Abstract
Background and Aims
In tropical lowland rain forest (TLRF) the leaves of most monocots differ from those of most dicots in two ways that may reduce attack by herbivores. Firstly, they are tougher. Secondly, the immature leaves are tightly folded or rolled until 50–100 % of their final length. It was hypothesized that (a) losses of leaf area to herbivorous invertebrates are generally greatest during leaf expansion and smaller for monocots than for dicots, and (b) where losses after expansion are appreciable any difference between monocots and dicots then is smaller than that found during expansion.
Methods
At six sites on four continents, estimates were made of lamina area loss from the four most recently mature leaves of focal monocots and of the nearest dicot shoot. Measurements of leaf mass per unit area, and the concentrations of water and nitrogen were made for many of the species. In Panama, the losses from monocots (palms) and dicots were also measured after placing fully expanded palm leaflets and whole dicot leaves on trails of leaf-cutter ants.
Key Results
At five of six sites monocots experienced significantly smaller leaf area loss than dicots. The results were not explicable in terms of leaf mass per unit area, or concentrations of water or nitrogen. At only one site was the increase in loss from first to fourth mature leaf significant (also large and the same in monocots and dicots), but the losses sustained during expansion were much smaller in the monocots. In the leaf-cutter ant experiment, losses were much smaller for palms than for dicots.
Conclusions
The relationship between toughness and herbivory is complex; despite the negative findings of some recent authors for dicots we hypothesize that either greater toughness or late folding can protect monocot leaves against herbivorous insects in tropical lowland rain forest, and that the relative importance varies widely with species. The difficulties of establishing unequivocally the roles of leaf toughness and leaf folding or rolling in a given case are discussed.
Key words: anti-herbivore defences, dicots, herbivory, leaf folding, leaf rolling, leaf toughness, monocots, palms, tropical rain forest
INTRODUCTION
In tropical lowland rain forest (TLRF) the leaves of monocots differ from those of dicots (eudicots and magnoliids) in two ways that are likely to reduce losses to herbivores. In general they are tougher, and a majority of them have the leaves tightly folded or rolled until a late stage of development. Dominy et al. (2008) found in a study of mature leaves spread across three rain forest regions that toughness (either punch strength or fracture toughness) was greater in monocots. The same authors showed for Australian rain forest that the difference in punch strength between monocots and dicots was relatively greater for immature leaves (with about 30 % mature area on average) than for fully expanded and fully toughened leaves. Critical studies on the fracture toughness of dicot leaves have shown that the chief determinant is generally the extent of development of fibres around the vascular bundles (Lucas et al., 1995, 2000), though other tissues may be important in a minority of species (Read et al., 2000). Among monocots in TLRF greater toughness is characteristic of not only stiff-leaved plants, such as many palms, but also monocots with water-rich leaves that readily roll up under drying conditions, such as many gingers.
For dicots in TLRF the classic study of Coley (1983) found that greater toughness (punch strength) was strongly negatively correlated with the rate of leaf area loss, and several later authors have found the same correlation, although some others have found no correlation (reviewed by Dominy et al., 2008). Notwithstanding the negative findings of some recent authors, we hypothesize that in TLRF monocots will be found to suffer, on average, less extensive loss of leaf area than dicots.
Grubb and Jackson (2007, tables 1 and 2) reported that, when TLRF on all continents is considered, species with the leaves tightly folded or rolled until >50 % of mature length are found in 56 % of families of monocots, but in only 3·3 % of families of dicots. At the species level the difference is even greater as in the majority of major monocot families the leaves of every species are tightly folded or rolled to a late stage, while in the majority of the dicot families involved only a minority of species has the leaves folded or rolled until >50 % of mature length. Authoritative reviews of invertebrate herbivory and plant defence in TLRF have failed to mention the possibility that tight folding or rolling of leaves to a late stage of development could be an effective defence against herbivores (Coley and Barone, 1996; Coley and Kursar, 1996). However, it has been shown for both tropical and temperate plants that, although most losses to herbivorous insects occur while the leaf is expanding, the losses are very small when the young leaf is still folded or rolled (Jackson, 1995; Jackson et al., 1999). Grubb and Jackson (2007) hypothesized that the late folding and rolling of the leaves of monocots in TLRF, while possibly of value in increasing structural stiffness as suggested by King et al. (1996), is of equal or greater importance in reducing attack by invertebrate herbivores.
Table 1.
Summary of mean values (±s.e.) for characteristics of mature leaves of monocots and dicots at various sites
| Dry mass per unit leaf area (mg cm−2) | Nitrogen concentration (mg g−1 dry mass) | Water concentration (g g−1 dry mass) | |
|---|---|---|---|
| Australia: low-rainfall site | |||
| Monocots | 6·70 ± 2·15 | 23·9 ± 3·59 | 4·0 ± 1·1 |
| Dicots | 5·17 ± 0·50 | 28·2 ± 4·78 | 3·0 ± 0·5 |
| Australia: high-rainfall site | |||
| Monocots | 5·35 ± 0·19*** | 18·3 ± 0·90 | 3·3 ± 0·6 |
| Dicots | 7·0 ± 0·31 | 19·1 ± 1·1 | 2·1 ± 0·1 |
| Singapore | |||
| Monocots | ND | 25·7 ± 3·03 | ND |
| Dicots | ND | 17·9 ± 1·18 | ND |
| Uganda | |||
| Monocots | 3·88 ± 0·28* | 26·3 ± 2·01 | 4·2 ± 1·1 |
| Dicots | 5·18 ± 0·37 | 26·1 ± 1·35 | 2·4 ± 0·25 |
| Costa Rica | |||
| Monocots | 4·86 ± 0·53 | 19·5 ± 2·36* | 3·4 ± 0·43 |
| Dicots | 3·78 ± 0·23 | 27·2 ± 1·9 | 3·2 ± 0·37 |
| Panama | |||
| Monocots | 4·34 ± 0·35 | ND | 1·8 ± 0·48 |
| Dicots | 5·16 ± 0·49 | ND | 2·4 ± 0·45 |
Primary data are given in Appendix 2.
The cases where monocots and dicots at a site were statistically different (two-tailed t-test) are indicated: *P < 0·05; ***P < 0·001.
ND, not determined.
In this paper, tests of two hypotheses are presented.
Most of the loss of lamina area to herbivorous invertebrates occurs during leaf expansion, and the proportion lost is smaller for monocots than for nearest-neighbour dicots.
At any site where there is appreciable loss of lamina area to herbivorous invertebrates after leaf expansion, the difference between monocots and nearest-neighbour dicots in the proportion lost to herbivorous invertebrates during expansion (i.e. when the laminae are rolled or folded to a late stage) is greater than any difference found once the laminae are fully expanded.
