Abstract
BACKGROUND
In mouse endometrium, glycogen synthase kinase-3ß (GSK3ß) is a key enzyme controlling nuclear localization of cyclin D1. We developed a functional model of xenografted human endometrium to test whether similar mechanisms are operative in the human by using Lithium chloride (LiCl), an inhibitor of GSK3ß.
METHODS
Human endometrial samples were obtained from normal volunteers, then implanted under the kidney capsule of nude mice, and treated with estradiol-17ß (E2) or LiCl. Xenografts were assessed for protein expression of MKI-67, mini-chromosome maintenance protein-2, estrogen receptor (ER), progesterone receptor (PR) and cyclin D1.
RESULTS
Both E2 and LiCl induced a robust proliferative response in the epithelium. Only lithium treatment produced clear nuclear localization of cyclin D1 consistent with the proliferative response observed. Regenerated endometrium had detectable ER and PR expression.
CONCLUSION
Xenografted human endometrium provides a dynamic model of uterine biology. Administration of LiCl in the absence of E2 induced epithelial proliferation, supporting the hypothesis that human and murine endometrial proliferation may share key regulatory pathways. These data suggest a possible link between the increased menstrual disturbances in women with affective disorders taking lithium and the consequent potential for the development of endometrial proliferative disorder.
Keywords: estrogen, progesterone, cell cycle, endometrium, lithium
Introduction
In women, hormonal states that result in unopposed estrogen stimulation are associated with menstrual irregularities and an increased risk of endometrial hyperplasia and adenocarcinoma (Deligdisch, 2000). Uterine corpus cancer is the most common gynecologic malignancy in Western countries (Bray et al., 2005; Jemal et al., 2008). Although progestins have been extensively used in treating endometrial hyperplasia and selected cases of endometrial carcinoma (Kim et al., 1997), molecular mechanisms of sex steroids interaction in the human endometrium remain incompletely understood. Estradiol-17ß (E2) and progesterone (P4) coordinately regulate cyclic endometrial regeneration. E2 induces cell proliferation of the uterine epithelium, although P4 inhibits this cell proliferation and triggers differentiation.
In the mouse, endometrial epithelial cell proliferation is regulated by E2 activation of the canonical cell-cycle machinery. This is accomplished by the inactivation of a tumor-suppressor protein pRB and related family members via phosphorylation by cyclin/cyclin dependent kinases (Hatakeyama et al., 1994). In particular, E2 treatment causes the nuclear accumulation of cyclin D1 that, together with its partners CDK4 and CDK6, phosphorylates pRb at specific sites with the resultant progression of cells towards S-phase (Tong et al., 2008). The cellular localization of cyclin D1 is governed by glycogen synthase kinase-3ß (GSK3ß) (Takahashi-Yanaga and Sasaguri, 2008). This key mediator of intracellular signaling is also involved in the canonical Wnt signaling pathway where it interacts with ß-Catenin and was recently found to play a role in regulation of uterine epithelial cell proliferation (Hou et al., 2004; Wang et al., 2007). GSK3ß phosphorylates cyclin D1 on Thr286 increasing its efflux from the nucleus. GSK3ß enzyme activity is inhibited by E2 treatment through an inhibitory phosphorylation by AKT1 at Ser9 (Diehl et al., 1998; Alt et al., 2000). AKT1, in turn, is activated by phosphorylation in response to PI3-kinase activation by an IGF-1 receptor mediated mechanism that is up-regulated by E2. Studies indicate that whereas the binding of E2 to its receptor (ERα) is required for the stimulation of cell proliferation, once the pathway is started it becomes ER-independent (Hall et al., 2001; Levin, 2005). Consistent with this observation, inhibition of GSK3ß in the absence of E2 induces DNA synthesis in the mouse uterine epithelium (Zhu and Pollard, 2007). This pathway is inhibited by P4 that acts to block E2-induced AKT1 activation with the consequent maintenance of activated GSK3ß and nuclear exclusion of cyclin D1.
Mechanistic studies of the sex steroid hormone regulation of human endometrial epithelial cell proliferation are hindered by the inability to safely administer potentially toxic agents to volunteers. To overcome these problems, researchers have used human endometrial tissue xenografts to examine hormonal regulation of gene expression (Satyaswaroop and Tabibzadeh, 2000), endometriosis (Fasciani et al., 2003) and uterine stroma-epithelial interactions (Kurita et al., 2005). In this study, we modified a previously published protocol that entailed collecting endometrial biopsies from healthy volunteers, recombining endometrial glands and stroma in collagen gels, and transplanting the xenografts under the kidney capsule of nude mice (Cunha and Young, 1992; Cooke et al., 1997) to study the regulation of cell proliferation. We sought to test the hypothesis that, as in the mouse, inhibition of GSK3ß in the absence of E2 is sufficient to cause a proliferative response in human endometrium.
