Abstract
Background and Aims
Floral rewards may be associated with certain morphological floral traits and thus act as underlying factors promoting selection on these traits. This study investigates whether some traits that are under pollinator-mediated selection (flower number, stalk height, corolla diameter, corolla tube length and corolla tube width) in the Mediterranean herb E. mediohispanicum (Brassicaceae) are associated with rewards (pollen and nectar).
Methods
During 2005 the phenotypic traits and the visitation rate of the main pollinator functional groups were quantified in 720 plants belonging to eight populations in south-east Spain, and during 2006 the same phenotypic traits and the reward production were quantified in 400 additional plants from the same populations.
Key Results
A significant correlation was found between nectar production rate and corolla tube length, and between pollen production and corolla diameter. Visitation rates of large bees and butterflies were significantly higher in plants exhibiting larger flowers with longer corolla tubes.
Conclusions
The association between reward production and floral traits may be a factor underlying the pattern of visitation rate displayed by some pollinators.
Key words: Erysimum, floral traits, nectar, pollen, pollinator visitation rate, reward
INTRODUCTION
Pollinators shape the evolution of flowers by influencing plant fitness and exerting significant selection pressure on several floral traits (Lloyd and Barrett, 1986; Harder and Barrett, 2006). Two key components determine the role played by pollinators as selective agents, their flower visitation rate and their pollinating effectiveness (Fenster et al., 2004). To increase pollinator visitation rate plants have developed a variety of rewards, such as nectar, pollen, floral oils, scents and resin (Faegri and van der Pijl, 1979; Simpson and Neff, 1983). Pollinators may discriminate between conspecific plants based on the quantity and quality of these rewards (Cunningham et al., 1998; Scheiner et al., 1999; Waddington, 2001; Plepys et al., 2002). Thus, reward production per flower strongly influences pollinator visitation rate (Thomson et al., 1989; Real and Rathcke, 1991; Mitchell, 1992; Hodges, 1995; Klinkhamer and van der Lugt, 2004), flower handling time (Zimmerman, 1983; Galen and Plowright, 1985; Neff and Simpson, 1990; Cresswell, 1999), which is correlated to pollen deposition (Thomson and Plowright, 1980), as well as departure decisions (number of flowers visited per individual plant), and the distance and direction of movements within and between individual plants (Pyke, 1978; Heinrich, 1979; Pleasants and Zimmerman, 1979; Kadmon and Shmida, 1992; Ohashi and Yahara, 2001). As a consequence of pollinator discrimination, producing more or better flower rewards may increase a plant's reproductive success (Zimmerman, 1983; Real and Rathcke, 1991; Mitchell and Waser, 1992; Mitchell, 1993; Hodges, 1995; Cresswell, 1999).
Rewards may also act as an underlying factor promoting selection for certain floral traits (Stanton and Preston, 1988; Young and Stanton, 1990; Stanton and Young, 1994; Armbruster et al., 2005; Fenster et al., 2006). This is possible when there is a link between reward quantity or quality, the value of the selected trait and the pollinator visitation rate (Ashman and Stanton, 1991; Campbell et al., 1991; Cohen and Shmida, 1993; Blarer et al., 2002; Fenster et al., 2006). Under these circumstances, some pollinators can learn to discriminate amongst plants based on floral traits that are related to reward abundance or quality (Smithson and Macnair, 1997; Blarer et al., 2002; Schaefer et al., 2004; Internicola et al., 2007).
Erysimum mediohispanicum (Brassicaceae) is a generalist-pollinator plant species. In south-eastern Spain it is visited by more than 100 pollinator species belonging to various functional groups, mostly bee-flies, large bees, small bees and small beetles (Gómez et al., 2007), and including species that collect pollen, nectar, and both these rewards (Gómez, 2003, 2005). Previous studies have demonstrated that pollinators exert significant selection on five plant traits: the number of flowers, the height of the flowering stalk, the diameter of the corolla, the length of the corolla tube, and the width of the corolla tube (Gómez, 2003, 2008; Gómez et al., 2006). The goal of this study was to investigate whether these pollinator-selected traits are associated with reward production in E. mediohispanicum, as a potential explanation for the observed selection. To accomplish this, we determined both (1) the relationship between floral rewards and the above-mentioned plant traits in eight plant populations, and (2) the effect of those phenotypic traits on the visitation rate of the main pollinators.
METHODS
Study system
Erysimum mediohispanicum Polatschek is a biennial monocarpic herb that occurs in two separate areas of the Iberian Peninsula, one in the north-east and the other in the south-east. Plants usually grow for 2–3 years as vegetative rosettes, and then die after producing one to eight reproductive stalks. Individual plants may bear between a few tens and several hundred hermaphroditic, slightly protandrous, bright-yellow flowers (Gómez, 2003). Erysimum mediohispanicum flowers have a tetradynamous androecium with four long and two short stamens. Nectar is produced in four nectar glands located at the sepal base. The species is self-compatible, but requires pollen vectors to produce full seed set (Gómez, 2005).
The study was conducted in 2005 and 2006 in the Sierra Nevada high mountains (Granada province, SE Spain), spanning the complete altitudinal range of E. mediohispanicum (1600–2300 m). In this area, E. mediohispanicum is found in two main habitats, the understory of pine forests (Pinus nigra and P. sylvestris), and montane species-rich shrublands, composed mainly of Berberis vulgaris, Juniperus communis, Astragalus granatensis, Vella spinosa and Ononis aragonensis. Plants flower from late-May to late-June, depending on the altitude (Gómez et al., 2007).
Eight populations were selected within a 5 × 2 km area (Table 1). Populations were at least 200 m apart from each other, with a mean interpopulation distance of 818 ± 82 m (± s.e.). Gaps between populations contained no E. mediohispanicum individuals. These populations differ both in pollinator abundance (Gómez et al., 2007) as well as in pollinator composition (Gómez et al., unpubl. res.).
Table 1.