In the interpretation of the results, the measurements of punch strength and fracture toughness reported by Dominy et al. (2008) and original data on leaf mass per unit area and the concentrations of nitrogen and water were used. Also reported are the relative losses from palm leaflets and dicot leaves left on trails of leaf-cutter ants. In the Discussion, the problems of demonstrating that any reduction in leaf-area loss from monocots during expansion is a result of the folding or rolling is considered.
MATERIALS AND METHODS
Study sites
Details of four of our six sites are shown in Table 1 of Dominy et al. (2008): the Australian low-rainfall and high-rainfall sites and the Singaporean and Panamanian sites.
The Ugandan site was in Kibale Forest National Park (30°21′E, 0°34′N; altitude approx. 1500 m), where the mean annual rainfall is 1500 mm, and there is one dry month sensu Walter (1971). Species nomenclature for this site follows the Flora of Tropical East Africa except that Leptaspis zeylanica Nees ex Steud., not L. urceolata R. Br., is recognized From families not yet covered by the Flora, Dracaena fragrans (L.) Ker Gawl, D. laxissima Engl., Palisota mannii C.B. Cl. and Pollia condensata C.B. Cl. were used
The Costa Rican site was at La Selva Biological Station (84°00′W, 10°26′N; altitude approx. 100 m), where the mean annual rainfall is 4000 mm, and there is no ‘dry month’. Species nomenclature for this site follows the La Selva Florula (www.ots.ac.cr/local/florula2005). The families and orders recognized follow Stevens (2007).
Sampling leaves and recording percentage area lost
Observers walked along a path through the forest, or on a bearing within the understorey, making records for one individual of each species of monocot tested using the first individual encountered within 2 m of the centre of the path (or the bearing) until all had been covered. Records were then made for the next individual of each species encountered regardless of order until all species had been sampled for a second time. This procedure was followed until ten individuals of each study species had been recorded. For each individual monocot the following records were made. The percentage of lamina area missing was estimated separately for each of the first four fully expanded and fully toughened leaves on the scale: 0, <1, 1–5, 5–10, 10–15, 15–25, 25–50, 50–75, 75–100. The same was done for the dicot shoot nearest to the main axis of the monocot. For each of the leaves recorded, the actual value for area missing was assumed to be the geometric mean of the upper and lower limits of the interval concerned, i.e. 0, 0·32, 2·24, 7·1, 12·2, 19·4, 35·4, 61·2 and 86·6. A variant of this procedure was used at the two Australian sites; the percentage area missing from each of the four leaves per plant was estimated to one digit rather than in classes. In almost all cases the area recorded as ‘missing’ was wholly missing, but in a very few cases (notably Calathea latifolia in Panama) it was missing only on one side as a result of scraping by an invertebrate.
The data were arcsine square-root transformed, and subjected to a nested generalized linear model (leaves within species within sites). The significance of differences between specific classes of leaves was determined using a two-tailed t-test on the response variable (proportion of lamina area lost by one class of leaf minus the proportion lost by another class).
Sites used within the forests and numbers of species studied
All the species used are listed in Appendix 1. At the two Australian sites and the Singaporean site, records were made in January 1999. At the Australian low-rainfall site six monocot species were studied (two light-demanding and four shade-tolerant). They were sampled along a path in tall forest; the extent of opening of the canopy was often sufficiently great to allow the establishment of the light-demanding species. At the Australian high-rainfall site, five monocot species were studied: one light-demanding species at the forest edge, and four shade-tolerant species on a bearing in the understorey of tall forest. At the Singaporean site five shade-tolerant monocot species were studied, all sampled on narrow paths through the understorey of tall forest.
At the Ugandan site, records were made in September 2001. Ten monocot species were studied (two light-demanding and eight shade-tolerant). They were sampled in tall forest beside narrow paths; in places the extent of opening of the canopy was sufficient to allow the establishment of the light-demanding species.
At the Costa Rican site, records were made in January 2000; eight shade-tolerant monocot species were studied, all sampled along a narrow path through tall forest. At the Panamanian site, records were made in January 2000 for three species (the light-demanding Calathea and Heliconia metallica, and the shade-tolerant Desmoncus; n = 5) and April–May 2001 for the other five species (two light-demanding and three shade-tolerant; n = 10–15). These species were studied in tall forest along paths of varying width; in places the extent of opening of the canopy was sufficient to allow the establishment of the light-demanding species.
An important feature of the sampling method is that all of the monocots studied occurred widely through the forest; monocots confined to specialized habitats within the forest landscape such as Pandanus ugandensis and Phoenix reclinata, which occur in the study area in Uganda but are confined to swamps or river-edges, were not included. The Australian low-rainfall site and Costa Rican site have only gentle slopes, and the study species occur right across them. The Australian high-rainfall site and the Singaporean and Panamanian sites have more marked topography, and a sophisticated study would probably show that some of the monocots are more common at uphill or downhill sites, but all of them were seen to be widely distributed from ridges to lower slopes. The Ugandan site is the only one where the monocots are all confined to part of the area inhabited by the dicots in the study. The monocots are certainly not plants of the valley-floor swamps, but are confined to lower-slope sites; the dicots that occur on these sites also extend to upper slope sites and were not studied there.
Measurements of dry mass per unit area of lamina and the concentrations of nitrogen and water
In order to see how far, if at all, differences in the losses from the laminae of monocots and dicots might be explained by leaf properties other than toughness, leaf dry mass per unit fresh area (LMA) and the concentrations of water and nitrogen were measured. For all three variables, fully expanded and toughened leaves were used; those used for LMA and N determinations were put into an oven for about 24 h within about 6 h of collection from the parent plant. LMA was calculated using leaf areas determined with a Delta-T leaf area meter and cut-out outlines of leaves (Australia) or a LICOR area meter with either fresh leaves (Costa Rica) or cut-out outlines of leaves (Panama), or by weighing cut-out outlines against a standard area (Uganda). The total nitrogen concentration was determined using an automated Kjeldahl method in Cambridge, UK; as a check samples of a well-mixed leaf powder for which Dr E. V. J. Tanner had obtained analyses from a number of laboratories were included in the analyses. Water concentration at saturation was determined on leaves left to hydrate overnight in a saturated atmosphere with their bases in liquid water. In each case, three replicates were used to obtain a mean value unless indicated otherwise.
For the Australian low-rainfall site, all the monocot species shown in Appendix 1 were compared with the two overwhelmingly most abundant dicots (Castanospora alphandii and Hodgkinsonia frutescens) and six other dicots common at the site and characteristic of the forest-type (n = 2 for all species). For the Australian high-rainfall site, four of the monocot species in Appendix 1 (not Pothos) were compared with 41 shade-tolerators (n = 2 or 3).
