Abstract
Background and Aims
There has been little previous work on the toughness of the laminae of monocots in tropical lowland rain forest (TLRF) despite the potential importance of greater toughness in inhibiting herbivory by invertebrates. Of 15 monocot families with >100 species in TLRF, eight have notably high densities of fibres in the lamina so that high values for toughness are expected.
Methods
In north-eastern Australia punch strength was determined with a penetrometer for both immature leaves (approx. 30 % final area on average) and fully expanded, fully toughened leaves. In Singapore and Panama, fracture toughness was determined with an automated scissors apparatus using fully toughened leaves only.
Key Results
In Australia punch strength was, on average, 7× greater in shade-tolerant monocots than in neighbouring dicots at the immature stage, and 3× greater at the mature stage. In Singapore, shade-tolerant monocots had, on average, 1·3× higher values for fracture toughness than neighbouring dicots. In Panama, both shade-tolerant and gap-demanding monocots were tested; they did not differ in fracture toughness. The monocots had markedly higher values than the dicots whether shade-tolerant or gap-demanding species were considered.
Conclusions
It is predicted that monocots will be found to experience lower rates of herbivory by invertebrates than dicots. The tough monocot leaves include both stiff leaves containing relatively little water at saturation (e.g. palms), and leaves which lack stiffness, are rich in water at saturation and roll readily during dry weather or even in bright sun around midday (e.g. gingers, heliconias and marants). Monocot leaves also show that it is possible for leaves to be notably tough throughout the expansion phase of development, something never recorded for dicots. The need to broaden the botanist's mental picture of a ‘tough leaf’ is emphasized.
Key words: Dicots, fracture toughness, herbivory, leaves, monocots, punch strength, tropical rain forest
INTRODUCTION
There is a long history of work on the ‘toughness’ of leaves as a protection against herbivory that stretches back to Williams (1954), and has been summarized by Wright and Vincent (1996), Lucas et al. (2000), Sanson et al. (2001), Read and Stokes (2006) and Sanson (2006). There have been two major approaches to measuring the ‘toughness’ of leaves (Lucas et al., 2000; Read and Sanson, 2003). A ‘penetrometer’ measures punch strength, the force needed to pass a rod of given diameter through the lamina; this property involves not only the properties of the materials in the leaf but also their arrangement and amount. An apparatus based on scissors or a guillotine measures work to shear, from which it is possible to derive specific work to shear (fracture toughness) which is work to shear per unit thickness. Choong et al. (1992) found a significant positive correlation between punch strength and fracture toughness (r2 = 0·420; P < 0·01) and not surprisingly a stronger one when punch strength was divided by lamina thickness (r2 = 0·555; P < 0·001). The chief determinant of fracture toughness in leaves is generally the extent of development of fibres around the vascular bundles (Lucas et al., 1995, 2000), though other tissues may be important in a minority of species (Read et al., 2000). There is still uncertainty as to whether punch strength or fracture toughness has the closer correlation, in general, with inhibition of herbivory by invertebrates (Lucas et al., 2000; Read and Stokes, 2006; Sanson, 2006). In this paper, following Read and Stokes (2006), we use the unqualified term ‘toughness’ to include measurements of both punch strength and fracture toughness.
There have been extensive studies of both punch strength and fracture toughness for dicots (eudicots and magnoliids) in tropical lowland rain forest (TLRF), but not for monocots. All of the earlier measurements were of punch strength, and made in the context of understanding the inhibition of herbivory, particularly by invertebrates (Coley and Barone, 1996; Coley and Kursar, 1996). More recently there has been a number of studies on the fracture toughness of dicots (Lucas et al., 1991; Choong et al., 1992; Turner et al., 1993, 2000; Yamashita, 1996; Dominy et al., 2003; Iddles et al., 2003; Read and Sanson, 2003; Read and Stokes, 2006; Read et al., 2006; Eichhorn et al., 2007). In contrast, we have traced no study of fracture toughness in monocots of TLRF, and only two studies of punch strength; both concern palms. Bernays (1991) found that the mean punch strength for eight unnamed palms was 1·6× the mean for 89 unnamed species of woody dicot species. Braker and Chazdon (1993) recorded mean punch strength for three understorey palms in Costa Rica. The lack of information on toughness of tropical monocots contrasts with the results of critical studies of temperate grasses obtained by Wright and Illius (1995) and Henry et al. (1996, 2000).
As pointed out by Edwards et al. (2000), botanists often make a subjective estimate of ‘toughness’ or ‘sclerophylly’ by testing stiffness (resistance to bending). By this approach most palm leaves seem ‘tough’ because they are stiff; also they do not change shape readily when they begin to dry. However, other monocots in TLRF have relatively closely set veins and a marked development of fibres along the vascular bundles, but lack stiffness and indeed may roll readily under drying conditions, e.g. aroids, gingers, heliconias and marants. Of the 15 families of monocots with >100 species in TLRF, eight have notably high densities of fibres in the laminae of most species (Table 1). Clearly there is a strong likelihood that the leaves of all these plants will have relatively high values for fracture toughness and punch strength. In the context of herbivory by invertebrates, particular interest attaches to the toughness of immature leaves. For dicots the losses to invertebrates are generally greater during the expansion phase than the mature phase (Coley and Barone, 1996; Coley and Kursar, 1996).
Table 1.
Families of monocots with 100 or more species in TLRF arranged firstly in decreasing order of degree of development of fibres in the lamina, and then alphabetically
| Family (order) | Extent of development of fibres* | Reference |
|---|---|---|
| Arecaceae (Arecales) | +++ | Tomlinson (1961); Knapp-Zinn (1973) |
| Bromeliaceae (Poales) | +++ | Tomlinson (1969) |
| Cyclanthaceae: Carludovicioideae (Pandanales) | +++ | Tomlinson and Wilder (1984); Wilder (1985a, b) |
| Pandanaceae (Pandanales) | +++ | North and Willis (1970, 1971); Tomlinson and Wilder (1984) |
| Cyclanthaceae: Cyclanthoideae (Pandanales) | ++ | Tomlinson and Wilder (1984); Roth (1990) |
| Cyperaceae (Poales) | ++ | Metcalfe (1971) |
| Marantaceae (Zingiberales) | ++ | Tomlinson (1969) |
| Poaceae (Poales) | ++ | Metcalfe (1960) |
| Zingiberaceae (Zingiberales) | ++ | Tomlinson (1969) |
| Araceae (Alismatales) | + | Solereder and Meyer (1928–33); Roth (1990) |
| Costaceae (Zingiberales) | + | Tomlinson (1969) |
| Heliconiaceae (Zingiberales) | + | Tomlinson (1969) |
| Orchidaceae (Asparagales) | + | Solereder and Meyer (1928–33) |
| Commelinaceae (Commelinales) | – | Tomlinson (1969) |
| Dioscoreaceae (Dioscoreales) | – | Ayensu (1972) |
| Smilacaceae (Liliales) | – | Solereder and Meyer (1928–33) |
* Key to degrees of development of fibrous tissue: ++ + , substantial strands of fibres along vascular bundles, girders of fibres from vascular bundles to one or both of the epidermides, and strands of fibres not associated with vascular tissues running through the leaf, often under the epidermis; ++ , substantial strands of fibres along vascular bundles, and girders of fibres from vascular bundles to one or both of the epidermides; +, most species with strands of fibres along vascular bundles, some without; –, most species with little or no development of fibre-strands along vascular bundles, a few with substantial strands.
The study presented here sought to answer two questions. (1) In TLRF, do monocot leaves on average have higher or lower values than dicots for punch strength and fracture toughness? This question was addressed by working in three widely separated parts of the wet tropics (north-eastern Australia, Singapore and Panama) and making determinations for mature leaves of species in a wide range of monocot families, and for their neighbouring dicots. (2) Is the relative toughness of monocot and dicot leaves approximately the same when immature as when mature? This question was answered for species in north-eastern Australia.
In the Discussion, it is first emphasized that it is necessary to confine any comparison of monocots and dicots to either shade-tolerators or light-demanders, and that it is desirable to make phylogenetically controlled contrasts between shade-tolerant and light-demanding species within either monocots or dicots wherever possible. Secondly, the contrast between monocots and dicots in the development of toughness during and after leaf expansion is reviewed. Thirdly, the relationship between punch strength and fracture toughness, and the relative strengths of correlation between those two properties and the rate of loss of leaf area to invertebrate herbivores is considered. Fourthly, the range in toughness of mature monocot leaves, and the need to recognize that monocots in TLRF include a type of tough leaf quite different from that considered in the classical literature is emphasized. In this final section use is made of data on the water contents of leaves given in a companion paper on the extent of leaf area loss to invertebrate herbivores suffered by monocots and dicots at six different TLRF sites (Grubb et al., 2008).
MATERIALS AND METHODS
Study sites
The locations are set out in Table 2, together with rainfall data and the sources of species nomenclature. The families and orders recognized follow Stevens (2007).
Table 2.
Characteristics of the sites at which leaves were sampled for determination of punch strength or fracture toughness, and the sources of species nomenclature
| Site | Latitude, longitude, altitude | Mean rainfall data* | Source of species nomenclature |
|---|---|---|---|
| Australian low-rainfall | |||
| Wongabel State Forest | 145°26'E, 17°18'S, approx. 750 m | 1400 mm year–1, 4 dry months | Bostock and Holland (2007) |
| Australian high-rainfall | |||
| Wooroonooran National Park | 145°43'E, 17°23'S, approx. 800 m | 3500 mmNo dry month | Bostock and Holland (2007) |
| Singaporean site | |||
| Bukit Timah Nature Reserve | 103°47'E, 1°21'N, approx. 100 m | 2400 mm year–1, no dry month | Turner (1995) |
| Panamanian site | |||
| Barro Colorado Natural Monument | 79°51'W, 9°9'N, approx. 50 m | 2650 mm year–1, 2 dry months | Henderson et al. (1995) for palms, otherwise Croat (1978) and updates in Condit et al. (1995) |
* Number of dry months as defined by Walter (1971).