Materials and Methods
Human endometrial tissue acquisition
Human endometrial tissue was acquired by biopsy from healthy female volunteers, ages 18–45, recruited from the community. Inclusion criteria for the study required regular menstrual cycles and no history of gynecological disease. Women who were breast-feeding or using hormonal contraception within 3 months of study entry were excluded. Screening done specifically for study purposes included negative urine pregnancy test, normal pap smear and a normal saline hysterosonogram. Endometrial biopsies were timed to be performed in the proliferative phase, days 9–12 after the first day of menses. All biopsies were performed by aspiration using a Pipelle catheter (Unimar, Wilton, CT, USA) with the aim of sampling fundal endometrium. The protocol was approved by the Institutional Review Board, Montefiore Medical Center and by the General Clinical Research Center Advisory Committee, Albert Einstein College of Medicine in accordance with the Declaration of Helsinki for Medical Research. Informed consent was obtained from all participants.
Preparation of endometrial cell extracts
The aspirated endometrial tissue was immediately washed in prewarmed McCoy's 5A medium (Invitrogen, Carlsbad, CA, USA) to remove blood and mucus. Subsequently, the tissue was minced for 5 min into 2–3 mm pieces and digested with collagenase type I (1.5 mg/ml; Worthington, Freehold, NJ, USA) and DNAase I (1.0 U/ml; Sigma Aldrich, St Louis, MO, USA) in McCoy's 5A medium. The cell suspension was placed in a shaking water bath at 37°C for 20 min. This procedure was performed three times with intermittent pipetting. The digested tissue suspension was subjected to centrifugation at 100 × g for 5 min. Next, using an established protocol of separating stroma from epithelium (Osteen et al., 1989), the suspension was filtered through a sterilized 85 µm nylon mesh filter (Small Parts, Miramar, FL, USA).
Retained tissue from the first filtration was digested with hyaluronidase (0.5%; Sigma), protease (0.5%; Sigma) and DNase I in McCoy's medium for 20 min. In this fashion, the epithelial cells are segregated as organoids (Fig. 1A) containing several glands in an effort to preserve physiological tissue orientation (Kurita et al., 2005). This method had been shown to yield pure isolates of stromal and epithelial compartments of human endometrium (Parekh et al., 2002).
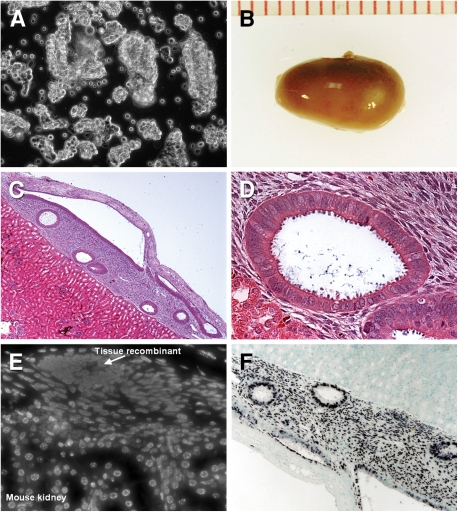
Figure 1.
Human endometrial tissue engrafted under the mouse renal capsule organizes into histologically normal uterine tissue.
(A) Phase contrast microscopy of the human endometrial epithelial cells isolated as organoids to preserve the physiological polarity of the tissue. (B) Macroscopic findings of the transplanted site of nude mice kidney harvested 6 weeks after transplantation. (C and D) H&E staining demonstrates several histologically normal uterine gland and stroma of human origin adjacent to mouse kidney parenchyma. (E) The species origin of the xenograft confirmed by staining with Hoechst 33258 dye shows characteristic punctuated nuclei in mouse cells and homogenous pattern in human cells. (F) ISH for human Alu-specific probe shows intense staining of the xenograft glands and stroma and no staining of the mouse parenchyma. Original magnification: (A) ×20, (C) ×5, (D) ×40, (E) ×20, (F) ×10.
The filtrate from the first filtration containing the stromal cells was then separated from the remaining red blood cells with one-layer Percoll (Sigma) density gradient. In order to obtain a single cell preparation of stromal cells, the suspension was then passed through a sterilized 20 µm nylon mesh filter (Small Parts). In general, one endometrial biopsy yielded ∼10–20 million of each epithelial and stromal cells sufficient for grafting 10–20 hosts.
