Abstract
Purpose
To determine the reproducibility of the pattern electroretinogram with the new Pattern Electroretinogram for Glaucoma (PERGLA) recording paradigm in glaucoma patients with a range of severity.
Design
Experimental study.
Participants
Fifty-three glaucoma patients were recruited for the study (mean age ± standard deviation [SD], 69 ±11 years). Their mean deviation (MD) global indices on static automatic perimetry ranged from 2.16 to –31.36 decibels (mean MD, −9.05).
Intervention
All patients had pattern electroretinogram recordings done 5 times by the same operator, on 5 different days with the standardized PERGLA paradigm.
Main Outcome Measures
Pattern electroretinogram amplitude (microvolts), phase (π radians), response variability (coefficient of variation [CV] = SD/mean × 100) of amplitude and phase of 2 partial averages that build up the pattern electroretinogram waveform, interocular asymmetry in amplitude and phase (in terms of the CV generated by the pattern electroretinogram software), signal-to-noise (S/N) ratio, SDs, CV, and intraclass correlation coefficient (ICC). All analyses were done on one eye of each subject, except when interocular asymmetry was studied.
Results
The CVs of intrasession variabilities in amplitude and phase were 12.08% and 2.20%, respectively, and those of intersession variabilities were 20.82% and 4.17%. The pattern electroretinogram produced intersession ICCs in amplitude and phase of 0.791 and 0.765, respectively. These ICCs were significantly higher than the ICCs for pattern electroretinogram interocular asymmetry in amplitude and phase (0.659 [P<0.05] and 0.571 [P<0.05], respectively). On average, the pattern electroretinogram S/N ratio in glaucomatous patients was about 5:1.
Conclusions
The reproducibility of PERGLA in glaucomatous patients is sufficiently good for it to be considered a useful complementary clinical tool. Being more reproducible, direct measures of amplitude and phase should be more useful in monitoring progression than interocular asymmetry comparisons. Ophthalmology 2008;115: 957–963 © 2008 by the American Academy of Ophthalmology.
It is generally agreed that the pattern electroretinogram response originates from the ganglion cell layer or, at least, the inner/proximal retina.1 The pattern electroretinogram has been shown to be abnormal in patients with established glaucoma and, sometimes, in ocular hypertension, which suggests its potential usefulness in the diagnosis and management of glaucoma.2–4 In fact, pattern electroretinogram amplitude reduction sometimes precedes other signs of glaucomatous damage.5–10 Even though most studies focused on the reduction of pattern electroretinogram amplitude in glaucoma, some studies suggested that other parameters, like pattern electroretinogram phase and latency, as well as interocular asymmetry in any of these parameters, could also be altered in glaucomatous and suspect patients.8,11–13
Recently, the pattern electroretinogram was shown to be as successful as frequency-doubling technology and short-wavelength automated perimetry in detecting disease before standard automated perimetry and identifying those likely to show future development of glaucoma and progression as judged by current standard methods.14 The pattern electroretinogram might even have a better sensitivity and specificity than short-wavelength automated perimetry in recognizing the presence of glaucoma.5 An abnormal pattern electroretinogram correlates with risk factors,8,15–18 possibly representing cases of subclinical disease, and it has also been thought, possibly, to predict progression.8 Pattern electroretinogram responses can improve upon lowering the intraocular pressure (IOP), which suggests that some retinal ganglion cells might not be functioning normally but are still alive in some glaucomatous eyes.19
However, despite these reasons for renewed scientific interest in the pattern electroretinogram, its clinical use in ophthalmology has not gained widespread acceptance. Both the recording and interpretation of the pattern electroretinogram are challenging, and the pattern electroretinogram response varies.20–28 Several contradictory studies have been published regarding pattern electroretinogram reproducibility.20–36
Recently, a new pattern electroretinogram paradigm tailored for use in glaucoma (PERGLA) was introduced to render the pattern electroretinogram testing more user-friendly and clinically useful by easing its execution and interpretation.24,37 PERGLA is automated and uses a fast paradigm with skin electrodes. On normal subjects, it yields results as reliable as the best previously reported with the traditional protocols.24 In glaucoma, however, the reproducibility, or test–retest variability, of PERGLA has not been studied. The reproducibility and range of values in normal subjects are useful in making a diagnosis, but reproducibility in glaucoma must also be evaluated if PERGLA is to be used to judge progression.
For this reason, we studied the reproducibility (intersession and intrasession–intratest variability) of PERGLA in stable glaucoma patients, with a standardized protocol and a single operator.
