) using a common pollen allele frequency pool for each field.
To determine whether estimates of the multi-locus outcrossing rates were significantly different from 1·0, the distribution of 1000 bootstrap values were examined following methods outlined in Eckert and Barrett (1994). Using this method, parameter estimates for each array were considered to be significantly <1·0 if 100[1 – (αPC/2)] % of the bootstrap values were all <1·0 (where αPC represents the Type 1 error rate per contrast). Similarly, for each density array, whether the difference between the multi-locus and single-locus estimators of mating was significantly different from zero was tested. Overall differences in outcrossing rate between the density treatments were tested using ANOVA in SAS. The outcrossing rates for each density treatment were weighted by the inverse of the bootstrap variance estimates, to account for the sampling error in each estimate (see Barrett et al., 1994).
Isolation experiment
To determine the reproductive success of isolated plants in the field, solitary individuals were planted at KSR during the summer of 2006. The seed for this experiment was collected in October 2005 from open-pollinated plants within the vicinity of KSR. Following stratification and germination procedures suggested in Willemsen (1975), seeds were grown from 40 maternal families in June 2005 in a glasshouse at the University of Toronto. Before buds were visible, 30 plants were transplanted into isolated habitats at KSR. The individuals were planted in old-field habitat, in a small patch (approx. 0·5 × 0·5 m), which was cleared of surrounding vegetation. Plants were watered upon transplanting, again 3 d later, and 1 week later to ensure successful establishment. The 30 sites were at least 300 m from known A. artemisiifolia populations, and in almost all cases were separated by forest and other woody vegetation. Plants were visited throughout flowering to ensure both female and male flowers were present. At the end of the flowering season all seed was harvested from each plant to estimate seed production.
Self-incompatibility experiment
Self-incompatibility in A. artemisiifolia was tested by comparing self- and cross-pollinated flowers for both pollen-tube growth and seed set. During autumn 2006, an additional 50 plants were grown using the same batch of seeds and germination conditions as described above. Plants were grown in a glasshouse using an artificial light regime to mimic the natural conditions during flowering in Southern Ontario (14 h light/10 h dark). Just before flowering, plants were randomly assigned to one of three treatments: cross-pollen donors (30 plants), cross-pollen recipients (10 plants) and self-pollen recipients (10 plants). For each plant, five flowers were brushed with either self-pollen or a mixture of pollen from five donor plants. It was necessary to use separate plants for the self- and cross-pollen treatments, to prevent the possibility of cross contamination between pollination treatments. Pollen donors were maintained in one glasshouse, cross-pollen recipients in a separate house, and each self-pollen recipient plant was isolated in its own glasshouse to prevent cross contamination. Cross-pollen recipients were emasculated continuously throughout flowering to avoid any self-pollen deposition. For the self-pollen recipients, covers created out of three layers of spun-fibre material were placed over female flowers to control the time of self-pollination. Twenty-four hours after pollination, styles were fixed in alcohol and examined for pollen tube growth. The aniline blue staining method and fluorescence microscopy were used to examine and count pollen germination and pollen-tube penetration. The remaining flowers were allowed to develop seed. Seed set was compared between plants in each treatment and the resulting data analysed with generalized linear models (Genmod procedure of SAS, release 9·1; SAS Inst. Inc., 2002; Allison, 1999) with logit transformations to accommodate the binomial distribution of data.
