Abstract
Background and Aims
The herbicide quinclorac has been reported to inhibit incorporation of glucose both into cellulose and other cell wall polysaccharides. However, further work has failed to detect any apparent effect of this herbicide on the synthesis of the wall. In order to elucidate whether quinclorac elicits the inhibition of cellulose biosynthesis directly, in this study bean cell calli were habituated to grow on lethal concentrations of the herbicide and the modifications in cell wall composition due to the habituation process were analysed.
Methods
Fourier transform infrared spectroscopy associated with multivariate analysis, cell wall fractionation techniques, biochemical analyses and the immunolocation of different cell wall components with specific monoclonal antibodies were used to characterize the cell walls of quinclorac-habituated cells.
Key Results
Quinclorac-habituated cells were more irregularly shaped than non-habituated cells and they accumulated an extracellular material, which was more abundant as the level of habituation rose. Habituated cells did not show any decrease in cellulose content, but cell wall fractionation revealed that changes occurred in the distribution and post-depositional modifications of homogalacturonan and rhamnogalacturonan I during the habituation process. Therefore, since the action of quinclorac on the cell wall does not seem to be due to a direct inhibition of any cell wall component, it is suggested that the effect of quinclorac on the cell wall could be due to a side-effect of the herbicide.
Conclusions
Long-term modifications of the cell wall caused by the habituation of bean cell cultures to quinclorac did not resemble those of bean cells habituated to the well-known cellulose biosynthesis inhibitors dichlobenil or isoxaben. Quinclorac does not seem to act primarily as an inhibitor of cellulose biosynthesis.
Key words: Quinclorac, herbicide, Phaseolus vulgaris, cell culture habituation, primary cell wall, cellulose, FTIR spectroscopy
INTRODUCTION
Quinclorac (3,7-dichloro-8-quinolinecarboxylic acid) is a quinolinecarboxylic auxin herbicide used in crops of rice, barley, sorghum, etc. in order to control mono- and dicotyledonous weeds (Grossmann, 2000). However, the basis of its selectivity remains unknown and there is controversy concerning the mode action of quinclorac. In sensitive species, the compound induces the 1-aminocyclopropane-1-carboxylic (ACC) synthase enzyme in the ethylene biosynthetic pathway, which activates epinastic growth and triggers the synthesis of abscisic acid (ABA), which causes stomatal closure (Hansen and Grossmann, 2000). This closure limits photosynthetic activity and biomass production, leading to an over-production of reactive oxygen species and causing tissue damage and cell death (Grossmann et al., 2001). In addition, the higher production of ACC causes an accumulation of cyanide, a co-product of ethylene synthesis during the oxidation of ACC by ACC oxidase (Grossmann and Kwiatkowski, 2000).
Previous studies have shown that quinclorac inhibits, in a dose- and time-dependent manner, the incorporation of glucose into the cell wall, specifically in cellulose, glucuronoarabinoxylans and mixed-glucan in maize roots (Koo et al., 1996) and other sensitive monocots (Koo et al., 1997), suggesting that quinclorac should be included among the cellulose biosynthesis inhibitors, together with dichlobenil (2,6-dichlorobenzonitrile) and isoxaben (N-3[-(1-ethyl-1-methylpropyl)-5-isoxazolyl]-2,6-dimethoxybenzamide). However, subsequent studies revealed that 24-h exposure to quinclorac does not modify cellulose synthesis in maize or barnyard grass Echinochloa crus-galli (Tresch and Grossman, 2003). Nonetheless, in the latter work the authors based their conclusions on a non-quantitative immnunocytochemical assay that makes it difficult to detect possible faint decreases in cellulose contents.
It is also likely that the effect of quinclorac on the cell wall would be a secondary consequence of its auxin activity, in a similar way to that of 2,4-D (2,4-dichlorophenoxyacetic acid), which modifies the expression of genes related to cell wall metabolism (Raghavan et al., 2005). In addition, most of the studies have been carried out with type-II cell wall plants, such as monocots, and hence the effect of the herbicide on type-I cell walls has not been evaluated. All the above demonstrates that the mode of action of quinclorac remains to be elucidated.
The characterization of cell cultures habituated to grow under lethal concentrations of a herbicide has been very useful in the study of the long-term effects of exposure to cellulose biosynthesis inhibitors (i.e. isoxaben or dichlobenil), and has provided a better understanding of their mode of action. Globally, cell cultures habituated to dichlobenil (Shedletzky et al., 1990, 1992; Wells et al., 1994; Nakagawa and Sakurai, 1998, 2001; Sabba et al., 1999; Encina et al., 2001, 2002; Alonso-Simón et al., 2004; García-Angulo et al., 2006) or to isoxaben (Díaz-Cacho et al., 1999; Sabba and Vaughn, 1999; Manfield et al., 2004) show a common pattern of cell wall modifications, with a noticeable reduction of cellulose and hemicellulose contents and an enrichment in pectins. These results suggest the replacement, at least partially, of the cellulose–hemicellulose network by a more extensive and cross-linked pectin network.
Accordingly, the habituation of cell cultures to lethal concentrations of quinclorac and their comparison with cells habituated to other previously characterized cellulose biosynthesis inhibitors should allow us to gain further insight into the architecture of quinclorac-habituated cell walls and the long-term effects of this herbicide.
Here, for the first time, we have habituated cultured cells to grow under lethal concentrations of quinclorac. Bean calli were used as a model in order to compare the modifications related to quinclorac habituation with those caused by dichlobenil (Encina et al., 2001) and isoxaben (Díaz-Cacho et al., 1999) habituation in an attempt to broaden our knowledge about the action of quinclorac, in particular as regards cell wall modifications.
MATERIAL AND METHODS
Plant material
Bean (Phaseolus vulgaris L. ‘Canellini’) calli were obtained and subcultured as described by Encina et al. (2001) in Murashige and Skoog (Murashige and Skoog, 1962) solid growth medium supplemented with sucrose (30 g L−1), 10 µm 2,4-D (2,4-dichlorophenoxyacetic acid) and agar (8 g L−1). The manipulation of cell cultures was always performed on a clean bench and all instruments and growth media used were sterilized by dry-heating or autoclave.
Quinclorac toxicity in callus cells
The toxicity of quinclorac was evaluated on the basis of callus dry weight (DW) gain, and was expressed depending on the I50 value, which is defined as the herbicide concentration that inhibits DW gain by 50 % in comparison with untreated cells. I10 and I90 values were also used and were defined by analogy with I50.
Quinclorac was dissolved in dimethylsulfoxide (DMSO), and its highest concentration was never sufficiently high to affect the cultured cells (data not shown).
