Abstract
The lung is one of the organs to which cancers from solid tumors frequently metastasize. Multiple tumors in the lung are usually treated by systemic chemotherapy because of the lack of efficient methods of targeting antitumor agents to the lung. Although intratracheal administration is an ideal route for targeting multiple lung tumors, antitumor agents are often harmful to the organ or induce inflammation. Mesenchymal stem cells (MSCs), nonhematopoietic stem cells capable of differentiating into various mesoderm-type cells, have a propensity to migrate to and proliferate in tumor tissues after systemic administration. We intratracheally injected MSCs expressing CX3CL1 (MSC/RGDFKN) into the lung of lung tumor–bearing mice with multiple metastases of C26 or Lewis lung carcinoma (LLC). Antitumor effects were evaluated by counting the number of lung metastases and survival. We demonstrated the tropism of mouse MSCs to lung tumor tissues after intratracheal administration of GFP-positive MSCs. Intratracheal injection of MSC/RGDFKN strongly inhibited growth of lung metastases of C26 or LLC, and thus prolonged survival. Intratracheal injection of MSC/RGDFKN did not induce an inflammatory reaction in the lung. These results suggest that MSCs expressing antitumor agents can be delivered intratracheally into multiple lung tumor tissues without causing inflammation.
INTRODUCTION
Advanced solid tumors eventually metastasize to other organs (1). The lung is one of the organs to which many types of tumors metastasize and is sometimes the only organ to which a primary tumor metastasizes (2). Systemic chemotherapy is commonly used in the treatment of metastatic lung tumors, because methods for efficient targeting of antitumor agents to the lung have yet to be established.
Intratracheal administration is an ideal route for drug delivery to the lung, because the drug reaches the target organ efficiently without entering the systemic circulation. Indeed, drugs such as steroids and anticholinergic agents have been administered intratracheally to the lung by inhalation in patients with bronchial asthma and chronic obstructive pulmonary disease (3,4). However, to date, antitumor agents have not been administered intratracheally because they are often harmful to the lung.
Bone marrow–derived mesenchymal stem cells (MSCs) are pluripotent cells, capable of differentiating into cells of various tissue lineages (5). MSCs can be expanded easily in vitro, can be transduced with various genes, and retain an extensive multipotent capacity for differentiation. Because of these characteristics, MSCs have attracted a great deal of interest with regard to their potential use in tissue repair and gene therapy (6). Importantly, MSCs can migrate selectively through the systemic circulation and proliferate in solid tumors (7–9). These features have led to the suggestion that MSCs may be useful as cellular vehicles for gene delivery to multiple tumor sites. With the use of this strategy in administering intravenous injections of gene-modified MSCs, antitumor effects have been demonstrated in multiple lung, brain, and subcutaneous tumors (7–10). However, only a small fraction of the injected MSCs migrated to the tumor sites. MSCs may also express transduced antitumor agents in normal organs, because these cells circulate in the bloodstream and come into contact with normal organs (11).
Recently, intratracheal injection has been used to administer several types of cells to the lung to repair damaged lungs (12–14). Injected cells survived in the lung tissues and showed treatment effects. Previously, we demonstrated that intravenous injection of MSCs expressing CX3CL1 (fractalkine) in mice bearing multiple lung tumors induced strong antitumor effects (15).
In this study, we administered MSCs expressing CX3CL1 intratracheally to lung tumor–bearing mice. Intratracheal administration of MSCs expressing CX3CL1 resulted in a strong inhibitory effect on lung metastases and prolonged the survival of tumor-bearing mice without apparent adverse effects.
MATERIALS AND METHODS
Cell Culture and Mice
The murine colon adenocarcinoma cell line C26 (H-2d), Lewis lung carcinoma cell line LLC (H-2b), and fibroblast cell line BLKCL4 (H-2b) were obtained from the Cell Resource Center for Biomedical Research (Tohoku University, Sendai, Japan). These cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). The murine fibroblast cell line BALB 3T3 (H-2d) was obtained from the Health Science Research Resources Bank (National Institute of Biomedical Innovation, Osaka, Japan) and propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. Female C57BL/6(H-2b) and BALB/c (H-2d) mice, 6–8 wks old, were purchased from Charles River, Japan (Atsugi, Japan). Green fluorescent protein (GFP)-expressing mice [C57BL/6-TgN (ACTbEGFP)] were kindly provided by Dr. Okabe (Osaka University, Osaka, Japan). All animal experiments were approved by the Institutional Review Board for Animal Experiments of Tohoku University.