Animals and treatments
Adult female nude mice were obtained from Taconic Farms (Germantown, NY, USA) and were maintained in accordance with the NIH Guide for Care and Use of Laboratory Animals. The Animal Institute Committee of Albert Einstein College of Medicine approved all procedures and protocols. Tissue recombinants were engrafted under the kidney capsule using a modified protocol described by Cunha (Cunha and Young, 1992; Cooke et al., 1997; http://mammary.nih.gov/tools/mousework/Cunha001/index.html). Briefly, freshly isolated uterine stroma and epithelium were recombined onto 30 µl of rat-tail collagen gel (BD Biosciences, Bedford, MA, USA) by placing three to five epithelial organoids and approximately 500 000 stromal cells per tissue recombinant. The recombinants were allowed to gel under room temperature. Kidney grafting was conducted via a dorsal incision under tribromoethanol (2.5%) anesthesia. One xenograft was implanted under the kidney capsule of each adult intact 8–10-week-old female nude mice. After allowing 6 weeks for tissue outgrowth, the hosts were ovariectomized via a dorsal incision. In most experiments, mice were then divided into groups of two to five animals per group according to the following treatments: (a) 5 days of daily subcutaneous injections of 125 ng of E2 (0.1 ml in peanut oil), starting 2 weeks after ovariectomy, (b) 20 days of drinking tap water with 0.05% lithium chloride (LiCL) (Sigma), (c) oil injections only and drinking plain tap water (controls). A concentration of lithium of 0.05% in drinking water has been demonstrated to result in proliferation of eutopic uterine epithelium in mice (Gunin et al., 2004). Animals were euthanized 18 h after the last E2 injection based on known kinetics of maximal proliferative response in xenografted endometrium (Kurita et al., 2005). Each experiment was repeated at least twice.
Histology and immunohistochemistry
At the end of the experimental protocol, the animals were killed in a carbon dioxide chamber in accordance with the guidelines on euthanasia of laboratory animals (AVMA, 2001). Xenografts were harvested, fixed with peroxidate–lysine–2% paraformaldehyde–0.05% glutaraldehyde, and processed for paraffin embedding. Cross sections (5-μm thickness) were mounted on glass slides and stained with hematoxylin and eosin. Two methods were used to confirm the species of origin of the tissue harvested from xenotransplantation. Staining with Hoechst dye produces characteristic punctuated nuclear staining in mouse tissues and homogenous staining of human nuclei (Cunha and Vanderslice, 1984). After treating the tissue with 1 mg/ml Hoechst 33258 dye (Sigma) for 1 min, the slides were washed and mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA) and examined by fluorescent microscopy. To further confirm the human origin of cells, in situ hybridization (ISH) for a human specific probe (Alu) was performed: after deparaffinization, sections were incubated with digoxigenin-labeled Alu probe (Invitrogen) at 37°C overnight. Hybridization was detected by using the anti-digoxigenin-AP antibody and developed by NTP/PCIP (Roche Applied Science, Indianapolis, IN, USA) as previously described (Pan et al., 2006).
Two markers were used as surrogates for cell proliferation: the antigen identified by monoclonal antibody Ki-67 (MKI67) and mini-chromosome maintenance deficient 2, mitotin (S. cerevisiae) (MCM2). Sex-steroid receptor (estrogen receptor, ERα, and progesterone receptor, PR) expression was assessed as a surrogate for hormonal receptivity of the xenografted endometrium. Monoclonal antibodies were used at the following concentrations: MKI67 (1:200, MB67, Neomarkers, Fremont, CA, USA), and cyclin D1 (1:100, Lab Vision, Fremont, CA, USA) and ERα (1:300, sc-8005, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Polyclonal antibodies were purchased from Santa Cruz Biotechnology and were used to detect expression of MCM2 (goat IgG, 1:300; sc-9839) and PR (rabbit IgG, 1:300; sc-538).
Immunohistochemical procedures were performed as previously described (Tong and Pollard, 1999). Briefly, 5 µm paraffin sections were deparaffinized and subjected to antigen retrieval by boiling the samples in 0.01 M sodium citrated buffer. The primary antibody was applied either for 60 min at room temperature (MKI67) or overnight at +4°C. The incubation times were kept consistent per antibody to optimize the staining, after which sections were washed in phosphate-buffered saline and then incubated with biotin-conjugated secondary antibodies (Vector Laboratories). Positive signals were visualized as brown precipitates utilizing 3,3′-diaminobenzidine tetrahydrochloride (Sigma). Sections were counterstained with hematoxylin. Negative controls included replacement of the primary antibody with either non-specific rabbit, goat or mouse immunoglobulin or saline. Immunopositive glandular epithelial cells were counted through the microscope at a magnification of ×100 with a mechanical tabulator. As described by others (Brenner et al., 2003), the protein expression was calculated as the labeling index, the proportion (%) of positively stained nuclei over the total number of nucleated cells present. Statistical comparisons were performed by Student's t test.
Results
Engraftment of human endometria under the kidney capsule had an approximately 50% success rate in our hands and is easily identified by the relatively avascular region on the surface (Fig. 1B). After 6 weeks of outgrowth, transverse histological sections revealed a morphological, vascularized endometrium with characteristic epithelial structures penetrating into a stroma that is clearly demarked from the underlying mouse kidney (Fig. 1C, D). Staining with Hoechst 33258 that gives characteristic nuclear morphology for mouse and human nuclei was used to determine the extent of contribution from mouse cells and to confirm the human origin of the graft and it limits (Cunha and Vanderslice, 1984). This showed that the xenotransplanted tissues were largely made up of human cells with only very limited hematopoietic infiltrate from the mouse (Fig. 1E). This relative lack of mouse contribution was confirmed by ISH for the human-specific Alu probe that revealed intense hybridization to the nuclei of the xenograft glands and stroma with none in the mouse tissue (Fig. 1F).