Materials and Methods
Subjects
Fifty-three glaucoma patients were recruited for the study. Their mean age (± standard deviation [SD]) was 69±11 years (median, 71; range, 40–91); 28 were male and 25 female. The study group included 23 Caucasians, 17 Hispanics, 11 African Americans, and 2 Asians. Their mean deviation (MD) on standard automated perimetry ranged from 2.16 to −31.36 decibels (mean MD, 9.05). Each subject had a complete ophthalmologic examination, including visual acuity (VA), slit-lamp examination, IOP measurement, visual field examination with the Humphrey Visual Field Analyzer (Carl Zeiss Meditec, Dublin, CA), and dilated fundus examination. Within 8 weeks, each subject had 5 pattern electroretinogram recordings on 5 different days. To be included as a subject, the patient had to (1) be at least 18 years old; (2) have a clinical diagnosis of glaucoma or emerging glaucoma; (3) be reasonably assumed, from clinical evaluation, to be stable; (4) have best-corrected VA of 20/40 or better; (5) have <5 diopters (D) of spherical and <3 D of cylindrical refractive error; (6) have a ≥ 2-mm pupil diameter; (7) have no history of ocular or neurologic disease or surgery that might produce test results or vision changes that confound recognition of test results due solely to glaucoma; (8) have no history of amblyopia; (9) have the mental and physical capacity to undergo the tests; and (10) agree to participate as a subject in the scientific study. An effort was made to have a good distribution of early, moderate, and advanced glaucoma, as well as glaucoma suspects. Patients were recruited from a hospital-based outpatient glaucoma ophthalmology practice (Anne Bates Leach Eye Hospital, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida).
The study followed the tenets of the Declaration of Helsinki, and the protocol was approved by the human subjects institutional review board of the University of Miami, Miller School of Medicine. Informed consent to participate was obtained from all subjects and documented with their signatures.
Pattern Electroretinogram Recording
The pattern electroretinograms were recorded by means of the recently described PERGLA paradigm.24 The testing strategy was incorporated in a commercially available system (Glaid, Lace Elettronica, Pisa, Italy). In accordance with the PERGLA technique, both eyes were recorded simultaneously by means of skin electrodes on the lower eyelids, reference electrodes on the temples, and a common ground electrode on the forehead. Before positioning the skin electrodes, the skin was gently cleansed with electrode skin preparation pads to reduce skin impedance to <5000 ohms. The skin electrodes (9-mm-diameter discs coated with conductive cream) were placed as consistently as possible from one visit to another, in keeping with the standard placement previously described.24 In summary, the central forehead electrode was placed 3 cm above the bridge of the nose, temporal electrodes were placed 3 cm lateral to the lateral canthus, and the upper margin of the lower eyelid electrodes was kept 5 mm below the eyelashes. The pattern stimulus consisted of black-and-white horizontal lines with a square wave profile (1.6 cycles per degree, 95% contrast, 40 candelas/m2 of mean luminance), alternating in counterphase at 8.14 hertz (16.28 reversals per second) and displayed on a television monitor (14.1-cm-diameter circular field) in a Ganzfeld bowl, which was used to provide a fixed amount of background adaptation and minimize the effects of stray light.34 The pattern covered a circular retinal area of 25° diameter centered on the fovea. Signals were band pass filtered (1–30 hertz), amplified (100,000 fold), and averaged.
The pattern electroretinogram waveform was obtained by averaging 2 separate sequential series of 300 artifact-free pairs of reversals (sweeps) of 122.8 milliseconds. For each block, the first 30 pairs of reversals, or sweeps, were rejected from the average to eliminate the spurious effect of the stimulus onset and allow steady-state recording. During the recording, subjects were allowed to blink freely, but sweeps contaminated by blinking or gross eye movement were automatically rejected, as judged by a threshold voltage over 25 µV. Subjects were recorded with undilated pupils and with the appropriate lens correction for the viewing distance (30 cm). The subjects received no anesthetic or other eyedrops before pattern electroretinogram recording to prevent any corneal surface alterations. Each subject was asked to fix his gaze on a target at the middle of the circular field stimulus. Because of the relatively fast alternating stimuli, the electrical response was a steady-state waveform. It was automatically analyzed by a discrete Fourier transform, to isolate the sinusoidal component at the reversal frequency of 16.28/second and to extract both the amplitude (microvolts) and the phase lag (π radians).38,39 The pattern electroretinogram amplitude represents half of the peak-to-trough amplitude, and the pattern electroretinogram phase is recorded relative to the reversal period.
Statistical Analysis
Intersession Test–Retest Variability
The pattern electroretinogram was recorded 5 times on 5 different days on each subject by the same operator within an 8-week period. The mean amplitude and mean phase lag of the 5 recordings (600 sweeps each) were calculated, along with the variances (and SDs) around the mean for each subject.
Intrinsic (Intratest) Variability
Intrinsic variability was defined as the SD of 2 consecutive partial averages of 300 sweeps, divided by √2. Therefore, intrinsic variability is a form of intrasession–intratest variability. Because each net pattern electroretinogram measurement was calculated as the average of 2 sets of 300 sweeps and test–retest variability was calculated on a total of 600 sweeps, an adjustment factor (√2) permitted an appropriate comparison of the magnitude of the 2 variabilities.
Interocular Asymmetry
Because glaucoma may be of asymmetric severity and may also progress asymmetrically,40 the differences in pattern electroretinogram amplitude and phase between the two eyes (interocular asymmetry) has been suggested as a potential criterion to recognize the presence of ganglion cell or axon injury and, perhaps, also to evaluate changes in the retinal ganglion cell function.41,42 Ventura et al8 have shown that the average interocular asymmetries in pattern electroretinogram amplitude increased with the severity of the disease at early stages (i.e., for glaucoma suspects and those with early manifest glaucoma). Therefore, we also evaluated the reproducibility of the interocular asymmetry, which is reported automatically by the commercial pattern electroretinogram computer software system (Glaid) as a coefficient of variation (CV) between the two eyes.