Quinclorac habituation
Calli were subcultured in increasing concentrations of quinclorac, starting at a value equal to I50 (10 µm), until cells that were able to grow on lethal quinclorac concentrations were obtained. Habituated cells were denoted as Qnm, where n indicates the herbicide concentration expressed in μm, and m refers to the number of subcultures in which the cells had been growing in such a quinclorac concentration. The highest concentration reached in the habituation process was 30 µm (Q30).
All different cells lines (non-habituated and habituated to different quinclorac concentrations) were regularly subcultured every 30 d.
Cell wall extraction
Callus cells were homogenized in liquid nitrogen with a mortar and pestle, washed twice with cold 100 mm potassium phosphate buffer, pH 7·0, and treated with type VI α-amylase from hog pancreas (Sigma) for 4 h at 37 °C. The suspension was centrifuged and the pellet was washed with distilled water (×3). The resulting pellet was washed with acetone (×3), methanol : chloroform (1 : 1; v/v; ×3), diethylether (×2) and air-dried (Talmadge et al., 1973).
Cell wall fractionation
Cell wall fractionation was a modification of the technique described by Selvendran and O'Neill (1987). Dry cell walls were extracted at room temperature with 50 mm cyclohexane-trans-1,2-diamine-N,N,N′,N′-tetraacetic acid sodium salt (CDTA) at pH 6·5 for 8 h (×2) and washed with distilled water. Then, 50 mm Na2CO3 plus 20 mm NaBH4 was added and the mixture was incubated at room temperature for 18 h and washed with distilled water. The extracts were combined with their respective washings, dialysed and lyophilized. Pectic fractions were referred to as CDTA and carbonate, respectively. After carbonate extraction the residue was incubated for 18 h in 4 m KOH containing 20 mm NaBH4 and washed with distilled water, in order to extract hemicelluloses. The extract was combined with its washing, neutralized with acetic acid, dialysed and lyophilized. The hemicellulosic fraction was referred to as the KOH fraction. The residue after KOH extraction was suspended in water and adjusted to pH 5·0 with acetic acid. After centrifugation, the supernatant was filtered and lyophilized; this was referred to as the supernatant-cellulose residue (SnCR) fraction. The residue was hydrolysed for 2·5 h at 121 °C with 2 m trifluoroacetic acid (TFA) and, after centrifugation, the supernatant was collected and referred to as the TFA fraction.
Cell wall analyses
Cellulose amounts in cell walls were quantified with the Updegraff (1969) method under the hydrolytic conditions described by Saeman et al. (1963). Released sugars were determined using the anthrone assay (Dische, 1962) and are expressed as glucose equivalents. Total sugars were determined with the phenol–sulphuric acid assay (Dubois et al., 1956) and uronic acids with the m-hydroxybiphenyl assay (Blumenkrantz and Asboe-Hansen, 1973), using glucose and galacturonic acid as standards, respectively.
Xyloglucan (XyG) contents were determined with the Kooiman assay (Kooiman, 1960), using bean xyloglucan as standard (Encina et al., 2001).
Neutral sugar analysis was performed as described by Albersheim et al. (1967). Lyophilized samples of each fraction were hydrolysed with 2 m trifluoroacetic acid (TFA) at 121°C for 1 h and the resulting sugars were derivatized to alditol acetates and analysed by gas chromatography using a Supelco SP-2330 column.
Microscopy
Cells were fixed in 2·5 % (w/v) paraformaldehyde in 0·1 m phosphate buffer, pH 7·5, at 4 °C overnight. After washing with phosphate buffer (PBS), cells were dehydrated in an ethanol series, then placed in gelatine capsules containing resin (LR White, London Resin, Reading, UK) and allowed to polymerize at 37 °C for 5 d. Sections of 1 µm thickness were obtained on an Ultracut LKB 2088 microtome (Reichart-Jung, Austria) and applied to multi-well slides (ICN Biomedicals, Cleveland, OH, USA) coated with Vectabond reagent (Vector Laboratories, Burlingame, CA, USA). Sections were incubated for 2 h with 5 % milk powder in PBS (MPBS) for blocking, and then in MPBS containing the primary antibody at a 1/10 dilution. After exhaustive washes with PBS, the sections were incubated in darkness for 2 h with a 1/100 dilution of an anti-rat immunoglobulin G linked to fluorescein isothiocyanate (FITC; Sigma) in MPBS at room temperature. Finally, the sections were washed with PBS and mounted in a glycerol/PBS-based antifade solution (Citifluor AF1; Agar Scientific, London, UK). Cellulose was localized in sections using 0·005 % (w/v) calcofluor white (fluorescent brightener 28, Sigma). The sections were observed under an Olympus BH-2 microscope equipped with epifluorescence light and LP470 filters (360–460 nm) for calcofluor and LP520 (450–490 nm) for FITC.
FTIR spectroscopy
Tablets for Fourier transform infrared spectroscopy (FTIR) were prepared in a Graseby-Specac Press, using cell walls (2 mg) mixed with KBr (1 : 100 w/w). Ten spectra were obtained from each tablet on a Perkin-Elmer instrument at a resolution of 1 cm−1 and the average spectrum was used thereafter. A window between 800 and 1800 cm−1, which contains information of characteristic polysaccharides, was selected in order to monitor cell wall structure modifications. All spectra were normalized and baseline-corrected with the Perkin-Elmer Spectra v. 5·3. software. The data were then exported to Microsoft Excel 2000 and all spectra were area-normalized.
Data analysis
Cluster analysis of FTIR spectra was performed using the Ward method, and the Pearson coefficient was selected as a distance measurement. Further principal component analysis (PCA) was performed using a maximum of five principal components.
In order to compare the cellulose amount between cell lines, a one-way ANOVA followed by the Tukey test was used after the data had been tested for normality. The variance analysis was accomplished taking as the cellulose content for non-habituated (Nh) cells the average value obtained from all the Nh cell lines tested (Nh88 to Nh133). The level of significance was tested at P < 0·05.
When indicated, a Student's t-test (P <0·05) was used to test differences between means. Data are expressed as mean ± s.d. All analyses were carried out using the Statistica software package.
RESULTS
Effect of quinclorac on callus growth
The effect of increasing concentrations of quinclorac on dry weight gain after 30 d of culture of non-habituated calli was evaluated. The growth of non-habituated cells was progressively decreased by quinclorac concentrations equal or greater than 5 µm; the I50 was 10 µm and concentrations higher than 20 µm inhibited DW gain by more than 90 % (data not shown).
Non-habituated cells were repeatedly subcultured in increasing concentrations of quinclorac, starting at 10 µm. Cells were grown for at least 10 subcultures of 30 d in a given concentration of the herbicide prior to incrementing that concentration up to 30 µm quinclorac. The growth rate of the habituated calli decreased progressively as the herbicide concentration was increased, being reduced by 50 % in Q30 cells as compared to non-habituated calli (Table 1). The DW/FW ratio tended to increase during the habituation process, while the cell wall DW/cell DW ratio did not vary significantly among samples.
