Abstract
Background
In the United States, a black-to-white disparity in age-standardized breast cancer mortality rates emerged in the 1980s and has widened since then.
Methods
To further explore this racial disparity, black-to-white rate ratios (RRsBW) for mortality, incidence, hazard of breast cancer death, and incidence-based mortality (IBM) were investigated using data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program on 244 786 women who were diagnosed with breast cancer from January 1990 through December 2003 and followed through December 2004. A counterfactual approach was used to examine the expected IBM RRsBW, assuming equal distributions for estrogen receptor (ER) expression, and/or equal hazard rates of breast cancer death, among black and white women.
Results
From 1990 through 2004, mortality RRBW was greater than 1.0 and widened over time (age-standardized breast cancer mortality rates fell from 36 to 29 per 100 000 for blacks and from 30 to 22 per 100 000 for whites). In contrast, incidence RRBW was generally less than 1.0. Absolute hazard rates of breast cancer death declined substantially for ER-positive tumors and modestly for ER-negative tumors but were persistently higher for blacks than whites. Equalizing the distributions of ER expression in blacks and whites decreased the IBM RRBW slightly. Interestingly, the black-to-white disparity in IBM RRBW was essentially eliminated when hazard rates of breast cancer death were matched within each ER category.
Conclusions
The black-to-white disparity in age-standardized breast cancer mortality was largely driven by the higher hazard rates of breast cancer death among black women, diagnosed with the disease, irrespective of ER expression, and especially in the first few years following diagnosis. Greater emphasis should be placed on identifying the etiology of these excess hazards and developing therapeutic strategies to address them.
CONTEXT AND CAVEATS
Prior knowledge
Since the 1980s, a widening disparity in age-adjusted mortality rates for black and white women diagnosed with breast cancer has emerged.
Study design
Based on data from the National Cancer Institute's Surveillance, Epidemiology, and End Results program, the authors calculated temporal trends in black-to-white ratios of mortality, incidence, hazard for death from breast cancer, and incidence-based mortality (IBM), with some analyses stratified by estrogen receptor (ER) status and age.
Contribution
The work indicates that the black-to-white disparity in breast cancer mortality is largely due to a higher hazard of death in black women diagnosed with the disease irrespective of ER status.
Implications
Research into the reasons for relatively poor outcomes for black women with breast cancer is warranted.
Limitations
IBM analyses only captured the experience of women with breast cancer in the first five years following diagnosis.
From the Editors
Over the last 15 years, age-standardized breast cancer mortality rates have improved in the United States (1–3). However, they have remained higher and declined more slowly among black women than among white women (4–11). In 2004, the disparity was 29 vs 22 breast cancer deaths per 100 000 woman-years in blacks and whites, respectively. Both biological and nonbiological factors have been proposed to contribute to this disparity, but the underlying causes remain unclear.
In a previous population-based study using age–period–cohort models, our group found that trends in age-specific mortality of breast cancer were largely driven by calendar period effects (5), reflecting changing practice patterns for early detection and/or treatment (12). These results suggested that differences in screening policies as well as response to or access to novel medical interventions could account for the racial differences in breast cancer mortality. Another study from our group that analyzed survivorship of case subjects diagnosed between 1990 and 2002 (1) showed that breast cancer mortality rates declined more over time in women with estrogen receptor–positive (ER+) tumors than in women with estrogen receptor–negative (ER−) tumors. All of these studies are consistent with the possibility that differences in population-based mortality rates between blacks and whites may reflect not only race-specific trends in incidence rates but also differential outcomes after diagnosis.
To elucidate the potential impact of racial differences in incidence rates, tumor characteristics, and outcomes after diagnosis on the observed racial gap in breast cancer mortality, we investigated temporal trends in black-to-white rate ratios for both breast cancer incidence and mortality. We also examined the absolute annual hazard of breast cancer death among black and white women living with breast cancer according to ER status of the tumor to explore if absolute hazard rates differ among black and white women with ER+ and ER− tumors and whether these differences could explain the racial disparity in breast cancer mortality. These analyses of absolute hazard rates were complemented by an examination of black-to-white hazard rate ratios for ER+ and ER− tumors that adjusted for several potential confounders.