Isolation and Culture of Mouse MSCs
BALB/c, C57BL/6, and GFP-expressing mice (6–10 wks old) were killed by cervical dislocation, and the femurs and tibias were removed and carefully cleaned of all connective tissue. The bone marrow was flushed out with culture medium and expelled from a 5-mL syringe through a 25-gauge needle. The bone marrow suspension was then transferred to 6-well plates at a concentration of 1.5 × 106 nucleated cells per cm2. The cells were cultured in low-glucose DMEM (Gibco-BRL, Life Technologies, Grand Island, NY, USA) with 10% FBS (Gibco-BRL). After 72 h, nonadherent cells were removed, and fresh medium was added. When the adherent cells reached 70%–80% confluence, cells were trypsinized, harvested, and expanded. When a homogenous cell population was obtained, after 3–5 passages, these cells were used for the subsequent experiments.
The induction of adipogenic differentiation from MSCs was performed as reported by Arai et al. (15). MSCs were cultured with 0.5 mmol/L 3-isobutyl-1-methylxanthine (IBMX; Sigma, St. Louis, MO, USA), 1 μmol/L dexamethasone, 10 mg/mL insulin, and 10% FBS in DMEM (adipogenic induction medium) for 1 wk. The medium was then changed to adipogenic maintenance medium (10% FBS DMEM containing 1 μmol/L dexamethasone and 10 mg/mL insulin) and culture was continued for an additional 4 d. The cells were fixed in 10% formalin for 10 min, and stained for 20 min with fresh Oil Red O solution (Sigma, St. Louis, MO, USA). Osteogenic differentiation was induced by culturing cells for 3 wks in DMEM, supplemented with 10% FBS, 0.2 μmol/L ascorbic acid (Sigma), 10 mmol/L β-glycerophosphate (Sigma), and 0.1 μmol/L dexamethasone (Sigma). The medium was changed every 3 d. Osteogenic differentiation was detected by staining with Alizarin Red S (Sigma).
Adenoviral Vectors
Genetically modified adenoviral vectors with an integrin-binding motif (Arg-Gly-Asp) in the HI loop of the fiber knob, carrying the mouse fractalkine (Ad-RGDFKN) and β-galactosidase genes (Ad-RGDLacZ) were constructed as reported previously (16,17). These recombinant adenoviral vectors were propagated from 293 cells and purified by CsCl gradient centrifugation. The total numbers of viral particles in the viral samples were measured by OD260 (1 OD260 = 1012 particles). We quantified the titers (expressed as pfu/mL) of the viral stocks by plaque-forming assay using 293 cells.
Expression of Fractalkine on MSCs
The Ad-RGDFKN–transduced MSCs (MSC/RGDFKN) were washed with PBS and lysed in RIPA buffer (10 mmol/L Tris-HCl, pH7.4, 0.15 mol/L NaCl, 5 mmol/L EDTA, 1% DOC, 0.1% SDS, 1 mmol/L PMSF, and 1 mmol/L DTT) containing protease inhibitors (Roche Molecular Biochemicals, Indianapolis, IN, USA) to determine fractalkine expression 48 h after Ad-RGDFKN infection (100 MOI). The cell lysates were cleared by centrifugation (20,000g, 30 min, 4°C). Recombinant mouse fractalkine (50 ng) encoding the extracellular domain (R&D Systems Inc., Minneapolis, MN, USA) and 30 μg of total protein from the Ad-RGDFKN–transduced MSCs were loaded per lane on 10% Bis-Tris gels (Invitrogen, Carlsbad, CA, USA), and transferred onto PVDF membranes (Invitrogen). The membranes were incubated with rat antimouse fractalkine mono-clonal antibody (R&D systems) and stained with a secondary antibody (goat antirat IgG-HRP; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Detection of specific signals was performed using the ECL detection system (Amersham Pharmacia Biotech AB, Uppsala, Sweden), in accordance with the manufacturer’s protocol.