Selection of species
All of the species chosen, both monocot and dicot, have wide-ranging distributions with respect to topography within the forests concerned. Thus our samples form an appropriate basis for comparing monocots and dicots in a given forest-type. However, it was long ago established for dicot trees in TLRF that punch strength is appreciably greater, on average, in shade-tolerant species than in species which need canopy gaps for establishment (Coley, 1983). Therefore, care is taken in this paper to confine the comparisons of monocots and dicots to shade-tolerant species at the sites where there were very few light-demanding monocots to sample. At the Panamanian site it was feasible to compare monocots and dicots using both shade-tolerant and light-demanding species.
Selection of material
Dominy et al. (2003) showed for dicot trees in the same forest as that studied by Coley (1983) that, on average, the fracture toughness is 1·7× greater in sun-exposed leaves than in highly shaded leaves, although they also found that species varied greatly so that the difference between exposed and shaded leaves ranged from a factor of ×3·7 to there being no significant difference. All of the monocot leaves sampled in the present study were collected within a few metres of the ground, and therefore values for shaded dicot leaves were used.
Measurements of punch strength for the Australian species
A penetrometer of the design of Sands and Brancatini (1991) was used, and in almost all cases three leaves (each from a different plant) were used per species at each stage of development studied. Measurements were made in January 1999 and August 2003. In 1999 measurements were made near the top, middle and bottom of the leaf blade, and the mean taken. At the low-rainfall site, Castanospora alphandii and Hodgkinsonia frutescens were, respectively, the commonest sapling and commonest shrub; at the high-rainfall site Wilkiea angustifolia was judged subjectively to be the toughest-leaved dicot in the forest. For most species, tests were made on both fully expanded but untoughened leaves, and on fully toughened leaves.
In August 2003, measurements were made on immature leaves that had expanded to varying extents depending on the species, and on the first fully expanded and toughened leaf on the same shoot. The immature leaves of the monocots and of the one dicot with relatively late-folded leaves (Argyrodendron trifoliolatum) were carefully unrolled or unfolded before measurements were made. The length (l) and breadth (b) were recorded for each leaf in millimetres, and the area of the immature leaf as a percentage of the area of the first fully expanded leaf was estimated from the respective values for lb; the mean value for this estimate was calculated for each species. By chance, immature leaves of monocots that were estimated to have 15–75 % of final lamina area and dicots with 1·6–67 % were found. The ultimate objective of the present study was to compare the leaves of monocots and dicots both when immature and when mature, and to do this in a way that was sensitive to any generalized difference there might be between the two groups in the relationship between punch strength and the extent of lamina expansion. To achieve this the following were investigated: (a) whether for dicots punch strength remained essentially constant during lamina expansion, as reported by Kursar and Coley (1992) for four of five dicots studied in TLRF on Panama; and (b) whether or not the same was true for monocots. All values for fully toughened leaves of Freycinetia excelsa in 1999 and Pandanus monticola in 2003 were beyond the limits of the apparatus (150 g cm–2 in 1999 and 155 g cm–2 in 2003), and were treated as 150 and 155 g cm–2, respectively, for statistical analysis.
Measurements of fracture toughness for the Singaporean and Panamanian species
A portable universal tester of the design of Darvell et al. (1996) was used. Measurements were made in August–October 2001 in Panama, and in August–September 2003 in Singapore. Only fully toughened leaves were tested. The mean fracture toughness for a species was calculated from four (sometimes three) mature leaves from an individual plant; each leaf was fractured with a single transverse cut perpendicular to the midrib equidistant between the base and apex.
RESULTS
Measurements of punch strength on leaves at different stages of development
In the initial study at two Australian sites, the punch strength of fully expanded leaves was markedly higher for monocots than dicots, both before and after they became fully toughened (Appendix 1). In that study, few dicots were tested, and the monocots were a mixture of shade-tolerant and gap-demanding species. However, the difference between monocots and dicots was confirmed in a second study of fully hardened leaves, which used only shade-tolerant species and in which the dicots were from a wide range of families (t = 7·99, P < 0·001; Appendix 2).
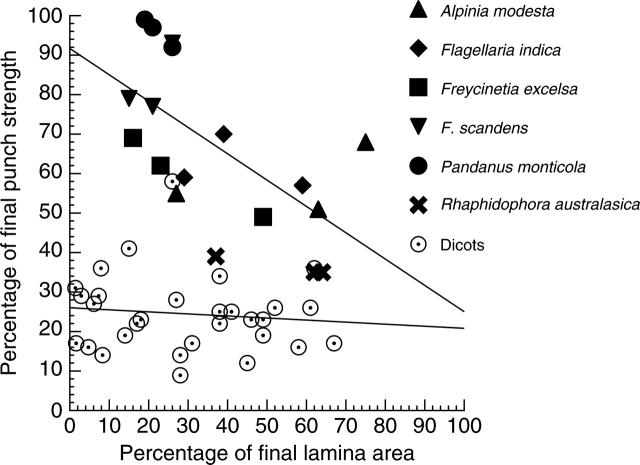
When, for dicots, all the individual values for percentage final punch strength are plotted against percentage final lamina area no trend toward increased punch strength in more expanded leaves is found (Fig. 1). There was relatively little variation in the extent to which punch strength increased between the immature and mature states: eight of 11 species increased by a factor of 3·6–5·4, the remaining three by factors of 1·5, 2·5 and 6·4 (cf. Appendix 2).
Fig. 1.
Percentage final punch strength as a function of percentage final lamina area for individual leaves of ten species of dicots (open circles) and six species of monocots (different symbol for each species) at the Australian high-rainfall site. The regressions are y = 90·6 – 0·664x (r = 0·629), and y = 26·0 – 0·052x (r = 0·105).
For monocots, there appears to be a decrease in the percentage final punch strength with increase in percentage final lamina area (Fig. 1). However, this result is an artefact which arises from the finding, by chance, of relatively smaller leaves on the species which have higher values for percentage final punch strength when immature. An analysis of variance shows that there is no effect of percentage final lamina area on percentage final punch strength, only an effect of species [F (percentage final lamina area) = 0·27, P = 0·61; F (species) = 24·18, P < 0·0001]. Figure 1 shows that there is no consistent trend for a reduction in punch strength with increasing lamina area for single species. The factors by which punch strength increased between immature and mature leaves were much lower than for dicots; they ranged from 1·1–1·9 for the one Zingiberaceae, three pandans and Flagellaria, to 2·8 for the one Araceae. In the 1999 study, similar increases in punch strength were found between the fully expanded but not yet fully toughened leaves and the fully mature leaves: 1·3–1·6 for four Zingiberaceae, a palm and a pandan, and 2·0–3·1 for two Araceae and Cordyline – based on data in Appendix 1.
If it is concluded that the stage in expansion when punch strength is measured is immaterial for dicots, and probably so for monocots, then it is reasonable to use all the results for immature leaves to compare monocots and dicots. In that case the monocots had 7·1× greater punch strength than dicots while still expanding, and only 2·8× greater when fully expanded and toughened.
Measurements of fracture toughness on fully expanded and fully toughened leaves
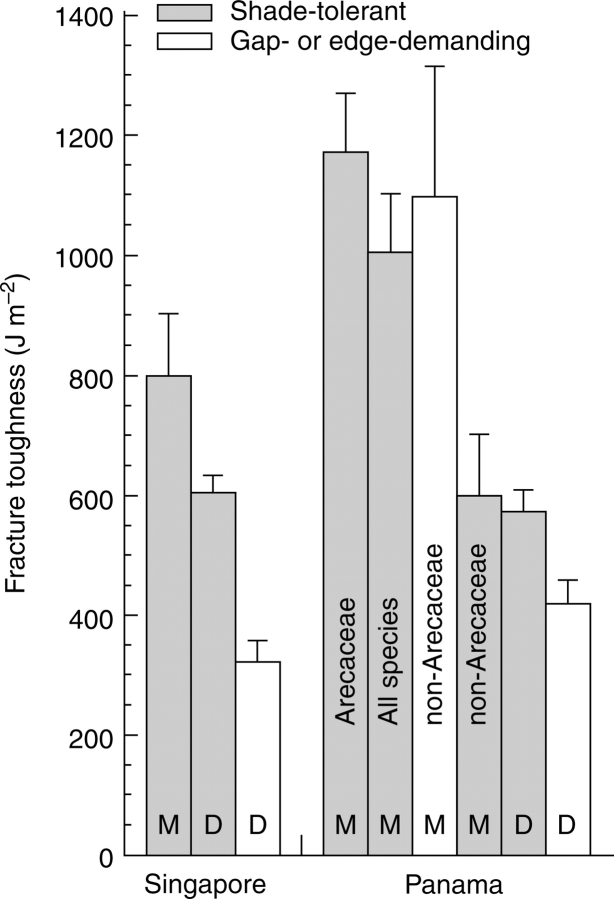
In Singapore the dicots, considered without respect to phylogeny, showed significantly and markedly higher fracture toughness in the shade-tolerators than in the light-demanders: 605 ± 28 vs. 322 ± 36 J m–2 (t = 8·93, P < 0·0001; Fig. 2 and Appendix 3). In phylogenetic contrasts, comparing genera within one family (or within two very closely related families) the shade-tolerators had higher values in five out of seven cases and there was one tie (Table 3); in one comparison among species within a genus (Clerodendrum) the shade-tolerator had a higher value, and in another (Ficus) the shade-tolerators did not have a higher value. All of the monocots tested were shade-tolerant; they had significantly higher fracture toughness than the shade-tolerant dicots: 798 ± 105 vs. 605 ± 28 J m–2 (t = 7·60, P < 0·0001; Fig. 2 and Appendix 3). Metcalfe and Grubb (1995) noted that there is a dearth of light-demanding species – whether monocot or dicot – among the herbs, shrubs, climbers and tall trees (as opposed to short and mid-height trees) at the Singaporean site; it was not practicable to make a comparison of shade-tolerant and light-demanding monocots.
