Abstract
Natural killer (NK) cells were viewed traditionally as cytotoxic effector cells whose rapid killing of infected and transformed cells without preactivation provides a first line of defense prior to the initiation of an adaptive immune response against infection and tumor development. However, it has become clear that NK cells interact with various components of the immune system, and therefore have the potential to function as regulatory cells. While NK cells can assist in dendritic cell (DC) maturation and T-cell polarization, increasing evidence indicates that NK cells can also prevent and limit adaptive (auto) immune responses via killing of autologous myeloid and lymphoid cells. Investigating immunoregulatory NK-cell functions might generate exciting insights into the reciprocal regulation between NK-cell–mediated innate immunity and adaptive immune responses, improve our capacity to monitor these cells as surrogate markers for disease activity and treatment responses in autoimmune diseases, and, perhaps, provide new prospects for NK cell-directed therapies.
PHENOTYPES AND FUNCTIONS OF NK CELLS IN HUMANS
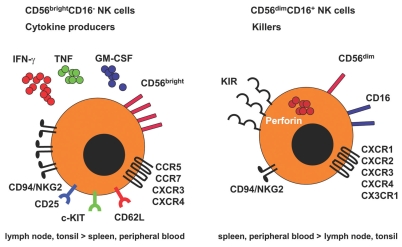
Although NK cells were described originally as a homogenous population of innate lymphocytes, there is increasing evidence that NK cells include distinct subsets with disparate functions, locations, and developmental origins. Human NK cells in peripheral blood can be divided into at least two functional subsets based on the expression of CD56 and CD16 (Figure 1). CD56dimCD16+ NK cells constitute about 90% of total blood NK cells, efficiently kill target cells, and secrete only low levels of cytokines. In contrast, CD56brightCD16− NK cells constitute <10% of total blood NK cells, but are enriched in secondary lymphoid tissues (1). In contrast to the CD56dimCD16+ NK-cell subset, activated CD56brightCD16−cells produce many cytokines, including IFN-γ, TNF, and GM-CSF, but acquire cytotoxicity only after prolonged activation (2). Therefore, cytokine secretion and cytotoxic effector functions are separated in human NK-cell subpopulations.
Figure 1.
Human NK cells in peripheral blood can be divided into at least two subsets based on the expression of CD56 and CD16. The major subset of CD56dimCD16+ NK cells constitutes ~90% of total blood NK cells, kills target cells upon proper recognition, and secretes only low levels of cytokines. CD56brightCD16− NK cells (<10% of total blood NK cells, but 75% of NK cells in secondary lymphoid tissues), in contrast, produce many cytokines, including IFN-γ, TNF, and GM-CSF, upon stimulation by proinflammatory cytokines, but acquire cytotoxicity only after prolonged activation. In addition, both subsets differ from each other with respect to expression of inhibitory and activating receptors. Although CD56brightCD16− NK cells express high levels of the inhibitory CD94/NKG2A complex recognizing HLA-E, they do not express MHC class I allele-specific killer cell Ig-like receptors that are, in contrast, expressed by CD56dimCD16+ NK cells. Moreover, only CD56brightCD16− NK cells express homing markers for secondary lymphoid organ (SLO), such as CCR7 and CD62L, and immature lymphocyte markers, such as c-KIT.
CD56brightCD16− and CD56dimCD16+ NK cells also differ from each other in the expression of inhibitory and activating NK-cell receptors, as well in the expression of adhesion molecules and chemokine receptors which facilitate homing to lymphoid tissues and sites of inflammation. NK-cell activation during target cell recognition is controlled by germ line encoded activating and inhibitory receptors on these innate lymphocytes. Among these, triggering of killer immunoglobulin-like receptors (KIRs) as well as of the CD94/NKG2A receptor on NK cells by classical and nonclassical MHC class I molecules delivers negative signals that prevent target cell killing (3). In contrast, engagement of activating receptors such as CD16 (FcgRIII), NK cell protein 30 (NKp30), NKp44, NKp46, DNAM-1, and NKG2D by antibody opsonization or ligands whose expression is upregulated following infection, transformation, or cellular stress, provides an NK-cell activating signal (4,5). Of the above-mentioned inhibitory receptors, cytokine-secreting CD56brightCD16− NK cells express high levels of the inhibitory CD94/NKG2A complex recognizing HLA-E, but lack MHC class Ia allele-specific killer cell Ig-like receptors, which are, in contrast, expressed by CD56dimCD16+ NK cells. Both NK-cell subsets express the activating receptors NKG2D as well as NKp30 and NKp46, but expression of the antibody-dependent cellular cytotoxicity mediating receptor CD16 (FcgRIII) is confined to the CD56dimCD16+ subset (6). Therefore, cytokine secreting CD56brightCD16− NK cells express a more rudimentary repertoire of inhibitory NK-cell receptors than cytotoxic CD56dimCD16+ NK cells.