In vivo Migration Assay
To examine the ability of MSCs to migrate to tumor tissues in vivo, 7 d after intratracheal injection of GFP-positive MSCs into mice with or without established LLC pulmonary metastases, organs (lung, liver, kidney, spleen, and bone marrow) were fixed with 4% paraformaldehyde and embedded in paraffin. Sections were treated by an autoclave-based antigen-retrieval technique with 10 mmol/L citrate buffer, pH 6.0, at 120°C for 10 min. After nonspecific staining and endogenous peroxidase activity were blocked, sections were incubated for 1 h with rabbit polyclonal anti-GFP antiserum (Molecular Probes, Invitrogen) and Simple Stain Mouse MAX PO (Nichirei, Tokyo, Japan), and labeling was visualized with AEC reagents (Nichirei). The specimens were incubated with hematoxylin for nuclear counterstaining.
Treatment of Lung Metastases with MSC/RGDFKN
To establish experimental lung metastases, 5 × 105 tumor cells in 0.2 mL of PBS were injected into the lateral tail vein of the mice (LLC into C57BL/6, C26 into BALB/c) (d 0). Five d later, the mice were randomly divided into five groups. Mice in the first group received an intratracheal (i.t.) injection of 5 × 105 MSC/RGDFKN, those in the second group received an i.t. injection of 5 × 105 Ad-RGDLacZ–transduced MSCs (MSC/RGDLacZ) as a control for Ad-RGDFKN, and those in the third group received an i.t. injection of 5 × 105 Ad-RGDFKN–transduced fibroblast cells as a control for MSCs. Mice in the fourth group received an i.t. injection of 5 × 107 pfu Ad-RGDFKN vector, without any cells. Mice in the fifth group received an i.t. injection of PBS alone. Loss of consciousness was induced by intraperitoneal injection of ketamine, and then the trachea was exposed with a midline incision and cannulated with a 24-guage catheter. After 0.2 mL of PBS containing MSCs was injected into trachea through a catheter, the incision was closed by sewing. Eight mice in each group were killed on d 12, and the lungs were fixed in Bouin’s solution. The metastatic colonies were easily identified macroscopically as white nodules on the lung surface. The other eight mice in each group were monitored until death for the survival assay. Survival curves were drawn by use of the Kaplan–Meier method.
Bronchoalveolar Lavage Fluid Collection
Mice were subjected to brief anesthesia by intraperitoneal injection of ketamine and xylazine. After mice lost consciousness, the trachea was exposed with a midline incision and cannulated with a 24-gauge catheter. After the mice were killed by exsanguination, the lungs were lavaged three times with sterile 0.9% NaCl at a volume of 0.75 mL/wash. The average fluid recovery was greater than 80%. The bronchoalveolar lavage fluid (BALF) was centrifuged (300g, 10 min, 4°C), and the supernatants were stored at −20°C until used for protein analysis. The protein concentrations in the recovered BALF were determined using the Pierce microBCA assay (Pierce, Rockford, IL, USA) with bovine serum albumin as a standard.
Cells from BALF samples were resuspended in 0.5 mL of normal saline. The cells were diluted 1:1 with Turk’s solution and counted with a hemocytometer. Cell differentials were determined on slides prepared in a Cytospin 3 (Shandon, Pittsburgh, PA, USA) centrifuge (113g, 2 min) and stained with a Diff-Quick staining kit (International Reagents, Kobe, Japan).
Statistical Analysis
The results are expressed as means ± standard error (SE) or as means ± standard deviation (SD). Statistical comparisons were performed using the two-tailed Student t test, and a value of P < 0.05 was deemed statistically significant. For the survival data, the log-rank test was used to assess differences among the five treatment groups, and P < 0.05 was deemed statistically significant.
RESULTS
Fractalkine Expression in MSCs/RGDFKN
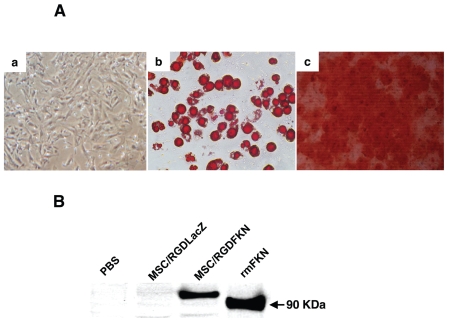
MSCs cultured at passage five differentiated readily into adipocytes when incubated in adipogenic maintenance medium (Figure 1Ab) and differentiated into osteoblasts following supplementation of the medium with osteogenic induction medium (Figure 1Ac). The MSCs used in the present study retained their differentiation capability. The expression of fractalkine by MSC/RGDFKN in vitro was confirmed by RT-PCR (data not shown) and by Western blotting analysis (Figure 1B). Although 100 kDa fractalkine was detected in MSC/RGDFKN, recombinant fractalkine yielded a protein band of ~90 kDa, because this recombinant protein consists of only the extracellular domain. As expected, no bands were detected in control MSC/RGDLacZ or non-transduced MSCs.