Fig. 2.
Mean values (±s.e.) for fracture toughness of the lamina of fully expanded and toughened leaves: Singapore – nine shade-tolerant monocots, 46 shade-tolerant dicots and 16 light-demanding monocots; Panama – 15 Arecaceae (all shade-tolerant, excluding four introduced species), 21 shade-tolerant monocots (15 Arecaceae and 6 non-Arecaceae), eight light-demanding non-Arecaceae, six shade-tolerant non-Arecaceae, 46 shade-tolerant dicots and 21 light-demanding dicots; original data in Appendix 3. M, Monocot; D, dicot.
Table 3.
Phylogenetically controlled contrasts in fracture toughness of leaf laminae between shade-tolerant (ST) and light-demanding (LD) trees – the value given is the quotient of the logarithmic mean for the ST species over that for the LD species
| Singapore | |
| Cannabaceae | |
| Gironniera (1)/Trema (2) | 1·28 |
| Fabaceae | |
| Albizia (1) + Milletia (1) + Parkia (1) + Sinodora (1)/Archidendron (1) | 1·15 |
| Loganiaceae | |
| Strychnos (1)/Fagraea (1) | 0·949 |
| Melastomataceae | |
| Pternandra (1)/Melastoma (1) | 1·00 |
| Moraceae | |
| Artocarpus (2) + Ficus (2)/Ficus (1) | 1·04* |
| Phyllanthaceae + Euphorbiaceae | |
| Aporusa (2) + Baccaurea (1)/Endospermum (1) + Macaranga (3) + Sapium (1) | 1·16 |
| Verbenaceae | |
| Clerodendrum (1)/Clerodendrum (1) | 1·14 |
| Panama | |
| Anacardiaceae | |
| Astronium (1)/Anacardium (1) + Spondias (2) | 0·937 |
| Annonaceae | |
| Guatteria (1) + Unonopsis (1) + Xylopia (1)/Annona (1) | 1·01 |
| Burseraceae | |
| Protium (2) + Tetragastris (1)/Trattinickia (1) | 1·14 |
| Malvaceae | |
| Quararibea (1) + Sterculia (1)/Apeiba (1) + Guazuma (1) + Luehea (1) + Pseudobombax (1) | 1·12 |
| Moraceae | |
| Brosimum (1) + Perebea (1) + Poulsenia (1)/Ficus (1) | 1·16 |
| Rubiaceae | |
| Genipa (1) + Psychotria (9)/Psychotria (4) | 0·992† |
| Urticaceae | |
| Pourouma (1)/Cecropia (2) | 1·30 |
The number in parenthesis after the generic name is the number of species for which values are available – the original data are in Appendix 3.
* 0·999 if only the Ficus species are considered.
† 0·994 if only the Psychotria species are considered.
In Panama, the dicots, considered without respect to phylogeny, again showed significantly higher fracture toughness in the shade-tolerators than in the light-demanders, but the difference was not as marked as in Singapore: 572 ± 37 vs. 418 ± 41 J m–2 (t = 10·21, P < 0·0001; Fig. 2 and Appendix 3). In phylogenetic contrasts, comparing genera within one family, the shade-tolerators had higher values in five out of seven cases and there was one tie (Table 3); for the 13 species of Psychotria tested (nine shade-tolerant and four gap-demanding) there was a tie. Only in Panama was it possible to make a useful comparison between shade-tolerant and light-demanding monocots, and this did not involve any phylogenetically controlled comparison. There was no significant difference: shade-tolerators 1006 ± 96 vs. gap-demanders 1097 ± 218 J m−2 (t = 0·45, P = 0·66; Fig. 2 and Appendix 3); for this comparison the four palm species marked ‡ in Appendix 3 were omitted. For both shade-tolerant and light-demanding species the monocots had much greater mean values for fracture toughness than the dicots: 1006 ± 96 vs. 572 ± 37 (t = 10·51, P < 0·0001), and 1097 ± 218 vs. 418 ± 41 J m–2 (t = 5·04, P < 0·002), respectively (Fig. 2).
Although palms, as a whole, had the highest fracture toughness values, it is notable that at both the Singaporean and Panamanian sites members of the Marantaceae had much higher values than some accompanying palms (Appendix 3). Other members of the Zingiberales also had notably high values: Elatteriopsis curtisii (Zingiberaceae) in Singapore and Heliconia catheta and H. mariae (Heliconiaceae) in Panama (Appendix 3).
DISCUSSION
Comparison of monocot and dicot leaves when fully expanded and fully toughened
Any satisfactory comparison must allow for the marked difference in toughness generally found within dicots between shade-tolerant and light-demanding species. However, the matter is more complicated than has been appreciated until now. The present results show that this widely recognized difference is not always found within genera. For the 13 Psychotria species studied in Panama the fracture toughness values for the four light-demanders are embedded within the range found for the shade-tolerators (Appendix 3). A similar result was found in Ficus in Singapore where only three species were studied (Appendix 3). The situation is reminiscent of that found by Grubb and Metcalfe (1996) in TLRF in NE Queensland in respect of seed dry mass; it was generally higher in shade-tolerators than in light-demanders if genera were compared within families, but not when species were compared within genera. Clearly new research is needed to see if the pattern found in Psychotria is widespread within genera that have considerable numbers of species in both shade-tolerant and light-demanding categories. It has long been known that there are wide, overlapping ranges of punch strength among both shade-tolerators and light-demanders in TLRF (Coley, 1983), but there has been no study of the significance of the range in each category, just as the wide range of foliar nitrogen concentration has been largely ignored (cf. Grubb, 2002). Valladares et al. (2000) reported on the morphological and physiological responses to shade of 16 Psychotria species in the Panamanian forest studied. They gave mean values for leaf longevity of nine of the species for which fracture toughness is available. The longevity values are positively correlated with fracture toughness values for three light-demanding species (r = 0·912), and at a given fracture toughness the longevity values for four shade-tolerant species are much higher than for the one relevant light-demander (approx. 650–900 d vs. approx. 200 d). The longevity values for the two shade-tolerators with relatively high values for fracture toughness are surprisingly low, and fall on the line for the light-demanders, so that for the shade-tolerators there is a negative correlation overall (r = –0·661). The explanation is not at all obvious.
The present results for dicots in Panama show that a satisfying comparison of shade-tolerant and light-demanding monocots in respect of fracture toughness must involve both intergeneric and intrageneric tests. In a comparison made without respect to phylogeny it was found that, on average, there was no difference within the monocots between shade-tolerators and light-demanders (Fig. 2 and Appendix 3). However, the value of the comparison is limited. Palms made up a large majority of the shade-tolerators, palms were not represented among the gap-demanders, and palms, on average, had significantly greater fracture toughness than other monocots (1270 ± 99 vs. 883 ± 145 J m–2; t = 12·81, P < 0·0001). For the non-palm monocots in the present sample, it was found that the shade-tolerators had markedly lower fracture toughness than the gap-demanders: 598 ± 104 vs. 1097 ± 218 J m–2 (Wilcoxon Signed-Rank test, t = 10·5, P = 0·03). The value of this comparison is limited by the over-representation of the Poaceae in the shade-tolerant sample. Clearly new comparisons of shade-tolerators and gap-demanders within such families as Marantaceae and Zingiberaceae are needed.
This section concludes by emphasizing that, when only shade-tolerant species are used, it is consistently found that the monocots have tougher leaves on average than the dicots. That is true for punch strength in the Australian high-rainfall forest, and for fracture toughness in the Singaporean and Panamanian forests.
Difference between monocot and dicot leaves when still expanding
The conclusion for shade-tolerant dicots in TLRF that there is no appreciable increase in punch strength during the expansion phase agrees with the findings of Kursar and Coley (1992) for three of the four shade-tolerant species they studied in Panama. Their fourth species seemed to show a small but significant increase in the 10 d before expansion ceased, and that may well happen in some Australian species; by chance the present study did not include any dicot species with leaves having >67 % of final leaf area. Brunt et al. (2006) found evidence that toughness began to increase before expansion was complete in Nothofagus moorei, a tree of Australian subtropical montane rain forest. The limited data in the present study suggest that in monocots too the punch strength of the lamina does not change appreciably during expansion, but further work is needed to test that conclusion. Monocots differ from dicots through having absolutely greater punch strength while expanding; they differed by a factor of about 7 in the present study of shade-tolerant species, but the factor would probably be greater if light-demanding species were studied because it is certain for dicots that the punch strength of light-demanders is appreciably lower than that of shade-tolerators, while for monocots the limited evidence suggests only a small difference between light-demanders and shade-tolerators (see above).
It seems most likely that the difference between monocots and dicots arises from the early development of fibres along the vascular bundles, and from the vascular bundles being relatively close. For both monocot and dicot leaves there is a need for new research on the changes during and after expansion in biomechanical properties, and in the major chemical fractions of the cell walls, along the lines of the classic study by Taylor (1971a, b) on leaves of mango.
