Abstract
Aberrant coronary vascular smooth muscle cell (CSMC) proliferation represents a pivotal event underlying intimal hyperplasia, a phenomenon impairing the long term efficacy of bypass surgery and angioplasty procedures. Consequently research has become focused on efforts to identify molecules that are able to control CSMC proliferation. Here, the down regulation of CSMC growth was investigated by small interfering RNAs (siRNAs) targeted against E2F1, cyclin E1 and E2, genes whose contribution to CSMC proliferation is only now emerging.
Chemically synthesized siRNAs were delivered by two different transfection reagents to asynchronous and synchronous growing human CSMCs cultivated either in normo or hyper-glycemia. The depletion of each of the three target genes affected the expression of the other two genes demonstrating a close regulatory control. The clearest effects associated with the inhibition of E2F1-cyclinE1/E2 circuit were the reduction in the phosphorylation levels of the retinoblastoma protein pRB and a decrease in the amount of cyclin A2. At the phenotypic level the down modulation of CSMC proliferation resulted in a decrease of S phase matched by the increase of G1-G0 phase cell amounts. The anti-proliferative effect was cell-donor and transfectant independent, reversible, effective in a-synchronous and synchronous growing CSMCs. Importantly it was also evident in hyper-glycemia, a condition that underlies the diabetic situation. No significant a-specific cytotoxicity was observed.
Our data prove, for the first time, the interrelation among E2F1-cyclinE1-cyclin E2 and the pivotal role exerted in CSMC proliferation. Additionally, our work validates the concept of utilizing anti E2F1-cyclinE1-cyclin E2 siRNAs to develop a potential novel therapy to control intimal hyperplasia.
Keywords: CSMC proliferation, siRNAs, cyclin E1, cyclin E2, E2F1
Introduction
In adult blood vessel vascular smooth muscle cells are mainly involved in controlling vessel tone to regulate blood flow distribution and blood pressure. In this condition, the cellproliferation rate is very low. However, in response to external stimuli induced, for example, by vascular injury, the growth rate dramatically increases and is accompanied by a number of other cellular modifications collectively known as “phenotypic modulation” (1). Whereas augmented growth rate represents a relevant physiological process in vascular repair, its aberrant increase has also been implicated in the pathogenesis of different hyper-proliferative vascular diseases such as vein graft occlusion, coronary by-pass surgery, in-stent restenosis (ISR), atherosclerosis and hypertension (2). In these conditions, vascular smooth muscle cell proliferation, together with a number of other patho-phisiological events (3), is responsible for the thickening of the intimal vessel layer (intimal hyperplasia - IH) with the consequent reduction of blood flow. In the case of coronary ISR (3), the down regulation of coronary smooth muscle cells (CSMC) growth by potent anti-proliferative and pro-apoptotic drugs released from endo-luminal stents (drug eluting stent, DES) has been shown to significantly reduce IH (4). This benefit is however limited to low risk patients i.e. those with discrete, de novo lesions in native coronary vessels. In high-risk patients, such as diabetics, IH occurrence is significantly higher (5). Moreover, the DES in current use has been associated with an increased risk of late stent thrombosis (6). Thus, the understanding of the molecular mechanisms controlling vascular smooth muscle cells proliferation, a pivotal event in IH generation, is crucial in ISR as well as in the other hyper-proliferative vascular diseases.
E2F1 is a transcription factor directly implicated in the regulation of many cellular processes including cell proliferation (7). Upon retinoblastoma protein (pRb) phosphorylation by cyclin dependent kinase (Cdk) bound cyclin D, E2F1 is released from the pRb-E2F1 complex. The free E2F1 induces the transcription of cyclin E1 which, bound to its Cdk, phosphorylates pRb and further increases the amount of free E2F1, which in turn induces the transcription of many S-phase genes. The existence of this E2F1-cyclinE1 positive feed back loop promoting cell growth in different cell types is well known (8). However, despite recent data on the contribution of E2F1 and cyclin E1 to CSMC proliferation (9,10), specific information on their interrelationship is missing. Importantly, nothing is known about the role of cyclin E2, the second component of the cyclin E family (11), to the E2F-1/cyclinE1 feed back loop and thus to CSMC proliferation. To investigate these aspects, E2F1, cyclin E1 and E2 were depleted in cultured human CSMCs by means of specific siRNAs. The effects on CSMC proliferation where evaluated in a-synchronous and synchronous growing CSMCs obtained from different donors using different transfectants and culturing conditions. In addition, the consequence of the depletion of each of the target genes on the other two genes was also investigated, and the effects on the other cell cycle relevant genes related to the E2F1-cyclin E loop determined.
Material and methods
Cell culture and siRNA selection
Primary cultures of human CSMC (Promocell, Heidelberg, Germany), isolated from two different donors (donor 1: 58 years old female Caucasian, donor 2: 35 years old male Caucasian) were used. For all experiments, cells in the third passage were considered. CSMCs were cultured as described (12). siRNA directed against cyclin E1 (siCycE1-1415 sense 5’GAGCGGUAAGAAGCAGAGCdTdT3’, siCycE1-827 sense 5’GAGGAAAUCUAUCCUCCAAdTdT3’,) cyclin E2 (siCycE2-647 sense 5’GCUUGCAGUGAAGAGGAUAdTdT3’, siCycE2-946 sense 5’GGAGUGGGACAGUAUUUCAdTdT3’) and E2F1 (siE2F1-1324 sense 5’GAGGAGUUCAUCAGCCUUUdTdT3’; siE2F1-1117 sense 5’GCCACCAUAGUGUCACCACdTdT3’) were chemically synthesized (Eurogentec S.A., Belgium) and selected according to previously reported guide lines (13). Additionally, a control siRNA directed against the luciferase gene (siRNAGL2 sense 5’CGUACGCGGAAUACUUCGAdTdT) was used.