Location and characteristics of the eight Erysimum mediohispanicum populations studied in the Sierra Nevada during 2005 and 2006
| Population | Latitude (N) | Longitude (W) | Habitat | Altitude | Abundance (± s.e.)* | Sobs (95 % CI)† | Most abundant flower visitors‡ |
|---|---|---|---|---|---|---|---|
| 08 | 37°8·00′ | 3°25·91′ | Shrubland | 1690 | 0·77 ± 0·07c | 33ab (22·6–41·4) | Large bees, bee-flies |
| 21 | 37°8·07′ | 3°25·71′ | Forest | 1723 | 1·60 ± 0·14a | 37b (27·3–46·7) | Small bees, bee-flies |
| 01 | 37°8·00′ | 3°25·69′ | Forest | 1750 | 0·64 ± 0·07c | 36cd (25·4–46·7) | Large bees, bee-flies, beetles |
| 22 | 37°7·86′ | 3°25·70′ | Forest | 1802 | 0·77 ± 0·07c | 32cd (22·7–41·3) | Bee-flies, small bees |
| 23 | 37°7·74′ | 3°25·58′ | Shrubland | 1874 | 0·97 ± 0·12b,c | 39ce (29·1–48·9) | Large bees, beetles |
| 24 | 37°7·51′ | 3°26·14′ | Forest | 1943 | 0·73 ± 0·10c | 30abc (20·1–39·5) | Large bees, beetles, small bees |
| 25 | 37°7·27′ | 3°26·05′ | Shrubland | 2064 | 0·82 ± 0·10c | 32de (22·8–41·2) | Large bees, small bees, beetles |
| 02 | 37°7·33′ | 3°25·86 | Shrubland | 2099 | 1·30 ± 0·12ab | 41ad (31·1–50·9) | Beetles |
* Abundance is expressed as visits per flower h–1, and was compared between populations by one-way random ANOVA (Gómez et al., 2007).
† Sobs is the observed number of pollinator species per population (observed species richness), and was compared between populations by rarefaction (Gómez et al., 2007).
‡ Based in the relative abundance of each functional group (Gómez et al., unpubl. res.).
Ninety plants per population in 2005 (720 plants in total) and fifty plants per population in 2006 (400 plants in total) were marked at the onset of the flowering period using an aluminum tag attached to the base of the flowering stalks.
Plant phenotype
For each tagged plant (n = 1120 plants) the following phenotypic traits were determined, all of which have been shown to be under pollinator-mediated selection in previous studies (Gómez, 2003, 2008; Gómez et al., 2006). (1) Stalk height: measured as the height of the tallest stalk (the distance from the ground to the top of the highest open flower, to the nearest 0·5 cm). (2) Flower number: the total lifetime number of flowers produced by each plant. (3) Corolla diameter: quantified as the distance between the edge of two opposite petals (± 0·1 mm of error; Gómez et al., 2006). (4) Corolla tube length: measured as the distance between the corolla tube aperture and the base of the sepals (Gómez et al., 2006). (5) Corolla tube width: measured as the width of the aperture of the corolla tube; this trait was estimated by subtracting from the corolla diameter the length of a petal × 2.
In addition, the size of each tagged plant was also measured, as a way to control for plant condition. Plant size was a compound trait determined by three individual traits: stalk height (see above), number of stalks and stalk diameter (quantified at the base of the tallest stalk; see Gómez et al., 2006 for details).
Flower reward
Nectar and pollen production were quantified of the plants tagged in the eight populations during 2006 (n = 400 plants). It was decided to use a different group of plants to quantify reward production because the manipulation associated with the quantification procedure would negatively affect the estimates of pollinator visitation rate. This would happen because E. mediohispanicum is monocarpic, flowering only once, and produces from 10 to 200 flowers arranged in 1–3 flowering stalks (Gómez et al., 2006, 2007). This means that the exclusion of insects from any flowering stalk or group of flowers in order to quantify the nectar production rate would affect the visitation rate to the other flowers (see Gómez, 2005).
Pollen production was quantified as the total volume of pollen produced per flower in each of the 400 tagged plants. Two flower buds per plant were taken and preserved in 70 % ethanol in order to estimate pollen production (Kearns and Inouye, 1993). Three of the six anthers per flower were placed in a vial with ethanol and sonicated for 3 min to dislodge pollen grains (Kearns and Inouye, 1993). A known volume of saline solution was then added to the vial and the number of pollen grains per volume was measured in a Multisizer particle counter (Global Medical Instrumentation, Inc., Ramsey, Minnesota). Pollen grain diameter was measured on pollen slides at 400 ×. From these two measures the total pollen volume per flower was obtained. Nectar production rate was measured as the volume of nectar produced by newly opened flowers in 24 h. Cellophane bags were used to cover 3–5 closed flowers of each tagged plant in order to prevent pollinator visitation. After 24 h, nectar volume was measured in two newly opened flowers per plant using calibrated 0·1 µL micropipettes (Kearns and Inouye, 1993).
Pollinator visitation rate
The visitation rate of the main pollinator functional groups to each tagged plant was determined in 2005 (n = 720 plants). For this, 5–7 pollinator censuses were conducted per population throughout the peak bloom period (10–15 d per population), noting the number of open flowers on each tagged plant and the number of pollinators that landed on the flowers during 5-min intervals. Each census lasted for 450 min, and more than 1500 min of observations were conducted per population. The pollinator abundance was quantified as the number of pollinators visiting each tagged plant per 5 min (Gómez et al., 2007).
The visitors to flowers of E. mediohispanicum were assigned to the following eight functional groups according to similarity in size, proboscis length, foraging behaviour and feeding habits (Fenster et al., 2004; Wilson et al., 2004). (1) Large bees: mostly pollen- and nectar-collecting females measuring 10 mm in body length or larger. (2) Small bees: mostly pollen- and nectar-collecting females smaller than 10 mm. (3) Wasps: aculeate wasps, large parasitic wasps and cleptoparasitic bees collecting only nectar. (4) Bee-flies: long-tongued, mostly nectar-collecting Bombyliidae. (5) Hover-flies: nectar- and pollen-collecting Syrphidae and short-tongued Bombyliidae. (6) Beetles: including species collecting nectar and/or pollen. (7) Butterflies: mostly Rhopalocera, all nectar collectors. (8) Others: nectar-collecting ants, small flies, small parasitic wasps, bugs and grasshoppers.
Data analysis
Among-population differences in reward were quantified with one-way ANOVAs, introducing population as a random factor. Variance components were determined with restricted maximum likelihood (REML) for unbalanced data using the Variability Chart Platform in JMP 7·0 (SAS Institute, Inc., 2007).
The relationship between plant phenotype and pollinator visitation rate was explored by multiple Poisson regressions, including as dependent variable the number of insects per plant, and as independent variables all plant phenotypic traits, the population and the interaction between population and phenotypic traits. We included logarithm as the link function between the dependent and the independent variables. In this analysis, we pooled together tagged plants of all eight populations studied during 2005. When the interaction between plant population and phenotypic traits was significant, separate multiple regressions for each population were performed.