The relationship between punch strength and fracture toughness
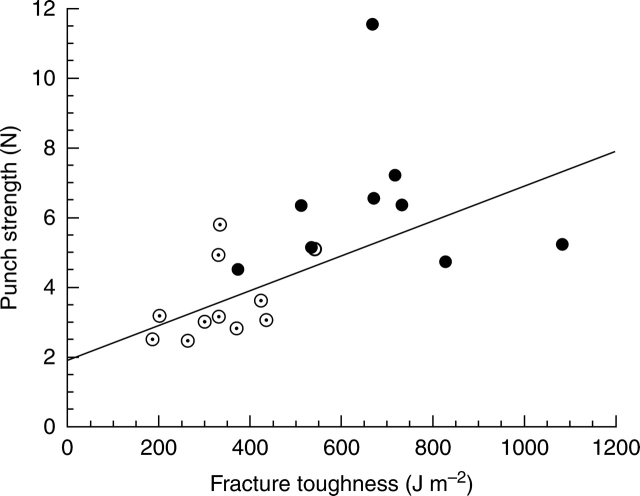
Choong et al. (1992) found a close relationship between fracture toughness and punch strength only after correcting for lamina thickness. The authors do not have values for both variables at any of the sites, but for the Panamanian forest the values for punch strength published by Coley (1983) and the present values for fracture toughness can be compared (Fig. 3). There is no significant relationship between the two variables if only the nine shade-tolerant species are considered (r = 0·007; P = 0·99), and a weak correlation if only the 11 light-demanding species are considered (r = 0·460; P = 0·15), but there is a significant and marked correlation if all species are considered together (r = 0·560; P = 0·01). The correlation becomes appreciably stronger if one shade-tolerant species (Calophyllum longifolium) is omitted (r = 0·639; P = 0·003). This finding parallels that of Coley (1988) who obtained significant positive correlations between leaf life span and the concentrations of tannins and fibres when using a data set comprising both strongly shade-tolerant and light-demanding species, but no significant correlation within either group when considered alone even though there were 21 species in the first group and 20 in the second.
Fig. 3.
The relationship between punch strength determined by Coley (1983) and fracture toughness (values from Appendix 3) for mature leaves of 20 dicot tree species: 11 light-demanding (open circles) and nine shade-tolerant (closed circles); the regression line (y = 2·44 + 0·0042x, r = 0·634) is for the two groups of species together, omitting the outlier (Calophyllum longifolium).
As punch strength is not simply related to fracture toughness in dicots, it is not expected that the difference in fracture toughness between monocots and dicots during expansion will necessarily parallel closely the difference in punch strength.
Relevance to extent of herbivory
Leaf properties can only ever be a partial explanation of the extent of loss to invertebrate herbivores. Once an animal has evolved the ability to attack a certain type of leaf, the extent of damage is largely a function of how far the herbivore's population is kept down by predators, parasitoids and disease (Strong et al., 1984). This is well shown by the way in which certain palms become badly damaged when in plantations rather than scattered in the forest (Cock et al., 1987). A leaf property such as toughness can be effective in two ways: in making evolution of a specialist herbivore difficult, and in reducing attack by generalists. The latter seems more likely to be important in TLRF, as generalists have been found to dominate the herbivorous insect community (Novotny and Basset, 2005).
It is a fact that almost all those who have considered extent or rate of loss of area to invertebrate herbivores in relation to leaf ‘toughness’ in both tropical and temperate vegetation have used what we now call punch strength as their measure of ‘toughness’ (Williams, 1954; Tanton, 1962; Feeny, 1970; Coley, 1983; Lowman and Box, 1983; Raupp, 1985; Aide and Londoño, 1989; Ernest, 1989; Nichols-Orians and Schulz, 1990; Sagers, 1992; Braker and Chazdon, 1993; Feller, 1995; Filip et al., 1995; Jackson, 1995; Coley and Barone, 1996; Coley and Kursar, 1996; Jackson et al., 1999; Blundell and Peart, 1998; Howlett and Davidson, 2001; Marquis et al., 2001; Spiller and Agrawal, 2003; Xiang and Chen, 2004). In contrast, those authors reporting critical, wider-ranging studies on the mechanical properties of leaves, in particular fracture toughness, have not also recorded rates of loss of leaf area in their study species, although the papers by Choong (1996), Iddles et al. (2003), Clissold et al. (2006) and Eichhorn et al. (2007) are exceptions. Peeters et al. (2007) recorded toughness measurements and related them to the densities of herbivorous insect guilds.
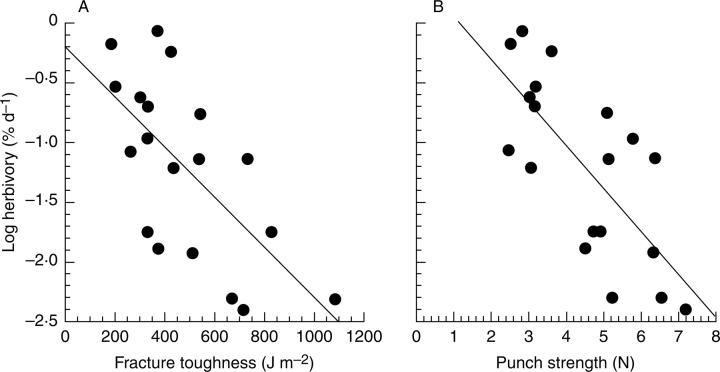
For the forest in Panama, it is possible to compare the correlations between log rate of loss of area from mature leaves with (a) punch strength and (b) fracture toughness. Coley (1983) provided the data for (a) and the authors have the data for (b). Of course, it would be better to have data on (a) and (b) from the same leaves, but a preliminary comparison is worthwhile. The correlation is tighter for punch strength than for fracture toughness (r = 0·727 vs. 0·660; P = 0·0003 vs. 0·0021; Fig. 4). Calophyllum longifolium which had zero loss to herbivores and a heavily biasing influence was left out of this analysis; if it were retained and the rate treated as 0·01 % per day, the difference between the two correlations would be even greater (punch strength: r = 0·796, P < 0·0001; fracture toughness: r = 0·655, P = 0·0017). In the present study, Coley (1983) has been followed in using the log rate of herbivory, but arguably the relationship between the rate of herbivory and punch strength found in her classic study is not well characterized by a regression. Rather punch strength sets an upper limit to the probable rate of herbivory; the actual rate varies greatly at low values of punch strength, often being far below the limit set by punch strength (Fig. 3 of Grubb et al., 2008).
Fig. 4.
The relationship between log rate of loss of leaf area to herbivores (y, determined by Coley, 1983) and (A) fracture toughness (x1, Appendix 3), and (B) punch strength (x2, Coley 1983). Regressions are: y = 0·455 – 0·00482x1 (r = 0·660), and y = 0·848 – 0·812x2 (r = 0·727).
In a number of more recent studies on dicots in the wet and seasonally dry tropics there has been no evidence of lower rates of loss from tougher leaves. Eichhorn et al. (2007) measured the rate of loss over 6 months from seedlings of five species of Dipterocarpaceae in TLRF in Sabah, and found a negative correlation with the concentration of phenolics but a positive correlation with fracture toughness. Blundell and Peart (1998) had earlier found no relationship between herbivory and toughness in four species of Dipterocarpaceae, and Howlett and Davidson (2001) in two species of the same family, likewise Braker and Chazdon (1993) with three species of palms. Filip et al. (1995) found no significant correlation between toughness and the rate of herbivory in a tropical dry forest. Two studies based on intraspecific comparisons yielded no significant correlation between toughness and extent or rate of herbivory: those of Ernest (1989) and Feller (1995). These results have to be balanced against the positive correlations between greater toughness and less extensive herbivory reported from intraspecific comparisons by Aide and Londoño (1989), Nichols-Orians and Schulz (1990), Sagers (1992), Jackson (1995) and Spiller and Agrawal (2003), and from the comparison of four species of Ficus by Xiang and Chen (2004). The only study to yield a positive correlation between toughness and extent of herbivory based on comparisons of a large number of species (25) is that of Marquis et al. (2001) for a cerrado (savanna) site in Brazil. Here toughness and herbivory increased in the order herbs < shrubs < trees. Such a result might depend on a parallel trend in the number of herbivorous species, as is found in other vegetation-types (Strong et al., 1984). There is no correlation between toughness and herbivory among the ten tree species studied by Marquis et al. (2001).
Notwithstanding the results of Eichhorn et al. (2007) and some other authors, we hypothesize that in TLRF the greater toughness of the leaves of monocots, especially the much greater toughness at the immature stage, is associated with smaller losses of leaf area to insects. This hypothesis is tested in a companion paper (Grubb et al., 2008), which also considers the independent hypothesis that the much greater incidence in monocots of tight folding or rolling of leaves to a late stage in development will deter herbivorous invertebrates (Grubb and Jackson, 2007).
The range in toughness of fully mature leaves of monocots
In the light of work with dicots (Lucas et al., 1995, 2000), the range in toughness among monocots would be expected to parallel the degree of development of fibres in the leaves. To an extent that appears to be the case, even when the crude categorizations for fibre-richness given in Table 1 are used. Most of the Arecaceae, Cyclanthaceae and Pandanaceae (all given a rating of +++ in Table 1) have relatively high values for fracture toughness or punch strength, while the Araceae (rated +) and Dioscoreaceae (rated –) have relatively low values (Appendices 1–3). However, some of the highest values for fracture toughness are for species of Marantaceae and Zingiberaceae (both rated ++ ) and Heliconiaceae (+). Clearly more sophisticated studies are needed to test the correlation between fracture toughness and development of fibres in the monocots of TLRF.
A new kind of tough leaf
The ‘sclerophyll’, which is characteristic of trees and tall shrubs in all five regions of the world with a Mediterranean type of climate, has been regarded by botanists since around 1900 as the type of leaf that is especially tough as well as hard and stiff. From the 1930s it came to be realized that scleromorphic leaves are also characteristic of forest and scrub communities of the wet tropics on exceptionally nutrient-poor soils (Grubb, 1986). In the last 15 years it has become clear that the Mediterranean-type sclerophylls, on average, do not have especially high fracture toughness when compared with leaves of trees of TLRF on average soils (Turner et al., 1993) or even when compared with some mesomorphic evergreen leaves of the transition between Mediterranean-climate forest and warm temperate rain forest (Edwards et al., 2000). The botanists' idea of sclerophylly is now known to correlate best with high values for dry mass per unit leaf area, leaf thickness and stiffness (Edwards et al., 2000; Read and Sanson, 2003; Read et al., 2006). Among the sclerophylls, those with a marked development of fibres along the vascular bundles have higher fracture toughness than those with minimal development of such fibres, e.g. among species of the Mediterranean Basin Laurus nobilis and Quercus ilex versus Arbutus andrachne and A. unedo (Turner et al., 1993). A properly revised perspective of the ‘tough leaf’ among dicots is that it can be found among evergreen species in the whole gamut of vegetation-types from TLRF on both average soils and very poor soils through to Mediterranean-climate forests (Turner et al., 1993, 2000; Edwards et al., 2000; Read et al., 2005, 2006).