Finally, we used a novel counterfactual approach to explore whether (and to what extent) differences in ER status and/or hazard rates of breast cancer death among black and white women could have accounted for the observed widening racial gap in the age-standardized breast cancer mortality rate. The counterfactual approach aims to predict the “unobserved” outcomes that would be expected under different scenarios, assuming that an underlying statistical model describing the outcomes is valid. The counterfactual results may help guide causal inferences. This method has been increasingly used in medical and epidemiological studies to inform public health policy decisions (13,14). Our models were developed using data from the National Cancer Institute's (NCI) Surveillance, Epidemiology, and End Results (SEER) Registry. The broad coverage of this registry allows for estimates that are nationally representative, and the individual-level and ecological data collected by this registry make it possible to control for potential confounders.
Methods
Population Data
Breast cancer incidence case and population data were obtained from the NCI SEER 9 Registries Database for the years 1973–2004 (15). These registries cover approximately 10% of the US population (Table 1). Crude incidence rates were age adjusted to the 2000 US standard population. Age-standardized mortality rates investigated in this study were supplied to the SEER Program by the National Center for Health Statistics (16). Demographic and tumor characteristics recorded by SEER included age at diagnosis, American Joint Committee on Cancer (AJCC) stage (17), tumor grade, ER expression, County Attribute 2000’s for percentage of people below the poverty level (18), and marital status. AJCC stage (I, II, III, and IV) is based on tumor size (in centimeters), axillary lymph node status (positive or negative), and distant metastasis (present or absent). Tumor grade was dichotomized into low and high. Low grade consisted of well-differentiated or moderately differentiated grades, whereas high grade was defined as poorly differentiated or undifferentiated grade. SEER recorded ER status as not done, positive, negative, borderline (undetermined whether positive or negative), ordered but results not on chart, unknown, or no information. Because SEER did not collect information on ER status until 1990, our primary analyses were limited to the time period January 1990 through December 2004. SEER also did not record the method of hormone receptor assay, but immunohistochemistry was likely used during the study period (19). For descriptive purposes, for each demographic or tumor characteristic, variables that did not match the above-mentioned categories were classified as “other or unknown.”
Table 1.
Descriptive statistics for 244 786 women with invasive female breast cancer in National Cancer Institute’s Surveillance, Epidemiology, and End Results 9 Registry Database diagnosed from January 1990 to December 2003 and followed through 2004*
| Variable | All case subjects |
Blacks |
Whites |
P† for heterogeneity |
|||
| n | % | n | % | n | % | ||
| Age | <.001 | ||||||
| <50 | 56 627 | 23.1 | 6786 | 33.4 | 44 701 | 21.5 | |
| 50–70 | 105 062 | 42.9 | 8624 | 42.5 | 88 806 | 42.7 | |
| ≥70 | 83 097 | 33.9 | 4894 | 24.1 | 74 428 | 35.8 | |
| AJCC stage | <.001 | ||||||
| I | 107 242 | 43.8 | 6208 | 30.6 | 93 587 | 45.0 | |
| II | 81 862 | 33.4 | 7694 | 37.9 | 68 302 | 32.8 | |
| III | 15 018 | 6.1 | 2039 | 10.0 | 12 028 | 5.8 | |
| IV | 10 647 | 4.3 | 1444 | 7.1 | 8540 | 4.1 | |
| Other or unknown | 30 017 | 12.3 | 2919 | 14.4 | 25 478 | 12.3 | |
| Tumor size | <.001 | ||||||
| ≤2.0 cm | 139 543 | 57.0 | 8746 | 43.1 | 121 467 | 58.4 | |
| >2.0 cm | 75 576 | 30.9 | 8345 | 41.1 | 61 882 | 29.8 | |
| Other or unknown | 29 667 | 12.1 | 3213 | 15.8 | 24 586 | 11.8 | |
| Lymph nodes | <.001 | ||||||
| Negative | 129 680 | 53.0 | 8615 | 42.4 | 111 837 | 53.8 | |
| Positive | 66 645 | 27.2 | 6586 | 32.4 | 55 440 | 26.7 | |
| Other or unknown | 48 461 | 19.8 | 5103 | 25.1 | 40 658 | 19.6 | |
| Tumor grade | <.001 | ||||||
| Low | 117 083 | 47.8 | 6955 | 34.3 | 101 753 | 48.9 | |
| High | 77 460 | 31.6 | 8620 | 42.5 | 63 489 | 30.5 | |
| Other or unknown | 50 243 | 20.5 | 4729 | 23.3 | 42 693 | 20.5 | |
| Estrogen receptor | <.001 | ||||||
| Not done | 13 555 | 5.5 | 1393 | 6.9 | 11 403 | 5.5 | |