SiRNA transfection
CSMCs were seeded at a density of 3,3x 103 cells/cm2 in 6 cm diameter plastic dishes in the presence of 4 ml of complete medium containing 15% FCS. In the experiments performed with a-synchronized cells, transfections were conducted the day after seeding, while in the case of synchronized cells, a starvation time of 48 hours in serum free medium preceded transfection. Five μg of each siRNAs were mixed at different weight ratios with the selected liposomes (Cellfectin, Invitrogen, Basel, Switzerland; Metafectene, Biontex Laboratories GmbH, Munich, Germany) in 200 μl of serum free medium and complexes allowed to form for 20 minutes at room temperature. Afterwards, 1,3 ml of serum free medium were added to the 200 μl, mixed and applied to the cells previously washed with Phosphate-Buffered Saline (PBS) to reach an siRNA concentration of 230 nM. After two hours at 37 °C, transfection medium was removed, cells were washed with PBS and 4 ml of complete medium or PBS were added to the cells, depending on whether they were used for proliferation inhibition experiments or for uptake studies, respectively.
Uptake studies
Transfections were performed using the siRNAGL2 control siRNA carrying the 5’ end of the sense strand labeled by fluorescein isothiocynate (FITC). Living cells were observed under a fluorescence microscope (Leica Mycrosystem) and photographed. Subsequently, cells were trypsinized, resuspended in 500 μl of PBS and the number of FITC positive cell evaluated by flow cytometry (FACScanto, Becton Dickinson, DIVA software).
Western blot
Protein extraction and immunoblotting were performed as described (14) using 20 μg of complete cell lysate. The antibodies mouse anti-CdK 2 (1 μg/ml), mouse anti-p16INK4 (1 μg/ml) and rabbit anti-cyclin E2 (1 μg/ml) were purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA), BD Becton Dickinson Biosciences (San Jose, California, USA) and Lab Vision (Fremont, California, USA), respectively.
Q-RT PCR
Total RNA extraction, the evaluation of RNA quality, integrity and quantification, reverse transcription and PCR amplification were performed as described (15) except that the primers for cyclin E2, 2′,5′-oligoadenylate synthetase 1 (OAS1) and 28S ribosomal RNA were Fw 5’CTTCCAAACTTGAGGAAATC3’ / Rv 5’TCCATCCTTAAGATATCCTC3’, Fw 5’TCCAAGGTGGTAAAGGGTGG3’ / Rv 5’AGGTCAGCGTCAGATCGGC3’, Fw 5’TGGGAATGCAGCCCAAAG3’ / Rv 5’CCTTACGGTACTTGTTGACTATGC3’, respectively. The annealing temperatures for each primer couple were 56 °C, 62 °C and 60 °C, respectively. All amplification reactions were conducted in triplicate, utilizing SYBRGreen Master Mix buffer (Applied Biosystems) and 1 μl of cDNA. The relative amounts of the mRNA of target genes were normalized by 28s rRNA content according to Pfaffl(16).
Cytotoxicity, apoptosis cell cycle and migration tests
Cytotoxicity was evaluated by lactate dehydrogenase (LDH) assay kit according to manufacturer instructions (BioVision Prod., Mountain View CA). As positive control, triton X-100-treated cells (1% of final concentration), were considered. Apoptosis was evaluated by the Annexin V test (Bender – Med System, Burlingame, CA) as described (14) followed by flow cytometry (FACS Canto, BD, DIVA software). Cell cycle phase evaluation and BrdU incorporation were performed as described (12) by flow cytometry. The amount of incorporated BrdU in siRNA treated cells over time was described by the following equation:
where t is time (in days) while a, b and c are fitting parameters which corresponded to 9.2 ± 1.2/-57.5 ± 9.0/135.0 ± 13.0 for siE2F1 1324 treated cells, to 7.2 ± 1.6/-50.7 ± 11.6/156.0 ± 17.4 for siCyE1 1415 treated cells and 7.4 ± 0.1/-48.9 ± 0.5/136.5 ± 0.8 for siCyE2 647 treated cells. The fitting parameters define the behavior of BrdU incorporation in all the different conditions considered.
CSMC migration assay was performed by the fluorescence-assisted trans-migration motility assay as described (17). In brief, three days after transfection CSMCs were trypsinized, stained by the vital dye Fast DiI TM (Molecular Probes, Inc) and seeded on the upper side of Transwell-like inserts (HTS FluoroBlockTM inserts, Becton Dickinson) with a porous PET membrane (8 μM pores) previously coated by collagen IV; the amount of cells migrated from the top side to the bottom side of the porous membrane was detected by Tecan Infinite 200 microplate fluorometer (Tecan Group Ltd, Mannerdorf, Switzerland) up to 5 hours post seeding.
Statistics
Values are expressed as mean ± SEM. Statistical significance was determined by one-way analysis of variance and the appropriate t test; a value of p<0.05 was considered to be statistically significant.