Among the monocots of TLRF with high values for punch strength and fracture toughness, the Heliconiaceae, Marantaceae and Zingiberaceae (all Zingiberales) possess a new kind of tough leaf. They differ in five ways from those scleromorphic dicots with genuinely tough leaves found in the same kind of forest: they are typically much larger, have a greater incidence of water-storage tissue under the epidermides, higher saturated water concentrations, a lack of stiffness and a tendency to wilt readily under drying conditions.
The majority of the leaves of dicot trees in TLRF fall into the mesophyll size-class of Raunkiaer, as amended by Webb (1959), i.e. 4500–18 225 mm2. In contrast, a high proportion of the Zingiberales have leaves in the macrophyll and megaphyll classes (18 225–164 025 and >164 025 mm2). The families of TLRF in the Zingiberales with the largest leaf laminae are the Musaceae (up to at least 4000 × 1000 mm in Ensete ventricosum; Palgrave, 1983) and Strelitziaceae (up to at least 4000 mm long in Ravenala madagascariensis; Brickell, 2003). There are no measurement of lamina toughness for these families but, on the basis of their anatomy (Tomlinson, 1961), they would be expected to comply with the present findings for large-leaved Heliconiacae.
Among dicots in TLRF the incidence of a colourless hypodermis is modest, 25 % or less of the species (Grubb et al., 1975; Choong et al., 1992), but it is very general in the Zingiberales, and such tissue generally occupies 20–45 % of the lamina thickness (Solereder and Meyer, 1928–33; Tomlinson, 1969; Roth, 1990). No data have been found on the saturated water concentrations of the leaves of the TLRF dicots shown by Turner et al. (1993, 2000) to have the highest recorded values for fracture toughness. However, among the dicots in high-rainfall forest in Australia, the five species found to have the greatest punch strength at maturity (45–54 g cm–2; Appendix 2) are known to have saturated water concentrations of 1·1–1·9 g g–1 dry mass (Appendix 2 of Grubb et al., 2008). In contrast, the ten species of Zingiberales for which toughness measurements are given in Appendices 1–3 had saturated water concentrations in the range 2·3–5·1 g g–1 (mean 3·8 ± 0·82 g g–1 s.e.; appendix 2 of Grubb et al., 2008). Sclerophylls in general do not change shape easily when partially dried out (Grubb, 1986), and that statement certainly applies to those that are genuinely tough. In contrast, leaves of most Zingiberales do not combine toughness with stiffness, and many roll up readily under drying conditions. This behaviour has been seen at various sites in TLRF during rainless spells, and even in bright sunshine around midday in large canopy gaps and at forest edges when the soil has not dried out.
The palms of TLRF are much closer in form and behaviour to the tough dicots in the same forest, despite the typically large size of their leaves. These have only weakly developed colourless water-storage tissue or none (Tomlinson, 1961), and the saturated water concentration is in the same range as that of the tough dicots in TLRF (1·5–1·7 g g–1 dry mass, n = 4; appendix 2 of Grubb et al., 2008). The leaves are mostly stiff and do not change shape readily as they dry out. The Cyclanthaceae and the Pandanaceae (both in the Pandanales) are intermediate between palms and the Zingiberales through being rich in fibres (Table 1) and relatively stiff, but rich in water: 3·1–3·7 g g–1 (n = 3; Grubb et al., 2008).
The tough leaves of such monocots in TLRF as those of Zingiberales and Pandanales are new in kind in a further sense. Coley and Barone (1996, p. 319) wrote that ‘toughness is not compatible with leaf expansion’, but the monocots in question show that that is not true. The present results from Australian TLRF show that in leaves of Zingiberales and Pandanales with only 15–60 % of final lamina area the punch strength was already greater (by a factor of 2·1 on average) than in the fully expanded and fully toughened leaves of the neighbouring shade-tolerant dicots (cf. Appendix 2).
Turner et al. (1993) and Edwards et al. (2000) revised the botanist's concept of the tough leaf by showing that among dicots rain forest trees commonly have leaves that are as tough as, or indeed tougher than, sclerophylls of the Mediterranean-climate regions. Now, we suggest that botanists and ecologists should pay more attention to monocots in this context, and make a further adjustment to their concept of the ‘tough leaf’.
CONCLUSIONS
As forecast on the basis of the abundance of fibres in their leaves, monocots in TLRF do indeed have greater punch strength and fracture toughness than the dicots with which they live. Many of the very tough monocot leaves are quite unlike the tough dicot sclerophylls of TLRF in being rich in water, and changing shape easily. Further work is needed to establish: (a) whether or not there is in monocots as in dicots, on average, greater toughness in shade-tolerators than in light-demanders, and (b) the anatomical and biochemical basis of the variation in fracture toughness and punch strength among the monocots. We forecast that the greater toughness of the monocots will be associated with smaller losses of leaf area to herbivorous invertebrates.
ACKNOWLEDGEMENTS
We thank Jenny Read for constructive criticism of an early draft of this report, two anonymous referees and Ignacio Barberis for scrupulously careful checking of our final text. Our work was supported by the British Ecological Society (D.J.M.), Carlsberg Foundation (J.-C.S.), Croucher Foundation (P.W.L.), Danish Natural Science Research Council (J.-C.S.), Explorers Club (N.J.D.), Mellon Foundation (P.J.G.), National Geographic Society (P.W.L.), National Institutes of Health (N.J.D.), Research Grants Council of Hong Kong (P.W.L.), Sigma Xi (N.J.D.), Smithsonian Tropical Research Institute (N.J.D.), Tropical Biology Association (P.J.G. and D.J.M.) and University of Cambridge (P.J.G.).
APPENDIX 1
Table A1.
Mean toughness (punch strength) values determined with a penetrometer for fully expanded leaves at two sites in Australian TLRF; species are ordered by the mean value for fully toughened leaves
| Family | Toughness of fully expanded but not fully toughened leaves (g cm–2) | Toughness of fully toughened leaves (g cm–2) | |
|---|---|---|---|
| Low-rainfall site | |||
| Monocots | |||
| Pothos longipes | Araceae | 52 | 106 |
| Pleuranthodium racemigerum | Zingiberaceae | 79 | 109 |
| Alpinia caerulea | Zingiberaceae | 88 | 110 |
| Alpinia modesta | Zingiberaceae | 80 | 111 |
| Alocasia brisbanensis | Araceae | 39 | 122 |
| Calamus caryotoides | Arecaceae | 83 | 130 |
| Mean (s.e.m.) | 70 (8·1) | 115 (3·8) | |
| Dicots | |||
| Hodgkinsonia frutescens | Rubiaceae | 18 | 33 |
| Castanospora alphandii | Sapindaceae | 25 | 40 |
| Mean (s.e.m.) | 22 (3·5) | 37 (3·5) | |
| High-rainfall site | |||
| Monocots | |||
| Alpinia arctiflora | Zingiberaceae | 50 | 72 |
| Cordyline cannifolia | Laxmanniaceae | 48 | 95 |
| Calamus australis | Arecaceae | ND | 127 |
| Freycinetia excelsa | Pandanaceae | 101 | >150 |
| Mean (s.e.m.) | 66 (17) | 111 (17) | |
| Dicots | |||
| Cryptocarya pleurosperma | Lauraceae | 24 | 48 |
| Wilkiea angustifolia | Atherospermataceae | 87 | |
| Mean (s.e.m.) | ND | 68 (20) |
Note: Alpinia arctiflora, A. caerulea and Pleuranthodium racemigerum all need tree-fall gaps or forest edges for establishment; the other species are shade-tolerant at establishment.
ND, not determined.
APPENDIX 2
Table A2.
Mean toughness (punch strength) values determined with a penetrometer for immature leaves and for fully expanded and toughened leaves at the Australian high-rainfall site
| Plant species | Family | Immature lamina area as % of mature lamina area (mean) | Mean toughness when immature (g cm–2) | Mean toughness when mature (g cm–2) |
|---|---|---|---|---|
| Monocots | ||||
| Rhaphidophora australasica | Araceae | 50 | 26 | 73 |
| Freycinetia scandens | Pandanaceae | 21 | 66 | 80 |
| Alpinia modesta | Zingiberaceae | 55 | 58 | 109 |
| Flagellaria indica | Flagellariaceae | 42 | 86 | 138 |
| Freycinetia excelsa | Pandanaceae | 29 | 92 | 153 |
| Pandanus monticola | Pandanaceae | 22 | 142 | >155 |
| Mean | 37 | 78 | 118 | |
| s.e.m. | 5·9 | 16 | 15 | |
| Dicots | ||||
| Prunus turneriana | Rosaceae | 16 | 7 | 29 |
| Atractocarpus hirtus | Rubiaceae | 22 | 20 | 29 |
| Cupaniopsis flagelliformis | Sapindaceae | 26 | 7 | 32 |
| Pouteria castanosperma | Sapotaceae | 20 | 15 | 38 |
| Ardisia bifaria | Myrsinaceae | 55 | 10 | 39 |
| Opisthiolepis heterophylla | Proteaceae | 20 | 12 | 43 |
| Neolitsea dealbata | Lauraceae | 17 | 7 | 45 |
| Beilschmiedia tooram | Lauraceae | 19 | 10 | 46 |
| Cryptocarya mackinnoniana | Lauraceae | 45 | 12 | 51 |
| Franciscodendron laurifolium | Malvaceae | 36 | 13 | 52 |
| Argyrodendron trifoliolatum | Malvaceae | 43 | 10 | 54 |
| Mean | 29 | 11 | 42 | |
| s.e.m. | 4·1 | 1·2 | 2·7 |
All species can become established in the shade. The leaves of the monocots and the Argyrodendron were unrolled or unfolded before measurements were made – the leaves of the other dicots were neither rolled nor folded at the stage when measurements were made.