Table 1.
Relative growth rate, DW/FW and cell wall DW/cell DW ratios of non-habituated calli (Nh) and calli habituated to different quinclorac concentrations (Q). The subscript indicates the herbicide concentration in μm and the superscript indicates the number of subcultures for which the cells were subjected to that quinclorac concentration. The growth rate and the other ratios were determined after 30 d of subculture
| Cell type | Growth rate (month−1)† | DW/FW | Cell wall DW/cell DW |
|---|---|---|---|
| Nh126 | 0·90 ± 0·19 | 0·045 ± 0·003 | 0·25 ± 0·06 |
| Q1044 | 0·72 ± 0·15* | 0·035 ± 0·002* | 0·23 ± 0·04 |
| Q1544 | 0·59 ± 0·10* | 0·044 ± 0·001 | 0·21 ± 0·01 |
| Q2024 | 0·69 ± 0·18* | 0·055 ± 0·008* | 0·24 ± 0·01 |
| Q3010 | 0·47 ± 0·15* | 0·050 ± 0·003* | 0·25 ± 0·03 |
Values represent means of at least five measurements ± s.d.
†Relative growth rate was determined by the formula (FWf–FWi)/FWi, where FWi is the initial fresh weight, and FWf is the final fresh weight of the cells.
*Significant difference with respect to non-habituated cells as evaluated by Student's t-test (P < 0·05).
Morphological characteristics of non-habituated and quinclorac-habituated cells
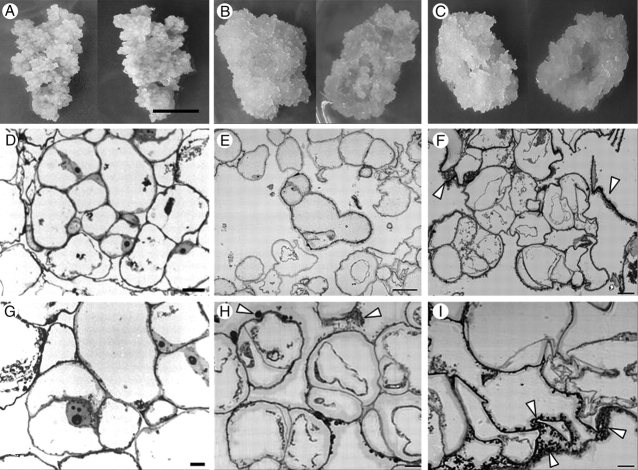
Quinclorac-habituated calli had a similar colour and texture to those of non-habituated calli (Fig. 1). However, they grew to form hollow protuberances that seemed to repel the surface of the medium. Several differences were observed by means of light microscopy. Although cells habituated to lower concentrations of quinclorac (10 and 15 µm) were similar to non-habituated cells, showing an isodiametric shape, as the quinclorac concentration increased (20 and 30 µm), cells became more irregular shaped. Most of the habituated cell types accumulated some extracellular material (arrowheads in Fig. 1), this being more abundant in cultures with higher levels of habituation.
Fig. 1.
(A, D, G) Non-habituated, (B, E, H) Q1044 and (C, F, I) Q3010 cells (see Table 1 for notation of calli). (A–C) Callus cells, from above (right) and below (left); scale bar in (A) = 1 cm. (D–F) Sections observed by light microscopy at low magnification and (G–I) at high magnification; scale bars: (D, E, G) = 50 µm, (F) = 30 µm and (H, I) = 10 µm. Arrowheads show the extracellular material found in habituated cells.
FTIR spectroscopy and cellulose content monitoring
Modifications in the cell wall composition and structure were preliminarily characterized with FTIR spectroscopy associated with multivariate analyses. FTIR spectra of cell walls were obtained from non-habituated cells and cells habituated to a range of quinclorac concentrations, and with a different number of subcultures in such concentrations. The FTIR spectra apparently did not reveal noticeable differences between cell walls from non-habituated and habituated cells (data not shown). Nevertheless, the cluster analysis clearly separated non-habituated and habituated spectra into two different groups (Fig. 2A). When principal component analysis was applied, the same two groups were found, separated along PC1, which absorbed 42·3 % of the total variance (Fig. 2B). The loading factors for PC1 (Fig. 2C) showed a positive correlation with peaks corresponding to pectins at 830 (Kačuraková et al., 2002), 1014 (Coimbra et al., 1999), 1070 (Kačuraková et al., 2000), 1104 (Coimbra et al., 1999) and 1240 cm−1 (Kačuraková et al., 2000). This indicates that the spectra located on the positive side of PC1 belonged to cell walls with a higher pectin content than those on the negative side of the same PC. In addition, some negative peaks in wave numbers related to proteins also appeared at 1550 and 1650 cm−1 (Séné et al., 1994), pointing to a higher protein level in the cell walls whose spectra were located on the negative side of PC1. Thus, globally, PCA applied to FTIR cell wall spectra indicated that habituated cells had a higher content of proteins and lower amounts of pectin in their cell walls than non-habituated cells.
Fig. 2.