Figure 1.
Differentiation of MSCs and fractalkine expression in MSC/RGDFKN. (A) Differentiation of mouse MSCs in vitro. MSCs were cultured in nondifferentiating medium (a); MSCs cultured in adipogenic media for 14 d were stained with Oil Red O (b); MSCs cultured in osteogenic media for 3 wks were stained with Alizarin Red S (c). (B) Western blotting analysis of fractalkine in MSCs transduced with Ad-RGDFKN or Ad-RGDLacZ. Equal amounts of protein (30 μg per lane) were subjected to Western blotting analysis as described in Materials and Methods. The recombinant extracellular domain of mouse fractalkine (rmFKN) (~90 kDa, 50 ng per lane) as a positive control was also detected by the same antibody.
Tropism of MSCs for Tumors after Intratracheal Injection
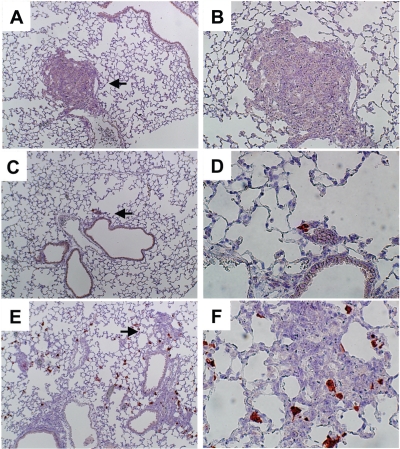
We reported previously that MSCs migrate to tumors in the lung after injection via the tail vein (15). In this study we performed in vivo migration assays of MSCs into the tumor tissues in the lung to assess the tropism of MSCs. GFP-positive MSCs were injected into the trachea of C57BL/6 mice bearing LLC lung metastases. GFP-positive MSCs were found primarily within and around tumor tissues (Figure 2E, F), and very few were found in the normal lung area. We found very few GFP-positive MSCs in the lung in non–tumor-bearing mice (Figure 2C, D). No GFP-positive cells were detected in areas of the tumors in mice injected with PBS alone (Figure 2A, B) or in the liver, spleen, or bone marrow 7 d after MSC injection (data not shown). We did not perform in vivo migration assays in C26 lung metastases because GFP mice were C57BL/6. These results suggested that the MSCs have specific migratory activity toward tumor tissues after intratracheal injection.
Figure 2.
Tropism of MSCs for tumor cells in vivo. Lungs with or without metastases of LLC were removed 7 d after intratracheal injection of MSCs derived from GFP-transgenic mice. GFP-positive cells were detected with anti-GFP antibody. Lung tissues with (A, B, E, and F) or without pulmonary metastases (C and D) are shown. Left panels are lower magnification (100×); right panels are higher magnification (200×); arrows indicate the part at higher magnification. Panels C, D, E, and F are the lungs after MSC injection. Panels A and B are the lungs after PBS injection.
Effects of Intratracheal Administration of MSC/RGDFKN on C26 Lung Metastases
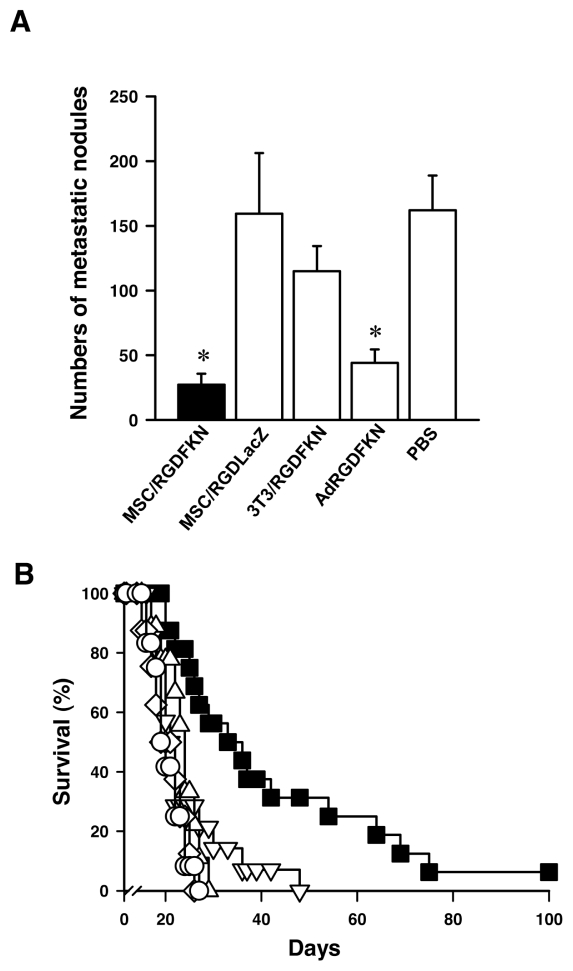