APPENDIX 3
Table A3.
Mean fracture toughness values of the lamina determined for species in various functional groups in Singapore and Panama
| Family | Fracture toughness of the lamina (J m–2) | |
|---|---|---|
| Singapore | ||
| Monocots (all shade-tolerant) | ||
| Schismatoglottis wallichii | Araceae | 308 |
| Moliniera latifolia | Hypoxidaceae | 475 |
| Tacca integrifolia | Dioscoreaceae | 568 |
| Calamus oxleyanus | Arecaceae | 750 |
| Amischotolype gracilis | Commelinaceae | 782 |
| Elettariopsis curtisii | Zingiberaceae | 908 |
| Caryota mitis | Arecaceae | 1030 |
| Hanguana malayana | Hanguanaceae | 1052 |
| Stachyphrynium griffithii | Marantaceae | 1311 |
| Mean (s.e.m.) | 798 (105) | |
| Gap- or edge-demanding dicots | ||
| Sapium discolor* | Euphorbiaceae | 81 |
| Trema cannabina* | Cannabaceae | 122 |
| Clerodendrum villosum | Verbenaceae | 211 |
| Archidendron clypearia | Fabaceae | 229 |
| Trema tomentosa* | Cannabaceae | 266 |
| Dillenia suffruticosa* | Dilleniaceae | 283 |
| Macaranga heynei* | Euphorbiaceae | 285 |
| Ploiarium alternifolium* | Pentaphylaceae | 307 |
| Adinandra dumosa | Pentaphylaceae | 339 |
| Macaranga gigantea* | Euphorbiaceae | 343 |
| Melastoma malabathricum | Melastomataceae | 357 |
| Macaranga conifera | Euphorbiaceae | 359 |
| Ficus aurantiaca | Moraceae | 368 |
| Endospermum diadenum | Euphorbiaceae | 378 |
| Fagraea fragrans | Loganiaceae | 528 |
| Myrica esculenta* | Myricaceae | 689 |
| Mean (s.e.m.) | 322 (36) | |
| Shade-tolerant dicots | ||
| Parkia speciosa | Fabaceae | 234 |
| Pellacalyx saccardianus | Rhizophoraceae | 253 |
| Strombosia javanica | Olacaceae | 289 |
| Ficus grossularioides | Moraceae | 303 |
| Pternandra echinata | Melastomataceae | 357 |
| Dysoxylum cauliflorum | Meliaceae | 380 |
| Strychnos axillaris | Loganiaceae | 384 |
| Garcinia parvifolia | Clusiaceae | 403 |
| Prunus polystachya | Rosaceae | 404 |
| Milletia atropurpurea | Fabaceae | 439 |
| Ficus fistulosa | Moraceae | 443 |
| Clerodendrum laevifolium | Verbenaceae | 457 |
| Gynotroches axillaris | Rhizophoraceae | 458 |
| Aporusa frutescens | Phyllanthaceae | 539 |
| Palaquium microcarpum | Sapotaceae | 549 |
| Artocarpus elasticus | Moraceae | 550 |
| Bhesa paniculata | Celastraceae | 561 |
| Calophyllum pulcherrimum | Clusiaceae | 566 |
| Xylopia malayana | Annonaceae | 573 |
| Timonius wallichianus | Rubiaceae | 581 |
| Santiria apiculata | Burseraceae | 585 |
| Ochnastachys amentacea | Olacaceae | 597 |
| Baccaurea parviflora | Phyllanthaceae | 599 |
| Artocarpus integer | Moraceae | 609 |
| Diospyros buxifolia | Ebenaceae | 633 |
| Elaeocarpus ferrugineus | Elaeocarpaceae | 641 |
| Palaquium gutta | Sapotaceae | 654 |
| Castanopsis lucida | Fagaceae | 660 |
| Albizia splendens | Fabaceae | 662 |
| Rhodamnia cinerea | Myrtaceae | 663 |
| Streblus elongatus | Moraceae | 668 |
| Aporusa benthamiana | Phyllanthaceae | 690 |
| Lithocarpus conocarpus | Fagaceae | 700 |
| Eugenia grandis | Myrtaceae | 706 |
| Buchanania arborea | Anacardiaceae | 713 |
| Dacryodes buxifolia | Burseraceae | 713 |
| Garcinia griffithii | Clusiaceae | 732 |
| Campnosperma auriculatum | Anacardiaceae | 759 |
| Anisophyllea disticha | Anisophylleaceae | 771 |
| Gironniera parvifolia | Cannabaceae | 788 |
| Nephelium lappaceum | Sapindaceae | 791 |
| Durio zibethinus | Malvaceae | 805 |
| Mangifera indica | Anacardiaceae | 862 |
| Sindora wallichiana | Fabaceae | 1005 |
| Xanthophyllum maingayi | Polygalaceae | 1025 |
| Fibraurea tinctoria | Menispermaceae | 1061 |
| Mean (s.e.m.) | 605 (28) | |
| Panama | ||
| Gap- or edge-demanding non-palm monocots | ||
| Costus laevis | Costaceae | 370 |
| Costus guinaiensis | Costaceae | 510 |
| Heliconia latispatha | Heliconiaceae | 618 |
| Heliconia mariae | Heliconiaceae | 995 |
| Calathea lutea | Marantaceae | 1119 |
| Heliconia catheta | Heliconiaceae | 1307 |
| Carludovica palmata | Cyclanthaceae | 1695 |
| Pleiostachya pruinosa | Marantaceae | 2164 |
| Mean (s.e.m.) | 1097 (218) | |
| Shade-tolerant non-palm monocots | ||
| Philodendron pterotum | Araceae | 151 |
| Chusquea simpliciflora | Poaceae | 479 |
| Streptochaeta spicata | Poaceae | 652 |
| Streptochaeta sodiroana | Poaceae | 662 |
| Pharus latifolius | Poaceae | 795 |
| Streptogyne americana | Poaceae | 850 |
| Mean (s.e.m.) | 598 (104) | |
| Palms (all shade-tolerant) | ||
| Desmoncus orthocanthos | Arecaceae | 572 |
| Chamaedorea tepejilote | Arecaceae | 590 |
| Geonoma interrupta | Arecaceae | 673 |
| Synecanthus warscewiczianus | Arecaceae | 869 |
| Bactris barronis | Arecaceae | 928 |
| Bactris coloradonis | Arecaceae | 1022 |
| Socratea exorrhiza | Arecaceae | 1033 |
| Attalea butyracea | Arecaceae | 1175 |
| Wettinia panamensis‡ | Arecaceae | 1265 |
| Bactris major | Arecaceae | 1329 |
| Chamaedorea warscewiczii‡ | Arecaceae | 1345 |
| Cryosophila warscewiczii | Arecaceae | 1422 |
| Oenocarpus mapora | Arecaceae | 1501 |
| Bactris coloniata | Arecaceae | 1506 |
| Elaeis oleifera | Arecaceae | 1562 |
| Astrocaryum standleyanum | Arecaceae | 1573 |
| Geonoma cuneata | Arecaceae | 1776 |
| Phytelephas seemannii‡ | Arecaceae | 1868 |
| Euterpe precatoria‡ | Arecaceae | 2116 |
| Mean (s.e.m.) | 1270 (99) | |
| Gap- or edge-demanding dicots | ||
| Trema micrantha§ | Cannabaceae | 186 |
| Cordia alliodora†,§ | Boraginaceae | 201 |
| Psychotria pubescens | Rubiaceae | 234 |
| Terminalia amazonica | Combretaceae | 241 |
| Trattinnickia aspera†,§ | Burseraceae | 263 |
| Guazuma ulmifolia | Malvaceae | 280 |
| Ficus insipida† | Moraceae | 290 |
| Luehea seemannii†,§ | Malvaceae | 301 |
| Apeiba tibourbou§ | Malvaceae | 331 |
| Sapium aucuparium§ | Euphorbiaceae | 332 |
| Cecropia insignis†,§ | Urticaceae | 333 |
| Cecropia obtusifolia†,§ | Urticaceae | 370 |
| Spondias radlkoferi†,§ | Anacardiaceae | 424 |
| Zanthoxylum belizense§ | Rutaceae | 435 |
| Annona spraguei§ | Annonaceae | 545 |
| Psychotria brachiata | Rubiaceae | 585 |
| Psychotria racemosa | Rubiaceae | 596 |
| Anacardium excelsum† | Anacardiaceae | 628 |
| Pseudobombax septenatum | Malvaceae | 682 |
| Psychotria poeppigiana | Rubiaceae | 686 |
| Spondias mombin† | Anacardiaceae | 841 |
| Mean (s.e.m.) | 418 (41) | |
| Shade-tolerant dicots | ||
| Platypodium elegans | Fabaceae | 202 |
| Psychotria horizontalis | Rubiaceae | 206 |
| Psychotria chagrensis | Rubiaceae | 217 |
| Psychotria limonensis | Rubiaceae | 240 |
| Psychotria marginata | Rubiaceae | 311 |
| Lacmellea panamensis† | Apocynaceae | 340 |
| Serjania mexicana† | Sapindaceae | 362 |
| Virola surinamensis† | Myristicaceae | 364 |
| Virola sebifera†,§ | Myristicaceae | 373 |
| Virola multiflora† | Myristicaceae | 376 |
| Tabernaemontana arborea | Apocynaceae | 378 |
| Unonopsis pittieri | Annonaceae | 381 |
| Symphonia globulifera† | Clusiaceae | 397 |
| Astronium graveolens† | Anacardiaceae | 405 |
| Pourouma bicolor† | Urticaceae | 410 |
| Genipa americana | Rubiaceae | 413 |
| Dendropanax arboreus† | Araliaceae | 432 |
| Psychotria graciliflora | Rubiaceae | 450 |
| Tachigalia versicolor† | Fabaceae | 461 |
| Garcinia intermedia | Clusiaceae | 463 |
| Hasseltia floribunda | Salicaceae | 495 |
| Xylopia macrantha | Annonaceae | 506 |
| Protium panamense† | Burseraceae | 509 |
| Protium tenuifolium | Burseraceae | 512 |
| Trichilia tuberculata | Meliaceae | 521 |
| Aspidosperma cruenta† | Apocynaceae | 535 |
| Hirtella triandra§ | Chrysobalanaceae | 537 |
| Piper reticulatum† | Piperaceae | 545 |
| Brosimum alicastrum | Moraceae | 609 |
| Calophyllum longifolium†,§ | Clusiaceae | 669 |
| Guarea grandiflora§ | Meliaceae | 669 |
| Psychotria psychotrifolia | Rubiaceae | 678 |
| Sterculia apetala | Malvaceae | 704 |
| Cupania rufescens | Sapindaceae | 712 |
| Poulsenia armata†,§ | Moraceae | 717 |
| Tetragastris panamensis§ | Burseraceae | 732 |
| Chrysophyllum cainito† | Sapotaceae | 740 |
| Chrysophyllum argenteum† | Sapotaceae | 758 |
| Psychotria brachybotrya | Rubiaceae | 819 |
| Quararibea asterolepis§ | Malvaceae | 829 |
| Perebea xanthochyma† | Moraceae | 837 |
| Beilschmiedia pendula | Lauraceae | 925 |
| Guatteria dumetorum†,§ | Annonaceae | 1085 |
| Psychotria capitata | Rubiaceae | 1129 |
| Psychotria deflexa | Rubiaceae | 1144 |
| Dipteryx panamensis | Fabaceae | 1232 |
| Mean (s.e.m.) | 572 (37) |
Values for the fracture toughness of the veins and midrib are available from Dr N. J. Dominy. All values are original unless otherwise stated.