| Positive | 153 068 | 62.5 | 9293 | 45.8 | 132 922 | 63.9 | |
| Negative | 45 239 | 18.5 | 6000 | 29.6 | 35 916 | 17.3 | |
| Borderline | 1483 | 0.6 | 149 | 0.7 | 1267 | 0.6 | |
| Results not on chart | 8283 | 3.4 | 1062 | 5.2 | 6914 | 3.3 | |
| Other or unknown | 23 158 | 9.5 | 2407 | 11.9 | 19 513 | 9.4 | |
| Persons below poverty level | <.001 | ||||||
| <10% | 144 929 | 59.2 | 5393 | 26.6 | 133 216 | 64.1 | |
| 10–19% | 95 580 | 39.0 | 17 880 | 88.1 | 70 767 | 34.0 | |
| ≥20% | 4277 | 1.7 | 31 | 0.2 | 3 952 | 1.9 | |
| Marital status | <.001 | ||||||
| Never married | 26 626 | 10.9 | 4652 | 22.9 | 19 936 | 9.6 | |
| Married | 133 828 | 54.7 | 7140 | 35.2 | 116 508 | 56.0 | |
| Separated | 1404 | 0.6 | 358 | 1.8 | 944 | 0.5 | |
| Divorced | 22 716 | 9.3 | 3008 | 14.8 | 18 546 | 8.9 | |
| Widowed | 52 363 | 21.4 | 4121 | 20.3 | 45 747 | 22.0 | |
| Other or unknown | 7 849 | 3.2 | 1025 | 5.0 | 6254 | 3.0 | |
Among 244 786 case subjects, 20 304 (8.3%) were black and 207 935 (84.9%) were white. Mean age of women in years (standard error of the mean) was 61.9 (0.03), 57.6 (0.10), and 62.7 (0.03) among all women, blacks, and whites, respectively. The corresponding median ages were 62 years, 56 years, and 63 years. N = number (or count); % = percentage of total cases. AJCC = American Joint Committee on Cancer.
P values from χ2 test (two-sided) for heterogeneity comparing black and white women.
Black-to-White Breast Cancer Incidence and Mortality Rate Ratios
We calculated crude black-to-white rate ratios (RRsBW) for breast cancer mortality and incidence. These ratios provided empirical measures of racial disparity between blacks and whites. Mortality RRBW was calculated for three age groups (<50, 50–69, and ≥70 years), selected to broadly approximate mammography screening prevalence: age less than 50 years (low to no screening), ages 50–69 years (likely screened), and age 70 and more years (low to no screening). We used linear regression with inverse variance to assess trends over time in the mortality RRBW. The weights were obtained using the delta method (20) under the assumption that the numbers of deaths per year followed a Poisson distribution. We used the same approach to study the incidence RRBW.
Hazard Rates of Breast Cancer Death Among Women Living With the Disease
We characterized the absolute annual hazard rates of death from breast cancer among women diagnosed from January 1990 through December 2003 and followed through December 2004. This analysis was based on individual follow-up of 244 786 case subjects (Table 1), stratified by race, period of diagnosis (1990–1996 and 1997–2004), and ER status. We used spline functions to estimate the hazard curves, as described previously (1,21). Hazard rate curves show the instantaneous rate of death (percentage dying per year) due to breast cancer in a specified time interval after initial diagnosis among women who are alive at the beginning of that time interval. This essentially model-free approach reveals the shape of the hazard curve, and it can accommodate adjustment for potential confounders through stratification. However, hazard rate curves do not provide a concise summary estimate of the hazard rate ratio.
Therefore, we used Cox models (22) to obtain a detailed characterization of the hazard RRBW. For this analysis, we stratified the case subjects according to single year of diagnosis and ER status and estimated the relative hazard for blacks vs whites adjusted for age at diagnosis (<50, 50–69, and ≥70), AJCC stage (I, II, III, and IV), tumor grade (low and high), County Attribute for percentage of people below the poverty level (<10%, 10%–19%, and ≥20%) (18), and marital status (never married, married, separated, divorced, widowed, and unknown). The calendar year and ER status–specific hazard RRsBW were then analyzed using the same approach as for the mortality RRBW and incidence RRBW, that is, weighted regression analysis.
To verify the assumption of proportional hazards in Cox regression analyses, we used Poisson regression to estimate absolute monthly hazard rates of death from breast cancer, stratified by ER status, age group, and calendar period, and adjusted for stage and grade. In these models, we fitted separate baseline hazard rates for blacks and whites using quadratic spline functions and graphically inspected these curves for proportionality.