Results
Uptake studies (supplementary material 1) indicated that both transfection reagents considered (Cellfectin and Metafectene) had an excellent transfection efficacy in CSMCs as evaluated by using a 5’fluorescein (FITC) labeled siRNAGL2 (siGL2, control siRNA directed against the luciferase gene).
siRNA effects on the protein and mRNA levels of the respective targets
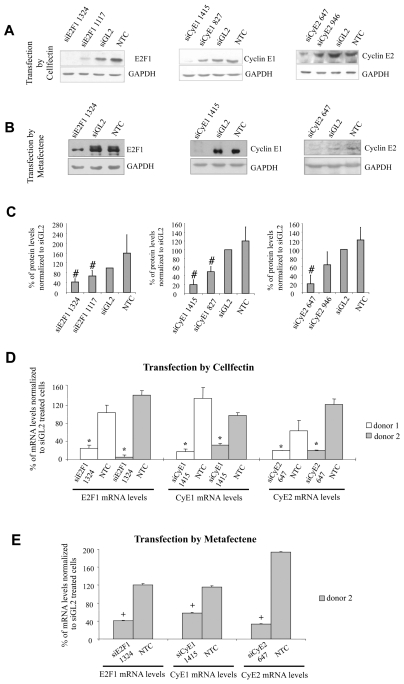
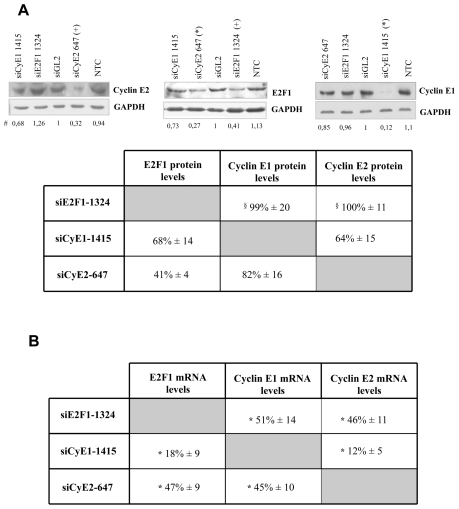
Our experiments were performed using CSMCs obtained from two independent donors. For each target gene, two siRNAs were synthesized without any chemical modifications. Three days after transfection, using Cellfectin on CSMCs of donor 1, the selected siRNAs (230 nM) could be demonstrated to reduce the protein levels of each target (Figure 1A). Comparable results were obtained using the most active siRNAs (siRNAE2F1-1324, siRNACycE1-1415 and siRNA-CycE2-647 directed against E2F1, cyclin E1 and cyclin E2, respectively), in the presence of the transfection reagent Metafectene (Figure 1B). The cumulative data obtained from Cellfectin and Metafectene transfections are reported in Figure 1C. Importantly these results could be repeated with donor 2 CSMCs (data not shown).
Figure 1. siRNA effects on the protein and mRNA levels of the respective targets.
A) Two siRNAs for each target gene were delivered to CSMCs from donor 1 by Cellfectin and the protein levels evaluated three days after transfection; representative blots are reported; B) the most effective siRNA among each couple were delivered by Metafectene and the protein levels evaluated (CSMCs from donor 1), shown are representative blots; C) cumulative data obtained from both Cellfectin and Metafectene transfection are indicated; data, reported as mean ± SEM, n = 5, were normalized to GAPDH and expressed as ratio of siGL2 treatment, #p< 0.02 compared to control; D) target mRNA levels were evaluated after siRNA delivery either by Cellfectin (CSMCs from donor 1 and 2) or E) Metafectene (CSMCs from donor 2); 28S transcript levels served as normalizator; NTC = non treated cells, siGL2 = control siRNA treated cells, data are expressed as mean ± SEM, n = 4. * p< 0.001 and + p< 0.05 compared to control.
Inhibition of target gene expression was confirmed at the mRNA level for both transfectants (Figure 1D–E). Comparable results were obtained, from the qualitative point of view, with the less active siRNAs (supplementary material 2) siRNAE2F1-1117, siRNACycE1-827 and siRNACycE2-946.
siRNA effects on CSMC death, proliferation and migration
Three days after transfection, the selected siRNAs were able to significantly reduce CSMC number (supplementary material 3) down to 57%, 84% and 83% for siRNAE2F1-1324, siRNA-CycE1-1415 and siRNACycE2-647, respectively, compared to controls. This observation, together with the clear effects on target gene expression reported above, was not accompanied by an increase in OAS-1 gene expression and therefore could not be ascribed to an a-specific induction of the interferon response (18) (supplementary material 4A). Additionally, neither significant cytotoxicity nor apoptosis were induced (supplementary material 4B–C). Similar results were observed at shorter (2 days) and longer (5 days) time points after transfection (data not shown).
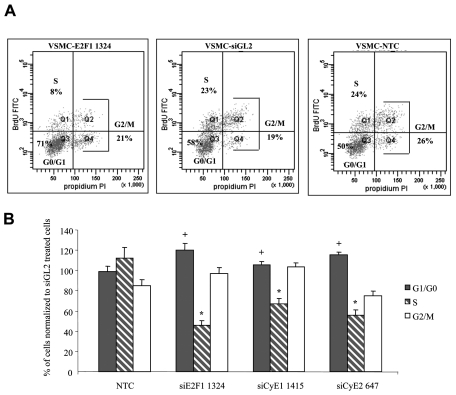
Consistently with the reduction in cell number, a clear reduction in cell proliferation was observed. Representative dot plots for donor 1 (Figure 2A) and the cumulative data obtained for donor 1 and 2 CSMCs using Cellfectin as transfectant (Figure 2B), indicated a marked reduction of S-phase and a parallel increase of G1/G0 phase cells. Comparable results were obtained using Metafectene as transfectant (data not shown).