We next examined the antitumor effects of MSC/RGDFKN in the C26 lung metastasis model. MSC/RGDFKN (5 × 105) were injected through the trachea once on d 5 after injection of C26 cells via the tail vein. Control mice received PBS or MSC/RGDLacZ as a control for Ad-RGDFKN, BALB 3T3/RGDFKN as a control for MSCs, or 5 × 107 pfu of Ad-RGDFKN, a dose equivalent to that for infection of MSCs ex vivo. The mice treated with MSC/RGDFKN developed fewer and smaller metastatic nodules on the lung surface than those in the other treatment groups. The group treated with MSC/RGDFKN was shown to have a significantly reduced number of lung metastases compared with the controls (Figure 3A; 27.2 ± 8.4 in the MSC/RGDFKN group, 162.0 ± 26.9 in the PBS group; P < 0.01). The effect of MSC/RGDFKN treatment was stronger than that of Ad-RGDFKN treatment, although Ad-RGDFKN (44.0 ± 10.4 metastatic nodules) had a significant effect compared with the three other groups (P < 0.01). Mice treated with either MSC/RGDLacZ or BALB 3T3/RGDFKN showed no reduction in lung metastases compared with the PBS group (Figure 3A).
Figure 3.
Inhibition of C26 lung metastases by single intratracheal injection of MSC/RGDFKN. (A) Numbers of pulmonary metastatic nodules in each treatment group. The numbers of metastatic nodules on the lung surface were counted macroscopically. The data represent the means ± SD of results from eight mice. *P < 0.01. (B) Survival curves for the mice in each treatment group. Mice with established C26 pulmonary metastases were treated as described in Materials and Methods. Survival curves were drawn by the Kaplan–Meier method (n = 8 in each treatment group). MSC/RGDFKN (▪), MSC/RGDLacZ (○), 3T3/RGDFKN (▵), Ad-RGDFKN vector (▿), PBS (⋄).
Mice treated with MSC/RGDFKN lived significantly longer than those in any of the other groups (P < 0.005; Figure 3B). The median survival time of the MSC/RGDFKN group was the longest (33 d) among the treatment groups (19–23 d). The mice treated with 5 × 107 pfu Ad-RGDFKN did not live significantly longer than those treated with PBS, MSC/RGD-LacZ, or BALB 3T3/RGDFKN.
Effects of Intratracheal Administration of MSC/RGDFKN on LLC Lung Metastases
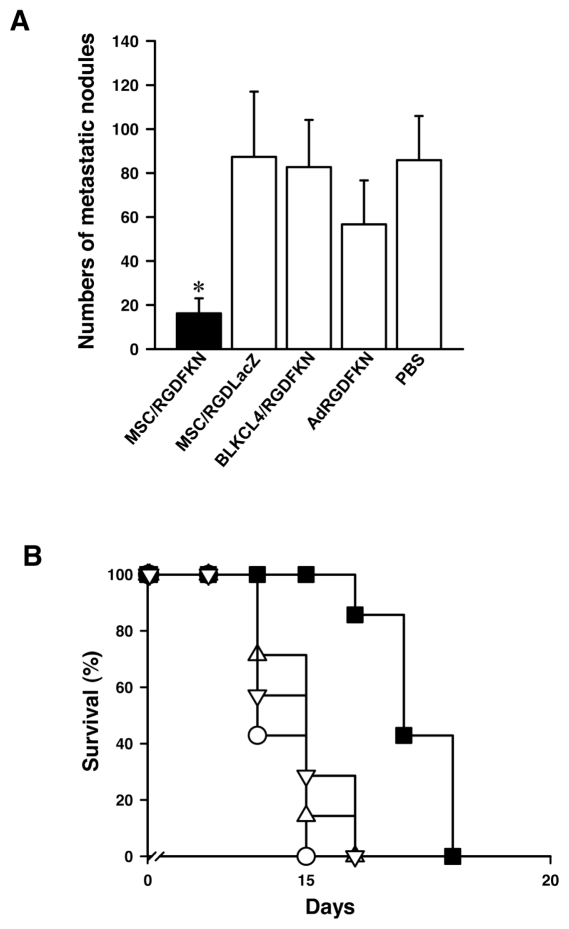
We also examined the in vivo effects of MSC/RGDFKN in the LLC lung metastasis model. Again, mice treated with MSC/RGDFKN developed fewer and smaller metastatic nodules on the lung surface than those in the other groups (Figure 4A). The number of metastatic nodules in mice treated with MSC/RGDFKN was markedly reduced by 81%, compared with the PBS controls (P < 0.01; Figure 4A). Although treatment with Ad-RGDFKN reduced metastatic nodules by 34%, this reduction was statistically insignificant (P = 0.81). Mice treated with either MSC/RGDLacZ or BLKCL4/RGDFKN showed no reduction in lung metastases (Figure 4A). Mice treated with MSC/RGDFKN lived significantly longer than those in any of the other groups (P < 0.005; Figure 4B). The median survival time of the MSC/RGDFKN group was the longest (17 d) among the treatment groups (10–15 d).