The relationship between plant traits and reward (pollen and nectar separately) was analysed using general linear modelling (GLM) in 2006. Plant traits were included as independent variables, and nectar and pollen as dependent variables. Variables were transformed prior to analysis when necessary. Population and population × plant trait interactions were included as random variables. Leverage analyses on reward residuals were used to determine the proportion of variance in reward production explained by each significant plant trait (Rawling et al., 1998).
RESULTS
Spatial variation in reward production and plant phenotype
Erysimum mediohispanicum produces, on average, 0·136 ± 0·010 µL of nectar per 24 h and 70323 ± 3037 pollen grains per flower (all values are given as ± s.e.). A significant between-population difference was found in both pollen and nectar production rate (Fig. 1). Most of the variation in nectar production rate was found within populations (85·1 %), rather than among populations (14·9 %). In contrast, most of the variation in pollen production was found among populations (61·5 %) rather than within populations (38·5 %).
Fig. 1.
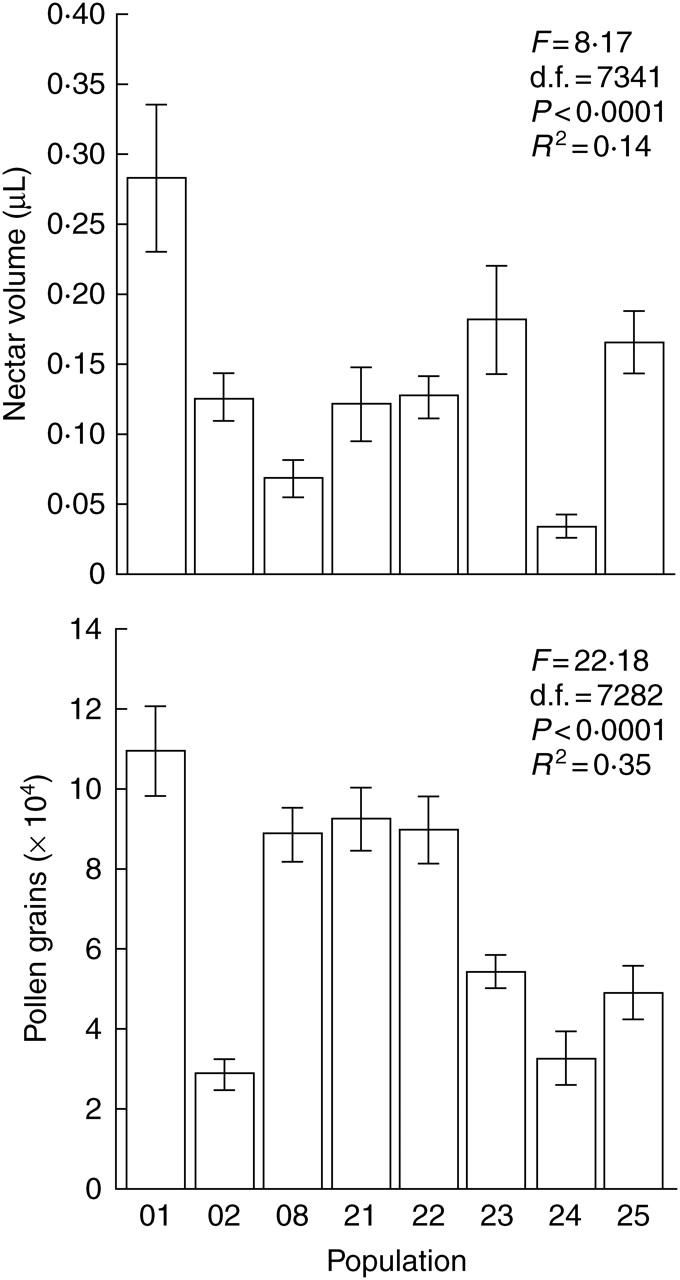
Differences in per-flower pollen and nectar production rate among Erysimum mediohispanicum populations. Results from random one-way ANOVAs are shown.
There was no correlation between nectar and pollen production in any of the populations (P > 0·05 in all cases, Pearson correlation coefficient). There was a significant negative effect of altitude on pollen production (β = −0·85 ± 0·01, F = 16·31, P < 0·0001, R2 = 0·68). In contrast, nectar production rate was not affected by altitude (F = 0·005, P = 0·98, linear regression).
There was significant interpopulation variation in plant traits (Table 2). Populations 02 and 21 were characterized by tall plants producing many flowers of large size, whereas in contrast population 08 was characterized by plants with deep corolla tubes.
Table 2.
Among-population variation in main phenotypic traits of Erysimum mediohispanicum during 2006
| Population | Stalk height (cm) | Number of flowers | Corolla tube width (mm) | Flower diameter (mm) | Corolla tube length (mm) |
|---|---|---|---|---|---|
| 08 | 39·85 ± 1·66 | 56·69 ± 8·86 | 0·77 ± 0·22 | 11·52 ± 0·30 | 11·22 ± 0·21 |
| 21 | 55·07 ± 1·73 | 90·14 ± 9·26 | 0·51 ± 0·23 | 12·46 ± 0·31 | 10·92 ± 0·22 |
| 01 | 40·95 ± 1·84 | 52·26 ± 9·83 | 0·66 ± 0·25 | 10·92 ± 0·34 | 10·10 ± 0·24 |
| 22 | 33·19 ± 1·64 | 34·78 ± 8·77 | 0·34 ± 0·26 | 11·83 ± 0·36 | 10·57 ± 0·25 |
| 23 | 40·86 ± 1·71 | 74·36 ± 9·15 | 0·00 ± 0·25 | 9·28 ± 0·34 | 11·10 ± 0·24 |
| 24 | 31·54 ± 1·94 | 34·29 ± 10·38 | 0·47 ± 0·30 | 10·53 ± 0·41 | 10·50 ± 0·29 |
| 25 | 32·30 ± 1·79 | 59·73 ± 9·59 | 0·53 ± 0·24 | 11·65 ± 0·32 | 10·79 ± 0·22 |
| 02 | 40·54 ± 1·75 | 116·56 ± 9·36 | 0·78 ± 0·23 | 12·49 ± 0·31 | 11·05 ± 0·22 |
| F-ratio | 18·64*** | 8·89*** | 6·23*** | 10·36*** | 2·62** |
| R2 | 0·26 | 0·14 | 0·13 | 0·16 | 0·06 |
F-ratios refer to one-way ANOVAs; ** P < 0·01, *** P < 0·0001.
Sample size is 400 (50 plants per population) for all traits.