CD56brightCD16− NK cells constitutively express CD62L (L-selectin) and CCR7, which allows their recruitment from the blood into lymphoid tissues and sites of inflammation (7,8). In fact, the CD56brightCD16− subset is markedly enriched in secondary lymphoid tissues, constituting up to 75% of NK cells in lymph nodes and 50% in the spleen (9). Even so, only 5% of lymph node mononuclear cells are NK cells, as lymph nodes are suggested to harbor 40% of all human lymphocytes; whereas probably only 2% of all lymphocytes circulate through peripheral blood at any given moment, CD56brightCD16− NK cells in secondary lymphoid organs constitute a significant pool of innate effector cells in humans. It is also noteworthy that the infiltrating NK cells in a number of human inflammatory lesions were found to be mainly CD56brightCD16− NK cells, although the CD56dimCD16+ subset expresses chemokine receptors that could also guide them to these sites (10–13). The homing cells were negative for perforin and had an increased capability to produce IFN-γ upon stimulation with a combination of IL-12 and IL-18, suggesting a prominent role for CD56brightCD16− NK cells both in secondary lymphoid tissues and at sites of infection and inflammation.
IMMUNOREGULATORY NK-CELL FUNCTIONS
Dendritic cells (DCs) are potent antigen-presenting cells that initiate and regulate immune responses. They are potent stimulators of naïve T cells, key inducers of primary immune responses, and play a central role in the development and maintenance of T-cell tolerance. NK cells are found in close association with DCs in both secondary lymphoid tissues and at inflammatory sites (14–17). DCs can activate resting NK cells directly, by cell-to-cell contact; and indirectly, via the secretion of cytokines such as type I IFNs, IL-12, IL-15, and IL-18. Among the soluble factors, IL-12 has been observed to induce IFN-γ secretion and proliferation repeatedly, and was thought to be the most pivotal signal-enhancing factor for NK-cell effector functions in humans and in mice (15,18–21). IL-15 appears to determine NK cell differentiation and survival, thereby possibly promoting NK-cell expansion (15,22,23). In addition, type I IFNs were suggested primarily to augment NK-cell cytotoxicity (24), but IL-12 can also contribute to enhancing this NK-cell function (21). Thus, different DC cytokines trigger different NK-cell–effector functions, enabling DC subsets or differently matured DCs to elicit a spectrum of NK-cell responses.
After in vivo stimulation with TLR ligands or bacterial and viral infection, it was shown that myeloid CD11chigh DCs need to prime NK cells via presentation of IL-15 to produce IFN-γ and become cytotoxic against an MHC class I low cell line expressing NKG2D ligands. In humans, we have shown recently that IL-15 receptor α colocalizes at the synapse between DCs and NK cells and contributes to NK-cell survival (22). While cytokines play a dominant role in human NK activation by DCs, IFN-α treatment of DCs additionally upregulates the NKG2D ligands, which seem to activate resting NK cells in a cell-contact-dependent manner (25), suggesting that cell contact might contribute additionally to NK activation by human DCs under certain inflammatory conditions.