Figure 4.
Inhibition of LLC lung metastases by single intratracheal injection of MSC/RGDFKN. (A) Numbers of pulmonary metastatic nodules in each treatment group. The data represent the means ± SD of results from eight mice. *P < 0.01. (B) Survival curves for the mice in each treatment group. Survival curves were drawn by the Kaplan–Meier method (n = 8 in each treatment group). MSC/RGDFKN (▪), MSC/RGDLacZ (○), 3T3/RGDFKN (▵), Ad-RGDFKN vector (▿).
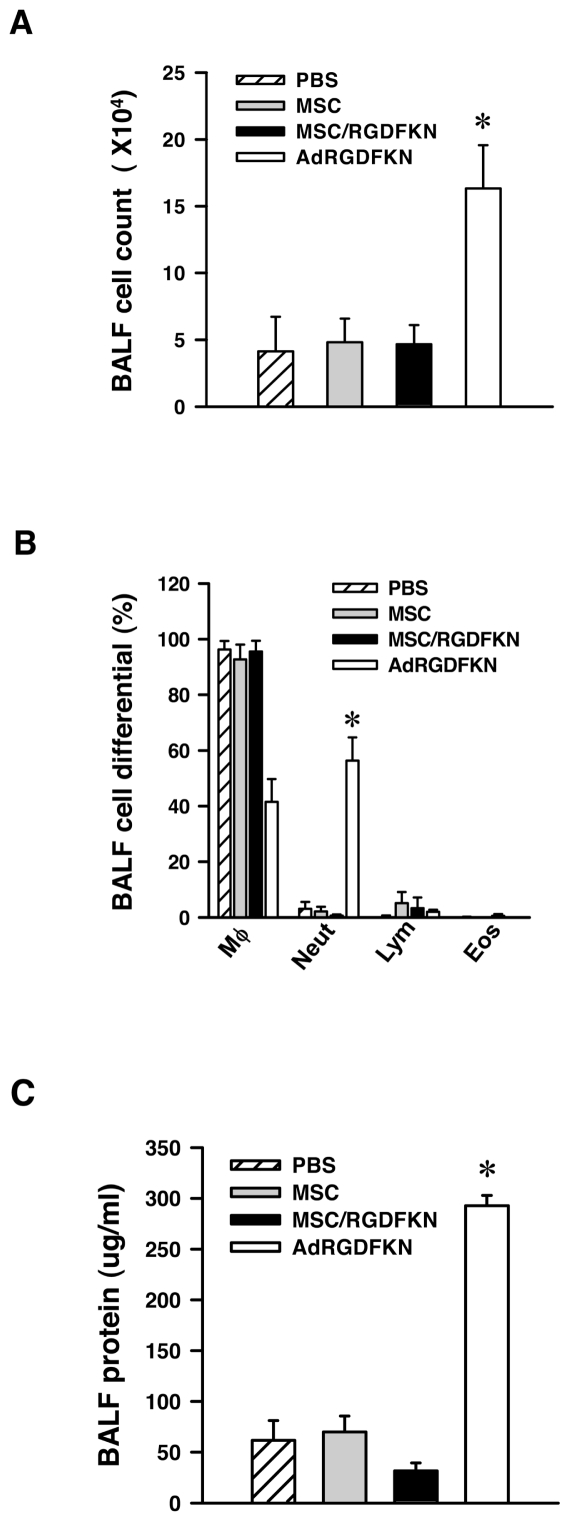
Intratracheal Delivery of MSC/RGDFKN is Less Inflammatory than Ad-RGDFKN
To evaluate lung inflammation, BALF was collected 1 d after intratracheal administration of MSC/RGDFKN or the adenoviral vector Ad-RGDFKN. We found that cellular infiltration was the same among MSCs, MSC/RGDFKN, and PBS groups. The mice receiving the Ad-RGDFKN vector showed a significantly greater cell number in BALF compared with the other groups (P < 0.01; Figure 5A). We further analyzed the cellular composition of the BALF after MSC/RGDFKN injection. Mice in the Ad-RGDFKN group showed a marked increase in the number of neutrophils compared with the other groups (P < 0.01; Figure 5B). As shown in Figure 5C, the protein level in the BALF of mice injected with MSC/RGDFKN was unchanged, whereas that in the Ad-RGDFKN group was increased to a greater extent than in the other groups (P < 0.01). Treatment with MSC/RGDFKN did not induce airway inflammation in the murine lung metastasis model.
