Abstract
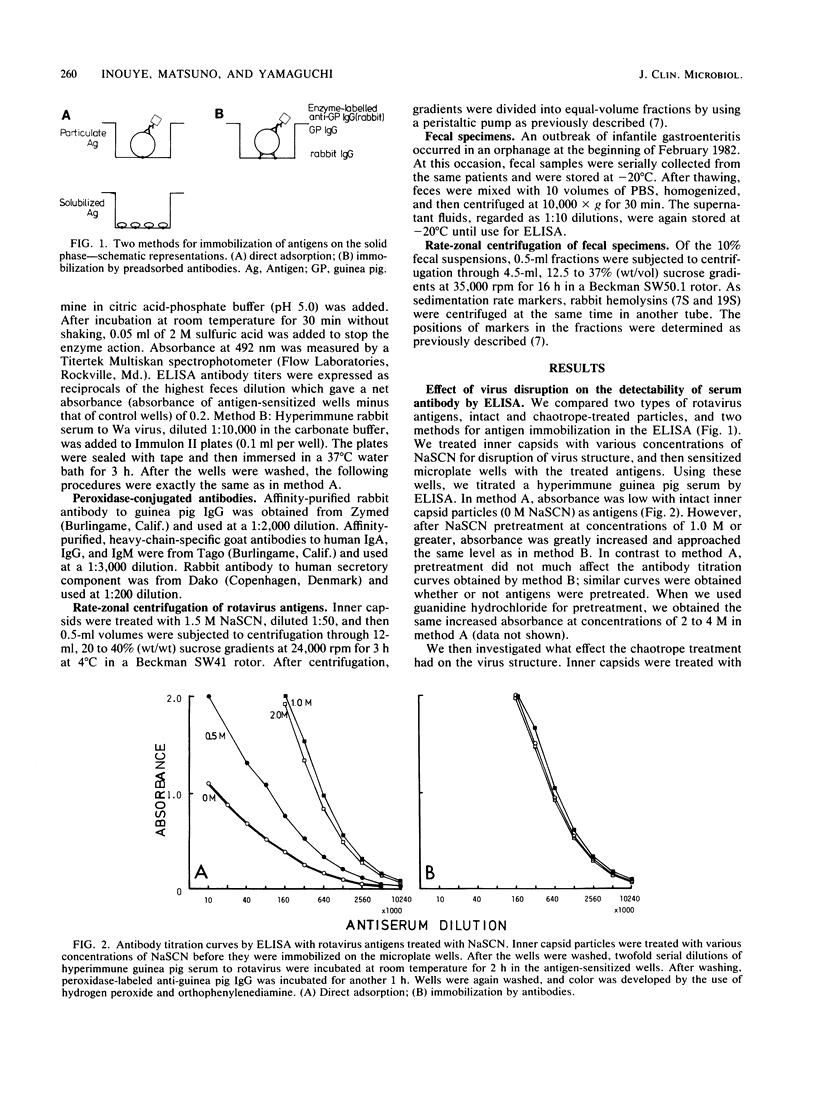
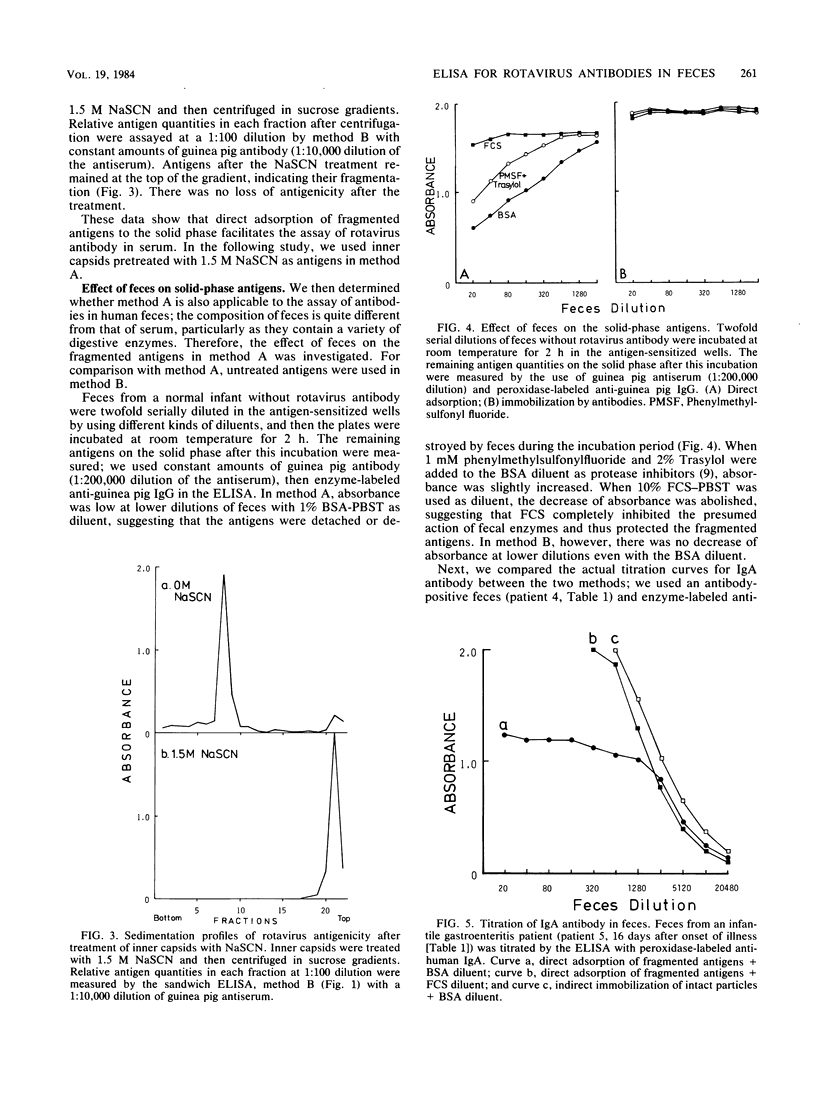
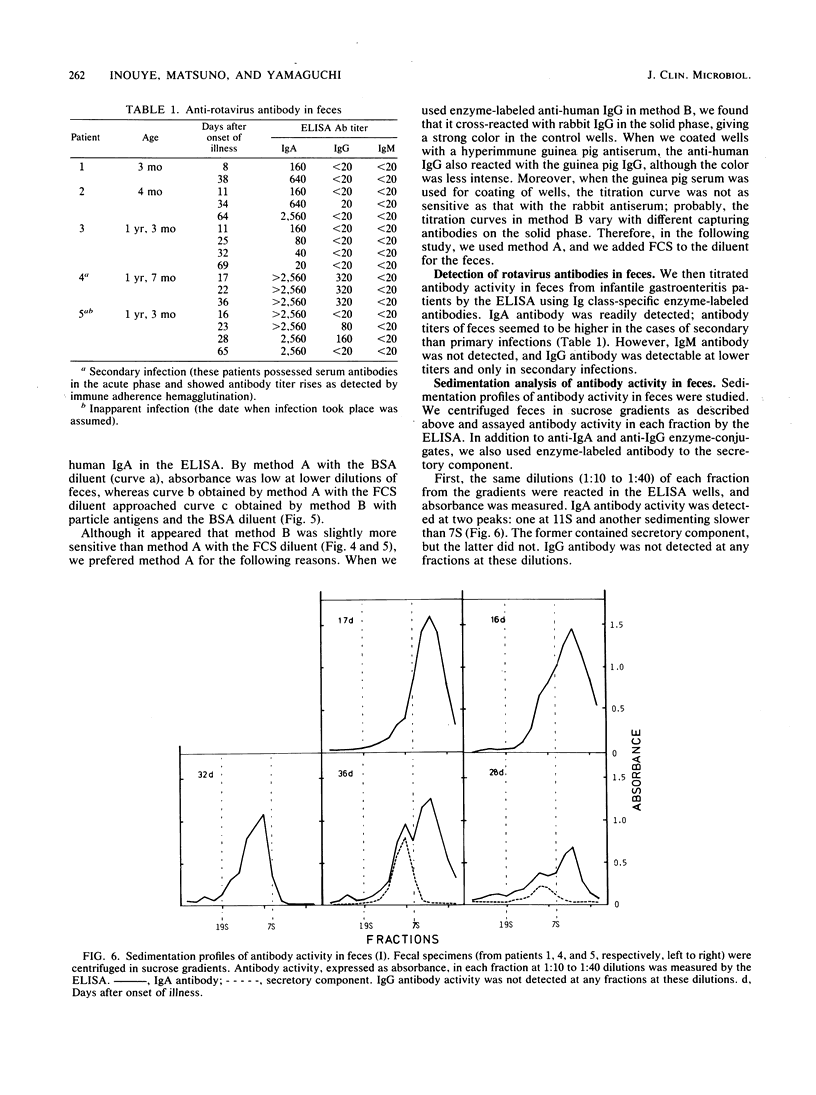
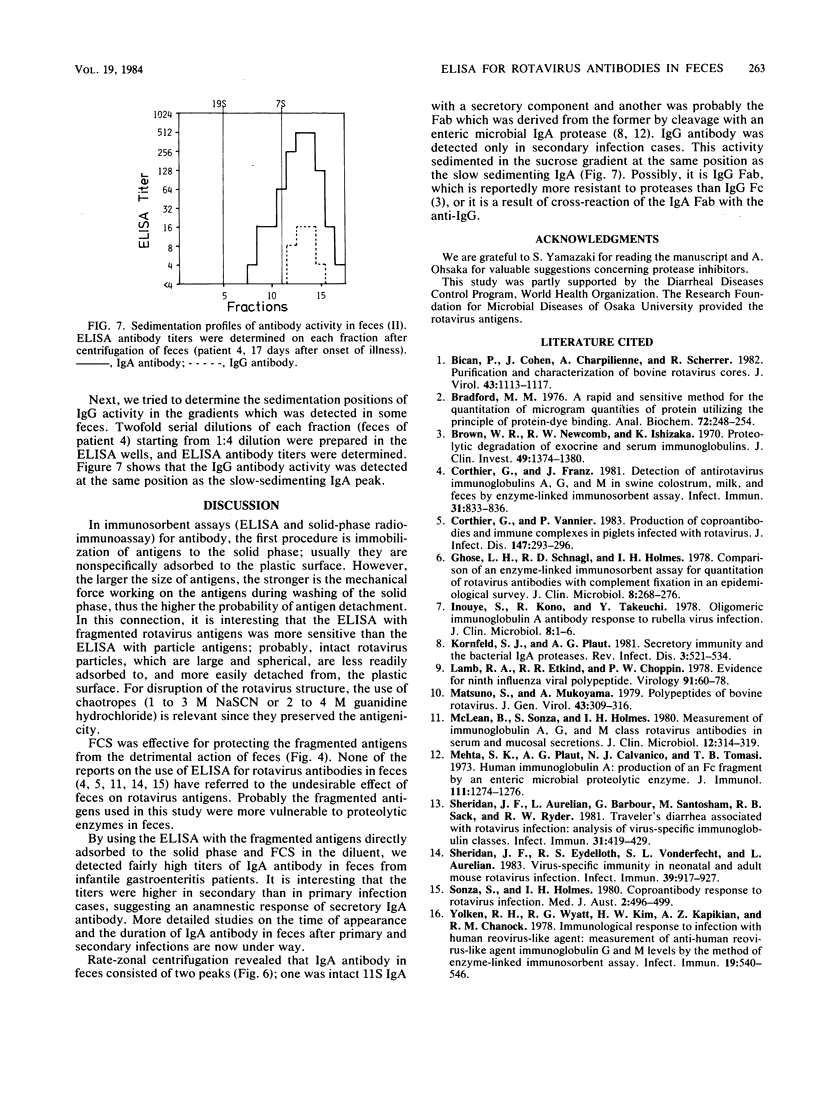
Sensitivity of the enzyme-linked immunosorbent assay for detecting serum antibodies to rotavirus was greatly enhanced when rotavirus particles were fragmented by chaotropic agents (NaSCN or guanidine hydrochloride) before adsorption of the antigens to the solid phase. For detecting fecal antibodies, the addition of fetal calf serum to the diluent was further needed to protect the antigens from the proteolytic activity of feces. With this technique, we readily detected immunoglobulin A antibody in feces from infantile gastroenteritis patients. Rate-zonal centrifugation of feces revealed that immunoglobulin A antibody activity sedimented with two peaks: one at 11S with a secretory component and another sedimenting slower than 7S, presumably as Fab portions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bican P., Cohen J., Charpilienne A., Scherrer R. Purification and characterization of bovine rotavirus cores. J Virol. 