Breast Cancer Incidence–Based Mortality
Incidence-based mortality (IBM) is a statistical tool for calculating population-based mortality rates according to tumor characteristics (in this case, ER expression) that are recorded in registry data. IBM provides an estimate of the cross-sectional mortality rate in the population as a whole for a specific tumor type in a given calendar period (23); similar to aggregate mortality rates, it is a statistical measure that reflects the combined impact of cancer incidence, case ascertainment, and treatment.
For this study, we calculated IBM according to the combination of race (black or white) and breast cancer ER status (ER+, ER−, or ER other or unknown) among women aged 25–84 years who died of breast cancer. Among the women who died from breast cancer, ER expression of the primary tumor was known for 81% of those diagnosed since 1990 (Table 1). Therefore, we computed IBM rates for the calendar years 1994 through 2003 so that we could stratify the rates according to ER expression using data ascertained from 1990. For example, the numerator of the rate for 1994 reflected breast cancer deaths that occurred in 1994 among women diagnosed during 1990–1994 at ages 20–79 years, and the rate for 1995 reflected deaths in 1995 among women diagnosed during 1991–1995. The denominators of the IBM rates were obtained from the midyear population counts. We used a bootstrap procedure to account for both uncertainty in the hazard rates of breast cancer death and random fluctuations in observed breast cancer incidence counts. The key assumptions of the IBM analyses are that women who have been diagnosed with breast cancer are subject to competing risks of death from breast cancer and death from all other causes. Furthermore, unbiased estimates of these risks can be obtained from registry data. Within this framework, we used a non-parametric approach that finely stratified the hazard estimates by age, period, and ER-status using the large cohort of cases in the SEER 17 database.
IBM rates should be interpreted as a characteristic of an entire population rather than of individuals, because the time scale is calendar time rather than time since diagnosis, and the denominator includes all women in the population whether or not they have breast cancer. Furthermore, the IBM rates in this study should be interpreted as a leading indicator of mortality trends based on early deaths among recently diagnosed cases; actual breast cancer mortality rates are substantially higher than the calculated IBM rates. A technical description of the IBM computations is given (see Supplementary Appendix A, which is available online).
Finally, we used a counterfactual approach to study whether IBM RRBW would have been different if the proportions of tumors according to ER status, and/or the hazard rates of breast cancer death, had been similar among black and white women with breast cancer. For example, we computed the IBM in black women using data resembling the ER−/ER+ proportions in white women to estimate the potential influence of the disproportional ER expression among black and white women on breast cancer IBM RRBW.
Results
There were 244 786 female breast cancer cases in SEER's Registries Database (Table 1) who were diagnosed during our study period (1990–2003) and followed through 2004. Black and white women comprised 8.3% and 84.9%, respectively, of the breast cancer cases in SEER. Compared with whites, blacks were younger at diagnosis and had tumors with higher AJCC stages, larger sizes, higher grade, more positive nodes, and had more ER− disease.
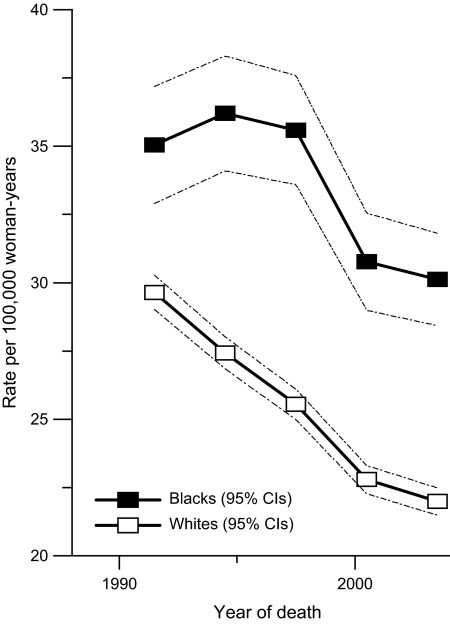
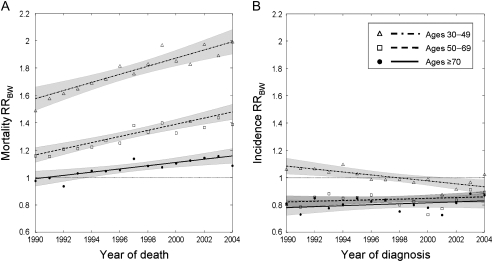
From 1990 through 2004, age-standardized breast cancer mortality rates decreased for both white and black women (Figure 1), however, it was persistently higher for the latter (F = 125.4, P < .001). The corresponding black-to-white breast cancer mortality rate ratio also varied over time according to age (Figure 2, A, P = .01 for age × race interaction), with the sharpest increases occurring among women younger than 50 years (secular rise of 0.03 per year, 95% confidence interval [CI] = 0.02 to 0.04 per year).