Figure 5.
Inflammatory reaction in the lung after MSC/RGDFKN injection. C57BL/6 mice (n = 4/group) with LLC pulmonary metastases received PBS, MSCs, MSC/RGDFKN, or Ad-RGDFKN by intratracheal administration. After 24 h, mice were killed and the BALF was recovered. Induced lung inflammation was monitored by counting total cells (A) and cell differential (B) and by measuring protein levels (C) in the BALF. Data are expressed as means ± SE. *P < 0.01.
DISCUSSION
In this study, we demonstrated that intratracheal administration of MSCs expressing CX3CL1 (MSC/RGDFKN) suppressed the growth of multiple lung metastases and prolonged survival in mice. MSCs migrated to the tumor tissues in the lung after intratracheal injection. We also confirmed that MSC/RGDFKN did not cause lung inflammation, in contrast to injection of Ad-RGDFKN vector, which was associated with marked inflammation in the lungs.
Genetically modified MSCs are a potential treatment of a variety of disorders, because modified MSCs have inherent MSC features that are relevant to the disease (19,20) and because MSCs can be used as a cellular vehicle to synthesize or deliver a protein of therapeutic value to the target organ (7–10). A major hurdle to using MSCs modified by gene therapy is finding a way to achieve organ specificity, to reduce or avoid the unnecessary burden of genetically modified cells at other sites. In the present study using a murine lung metastasis model system, we demonstrated that direct transplantation into the respiratory tract is a feasible approach. We found abundant GFP-positive MSCs around tumors or in tumor tissues after intratracheal injection. MSCs transplanted into the lung remained in the lung tumor tissues for at least 7 d, suggesting tropism to metastatic lung tumors. Our previous study showed that MSCs migrated to lung tumors and showed high-level expression of CX3CL1 in lung tumor tissues after systemic administration of MSC/RGDFKN. Thus, we believe intratracheally injected MSC/RGDFKN expressed CX3CL1 in the tumor milieu and induced antitumor immune responses, as we demonstrated previously (15,21).
An adenoviral vector with a natural affinity for airway epithelia has been applied to gene therapy for lung disease but caused strong immune reactions in both animal models and humans (22–25). Thus, adenoviral vectors cannot be administered repeatedly. In this study, intratracheal administration of an adenoviral vector expressing CX3CL1 was partially effective in the lung metastatic model but induced an immune reaction in recipient mice, as reported previously (24,25). On the other hand, MSCs expressing CX3CL1 did not cause an inflammatory reaction in recipient mice. Thus, modified MSCs can be administered repeatedly to the lung, because they did not induce an immune reaction. MSC-based gene therapy may be useful for gene transfer not only to lung tumors, but also to lung epithelia, because MSCs did not induce an inflammatory reaction.
A striking finding in the present study was that the intratracheal administration of MSC/RGDFKN into tumor-bearing mice was more effective than Ad-RGDFKN injection with regard to prolonging survival, although the Ad-RGDFKN vector apparently suppressed the number of lung metastases as effectively as MSC/RGDFKN. A possible explanation is that because MSC/RGDFKN remained in or around metastatic tissue and expressed CX3CL1 for longer than the Ad-RGDFKN vector, a stronger immune reaction was induced in mice treated with MSC/RGDFKN.