RESULTS
Density arrays
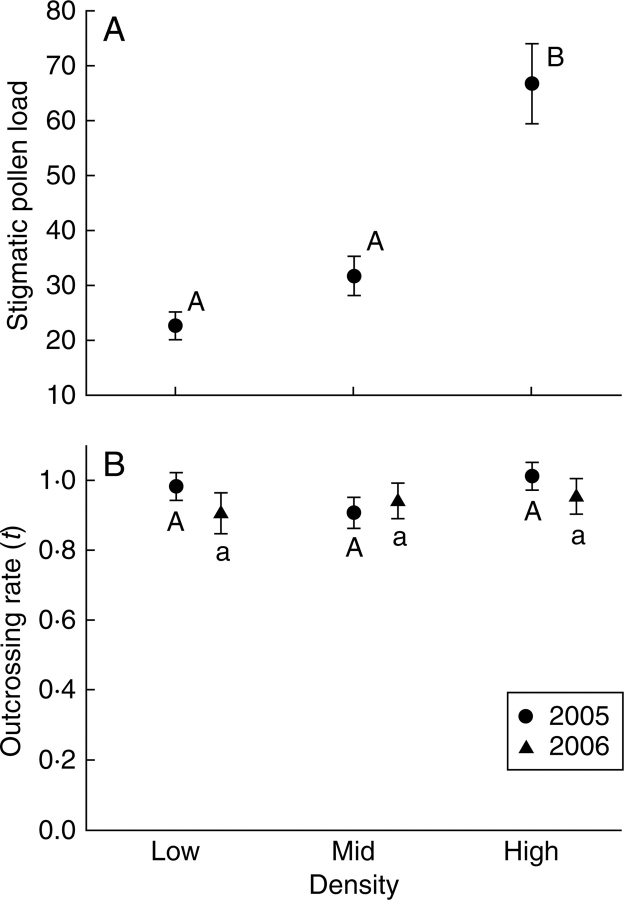
In 2005 pollen receipt varied significantly between plants in the three density arrays (F2,62·2 = 22·60, P < 0·0001; Fig. 1A). Stigmas on plants in low and mid-density arrays captured equivalent amounts of pollen (t54·3 = 2·03, P > 0·05). In contrast, stigmas on plants in high density arrays captured significantly more pollen than those in either low or mid-densities (high vs. low t63·1 = 6·54, P < 0·001; high vs. mid t71·2 = 4·52, P < 0·001; Fig 1A). There was no significant difference between the same density arrays across the three different fields (F6,60·9 = 1·17, P > 0·3).
Fig. 1.
Influences of plant density (low, mid, high) on (A) mean (± s.e.) number of pollen grains on stigmas and (B) multi-locus outcrossing rate (t ± s.e.) for 2005 and 2006 in experimental field arrays of Ambrosia artemisiifolia. Letters indicate the outcomes of Dunn–Šidák multiple comparisons. See text for statistical details.
Multi-locus outcrossing rates for all arrays were close to 1·0 in both 2005 and 2006 (Fig. 1B). Based on the distribution of bootstrap values, one of the 26 arrays had an outcrossing rate that differed significantly from 1·0 (2005, one mid density array). For all arrays, the differences between the multi-locus and single-locus estimators of outcrossing rate were not significantly different from zero.
There were no significant differences among the outcrossing rates of the three density arrays in either year (2005: F2,10 = 3·29, P > 0·05; 2006: F2,7 = 0·44, P > 0·6; Fig. 1B). In 2005 there was no significant difference in outcrossing rate between the same density arrays among the three fields (F3,10 = 1·28, P > 0·3).
Seed production of isolated plants
Plants in isolated locations at KSR set between 0 and 254 seeds (mean 58·04 ± s.e. 15·11), well below the maximum potential seed set since the present observations indicated that the plants produced hundreds of female flowers.
Testing for self-incompatibility
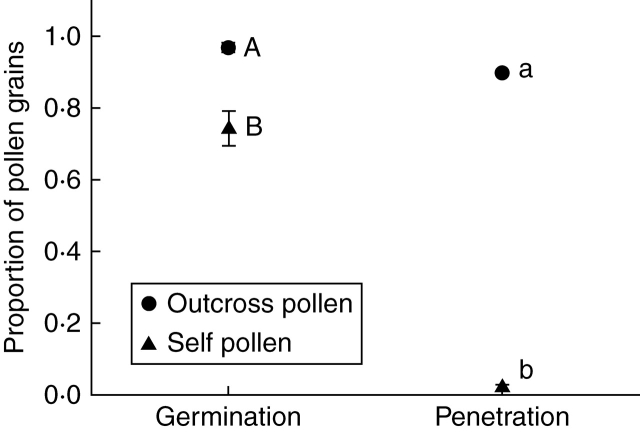
A significant difference in successful pollen tube penetration of the stigmatic tissue was found between plants that received cross- versus self-pollen (F1,11·1 = 1721·53, P < 0·0001; Fig. 2). The two classes of unsuccessful penetration (no germination, and germination but no penetration) also yielded significant differences between the two treatments (no germination: self vs. cross F1,10·4 = 23·00, P < 0·001; germination: self vs. cross F1,9·82 = 157·73, P < 0·0001; Fig. 2). Qualitative differences in the growth of pollen tubes between cross- versus self-pollen can be clearly seen in Fig. 3, where cross-pollen tubes grow straight and long and penetrate the stigma (Fig. 3A), whereas self-pollen tubes are unable to penetrate the stigma and there is formation of callose (Fig. 3B) typical of a self-incompatibility reaction (de Nettancourt, 1977).