Figure 1.
Age-standardized breast cancer mortality rates. Age-standardized breast cancer mortality rates in the National Cancer Institute's Surveillance, Epidemiology, and End Results 9 are plotted for black (solid squares) and white (open squares) women between the years 1990 and 2004 by 3-year period. Dashed lines indicate 95% confidence intervals (CIs).
Figure 2.
Black-to-white breast cancer mortality and incidence rate ratios (RRsBW) for three age groups. A) Mortality RRBW. B) Incidence RRBW. Observed values (dots, age ≥70 years; squares, ages 50–69 years; and triangles, ages 30–49 years) are shown along with the corresponding fitted trends (solid line, dashed line, and dot–dash line, respectively) with 95% confidence intervals (shaded areas). Broken black line indicates an RRBW = 1.
In contrast, the breast cancer age-specific incidence rate ratio remained relatively stable over time (Figure 2, B). Throughout the study period, breast cancer incidence rates were lower for black women of all ages (incidence RRBW <1.0), except for the moderately higher rates that were seen among women who were younger than 50 years and were diagnosed before 1996.
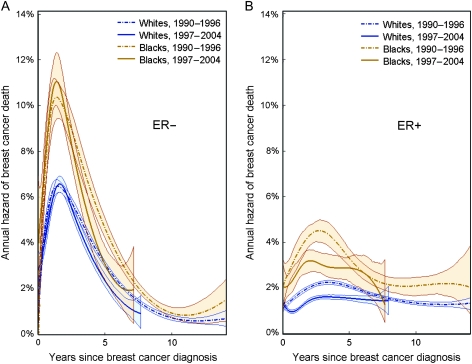
We calculated annual hazard rates of breast cancer death among women living with the disease, stratified by race, period of diagnosis, and ER status (Figure 3). During the time periods 1990–1996 and 1997–2004, absolute annual hazard rates of breast cancer death were statistically significantly higher for black women with ER− tumors and black women with ER+ tumors compared with whites with the same tumor status. For example, between the years 1997 and 2004, absolute hazard rates in black women with ER− tumors peaked at 11% per year (95% CI = 10.0% to 12.3% per year) approximately 1.5 years after diagnosis. In contrast, among white women, the peak hazard rate occurred at the same time after diagnosis but was only 6.6% per year (95% CI = 6.2% per year to 6.9% per year). The absolute hazard rates for patients with ER+ tumors were substantially lower, but in these patients as well, the hazard rates for black women were approximately twice as high as the corresponding hazard rates in white women (Figure 3, B). A statistically significant decline in hazard rates was observed between the two examined calendar periods (1990–1996 and 1997–2004) for both white and black women with ER+ tumors. Specifically, there was a 31.6% decline in the peak hazard rate in whites (95% CI = 28.1% to 32.7%) and a 27.7% decline (95% CI = 26.7% to 28.8%) in the peak for blacks. In contrast, there was a smaller relative decline in the hazard rates for both black and white women with ER− tumors, and this decline occurred after the early hazard peak (Figure 3, A).
Figure 3.
Annual hazard rates of breast cancer death (percentage of breast cancer dying per year among women living with the disease) are plotted against years since diagnosis for black women (orange) and white women (blue) for two calendar periods: 1990–1996 (broken lines) and 1997–2004 (solid lines). A) Hazard rates among case subjects with estrogen receptor–negative (ER−) tumors. B) Hazard rates among case subjects with estrogen receptor–positive (ER+) tumors.