Results
PERGLA produces values for amplitude and phase and, for each, the intrinsic (intratest) variability and interocular differences. We evaluated reproducibility for both intersession test–retest and intrinsic (intratest) of both amplitude and phase in 3 ways: the SD of repeat measurements, CV, and intraclass correlation coefficient (ICC).
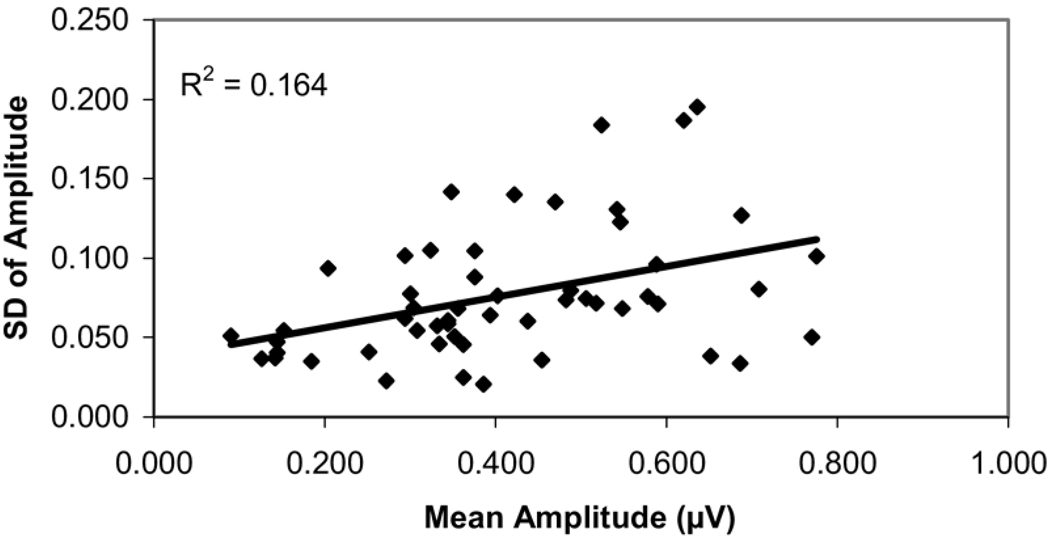
Figure 1 shows the intersession test–retest variability of pattern electroretinogram amplitude, expressed in terms of the SD of the results obtained for each glaucoma subject during the 5 sessions. The results were plotted against their mean amplitude to illustrate whether variability relates to the PERGLA amplitude, which in turn may relate to the severity of the glaucomatous disease (i.e., cupping and field loss).8,18,34,43,44 Thus, plotting SD against their mean amplitude also might show any possible relationship of the variability of PERGLA to the severity of glaucomatous disease. A Pearson correlation coefficient was calculated to determine the strength of the link between the magnitude of amplitude and variability of the measurement. The same approach was used for phase.
Figure 1.
Scatter plot showing the intersession test–retest variability of pattern electroretinogram amplitude, expressed as standard deviation (SD) of amplitude on 5 repeat sessions per glaucoma patients against their mean amplitude value, measured by the Pattern Electroretinogram for Glaucoma paradigm.
The correlation coefficient between the mean amplitude and variability (represented as the SD of repeat measurements) was r2 = 0.164 (P = 0.003). We explored whether this weak but statistically significant correlation existed over the entire range or might be explained mainly by the reduced variability in a limited region where the measurement values were small. The variability may become smaller simply because the electrophysiologic signal is close to the noise level and, hence, relatively invariant upon repeat measurement. The amplitude noise level with the PERGLA recording paradigm is, on average, 0.084±0.03 µV.24 An amplitude of 0.144 µV (mean noise + 2 SDs) should be the lower limit of the clinically useful dynamic range, and the test–retest variability near these low amplitude values should diminish where measurement values include a large element of noise and a small element of electrophysiologic response (signal). If we exclude these lower amplitudes (≤0.144 µV) from the analysis, the correlation of variability (expressed as SD) with the mean measurement is indeed smaller but still statistically significant (r2 = 0.104, P = 0.026). To further support the anchoring effect produced by the small amplitude values (i.e., those closer to the noise level), there was no relationship of SD to severity of disease between 0.3 and 0.8 µV (r2 = 0.064, P = 0.11). For phase, the correlation was r2 = 0.026 (P = 0.25), so reproducibility was not affected by the extent of the phase lag.