Fig. 2.
Relationship of proportion of pollen grains (± s.e.) that fall into each subclass (germination, penetration of the style) for Ambrosia artemisiifolia plants that received outcross pollen and self pollen. Letters indicate the outcomes of Dunn–Šidák multiple comparisons within each subclass between the two treatments, with capital letters for germination, and lower-case letters for penetration. See text for statistical details.
Fig. 3.
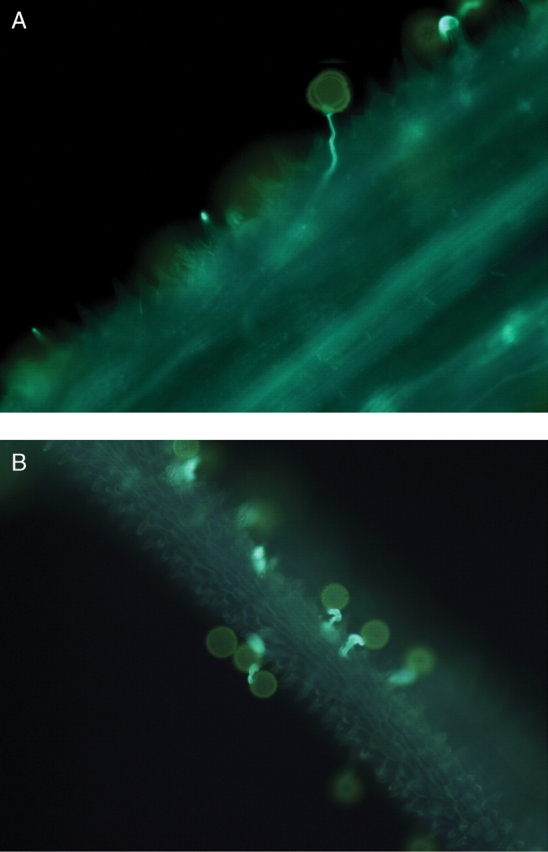
Pollen grains and tubes of Ambrosia artemisiifolia under a fluorescence microscope showing pollen tube growth for (A) cross-pollen and (B) self-pollen at ×200 magnification. Self-pollen tubes do not penetrate the style due to the formation of callose, while cross-pollen tubes penetrate the style, growing long and straight towards the ovule.
There was a significant difference in the proportion of seed set between plants that received cross- versus self-pollen (χ2 = 79·55, P < 0·0001). Plants that received cross-pollen set 0·56 ± 0·03 (mean ± s.e.) proportion seed; whereas plants that received self-pollen set 0·04 ± 0·02 (mean ± s.e.).
DISCUSSION
Our results clearly demonstrate that Ambrosia artemisiifolia is a highly outcrossing, self-incompatible plant. Provided that population densities are sufficient, seed set is generally high. Isolated plants in the present study experienced reduced seed set, although they still set some seed. Our glasshouse experiment demonstrated that plants exposed to only self-pollen set very few seeds relative to those that were cross-pollinated. Furthermore, fluorescence microscopy provided evidence that A. artemisiifolia is self-incompatible based on observations of pollen germination and pollen tube growth. These findings were unexpected and contrary to previous reports that the species is self-compatible (e.g. Jones, 1936; Bassett and Crompton, 1975).
A significant effect of plant density on stigmatic pollen loads was detected with plants in high-density patches capturing more pollen grains. However, because flowers of A. artemisiifolia are uni-ovulate, plants at lower densities still captured sufficient pollen for high seed set. The measures of stigmatic pollen loads did not differentiate between self- and cross-pollen, so it is likely that a substantial portion of the pollen captured was self-pollen. In contrast to most wind-pollinated plants where pollen is held in the anthers until it is removed by the wind, studies on the release of pollen in A. artemisiifolia indicate that as anthers swell and dehisce, pollen first falls passively downwards to vegetation and is then swept away by the wind (Bianchi et al., 1959). This process causes deposition of large quantities of self-pollen on stigmas within the same plant (geitonogamy). Opportunities for geitonogamous pollination are exacerbated by the inflorescence architecture of A. artemisiifolia. Male flowers are typically positioned above female flowers and despite some protandry there is considerable overlap between the sex functions. Self-incompatibility in A. artemisiifolia may therefore function largely to limit the deleterious consequences of inbreeding that arise from high levels of geitonogamous pollination.