Flower phenotype–reward relationship
The statistical models showed that two phenotypic traits were related to floral reward (Table 3). Corolla tube length was positively and significantly related with flower nectar (β = 0·238 ± 0·115; Table 3), explaining 18 % of its variance (leverage plot). The absence of a significant interaction term between this trait and population suggests that this association was similar across the eight populations. Similarly, corolla diameter was positively related with pollen production (β = 2·191 ± 0·769; Table 3), explaining 12 % of its variance (leverage plot). This relationship also seemed to be similar across populations, since the significant interaction found between this trait and population (Table 3) was exclusively due to changes in slope intensity. It is interesting to note that plant size was not associated with any reward trait.
Table 3.
Summary of the GLMs fitting flower reward and flower phenotype in Erysimum mediohispanicum. All phenotypic traits were log-transformed prior to analysis
| Log Nectar |
Log Pollen |
|||||
|---|---|---|---|---|---|---|
| β ± s.e. | t | P | β ± s.e. | t | P | |
| Stalk height | –0·039 ± 0·048 | –0·81 | 0·4210 | –0·456 ± 0·298 | –1·53 | 0·1276 |
| Number of flowers | 0·009 ± 0·023 | 0·38 | 0·7024 | 0·040 ± 0·155 | 0·26 | 0·7965 |
| Corolla tube width | 0·020 ± 0·029 | 0·70 | 0·4848 | –0·371 ± 0·210 | –1·77 | 0·0794 |
| Corolla diameter | 0·016 ± 0·115 | 0·14 | 0·8874 | 2·191 ± 0·769 | 2·85 | 0·0051 |
| Corolla tube length | 0·238 ± 0·115 | 2·08 | 0·0395 | 0·287 ± 0·761 | 0·38 | 0·7072 |
| Plant size | 0·007 ± 0·005 | 1·50 | 0·1346 | 0·041 ± 0·031 | 1·25 | 0·2133 |
| Population | 7·10 | <0·0001 | 8·165 | <0·0001 | ||
| Stalk height × Population | 0·72 | 0·6495 | 0·914 | 0·4978 | ||
| Number of flowers × Population | 0·82 | 0·5666 | 1·310 | 0·2510 | ||
| Corolla tube width × Population | 0·74 | 0·6332 | 0·751 | 0·6293 | ||
| Flower diameter × Population | 0·59 | 0·7567 | 2·682 | 0·0128 | ||
| Corolla tube length × Population | 1·07 | 0·3808 | 0·935 | 0·4818 | ||
| R2 | 0·13 | 0·40 | ||||
Sample size is 400 (50 plants per population).
Phenotypic traits and pollinator visitation rate
Four pollinator functional groups (butterflies, large bees, bee-flies and hoverflies) were associated with the same plant phenotypic traits in all eight of the studied populations during 2005, since no significant population × trait interaction terms were found (Table 4). Two pollinator groups, butterflies and large bees, were associated with highly rewarding plants. Thus, large bees visited plants with many flowers (estimate + 1 s.e. = 0·01 ± 0·001, χ2 = 26·51, P < 0·0001), large flowers (0·20 ± 0·08, χ2 = 6·68, P < 0·001) and long corolla tubes (0·18 ± 0·05, χ2 = 10·40, P < 0001). Similarly, the visitation rate of butterflies was significantly higher in plants with large corolla diameter (0·35 ± 0·15, χ2 = 5·42, P < 0·05; multiple Poisson regression). In contrast, bee-flies tended to visit plants with tall stalks (0·03 ± 0·001, χ2 = 32·29, P < 0·0001), whereas hoverflies visited mostly plants with many flowers (0·01 ± 0·001, χ2 = 21·14, P < 0·0001) and short corolla tubes (−0·45 ± 0·14, χ2 = 10·63, P < 0·0001).
Table 4.
Summary of multiple-response-curves fitting of each pollinator group to Erysimum mediohispanicum phenotypic traits at the individual plant level. Equations refer to the best prediction of visitation rate for each pollinator functional group (see Appendix for details)
| Functional groups | Populations | Best fitted predictive equation |
|---|---|---|
| Large bees | All | 0·01Flower number + 0·20Corolla diameter + 0·18Corolla tube length |
| Bee-flies | All | 0·03Stalk height |
| Butterflies | All | 0·35Corolla diameter |
| Hoverflies | All | 0·01Flower number – 0·45Corolla tube length |
| Small bees | Em21 | 0·01Flower number – 0·23Corolla tube length |
| Em01 | 0·01Flower number – 0·08Stalk height | |
| Em22 | 0·02Flower number – 0·07Stalk height | |
| Em23 | 0·01Flower number + 0·74Corolla diameter | |
| Em24 | 0·02Flower number | |
| Em25 | 0·04Flower number | |
| Em02 | 0·01Flower number – 0·07Stalk height + 0·46Corolla tube length | |
| Beetles | Em21 | –0·67Corolla tube length |
| Em01 | 0·01Flower number – 0·05Stalk height + 1·64Corolla diameter + 0·50Corolla tube length | |
| Em23 | 0·01Flower number | |
| Em24 | 0·02Flower number + 1·27Corolla tube length | |
| Em25 | 0·02Flower number – 0·07Stalk height | |
| Em02 | 0·01Flower number + 0·87Corolla diameter | |
| Wasps | Em23 | 2·08Corolla diameter |
| Em24 | 0·04Corolla diameter | |
| Em02 | –0·84Stalk height + 3·27Corolla diameter – 3·15Corolla tube length | |
| Others | Em08 | 0·13Flower number – 0·36Stalk height |
| Em01 | 0·02Flower number | |
| Em25 | 0·05Flower number – 1·83Corolla tube length | |
| Em02 | –0·26Stalk height |
Visitation rates were fitted to Poisson distributions because they were estimated as number of insects per plant.
Sample size is 720 (90 plants per population).
For functional groups showing a significant phenotypic trait × population interaction term, a separate Poisson multiple regression was performed for each population (see Appendix for details). These analyses suggest that these pollinator groups have different preference patterns in different plant populations. Even so, some general patterns can be derived from the results, since small bees mostly visited plants with many-flowers (seven populations) and short stalks (three populations), and small beetles tended to visit plants with many flowers (four populations; Table 4).
DISCUSSION
Nectar and pollen production showed high variation in E. mediohispanicum. Interestingly, within-population variation was much higher for nectar than for pollen production. In contrast to other morphological floral traits, nectar is a physiological trait and its production is thus affected by the condition of the plants. Consequently, intrapopulation variation in nectar is frequent in many plant species (Real and Rathcke, 1991; Hodges, 1995; Boose, 1997).