Figure 2. siRNA effects on cell proliferation.
A) Cell cycle phase distribution data were obtained three days after siRNA transfection: a representative example relative to siE2F1-1324 treated CSMCs (donor 1) and its control is reported, BrdU FITC: Ig anti-BrdU labeled by FITC; PI: propidium iodide staining; B) cumulative data for donors 1 and 2 indicate, for all siRNAs, a decrease of S-phase and an increase of G1-G0 phase cells; data for each cell cycle phase are reported as % of the values measured in siGL2 treated cells and are expressed as means ± SEM, n=9. * p< 0.001 and + p< 0.05 compared to control.
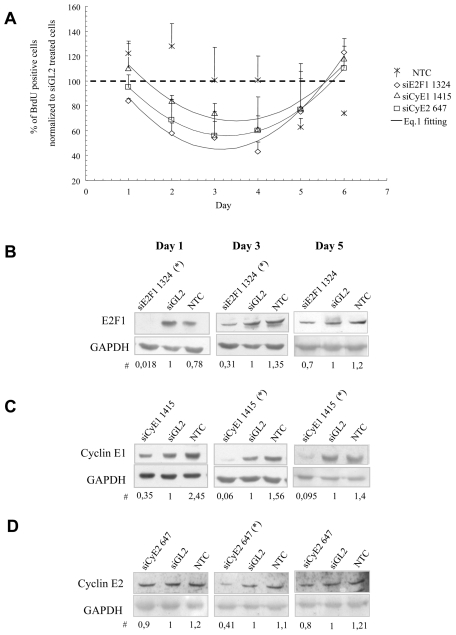
The time course effects of the selected siRNAs on cell proliferation (BrdU incorporation), indicated a reversible inhibition of CSMC proliferation (Figure 3A). Despite a somewhat quantitative difference among the three siRNAs, as indicated by eq. 1 parameters (see material and methods), the maximum of BrdU incorporation inhibition (occurring in correspondence of parabola minimum, i.e for t = −b/(2*a)) was similar for all the siRNAs considered and occurred just after day 3 (3.1 days for siE2F1 1324, 3.5 days for siCyE1 1415 and 3.3 days for siCyE2 647). Notably, the decrease of BrdU incorporation was paralleled by a consistent reduction in the target protein levels (Figure 3 B–D).
Figure 3. Time course effects of the selected siRNAs on CSMC proliferation.
A) Inhibition of CSMC proliferation, as evaluated by BrdU incorporation, lasted up to day five post transfection; the kinetic of cell proliferation reduction for siRNA treated cells was mathematically evaluated by equation 1 (black lines); cumulative data from both donors are expressed as mean ± SEM, n = 6; B–D) proliferation inhibition was paralleled by a concomitant reduction of target proteins: shown are representative blots from donor 1 performed using Cellfectin as transfectant; # all the numbers reported below the blots derive from band quantification normalized to GAPDH and are expressed as ratio of siGL2 treatment; * p<0.02 compared to controls, n=4; NTC = non treated cells, siGL2 = control siRNA treated cells.
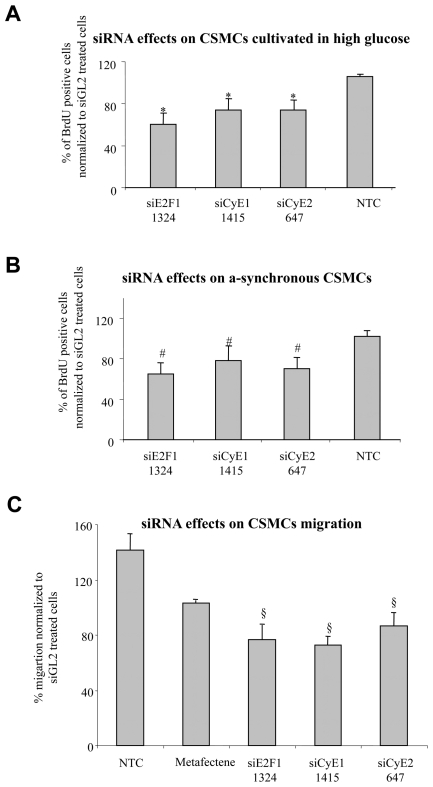
Augmented CSMC proliferation in diabetics represents a relevant element responsible for the increased risk of developing severe hyper-proliferative vascular diseases (19). Thus, our siRNAs were tested in CSMCs cultured in 22 mmol/L glucose (Figure 4A), a concentration commonly adopted to mimic the condition of poorly controlled diabetes (14). Notably, under this condition, BrdU incorporation into CSMCs exceed that of CSMC cultured in normal glucose of 21% ± 12. Cumulative data from donor 1 and 2 CSMCs indicate the anti-proliferative efficacy of the selected siRNAs under a high glucose concentration (approximately four-fold higher than the normal one corresponding to 5 mmol/L).
Figure 4. siRNA effects on CSMC proliferation in condition of hyperglicemia or in asynchronous cells and on CSMC migration.