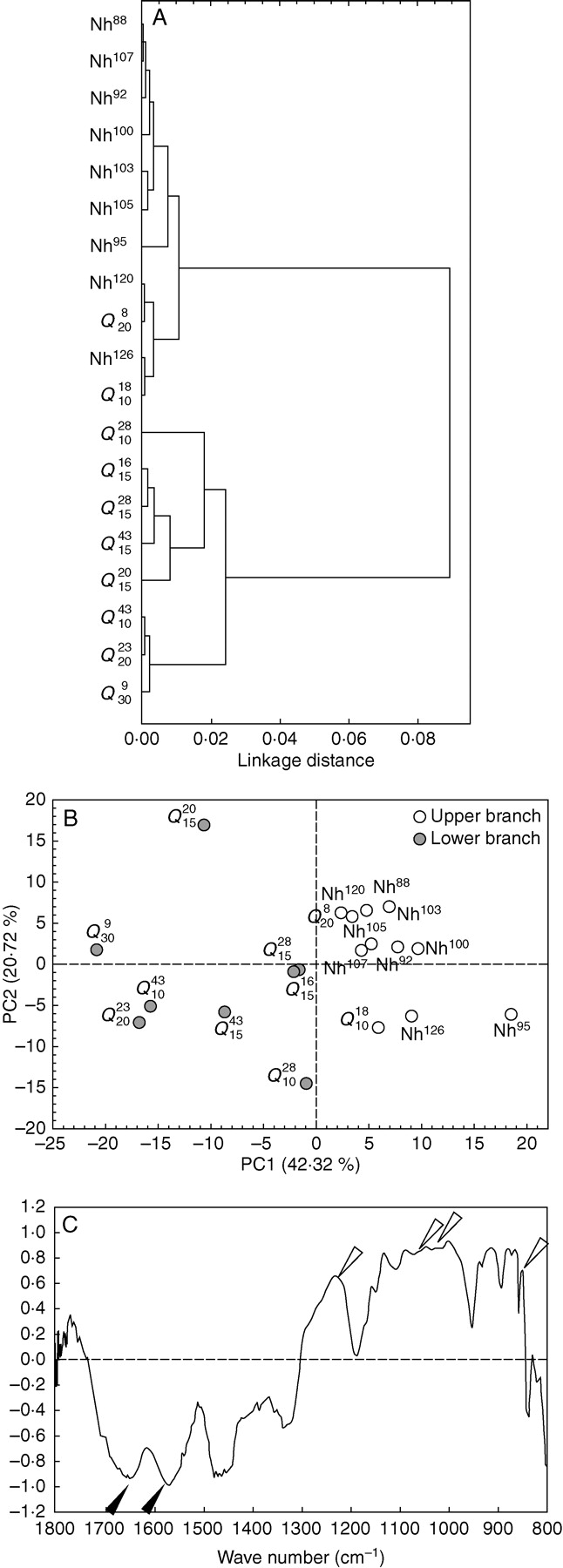
Multivariate analyses performed on FTIR spectra from non-habituated and quinclorac-habituated cell walls. (A) Dendrogram obtained by cluster analysis. (B) Representation of PC1 and PC2 from principal component analysis; spectra from the upper and lower branches of the dendrogram from cluster analysis in (A) are indicated. (C) Loading factors for PC1 of PCA represented in (B); open arrowheads show wave numbers related to pectins, solid arrowheads show those related to proteins.
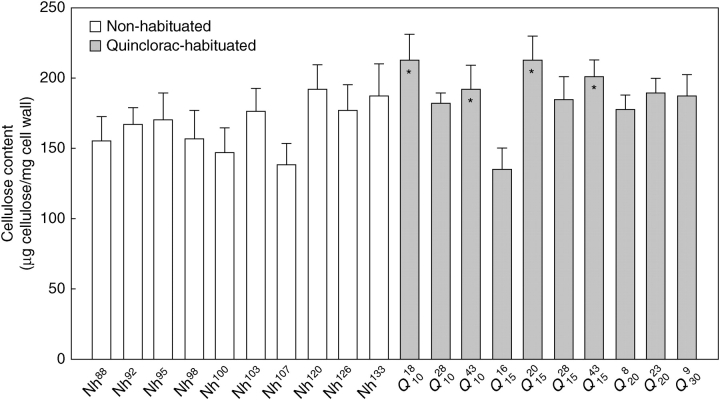
When cellulose was quantified with the Updegraff method, the ANOVA analysis did reveal significant differences between the cellulose contents of the cell lines tested (F = 12·63; P < 0·005; Fig. 3). However, no trends in changes in cellulose content along the habituation process were found. Because the cellulose content varied greatly within non-habituated and habituated cell lines, the post-hoc test did not allow establishment of a relationship between quinclorac-habituation and increment or reduction in cell wall cellulose content.
Fig. 3.
Cellulose contents of non-habituated and quinclorac-habituated cells (mean of three replicates ± s.d). Asterisks indicate those quinclorac-habituated cells that significantly differed from non-habituated cells (P < 0·05; one-way ANOVA followed by Tukey test).
Cell wall fractionation
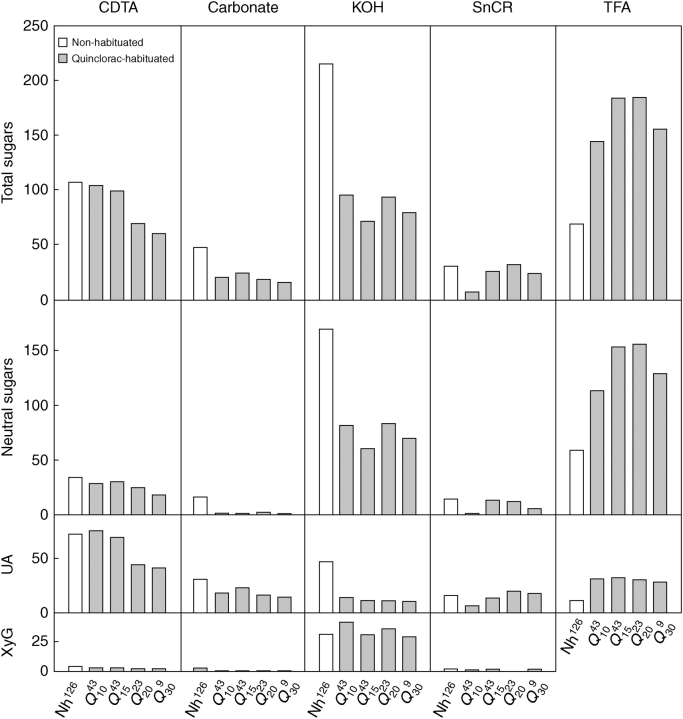
Non-habituated and habituated cell walls were fractionated in order to gain a better understanding of the cell wall modifications associated with quinclorac habituation (Fig. 4). The pectic (CDTA and carbonate) and hemicellulosic (KOH) fractions from habituated cell walls had lower contents of both neutral sugars and uronic acids than non-habituated cell walls. Thus, a clear trend during habituation was the decrease in CDTA, carbonate and KOH-extracted polysaccharides, especially in this latter fraction. The reduction in the amount of KOH-extracted polysaccharides was not related to a reduction in xyloglucan.
Fig. 4.
Total sugars, neutral sugars, uronic acids (UA) and xyloglucan (XyG) contents in cell wall fractions obtained from non-habituated cells (Nh126) and from cells habituated to 10 (Q1043), 15 (Q1543), 20 (Q2023) and 30 (Q309) μm quinclorac. Each bar represents the average of at least three measurements and all values are μg per mg of cell wall.
Parallel to these results, an enrichment in TFA-extracted polysaccharides was noted during the habituation, to the extent that this was the main fraction encountered in habituated cell walls. Also noticeable was the high amount of uronic acids still extracted in TFA fractions, comparable with the amount extracted with carbonate.
According to the uronic acid/total sugar ratio, no significant changes in the proportion of uronic acids occurred during habituation to quinclorac, the values ranging from 0·38 for non-habituated cells to 0·31 for Q20.