This study showed that corolla tube length was positively related to nectar production rate, whereas corolla diameter was positively related to pollen production. These phenotypic relationships have been found in other plant species (Plowright, 1981; Stanton and Preston, 1988; Harder and Cruzan, 1990; Young and Stanton, 1990; Dafni, 1991; Navarro, 1996; Worley and Barrett, 2000; Kaczorowski et al., 2005; Fenster et al., 2006; Ornela et al., 2007; but see Zimmerman and Pyke, 1986). Several non-exclusive proximate causes could explain the observed phenotype–reward associations. For example, larger flowers have deeper corollas, hold larger nectar glands and provide more space for nectar accumulation in Nicotiana alata (Kaczorowski et al., 2005). Navarro (1996) suggested that the significant association between flower size and nectar production in Petrocoptis grandiflora could result from larger flowers producing or accumulating more photosynthates (see also Harder and Cruzan, 1990). Similarly, Ornela et al. (2007) have recently shown that, in hummingbird-pollinated plants, there has been a correlated evolution between nectar production and corolla tube length. These authors propose that this correlation occurs because flowers with longer corollas will be able to hold more nectar or have larger nectaries. Finally, deep corolla tubes would slow down nectar evaporation, resulting in a higher amount of nectar in flowers with a deeper corolla (Pleasant, 1983).
This study has shown that these two plant traits, floral size and corolla tube length, are positively associated with the visitation rate of one group of efficient pollinators, the large bees. In general, the results agree with the foraging patterns and behaviour reported for the same pollinator groups in other studies. Thus, many studies have shown that long-tongued pollinators, such as large bees, prefer to forage in plants with deep corollas and large flowers (Inouye, 1980; Galen et al., 1987; Campbell, 1991; Goulson, 1999; Corbet, 2000; Gómez and Zamora, 2000; Szucsich and Krenn, 2002; Martin, 2004; Wilson et al., 2004; Ishii and Harder, 2006). In contrast, short-tongued pollinators, such as hoverflies, prefer to forage in plants with a short corolla, since they find easier access to nectar and pollen (Gilbert, 1981; Branquart and Hemptinne, 2000; Colley and Luna, 2000). Even though we were unable to quantify rewards and pollinator attraction in the same plants due to the destructive methodology used to study reward production (see Methods), our findings suggest that large bees could use some E. mediohispanicum floral traits as cues to identify appropriate reward production. In fact, it has been repeatedly shown that this kind of pollinator can learn to discriminate amongst flowers based on reward availability (von Frisch, 1965; Hammer and Menzel, 1995; Goulson, 1999; Waddington, 2001; Makino and Sakai, 2007). It is remarkable that the other flower visitors were not attracted by these two reward-related phenotypic traits, despite the fact that some pollinators (like bee-flies or butterflies) are mostly nectarivorous. Based on our analysis of visitation rates, we presume that most of these pollinators would display a size-based rather than a reward-based foraging behaviour (sensu Makino and Sakai, 2007), being attracted to plants having a larger flower display, irrespectively of the reward of each individual flower.
Previous studies have demonstrated the occurrence of selection on E. mediohispanicum corolla diameter and corolla tube length, with plants having larger flowers and longer corollas producing more seeds and seedlings (Gómez, 2003, 2008; Gómez et al., 2006). Interestingly, this study has found that these traits are positively associated with reward production. In addition, since pollen production contributes to plant fitness, this outcome suggests that flower size can be selected in E. mediohispanicum not only through female function (seed production), but also through male fitness. It is interesting to note that the selection caused by the association between reward and phenotype does not affect other plant traits also under pollinator-mediated selection in E. mediohispanicum, such as stalk height, number of flowers per individual and corolla tube width (Gómez, 2003; Gómez et al., 2006, unpubl. res.). Other mechanisms might drive selection for these traits. For example, a positive relationship between floral display (measured as number of open flowers per individual) and number of pollinators attracted has been found in numerous species (Mitchell et al., 2004; Grindeland et al., 2005). Increased pollinator attraction in multi-flowered plants has been related to increased male function via pollen dispersal (Biernaskie and Cartar, 2004). This mechanism may cause pollinator-mediated selection on flower number even if this trait does not act as a per-flower indicator of reward. In this scenario, floral display size positively correlates with overall reward size (Makino and Sakai, 2007). Consequently, pollinators preferring plants with many flowers are ensuring a copious amount of pollen and nectar during their foraging bouts since, upon landing on an E. mediohispanicum individual, they usually visit several flowers before moving onto another plant (authors' unpubl. data). In fact, we found that large bees also preferred plants with many flowers, maximizing in this way their nectar and pollen intake rate.
In summary, this study has shown the occurrence of significant associations between some E. mediohispanicum floral traits and reward production. Unfortunately, since reward and pollinator visitation were determined on different plants in different years, we need to be cautious in concluding that the E. mediohispanicum floral traits act as reward signals. Further experimental studies are necessary to determine if they represent true reward signals, explaining the previously demonstrated pollinator-mediated selection on the floral traits. Exploring pollinator behavioural responses to the experimental manipulation of both reward and plant phenotype will help to clarify the role of floral traits as reward signals in E. mediohispanicum.
ACKNOWLEDGEMENTS
We thank James Cresswell and three anonymous reviewers for helpful comments on the manuscript. The Ministerio de Medio Ambiente and Consejería de Medio Ambiente of the Junta de Andalucía granted permission to work in the Sierra Nevada National Park. This study was partially supported by the Spanish MCyT (GLB2006–04883/BOS) and Junta de Andalucía PAI (RNM 220 and CVI 165).