A) Three days after transfection, selected siRNAs were able to reduce the proliferation of CSMCs cultivated in conditions of high glucose (22 mmol/L) compared to controls (*p<0.05); B) selected siRNAs were able to reduce the proliferation of a-synchronous CSMCs, (#p<0.05, comparing siRNA-treated to control a-synchronous cells); NTC = non treated cells, siGL2 = control siRNA treated cells; cumulative data from both donors are expressed as mean ± SEM, n = 4. C) Three days after transfection, the migration rates of siRNA treated cells were tested as detailed in M&M; the selected siRNAs were able to reduce the migration of synchronous CSMCs cultivated in conditions of normal glucose compared to controls (§p<0.04); cumulative data from both donors are expressed as mean ± SEM, n = 15
The experiments so far presented were conducted on starved CSMCs (synchronous cells) to which growth factors (serum) were added just after the end of siRNA administration. This experimental set up was intended to mimic the condition of in vivo resting CSMCs exposed to blood growth factors and anti-proliferative drugs at the time of re-canalization interventions, a condition typically occurring during ISR treatment. However, the presence of variable amounts of proliferating CSMCs prior to re-canalization interventions (20), prompted us to investigate siRNA efficacies on actively growing cells (a-synchronous cells). Cumulative data from donor 1 and 2 CSMCs confirmed the anti-proliferative effects of the selected siRNAs also in a-synchronous cells (Figure 4 B).
In addition to the augmented CSMC proliferation, increased CSMC migration represents a relevant element responsible for the development of hyper-proliferative vascular diseases (3). We therefore evaluated the anti-migratory potential of the selected siRNA by using the fluorescence-assisted trans-migration motility assay (17). As reported in Figure 4C, our siRNAs were able to reduce the migration of synchronized CSMCs compared to controls.
Effects of cyclin E1, 2 and E2F1 depletion on the expression levels of each other
To elucidate the mechanisms leading to the observed impairment of the G1-S phase transition and to define the interrelation among E2F1-cyclin E1/E2, we studied the effects of the targeting of each of the E2F1-cyclin E1/E2 on the other two (Figure 5). Cyclin E1 depletion resulted in the decrease of both cyclin E2 and E2F1 protein (Figure 5A) and mRNA levels (Figure 5B). Cyclin E2 depletion gave comparable result on the protein and mRNA levels of cyclin E1 and E2F1 (Figure 5A). Three days after siE2F1-1324 transfection, E2F1 depletion generated a considerable reduction in cyclin E1/E2 mRNA levels but not in the protein levels (Figure 5A). However, cyclin E1/E2 protein levels decreased at day five post transfection (approximately down to 60% of control). Notably, the less active siRNAs gave, from the qualitative point of view, similar results (data not shown).
Figure 5. Interrelation among E2F1, cyclin E1 and E2 in CSMCs.
Three days after trasfection, each of the target gene was depleted by the respective siRNAs and the protein levels (A) of the other two target genes evaluated: in the upper panel representative blots out of three independent experiments are reported (CSMCs from donor 2), in the lower panel the total data are shown (CSMCs from donors 1 and 2); # all the numbers reported below the blots are derived from band quantification normalized to GAPDH and are expressed as ratio of siGL2 treatment; § protein levels were reduced at day five post transfection; B) the mRNA levels corresponding to the data shown in A) are reported (28S transcript levels served as normalizator); cumulative data from both donors are expressed as mean ± SEM, n = 6. * p< 0.05 compared to control.
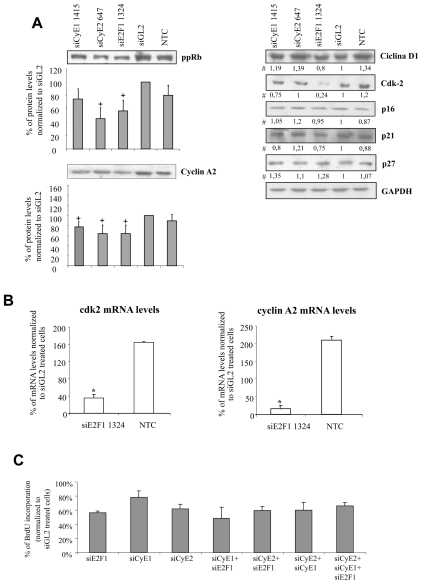
Finally, upon depletion of each of the E2F1-cyclin E1/E2 target proteins, a reduction in cyclin A2 protein level was observed (Figure 6A). In addition, the levels of the hyper-phosphorylated form of the protein pRb displayed an evident reduction in E2F1/cyclin E2 depleted cells; a tendency to the reduction was observed in cyclin E1 depleted cells. Only in the case of E2F1 depletion was a clear decrease of Cdk2 observed. We could also demonstrate that siE2F1-1324 treatment produced the transcriptional down regulation of cyclin A2 and Cdk2 (Figure 6B). By contrast, no major variations were detected for cyclin D1, p21cip1, p16INK4 and p27kip1 protein levels (Figure 6A).
Figure 6. Expression levels of different cell cycle regulators following E2F1, cyclin E1/E2 depletionand effect of the combined administration of different siRNAs on BrdU incorporation.