DC-NK cell interactions are bidirectional. Activated NK cells also can mature DCs, via TNF and IFN-γ production and additional cell-to-cell contact-dependent signals (26,27), which promote priming and expansion of CD8+ T cells (28). On the other hand, activated NK cells can kill autologous immature myeloid DCs via NKp30-, NKp46-, and DNAM-1–mediated recognition (29–31). Resistance to NK lysis was achieved via upregulation of MHC class I molecules upon DC maturation, in particular, up-regulation of HLA-E (32), suggesting that NK cells expressing the inhibitory receptor for HLA-E recognition, that is, CD94/NKG2A, are particularly important in editing nonimmunogenic DCs. In addition to DCs, other myeloid cells also have been found to be susceptible to NK-cell cytotoxicity. Along these lines, activated macrophages have been found to be susceptible to NKG2D-dependent cytotoxicity by NK cells (33), and we have shown recently that human microglial cells, resident macrophage-like antigen presenting cells (APCs) of the central nervous system (CNS), are lysed by activated NK cells via NKG2D- and NKp46-mediated recognition (34). Similar to immature DCs, resting microglia are susceptible to NK-cell–mediated cytotoxicity while microglial activation and up-regulation of MHC class I molecules protected these CNS-resident APCs from being killed by NK cells (34). We suggest that this mechanism reduces the pool of immature DCs and resting microglial cells during immune activation, but allows fully activated APCs to present antigens to infiltrating T cells and to initiate a limited immune response. In contrast to DCs and microglia, monocyte-derived macrophages are resistant to autologous NK-cell cytotoxicity, unless they become activated by high doses of lipopolysaccharide (LPS), and express NKG2D ligands (NKG2DL) (33). This indicates that the stimulatory signal resulting from NKG2D–NKG2DL interactions overcomes the inhibitory signal provided by MHC class I ligands during recognition of activated macrophages by NK cells. The latter study also indicates fundamental differences between different subsets of myeloid cells in their susceptibility toward NK-cell–mediated killing. Although all of the above mentioned studies were performed in vitro, and definite evidence that these mechanisms are also operative in vivo is still lacking, the results obtained suggest that activated NK cells might play an important regulatory role by selectively editing APCs during the course of immune responses.
In addition to regulation of myeloid cells by NK cells, several studies also have shown the importance of NK cells for promoting Th1 polarization of CD4+ T cells through production of IFN-γ, a cytokine that not only initiates, but also reinforces Th1 differentiation. During experimental Leishmania major infection, NK cells are recruited rapidly to lymph nodes where they are found in close contact to the same DCs as antigen-specific CD4+ T cells providing the IFN-γ required for the induction of Th1 polarization (17). A similar effect was observed in allogeneic immune responses during which NK cells produce high levels of IFN-γ that are sufficient to mediate T-cell polarization via acting on naïve T cells directly, and by enhancing DC maturation (35–37). In addition to their function in T-cell polarization, NK cells expressing OX40 ligand and CD86 upon ligation of the activating FcgRIII (CD16) are capable of inducing IFN-γ production and proliferation of autologous T cells (38). Moreover, a supportive role of NK cells in B-cell activation and the promotion of isotype class switching has also been documented (39–41). These studies indicate that NK cells, particularly the cytokine secreting CD56brightCD16− subset (37), can influence T-cell polarization during primary immune responses.
In addition to shaping T-cell responses, NK cells can, however, also dampen these. Following T-cell activation, T cells upregulate NKG2D ligands and become susceptible to autologous NK-cell–mediated cell lysis in vitro (42–44). In this context, Lu et al. (45) demonstrated that the interaction between the mouse homologue of the human MHC class Ib molecule HLA-E, Qa-1-Qdm, on activated T cells and CD94/NKG2A inhibitory NK-cell receptors, protects activated CD4+ T cells from perforin-mediated NK-cell cytotoxicity. Ab-dependent blockade of this Qa-1-NKG2A interaction resulted in potent NK-dependent elimination of activated autoreactive T cells and amelioration of experimental autoimmune encephalomyelitis (EAE) in myelin oligodendrocyte glycoprotein (MOG) 35–55 immunized C57BL/6 mice (45). Thus, NK cells contribute to the resolution of adaptive immune responses via deletion of activated T cells in vivo. The latter study provided a mechanistic insight into the finding that NK-cell depletion profoundly influences the outcome of T-cell–mediated autoimmune disease models as outlined below.