Neutral sugar analysis of cell wall fractions afforded two significant results (Fig. 5). The KOH fraction from non-habituated cells was mainly composed of Ara followed by Xyl, Glc and Gal, pointing to the presence of high amounts of arabinans and xyloglucan. A noticeable decrease in Ara was detected in habituated cell walls, indicating a relationship between quinclorac habituation and the reduction in KOH-extracted arabinans.
Fig. 5.
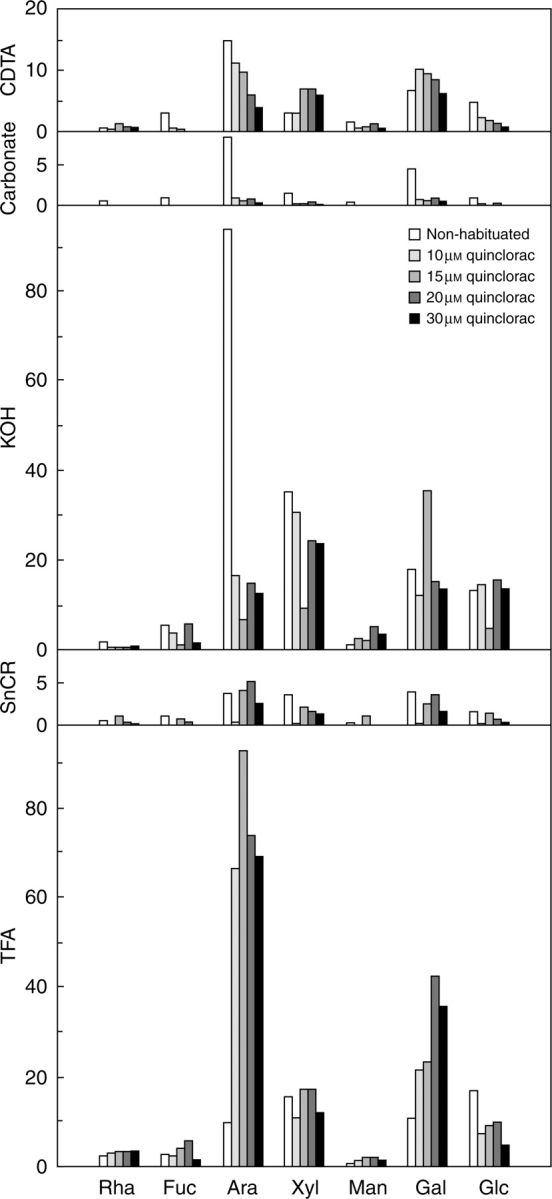
GC analysis of neutral sugars from each cell wall fraction. The samples were the same as those shown in Fig. 4, obtained from non-habituated cells (Nh126), and cells habituated to 10 (Q1043), 15 (Q1543), 20 (Q2023) and 30 (Q309) μm quinclorac. Rha, rhamnose; Fuc, fucose; Ara, arabinose; Xyl, xylose; Man, mannose; Gal, galactose; Glc, glucose. Each bar represents the average of at least three measurements and all values are μg per mg of cell wall.
The neutral sugar composition of the TFA fraction was similar to that of the KOH fraction, with the exception of an increased Rha content. In parallel with the decrease in Ara found in the KOH fraction, a noteworthy enrichment in Ara, and to a lesser extent Gal, was observed in the TFA fraction from habituated cell walls.
Bulk analysis of Ara indicated that the differences among the cell lines were due to changes in extractability, since the net content in Ara expressed with respect to total sugars hardly varied among the different cell lines (from approx. 28 % for Nh to 24 % for Q10). In contrast, a net enrichment in Gal was detected as the habituation level rose (from approx. 9 % of total sugars for Nh to 18 % of total sugars for Q10).
Immunolocation of cell wall components
In order to determine whether habituation to quinclorac modified the polymer distribution in the cell wall, sections were obtained from non-habituated and quinclorac-habituated cells and probed with a set of monoclonal antibodies (Fig. 6). Homogalacturonan (HG) domains were analysed using JIM5 and JIM7, which recognize low and high methyl-esterified HG, respectively (Willats et al., 2000). Labelling with JIM5 was very faint in the case of the habituated cells, in contrast with their non-habituated counterparts (Fig. 6A–D), while JIM7 labelling was intense in both non-habituated and habituated cell walls (Fig. 6E–H). It is also remarkable that the extracellular material in habituated cells was labelled with JIM7, as observed on comparing JIM7 labelling with the results of calcofluor staining (Fig. 6H and inset).
Fig. 6.
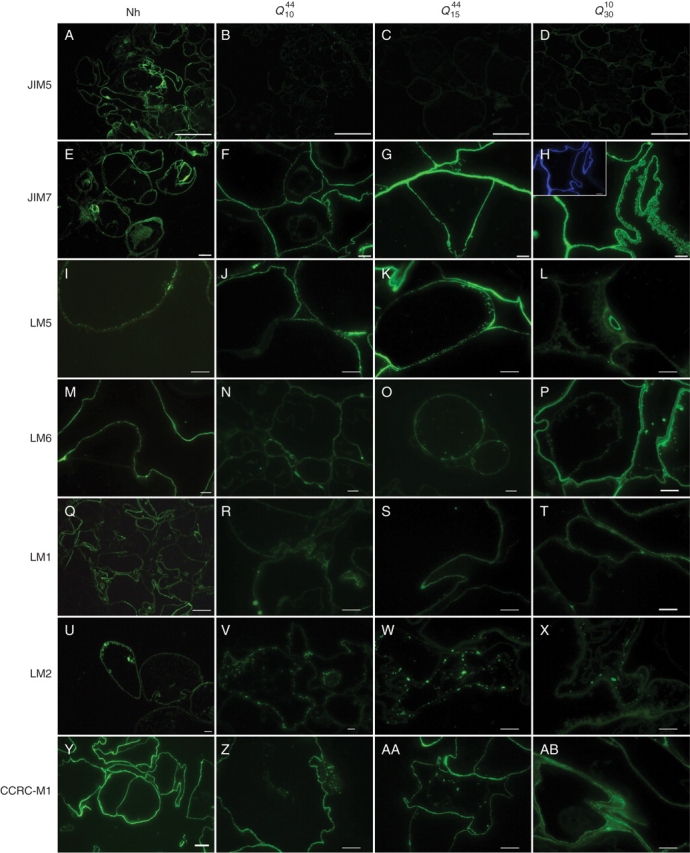
Immunolocation of cell wall polysaccharides and proteins in sections of (A, E, I, M, Q, U, Y) Nh126, (B, F, J, N, R, V, Z) Q1044, (C, G, K, O, S, W, AA) Q1544, and (D, H, L, P, T, X, AB) Q3010. Sections were probed with a monoclonal antibody specific for homogalacturonan with a low degree of methyl esterification (JIM5, A–D), homogalacturonan with a high degree of methyl esterification (JIM7, E–H), galactan from rhamnogalacturonan I or AGPs (LM5, I–L), arabinan from rhamnogalacturonan I (LM6, M–P), extensin (LM1, Q–T), AGPs (LM2, U–X), and fucosylated xyloglucan (CCRC-M1, Y–AB). Inset in (H) shows calcofluor staining of the same section shown in (H). Scale bars: (A) = 100 µm; (B–D) = 7 µm; (Y) = 2 µm; all others = 10 µm.