A) Three days after transfection, the protein levels of different cell cycle regulators were measured in protein extracts obtained from CSMCs of donor 2: shown are representative blots; # all the numbers reported below the blots derive from band quantification normalized to GAPDH and are expressed as ratio of siGL2 treatment; for ppRb (hyper-phosphorylated pRB) and cyclin A2, whose phosphorylation status and protein levels, respectively, were modified, cumulative data are reported as ratio to GAPDH; data are expressed as means ± SEM, n=3, + p< 0.05 compared to control; B) mRNA levels of cyclin A2 and Cdk2 were measured in CSMC treated with siE2F1-1324 in total RNA extracts obtained from both donors (28S transcript levels served as normalizator); results are expressed as means ± SEM, n=6, * p< 0.05 compared to control. C) CSMCs were treated by a mix of the different siRNAs (115 or 76 nM each in the case of a combination of two or three siRNA, respectively) and CSMC proliferation evaluated by BrdU incorporation; the effects of the combined administration were compared to an equimolar concentration of each of the single siRNAs (230 nM each) three days after transfection; NTC = non-treated cells, siGL2 = control siRNA treated cells; cumulative data from donor 1 and 2 CSMCs are expressed as means ± SEM, n=3.
Combined administration of siRNAs
Our demonstration of the relationship between E2F1-cyclinE1/E2 and the contribution of these proteins to the proliferation of CSMCs, lead us to investigate whether the contemporary targeting of these genes might have produced a more effective inhibition of CSMC proliferation. However, using a final siRNA concentration of 230 nM, no appreciable differences were observed for all the combinations tested (Figure 6C). Similar results were observed by increasing the total siRNA concentration up to 320 nM (data not shown). At higher siRNA concentrations, the transfection-induced cytotoxicity interfered with experimentation.
Discussion
The data presented indicates the potency of the selected siRNAs in reducing the target mRNA and protein levels (Figure 1). Notably, similar efficacies were observed for CSMCs obtained from two independent donors. This fact suggests a patient-independent effect that increases the significance of our findings. It was also clear, from the qualitative point of view, that comparable effects were obtained using either the most or the less active siRNAs (supplementary material 2). This makes the possibility that the observed results depends on an siRNA off-targeting effect very unlikely. Additionally, the activity of the selected siRNAs was not dependent on the transfectants (Figure 1), an observation that favors the concept of a pure siRNA-mediated mechanism of action. This supports the possibility that the selected siRNAs may maintain their efficacy also when delivered by more complex systems, i.e. those required for in vivo delivery (21,22).Indeed, in vivo, to overcome siRNA wash out by the blood, a robust method of delivery is necessary. In addition, given the relative short effects of the siRNAs (up to five days, Figure 3) a prolonged delivery is of utmost importance since it is commonly accepted that in vivo the anti-proliferative effect should be exerted for some months to down modulate IH developing in the course of ISR .
The target gene inhibition resulted in a down regulation of CSMC proliferation characterized by a significant reduction in the amount of S-phase cells with an increase of G1-G0 phase cells (Figure 2). Beside being patient-independent, the extent of this phenomenon is extremely encouraging for future in vivo application. Of interest is the observation that the siRNA effect is reversible (Figure 3A) and is neither associated to significant cytotoxicity nor apoptosis (supplementary material 4 B–C). Thus, the impact on CSMC biology is specific, an observation that is also supported by the failure to detect any evidence of induction of the interferon response (supplementary material 4 A) (18). This feature may confer to our siRNAs an advantage over the commonly used drugs for ISR, a hyper-proliferative vascular disease, commonly treated by stents able to release drugs such as rapamycin (23). This kind of molecule, beside having an anti-proliferative action, displays a potent apoptotic effect (24) which in the long term can cause excessive cell death, a fact which might contribute to explaining its reduced efficacy in high risk patients (5) and the recently reported problems of late stent thrombosis (6).
In conditions of high glucose concentrations, CSMC grow faster that under normal glucose concentration (25). This observation is a contributing factor in understanding why in diabetics hyper-proliferative vascular diseases such as ISR develop faster and more frequently than in normo-glycemic patients (26). To explore the effectiveness of our siRNAs in the unfavorable conditions of high glucose concentration, siRNA anti-proliferative efficacy was tested in CSMC grown in the presence of 22 mmol/L glucose (Figure 4A). This glucose concentration was chosen to mimic the conditions associated with a poorly-controlled diabetes (14). While we accept that the simulation of the diabetic condition we adopted may not completely reproduce the complexity of the diabetic patient, the data we present does indicate the efficacy of the selected siR-NAs in down-modulating CSMC growth in hyper-glycemia thus extending their potential effectiveness to diabetics.
Another positive feature of our approach is represented by the fact that the selected siRNAs achieve CSMC proliferation inhibition also when applied to a-synchronous growing cells (Figure 4B). We believe this information is potentially relevant for future in vivo applications where drugs will encounter both resting and actively growing CSMCs in the vessel wall (20). Our study also revealed that E2F1 knock down reduces CSMC migration (Figure 4 C), a patho-physiological event which greatly contributes to the development of hyper-proliferative vascular diseases (3). This data is in agreement with the observed reduced migration in E2F1 knock-out keratinocytes (27). Notably, the impaired migration detected upon cyclin E1/2 knock down (Figure 4C) may be dependent on the concomitant down regulation of E2F1 expression (Figure 5). Future investigations will further clarify the molecular mechanisms ruling this phenomenon.
Here we observe that the transient depletion of cyclin E1 or E2 reduces the growth rate both in a-synchronous (Figure 4B) and synchronous CSMCs (cell re-entering cell cycle) (Figure 2). However, cyclin E1 or E2 knock out mouse embryonic fibroblasts (MEFs) can grow and develop as efficiently as wild type MEFs and can retain a normal capacity to re-enter the cell cycle from quiescence (28). This discrepancy with our data may be due to the fact that cyclin E1 or E2 knock out MEFs adapted to cyclin E1/E2 absence. In contrast, in our CSMCs, the transient depletion was able to subvert the proliferation processes. Additionally, differences among species (mouse and human) cannot be excluded. In this sense, in contrast to MEFs, cyclin E2 knock-out Xenopus laevis cells cannot develop and proliferate (29).