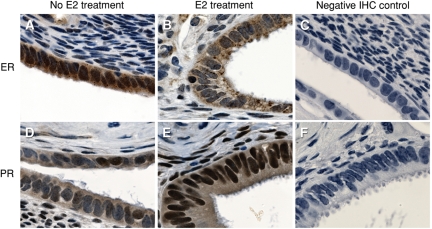
The endometrium is a classical sex steroid hormone target tissue characterized by ER and PR expression. Immunohistochemistry (IHC) for ERα using anti ERα antibodies revealed strong expression of ERα in the human xenotransplants following ovariectomy of the host mice. This was especially strong in the epithelial compartment with lesser expression in the stroma (Fig. 2). E2 treatment of the ovariectomized mice produced a relative down-regulation of ERα immunohistochemical staining (Fig. 2B) as compared with control (Fig. 2A). This treatment with E2 also resulted in an up-regulation of PR in both the stroma and epithelium of the xenografts (Fig. 2E) as compared with control (Fig. 2D). This hormone receptor expression suggests that the tissue will be responsive to these hormones. This can be seen by the induction of a characteristic stromal edema following E2 treatment of the ovariectomized mice (Fig. 2B, E).
Figure 2.
Human xenografts exhibit robust ER and PR expression.
Xenograft in an ovariectomized mouse without any hormonal treatment shows robust expression of ER by IHC (A). ER expression is down-regulated after E2 treatment (B). Negative IHC control with normal mouse (C) and rabbit (F) IgG. PR IHC of a xenotransplant in a mouse treated with E2 (D) demonstrates edema associated with E2 treatment and up-regulation of PR as compared with ovariectomized control (E). Brown staining indicates positive cells; two replicate experiments were conducted with same results. Original magnification ×100.
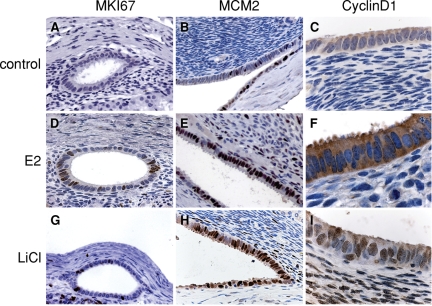
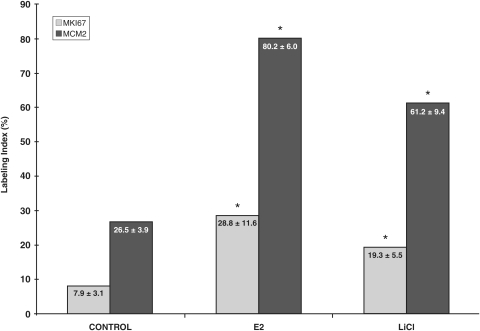
Ovariectomized mice had a low level of cell proliferation in both the stromal and epithelial compartment as assessed by the surrogate proliferation markers, MKI67 and MCM2. E2 treatment induced a characteristic proliferative response in the epithelium that was significantly elevated by about 3.5-fold over the ovariectomized controls as assessed by MKI67 or MCM2 staining (Figs. 3A, D and 4). Notably, MCM2 IHC revealed a greater proportion of proliferating cells (∼80%) than did MKI67 (∼30%) staining, although the proportional increment over controls was similar for these two markers (Figs. 3B, E and 4). Interestingly, this MKI67 and MCM2 nuclear expression was restricted to the epithelial compartment, with little in the stroma (Fig. 3).
Figure 3.
Human xenografts respond to E2 and LiCl treatment with DNA synthesis.
IHC staining of cell-cycle markers in human endometrial xenografts harvested from the host that were treated with 5 days of daily subcutaneous injections of 125 ng of 17B-estradiol (E2), 20 days of drinking tap water with 0.05% LiCl, or injected with oil only and were drinking plain tap water (control). As compared with controls (A–C), protein expression of MKI67, MCM2, and cyclin D1 was greater in E2 (D–F) and LiCl (G–I) treated xenografts. Brown staining indicates positive cells; three replicate experiments were conducted with same results. Original magnification: (C), (F), (I) ×100; otherwise ×40.
Figure 4.
Epithelial labeling index for MKI67 and MCM2.
Results of three replicate experiments are summarized and are expressed as mean ± standard deviation. * Signifies P < 0.01, compared with control.
To test the hypothesis that E2 signaling was through GSK-3β inhibition, we treated the ovariectomized xenotransplanted mice by oral administration of 0.05% LiCl, a relatively specific inhibitor of GSK3ß at this concentration (Rossig et al., 2002; Gunin et al., 2004) in the absence of E2. Xenotransplanted mice treated with LiCl for 20 days resulted in a statistically significant increase in the proportion of proliferating cells compared with control, as assessed by MKI67 and MCM2 staining (Figs. 3G, H and 4). The numbers of proliferating cells was similar to that observed with chronic E2 treatment.