LM5 and LM6 recognize β-1,4-galactans and α-1,5-arabinans, respectively, from rhamnogalacturonan I (both antibodies) and AGPs (LM6; Jones et al., 1997; Willats et al., 1998; Willats and Knox, 1999). The LM5-labelled epitopes were located on the inner side of cell walls in all cells, and were apparently more abundant in habituated cells (Fig. 6I–L). The labelling with LM6 was fainter in Q10 and Q15, whereas its intensity was similar to that of non-habituated cells in Q30 (Fig. 6M–P).
With regard to cell wall proteins, LM1 and LM2 were used to study the distribution of extensin and the carbohydrate part of some AGPs, respectively (Smallwood et al., 1995, 1996). The distribution of the LM1 epitope was homogeneous for non-habituated cells and scarcer in the case of quinclorac-habituated cells, where it was restricted to junction areas or to some points in the cell wall (Fig. 6Q–T). In the case of LM2, the labelling was located at certain points of the protoplast close to the inner side of the plasma membrane of non-habituated cells, whereas quinclorac-habituated cells were also labelled in the protoplast, although not necessarily close to the plasmalemma (Fig. 6U–X).
An antibody specific for fucosylated xyloglucan (CCRC-M1; Pulhman et al., 1994) was also used. The level of labelling was very similar in both non-habituated and quinclorac-habituated cells, and was uniformly distributed in the cell wall (Fig. 6Y–AB).
DISCUSSION
Bean calli capable of growing under lethal concentrations of quinclorac (up to 30 µm, Table 1) were obtained by stepwise subculture in increasing concentrations of the herbicide. Habituated calli grew slower than their non-habituated counterparts, but their growth was considerably higher than that shown by non-habituated cells in the presence of the same concentration of quinclorac (i.e. in non-habituated calli cultured on 20 µm quinclorac the growth rate was reduced by 90 %, and at 30 µm the non-habituated cells died, whereas the quinclorac-habituated calli grew at a rate higher than 50 % of the controls). A similar reduction in growth rate has been observed in cell cultures habituated to other herbicides (Shedletzky et al., 1990; Díaz-Cacho et al., 1999; Encina et al., 2001, 2002).
The colour and hardness of the quinclorac-habituated calli were similar to those of the non-habituated calli growing in the absence of herbicide. However, during growth the habituated calli formed hollow protuberances that repelled the surface of the medium (Fig. 1), as previously observed in isoxaben- and dichlobenil-habituated bean calli (Díaz-Cacho et al., 1999; Encina et al., 2001). This is most likely due to their modes of growth; as the callus grows, cells are pushed upwards and outwards from the surface of the medium such that herbicide gradients are set up between the cells and the growth substrate, and this would favour the growth of cells located farther from the medium, which would then be exposed to a lower herbicide concentration.
Habituated cells were more irregularly shaped and some material was deposited outside as the concentration of quinclorac was increased. However, they did not show thicker cell walls and there were no differences in their cell wall DW/cell DW ratios, unlike dichlobenil-habituated cells (Encina et al., 2001, 2002; García-Angulo et al., 2006).
In contrast to previously described results in corn (Koo et al., 1996) and other monocots (Koo et al., 1997), where quinclorac was seen to inhibit the incorporation of glucose into cellulose and hence the synthesis of this polysaccharide, the habituated cells studied here did not show decreased cellulose contents. The FTIR spectra of non-habituated cell walls were not markedly different from those of quinclorac-habituated walls. However, multivariate analyses of the spectra, both in cluster and principal component analysis, separated two groups of spectra corresponding to two levels of habituation to quinclorac, the most remarkable differences being the lower content of pectins and the higher amount of cell wall proteins in quinclorac-habituated cells, with no apparent changes in cellulose or xyloglucan contents. This contrasts with previous results on the habituation of bean cells to other cellulose biosynthesis inhibitors, in which a reduction in cellulose content was reported even at low habituation levels (Encina et al., 2001; Alonso-Simón et al., 2004).
Additional analyses of non-cellulosic cell wall polysaccharides shed further light on the results obtained with FTIR spectroscopy. Thus, a decrease in the sugars of the pectic fractions was observed, although this could have been due to a lower incorporation into the cell wall rather than to a true inhibition of their synthesis, since the material accumulated outside habituated cells was recognized by antibodies specific for HG with a high degree of methyl-esterification (JIM7). Pectins are synthesized in the Golgi apparatus as highly methyl-esterified polysaccharides (Willats et al., 2001), and are then de-esterified after their deposition in the cell wall. HG molecules are cross-linked with each other through non-esterified regions (Jarvis, 1984). Therefore, the presence of highly methyl-esterified pectins outside cells could be indicative of a deficient de-esterification, which in turn would partly prevent the correct integration and persistence of some of the HG molecules in the cell wall. In this way, HG would not bind efficiently to other polysaccharides in the cell wall matrix and it would be much easier for them to become detached from such polysaccharides. This putative incorrect de-esterification would be confirmed by the faint labelling of cell sections probed with JIM5, an antibody specific for low-esterified HG. Dichlobenil-habituated cells do not show this effect: indeed, the pectin content of cell walls increases with habituation and the labelling with JIM5 is constant (Sabba et al., 1999; García-Angulo et al., 2006).
GC analysis of the KOH- and TFA-extracted polysaccharides indicated a shift in the extractability of arabinans and galactans from the KOH fraction in non-habituated cell walls relative to the TFA fraction in habituated cell walls. This shift suggests that as a consequence of habituation to quinclorac a pool of RGI had become very tightly bound to cellulose through their neutral side-chains. These side-chains would be the equivalent of those tightly bound to cellulose recently described in potato and sugar beet (Zykwinska et al., 2005, 2007). Thus, an alteration in the arrangement of the cellulose/pectin network arises as one of the main changes in cell walls habituated to quinclorac. Since the total amount of Ara did not differ markedly between cell walls, changes in the association between pectins and cellulose do not seem to be related to differences in the proportion of neutral side-chains. Alternatively, they could indicate differences in cellulose crystallinity or surface availability (Vincken et al., 1995).