Controlled pollinations in the glasshouse demonstrated that A. artemisiifolia has a self-incompatibility system. Seven of the ten plants set no seed following self-pollination, with three plants setting small amounts of seed. There may be some leakiness in the SI system, as is commonly observed in self-incompatible species (de Nettancourt, 1977; Levin, 1996; Stephenson et al., 2000) and has been reported in other species of Asteraceae (Ferrer and Good-Avila, 2007). It is possible that previous reports of self-compatibility in A. artemisiifolia (Jones, 1936; Bassett and Crompton, 1975) involved plants with weak self-incompatibility. However, neither of these studies was designed to specifically test self-incompatibility, and so it is not possible to evaluate the rigour of their experimental designs or the validity of the results. Surveys of Asteraceae indicate that many species are partially self-incompatible (Ferrer and Good-Avila, 2007), and, in some cases, this may have resulted from selection for reproductive assurance in colonizing populations or other demographic factors (Hiscock, 2000, Cheptou et al., 2001, 2002; but see Brennan et al., 2005). Further studies of the incompatibility status of A. artemisiifolia populations would certainly be warranted, especially in the invasive range (see Genton et al., 2005) where demographic factors associated with repeated colonizing events may possibly favour the breakdown of self-incompatibility.
The relative costs and benefits of self-compatibility versus self-incompatibility in colonizing species depend on a variety of demographic and life-history conditions including plant density, propagule number, life-span, seed dormancy and the capacity for clonal reproduction. Pannell and Barrett (1998) investigated the effects of different life-history traits on the reproductive success of self-compatible and self-incompatible phenotypes in a metapopulation context. Their results suggest that the advantage of self-compatible phenotypes through reproductive assurance is diminished if plants can survive unfavourable conditions in a dormant seed bank. Germination studies in A. artemisiifolia indicate that seeds can remain viable for 39 years or more when buried in the soil (Toole and Brown, 1946). Pannell and Barrett (1998) also found that enhanced seed productivity can offset the disadvantage possessed by self-incompatible plants. According to Dickerson and Sweet (1971), a small A. artemisiifolia plant produces about 3000 seeds, while large plants can produce up to 62 000 seeds. Thus, although A. artemisiifolia is an annual species with no vegetative propagation, its prolific seed production, seed dormancy and high population densities may offset the cost of being self-incompatible allowing successful colonization.
Ambrosia artemisiifolia is an aggressive weed in North America, and invasive in Europe. Records from Europe suggest an early origin in France during the 18th century, and substantial spread since introduction as a seed contaminant in crops from North America (Chauvel et al., 2006). Both herbarium records (Chauvel et al., 2006) and a recent molecular study suggest multiple independent introductions (Genton et al., 2005) with no loss of genetic diversity in introduced populations compared with native North American populations. This result would be unexpected in a selfing colonist because uniparental reproduction commonly leads to severe genetic bottlenecks during range expansion in invading species (reviewed in Novak and Mack, 2005; Barrett et al., 2008). However, their finding of similar levels of genetic diversity in the introduced and native range of A. artemisiifolia is consistent with what one might expect for a highly outcrossing, self-incompatible colonist that has spread through multiple introductions. Extensive pollen dispersal through wind pollination is more likely to foster outcrossing among separate introductions and the maintenance of genetic diversity in the introduced range.