NK-CELL FUNCTION IN EXPERIMENTAL AUTOIMMUNE DISEASES
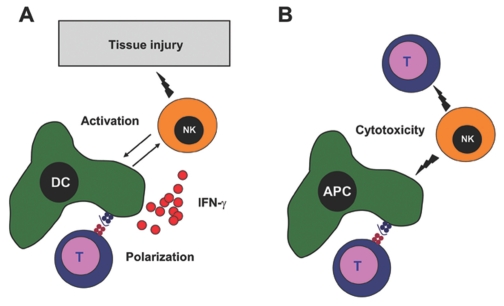
Because of the lack of mouse strains that are selectively deficient in NK cells, the study of NK-cell function in vivo has been challenging in the past (46). Animal models of autoimmune diseases provide evidence for both disease-accelerating and disease-protective effects of NK cells (Figure 2). The contradictory results achieved by NK-cell depletion are best illustrated in EAE, the animal model of multiple sclerosis (MS). It has been suggested that NK cells could be pathogenic by shaping Th1-polarized adaptive immune responses and by activating CNS-infiltrating DCs (47). Indeed, several mouse strains which have defective NK-cell functions, such as IL-18 deficient mice, or that are devoid of NK cells, such as T-bet deficient mice, are resistant to EAE induction (48,49). Surprisingly, most studies in the EAE model reported that NK cells protect from autoimmune-mediated tissue damage, presumably by editing initiator and effector cell populations (45,50–52). Antibody-mediated depletion of NK cells exacerbated EAE pathology in Lewis rats, SJL mice, and C57BL/6 mice (50,51,53), whereas adoptive transfer of in vitro-generated NK cells from the bone marrow decreased the severity of EAE in DA rats (54). Furthermore, it was observed that NK-cell depletion resulted in increased proliferation and IFN-γ production of T cells specific for the antigen that was used to induce EAE (50). Interestingly, opposite findings were observed in a different study using the same mouse strain immunized with the same myelin antigen (47). Contradictory findings on the effects of NK-cell depletion also were reported in animal models of type 1 diabetes (T1D). Whereas antibody-mediated NK-cell depletion was protective in one study (55), another report suggested that NK cells protect from T1D through secretion of perforin, IFN-γ, or immunoregulatory cytokines (56,57). Such apparently controversial findings might be explained, at least in part, by differences in the model system used to determine NK-cell function. For EAE induction, timing of myelin immunization and NK-cell activation/depletion could be critical for the outcome; NK cells could modulate and suppress T-cell polarization and effector function during the initiation of autoimmune responses against auto-antigens, but might contribute to immunopathology by directly causing tissue damage during progression of the established disease. In addition, using different tools and protocols to achieve NK-cell depletion could explain different outcomes. Unfortunately, no antibody targets NK cells specifically. The most frequently used antibodies for NK-cell depletion target NK1.1 and asialo-GM1, which also are expressed on natural killer T cells (NKT cells) and a subset of cytotoxic T cells, respectively. Moreover, antibody-mediated NK-cell depletion could be preceded by NK-cell activation and IFN-γ production, or might preferentially target distinct subsets of NK cells that mediate divergent effects on EAE initiation and progression. Studies in mice which allow selective in vivo ablation of NK cells (46) might be helpful in better characterizing the functional impact of NK cells on the initiation and progression of experimental autoimmune diseases in the future.
Figure 2.
Immunoregulatory Functions of NK cells. Depending on the model system, NK cells were found to either accelerate or inhibit the development of autoimmune diseases. (A) NK cells could contribute to autoimmune inflammation by shaping Th1-polarized adaptive immune responses, by activating DCs, and by causing tissue destruction directly. (B) On the other hand, NK cells could be protective by editing initiator and effector cell populations such as antigen-presenting cells (APC)—including DCs, macrophages, and microglia, and autoreactive T cells via their cytotoxicity.
NK-CELL (DYS)FUNCTION IN HUMAN AUTOIMMUNE DISEASES
Several studies reported that individuals with autoimmune diseases such as MS and systemic lupus erythematosus (SLE), have fewer blood NK cells compared with healthy subjects. Moreover, NK cells isolated from patients with MS were found not only to be decreased in frequency, but also to be impaired in effector function (58–68). Notably, patients with hemophagocytic lymphohistiocytosis (HLH), a rare condition characterized by impaired or absent function of the perforin/granzyme system in NK cells and cytotoxic T-cells, demonstrated decreased NK-cell activity. This resulted in increased T-cell and sustained-macrophage activation, and in the production of large quantities of the lymphokines IFN-γ, TNF-α, and GM-CSF, as well as of IL-1 and IL-6 (69). The macrophage activation syndrome (MAS), now considered a special form of HLH, occurs in children and adults with autoimmune diseases, especially systemic juvenile rheumatoid arthritis (sJRA) (70). Interestingly, patients with sJRA show decreased NK-cell frequencies and impaired NK-cell functions compared with children with other forms of juvenile rheumatoid arthritis such as pauciarticular and polyarticular JRA, even in the absence of MAS (71,72), which can, however, be triggered by environmental insults such as viral infections (73). Thus, patients with HLH and sJRA represent an example for an association of NK-cell dysfunction and excessive immune activation, which could contribute, potentially, to the initiation and maintenance of autoimmune responses.