Although CX3CL1 induced the antitumor effects through both natural killer cells and T lymphocytes (15,26), no up-regulation of lymphocytes was observed in BALF. This discrepancy can be attributed to the day when BAL was performed. BAL was performed only 1 day after MSC/RGDFKN, before antitumor immune reaction was induced.
MSC-based gene therapy for targeting tumors has been shown to be effective in various models, including multiple lung metastatic tumors, brain tumors, and subcutaneous tumors (7–10,15). Intravascular administration was used in all animal experiments. Although the tissue distribution of MSCs after systemic administration has been evaluated extensively, many MSCs were found in other organs (7). No adverse effects of modified MSCs were observed. MSCs pass through the systemic circulation after intravenous injection and may remain for short periods in normal organs. In addition, in nonhuman primates, engraftment of MSCs in normal organs was observed after systemic administration (11). Thus, systemic administration of engineered MSCs may result in undesired adverse effects in normal organs. In this context, intratracheal administration of engineered MSCs should be considered in treatment for multiple lung metastases and other lung diseases.
In conclusion, we demonstrated the suppression of lung metastases by intratracheal administration of MSCs expressing CX3CL1. The data presented in this study reemphasize the important role of MSC-based cell therapy in the suppression of lung metastases.
ACKNOWLEDGMENTS
This work was supported, in part, by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture, and Technology of Japan (No. 16022206).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Hill RP. Metastasis. In: Tannock IF, Hill RP, editors. The basic science of oncology. 2nd ed. New York: McGraw-Hill; 1992. pp. 178–95. [Google Scholar]
- 2.Crow J, Slavin G, Kreel L. Pulmonary metastasis: a pathologic and radiologic study. Cancer. 1981;47:2595–602. doi: 10.1002/1097-0142(19810601)47:11<2595::aid-cncr2820471114>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Lemanske RF, Jr, et al. Inhaled corticosteroid reduction and elimination in patients with persistent asthma receiving salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–603. doi: 10.1001/jama.285.20.2594. [DOI] [PubMed] [Google Scholar]
- 4.Pauwels RA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med. 1999;340:1948–53. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 5.Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;2005;306:330–5. doi: 10.1016/j.yexcr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Hamada H, et al. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005;96:149–56. doi: 10.1111/j.1349-7006.2005.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studeny M, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura K, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–64. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 9.Nakamizo A, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 10.Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–13. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 11.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 12.Kuang PP, et al. Engraftment of neonatal lung fibroblasts into the normal and elastase-injured lung. Am J Respir Cell Mol Biol. 2005;33:371–7. doi: 10.1165/rcmb.2004-0319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baber SR, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–8. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- 14.Serrano-Mollar A, et al. Intratracheal transplantation of alveolar type II cells reverse bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2007;176:1261–8. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- 15.Xin H, et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 16.Arai F, Ohneda O, Miyamoto T, Zhang XQ, Suda T. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med. 2002;195:1549–1563. doi: 10.1084/jem.20011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizuguchi H, et al. A simplified system for constructing recombinant adenoviral vectors containing heterologous peptides in the HI loop of their fiber knob. Gene Ther. 2001;8:730–5. doi: 10.1038/sj.gt.3301453. [DOI] [PubMed] [Google Scholar]
- 18.Gao JQ, et al. Antitumor effect by interleukin-11 receptor alpha-locus chemokine/CCL27, introduced into tumor cells through a recombinant adenovirus vector. Cancer Res. 2003;63:4420–5. [PubMed] [Google Scholar]
- 19.Noiseux N, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–50. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–45. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin H, et al. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur J Immunol. 2005;35:1371–80. doi: 10.1002/eji.200526042. [DOI] [PubMed] [Google Scholar]
- 22.Crystal RG, et al. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- 23.Crystal RG, et al. Evaluation of repeat administration of a replication deficient, recombinant adenovirus containing the normal cystic fibrosis transmembrane conductance regulator cDNA to the airways of individuals with cystic fibrosis. Hum Gene Ther. 1995;6:667–703. doi: 10.1089/hum.1995.6.5-667. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–15. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Ginkel FW, et al. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and beta-galactosidase. Hum Gene Ther. 1995;6:895–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- 26.Tang L, et al. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007;14:1226–34. doi: 10.1038/sj.gt.3302959. [DOI] [PubMed] [Google Scholar]