Self-incompatible plants may suffer reduced reproductive success when population density or size is low (‘Allee effect’; Allee, 1951). Animal-pollinated species may be more prone to Allee effects through insufficient pollen transfer or through the transfer of different species' pollen, particularly when rare plants are surrounded by other flowering species (Kunin, 1993, 1997) or patches are quite isolated (Aizen and Feinsinger, 1994). Because A. artemisiifolia is wind-pollinated and plants produce copious pollen, the effects of being relatively isolated may to some extent be diminished. Long-distance transport of pollen in A. artemisiifolia could alleviate some of the costs associated with growing sparsely. There are records of A. artemisiifolia pollen appearing in air samples hundreds of kilometres from the nearest population indicating long-distance transport of ragweed pollen (e.g. Lorenzo et al., 2006; Stach et al., 2007), although the duration of pollen viability is unknown. Low levels of seed set were recorded in isolated plants of A. artemisiifolia positioned among forest patches. However, whether this seed resulted from self-pollination in plants with leaky SI or from long-distance pollen transport is not known.
Pollen limitation has been well documented and discussed in animal-pollinated plants (for reviews, see Burd, 1994; Larson and Barrett, 2000; Ashman et al., 2004; Knight et al., 2005), but there is much less evidence on whether pollen availability limits reproduction in wind-pollinated plants. The dispersal of wind-borne pollen from point sources has a leptokurtic distribution, although the direction, speed and turbulence of wind and settling velocity of pollen also affects patterns of pollen deposition (Bateman, 1947; Gleaves, 1973; Dowding, 1987; Okubo and Levin, 1989; Giddings, 2000). Recent empirical work suggests that pollen capture and the proportion of fertilized ovules may decrease rapidly with increasing distance from pollen donors in wind-pollinated plants (Knapp et al., 2001; Davis et al., 2004; Stehlik and Barrett, 2006; Eppley and Pannell, 2007). The present density experiments indicated that pollen capture decreased with increasing distance, but this did not have an effect on outcrossing rates. Also, isolated plants set few seeds, suggesting that they may have suffered from pollen limitation. However, various features of pollen dispersal in A. artemisiifolia demonstrating long-distance transport (Raynor et al., 1968, 1970, 1973) suggest that pollen-limited reproduction may be relatively uncommon in A. artemisiifolia.
The present study illustrates the importance of understanding both demographic and genetic influences on mating patterns in plant populations. Because A. artemisiifolia is a weedy, colonizing species, the demographic conditions in which it occurs should have important influences on mating and fertility. Although the present study suggests that A. artemisiifolia may be obligately outcrossing due to self-incompatibility, various features of its ecology and reproductive biology may ameliorate the costs that are normally associated with self-incompatibility in colonizing annuals. Traits that could diminish costs associated with outcrossing include producing enormous quantities of wind-borne pollen, prolific seed production and the presence of a seed bank.
ACKNOWLEDGEMENTS
We thank Tammy Sage for advice in visualizing pollen tubes, Lul Hassan for help with electrophoresis, Bill Cole and Sherosha Raj for field assistance, and Rob Colautti and Mario Vallejo-Marín for comments on the manuscript. This research was supported by the Natural Sciences and Engineering Research Council of Canada, through a Canada Graduate Scholarship (J.F.), and funding from a Discovery Grant and the Canada Research Chair's Programme (S.C.H.B.).
LITERATURE CITED
- Abbott RJ, Forbes DG. Outcrossing rate and self-incompatibility in the colonizing species Senecio squalidus. Heredity. 1993;71:155–159. [Google Scholar]
- Aizen MA, Feinsinger P. Forest fragmentation, pollination, and plant reproduction in a chaco dry forest, Argentina. Ecology. 1994;75:330–351. [Google Scholar]