At the Singaporean site all the monocot species were shade-tolerant, and all the species in Appendix 1 were compared with the 14 shade-tolerant dicot species analysed by Grubb et al. (1994); n = 3 in all cases. Note that Ficus fistulosa and F. grossularioides were categorized as light-demanding by Grubb et al. (1994) but found to be shade-tolerant at establishment by Metcalfe et al. (1998). For the Ugandan site, analyses were made in 2001 of nitrogen (n = 1) for six out of ten monocots in Appendix 1 (one light-demanding and five shade-tolerant) and compared with 18 of the most abundant dicots in the understorey (n = 1 for all species). All but one of these species were analysed for leaf dry mass per unit area and water concentration (n = 3) in 1998.
For the Costa Rican site, all the monocot species were shade-tolerant; all of those in Appendix 1 (n = 3) were compared with 17 of the nearest-neighbour dicot species (n = 1 mostly, in some cases 2–5). For the Panamanian site, analyses of water concentration and leaf dry mass per unit area were made for five out of eight monocots in Appendix 1 (n = 3; two light-demanding and three shade-tolerant) and for 15 unidentified nearest-neighbour dicots of the five species (n = 3).
Comparison of losses from dicots and palms to leaf-cutter ants
On 12 days in October–November 2001, eight fully expanded leaves (or leaflets in the case of palms with divided leaves), in healthy, undamaged condition, of two palm and two dicot tree or shrub species were placed alternately at 15-cm intervals on one of two neighbouring active trails of Atta colombica at Gamboa, Panama. The mean areas (±s.e.) of the palm leaflets and dicot leaves put out were not significantly different: 173 ± 20 cm2 and 144 ± 24 cm2. Percentage loss of leaf area was estimated visually for each leaf after 24–25 h. On each day a new set of plant species was used, except that two palm species (Chamaedorea tepejilote and Bactris coloniata) had to be used twice. Both native and exotic palms were used to increase the number of palm species tested. Dicots were selected to sample a broad range of life histories among common native trees and shrubs. In all, 21 palm species and 24 dicots were used. The hypothesis that palms experienced a smaller percentage area loss than dicots using the one-tailed sign test was tested, comparing the mean value for palms with that for dicots for each experiment (n = 12).
RESULTS
Incidence of lamina area loss relative to leaf development
At five of the six sites (not the one in Uganda) the apparent slight increases in area lost from the lamina between the first mature leaf and the fourth are not significant for either monocots or dicots. This result is consistent with the first part of our first hypothesis: that the losses of leaf area occur mostly before leaf expansion is complete.
Proportion of lamina lost by monocots and by nearest-neighbour dicots
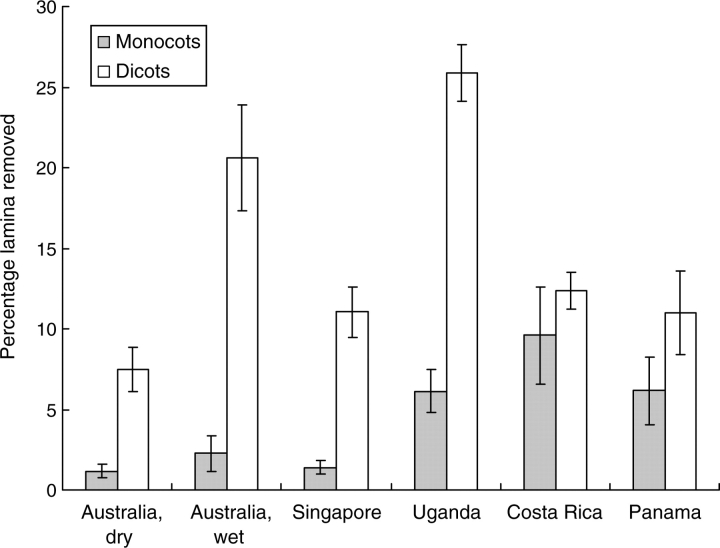
At five of the six sites the second part of our first hypothesis was upheld; the estimated percentage area missing from monocots was significantly and markedly smaller than that missing from dicots (Fig. 1; P < 0·01 for four sites, and P < 0·05 for that in Panama). At the Costa Rican site monocots also tended to lose less lamina area, but the difference was not significant (P > 0·05); invertebrates removed as high a proportion of leaf area from three of the study species as from nearest-neighbour dicots, or even a greater proportion (Appendix 1). The only analogous cases were found in the Panamanian sample.
Fig. 1.
The estimated mean percentage (±s.e.) of lamina area missing from the first four fully expanded and toughened leaves of focal monocot species and their nearest-neighbour dicots at four sites in the Old World and two in the New World. For original data see Appendix 1.
Relationship between lamina properties and area lost
There is no evidence that higher values for dry mass per unit leaf area, or lower values for nitrogen or water concentration, can explain the differences in extent of herbivory between monocots and dicots in the Old World. The present data on these variables for fully expanded and toughened leaves (Table 1) show few significant differences between monocots and dicots at a given site. In both cases where the difference is significant, the monocots would be expected to be more palatable (lower LMA at the Australian high-rainfall site and at the Ugandan site). Of the New World sites only the one in Costa Rica yielded a significant difference; the monocots had a significantly lower nitrogen concentration, but there was no significant difference between monocots and dicots in the extent of leaf area loss (Fig. 1).
Losses from monocots and dicots during expansion where there are significant losses after expansion
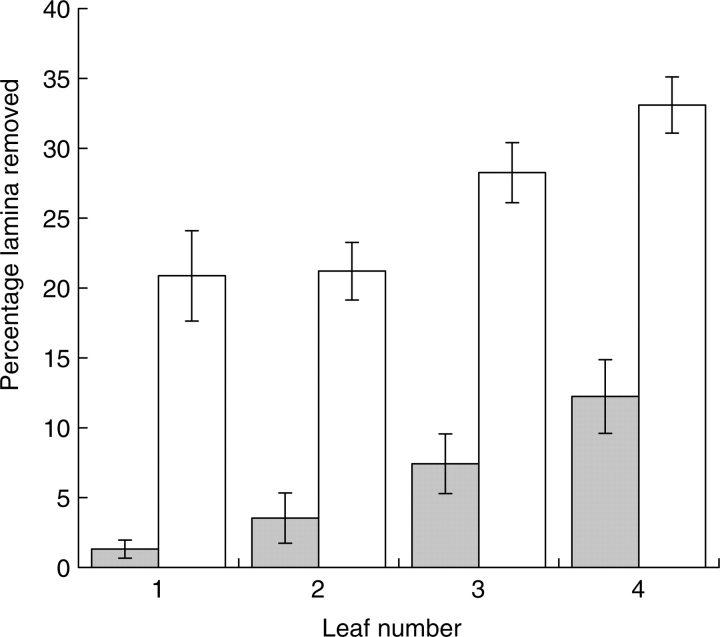
At the Ugandan site there were significant and marked increases in estimated percentage of lamina area missing between the first and fourth leaves for both monocots and dicots (Fig. 2). It was thus possible at this site to test the second hypothesis: that the difference between monocots and nearest-neighbour dicots in the proportion of leaf area lost to herbivorous invertebrates during expansion (i.e. when the laminae are rolled or folded to a late stage) is greater than any difference found once the laminae are fully expanded. Our hypothesis is supported; the losses from the first fully mature leaves were very much smaller for monocots than for dicots (Fig. 2; P < 0·05), while the mean increases between the first and fourth mature leaves (10·5 % monocots, 11·2 % dicots) were not significantly different (P > 0·05).