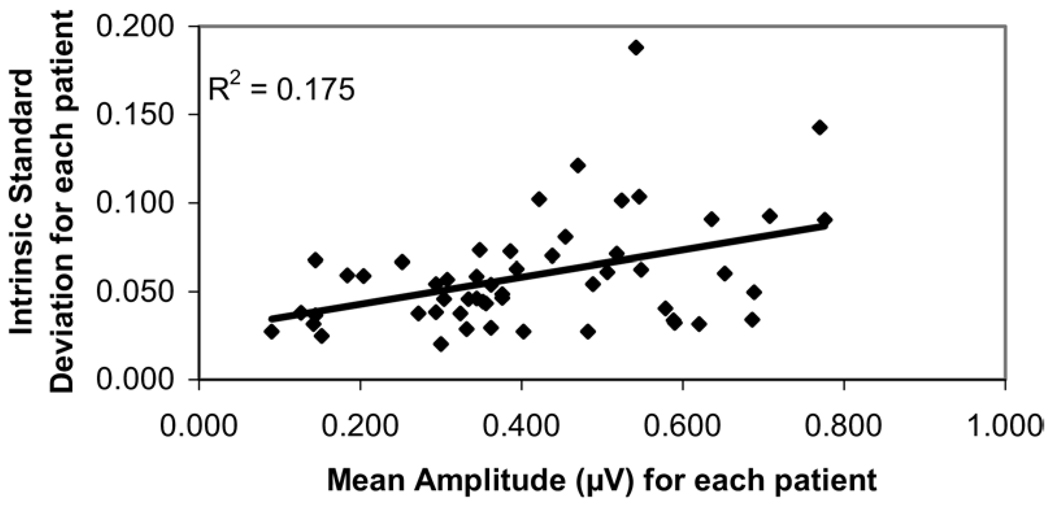
Figure 2 shows the intrinsic (intratest) variability of pattern electroretinogram amplitude, as described in the method section. To view the impact of amplitude on the variability, the intrinsic (intratest) variations were plotted against their mean amplitude. The same approach was used for phase. Their correlation coefficients were r2 = 0.175 (P = 0.002) and r2 = 0.0075 (P = 0.54) for the amplitude and phase variability, respectively.
Figure 2.
Scatter plot showing the intrinsic (intratest) variability of pattern electroretinogram amplitude, expressed as intratest standard deviation of amplitude per glaucoma patients against their mean amplitude value, measured by the Pattern Electroretinogram for Glaucoma paradigm.
Table 1 shows the intrinsic (intratest) and test–retest (intersession) CVs for the pattern electroretinogram amplitude and phase. Coefficients of variation for test–retest (intersession) are higher than those of intrinsic (intratest) variability for both amplitude and phase, suggesting that the between-days (between session) variability is greater than the inner variability due to the PERGLA instrument itself.
Table 1.
Summary of the Variability and Repeatability of the Pattern Electroretinogram Recorded According to the Pattern Electroretinogram for Glaucoma Paradigm
| Coefficient of Variation and Standard Deviation |
||
|---|---|---|
| Source of Variability | Amplitude | Phase |
| Intrinsic | 12.08 ± 6.90 | 2.02 ± 1.95 |
| Test–retest | 20.82 ± 10.84 | 4.17 ± 3.49 |
Table 2 lists the ICC for several pattern electroretinogram parameters (amplitude, phase, interocular asymmetry in amplitude, and interocular asymmetry in phase). Because amplitude and interocular asymmetry in amplitude have each been thought to be useful parameters to evaluate abnormality due to glaucoma, 2–4,8,11–13 we compared the ICC of the pattern electroretinogram amplitude value with the ICC of the interocular asymmetry in amplitude. The ICC of interocular asymmetry in amplitude (0.659; 95% confidence interval [CI], 0.5502–0.7605) was significantly worse (P<0.05) than the ICC for the amplitude value (0.791; 95% CI, 0.7104– 0.8592). Scientific evidence for the alteration of pattern electroretinogram phase in glaucoma is not as strong as that for amplitude,8,46 but pattern electroretinogram phase and pattern electroretinogram interocular asymmetry in phase could also be altered by glaucoma.8 Therefore, we compared the ICC of phase with the ICC of interocular asymmetry in phase. The ICC of interocular asymmetry in phase was significantly lower than the ICC for the phase value (phase ICC, 0.7650 [95% CI, 0.6781– 0.8408]; interocular asymmetry in phase ICC, 0.5714 [95% CI, 0.4516–0.6906]) (P<0.05).
Table 2.
Summary of the Intraclass Correlation Coefficient (ICC) of the Pattern Electroretinogram Recorded According to the Pattern Electroretinogram for Glaucoma Paradigm on Glaucomatous Patients
| ICC | |
|---|---|
| Amplitude | 0.7905 |
| Interocular asymmetry in amplitude | 0.6590 |
| Phase | 0.7650 |
| Interocular asymmetry in phase | 0.5714 |
Discussion
Reproducibility of Pattern Electroretinogram
Several contradictory reports have been published in the past on pattern electroretinogram reproducibility. The large test–retest variability reported in some studies (as high as 100% of coefficient of variation) entertained doubts concerning its clinical usefulness.20–36
Reproducibility is affected by details of the recording techniques, including the nature of the stimuli, as well as electrode sites and types.20–36 PERGLA was developed in hopes that attention to technical details, use of comfortable electrodes, and empirically optimized stimuli with signal averaging would permit the pattern electroretinogram to be clinically practical and more reproducible.24 The present study was undertaken to test in detail the reproducibility of the PERGLA method in glaucoma patients, a necessary condition for it to be useful in monitoring the course of the disease and for the diagnosis of early disease.