* Values taken from Choong et al. (1992); these are not used in Fig. 2.
† Values taken from Dominy et al. (2003).
‡ Species not present in Barro Colorado Natural Monument.
§ Species used by Coley (1983) in her comparison of ‘toughness’ values in ‘pioneer’ and ‘persistent’ species.
LITERATURE CITED
- Aide TM, Londoño EC. The effects of rapid leaf expansion on the growth and survivorship of a lepidopteran herbivore. Oikos. 1989;55:66–70. [Google Scholar]
- Ayensu ES. Anatomy of the monocotyledons. VI. Dioscoreales. Oxford: Clarendon Press; 1972. [Google Scholar]
- Bernays EA. Evolution of insect morphology in relation to plants. Philosophical Transactions of the Royal Society of London. 1991;B333:257–264. [Google Scholar]
- Blundell AG, Peart DR. Distance-dependence in herbivory and foliar condition for juvenile Shorea trees in Bornean dipterocarp rain forest. Oecologia. 1998;117:151–160. doi: 10.1007/s004420050643. [DOI] [PubMed] [Google Scholar]
- Bostock PD, Holland AE, editors. Census of the Queensland flora 2007. Brisbane: Queensland Herbarium, Environmental Protection Agency; 2007. [Google Scholar]
- Braker E, Chazdon RL. Ecological, behavioural and nutritional factors influencing use of palms as host plants by a neotropical grasshopper. Journal of Tropical Ecology. 1993;9:183–197. [Google Scholar]
- Brickell C, editor. The Royal Horticultural Society A–Z encyclopaedia of garden plants. (revd edn) 2 vols. London: Dorling Kindersley; 2003. [Google Scholar]
- Brunt C, Read J, Sanson GD. Changes in resource concentration and defence during leaf development in a tough-leaved (Nothofagus moorei) and soft-leaved (Toona ciliata) species. Oecologia. 2006;148:583–592. doi: 10.1007/s00442-006-0369-4. [DOI] [PubMed] [Google Scholar]
- Choong MF. What makes a leaf tough and how this affects the pattern of Castanopsis fissa leaf consumption by caterpillars. Functional Ecology. 1996;10:668–674. [Google Scholar]
- Choong MF, Lucas PW, Ong JSY, Pereira B, Tan HTW, Turner I. Leaf fracture-toughness and sclerophylly – their correlations and ecological implications. New Phytologist. 1992;121:597–610. [Google Scholar]
- Clissold F, Sanson GD, Read J. The paradoxical effects of nutrient ratios and supply rates on an outbreaking insect herbivore, the Australian plague locust. Journal of Animal Ecology. 2006;75:1000–1013. doi: 10.1111/j.1365-2656.2006.01122.x. [DOI] [PubMed] [Google Scholar]
- Cock MJW, Godfray HCJ, Holloway JD, editors. Slug and nettle caterpillars: the biology, taxonomy and control of the Limacodidae of economic importance on palms in South-east Asia. Wallingford, UK: Commonwealth Agricultural Bureaux; 1987. [Google Scholar]
- Coley PD. Herbivory and defensive characteristics of tree species in a lowland rain forest. Ecological Monographs. 1983;53:209–233. [Google Scholar]
- Coley PD. Effects of plant growth rate and leaf life-time on the amount and type of herbivore defense. Oecologia. 1988;74:531–536. doi: 10.1007/BF00380050. [DOI] [PubMed] [Google Scholar]
- Coley PD, Barone JA. Herbivory and plant defence in tropical forests. Annual Review of Ecology and Systematics. 1996;27:305–335. [Google Scholar]
- Coley PD, Kursar TA. Anti-herbivore defenses of young tropical leaves: physiological constraints and ecological trade-offs. In: Mulkey SS, Chazdon RL, Smith AP, editors. Tropical forest plant ecophysiology. New York, NY: Chapman & Hall; 1996. pp. 305–336. [Google Scholar]
- Condit R, Hubbell SP, Foster RB. Mortality-rates of 205 neotropical tree and shrub species and the impact of severe drought. Ecological Monographs. 1995;65:419–439. [Google Scholar]
- Croat TB. Flora of Barro Colorado Island. Stanford, CA: Stanford University Press; 1978. [Google Scholar]
- Darvell BW, Lee PKD, Yuen TDB, Lucas PW. A portable fracture toughness tester for biological materials. Measurement Science and Technology. 1996;7:954–962. [Google Scholar]
- Dominy NJ, Lucas PW, Wright SJ. Mechanics and chemistry of rain forest leaves: canopy and understorey compared. Journal of Experimental Botany. 2003;54:2007–2014. doi: 10.1093/jxb/erg224. [DOI] [PubMed] [Google Scholar]
- Edwards C, Read J, Sanson G. Characterising sclerophylly: some mechanical properties of leaves of heath and forest. Oecologia. 2000;123:158–167. doi: 10.1007/s004420051001. [DOI] [PubMed] [Google Scholar]
- Eichhorn MP, Fagan KC, Compton SG, Dent DH, Hartley SE. Explaining leaf herbivory rates on tree seedlings in a Malaysian rain forest. Biotropica. 2007;39:416–421. [Google Scholar]
- Ernest KA. Insect herbivory on a tropical understory tree – effects of leaf age and habitat. Biotropica. 1989;21:194–199. [Google Scholar]
- Feeny P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology. 1970;51:565–581. [Google Scholar]
- Feller IC. Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle) Ecological Monographs. 1995;65:477–505. [Google Scholar]
- Filip VR, Dirzo R, Maass JM, Sarukhan J. Within- and among-year variation in the levels of herbivory on the foliage of trees from a Mexican tropical deciduous forest. Biotropica. 1995;27:78–86. [Google Scholar]
- Grubb PJ. Sclerophylls, pachyphylls and pycnophylls: the nature and significance of hard leaf surfaces. In: Juniper B, Southwood R, editors. Insects and the plant surface. London: Edward Arnold; 1986. pp. 139–150. [Google Scholar]
- Grubb PJ. Leaf form and function – towards a radical new approach. New Phytologist. 2002;155:317–320. doi: 10.1046/j.1469-8137.2002.00491.x. [DOI] [PubMed] [Google Scholar]
- Grubb PJ, Jackson RV. The adaptive value of young leaves being tightly folded or rolled on monocotyledons in tropical lowland rain forest: an hypothesis in two parts. Plant Ecology. 2007;192:9317–9327. [Google Scholar]
- Grubb PJ, Metcalfe DJ. Adaptation and inertia in the Australian tropical lowland rain-forest flora: contradictory trends in intergeneric and intrageneric comparisons of seed size in relation to light demand. Functional Ecology. 1996;10:512–520. [Google Scholar]
- Grubb PJ, Grubb EAA, Miyata I. Leaf structure and function in evergreen trees and shrubs of Japanese warm temperate rain forest. I. The structure of the lamina. Botanical Magazine, Tokyo. 1975;88:197–211. [Google Scholar]
- Grubb PJ, Jackson RV, Barberis IM, Bee JN, Coomes DA, Dominy NJ, et al. Monocot leaves are eaten less than dicot leaves in tropical lowland rain forests: correlations with toughness and leaf presentation. Annals of Botany. 2008;101:1379–1389. doi: 10.1093/aob/mcn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A, Galeano G, Bernal R. Field guide to the palms of the Americas. Princeton, NJ: Princeton University Press; 1995. [Google Scholar]
- Henry DA, MacMillan RH, Simpson RJ. Measurement of the shear and tensile fracture properties of leaves of pasture grasses. Australian Journal of Agricultural Science. 1996;47:587–603. [Google Scholar]
- Henry DA, Simpson RJ, MacMillan RH. Seasonal changes and the effect of temperature and leaf moisture content on intrinsic shear strength of leaves of pasture grasses. Australian Journal of Agricultural Research. 2000;51:823–831. [Google Scholar]
- Howlett BE, Davidson DW. Herbivory on planted dipterocarp seedlings in secondary logged forests and primary forests of Sabah, Malaysia. Journal of Tropical Ecology. 2001;17:285–302. [Google Scholar]
- Iddles T, Read J, Sanson GD. The potential contribution of biomechanical properties to anti-herbivore defence in six Australian rainforest trees. Australian Journal of Botany. 2003;51:119–128. [Google Scholar]
- Jackson RV. Insect herbivory on tropical Alphitonia (Rhamnaceae) species. Townsville: James Cook University; 1995. PhD Thesis. [Google Scholar]
- Jackson RV, Kollmann J, Grubb PJ, Bee JN. Insect herbivory on European tall-shrub species: the need to distinguish leaves before and after unfolding or unrolling, and the advantage of longitudinal sampling. Oikos. 1999;87:561–570. [Google Scholar]
- Knapp-Zinn K. Anatomie des Blättes. II. Blattanatomie der Angiospermen. A. Entwicklungsgeschichte und topographische Anatomie des Angiospermenblattes. Encyclopaedia of plant anatomy 8. Berlin: Borntraeger; 1973. [Google Scholar]