Fig. 2.
The estimated mean percentage (±s.e.) of lamina area missing from the first four fully expanded and toughened leaves of monocots and nearest-neighbour dicots at the Ugandan site. Leaf 1 is the most recent to become fully toughened.
Losses from palm leaflets and dicot leaves placed on trails of leaf-cutter ants
The mean percentage losses were much smaller for palm leaflets (3·0 ± 1·8 %, s.e.) than for dicot leaves (61 ± 8·0 %; P = 0·00024).
DISCUSSION
Losses of leaf area from monocots and dicots
The hypothesis that monocots would be found to have smaller losses of leaf area than dicots proved to be correct at five out of six study sites. The same trend was found at the sixth site (in Costa Rica), but the difference was not significant. At the Panamanian site, the difference between monocots and dicots was less than at the four Old World sites. Possibly there is a higher incidence in the New World tropics of invertebrates able to overcome the defences of monocots. Certainly significant damage by invertebrates on palms and other monocots has been recorded at our Costa Rican site (Strong, 1977; Braker, 1991; Braker and Chazdon, 1993). However, some cases of severe damage to monocot leaves by invertebrates have been recorded from the Old World tropics too (Cock et al., 1987), and further sampling is needed to test whether or not there is a consistent difference between the Old and New World tropics.
Losses from leaves before and after expansion
The finding that at the majority of sites most losses of lamina area from both monocots and dicots occurred during the expansion phase agrees with the results of earlier work (Coley, 1983; Lowman, 1985; Coley and Barone, 1996; Coley and Kursar, 1996). At the one site where there were appreciable losses after expansion (in Uganda), a useful test of the difference between monocots and dicots in loss of leaf area could be made both during and after expansion. There was no difference after expansion, but a huge difference during the expanding phase (Fig. 2). This result provides a prima facie case for the value of late rolling in protection against herbivorous invertebrates, but other interpretations are possible as is emphasized below.
Interpreting relative losses from monocots and dicots
There was no case that the smaller losses from monocots might be a result of larger amounts of dry mass per unit area, or smaller amounts of water or nitrogen per unit dry mass. On the other hand, recent research has shown that insect attack may be related to the balance between nitrogen and other key nutrients in leaves rather than to the concentration of nitrogen as such (cf. Clissold et al., 2006), and new studies of this issue are needed for plants and insects in TLRF. Although some monocots in TLRF undoubtedly contain significant quantities of secondary metabolites that may deter herbivores, the general trend seems to be for a less marked development in monocots than in dicots (Hegnauer, 1963–86). Thus, there is a strong case for considering the impacts of leaf toughness and leaf rolling or folding.
The much smaller losses from mature palm leaflets than from mature dicot leaves placed on trails of leaf-cutter ants at our Panamanian site is consistent with the palm laminae having greater fracture toughness (appendix 3 in Dominy et al., 2008). On the other hand the fact that at the Ugandan site mature leaves of monocots and dicots suffered the same losses of leaf area raises a problem. If at that site the monocot leaves are tougher than those of the dicots, as found for all four sites where Dominy et al. (2008) made tests (two in Australia and one each Singapore and Panama), then the evidence is against the importance of toughness. There was no palm or pandan at the Ugandan study site, and possibly the monocot leaves were not tougher than the dicot leaves (cf. dicots and non-palm monocots among the shade-tolerators in Panama; Fig. 2 in Dominy et al. 2008). If so, the evidence would support the importance of leaf rolling as a protection.
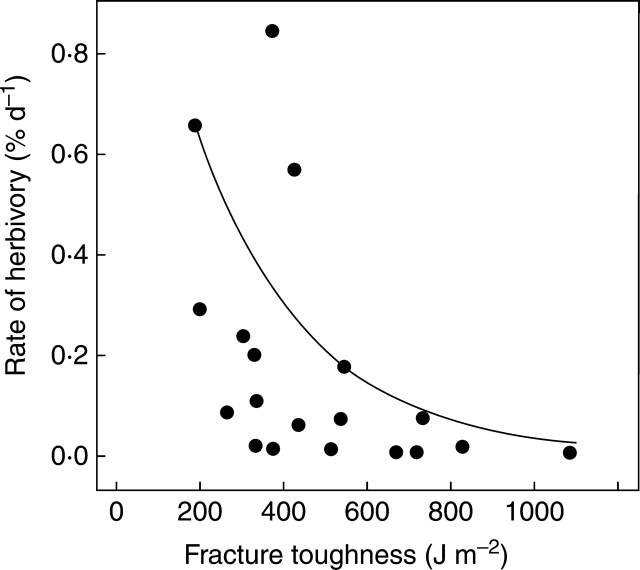
Inspection of the relationship between the rate of loss of leaf area from mature leaves of dicot trees and their punch strength found by Coley (1983) shows that it is one where punch strength appears to set an upper limit. Many species with low punch strength suffer rates of loss far below the limit, presumably because other defences (physical and/or chemical) are effective, or because the populations of the relevant herbivores are kept low by predators, parasitoids, disease or physical factors. Essentially the same relationship is found between rate of loss and fracture toughness, with the maximum rate of loss at 600 J m−2 reduced to about an order of magnitude lower than the maximum at 200 J m−2 (Fig. 3). Our working hypothesis for monocots is that values for fracture toughness above about 600 J m−2 are a strong disincentive to herbivores, and that late-rolling or -folding is likely to have the greater inhibitory effect at values <600 J m−2. Almost all the palms we tested had fracture toughness values >600 J m−2 (Appendix 3 of Dominy et al. 2008). We suggest that the late folding of palms and cyclanths is of value in reducing damage by at least some of the invertebrates which have evolved an ability to attack very tough unfolded laminae, e.g. caterpillars of the Limacodidae on palms (Cock et al., 1987).
Fig. 3.