Whereas the relevance of E2F1 in IH generation has been recently reported (10), its relation with cyclin E1/E2 in CSMCs has never been investigated. Our data support the direct transcriptional control of E2F1 on cyclin E2 and cyclin E1 (Figure 5B). Moreover, the fact that cyclin E2 depletion is paralleled by a reduction of E2F1-cyclin E1 mRNA levels (Figure 5B) and by the reduction of the amount of the hyper-phosphorylated form of pRb (Figure 6A), strongly supports the idea that cyclin E2 actively participates to the E2F1-cyclin E1 positive feed back loop (8) in human CSMCs. Furthermore, the observation that cyclin E1 depletion results in the transcriptional down regulation of cyclin E2-E2F1 further stresses the interrelation among these three genes, an observation also confirmed using the less active siRNAs (data not shown). In agreement with the mutual interaction among E2F1-cyclinE1/E2, we observed that the depletion of each of these genes reduced the expression of cyclin A2, a known transcriptional target of E2F1 (Figure 6A). Less evident, although present, was the effects on another E2F1 regulated gene, i.e.Cdk2. These observations together with the reduction in the amounts of the hyper-phosphorylated form of pRb are compatible with the G1-S phase block observed (Figure 2).
Surprisingly, the simultaneous targeting of two members of the E2F1-cyclin E1/2 circuit did not improve the anti-proliferative effects compared to the single targeting of each of them (Figure 6C). A competition between the different siRNAs might account for this observation (30). Alternatively, a sub-optimal amount of the siRNA used (115 nM each) cannot be excluded. This hypothesis is in line with the fact that when combining the three siRNA together (73 nM each), proliferation inhibition tended to be even less pronounced. Unfortunately, the in vitro set up did not allow to markedly increase the total siRNA concentrations due to an a-specific toxicity transfection-related (data not shown). Finally, as the targeting of each of the three members induces the down regulation of the expression of the other two (Figure 5), it is possible that an additional down regulation by specific siRNAs cannot further impair E2F1-cyclin E1/E2 circuit and thus cell proliferation. This hypothesis would strength the relevance and the effectiveness of targeting the E2F1-cyclin E1/E2 circuit for proliferation inhibition purposes.
In conclusion, our data prove, for the first time, the interrelation among E2F1-cyclinE1/E2 in human CSMCs. Importantly we also show that E2F1-cyclin E1/E2 circuit inhibition by siR-NAs resulted in a remarkable down regulation of human CSMCs proliferation. This effect is donor and transfectant independent, reversible, effective in a-synchronous and synchronous CSMCs, in normo-hyper-glycemia and does not induce cell death. Thus, our results provide the rationale for future experimentation in animal models aimed at the development of novel therapeutic approaches for hyper-proliferative vascular diseases.
Supplemental Data
ACKNOWLEDGEMENTS
We wish to thank Dr David Edwards (Senior Lecturer in Microbiology, University of Dundee, Scotland, UK) for critical reading of the manuscript. We also wish to thank Dr Cristina Zennaro (Renal child Foundation, G. Gaslini Children Hospital, Genoa, Italy) for excellent technical assistance. This work was in part supported by the “Fondazione Cassa di Risparmio of Trieste”, by the “Fondazione Benefica Kathleen Foreman Casali of Trieste” and by “Fondo Trieste”.
REFERENCES
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Jackson CL, Schwartz SM. Pharmacology of smooth muscle cell replication. Hypertension. 1992;20:713–736. doi: 10.1161/01.hyp.20.6.713. [DOI] [PubMed] [Google Scholar]
- 3.Edelman ER, Rogers C. Pathobiologic responses to stenting. Am J Cardiol. 1998;81:4E–6E. doi: 10.1016/s0002-9149(98)00189-1. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 5.Moussa I, Leon MB, Baim DS, O’Neill WW, Popma JJ, Buchbinder M, Midwall J, Simonton CA, Keim E, Wang P, Kuntz RE, Moses JW. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109:2273–2278. doi: 10.1161/01.CIR.0000129767.45513.71. [DOI] [PubMed] [Google Scholar]
- 6.Serruys PW, Daemen J. Are drug-eluting stents associated with a higher rate of late thrombosis than bare metal stents? Late stent thrombosis: a nuisance in both bare metal and drug-eluting stents. Circulation. 2007;115:1433–1439. doi: 10.1161/CIRCULATIONAHA.106.666826. [DOI] [PubMed] [Google Scholar]