- Allee WC. The social life of animals. Boston, MA: Beacon Press; 1951. [Google Scholar]
- Allison PD. Logistic regression using the SAS system – theory and application. Cary, NC: SAS Institute, Inc; 1999. [Google Scholar]
- Antonovics J, Levin DA. The ecological and genetic consequences of density-dependent regulation in plants. Annual Review of Ecology and Systematics. 1980;11:411–452. [Google Scholar]
- Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DR, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Baker HG. Self compatibility and establishment after long distance dispersal. Evolution. 1955;9:347–349. [Google Scholar]
- Barrett SCH, Harder LD, Cole WW. Effects of flower number and position on self-fertilization in experimental populations of Eichhornia paniculata (Pontederiaceae) Functional Ecology. 1994;8:526–535. [Google Scholar]
- Barrett SCH, Colautti RI, Eckert CG. Plant reproductive systems and evolution during biological invasion. Molecular Ecology. 2008;17:373–383. doi: 10.1111/j.1365-294X.2007.03503.x. [DOI] [PubMed] [Google Scholar]
- Bass DJ, Delpech V, Beard J, Bass P, Walls RS. Late summer and fall (March–May) pollen allergy and respiratory disease in Northern New South Wales, Australia. Annals of Allergy Asthma & Immunology. 2000;85:374–381. doi: 10.1016/S1081-1206(10)62549-5. [DOI] [PubMed] [Google Scholar]
- Bassett IJ, Crompton CW. Biology of Canadian weeds. II. Ambrosia artemisiifolia L. and A. psilostachya D.C. Canadian Journal of Plant Science. 1975;55:463–476. [Google Scholar]
- Bateman AJ. Contamination is seed crops. III. Relation with isolation distance. Heredity. 1947;1:303–336. [Google Scholar]
- Bazzaz FA. Ecophysiology of Ambrosia artemisiifolia – a successional dominant. Ecology. 1974;55:112–119. [Google Scholar]
- Bianchi DE, Schwemmin DJ, Wagner WHJ. Pollen release in the common ragweed (Ambrosia artemisiifolia) Botanical Gazette. 1959;120:235–243. [Google Scholar]
- Brennan AC, Harris SA, Hiscock SJ. Modes and rates of selfing and associated inbreeding depression in the self-incompatible plant Senecio squalidus (Asteraceae): a successful colonizing species in the British Isles. New Phytologist. 2005;168:475–486. doi: 10.1111/j.1469-8137.2005.01517.x. [DOI] [PubMed] [Google Scholar]
- Brunet J, Sweet HR. Impact of insect pollinator group and floral display size on outcrossing rate. Evolution. 2006;60:234–246. [PubMed] [Google Scholar]
- Burd M. Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. The Botanical Review. 1994;60:83–139. [Google Scholar]
- Chauvel B, Dessaint F, Cardinal-Legrand C, Bretagnolle F. The historical spread of Ambrosia artemisiifolia L. in France from herbarium records. Journal of Biogeography. 2006;33:665–673. [Google Scholar]
- Cheptou PO. Allee effect and self-fertilization in hermaphrodites: reproductive assurance in demographically stable populations. Evolution. 2004;58:2613–2621. doi: 10.1111/j.0014-3820.2004.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Cheptou PO, Lepart J, Escarre J. Inbreeding depression under intraspecific competition in a highly outcrossing population of Crepis sancta (Asteraceae): evidence for frequency-dependent variation. American Journal of Botany. 2001;88:1424–1429. [PubMed] [Google Scholar]
- Cheptou PO, Lepart J, Escarre J. Mating system variation along a successional gradient in the allogamous and colonizing plant Crepis sancta (Asteraceae) Journal of Evolutionary Biology. 2002;15:753–762. [Google Scholar]
- Davis HG, Taylor CM, Civille JC, Strong DR. An allee effect at the front of a plant invasion: Spartina in a Pacific estuary. Journal of Ecology. 2004;92:321–327. [Google Scholar]
- Dickerson CT, Sweet RD. Common ragweed ecotypes. Weed Science. 1971;19:64–66. [Google Scholar]
- Dowding P. Wind pollination mechanisms and aerobiology. International Review of Cytology. 1987;107:421–437. [Google Scholar]
- Eckert CG, Barrett SCH. Post-pollination mechanisms and the maintenance of outcrossing in self-compatible Decodon verticillatus (Lythraceae) Heredity. 1994;72:396–411. [Google Scholar]