The relationship between rate of loss of area to invertebrate herbivores and fracture toughness for mature leaves of 19 species of dicot trees in Panama; the line shows the upper 80 % quantile fitted by quantile regression implemented in R. Data on herbivory from Coley (1983) and on fracture toughness from Dominy et al. (2008).
It is difficult to disentangle the parts played by toughness and rolling or folding of leaves. The fact that, at the Ugandan site, there was a big difference in loss of leaf area during leaf expansion but not after expansion suggests the importance of leaf rolling. However, at the Australian site the mean difference in punch strength between the monocots and dicots was much greater during the expansion phase than after full expansion and full toughening: 7× versus 3× (Dominy et al., 2008). If that is also true at the Ugandan site, then the case for the importance of leaf rolling is weakened. It remains possible, though we think unlikely, that changes in concentrations of deterrent chemicals between the expanding and mature stages are dominant in controlling the extent of leaf loss. Only experiments, in which leaves are exposed unrolled at the immature stage, can give conclusive evidence on the importance of rolling. Experiments with chemical extracts in materials of known toughness are required to establish the relative importance of chemical and physical defences of leaves that have been prematurely unrolled.
CONCLUSIONS
Our hypothesis that monocot leaves would be found to lose less leaf area to herbivores than immediately neighbouring dicots was supported unequivocally at four old World sites, but the difference was less marked at one of two New World sites, and not significant at the other. However, in the leaf-cutter ant experiment in Panama losses were markedly smaller for palms than for dicots. Only new work can determine whether or not there is a general difference between Old and New World TLRF in the relative losses from monocots and dicots. It is difficult to determine how far the generally lower losses of leaf area from monocots can be explained by (a) greater toughness and (b) later-lasting folding or rolling, and certainly the problem needs to be approached experimentally. It seems likely that some monocots receive much more protection from folding or rolling than from greater toughness, while the reverse is true of others, and some species receive significant protection from both. Studies on losses to herbivores at all stages of the life cycle of monocots and dicots are needed to follow up the implication that in at least some TLRF lower levels of herbivory on leaves might help to explain the high abundance of monocots relative to dicots in the understorey and among the climbers and epiphytes.
ACKNOWLEDGEMENTS
We thank Glyn Jones for chemical analyses, and William Foster, Julian Hibberd, Jenny Read, Lawren Sack, Mark Westoby and two anonymous referees for constructive criticism of earlier drafts. Our work was supported by the British Ecological Society (D.J.M.), Carlsberg Foundation (J.C.S.), Croucher Foundation (P.W.L.), Danish Natural Science Research Council (J.C.S.), Explorers Club (N.J.D.), Fondo para el Mejoramiento de la Calidad Universitaria, Universidad Nacional de Rosario, Argentina (I.M.B.), Mellon Foundation (P.J.G.), National Geographic Society (P.W.L.), National Institutes of Health (N.J.D.), Research Grants Council of Hong Kong (P.W.L.), Sigma Xi (N.J.D.), Smithsonian Tropical Research Institute (N.J.D.), Tropical Biology Association (P.J.G. and D.J.M.) and University of Cambridge (P.J.G.).
APPENDIX 1
TABLE A1.
Mean estimated percentages of leaf area missing from the first four fully expanded and toughened leaves of focal monocots and nearest-neighbour dicots at six sites in TLRF
| Site and species | Family | Life form | Mean estimated percentage of leaf area missing from monocot | Mean estimated percentage of leaf area missing from nearest-neighbour dicots |
|---|---|---|---|---|
| Australia: low-rainfall site | ||||
| Pothos longipes | Araceae | C | 0 | 4·7 |
| Alpinia modesta | Zingiberaceae | H | 0·25 | 3·5 |
| Pleuranthodium racemigerum* | Zingiberaceae | TH | 0·50 | 11·2 |
| Alocasia brisbanensis | Araceae | TH | 2·0 | 5·1 |
| Alpinia caerulea* | Zingiberaceae | TH | 2·1 | 10·0 |
| Calamus caryotoides | Arecaceae | TC | 2·2 | 10·3 |
| Mean (s.e.m.) | 1·2 (0·4) | 7·5 (1·4) | ||
| Australia: high-rainfall site | ||||
| Freycinetia excelsa | Pandanaceae | TC | 0 | 14·7 |
| Calamus australis | Arecaceae | TC | 1·2 | 32·4 |
| Cordyline cannifolia | Laxmanniaceae | S | 1·5 | 18·9 |
| Alpinia arctiflora* | Zingiberaceae | TH | 2·1 | 14·5 |
| Pothos longipes | Araceae | C | 6·6 | 22·6 |
| Mean (s.e.m.) | 2·3 (0·9) | 20·6 (3·3) | ||
| Singapore | ||||
| Hanguana malayana | Hanguanaceae | H | 0·51 | 6·9 |
| Stachyphrynium griffithii | Marantaceae | TH | 1·0 | 11·0 |
| Schismatoglottis wallichii | Araceae | H | 1·1 | 9·6 |
| Molinieria latifolia | Hypoxidaceae | H | 1·5 | 11·2 |
| Tacca integrifolia | Dioscoreaceae | H | 2·9 | 16·6 |
| Mean (s.e.m.) | 1·4 (0·4) | 11·1 (1·6) | ||
| Uganda | ||||
| Marantochloa leucantha* | Marantaceae | S | 0·29 | 18·8 |
| Aframomum cf. angustifolium | Zingiberaceae | TH | 2·5 | 28·5 |
| Aframomum mildbraedii | Zingiberaceae | H | 3·4 | 19·3 |
| Palisota mannii | Commelinaceae | H | 3·7 | 20·1 |
| Leptaspis zeylanica | Poaceae | H | 4·2 | 26·2 |
| Dracaena fragrans | Ruscaceae | S | 5·1 | 34·7 |
| Dracaena laxissima | Ruscaceae | S | 8·7 | 27·8 |
| Costus sp.* | Costaceae | TH | 9·2 | 23·7 |
| Culcasia falcifolia | Araceae | C | 9·7 | 33·8 |
| Pollia condensata | Commelinaceae | H | 14·5 | 25·7 |
| Mean (s.e.m.) | 6·1 (0·7) | 25·9 (1·8) | ||
| Costa Rica | ||||
| Calathea cleistantha | Marantaceae | H | 2·6 | 6·8 |
| Renealmia pluriplicata | Zingiberaceae | H | 3·1 | 13·9 |
| Welfia regia | Arecaceae | T | 3·5 | 10·8 |
| Asplundia uncinata | Cyclanthaceae | H (+C) | 3·5 | 13·9 |
| Heliconia irrasa | Heliconiaceae | H | 6·5 | 17·8 |
| Geonoma congesta | Arecaceae | S | 13·4 | 12·8 |
| Spathiphyllum fulvovirens | Cyclanthaceae | H | 22·1 | 12·9 |
| Cyclanthus bipartitus | Araceae | TH | 22·2 | 10·2 |
| Mean (s.e.m.) | 9·6 (3·0) | 12·4 (1·1) | ||
| Panama | ||||
| Rhipidocladium racemiflorum | Poaceae | SCR | 0·14 | 6·3 |
| Pleiostachya pruinosa* | Marantaceae | TH | 0·76 | 0·88 |
| Chusquea simpliciflora | Poaceae | SCR | 1·8 | 7·2 |
| Heliconia metallica* | Heliconiaceae | H | 2·8 | 18·7 |
| Pharus latifolius | Poaceae | H | 6·1 | 19·2 |
| Heliconia catheta* | Heliconiaceae | H | 8·2 | 3·5 |
| Calathea latifolia* | Marantaceae | TH | 12·7 | 15·7 |
| Desmoncus orthocanthos | Arecaceae | C | 16·7 | 16·4 |
| Mean (s.e.m.) | 6·2 (2·1) | 11·0 (2·6) |
Life forms: T, tree; S, shrub; TC, tall climber (>20 m); C, climber; SCR, scrambler; TH, tall herb (2–4 m); H, herb (<2 m, generally 1·5 m or shorter).