Reproducibility with the PERGLA Paradigm for Recording
In glaucomatous patients, we obtained CVs in amplitude of 20.82% for test–retest (intersession) variability and 12.08% for intrinsic (intrasession–intratest) variability, about 2 to 2.5 times that reported on normal subjects with the same paradigm (8.2% ±5.0% for test–retest (intersession) variability and 6.9% ±4.9% for intrinsic variability (intratest– intrasession).24 That finding is not surprising because, by definition, the CV takes into account the mean value of the measurement, and the average amplitude in our glaucoma cohort is about 2 to 2.5 times smaller than that of normal subjects (i.e., 0.412 µV in this study on glaucoma and 1.1 µV in normals using PERGLA24). Indeed, the CV will tend to increase as the amplitude decreases in glaucomatous patients, even if the SD remains constant. Because the CV is often used in the pattern electroretinogram literature to express the degree of variability, we included it in this report, but the CVs should not be the sole basis for judging the reproducibility of a diagnostic test in a diseased population.
Overall, however, we obtained better CVs of amplitude than most previous reports on pattern electroretinogram reproducibility, close to those reported by Trick46,47 and Odom et al.29 Our better reproducibility could, in part, be explained by technical aspects of the recording (such as electrode placement), the optimized stimulus characteristics of PERGLA, and the analysis of steady-state responses rather than transient responses. Hess and Baker48 reported that steady-state recordings with a fast Fourier transformation could perform better than transient recordings because of lower variability and superior S/N ratio.
Sources of Variation in Pattern Electroretinogram Measurements
Size of rejection band, number of sweeps, reference electrode placement, electrode positioning, and type of electrode have all been shown to influence pattern electroretinogram variability.20,32,49–53 Gold foil electrodes can generate excessive tearing, blinking, and electrode movement artifacts that degrade the pattern electroretinogram.29,51–55 In our study, only skin electrodes were used, and their positioning was standardized according to the PERGLA paradigm.24 Also, when the forehead or ear is used as a reference, a response can be obtained from an occluded eye because of contamination from a cortically generated visual evoked potential by the other eye.52–54 The PERGLA paradigm places the reference electrodes temporally to the outer canthus, which, compared with the forehead or ear, minimizes contamination from other sources and minimizes variability in the measurement.32,54,55
Other sources of variation were avoided by not permitting use of anesthetic or dilating drops before the pattern electroretinogram was performed, as corneal surface irregularities produce scatter that could, in principle, change the contrast of the stimulus and the pattern electroretinogram response.56 Not only were these confounding factors eliminated, but doing PERGLA without dilation allowed us to evaluate PERGLA in a more clinically useful way (i.e., not requiring dilation). Further study would be required to determine whether any of these factors have a significant impact.
The fact that all measurements in this study were done by a single operator could cause an underestimate of the test– retest variability that might occur when different operators perform the test. On the other hand, the pattern electroretinogram operator had only brief training, which could generate more variability, as demonstrated by Odom et al.29 However, compared with the technique used by Odom, the PERGLA method has fewer details needing attention and may be less affected by training.24 Because pattern electroretinogram testing is usually done in an electrophysiology unit with one or more operators experienced in this testing, it can be hoped that variability would be at least as low as found in this study.
Intratest Variation
There is likely a systematic difference between the first 300 sweeps and the second, because habituation has been reported in retinal ganglion cell activity during steady-state pattern electroretinogram in normal subjects.57 Thus comparison of these halves of the test is not a perfect surrogate for an independent test conducted in the same session after a rest period.
Interocular Asymmetry
Interocular asymmetry may signify glaucoma even before pattern electroretinogram amplitude and phase are outside the normal range of values, provided that the normal range of asymmetry has been documented.8,24 However, to be a reliable early indicator of glaucoma and to be able to judge progression, it must also be reproducible. We found a significantly worse (lower) ICC for pattern electroretinogram interocular asymmetry in amplitude (and phase) than for the pattern electroretinogram amplitude (and phase) of a single eye. Therefore, interocular asymmetry is not likely to be as useful for monitoring progression, even if it turns out to be a useful parameter for detection of glaucoma.
Signal-to-Noise Ratio and Impact of Noise on Our Results
We obtained an average amplitude of 0.412 µV on glaucomatous patients. An average noise in amplitude of 0.084 µV has previously been reported for PERGLA,24 yielding an average S/N ratio of 4.9 if we apply that value in our study on glaucomatous patients. Assuming that the noise component is constant over the range of measurements, the S/N ratio would be expected to be lower in those with glaucoma than in normal subjects5–9,11,12 and become lower progressively as the disease is more severe and the signal is smaller. Not surprisingly, our estimate of the S/N ratio in a glaucomatous population is not as good as what was previously reported with the same paradigm on normal subjects. However, it is still in the higher range of what has been reported in the field of evoked potential recordings.24,58
The amplitude noise level with the PERGLA recording paradigm being on average 0.084±0.03 µV,24 an amplitude of 0.144 µV (mean + 2 SDs) is probably the lower limit of the clinically useful dynamic range of the pattern electroretinogram instrument with this paradigm. Estimates of measurement variability as these low amplitudes are approached may be dominated by the variability in noise rather than the variability of a small signal due to glaucomatous damage. If we exclude these lower amplitudes (≤0.144 µV) from the analysis, we obtain ICCs of 0.731 for amplitude and 0.737 for phase, which are slightly lower than those of all patients combined (ICCs of 0.791 and 0.765 for amplitude and phase, respectively) but still in an acceptable range.