The fact that LM1 and LM2 antibodies specific to diverse cell wall proteins showed less labelling in sections of habituated cells than in non-habituated ones contrasts with habituation to dichlobenil, in which an increase in labelling with anti-extensin antibodies has been observed in sections from habituated cells (Sabba et al., 1999) and in immunodot-assays with antibodies specific to extensin and AGPs in cell wall fractions (García-Angulo et al., 2006). However, PCA of the FTIR spectra indicated a higher proportion of cell wall proteins in the quinclorac-habituated cells. Accordingly, this increase in proteins would be due to proteins other than extensin and AGPs, or even AGPs not recognized by LM2.
In contrast to previous studies addressing the effect of this herbicide (Koo et al., 1996, 1997), quinclorac-habituated cells did not show decreased cellulose contents, but instead a reduced content in pectic polysaccharides, probably due to a deficient integration of pectins in the cell wall rather than to the inhibition of pectin synthesis. These results contrast with the changes promoted by the habituation of cells to well-known cellulose biosynthesis inhibitors (dichlobenil and isoxaben), which produce a marked decrease in cellulose and an increase in pectins. Thus, since the action of quinclorac on cell walls does not seem to be due to a direct inhibition of any of the cell wall components, we suggest that the changes could be due to a side effect of the herbicide, probably associated with the auxin-dependent regulation of hydrolytic enzymes or with the changes in ethylene and ABA metabolism, as previously pointed by Tresch and Grossmann (2003).
In summary, the long-term modifications of the cell wall caused by the habituation of bean cell cultures to quinclorac do not resemble those of bean cells habituated to the well-known cellulose biosynthesis inhibitors dichlobenil or isoxaben.
In the comparison of quinclorac-resistant and -susceptible plants, it has been reported that resistance is associated with a higher level of activity of constitutive anti-oxidative enzymes (Sunohara and Matsumoto, 2004), or to a greater ability to detoxify cyanide, a by-product of the reaction that converts ACC to ethylene (Abdallah et al., 2006). Further analyses will be conducted to elucidate whether any of these mechanism of tolerance are present in our quinclorac-habituated bean cells.
ACKNOWLEDGEMENTS
This work was supported by grants from the Junta de Castilla y León (LE 048A07) and a pre-doctoral grant from the University of León to Ana Alonso-Simón. We are grateful to J. P. Knox and W. G. T. Willats for the generous gifts of the antibodies, and to N. Skinner for correcting the English within the manuscript.
LITERATURE CITED
- Abdallah I, Fischer AJ, Elmore CL, Saltveit ME, Zaki M. Mechanism of resistance to quinclorac in smooth crabgrass (Digitaria ischaemum) Pesticide Biochemistry and Physiology. 2006;84:38–48. [Google Scholar]
- Albersheim P, Nevins DS, English PD. A method for the analysis of sugars in plant cell wall polysaccharides by gas liquid chromatography. Carbohydrate Research. 1967;5:340–345. [Google Scholar]
- Alonso-Simón A, Encina AE, García-Angulo P, Álvarez JM, Acebes JL. FTIR spectroscopy monitoring of cell wall modifications during the habituation of bean (Phaseolus vulgaris L.) callus cultures to dichlobenil. Plant Science. 2004;167:1273–1281. [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Analytical Biochemistry. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Coimbra MA, Barros A, Rutledge DN, Delgadillo I. FTIR spectroscopy as a tool for the analysis of olive pulp cell-wall polysaccharide extracts. Carbohydrate Research. 1999;317:145–154. [Google Scholar]
- Díaz-Cacho MP, Moral R, Encina A, Acebes JL, Álvarez JM. Cell wall modifications in bean (Phaseolus vulgaris L.) callus cultures tolerant to isoxaben. Physiologia Plantarum. 1999;107:54–59. [Google Scholar]
- Dische Z. Color reactions of carbohydrates. In: Whistler RL, Wolfrom ML, editors. Methods in carbohydrate chemistry. vol. 1. New York: Academic Press; 1962. pp. 475–514. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- Encina AE, Moral RM, Acebes JL, Álvarez JM. Characterization of cell walls in bean (Phaseolus vulgaris L.) callus cultures tolerant to dichlobenil. Plant Science. 2001;160:331–339. doi: 10.1016/s0168-9452(00)00397-6. [DOI] [PubMed] [Google Scholar]
- Encina AE, Sevillano JM, Acebes JL, Álvarez J. Cell wall modifications of bean (Phaseolus vulgaris) cell suspensions during habituation and dehabituation to dichlobenil. Physiologia Plantarum. 2002;114:182–191. doi: 10.1034/j.1399-3054.2002.1140204.x. [DOI] [PubMed] [Google Scholar]
- García-Angulo P, Willats WGT, Encina AE, Alonso-Simon A, Alvarez JM, Acebes JL. Immunocytochemical characterization of the cell walls of bean cell suspensions during habituation and dehabituation to dichlobenil. Physiologia Plantarum. 2006;127:87–99. [Google Scholar]
- Grossmann K. Mode of action of auxin herbicides: a new ending to a long, drawn out story. Trends in Plant Science. 2000;5:506–508. doi: 10.1016/s1360-1385(00)01791-x. [DOI] [PubMed] [Google Scholar]
- Grossmann K, Kwiatkowski J. The mechanism of quinclorac selectivity in grasses. Pesticide Biochemistry and Physiology. 2000;66:83–91. [Google Scholar]
- Grossmann K, Kwiatkowski J, Tresch S. Auxin herbicides induce H2O2 overproduction and tissue damage in cleavers (Galium aparine L.) Journal of Experimental Botany. 2001;52:1811–1816. doi: 10.1093/jexbot/52.362.1811. [DOI] [PubMed] [Google Scholar]
- Hansen H, Grossmann K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiology. 2000;124:1437–1448. doi: 10.1104/pp.124.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MC. Structure and properties of pectin gels in plant cell walls. Plant Cell and Environment. 1984;7:153–164. [Google Scholar]
- Jones L, Seymour GB, Knox JP. Localization of pectic galactan in tomato cells using a monoclonal antibody specific to (1–4)-β-galactan. Plant Physiology. 1997;113:1405–1412. doi: 10.1104/pp.113.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kačuraková M, Capek P, Sasinková V, Wellner N, Ebringerová A. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydrate Polymers. 2000;43:195–203. [Google Scholar]