1982 Sep;43(3):1113–1117. doi: 10.1128/jvi.43.3.1113-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Newcomb R. W., Ishizaka K. Proteolytic degradation of exocrine and serum immunoglobulins. J Clin Invest. 1970 Jul;49(7):1374–1380. doi: 10.1172/JCI106354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthier G., Franz J. Detection of antirotavirus immunoglobulins A, G, and M in swine colostrum, milk, and feces by enzyme-linked immunosorbent assay. Infect Immun. 1981 Feb;31(2):833–836. doi: 10.1128/iai.31.2.833-836.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthier G., Vannier P. Production of coproantibodies and immune complexes in piglets infected with rotavirus. J Infect Dis. 1983 Feb;147(2):293–296. doi: 10.1093/infdis/147.2.293. [DOI] [PubMed] [Google Scholar]
- Ghose L. H., Schnagl R. D., Holmes I. H. Comparison of an enzyme-linked immunosorbent assay for quantitation of rotavirus antibodies with complement fixation in an epidemiological survey. J Clin Microbiol. 1978 Sep;8(3):268–276. doi: 10.1128/jcm.8.3.268-276.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Kono R., Takeuchi Y. Oligomeric immunoglobulin A antibody response to rubella virus infection. J Clin Microbiol. 1978 Jul;8(1):1–6. doi: 10.1128/jcm.8.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. J., Plaut A. G. Secretory immunity and the bacterial IgA proteases. Rev Infect Dis. 1981 May-Jun;3(3):521–534. doi: 10.1093/clinids/3.3.521. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A. Polypeptides of bovine rotavirus. J Gen Virol. 1979 May;43(2):309–316. doi: 10.1099/0022-1317-43-2-309. [DOI] [PubMed] [Google Scholar]
- McLean B., Sonza S., Holmes I. H. Measurement of immunoglobulin A, G, and M class rotavirus antibodies in serum and mucosal secretions. J Clin Microbiol. 1980 Sep;12(3):314–319. doi: 10.1128/jcm.12.3.314-319.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. K., Plaut A. G., Calvanico N. J., Tomasi T. B., Jr Human immunoglobulin A: production of an Fc fragment by an enteric microbial proteolytic enzyme. J Immunol. 1973 Oct;111(4):1274–1276. [PubMed] [Google Scholar]
- Sheridan J. F., Aurelian L., Barbour G., Santosham M., Sack R. B., Ryder R. W. Traveler's diarrhea associated with rotavirus infection: analysis of virus-specific immunoglobulin classes. Infect Immun. 1981 Jan;31(1):419–429. doi: 10.1128/iai.31.1.419-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. F., Eydelloth R. S., Vonderfecht S. L., Aurelian L. Virus-specific immunity in neonatal and adult mouse rotavirus infection. Infect Immun. 1983 Feb;39(2):917–927. doi: 10.1128/iai.39.2.917-927.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S., Holmes I. H. Coproantibody response to rotavirus infection. Med J Aust. 1980 Nov 1;2(9):496–499. doi: 10.5694/j.1326-5377.1980.tb100710.x. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Kim H. W., Kapikian A. Z., Chanock R. M. Immunological response to infection with human reovirus-like agent: measurement of anti-human reovirus-like agent immunoglobulin G and M levels by the method of enzyme-linked immunosorbent assay. Infect Immun. 1978 Feb;19(2):540–546. doi: 10.1128/iai.19.2.540-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