The correlation between the SD of amplitude and the amplitude itself is low (r2 = 0.164 for test–retest variability and r2 = 0.17 for intrinsic variability). If the lower end of the measurement range (≤0.144 µV) is excluded, the correlation coefficients become even smaller, with r2 = 0.104 (P = 0.026) for test–retest variability and r2 = 0.146 (P = 0.007) for intrinsic variability. Even though the variability is thus weakly related to the severity of disease, the magnitude is not great enough to be of consequence for the clinical interpretation of the test. To further support that, we found no significant relationship of SD to amplitude value between 0.3 and 0.8 µV (r2 = 0.064, P = 0.11).
Overall, the PERGLA recordings are reproducible enough in glaucomatous patients to be considered a useful complementary clinical tool for recognizing the presence of glaucoma and monitor its progression. To document fully the potential usefulness of PERGLA for quantifying deterioration of disease, further studies are needed to show how each parameter (amplitude and phase) changes in concert with standardly defined degrees of worsening of the disease, as well as to determine the range of disease severity covered by the useful dynamic range of the pattern electroretinogram signal. Both amplitude and interocular asymmetry in amplitude have been proposed as useful in early diagnosis and in monitoring glaucoma.8 Because the amplitude parameter is more reproducible than the interocular asymmetry in amplitude, an abnormal interocular asymmetry should be taken as an early sign of glaucoma only if confirmed or in keeping with other clinical evidence. As with other clinical tests, interpretation must be done with a good understanding of the unique additional information that pattern electroretinogram technology provides, as well as its limitations.45
Acknowledgments
Supported in part by the National Eye Institute, Bethesda, Maryland (research grant no. RO1 EY014957 [VP], core grant no. P30 EY014801); an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York; an unrestricted donation from Zeiss-Meditec-Humphrey, Dublin, California; an unrestricted donation from Allergan, Inc., Irvine, California; an investigator-initiated grant from Pfizer, Inc., New York, New York; and a fellowship scholarship from Laval University.
Footnotes
Presented in part at: American Academy of Ophthalmology 109th annual meeting, October 2005, Chicago, Illinois.
All authors except Dr Porciatti have no financial or proprietary interest in any products or devices mentioned in the article. Dr Porciatti developed the Pattern Electroretinogram for Glaucoma paradigm and has a financial interest with Lace Elettronica, Pisa, Italy.
References
- 1.Zrenner E. The physiological basis of the pattern electroretinogram. Prog Retin Res. 1990;9:427–464. [Google Scholar]
- 2.Korth M. The value of electrophysiological testing in glaucomatous diseases. J Glaucoma. 1997;6:331–343. [PubMed] [Google Scholar]
- 3.Bach M. Electrophysiological approaches for early detection of glaucoma. Eur J Ophthalmol. 2001;11(suppl):S41–S49. doi: 10.1177/112067210101102s05. [DOI] [PubMed] [Google Scholar]
- 4.Ventura LM, Porciatti V. Pattern electroretinogram in glaucoma. Curr Opin Ophthalmol. 2006;17:196–202. doi: 10.1097/01.icu.0000193082.44938.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham SL, Drance SM, Chauhan BC, et al. Comparison of psychophysiological and electrophysiological testing in early glaucoma. Invest Ophthalmol Vis Sci. 1996;37:2651–2662. [PubMed] [Google Scholar]
- 6.Korth M, Horn F, Storck B, Jonas J. The pattern-evoked electroretinogram (PERG): age-related alterations and changes in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1989;227:123–130. doi: 10.1007/BF02169783. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer N, Tillmon B, Bach M. Predictive value of the pattern electroretinogram in high-risk ocular hypertension. Invest Ophthalmol Vis Sci. 1993;34:1710–1715. [PubMed] [Google Scholar]
- 8.Ventura LM, Porciatti V, Ishida K, et al. Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112:10–19. doi: 10.1016/j.ophtha.2004.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach M, Pfeiffer N, Birkner-Binder D. Pattern-electroretinogram reflects diffuse retinal damage in early glaucoma. Clin Vis Sci. 1992;7:335–340. [Google Scholar]
- 10.Ventura LM, Sorokac N, De Los Santos R, et al. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Invest Ophthalmol Vis Sci. 2006;47:3904–3911. doi: 10.1167/iovs.06-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papst N, Bopp M, Schnaudigel OE. Pattern electroretinogram and visually evoked cortical potentials in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1984;222:29–33. doi: 10.1007/BF02133774. [DOI] [PubMed] [Google Scholar]