APPENDIX
Summary of multiple-response-curves fitting of each pollinator group to Erysimum mediohispanicum phenotypic traits at the plant individual level. Figures are estimates ± s.e. from multiple Poisson regressions performed separately for each plant population
| Plant populations |
||||||||
|---|---|---|---|---|---|---|---|---|
| Em08 | Em21 | Em01 | Em22 | Em23 | Em24 | Em25 | Em02 | |
| Small bees | ||||||||
| Stalk height | 0·01 ± 0·02 | –0·01 ± 0·01 | –0·08 ± 0·03** | –0·07 ± 0·03* | –0·01 ± 0·02 | 0·00 ± 0·03 | –0·02 ± 0·03 | –0·07 ± 0·04* |
| Flower number | 0·00 ± 0·01 | 0·01 ± 0·01* | 0·01 ± 0·00* | 0·02 ± 0·01* | 0·01 ± 0·00**** | 0·02 ± 0·01**** | 0·04 ± 0·01*** | 0·01 ± 0·00**** |
| Corolla tube width | 0·19 ± 0·59 | 0·74 ± 0·23** | 1·67 ± 0·60** | –0·68 ± 0·64 | –0·99 ± 0·69 | –0·18 ± 0·55 | 0·71 ± 0·84 | 0·39 ± 0·46 |
| Corolla diameter | 0·08 ± 0·24 | –0·18 ± 0·11 | –0·14 ± 0·26 | 0·36 ± 0·33 | 0·74 ± 0·36* | 0·15 ± 0·25 | –0·11 ± 0·45 | –0·29 ± 0·25 |
| Corolla tube length | –0·24 ± 0·27 | –0·23 ± 0·09* | –0·12 ± 0·22 | 0·00 ± 0·23 | –0·10 ± 0·23 | 0·28 ± 0·19 | –0·15 ± 0·24 | 0·47 ± 0·20* |
| Beetles | ||||||||
| Stalk height | 0·01 ± 0·01 | 0·02 ± 0·02 | –0·05 ± 0·02**** | 0·01 ± 0·03 | 0·01 ± 0·02 | –0·07 ± 0·04 | –0·07 ± 0·03** | –0·03 ± 0·02 |
| Flower number | 0·01 ± 0·01 | 0·00 ± 0·01 | 0·01 ± 0·00**** | 0·01 ± 0·01 | 0·00 ± 0·00 | 0·02 ± 0·01** | 0·02 ± 0·01* | 0·01 ± 0·00* |
| Corolla tube width | –0·43 ± 0·47 | 0·59 ± 0·46 | 0·14 ± 0·44 | 0·40 ± 0·64 | 0·56 ± 0·51 | –0·21 ± 0·77 | 0·00 ± 0·48 | –0·34 ± 0·29 |
| Corolla diameter | 0·33 ± 0·23 | 0·20 ± 0·23 | 1·64 ± 0·55**** | –0·13 ± 0·30 | –0·31 ± 0·29 | –0·32 ± 0·43 | 0·23 ± 0·27 | 0·11 ± 0·15 |
| Corolla tube length | –0·23 ± 0·18 | –0·67 ± 0·22*** | 0·50 ± 0·14**** | 0·30 ± 0·20 | –0·02 ± 0·14 | 1·27 ± 0·37**** | –0·10 ± 0·20 | 0·00 ± 0·11 |
| Wasps | ||||||||
| Stalk height | 0·02 ± 0·02 | 0·11 ± 0·16 | 0·14 ± 0·21 | 0·02 ± 0·06 | 0·00 ± 0·08 | 0·84 ± 0·39**** | ||
| Flower number | 0·02 ± 0·01 | –0·05 ± 0·12 | 0·02 ± 0·07 | 0·01 ± 0·01 | 0·04 ± 0·02*** | 0·04 ± 0·05 | ||
| Corolla tube width | 0·86 ± 0·57 | 2·18 ± 3·18 | 0·77 ± 6·03 | 5·35 ± 1·88**** | 0·52 ± 1·30 | 1·79 ± 2·29 | ||
| Corolla diameter | –0·08 ± 0·26 | 1·14 ± 1·73 | –1·27 ± 2·93 | 2·08 ± 0·82*** | –0·96 ± 0·64 | 3·27 ± 1·72* | ||
| Corolla tube length | –0·28 ± 0·22 | –0·68 ± 1·17 | 0·05 ± 1·83 | 0·76 ± 0·61 | 0·51 ± 0·49 | –3·51 ± 1·72** | ||
| Other | ||||||||
| Stalk height | –0·36 ± 0·26*** | –0·04 ± 0·05 | 0·00 ± 0·03 | 0·14 ± 0·11 | –0·26 ± 0·14** | |||
| Flower number | 0·13 ± 0·09** | 0·04 ± 0·03 | 0·02 ± 0·01*** | 0·05 ± 0·03* | 0·01 ± 0·02 | |||
| Corolla tube width | 6·53 ± 4·58** | 2·24 ± 1·37 | 1·30 ± 0·82 | 0·60 ± 0·94 | –1·12 ± 0·84 | |||
| Corolla diameter | –1·45 ± 1·04 | –0·04 ± 0·56 | –0·53 ± 0·33 | 0·39 ± 0·64 | 0·77 ± 0·54 | |||
| Corolla tube length | 0·39 ± 0·87 | –0·31 ± 0·46 | 0·30 ± 0·30 | –1·84 ± 0·94** | –0·72 ± 0·57 | |||
Only pollinator functional groups showing a significant population × plant phenotype interaction term are shown.
Cells without data were a consequence of a singularity in the data matrix (with Hessian not positive definite), which produce failure in the convergence process. This was most likely due to a poor fit of the model or to linear dependencies among model covariates, probably as a consequence of low abundance of pollinators in those populations that generate over-dispersion.
* P <0·05, ** P <0·01, *** P <0·001, **** P <0·0001.