In the mouse, inhibition of GSK3ß results in the nuclear localization of cyclin D1 in the uterine epithelial cells (Zhu and Pollard, 2007). To determine if this is the case for the endometrial transplants we immunostained the human endometrial tissue with an anti-cyclin D1 antibody. The endometrial transplants from ovariectomized mice showed little cyclin D1 staining in any cell type (Fig. 3C). Treatment with E2 resulted in an up-regulation, although this was mostly but not exclusively cytoplasmic (Fig. 3F). In contrast, LiCl treatment produced robust nuclear cyclin D1 staining in the xenografted epithelium (Fig. 3I) compared with the ovariectomized control group.
Discussion
Significant advances have been made in recent years in the understanding of uterine physiology, especially as it pertains to the molecular switch from E2 to P4-dominated signaling (Tong et al., 2008). Although it has been enormously helpful to use the mouse as an informative model system amenable to biochemical (Tong and Pollard, 1999) and genetic (Niklaus and Pollard, 2006) analysis, it is necessary to have a similar understanding in humans. Ex-vivo studies of human endometrial biopsies from normal reproductive aged women have given us insight at both the transcript and protein level (Niklaus et al., 2007), but have significant limitations for mechanistic studies. For this reason, we have used and improved a model in which immuno-compromised mice are transplanted with disaggregated and recombined human epithelial glands and stroma (Cunha and Young, 1992).
In this work, we demonstrate that human endometrial tissue xenografts retain their hormonal receptivity and capacity to proliferate in response to estrogen treatment. We demonstrate that they express ER and PR in the appropriate tissue compartments. Furthermore, the response of the expression of ER and PR to E2 is similar to that found in normal human endometrium (Brenner and Slayden, 2005; Niklaus et al., 2007). Additionally, there is a profound stromal edema response to E2 that is also comparable to that observed in the menstrual cycle. The degree of the proliferative reaction we observed in our xenografts is similar to that found in the work of others who have extensively manipulated human xenografts to study epithelial-stromal interactions (Kurita et al., 2005). In addition to the classic use of MKI67 as an indicator of uterine proliferative activity, we observed an even stronger MCM2 response to exogenous E2. MCM proteins are part of the DNA replication licensing complex that marks the origin of DNA replication and are essential for S-phase entry. Others have noted a higher proportion of MCM2 as compared with MKI67 stained proliferating cells in studies that performed side-by-side comparison of these antibodies as markers of cellular proliferation. In several studies of premalignant and malignant lung cells, anti-MCM2 antibodies stain a higher proportion of cells than do anti-Ki-67 antibodies (Ramnath et al., 2001; Tan et al., 2001). This divergence of data is difficult to explain on a mechanistic level due to the fact that a biological function of the protein that possesses the Ki-67 epitope is unknown (Endl and Gerdes, 2000). Further, MCM2 has been demonstrated as a reliable marker for endometrial hyperplasia (Kato et al., 2003) and, most recently, breast carcinoma (Reena et al., 2008). This data suggests that MCM2 expression in the endometrium will be a useful indicator of competence for a cell to enter into DNA synthesis. To confirm this, sustained incorporation of BrDU will need to be performed, as this is a definitive marker of DNA synthesis. Nevertheless, the data taken together have demonstrated that these xenotransplants of endometrial tissue provide a pathophysiologically relevant model in which to study human endometrial biology.
Work from our group points to GSK3ß as a central point in regulating the molecular switch from E2 to P4 dominated signaling in uterine epithelium. The cascade of events that trigger cell proliferation is initiated by E2 and ultimately leads to cyclin D1 nuclear accumulation that, in turn, results in cell cycle progression (Tong and Pollard, 1999). The cellular localization of cyclin D1 is governed by GSK3ß which is constitutively active at rest, favoring transportation of cyclin D1 out of the nucleus through a phosphorylation of Thr286 that allows interaction with the nuclear exportin, CREM1 (Alt et al., 2000). Upon E2 stimulation, activation of PI3K results in activating phosphorylation of protein kinase B (AKT) that, in turn, causes an inhibitory phosphorylation of GSK3ß. This inactivated GSK3ß no longer phosphorylates cyclin D1 and thus it accumulates in the nucleus where it together with its CDK partners 4 and 6 phosphorylates pRB and progression of the cell cycle (Tong and Pollard, 1999). Although P4 treatment suppresses this pathway, inhibition of GSK3ß reverses the P4 effect and results in progress towards DNA synthesis (Chen et al., 2005).