- 12.Trick GL. Retinal potentials in patients with primary open-angle glaucoma: physiological evidence for temporal frequency tuning deficits. Invest Ophthalmol Vis Sci. 1985;26:1750–1758. [PubMed] [Google Scholar]
- 13.Ringens PJ, Vijfvinkel-Bruinenga S, van Lith GH. The pattern-elicited electroretinogram. I. A tool in the early detection of glaucoma? Ophthalmologica. 1986;192:171–175. doi: 10.1159/000309635. [DOI] [PubMed] [Google Scholar]
- 14.Bayer AU, Erb C. Short wavelength automated perimetry, frequency doubling technology perimetry, and pattern electroretinography for prediction of progressive glaucomatous standard visual field defects. Ophthalmology. 2002;109:1009–1017. doi: 10.1016/s0161-6420(02)01015-1. [DOI] [PubMed] [Google Scholar]
- 15.Nesher RN, Trick GL, Kass MA, Gordon MO. Steady-state pattern electroretinogram following long term unilateral administration of timolol to ocular hypertensive subjects. Doc Ophthalmol. 1990;73:101–109. doi: 10.1007/BF00146546. [DOI] [PubMed] [Google Scholar]
- 16.Siliprandi R, Bucci MG, Canella R, Carmignoto G. Flash and pattern electroretinograms during and after acute intraocular pressure elevation in cats. Invest Ophthalmol Vis Sci. 1988;29:558–565. [PubMed] [Google Scholar]
- 17.Trick GL, Bickler-Bluth M, Cooper DG, et al. Pattern reversal electroretinogram (PRERG) abnormalities in ocular hypertension: correlation with glaucoma risk factors. Curr Eye Res. 1988;7:201–206. doi: 10.3109/02713688808995749. [DOI] [PubMed] [Google Scholar]
- 18.Korth M, Horn F, Storck B, Jonas J. Pattern electroretinograms in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1987;28(suppl):129. [PubMed] [Google Scholar]
- 19.Ventura LM, Porciatti V. Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction. A pilot study. Ophthalmology. 2005;112:20–27. doi: 10.1016/j.ophtha.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holopigian K, Snow J, Seiple W, Siegel I. Variability of the pattern electroretinogram. Doc Ophthalmol. 1988;70:103–115. doi: 10.1007/BF00154741. [DOI] [PubMed] [Google Scholar]
- 21.Jacobi PC, Walter P, Brunner R, Krieglstein GK. Reproducibility and intraindividual variability of the pattern electroretinogram. Ger J Ophthalmol. 1994;3:216–219. [PubMed] [Google Scholar]
- 22.Bartel P, Becker P, Robinson E. The intrasession repeatability of pattern electroretinogram and the effects of digital filtering. Doc Ophthalmol. 1991;76:351–358. doi: 10.1007/BF00142673. [DOI] [PubMed] [Google Scholar]
- 23.Bach M, Hiss PJ, Rover J. Check-size specific changes of pattern electroretinogram in patients with early open-angle glaucoma. Doc Ophthalmol. 1988;69:315–322. doi: 10.1007/BF00154412. [DOI] [PubMed] [Google Scholar]
- 24.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111:161–168. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess RF, Baker CL, Jr, Verhoeve JN, et al. The pattern evoked electroretinogram: its variability in normals and its relationship to amblyopia. Invest Ophthalmol Vis Sci. 1985;26:1610–1623. [PubMed] [Google Scholar]
- 26.Schuurmans RP, Berninger T. Luminance and contrast responses recorded in man and cat. Doc Ophthalmol. 1985;59:187–197. doi: 10.1007/BF00160615. [DOI] [PubMed] [Google Scholar]
- 27.Korth M, Ilschner S. The spatial organization of retinal receptive fields in light and darkness as revealed by the pattern electroretinogram. Doc Ophthalmol. 1986;63:143–149. doi: 10.1007/BF00157124. [DOI] [PubMed] [Google Scholar]
- 28.Korth M, Rix R. Changes in spatial selectivity of pattern-ERG components with stimulus contrast. Graefes Arch Clin Exp Ophthalmol. 1985;223:23–28. doi: 10.1007/BF02150569. [DOI] [PubMed] [Google Scholar]
- 29.Odom JV, Holder GE, Feghali JG, Cavender S. Pattern electroretinogram intrasession reliability: a two center comparison. Clin Vis Sci. 1992;7:263–281. [Google Scholar]
- 30.Otto T, Bach M. Retest variability and diurnal effects in the pattern electroretinogram. Doc Ophthalmol. 1996–1997;92:311–323. doi: 10.1007/BF02584085. [DOI] [PubMed] [Google Scholar]
- 31.Otto T, Bach M. Reproducibility of the pattern electroretinogram [in German] Ophthalmologe. 1997;94:217–221. doi: 10.1007/s003470050105. [DOI] [PubMed] [Google Scholar]
- 32.Tan CB, King PJ, Chiappa KH. Pattern ERG: effects of reference electrode site, stimulus mode and check size. Electroencephalogr Clin Neurophysiol. 1989;74:11–18. doi: 10.1016/0168-5597(89)90046-4. [DOI] [PubMed] [Google Scholar]
- 33.van Lith G, Ringens P, de Heer LJ. Pattern electroretinogram and glaucoma. Dev Ophthalmol. 1984;9:133–139. doi: 10.1159/000409816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porciatti V, Falsini B, Brunori S, et al. Pattern electroretinogram as a function of spatial frequency in ocular hypertension and early glaucoma. Doc Ophthalmol. 1987;65:349–355. doi: 10.1007/BF00149941. [DOI] [PubMed] [Google Scholar]