LITERATURE CITED
- Armbruster WS, Antonsen L, Pelabon C. Phenotypic selection on Delachampia blossoms: honest signaling affects pollination success. Ecology. 2005;86:3323–3333. [Google Scholar]
- Ashman TL, Stanton M. Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana spp. spicata (Malvaceae) Ecology. 1991;72:993–1003. [Google Scholar]
- Biernaskie JM, Cartar RV. Variation in rate of nectar production depends on floral display size: a pollinator manipulation hypothesis. Functional Ecology. 2004;18:125–129. [Google Scholar]
- Blarer A, Keasar T, Shmida A. Possible mechanisms for the formation of flower size preferences by foraging bumblebees. Ethology. 2002;108:341–351. [Google Scholar]
- Boose DL. Sources of variation in floral nectar production rate in Epilobium canum (Onagraceae): implications for natural selection. Oecologia. 1997;110:493–500. doi: 10.1007/s004420050185. [DOI] [PubMed] [Google Scholar]
- Branquart E, Hemptinne JL. Selectivity in the exploitation of floral resources by hoverflies (Diptera: Syrphinae) Ecography. 2000;23:732–742. [Google Scholar]
- Campbell DR. Effects of floral traits on sequential components of fitness in Ipomopsis aggregata. American Naturalist. 1991;137:713–737. [Google Scholar]
- Campbell DR, Waser NM, Price MV, Lynch EA, Mitchell RJ. Components of phenotypic selection: pollen export and flower corolla width in Ipomopsis aggregata. Evolution. 1991;45:1458–1467. doi: 10.1111/j.1558-5646.1991.tb02648.x. [DOI] [PubMed] [Google Scholar]
- Cohen D, Shmida A. The evolution of flower display and reward. Evolutionary Biology. 1993;27:197–243. [Google Scholar]
- Colley MR, Luna JM. Relative attractiveness of potential insectary plants to aphidophagous hoverflies (Diptera: Syrphidae) Environmental Entomology. 2000;29:1054–1059. [Google Scholar]
- Corbet SA. Butterfly nectaring flowers: butterfly morphology and flower form. Entomologia Experimentalis et Applicata. 2000;96:289–298. [Google Scholar]
- Cresswell JE. The influence of nectar and pollen availability on pollen transfer by individual flowers of oil-seed rape (Brassica napus) when pollinated by bumblebees (Bombus lapidarius) Journal of Ecology. 1999;87:670–677. [Google Scholar]
- Cunningham JP, West SA, Wright DJ. Learning in the nectar foraging behaviour of Helicoverpa armigera. Ecological Entomology. 1998;23:363–369. [Google Scholar]
- Dafni A. Advertisement, flower longevity, reward and nectar production in Labiatae. Acta Horticulturae. 1991;288:340–346. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. 3rd ed. Oxford, UK: Pergamon Press; 1979. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Reviews of Ecology, Evolution & Systematics. 2004;35:375–403. [Google Scholar]
- Fenster CB, Cheely G, Dudash MR, Reynolds RJ. Nectar reward and advertisement in hummingbird-pollinated Silene virginica (Caryophyllaceae) American Journal of Botany. 2006;93:1800–1807. doi: 10.3732/ajb.93.12.1800. [DOI] [PubMed] [Google Scholar]
- von Frisch K. Tanzsprache un orientierung der Bienen. Berlin: Springer; 1965. [Google Scholar]
- Galen C, Plowright RC. The effects of nectar level and flower development on pollen carry-over in inflorescences of fireweed (Epilobium angustifolium) Canadian Journal of Botany. 1985;63:488–491. [Google Scholar]
- Galen C, Zimmer KA, Newport ME. Pollination in floral scent morphs of Polemonium viscosum: a mechanism for disruptive selection on flower size. Evolution. 1987;41:599–606. doi: 10.1111/j.1558-5646.1987.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Gilbert FS. Foraging ecology of hoverflies: morphology of the mouthparts in relation to feeding on nectar and pollen in some common urban species. Ecological Entomology. 1981;6:245–262. [Google Scholar]
- Gómez JM. Herbivory reduces the strength of pollinator-mediated selection in the Mediterranean herb Erysimum mediohispanicum: consequences for plant specialization. American Naturalist. 2003;162:242–256. doi: 10.1086/376574. [DOI] [PubMed] [Google Scholar]
- Gómez JM. Non-additivity effect of herbivores and pollinators on Erysimum mediohispanicum (Cruciferae) fitness. Oecologia. 2005;143:412–418. doi: 10.1007/s00442-004-1809-7. [DOI] [PubMed] [Google Scholar]
- Gómez JM. Sequential conflicting selection due to multispecific interactions triggers evolutionary trade-offs in a monocarpic herb. Evolution. 2008;62:668–679. doi: 10.1111/j.1558-5646.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Zamora R. Spatial variation in the selective scenarios of Hormathophylla spinosa (Cruciferae) American Naturalist. 2000;155:657–668. doi: 10.1086/303353. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Perfectti F, Camacho JPM. Natural selection on Erysimum mediohispanicum flower shape: insights into the evolution of zygomorphy. American Naturalist. 2006;168:531–545. doi: 10.1086/507048. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Bosch J, Perfectti P, Fernandez JD, Abdelaziz M. Pollinator diversity affects plant reproduction and recruitment: the tradeoffs of generalization. Oecologia. 2007;153:597–605. doi: 10.1007/s00442-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Goulson D. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspectives in Plant Ecology, Evolution and Systematics. 1999;2:185–209. [Google Scholar]
- Grindeland JM, Sletwold N, Ims RA. Effects of floral display size and plant density on pollinator visitation in a natural population of Digitalis purpurea. Functional Ecology. 2005;19:383–390. [Google Scholar]
- Hammer M, Menzel R. Learning and memory in the honeybee. Journal of Neuroscience. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. [Google Scholar]
- Harder LD, Cruzan MB. An evaluation of the physiological and evolutionary influences of inflorescence size and flower depth on nectar production. Functional Ecology. 1990;4:559–572. [Google Scholar]
- Heinrich B. Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia. 1979;40:235–245. doi: 10.1007/BF00345321. [DOI] [PubMed] [Google Scholar]
- Hodges SA. Influence of nectar production on hawkmoth behavior, self pollination, and seed production in Mirabilis multiflora (Nyctaginaceae) American Journal of Botany. 1995;82:197–204. [Google Scholar]
- Inouye DW. The effects of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia. 1980;45:197–201. doi: 10.1007/BF00346460. [DOI] [PubMed] [Google Scholar]
- Internicola AI, Page PA, Bernasconi G, Gigord LDB. Competition for pollinator visitation between deceptive and rewarding artificial inflorescences: an experimental test of the effects of floral colour similarity and spatial mingling. Functional Ecology. 2007;21:864–872. [Google Scholar]
- Ishii HS, Harder LD. The size of individual Delphinium flowers and the opportunity for geitonogamous pollination. Functional Ecology. 2006;20:1115–1123. [Google Scholar]