LiCl is an allosteric inhibitor of GSK3ß that inhibits its activity in a manner that is distinctly different to the mode of action of E2 (Rossig et al., 2002). In mice, LiCl had been demonstrated to augment the magnitude of the E2 proliferative effect (Gunin et al., 2004) as well as to result in endometrial proliferation in the absence of E2 (Zhu and Pollard, 2007). Although LiCl affects a multitude of signaling pathways, its mode of action on GSK3ß has been well correlated with the similar response to action of specific inhibitors of this enzyme (Fuentealba et al., 2007). Indeed, inhibition of GSK3ß using a more specific inhibitor SB415286 applied directly to the uterine luminal epithelium in mice also results in an E2-independent induction of DNA synthesis (Zhu and Pollard, 2007), indicating that it acts downstream of the ER. In the present study, we show that the human endometrium also responds to LiCl by DNA synthesis in an E2 independent manner.
One of the hypotheses that we tested was whether cyclin D1 expression in the nucleus of human uterine epithelial cells occurs co-incident with the proliferative response in a manner similar to the mouse. Although lithium treatment produced robust nuclear staining in the xenografted epithelium, E2-treated glands exhibited staining that was largely restricted to the cytoplasm. Thus, we were not able to confirm the pattern of nuclear localization of cyclin D1 that is characteristic for the mouse response of uterine epithelium to E2. However, differences in the respective treatments may account for the observed results. Lithium administration in drinking water that is consumed ad lib throughout the day is expected to produce a sustained serum concentration. In contrast, once-a-day injection of E2 is known to result in a transient peak of ER occupancy 2 h after administration (Martin et al., 1976). Consequently in the mouse uterine epithelium, there is a temporally brief window of nuclear expression of cyclin D1 that is maximal at 3–5 h after this E2 administration (Tong and Pollard, 1999; Tong et al., 2008). Thus, prolonged E2 exposure (5 days) used in this study might have resulted in our experiments missing the transient cyclin D1 nuclear influx or, alternatively, longer E2 exposure (14 days) as seen during the human menstrual cycle might be required for significant nuclear localization to be detected. Nevertheless, we have shown that GSK3ß inhibition is sufficient to induce human endometrial epithelial proliferation and future studies will determine if it is necessary for the E2-induced response in these cells.
A key rationale for this work is an attempt to verify whether the molecular mechanisms in response to E2 stimulation are identical or similar between the human and mouse endometrium. Studies of human endometrium have produced mixed results. Cell culture-models indicate that, similar to the Muridae, growth of human endometrial epithelium in response to E2 is mediated by the stromal cells via a presumably paracrine mechanism (Arnold et al., 2001; Pierro et al., 2001). Conversely, a study of chimeric tissue recombinants that combined human and mouse endometrial compartments concluded that the ER in the uterine stroma is not necessary for the E2 response in human uterine epithelium (Kurita et al., 2005). However, the chimeric model utilizes mouse uterine stroma from the immature neonatal tissue. It is conceivable that the mature adult mouse endometrium differs in hormonal and growth response from its immature antecedent. Similarly, although the disparate response in cyclin D1 localization in response to E2 may represent a mechanistic difference between the mouse and human, more work is needed to elucidate the central role of this machinery of hormonal regulation in humans.
The studies showing that inhibition of GSK3ß induces DNA synthesis in human endometrial cells may be especially important in light of the fact that lithium is a commonly taken drug. Lithium has been used for treatment of bipolar disorder since 1970s and has been prescribed to millions of women worldwide. Endometrial hyperplasia has been reported in women with affective disorders who had been treated with mood stabilizers (Joffe, 2007); yet, most of the attention regarding menstrual disturbances in women with affective disorders has focused on valproate, another frequently administered drug. Valproate had been postulated to cause hyperandrogenism in susceptible individuals and thereby trigger irregular menses (McIntyre et al., 2003). However, the increased prevalence of menstrual disturbances in women with bipolar disorder (up to 35–50%) has been reported in at least one longitudinal study without an association with valproate therapy, suggesting that menstrual disturbances may be a common side effect for both lithium and valproate (Rasgon et al., 2005). Valproate also possesses GSK3ß inhibitor properties (Chen et al., 1999) and has a wide range of indications, including epilepsy. More attention is needed to elucidate a possible link between the increased menstrual disturbances in women taking lithium and valproate, their possible common mediation via inhibition of GSK3ß, and the consequent potential for the development of endometrial proliferative disorders such as hyperplasia and carcinoma.
In summary, we have demonstrated that xenotransplantation of human endometrial tissue can provide an excellent model in which to study comparative endometrial biology. Treatment with lithium induces a proliferative response of the human xenograft that mimics the initial molecular control of endometrial proliferation in mice. This model thus offers significant promise. As recently illustrated by others, one of the potential applications of the xenograft model lies in the use of an identifiable marker, such as luciferase, that could be introduced via genetic manipulation and allows for non-invasive in-vivo assessment of the xenografted tissue (Masuda et al., 2007). Further, this paradigm allows for the assessment of potentially toxic agents that affect endometrial cell proliferation but cannot be given to human volunteers.