- Eppley SM, Pannell JR. Density-dependent self-fertilization and male versus hermaphrodite siring success in an androdioecious plant. Evolution. 2007;61:2349–2359. doi: 10.1111/j.1558-5646.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- Farris MA, Mitton JB. Population density, outcrossing rate, and heterozygote superiority in ponderosa pine. Evolution. 1984;38:1151–1154. doi: 10.1111/j.1558-5646.1984.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Ferrer MM, Good-Avila SV. Macrophylogenetic analyses of the gain and loss of self-incompatibility in the Asteraceae. New Phytologist. 2007;173:401–414. doi: 10.1111/j.1469-8137.2006.01905.x. [DOI] [PubMed] [Google Scholar]
- Genton BJ, Shykoff JA, Giraud T. High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Molecular Ecology. 2005;14:4275–4285. doi: 10.1111/j.1365-294X.2005.02750.x. [DOI] [PubMed] [Google Scholar]
- Giddings G. Modelling the spread of pollen from Lolium perenne: the implications for the release of wind-pollinated transgenics. Theoretical Applied Genetics. 2000;100:971–974. [Google Scholar]
- Gleaves JT. Gene flow mediated by wind-borne pollen. Heredity. 1973;31:355–366. [Google Scholar]
- Herlihy CR, Eckert CG. Experimental dissection of inbreeding and its adaptive significance in a flowering plant, Aquilegia canadensis (Ranunculaceae) Evolution. 2004;58:2693–2703. doi: 10.1111/j.0014-3820.2004.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ. Genetic control of self-incompatibility in Senecio squalidus L. (Asteraceae): a successful colonizing species. Heredity. 2000;85:10–19. doi: 10.1046/j.1365-2540.2000.00692.x. [DOI] [PubMed] [Google Scholar]
- Jones KL. Studies on Ambrosia. I. The inheritance of floral types in the ragweed, Ambrosia elatior L. American Midland Naturalist. 1936;17:673–699. [Google Scholar]
- Kalisz S, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Karron JD, Thumser NN, Tucker R, Hessenauer AJ. The influence of population-density on outcrossing rates in Mimulus ringens. Heredity. 1995;75:175–180. [Google Scholar]
- Knapp EE, Goedde MA, Rice KJ. Pollen-limited reproduction in blue-oak: implications for wind pollination in fragmented populations. Oecologia. 2001;128:48–55. doi: 10.1007/s004420000623. [DOI] [PubMed] [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, et al. Pollen limitation of plant reproduction: pattern and process. Annual Review of Ecology and Systematics. 2005;36:467–497. [Google Scholar]
- Kohn JR, Barrett SCH. Pollen discounting and the spread of a selfing variant in tristylous Eichhornia paniculata: evidence from experimental populations. Evolution. 1994;48:1576–1594. doi: 10.1111/j.1558-5646.1994.tb02197.x. [DOI] [PubMed] [Google Scholar]
- Kunin WE. Sex and the single mustard: population density and pollinator behavior effects on seed-set. Ecology. 1993;74:2145–2160. [Google Scholar]
- Kunin WE. Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. Journal of Ecology. 1997;85:225–234. [Google Scholar]
- Larson BMH, Barret SCH. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society. 2000;69:503–520. [Google Scholar]
- Levin DA. The evolutionary significance of pseudo-self-fertility. American Naturalist. 1996;148:321–332. [Google Scholar]
- Lorenzo C, Marco M, Paola DM, Alfonso C, Marzia O, Simone O. Long distance transport of ragweed pollen as a potential cause of allergy in central Italy. Annals of Allergy Asthma & Immunology. 2006;96:86–91. doi: 10.1016/s1081-1206(10)61045-9. [DOI] [PubMed] [Google Scholar]
- Lundholm JT, Aarssen LW. Neighbor effects on gender variation in Ambrosia artemisiifolia. Canadian Journal of Botany. 1994;72:794–800. [Google Scholar]
- McKone MJ, Tonkyn DW. Intrapopulation gender variation in common ragweed (Asteraceae, Ambrosia artemisiifolia L.), a monoecious, annual herb. Oecologia. 1986;70:63–67. doi: 10.1007/BF00377111. [DOI] [PubMed] [Google Scholar]
- Motten AF, Antonovics J. Determinants of outcrossing rate in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae) American Journal of Botany. 1992;79:419–427. [PubMed] [Google Scholar]
- Mulligan GA, Findlay JN. Reproductive systems and colonization in Canadian weeds. Canadian Journal of Botany. 1970;48:859–860. [Google Scholar]