All species are shade-tolerant except those marked with an asterisk, which need either a single-treefall gap or a greater increase in illumination.
APPENDIX 2
TABLE A2.
Primary data for leaf dry mass per unit area, nitrogen concentration and water concentration
| Family | Dry mass per unit leaf area (mg cm−2) | Nitrogen concentration per unit dry mass (mg g−1) | Water concentration per unit dry mass (g g−1) | |
|---|---|---|---|---|
| Australia: low rainfall site | ||||
| Monocots | ||||
| Alocasia brisbanensis | Araceae | 2·63 | 41·4 | 8·6 |
| Alpinia caerulea | Zingiberaceae | 4·35 | 21·1 | 4·0 |
| Alpinia modesta | Zingiberaceae | 4·00 | 20·6 | 3·2 |
| Calamus caryotoides | Arecaceae | 16·70 | 16·9 | 1·5 |
| Pothos longipes | Araceae | 8·33 | 22·6 | 1·9 |
| Pleuranthodium racemigerum | Zingiberaceae | 4·17 | 20·7 | 4·8 |
| Mean (s.e.) | 6·70 (2·15) | 23·9 (3·6) | 4·0 (1·1) | |
| Dicots | ||||
| Aglaia sapindina | Meliaceae | 5·56 | 1·9 | |
| Castanospora alphandii | Sapindaceae | 7·14 | 23·0 | 1·5 |
| Codiaeum variegatum | Euphorbiaceae | 4·17 | 20·0 | 4·1 |
| Dichapetalum papuanum | Dichapetalaceae | 5·88 | 20·0 | 1·8 |
| Hodgkinsonia frutescens | Rubiaceae | 3·23 | 51·0 | 5·8 |
| Neisosperma poweri | Apocynaceae | 5·00 | 3·3 | |
| Phaleria octandra | Thymelaeaceae | 6·67 | 26·0 | 2·4 |
| Sauropus macranthus | Phyllanthaceae | 3·70 | 29·0 | 2·9 |
| Mean (s.e.) | 5·17 (0·50) | 28·2 (4·8) | 3·0 (0·5) | |
| Australia: low rainfall site | ||||
| Monocots | ||||
| Alpinia arctiflora | Zingiberaceae | 5·00 | 19·9 | 4·0 |
| Calamus australis | Arecaceae | 5·88 | 19·8 | 1·5 |
| Cordyline cannifolia | Laxmanniaceae | 5·26 | 16·9 | 4·1 |
| Freycinetia excelsa | Pandanaceae | 5·26 | 16·6 | 3·4 |
| Mean (s.e.) | 5·35 (0·19) | 18·3 (0·9) | 3·3 (0·6) | |
| Dicots | ||||
| Aglaia australiensis | Meliaceae | 8·3 | 26 | 2·6 |
| Aglaia tomentosa | Meliaceae | 5·0 | 32 | 2·2 |
| Apodytes brevistylis | Icacinaceae | 5·0 | 18 | 3·5 |
| Argyrodendron peralatum | Malvaceae | 11·1 | 18 | 1·1 |
| Argyrodendron trifoliolatum | Malvaceae | 8·6 | 17 | 1·3 |
| Austromuellera trinervia | Proteaceae | 6·8 | 11 | 1·4 |
| Beilschmiedia brunnea | Lauraceae | 5·7 | 27 | 2·2 |
| Beilschmiedia tooram | Lauraceae | 10·3 | 14 | 1·2 |
| Bubbia semecarpoides | Winteraceae | 10·3 | 17 | 2·5 |
| Cardwellia sublimis | Proteaceae | 6·7 | 9·2 | 2·0 |
| Castanospora alphandii | Sapindaceae | 6·9 | 20 | 1·7 |
| Cryptocarya angulata | Lauraceae | 6·2 | 18 | 1·5 |
| Cryptocarya grandis | Lauraceae | 5·8 | 18 | 1·8 |
| Cryptocarya mackinnoniana | Lauraceae | 8·5 | 14 | 1·4 |
| Cryptocarya melanocarpa | Lauraceae | 4·9 | 20 | 1·5 |
| Cryptocarya pleurosperma | Lauraceae | 7·9 | 14 | 1·9 |
| Cupaniopsis flagelliformis | Sapindaceae | 5·7 | 24 | 1·4 |
| Daphnandra repandula | Atherospermataceae | 4·4 | 32 | 3·4 |
| Darlingia ferruginea | Proteaceae | 6·3 | 13 | 2·5 |
| Diploglottis bracteata | Sapindaceae | 6·8 | 17 | 1·7 |
| Doryphora aromatica | Atherospermataceae | 5·7 | 28 | 2·5 |
| Endiandra bessaphila | Lauraceae | 7·4 | 16 | 2·2 |
| Endiandra monothyra | Lauraceae | 8·3 | 19 | 1·3 |
| Flindersia bourjotiana | Rutaceae | 11·6 | 9·4 | 1·8 |
| Franciscodendron laurifolium | Malvaceae | 6·3 | 14 | 1·9 |
| Garcinia gibbsiae | Clusiaceae | 9·2 | 13 | 2·8 |
| Gillbeea adenopetala | Cunoniaceae | 6·0 | 14 | 2·3 |
| Goniothalamus australis | Annonaceae | 4·8 | 25 | 2·5 |
| Guioa lasioneura | Sapindaceae | 7·0 | 16 | 1·4 |
| Hypsophila dielsiana | Celastraceae | 9·7 | 20 | 3·0 |
| Litsea leefeana | Lauraceae | 6·0 | 16 | 2·2 |
| Melicope vitiflora | Rutaceae | 3·2 | 42 | 6·4 |
| Myristica globosa ssp. muelleri | Myristicaceae | 6·4 | 17 | 2·6 |
| Neolitsea dealbata | Lauraceae | 5·9 | 15 | 1·1 |
| Opisthiolepis heterophylla | Proteaceae | 7·8 | 15 | 2·3 |
| Pouteria castanosperma | Sapotaceae | 4·5 | 37 | 2·3 |
| Prunus turneriana | Rosaceae | 5·5 | 16 | 1·5 |
| Sloanea australis | Elaeocarpaceae | 5·9 | 21 | 1·8 |
| Synima macrophylla | Sapindaceae | 9·7 | 22 | 1·7 |
| Syzygium cormiflorum | Myrtaceae | 8·5 | 13 | 1·6 |
| Syzygium cryptophlebium | Myrtaceae | 4·8 | 15 | 1·4 |
| Mean (s.e.) | 7·0 (0·31) | 19·1 (1·1) | 2·1 (0·14) | |
| Singapore | ||||
| Monocots | ||||
| Hanguana malayana | Hanguanaceae | 16·0 | ||
| Moliniera latifolia | Hypoxidaceae | 22·1 | ||
| Schismatoglottis wallichii | Araceae | 32·3 | ||