Author's Role
J.W.P. designed the animal portion of research; A.J.P. and N.S. designed the human portion of research; A.J.P. and L.Z. performed the experiments; A.J.P., L.Z., N.S. and J.W.P. analyzed the data; A.J.P. wrote the paper with editing by L.Z., N.S. and J.W.P.
Funding
This research was supported by NIH grants T32 HD040135 (A.J.P.), K24 HD041978 (N.S.), NCI RO1 89617 (J.W.P.) and RR 95261064 (GCRC).
Acknowledgements
We thank Dr Takeshi Kurita for assistance with training in performance of renal subcapsular grafting and Drs Kevin Osteen, Kaylon Bruner-Tran and Leslie Gold for assistance with optimizing endometrial isolation. We also thank Dr Yan Deng for guidance with microphotography, Mr Jim Lee for excellent technical assistance with animal care and Ms. Donna Gerardi and Ms. Donna Bruno for outstanding support with graphic and figure design. We express gratitude to the Einstein GCRC staff and all the women who participated in this study.
References
- Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JT, Kaufman DG, Seppala M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16:836–845. doi: 10.1093/humrep/16.5.836. [DOI] [PubMed] [Google Scholar]
- AVMA. 2000 Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc. 2001;218:669–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- Bray F, Dos Santos Silva I, Moller H, Weiderpass E. Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiol Biomarkers Prev. 2005;14:1132–1142. doi: 10.1158/1055-9965.EPI-04-0871. [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD. Progesterone receptor antagonists and the endometrial antiproliferative effect. Semin Reprod Med. 2005;23:74–81. doi: 10.1055/s-2005-864035. [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD, Rodgers WH, Critchley HO, Carroll R, Nie XJ, Mah K. Immunocytochemical assessment of mitotic activity with an antibody to phosphorylated histone H3 in the macaque and human endometrium. Hum Reprod. 2003;18:1185–1193. doi: 10.1093/humrep/deg255. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Chen B, Pan H, Zhu L, Deng Y, Pollard JW. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase–>AKT–>GSK-3beta–>cyclin D1–>pRB pathway to block uterine epithelial cell proliferation. Mol Endocrinol. 2005;19:1978–1990. doi: 10.1210/me.2004-0274. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Nat Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Vanderslice KD. Identification in histological sections of species origin of cells from mouse, rat and human. Stain Technol. 1984;59:7–12. doi: 10.3109/10520298409113823. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P. Role of stroma in oestrogen-induced epithelial proliferation. Epithelial Cell Biol. 1992;1:18–31. [PubMed] [Google Scholar]
- Deligdisch L. Hormonal pathology of the endometrium. Mod Pathol. 2000;13:285–294. doi: 10.1038/modpathol.3880050. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endl E, Gerdes J. The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res. 2000;257:231–237. doi: 10.1006/excr.2000.4888. [DOI] [PubMed] [Google Scholar]
- Fasciani A, Bocci G, Xu J, Bielecki R, Greenblatt E, Leyland N, Casper RF. Three-dimensional in vitro culture of endometrial explants mimics the early stages of endometriosis. Fertil Steril. 2003;80:1137–1143. doi: 10.1016/s0015-0282(03)02164-2. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunin AG, Emelianov VU, Mironkin IU, Morozov MP, Tolmachev AS. Lithium treatment enhances estradiol-induced proliferation and hyperplasia formation in the uterus of mice. Eur J Obstet Gynecol Reprod Biol. 2004;114:83–91. doi: 10.1016/j.ejogrb.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M, Brill JA, Fink GR, Weinberg RA. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 1994;8:1759–1771. doi: 10.1101/gad.8.15.1759. [DOI] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18:3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Joffe H. Reproductive biology and psychotropic treatments in premenopausal women with bipolar disorder. J Clin Psychiatry. 2007;68:10–15. [PubMed] [Google Scholar]
- Kato K, Toki T, Shimizu M, Shiozawa T, Fujii S, Nikaido T, Konishi I. Expression of replication-licensing factors MCM2 and MCM3 in normal, hyperplastic, and carcinomatous endometrium: correlation with expression of Ki-67 and estrogen and progesterone receptors. Int J Gynecol Pathol. 2003;22:334–340. doi: 10.1097/01.pgp.0000092129.10100.5e. [DOI] [PubMed] [Google Scholar]
- Kim YB, Holschneider CH, Ghosh K, Nieberg RK, Montz FJ. Progestin alone as primary treatment of endometrial carcinoma in premenopausal women. Report of seven cases and review of the literature. Cancer. 1997;79:320–327. doi: 10.1002/(sici)1097-0142(19970115)79:2<320::aid-cncr15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, et al. The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation. 2005;73:313–322. doi: 10.1111/j.1432-0436.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Pollard JW, Fagg B. Oestriol, oestradiol-17beta and the proliferation and death of uterine cells. J Endocrinol. 1976;69:103–115. doi: 10.1677/joe.0.0690103. [DOI] [PubMed] [Google Scholar]