On the other hand, there are many studies which found no differences in NK-cell frequencies and functions in patients with common autoimmune diseases compared with healthy controls (74–77). These contradictory findings are difficult to interpret since all of the aforementioned studies differed widely in their criteria used to classify NK cells. Some of the older reports did not distinguish between NK cells and NKT cells and none of the above studies differentiated between CD56brightCD16− and CD56dimCD16+ NK cells. Moreover, the assays and protocols used to study NK cell frequencies and functions, as well as the patient populations studied, varied significantly (78). With these caveats in mind, genetic predisposition for autoimmune diseases has been linked to NK cells, and successful therapies of autoimmune diseases correlated with changes in the NK-cell compartment.
The genetic analysis of NK cells has focused mainly on KIR allele typing. KIRs are expressed on NK cells and subsets of T cells. The KIR genes are extremely polymorphic, probably more than the HLA loci, and the KIR gene complex is polygenic with varying numbers of inhibitory and activating receptors (79). HLA class I molecules serve as ligands for the KIR. Interactions of the independently segregating KIR and HLA loci are important for recognition of targets by NK cells. Several studies correlated incidence and progression of infectious and autoimmune diseases with the expression of particular KIRs, as well as with HLA–KIR combinations. For example, in patients with psoriasis and psoriatic arthritis, conditions that have long been associated with HLA-C, specifically with the C2 allotype gene HLA-Cw*06, it has been reported that activating KIR genes (usually KIR2DS1 and/or KIR2DS2) are at a higher frequency in patients than in healthy control individuals (80,81). These studies suggest that the overall balance of activating and inhibitory composite KIR–HLA genotypes contributes to the susceptibility toward autoimmune diseases. However, it remains to be defined whether these genotypes also translate into functional differences (82).
Stronger evidence for a regulatory role of NK cells in MS stems from immuno-monitoring of a phase II therapy trial in MS. The rationale for the use of a humanized IgG1 monoclonal antibody targeting the interleukin-2 receptor α chain (daclizumab) in patients with autoimmune diseases such as MS was to block the proliferation of antigen-activated autoreactive T cells. Indeed, results of four open-label studies of intravenous daclizumab in patients with active forms of either RRMS or secondary progressive MS (SPMS) have suggested beneficial effects of daclizumab for both add-on and monotherapy protocols as measured by magnetic resonance imaging (MRI) and clinical outcomes (83). Surprisingly, CD4+ and CD8+ T-cell counts were decreased only moderately in patients responding to therapy. In stark contrast, CD56brightCD16− NK cells increased in frequency in the blood of treated patients and the suppression of disease activity, as quantified by MRI, correlated with the expansion of blood NK cells (84). Notably, NK cells isolated from patients during, but not before, therapy killed autologous-activated T cells even without the need for NK-cell activation in vitro (84). Similar observations were made in patients who received the identical monoclonal antibody for the treatment of autoimmune uveitis (85). In addition, patients with MS receiving IFN-β therapy also show an increase in the frequency of CD56brightCD16− NK cells within 3 months of treatment (86). Altogether, these human studies suggest that NK cells exert beneficial functions in autoimmune diseases. The mechanisms that could mediate such immunoregulatory NK-cell functions remain, however, poorly understood.
Nevertheless, both genetic and immunotherapeutic observations point to an involvement of NK cells in autoimmune disease and therefore their role in these pathologies needs to be better understood.
CONCLUSION
NK cells are multicompetent lymphocytes with the ability to regulate innate and adaptive immune responses through their reciprocal interaction with antigen-presenting cells as well as T cells and B cells. Antibody-mediated NK-cell depletion profoundly affects the outcome of many experimental autoimmune diseases. The observation that NK cells are decreased in frequency or impaired in function in patients with autoimmune diseases are reminiscent of defective functions noted for regulatory T-cell populations such as CD4+CD25+ regulatory T (Treg) cells and CD1d-restricted NKT cells, in patients with autoimmune diseases (87–90).