- 35.Prager TC, Saad N, Schweitzer FC, et al. Electrode comparison in pattern electroretinography. Invest Ophthalmol Vis Sci. 1992;33:390–394. [PubMed] [Google Scholar]
- 36.Trick LR. Age-related alterations in retinal function. Doc Ophthalmol. 1987;65:35–43. doi: 10.1007/BF00162718. [DOI] [PubMed] [Google Scholar]
- 37.Yang A, Swanson WH. A new pattern electroretinogram paradigm evaluated in terms of user friendliness and agreement with perimetry. Ophthalmology. 2007;114:671–679. doi: 10.1016/j.ophtha.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fourier JB. The Analytical Theory of Heat [Freeman A, trans] New York: Dover; 1955. [original work published 1878]. [Google Scholar]
- 39.Grattan-Guinness I. Joseph Fourier 1768–1830: a survey of his life and work, based on a critical edition of his monograph on the propagation of heat, presented to the Institut de France in 1807. Cambridge, MA: MIT Press; 1972. [Google Scholar]
- 40.Zeyen TG, Caprioli J. Progression of disc and field damage in early glaucoma. Arch Ophthalmol. 1993;111:62–65. doi: 10.1001/archopht.1993.01090010066028. [DOI] [PubMed] [Google Scholar]
- 41.Gugleta K, Orgul S, Flammer J. Asymmetry in interocular pressure and retinal nerve fiber layer thickness in normaltension glaucoma. Ophthalmologica. 1999;213:219–223. doi: 10.1159/000027425. [DOI] [PubMed] [Google Scholar]
- 42.Tomita G, Nyman K, Raitti C, Kawamura M. Interocular asymmetry of optic disc size and its relevance to visual field loss in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 1994;232:290–296. doi: 10.1007/BF00194478. [DOI] [PubMed] [Google Scholar]
- 43.O’Donaghue E, Arden GB, O’Sullivan F, et al. The pattern electroretinogram in glaucoma and ocular hypertension. Br J Ophthalmol. 1992;76:387–394. doi: 10.1136/bjo.76.7.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wanger P, Persson HE. Pattern-reversal electroretinograms from normotensive, hypertensive and glaucomatous eyes. Ophthalmologica. 1987;195:205–208. doi: 10.1159/000309814. [DOI] [PubMed] [Google Scholar]
- 45.Harwerth RS, Carter-Dawson L, Smith EL, III, et al. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45:3152–3160. doi: 10.1167/iovs.04-0227. [DOI] [PubMed] [Google Scholar]
- 46.Trick GL. The pattern electroretinogram in glaucoma and ocular hypertension. In: Heckenlively JR, Arden GB, editors. Principles and Practice of Clinical Electrophysiology of Vision. St. Louis: Mosby; 1991. pp. 766–773. [Google Scholar]
- 47.Trick GL. Pattern electroretinogram: an electrophysiological technique applicable to primary open-angle glaucoma and ocular hypertension. J Glaucoma. 1992;1:271–279. [PubMed] [Google Scholar]
- 48.Hess RF, Baker CL., Jr The human pattern-evoked electroretinogram. J Neurophysiol. 1984;51:939–951. doi: 10.1152/jn.1984.51.5.939. [DOI] [PubMed] [Google Scholar]
- 49.Kakisu Y, Mizota A, Adachi E. Clinical application of the pattern electroretinogram with lid skin electrode. Doc Ophthalmol. 1986;63:187–194. doi: 10.1007/BF00157130. [DOI] [PubMed] [Google Scholar]
- 50.McCulloch DL, Van Boemel GB, Borchert MS. Comparisons of contact lens, foil, fiber and skin electrodes for patterns electroretinograms. Doc Ophthalmol. 1997–1998;94:327–340. doi: 10.1007/BF02580858. [DOI] [PubMed] [Google Scholar]
- 51.Leguire LE, Rogers GL. Pattern electroretinogram: use of noncorneal skin electrodes. Vision Res. 1985;25:867–870. doi: 10.1016/0042-6989(85)90195-6. [DOI] [PubMed] [Google Scholar]
- 52.Seiple WH, Siegel IM. Recording the pattern electroretinogram: a cautionary note. Invest Ophthalmol Vis Sci. 1983;24:796–798. [PubMed] [Google Scholar]
- 53.Peachey NS, Sokol S, Moskowitz A. Recording the contralateral PERG: effect of different electrodes. Invest Ophthalmol Vis Sci. 1983;24:1514–1516. [PubMed] [Google Scholar]
- 54.Berninger TA. The pattern electroretinogram and its contamination. Clin Vis Sci. 1986;1:185–190. [Google Scholar]
- 55.Odom JV, Maida TM, Dawson WW, Hobson R. Pattern electroretinogram: effects of reference electrode position. Doc Ophthalmol. 1987;65:297–306. doi: 10.1007/BF00149936. [DOI] [PubMed] [Google Scholar]
- 56.Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Res. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- 57.Porciatti V, Sorokac N, Buchser W. Habituation of retinal ganglion cell activity in response to steady state pattern visual stimuli in normal subjects. Invest Ophthalmol Vis Sci. 2005;46:1296–1302. doi: 10.1167/iovs.04-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Regan D. Human Brain Electrophysiology: Evoked Potential and Evoked Magnetic Fields in Science and Medicine. New York: Elsevier; 1989. pp. 56–57. [Google Scholar]