| Stachyphrynium griffithii | Marantaceae | 31·3 | ||
| Tacca integrifolia | Dioscoreaceae | 26·8 | ||
| Mean (s.e.) | 25·7 (3·0) | |||
| Dicots | ||||
| Baccaurea parviflora | Phyllanthaceae | 12·6 | ||
| Calophyllum ferrugineum | Clusiaceae | 11·3 | ||
| Canarium patentinervium | Burseraceae | 15·2 | ||
| Cinnamomum iners | Lauraceae | 20·9 | ||
| Cyathocalyx ridleyi | Annonaceae | 21·2 | ||
| Diospyros buxifolia | Ebenaceae | 18·2 | ||
| Ficus fistulosa | Moraceae | 22·0 | ||
| Ficus grossularioides | Moraceae | 23·2 | ||
| Gluta wallichii | Anacardiaceae | 18·2 | ||
| Hopea griffithii | Dipterocarpaceae | 16·8 | ||
| Knema communis | Myristicaceae | 16·7 | ||
| Pternandra echinata | Melastomataceae | 15·4 | ||
| Rhodamnia cinerea | Myrtaceae | 12·6 | ||
| Shorea curtisii | Dipterocarpaceae | 17·2 | ||
| Urophyllum hirsutum | Rubiaceae | 26·4 | ||
| Mean (s.e.) | 17·9 (1·2) | |||
| Uganda | ||||
| Monocots | ||||
| Aframomum angustifolium | Zingiberaceae | 3·33 | 20·4 | 3·1 |
| Dracaena laxissima | Ruscaceae | 4·50 | 27·7 | 3·2 |
| Leptaspis zeylanica | Poaceae | 3·50 | 22·6 | 1·7 |
| Marantochloa leucantha | Marantaceae | 24·8 | ||
| Palisota mannii | Commelinaceae | 4·61 | 34·5 | 8·3 |
| Pollia condensata | Commelinaceae | 3·44 | 27·6 | 4·6 |
| Mean (s.e.) | 3·88 (0·28) | 26·3 (2·0) | 4·2 (1·1) | |
| Dicots | ||||
| Aningeria altissima | Sapotaceae | 4·85 | 23·2 | 1·6 |
| Cassipourea ruwenzorensis | Rhizophoraceae | 6·54 | 27·2 | 1·5 |
| Chrysophyllum gorungosanum | Sapotaceae | 4·85 | 23·5 | 1·6 |
| Citropsis articulata | Rutaceae | 8·85 | 22·8 | 1·7 |
| Clausena anisata | Rutaceae | 3·24 | 22·3 | 2·5 |
| Diospyros abyssinica | Ebenaceae | 6·99 | 26·7 | 1·3 |
| Ficus asperifolia | Moraceae | 4·65 | 27·2 | 2·2 |
| Monodora myristica | Annonaceae | 3·83 | 20·9 | 2·8 |
| Myrianthus arboreus | Urticaceae | 4·98 | 25·1 | 2·4 |
| Newtonia buchananii | Fabaceae | 3·37 | 29·8 | 1·5 |
| Piper capense | Piperaceae | 2·92 | 37·0 | 5·4 |
| Psychotria lauracea | Rubiaceae | 5·21 | 23·5 | 4·0 |
| Symphonia globulifera | Clusiaceae | 5·10 | 15·6 | 2·7 |
| Tabernaemontana pachysiphon | Apocynaceae | 4·59 | 37·9 | 3·9 |
| Tarenna pavettoides | Rubiaceae | 4·41 | 32·6 | 2·9 |
| Teclea nobilis | Rutaceae | 7·75 | 28·9 | 1·5 |
| Trichilia dregeana | Meliaceae | 5·99 | 19·1 | 2·2 |
| Uvariopsis congolana | Annonaceae | 5·03 | 27·2 | 2·3 |
| Mean (s.e.) | 5·18 (0·37) | 26·1 (1·4) | 2·4 (0·25) | |
| Costa Rica | ||||
| Monocots | ||||
| Asplundia uncinata | Cyclanthaceae | 5·92 | 15·3 | 3·7 |
| Calathea cleistantha | Marantaceae | 2·65 | 24·8 | 5·1 |
| Cyclanthus bipartitus | Cyclanthaceae | 4·88 | 22·7 | 3·1 |
| Geonoma congesta | Arecaceae | 7·09 | 12·7 | 1·7 |
| Heliconia irrasa | Heliconiaceae | 3·60 | 20·1 | 3·7 |
| Renealmia pluriplicata | Zingiberaceae | 3·82 | 15·2 | 4·0 |
| Spathiphyllum fulvovirens | Araceae | 4·69 | 31·8 | 4·1 |
| Welfia regia | Arecaceae | 6·21 | 13·2 | 1·6 |
| Mean (s.e.) | 4·86 (0·53) | 19·5 (2·4) | 3·4 (0·43) | |
| Dicots | ||||
| Anaxagorea crassipetala | Annonaceae | 4·10 | 20·7 | 3·1 |
| Aphelandra storkii | Acanthaceae | 2·49 | 25·2 | 4·4 |
| Dichapetalum axillare | Dichapetalaceae | 3·88 | 18·0 | 2·4 |
| Dichapetalum nervatum | Dichapetalaceae | 6·25 | 27·9 | 2·1 |
| Forsteronia myriantha | Apocynaceae | 3·23 | 19·9 | 3·2 |
| Ocotea cernua | Lauraceae | 4·90 | 30·9 | 1·9 |
| Paullinia grandifolia | Sapindaceae | 4·81 | 26·5 | 2·0 |
| Pentaclethra macroloba | Fabaceae | 3·82 | 25·3 | 1·7 |
| Perebea angustifolia | Moraceae | 3·46 | 20·0 | 2·6 |
| Piper holdridgeianum | Piperaceae | 2·40 | 39·0 | 6·9 |
| Piper trigonum | Piperaceae | 2·82 | 39·3 | 6·1 |
| Psychotria buchtienii | Rubiaceae | 3·73 | 31·7 | 2·7 |
| Psychotria surensis | Rubiaceae | 3·61 | 27·9 | 2·7 |
| Siparuna thecaphora | Siparunaceae | 2·99 | 34·8 | 5·3 |
| Swartzia sp. A | Fabaceae | 3·66 | 38·6 | 2·9 |
| Trophis involucrata | Moraceae | 4·63 | 21·7 | 1·8 |
| Virola sebifera | Myristicaceae | 3·44 | 14·6 | 3·2 |
| Mean (s.e.) | 3·78 (0·23) | 27·2 (1·9) | 3·2 (0·37) | |
| Panama | ||||
| Monocots | ||||
| Chusquea simpliciflora | Poaceae | 4·74 | 1·2 | |
| Heliconia catheta | Heliconiaceae | 3·51 | 3·5 | |
| Pharus latifolius | Poaceae | 5·18 | 1·1 | |
| Pleiostachya pruinosa | Marantaceae | 4·76 | 2·3 | |
| Rhipidocladium racemiflorum | Poaceae | 3·50 | 1·0 | |