NK cells include distinct subsets with disparate repertoires, location, function, and developmental origin. In analogy to regulatory T-cell subsets, NK-cell sub-populations that have yet to be characterized might be particularly important in regulating autoimmune inflammation. We suggest that investigating immunoregulatory NK-cell functions in healthy individuals and patients with autoimmune diseases will generate exciting insights into the reciprocal regulation between NK-cell–mediated innate immunity and adaptive immune responses, improve our capacity to monitor these cells as surrogate markers for disease activity and treatment response, and, perhaps, provide new prospects for NK-cell–directed therapies.
ACKNOWLEDGMENTS
The authors are supported by the Dana Foundation’s Neuroimmunology program, the Burroughs Wellcome Fund, the Starr Foundation, the National Cancer Institute (R01CA108609 and R01CA101741), the National Institute of Allergy and Infectious Diseases (RFP-NIH-NIAID-DAIDS-BAA-06-19), the Foundation for the National Institutes of Health (Grand Challenges in Global Health), and an Institutional Clinical and Translational Science Award (to the Rockefeller University Hospital) to C Münz; by a Dana Foundation and Irvington Institute’s Human Immunology Fellowship and an Institutional Clinical and Translational Science Pilot and Collaborative Project Grant (to the Rockefeller University Hospital) to JD Lüneman.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Ferlazzo G, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–62. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 2.Strowig T, Brilot F, Münz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–91. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 5.Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15:226–33. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs R, et al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–7. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M. Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J Exp Med. 2005;202:1679–89. doi: 10.1084/jem.20051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mailliard RB, et al. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–53. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferlazzo G, Münz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–9. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 10.Pridgeon C, et al. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright, CD94bright, CD158negative phenotype. Rheumatology (Oxford) 2003;42:870–8. doi: 10.1093/rheumatology/keg240. [DOI] [PubMed] [Google Scholar]
- 11.Dalbeth N, et al. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–26. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 12.Ottaviani C, et al. CD56brightCD16− NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–28. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 13.Carrega P, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56brightCD16− cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–75. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 14.Buentke E, et al. Natural killer and dendritic cell contact in lesional atopic dermatitis skin—Malassezia-influenced cell interaction. J Invest Dermatol. 2002;119:850–7. doi: 10.1046/j.1523-1747.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferlazzo G, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606–11. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laffont S, Seillet C, Ortaldo J, Coudert JD, Guery JC. Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood. 2008;112:661–71. doi: 10.1182/blood-2007-10-120089. [DOI] [PubMed] [Google Scholar]
- 17.Bajenoff M, et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–31. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, et al. Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J Immunol. 2001;166:1590–600. doi: 10.4049/jimmunol.166.3.1590. [DOI] [PubMed] [Google Scholar]
- 19.Orange JS, Biron CA. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–56. [PubMed] [Google Scholar]
- 20.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–42. [PubMed] [Google Scholar]
- 21.Strowig T, et al. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma. PLoS Pathog. 2008;4:e27. doi: 10.1371/journal.ppat.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brilot F, Strowig T, Roberts SM, Arrey F, Münz C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J Clin Invest. 2007;117:3316–29. doi: 10.1172/JCI31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–17. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerosa F, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–34. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 25.Jinushi M, et al. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–9. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 26.Gerosa F, et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adam C, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for anti-tumor CTL induction. Blood. 2005;106:338–44. doi: 10.1182/blood-2004-09-3775. [DOI] [PubMed] [Google Scholar]