- Murawski DA, Hamrick JL. The effect of the density of flowering individuals on the mating systems of 9 tropical tree species. Heredity. 1991;67:167–174. [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4th edn. Chicago, IL: Irwin; 1996. [Google Scholar]
- de Nettancourt D. Incompatibility in angiosperms. Berlin: Springer-Verlag; 1977. [Google Scholar]
- Novak SJ, Mack RN. Genetic bottlenecks in alien plant species: influence of mating systems and introduction dynamics. In: Sax DF, Stachowicz JJ, Gaines SD, editors. Species invasions: insights into ecology, evolution and biogeography. Sunderland, MA: Sinauer Associates; 2005. pp. 201–228. [Google Scholar]
- Okubo A, Levin SA. A theoretical framework for data analysis of wind dispersal of seeds and pollen. Ecology. 1989;70:329–338. [Google Scholar]
- Pannell JR, Barrett SCH. Baker's law revisited: reproductive assurance in a metapopulation. Evolution. 1998;52:657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Payne WW. The morphology of the inflorescence of ragweeds (Ambrosia franseria: Compositae) American Journal of Botany. 1963;50:872–880. [Google Scholar]
- Price SC, Jain SK. Are inbreeders better colonizers? Oecologia. 1981;49:283–286. doi: 10.1007/BF00349202. [DOI] [PubMed] [Google Scholar]
- Raynor GS, Ogden EC, Hayes JV. Effect of a local source on ragweed pollen concentrations from background sources. Journal of Allergy. 1968;41:217–225. doi: 10.1016/0021-8707(68)90044-0. [DOI] [PubMed] [Google Scholar]
- Raynor GS, Ogden EC, Hayes JV. Dispersion and deposition of ragweed pollen from experimental sources. Journal of Applied Meteorology. 1970;9:885–895. [Google Scholar]
- Raynor GS, Ogden EC, Hayes JV. Variation in ragweed pollen concentration to a height of 108 meters. Journal of Allergy and Clinical Immunology. 1973;51:199–207. doi: 10.1016/0091-6749(73)90139-5. [DOI] [PubMed] [Google Scholar]
- Ritland K. Joint maximum likelihood estimation of genetic and mating structure using open-pollinated progenies. Biometrics. 1986;42:25–43. [Google Scholar]
- Soltis DE, Haufler CH, Darrow DC, Gastony GJ. Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffers, and staining schedules. American Fern Journal. 1983;73:9–27. [Google Scholar]
- Stach A, Smith M, Skjoth CA, Brandt J. Examining Ambrosia pollen episodes at Poznan (Poland) using back-trajectory analysis. International Journal of Biometeorology. 2007;51:275–286. doi: 10.1007/s00484-006-0068-1. [DOI] [PubMed] [Google Scholar]
- Stehlik I, Barrett SCH. Pollination intensity influences sex ratios in dioecious Rumex nivalis, a wind-pollinated plant. Evolution. 2006;60:1207–1214. [PubMed] [Google Scholar]
- Stephenson AG, Good SV, Vogler DW. Interrelationships among inbreeding depression, plasticity in the self-incompatibility system, and the breeding system of Campanula rapunculoides L. (Campanulaceae) Annals of Botany. 2000;85(Suppl. A):211–219. [Google Scholar]
- Sun M, Ritland K. Mating system of yellow starthistle (Centaurea solstitialis), a successful colonizer in North America. Heredity. 1998;80:225–232. [Google Scholar]
- Toole EH, Brown E. Final results of the Durvel buried seed experiment. Journal of Agricultural Research. 1946;72:201–210. [Google Scholar]
- van Treuren R, Bijlsma R, Ouborg NJ, Vandelden W. The effects of population size and plant-density on outcrossing rates in locally endangered Salvia pratensis. Evolution. 1993;47:1094–1104. doi: 10.1111/j.1558-5646.1993.tb02138.x. [DOI] [PubMed] [Google Scholar]
- Vaquero F, Vences FJ, Garcia P, Ramirez L, Delavega MP. Mating system in rye: variability in relation to the population and plant density. Heredity. 1989;62:17–26. doi: 10.1038/hdy.1989.3. [DOI] [PubMed] [Google Scholar]
- Wendel JF, Weeden NF. Visualization and interpretation of plant isozymes. In: Soltis DE, Soltis PS, editors. Isozymes in plant biology. Portland, OR: Dioscorides Press; 1991. pp. 5–45. [Google Scholar]
- Willemsen RW. Effect of stratification temperature and germination temperature on germination and induction of secondary dormancy in common ragweed seeds. American Journal of Botany. 1975;62:1–5. doi: 10.1002/j.1537-2197.1975.tb12333.x. [DOI] [PubMed] [Google Scholar]