- Masuda H, Maruyama T, Hiratsu E, Yamane J, Iwanami A, Nagashima T, Ono M, Miyoshi H, Okano HJ, Ito M, et al. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc Nat Acad Sci USA. 2007;104:1925–1930. doi: 10.1073/pnas.0604310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Mancini DA, McCann S, Srinivasan J, Kennedy SH. Valproate, bipolar disorder and polycystic ovarian syndrome. Bipolar Disord. 2003;5:28–35. doi: 10.1034/j.1399-5618.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- Niklaus AL, Pollard JW. Mining the mouse transcriptome of receptive endometrium reveals distinct molecular signatures for the luminal and glandular epithelium. Endocrinology. 2006;147:3375–3390. doi: 10.1210/en.2005-1665. [DOI] [PubMed] [Google Scholar]
- Niklaus AL, Aubuchon M, Zapantis G, Li P, Qian H, Isaac B, Kim MY, Adel G, Pollard JW, Santoro NF. Assessment of the proliferative status of epithelial cell types in the endometrium of young and menopausal transition women. Hum Reprod. 2007;22:1778–1788. doi: 10.1093/humrep/dem032. [DOI] [PubMed] [Google Scholar]
- Osteen KG, Hill GA, Hargrove JT, Gorstein F. Development of a method to isolate and culture highly purified populations of stromal and epithelial cells from human endometrial biopsy specimens. Fertil Steril. 1989;52:965–972. doi: 10.1016/s0015-0282(16)53160-4. [DOI] [PubMed] [Google Scholar]
- Pan H, Zhu L, Deng Y, Pollard JW. Microarray analysis of uterine epithelial gene expression during the implantation window in the mouse. Endocrinology. 2006;147:4904–4916. doi: 10.1210/en.2006-0140. [DOI] [PubMed] [Google Scholar]
- Parekh TV, Gama P, Wen X, Demopoulos R, Munger JS, Carcangiu ML, Reiss M, Gold LI. Transforming growth factor beta signaling is disabled early in human endometrial carcinogenesis concomitant with loss of growth inhibition. Cancer Res. 2002;62:2778–2790. [PubMed] [Google Scholar]
- Pierro E, Minici F, Alesiani O, Miceli F, Proto C, Screpanti I, Mancuso S, Lanzone A. Stromal-epithelial interactions modulate estrogen responsiveness in normal human endometrium. Biol Reprod. 2001;64:831–838. doi: 10.1095/biolreprod64.3.831. [DOI] [PubMed] [Google Scholar]
- Ramnath N, Hernandez FJ, Tan DF, Huberman JA, Natarajan N, Beck AF, Hyland A, Todorov IT, Brooks JS, Bepler G. MCM2 is an independent predictor of survival in patients with non-small-cell lung cancer. J Clin Oncol. 2001;19:4259–4266. doi: 10.1200/JCO.2001.19.22.4259. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Reynolds MF, Elman S, Saad M, Frye MA, Bauer M, Altshuler LL. Longitudinal evaluation of reproductive function in women treated for bipolar disorder. J Affect Disord. 2005;89:217–225. doi: 10.1016/j.jad.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Reena RM, Mastura M, Siti-Aishah MA, Munirah MA, Norlia A, Naqiyah I, Rohaizak M, Sharifah NA. Minichromosome maintenance protein 2 is a reliable proliferative marker in breast carcinoma. Ann Diagn Pathol. 2008;12:340–343. doi: 10.1016/j.anndiagpath.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J Biol Chem. 2002;277:9684–9689. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- Satyaswaroop PG, Tabibzadeh S. Progestin regulation of human endometrial function. Hum Reprod. 2000;15:74–80. doi: 10.1093/humrep/15.suppl_1.74. [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F, Sasaguri T. GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal. 2008;20:581–589. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Tan DF, Huberman JA, Hyland A, Loewen GM, Brooks JS, Beck AF, Todorov IT, Bepler G. MCM2–a promising marker for premalignant lesions of the lung: a cohort study. BMC Cancer. 2001;1:6. doi: 10.1186/1471-2407-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Nicklaus A, Zhu L, Pan H, Chen B, Aubuchon M, Santoro N, Pollard JW. Estrogen and progesterone regulation of cell proliferation in the endometrium of muridae and humans. In: Aplin JD, editor. The Endometrium. pp. 99–122. London: Informa Healthcare; 2008. [Google Scholar]
- Wang Y, Feng H, Bi C, Zhu L, Pollard JW, Chen B. GSK-3beta mediates in the progesterone inhibition of estrogen induced cyclin D2 nuclear localization and cell proliferation in cyclin D1-/- mouse uterine epithelium. FEBS Lett. 2007;581:3069–3075. doi: 10.1016/j.febslet.2007.05.072. [DOI] [PubMed] [Google Scholar]
- Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Nat Acad Sci USA. 2007;104:15847–15851. doi: 10.1073/pnas.0705749104. [DOI] [PMC free article] [PubMed] [Google Scholar]