- 29.Spaggiari GM, et al. NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur J Immunol. 2001;31:1656–65. doi: 10.1002/1521-4141(200106)31:6<1656::aid-immu1656>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Ferlazzo G, et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pende D, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–6. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 32.Della Chiesa M, et al. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657–66. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]
- 33.Nedvetzki S, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–85. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 34.Lünemann A, et al. Human NK cells kill resting but not activated microglia via NKG2D-and NKp46-mediated recognition. J Immunol. 2008;181:6170–7. doi: 10.4049/jimmunol.181.9.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Fontecha A, Carbone E. The social life of NK cells. Arch Immunol Ther Exp (Warsz) 2001;49(Suppl 1):S33–9. [PubMed] [Google Scholar]
- 36.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–7. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 37.Morandi B, Bougras G, Muller WA, Ferlazzo G, Münz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur. J. Immunol. 2006;3it6:2394–400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 38.Zingoni A, et al. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–24. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 39.Yuan D, Wilder J, Dang T, Bennett M, Kumar V. Activation of B lymphocytes by NK cells. Int Immunol. 1992;4:1373–80. doi: 10.1093/intimm/4.12.1373. [DOI] [PubMed] [Google Scholar]
- 40.Gao N, Dang T, Yuan D. IFN-gamma dependent and -independent initiation of switch recombination by NK cells. J Immunol. 2001;167:2011–8. doi: 10.4049/jimmunol.167.4.2011. [DOI] [PubMed] [Google Scholar]
- 41.Gao N, Jennings P, Yuan D. Requirements for the natural killer cell-mediated induction of IgG1 and IgG2a expression in B lymphocytes. Int Immunol. 2008;20:645–57. doi: 10.1093/intimm/dxn021. [DOI] [PubMed] [Google Scholar]
- 42.Rabinovich BA, et al. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol. 2003;170:3572–6. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 43.Cerboni C, et al. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK-cell lysis. Blood. 2007;110:606–15. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]
- 44.Roy S, et al. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180:1729–36. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 45.Lu L, et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walzer T, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–9. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkler-Pickett R, et al. In vivo regulation of experimental autoimmune encephalomyelitis by NK cells: alteration of primary adaptive responses. J Immunol. 2008;180:4495–506. doi: 10.4049/jimmunol.180.7.4495. [DOI] [PubMed] [Google Scholar]
- 48.Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J Immunol. 2000;165:3099–104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- 49.Bettelli E, et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–87. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto Y, et al. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681–8. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 52.Huang D, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 53.Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;163:24–30. doi: 10.1016/j.jneuroim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Smeltz RB, Wauben MH, Wolf NA, Swanborg RH. Critical requirement for aspartic acid at position 82 of myelin basic protein 73–86 for recruitment of V beta 8.2+ T cells and encephalitogenicity in the Lewis rat. J Immunol. 1999;162:829–36. [PubMed] [Google Scholar]
- 55.Poirot L, Benoist C, Mathis D. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc Natl Acad Sci U S A. 2004;101:8102–7. doi: 10.1073/pnas.0402065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beilke JN, Kuhl NR, Van Kaer L, Gill RG. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med. 2005;11:1059–65. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 57.Zhou R, Wei H, Tian Z. NK3-like NK cells are involved in protective effect of polyinosinic-polycytidylic acid on type 1 diabetes in nonobese diabetic mice. J Immunol. 2007;178:2141–7. doi: 10.4049/jimmunol.178.4.2141. [DOI] [PubMed] [Google Scholar]
- 58.Shibatomi K, et al. A novel role for interleukin-18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthritis Rheum. 2001;44:884–92. doi: 10.1002/1529-0131(200104)44:4<884::AID-ANR145>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 59.De Jager PL, et al. Cytometric profiling in multiple sclerosis uncovers patient population structure and a reduction of CD8low cells. Brain. 2008;131:1701–11. doi: 10.1093/brain/awn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benczur M, et al. Dysfunction of natural killer cells in multiple sclerosis: a possible pathogenetic factor. Clin Exp Immunol. 1980;39:657–62. [PMC free article] [PubMed] [Google Scholar]
- 61.Kastrukoff LF, et al. A role for natural killer cells in the immunopathogenesis of multiple sclerosis. J Neuroimmunol. 1998;86:123–33. doi: 10.1016/s0165-5728(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 62.Hirsch RL, Johnson KP. The effect of recombinant alpha 2-interferon on defective natural killer cell activity in multiple sclerosis. Neurology. 1985;35:597–600. doi: 10.1212/wnl.35.4.597. [DOI] [PubMed] [Google Scholar]