- Kaczorowski RL, Gardener MC, Holtsford TP. Nectar traits in Nicotiana section alatae (Solanaceae) in relation to floral traits, pollinators, and mating system. American Journal of Botany. 2005;92:1270–1283. doi: 10.3732/ajb.92.8.1270. [DOI] [PubMed] [Google Scholar]
- Kadmon R, Shmida A. Departure rules used by bees foraging for nectar: a field test. Evolutionary Ecology. 1992;6:142–151. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Boulder, CO: University Press of Colorado; 1993. [Google Scholar]
- Klinkhamer PGL, Van der Lugt PP. Pollinator service only depends on nectar production rates in sparse populations. Oecologia. 2004;140:491–494. doi: 10.1007/s00442-004-1569-4. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Barrett SCH. Floral biology, studies on floral evolution in animal-pollinated plants. New York: Academic Press; 1986. [Google Scholar]
- Makino TT, Sakai S. Experience changes pollinator responses to floral display size: from size-based to reward-based foraging. Functional Ecology. 2007;21:854–863. [Google Scholar]
- Martin NH. Flower size preferences of the honeybee (Apis mellifera) foraging on Mimulus guttatus. (Scrophulariaceae) Evolutioanry Ecology Research. 2004;6:777–782. [Google Scholar]
- Mitchell RJ. Adaptive significance of Ipomopsis aggregata nectar production: pollination success of single flowers. Ecology. 1992;73:633–638. [Google Scholar]
- Mitchell RJ. Adaptive significance of Ipomopsis aggregata nectar production: observation and experiment in the field. Evolution. 1993;47:25–35. doi: 10.1111/j.1558-5646.1993.tb01196.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Waser NM. Adaptive significance of Ipomopsis aggregata nectar production: pollination success of single flowers. Ecology. 1992;73:633–638. [Google Scholar]
- Mitchell RJ, Karron JD, Holmquist KG, Bell JM. The influence of Mimulus ringens floral display size on pollinator visitation patterns. Functional Ecology. 2004;18:116–124. [Google Scholar]
- Navarro L. Santiago de Compostela, Spain: Universidade de Santiago de Compostela; 1996. Biología reproductiva y conservación de dos endemismos del noroccidente Ibérico: Petrocoptis grandiflora Rothm y Petrocoptis viscosa Rothm (Caryophyllaceae) PhD Thesis. [Google Scholar]
- Neff JL, Simpson BB. The roles of phenology and reward structure in the pollination biology of wild sunflower (Helianthus annuus L. Asteraceae) Israel Journal of Botany. 1990;39:197–216. [Google Scholar]
- Ohashi K, Yahara T. Behavioral responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Ornela JF, Ordano M, de Nova AJ, Quintero ME, Garland T., Jr Phylogenetic analysis of interspecific variation in nectar of hummingbird-visited plants. Journal of Evolutionary Biology. 2007;20:1904–1917. doi: 10.1111/j.1420-9101.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- Pleasant JM. Nectar production patterns in Ipomopsis aggregata (Polemoniaceae) American Journal of Botany. 1983;70:1468–1475. [Google Scholar]
- Pleasants JM, Zimmerman M. Patchiness in the dispersion of nectar resources: evidence for hot and cold spots. Oecologia. 1979;41:283–288. doi: 10.1007/BF00377432. [DOI] [PubMed] [Google Scholar]
- Plepys D, Ibarra F, Fracnke W, Löfstedt C. Odour-mediated nectar foraging in the silver Y moth Autographa gamma (Lepidoptera: Noctuidae): behavioural and electrophysiological responses to floral volatiles. Oikos. 2002;99:75–82. [Google Scholar]
- Plowright RC. Nectar production in the boreal forest lily Clintonia borealis. Canadian Journal of Botany. 1981;59:156–160. [Google Scholar]
- Pyke GH. Optimal foraging: movement patterns of bumblebees between inflorescences. Theoretical Population Biology. 1978;13:72–98. doi: 10.1016/0040-5809(78)90036-9. [DOI] [PubMed] [Google Scholar]
- Rawling JO, Pantula SG, Dickey DA. Applied regression analysis, a research tool. New York: Springer; 1998. [Google Scholar]
- Real LA, Rathcke BJ. Individual variation in nectar production and its effect on fitness in Kalmia latifolia. Ecology. 1991;72:149–155. [Google Scholar]
- Schaefer HM, Schaefer V, Levey DJ. How plant–animal interactions signal new insights in communication. Trends in Ecology and Evolution. 2004;19:1–8. [Google Scholar]
- Scheiner R, Erber J, Page RE., Jr Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.) Journal of Comparative Physiology A. 1999;185:1–10. doi: 10.1007/s003590050360. [DOI] [PubMed] [Google Scholar]
- Simpson BB, Neff JL. Evolution and diversity of floral rewards. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York: Van Nostrand Reinold Co; 1983. pp. 142–159. [Google Scholar]
- Smithson A, Macnair MR. Negative frequency-dependent selection by pollinators on artificial flowers without rewards. Evolution. 1997;51:715–723. doi: 10.1111/j.1558-5646.1997.tb03655.x. [DOI] [PubMed] [Google Scholar]
- Stanton M, Young HJ. Selection for floral character associations in wild radish. Journal of Evolutionary Biology. 1994;7:271–285. [Google Scholar]
- Stanton ML, Preston RE. Ecological consequences and phenotypic correlates of flower size variation in wild radish, Raphanus sativus L. (Brassicaceae) American Journal of Botany. 1988;75:528–539. [Google Scholar]
- Szucsich NU, Krenn HW. Flies and concealed nectar sources: morphological innovations in the proboscis of Bombyliidae (Diptera) Acta Zoologica. 2002;83:183–192. [Google Scholar]
- Thomson JD, Plowright RC. Pollen carryover, nectar rewards, and pollinator behavior with special reference to Diervilla lonicera. Oecologia. 1980;46:68–74. doi: 10.1007/BF00346968. [DOI] [PubMed] [Google Scholar]
- Thomson JD, McKenna MA, Cruzan MB. Temporal patterns of nectar and pollen production in Aralia hispida: implications for reproductive success. Ecology. 1989;70:1061–1068. [Google Scholar]
- Waddington KD. Subjective evaluation and choice behavior by nectar- and pollen-collecting bees. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination: animal behavior and floral evolution. Cambridge: Cambridge University Press; 2001. pp. 41–60. [Google Scholar]
- Wilson P, Castellanos MC, Hogues JN, Thomson JD, Armbruster WS. A multivariate search for pollination syndrome among penstemons. Oikos. 2004;104:345–361. [Google Scholar]
- Worley AC, Barrett SCH. Evolution of floral display in Eichhornia paniculata (Pontederiaceae): direct and correlated responses to selection on flower size and number. Evolution. 2000;54:1533–1545. doi: 10.1111/j.0014-3820.2000.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Young HJ, Stanton ML. Influences of floral variation on pollen removal and seed production in wild radish. Ecology. 1990;71:536–547. [Google Scholar]
- Zimmerman M. Plant reproduction and optimal foraging: experimental nectar manipulations in Delphinium nelsonii. Oikos. 1983;41:57–63. [Google Scholar]
- Zimmerman M, Pyke GH. Reproduction in Polemonium: patterns and implications of floral nectar production and standing crops. American Journal of Botany. 1986;73:1405–1415. [Google Scholar]