- Kačuraková M, Smith AC, Gidley MJ, Wilson RH. Molecular interactions in bacterial cellulose composites studied by 1D FT-IR spectroscopy. Carbohydrate Research. 2002;337:1145–1153. doi: 10.1016/s0008-6215(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Koo SJ, Neal JC, DiTomaso JM. 3,7-dichloroquinolinecarboxylic acid inhibits cell-wall biosynthesis in maize roots. Plant Physiology. 1996;112:1383–1389. doi: 10.1104/pp.112.3.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SJ, Neal JC, DiTomaso JM. Mechanism of action and selectivity of quinclorac in grass roots. Pesticide Biochemistry and Physiology. 1997;57:44–53. [Google Scholar]
- Kooiman P. A method for the determination of amyloid in plant seeds. Recueil des Travaux Chimiquese de Pays-Bas. 1960;79:675–678. [Google Scholar]
- Manfield IW, Orfila C, McCartney L, Harholt J, Bernal AJ, Scheller HV, et al. Novel cell wall architecture of isoxaben-habituated Arabidopsis suspension-cultured cells: global transcript profiling and cellular analysis. Plant Journal. 2004;40:260–275. doi: 10.1111/j.1365-313X.2004.02208.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nakagawa N, Sakurai N. Increase in the amount of celA1 protein in tobacco BY-2 cells by a cellulose biosynthesis inhibitor, 2,6-dichlorobenzonitrile. Plant and Cell Physiology. 1998;39:779–785. doi: 10.1093/oxfordjournals.pcp.a029434. [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Sakurai N. Cell wall integrity controls expression of endoxyloglucan transferase in tobacco BY2 cells. Plant and Cell Physiology. 2001;42:240–244. doi: 10.1093/pcp/pce023. [DOI] [PubMed] [Google Scholar]
- Pulhman J, Bucheli E, Swain MJ, Dunning N, Albersheim P, Darvill AG, Hamn MG. Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. Generation of a monoclonal antibody to a terminal alfa-(1,2)-linked fucosyl containing epitope. Plant Physiology. 1994;104:699–710. doi: 10.1104/pp.104.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan C, Ong EK, Dalling MJ, Stevenson TW. Effect of herbicidal application of 2,4-dichlorophenoxyacetic acid in Arabidopsis. Functional and Integrative Genomics. 2005;5:4–17. doi: 10.1007/s10142-004-0119-9. [DOI] [PubMed] [Google Scholar]
- Sabba RP, Vaughn KC. Herbicides that inhibit cellulose biosynthesis. Weed Science. 1999;47:757–763. [Google Scholar]
- Sabba RP, Durso NA, Vaughn KC. Structural and immunocytochemical characterization of the walls of dichlobenil-habituated BY-2 tobacco cells. International Journal of Plant Science. 1999;160:275–290. [Google Scholar]
- Saeman JF, Moore WE, Millet MA. Sugar units present. In: Whistler RL, editor. Methods in carbohydrate chemistry. vol. 3. New York: Academic Press; 1963. pp. 54–69. [Google Scholar]
- Selvendran RR, O'Neill MA. Isolation and analysis of cell wall from plant material. In: Glich D, editor. Methods of biochemical analysis. vol. 32. Cambridge: Cambridge University Press; 1987. pp. 25–153. [DOI] [PubMed] [Google Scholar]
- Séné CFB, McCann MC, Wilson RH, Grinter R. Fourier-Transform Raman and Fourier-Transform Infrared Spectroscopy; an investigation of five higher plant cell walls and their components. Plant Physiology. 1994;106:1623–1631. doi: 10.1104/pp.106.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedletzky E, Shmuel M, Delmer DP, Lamport DTA. Adaptation and growth of tomato cells on the herbicide 2,6-dichlorobenzonitrile leads to production of unique cell walls virtually lacking a cellulose-xyloglucan network. Plant Physiology. 1990;94:980–987. doi: 10.1104/pp.94.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedletzky E, Shmuel M, Trainin T, Kalman S, Delmer DP. Cell wall structure in cells adapted to growth on the cellulose-synthesis inhibitor 2,6-dichlorobenzonitrile: a comparison between two dicotyledonous plants and a graminaceous monocot. Plant Physiology. 1992;100:120–130. doi: 10.1104/pp.100.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M, Martin H, Knox JP. An epitope of rice threonine- and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta. 1995;196:510–522. doi: 10.1007/BF00203651. [DOI] [PubMed] [Google Scholar]
- Smallwood M, Yates EA, Willats WGT, Martin H, Knox JP. Immunochemical comparison of membrane associated and secreted arabinogalactan-proteins in rice and carrot. Planta. 1996;198:452–459. [Google Scholar]
- Sunohara Y, Matsumoto H. Oxidative injury induced by the herbicide quinclorac on Echinochloa oryzicola Vasing. and the involvement of antioxidative ability in its highly selective action in grass species. Plant Science. 2004;167:597–606. [Google Scholar]
- Talmadge KM, Keegstra K, Bauer WD, Albersheim P. The structure of plant cell walls I. The macromolecular components of the walls of suspension-cultured sycamore cells with a detailed analysis of the pectic polysaccharides. Plant Physiology. 1973;51:158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch S, Grossmann K. Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots. Pesticide Biochemistry and Physiology. 2003;75:73–78. [Google Scholar]
- Updegraff DM. Semi-micro determination of cellulose in biological materials. Analytical Biochemistry. 1969;32:420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Vincken JP, de Keizer A, Beldman G, Voragen AGJ. Fractionation of xyloglucan fragments and their interaction with cellulose. Plant Physiology. 1995;108:1579–1585. doi: 10.1104/pp.108.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells B, McCann MC, Shedletzky E, Delmer D, Roberts K. Structural features of cell walls from tomato cells adapted to grow on the herbicide 2,6-dichlorobenzonitrile. Journal of Microscopy. 1994;173:155–164. [Google Scholar]
- Willats WGT, Knox JP. Immunoprofiling of pectic polysaccharides. Analytical Biochemistry. 1999;268:143–146. doi: 10.1006/abio.1998.3039. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Marcus SE, Knox JP. Generation of a monoclonal antibody specific to (1,5)-α-L-arabinan. Carbohydrate Research. 1998;308:149–152. doi: 10.1016/s0008-6215(98)00070-6. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Steele-King CG, McCartney L, Orfila C, Marcus SE, Knox JP. Making and using antibody probes to study plant cell walls. Plant Physiology and Biochemistry. 2000;38:27–36. [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology. 2001;47:9–27. [PubMed] [Google Scholar]
- Zykwinska AW, Ralet MC, Garnier CD, Thibault JF. Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiology. 2005;139:397–407. doi: 10.1104/pp.105.065912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zykwinska AW, Thibault JF, Ralet MC. Organization of pectic arabinan and galactan side chains in association with cellulose microfibrils in primary cell walls and related models envisaged. Journal of Experimental Botany. 2007;58:1795–1802. doi: 10.1093/jxb/erm037. [DOI] [PubMed] [Google Scholar]