- 63.Kreuzfelder E, et al. Enumeration of T, B and natural killer peripheral blood cells of patients with multiple sclerosis and controls. Eur Neurol. 1992;32:190–4. doi: 10.1159/000116820. [DOI] [PubMed] [Google Scholar]
- 64.Munschauer FE, Hartrich LA, Stewart CC, Jacobs L. Circulating natural killer cells but not cytotoxic T lymphocytes are reduced in patients with active relapsing multiple sclerosis and little clinical disability as compared to controls. J Neuroimmunol. 1995;62:177–81. doi: 10.1016/0165-5728(95)00115-9. [DOI] [PubMed] [Google Scholar]
- 65.Uchida A, Maida EM, Lenzhofer R, Micksche M. Natural killer cell activity in patients with multiple sclerosis: interferon and plasmapheresis. Immunobiology. 1982;160:392–402. doi: 10.1016/S0171-2985(82)80003-X. [DOI] [PubMed] [Google Scholar]
- 66.Vranes Z, Poljakovic Z, Marusic M. Natural killer cell number and activity in multiple sclerosis. J Neurol Sci. 1989;94:115–23. doi: 10.1016/0022-510x(89)90222-0. [DOI] [PubMed] [Google Scholar]
- 67.Kastrukoff LF, et al. Clinical relapses of multiple sclerosis are associated with ‘novel’ valleys in natural killer cell functional activity. J Neuroimmunol. 2003;145:103–14. doi: 10.1016/j.jneuroim.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Infante-Duarte C, et al. Frequency of blood CX3CR1-positive natural killer cells correlates with disease activity in multiple sclerosis patients. FASEB J. 2005;19:1902–4. doi: 10.1096/fj.05-3832fje. [DOI] [PubMed] [Google Scholar]
- 69.Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. 2006;38:20–31. doi: 10.1080/07853890500465189. [DOI] [PubMed] [Google Scholar]
- 70.Ravelli A. Macrophage activation syndrome. Curr Opin Rheumatol. 2002;14:548–52. doi: 10.1097/00002281-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Grom AA, et al. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr. 2003;142:292–6. doi: 10.1067/mpd.2003.110. [DOI] [PubMed] [Google Scholar]
- 72.Villanueva J, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7:R30–7. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly A, Ramanan AV. Recognition and management of macrophage activation syndrome in juvenile arthritis. Curr Opin Rheumatol. 2007;19:477–81. doi: 10.1097/BOR.0b013e32825a6a79. [DOI] [PubMed] [Google Scholar]
- 74.Hauser SL, Ault KA, Levin MJ, Garovoy MR, Weiner HL. Natural killer cell activity in multiple sclerosis. J Immunol. 1981;127:1114–7. [PubMed] [Google Scholar]
- 75.Rauch HC, Montgomery IN, Kaplan J. Natural killer cell activity in multiple sclerosis and myasthenia gravis. Immunol Invest. 1985;14:427–34. doi: 10.3109/08820138509047611. [DOI] [PubMed] [Google Scholar]
- 76.Rice GP, Casali P, Merigan TC, Oldstone MB. Natural killer cell activity in patients with multiple sclerosis given alpha interferon. Ann Neurol. 1983;14:333–8. doi: 10.1002/ana.410140312. [DOI] [PubMed] [Google Scholar]
- 77.Santoli D, et al. Cytotoxic activity and interferon production by lymphocytes from patients with multiple sclerosis. J Immunol. 1981;126:1274–8. [PubMed] [Google Scholar]
- 78.Segal BM. The role of natural killer cells in curbing neuroinflammation. J Neuroimmunol. 2007;191:2–7. doi: 10.1016/j.jneuroim.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parham P. The genetic and evolutionary balances in human NK cell receptor diversity. Semin Immunol. 2008;20:311–6. doi: 10.1016/j.smim.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nelson GW, et al. Cutting edge: heterozygote advantage in autoimmune disease: hierarchy of protection/susceptibility conferred by HLA and killer Ig-like receptor combinations in psoriatic arthritis. J Immunol. 2004;173:4273–6. doi: 10.4049/jimmunol.173.7.4273. [DOI] [PubMed] [Google Scholar]
- 81.Martin MP, et al. Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J Immunol. 2002;169:2818–22. doi: 10.4049/jimmunol.169.6.2818. [DOI] [PubMed] [Google Scholar]
- 82.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–14. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 83.Schippling DS, Martin R. Spotlight on anti-CD25: daclizumab in MS. Int MS J. 2008;15:94–8. [PubMed] [Google Scholar]
- 84.Bielekova B, et al. Regulatory CD56z(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56bright regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–91. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 86.Saraste M, Irjala H, Airas L. Expansion of CD56bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol Sci. 2007;28:121–6. doi: 10.1007/s10072-007-0803-3. [DOI] [PubMed] [Google Scholar]
- 87.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4+CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–41. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.La Cava A, Van Kaer L, Fu Dong S. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–7. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 90.Linsen L, Somers V, Stinissen P. Immunoregulation of autoimmunity by natural killer T cells. Hum Immunol. 2005;66:1193–202. doi: 10.1016/j.humimm.2006.02.020. [DOI] [PubMed] [Google Scholar]