- Kursar TA, Coley PD. Delayed greening in tropical leaves: an antiherbivore defense? Biotropica. 1992;24:256–262. [Google Scholar]
- Lowman MD, Box JD. Variation in leaf toughness and phenolic content among 5 species of Australian rain-forest trees. Australian Journal of Ecology. 1983;8:17–25. [Google Scholar]
- Lucas PW, Choong MF, Tan HTW, Turner IM, Berrick AJ. The fracture toughness of the leaf of Calophyllum inophyllum L. (Guttiferae) Philosophical Transactions of the Royal Society. 1991;B334:95–106. [Google Scholar]
- Lucas PW, Darvell BW, Lee PKD, Yuen TDB, Choong MF. The toughness of plant-cell walls. Philosophical Transactions of the Royal Society. 1995;B348:363–372. [Google Scholar]
- Lucas PW, Turner IM, Dominy NJ, Yamshita N. Mechanical defences to herbivory. Annals of Botany. 2000;86:913–920. [Google Scholar]
- Marquis RJ, Diniz IR, Morais H. Patterns and correlates of interspecific variation in foliar insect herbivory and pathogen attack in Brazilian cerrado. Journal of Tropical Ecology. 2001;17:127–148. [Google Scholar]
- Metcalfe CR. Anatomy of the Monocotyledons. I. Gramineae. Oxford: Clarendon Press; 1960. [Google Scholar]
- Metcalfe CR. Anatomy of the Monocotyledons. V. Cyperaceae. Oxford: Clarendon Press; 1971. [Google Scholar]
- Metcalfe DJ, Grubb PJ. Seed mass and light requirements for regeneration in Southeast Asian rain forest. Canadian Journal of Botany. 1995;73:817–826. [Google Scholar]
- Nicholls-Orians CM, Schulz JC. Interactions among leaf toughness, chemistry, and harvesting by attine ants. Ecological Entomology. 1990;15:311–320. [Google Scholar]
- North CA, Willis AJ. Contributions to the anatomy of Freycinetia species from the Solomon Islands. Botanical Journal of the Linnean Society. 1970;63:69–80. [Google Scholar]
- North CA, Willis AJ. Contributions to the anatomy of Sararanga (Pandanaceae) Botanical Journal of the Linnean Society. 1971;64:411–421. [Google Scholar]
- Novotny V, Basset Y. Review – host specificity of insect herbivores in tropical forests. Proceedings of the Royal Society, London. 2005;B272:1083–1090. doi: 10.1098/rspb.2004.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palgrave KC. Trees of southern Africa. Cape Town: Struik; 1983. [Google Scholar]
- Peeters P, Sanson G, Read J. Leaf biomechanical properties and the densities of herbivorous insect guilds. Functional Ecology. 2007;21:246–255. [Google Scholar]
- Raupp MJ. Effects of leaf toughness on mandibular wear of the leaf beetle Plagiodera versicolora. Ecological Entomology. 1985;10:73–79. [Google Scholar]
- Read J, Sanson GD. Characterizing sclerophylly: the mechanical properties of a diverse range of leaf types. New Phytologist. 2003;160:81–99. doi: 10.1046/j.1469-8137.2003.00855.x. [DOI] [PubMed] [Google Scholar]
- Read J, Stokes A. Plant biomechanics in an ecological context. American Journal of Botany. 2006;93:1546–1565. doi: 10.3732/ajb.93.10.1546. [DOI] [PubMed] [Google Scholar]
- Read J, Edwards C, Sanson GD, Aranwela N. Relationships between sclerophylly, leaf biomechanics and leaf anatomy in some Australian heath and forest species. Plant Biosystems. 2000;134:261–277. [Google Scholar]
- Read J, Sanson GD, Lamont BB. Leaf mechanical properties in sclerophyll woodland and shrubland on contrasting soils. Plant and Soil. 2005;276:95–113. [Google Scholar]
- Read J, Sanson GD, de Garine-Wichatitsky M, Jaffre T. Sclerophylly in two contrasting tropical environments: low nutrients vs. low rainfall. American Journal of Botany. 2006;93:1601–1614. doi: 10.3732/ajb.93.11.1601. [DOI] [PubMed] [Google Scholar]
- Roth I. Leaf structure of a Venezuelan cloud forest in relation to microclimate. Berlin: Borntraeger; 1990. [Google Scholar]
- Sands DPA, Brancatini V. A portable penetrometer for measuring leaf toughness in insect herbivory studies. Proceedings of the Entomological Society of Washington. 1991;93:786–788. [Google Scholar]
- Sagers CL. Manipulation of host plant quality: herbivores keep leaves in the dark. Functional Ecology. 1992;6:741–743. [Google Scholar]
- Sanson G. The biomechanics of browsing and grazing. American Journal of Botany. 2006;93:1531–1545. doi: 10.3732/ajb.93.10.1531. [DOI] [PubMed] [Google Scholar]
- Sanson G, Read J, Aranwela F, Clissold F, Peeters PJ. Measurement of leaf biomechanical properties in studies of herbivory: opportunities, problems and procedures. Austral Ecology. 2001;26:535–546. [Google Scholar]
- Solereder H, Meyer FJ. Systematische Anatomie der Monocotyledonen. Vols I, III, IV and VI. Berlin: Borntraeger; 1928. –1933. [Google Scholar]
- Spiller DA, Agrawal AA. Intense disturbance enhances plant susceptibility to herbivory: natural and experimental evidence. Ecology. 2003;84:890–897. [Google Scholar]
- Stevens PF. Angiosperm Phylogeny Website, Version 6, May 2006. 2007. updated continually. Available www.mobot.org/MOBOT/research/APweb/.mobot.org/MOBOT/research/APweb/ . Accessed 2 September 2007.
- Strong DR, Lawton JH, Southwood R. Insects on plants. Oxford: Blackwell Scientific Publications; 1984. [Google Scholar]
- Tanton MT. The effect of leaf ‘toughness’ on the feeding of larvae of the mustard beetle Phaedon cochliariae Fab. Entomology Experimental and Applied. 1962;5:74–78. [Google Scholar]
- Taylor FJ. Some aspects of the growth of mango (Mangifera indica L.) leaves. II. Methanol-soluble and cell wall-fractions. New Phytologist. 1971;a 70:567–579. [Google Scholar]
- Taylor FJ. Some aspects of the growth of mango (Mangifera indica L.) leaves. III. A mechanical analysis. New Phytologist. 1971;b 70:911–922. [Google Scholar]
- Tomlinson PB. Anatomy of the Monocotyledons. II. Palmae. Oxford: Clarendon Press; 1961. [Google Scholar]
- Tomlinson PB. Anatomy of the Monocotyledons. III. Commelinales-Zingiberales. Oxford: Clarendon Press; 1969. [Google Scholar]
- Tomlinson PB, Wilder GJ. Systematic anatomy of Cyclanthaceae (Monocotyledoneae) – an overview. Botanical Gazette. 1984;145:535–5439. [Google Scholar]
- Turner IM. The names used for Singapore plants since 1900. Gardens Bulletin, Singapore. 1995;47:1–287. [Google Scholar]
- Turner IM, Choong MF, Tan HTW, Lucas PW. How tough are sclerophylls? Annals of Botany. 1993;71:343–345. [Google Scholar]
- Turner IM, Lucas PW, Becker P, Wong SC, Yong JWH, Choong MF, Tyree MT. Tree leaf form in Brunei: a heath forest and a mixed dipterocarp forest compared. Biotropica. 2000;32:53–61. [Google Scholar]
- Valladares F, Wright JS, Lasso E, Kitajima K, Pearcy RW. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rain forest. Ecology. 2000;81:1925–1936. [Google Scholar]
- Walter H. The ecology of tropical and subtropical vegetation. Edinburgh: Oliver and Boyd; 1971. [Google Scholar]
- Webb LJ. A physiognomic classification of Australian rain-forest. Journal of Ecology. 1959;47:551–570. [Google Scholar]
- Wilder GJ. Anatomy of noncostal portions of lamina in the Cyclanthaceae (Monocotyledoneae). II. Regions of mesophyll, monomorphic and dimorphic ordinary mesophyll cells, mesophyll fibres and parenchyma-like dead cells. Botanical Gazette. 1985;a 146:213–231. [Google Scholar]
- Wilder GJ. The same. IV. Veins of interridge areas, expansion tissue and adaxial and abaxial ridges. Botanical Gazette. 1985;b 146:545–563. [Google Scholar]
- Williams LH. The feeding habits and food preferences of Acrididae and the factors which determine them. Transactions of the Royal Entomological Society of London. 1954;105:423–454. [Google Scholar]
- Wright W, Illius A. A comparative study of the fracture properties of five grasses. Functional Ecology. 1995;9:269–278. [Google Scholar]
- Wright W, Vincent JFV. Herbivory and the mechanics of fracture in plants. Biological Reviews. 1996;71:401–413. [Google Scholar]
- Xian H, Chen J. Interspecific variation of plant traits associated with resistance to herbivory among four Ficus species (Moraceae) Annals of Botany. 2004;94:377–384. doi: 10.1093/aob/mch153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N. Seasonality and the specificity of mechanical dietary patterns of two Malagasy lemur families (Lemuridae and Indriidae) International Journal of Primatology. 1996;17:355–387. [Google Scholar]