- 7.Attwooll C, Lazzerini DE, Helin K. The E2F family: specific functions and overlapping interests. EMBO J. 2004;23:4709–4716. doi: 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng Y, Eaton EN, Picon M, Roberts JM, Lundberg AS, Gifford A, Sardet C, Weinberg RA. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- 9.O’Sullivan M, Scott SD, McCarthy N, Figg N, Shapiro LM, Kirkpatrick P, Bennett MR. Differential cyclin E expression in human in-stent stenosis smooth muscle cells identifies targets for selective anti-restenosis therapy. Cardiovasc Res. 2003;60:673–683. doi: 10.1016/j.cardiores.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Giangrande PH, Zhang J, Tanner A, Eckhart AD, Rempel RE, Andrechek ER, Layzer JM, Keys JR, Hagen PO, Nevins JR, Koch WJ, Sullenger BA. Distinct roles of E2F proteins in vascular smooth muscle cell proliferation and intimal hyperplasia. Proc Natl Acad Sci U S A. 2007;104:12988–12993. doi: 10.1073/pnas.0704754104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zariwala M, Liu J, Xiong Y. Cyclin E2, a novel human G1 cyclin and activating partner of CDK2 and CDK3, is induced by viral oncoproteins. Oncogene. 1998;17:2787–2798. doi: 10.1038/sj.onc.1202505. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Schneider A, Engel S, Racchi G, Kandolf R, Kuhn A. Hammerhead ribozymes targeted against cyclin E and E2F1 co-operate to down regulate coronary smooth muscle cells proliferation. The Journal of Gene Medicine. 2005;7:1223–1234. doi: 10.1002/jgm.755. [DOI] [PubMed] [Google Scholar]
- 13.Poliseno L, Evangelista M, Mercatanti A, Mariani L, Citti L, Rainaldi G. The energy profiling of short interfering RNAs is highly predictive of their activity. Oligonucleotides. 2004;14:227–232. doi: 10.1089/oli.2004.14.227. [DOI] [PubMed] [Google Scholar]
- 14.Zanetti M, Stocca A, Dapas B, Farra R, Uxa L, Bosutti A, Barazzoni R, Bossi F, Giansante C, Tedesco F, Cattin L, Guarnieri G, Grassi G. Inhibitory effects of fenofibrate on apoptosis and cell proliferation in human endothelial cells in high glucose. J Mol Med. 2008;86:185–195. doi: 10.1007/s00109-007-0257-3. [DOI] [PubMed] [Google Scholar]
- 15.Baiz D, Pozzato G, Dapas B, Farra R, Scaggiante B, Grassi M, Uxa L, Giansante C, Zennaro C, Guarnieri G, Grassi G. Bortezomib arrests the proliferation of hepatocellular carcinoma cells HepG2 and JHH6 by differentially affecting E2F1, P21 and P27 levels. Biochimie. 2009;91:373–382. doi: 10.1016/j.biochi.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Michael W. Pfaffl. quantification strategies in real-time PCR. In: Bustin SA, editor. A–Z of quantitative PCR. International University Line (IUL); La Jolla, CA,USA: 2004. [Google Scholar]
- 17.Spessotto P, Lacrima K, Nicolosi PA, Pivetta E, Scapolan M, Perris R. Fluorescence-based assays for in vitro analysis of cell adhesion and migration. Methods Mol Biol. 2009;522:221–250. doi: 10.1007/978-1-59745-413-1_16. [DOI] [PubMed] [Google Scholar]
- 18.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 19.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 20.Glass CK, Witztum JL. Atherosclerosis. the road ahead Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 21.Grassi G, Farra R, Noro E, Voinovic D, Lapasin R, Dapas B, Alpar O, Zennaro C, Carraro M, Giansante C, Guarnieri G, Pascotto A, Rehimers B, Grassi M. Charactherization of nucleid acid molecule/liposome complexes and rheological effects on pluronic/alginate matrices. Journal of Drug Delivery Science and Technology. 2007;17:325–331. [Google Scholar]
- 22.Davia L, Grassi G, Pontrelli G, Lapasin R, Perin D, Grassi M. Mathematical modelling of NABD release from endoluminal gel paved stent. Comput Biol Chem. 2009;33:33–40. doi: 10.1016/j.compbiolchem.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 24.Roque M, Reis ED, Cordon-Cardo C, Taubman MB, Fallon JT, Fuster V, Badimon JJ. Effect of p27 deficiency and rapamycin on intimal hyperplasia: in vivo and in vitro studies using a p27 knockout mouse model. Lab Invest. 2001;81:895–903. doi: 10.1038/labinvest.3780298. [DOI] [PubMed] [Google Scholar]
- 25.Zhu L, Sun G, Zhang H, Zhang Y, Chen X, Jiang X, Jiang X, Krauss S, Zhang J, Xiang Y, Zhang CY. PGC-1alpha is a key regulator of glucose-induced proliferation and migration in vascular smooth muscle cells. PLoS ONE. 2009;4:e4182. doi: 10.1371/journal.pone.0004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dibra A, Kastrati A, Mehilli J, Pache J, Schuhlen H, von Beckerath N, Ulm K, Wessely R, Dirschinger J, Schomig A. Paclitaxel-eluting or sirolimus-eluting stents to prevent restenosis in diabetic patients. N Engl J Med. 2005;353:663–670. doi: 10.1056/NEJMoa044372. [DOI] [PubMed] [Google Scholar]
- 27.D’Souza SJ, Vespa A, Murkherjee S, Maher A, Pajak A, Dagnino L. E2F-1 is essential for normal epidermal wound repair. J Biol Chem. 2002;277:10626–10632. doi: 10.1074/jbc.M111956200. [DOI] [PubMed] [Google Scholar]
- 28.Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, Amati B. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003;22:4794–4803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotoh T, Shigemoto N, Kishimoto T. Cyclin E2 is required for embryogenesis in Xenopus laevis. Dev Biol. 2007;310:341–347. doi: 10.1016/j.ydbio.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Poliseno L, Evangelista M, Giacca M, rainaldi G. The analysis of dose response curve cimes in useful for the assembly of multi-siRNAs expressing cassettes. Gene Ther and Mol Biol. 2006;10:101–108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.