| Mean (s.e.) | 4·34 (0·35) | 1·8 (0·48) | ||
| Dicots | ||||
| Unidentified nearest-neighbour dicots (n =15), mean (s.e.) | 5·16 (0·49) | 2·4 (0·45) |
LITERATURE CITED
- Braker E. Natural-history of a neotropical gap-inhabiting grasshopper. Biotropica. 1991;23:41–50. [Google Scholar]
- Braker E, Chazdon RL. Ecological, behavioural and nutritional factors influencing use of palms as host plants by a neotropical grasshopper. Journal of Tropical Ecology. 1993;9:183–197. [Google Scholar]
- Clissold FJ, Sanson GD, Read J. The paradoxical effects of nutrient ratios and supply rates on an outbreaking insect herbivore, the Australian plague locust. Journal of Animal Ecology. 2006;75:1000–1013. doi: 10.1111/j.1365-2656.2006.01122.x. [DOI] [PubMed] [Google Scholar]
- Cock MJW, Godfray HCJ, Holloway JD. Slug and nettle caterpillars: the biology, taxonomy and control of the Limacodidae of economic importance on palms in South-east Asia. Wallingford, UK: Commonwealth Agricultural Bureaux; 1987. [Google Scholar]
- Coley PD. Herbivory and defensive characteristics of tree species in a lowland rain forest. Ecological Monographs. 1983;53:209–233. [Google Scholar]
- Coley PD, Barone JA. Herbivory and plant defence in tropical forests. Annual Review of Ecology and Systematics. 1996;27:305–335. [Google Scholar]
- Coley PD, Kursar TA. Anti-herbivore defenses of young tropical leaves: physiological constraints and ecological trade-offs. In: Mulkey SS, Chazdon RL, Smith AP, editors. Tropical forest plant ecophysiology. New York, NY: Chapman & Hall; 1996. pp. 305–336. [Google Scholar]
- Dominy NJ, Grubb PJ, Jackson RV, Lucas PW, Metcalfe DJ, Svenning J-C, Turner IM. In tropical lowland rain forests monocots have tougher leaves than dicots, and include a new kind of tough leaf. Annals of Botany. 2008;101:1363–1377. doi: 10.1093/aob/mcn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb PJ, Jackson RV. The adaptive value of young leaves being tightly folded or rolled on monocotyledons in tropical lowland rain forest: an hypothesis in two parts. Plant Ecology. 2007;192:317–327. [Google Scholar]
- Grubb PJ, Turner IM, Burslem DFRP. Mineral nutrient status of coastal hill dipterocarp forest and adinandra belukar in Singapore: analysis of soil, leaves and litter. Journal of Tropical Ecology. 1994;10:559–577. [Google Scholar]
- Hegnauer R. Chemotaxonomie der Pflanzen. Vols 2–7. Basel: Birkhäuser; 1963. –86. [Google Scholar]
- Jackson RV. Insect herbivory on tropical Alphitonia (Rhamnaceae) species. Townsville, Queensland, Australia: James Cook University; 1995. PhD Thesis. [Google Scholar]
- Jackson RV, Kollmann J, Grubb PJ, Bee JN. Insect herbivory on European tall-shrub species: the need to distinguish leaves before and after unfolding or unrolling, and the advantage of longitudinal sampling. Oikos. 1999;87:561–570. [Google Scholar]
- King MJ, Vincent JFV, Harris W. Curling and folding of leaves of monocotyledons – a strategy for structural stiffness. New Zealand Journal of Botany. 1996;34:411–416. [Google Scholar]
- Lowman MD. Temporal and spatial variability in insect grazing of the canopy of 5 Australian rainforest tree species. Australian Journal of Ecology. 1985;10:7–24. [Google Scholar]
- Lucas PW, Darvell BW, Lee PKD, Tuen TDB, Choong MF. The toughness of plant-cell walls. Philosophical Transactions of the Royal Society. 1995;B 348:363–372. [Google Scholar]
- Lucas PW, Turner IM, Dominy NJ, Yamashita N. Mechanical defences to herbivory. Annals of Botany. 2000;86:913–920. [Google Scholar]
- Metcalfe DJ, Grubb PJ, Turner IM. The ecology of very small-seeded shade-tolerant trees and shrubs in lowland rain forest in Singapore. Plant Ecology. 1998;134:131–149. [Google Scholar]
- Read J, Edwards C, Sanson GD, Aranwela N. Relationships between sclerophylly, leaf biomechanical properties and leaf anatomy in some Australian heath and forest species. Plant Biosystems. 2000;134:261–277. [Google Scholar]
- Stevens PF. Angiosperm Phylogeny Website. 2007. Version 6, May 2006 (updated continually), www.mobot.org/MOBOT/research/APweb/ . Accessed 2 April 2007.
- Strong DR. Rolled-leaf hispine beetles (Chrysomelidae) and Zingiberales host plants in Middle America. Biotropica. 1977;9:156–159. [Google Scholar]
- Walter H. The ecology of tropical and subtropical vegetation. Edinburgh: Oliver & Boyd; 1971. [Google Scholar]