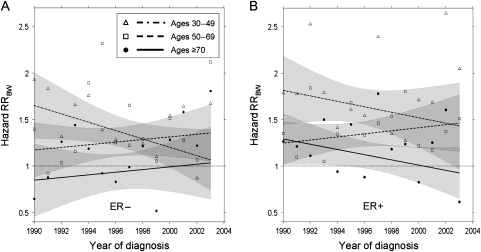
Next, we analyzed the hazard of breast cancer death RRBW for each calendar year of diagnosis among women with ER− and ER+ tumors adjusted for age at diagnosis, AJCC stage, tumor grade, and socioeconomic status (based on a County Attribute for percentage of people below the poverty level and marital status) (Figure 4). For women with ER− tumors (Figure 4, A), the risk of breast cancer death was generally higher among black women than white women (hazard RRBW >1.0 except for case subjects who were older than 70 years at diagnosis; for this group, the values were scattered above and below the referent line [RRBW = 1.0]). A statistically significant decline with time (P = .03) of hazard RRBW was seen in younger women (ages 30–49 years), but the observed values were generally greater than 1. Overall, the hazard RRBW for women with ER− tumors was 1.30 (95% CI = 1.11 to 1.50). For women with ER+ tumors (Figure 4, B), the risk of breast cancer death was higher among black women than white women (hazard RRBW > 1.0 for all age groups except for women older than 70 years at diagnosis in the years 1994, 1996, 2000, and 2003 [overall hazard RRBW = 1.40; 95% CI = 1.13 to 1.67]). No statistically significant temporal trend was seen in the hazard RRBW of the different age groups with ER+ tumors.
Figure 4.
Black-to-white breast cancer hazard rate ratios (RRsBW). Hazard RRsBW are plotted for three age groups. A) Estrogen receptor–negative (ER−) tumors. B) Estrogen receptor–positive (ER+) tumors. In each panel, observed values (dots, ages ≥70 years; squares, ages 50–69 years; and triangles, ages 30–49 years) are shown along with the corresponding fitted trends (solid line, dashed line, and dot–dash line, respectively) with 95% confidence intervals (shaded areas). Broken black line indicates an RRBW = 1.
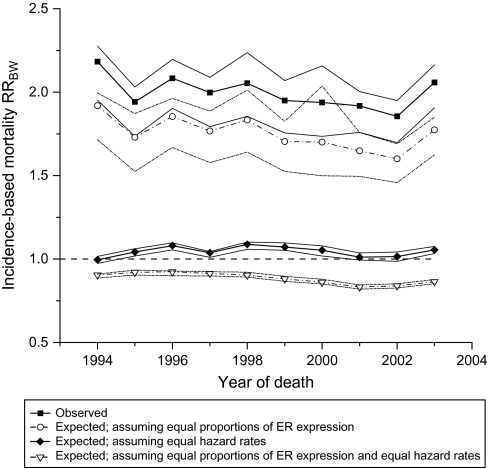
Finally, we investigated the RRBW of the expected IBM constructed using observed race-specific parameters (ER expression and hazard rates of breast cancer death) and also under different counterfactual scenarios (Figure 5). The observed IBM among all black case subjects was twice as high as that among all white case subjects throughout the study period. Equalizing proportions of case subjects with ER+ and ER− breast cancer slightly reduced the IBM RRBW from about 2.00 to approximately 1.75. In contrast, equalizing annual hazard rates eliminated the black-to-white racial disparity, that is, the IBM RRBW fell from approximately 2.00 to close to 1.00. Finally, the IBM RRBW was reversed (IBM RRBW was approximately 0.90) after equalizing both the proportion of ER− and ER+ case subjects and annual hazard rates of breast cancer death. A sensitivity analysis demonstrated that the results were essentially unchanged if the data were stratified by all the categories of ER expression from Table 1 (Supplementary Figure 1, available online).
Figure 5.
Black-to-white incidence–based mortality rate ratios (IBM RRsBW). Black-to-white IBM RRsBW with 95% confidence intervals were calculated and plotted under four different scenarios: 1) observed IBM RRsBW (solid squares), 2) expected IBM RRsBW after equalizing the proportions of estrogen receptor–negative and estrogen receptor–positive (ER− and ER+) breast cancers in black women to the proportions in white women (open circles), 3) expected IBM RRsBW assuming that annual hazard rates for death from ER+ and ER− breast cancers in blacks were equal to the corresponding rates in whites (solid diamonds), and 4) expected IBM RRsBW after equalizing the proportions of ER− and ER+ breast cancers and annual hazard rates for death from ER+ and ER− breast cancers (open triangles).
Discussion
In this study, we investigated aspects of the racial disparity in age-standardized breast cancer mortality rates that have been described as “a moral and ethical dilemma for our nation” (11). The gap between the breast cancer mortality rates in black women and white women emerged in the late 1970s and has continued to widen. Our analysis focused on the period since 1990, when ER status was first systematically recorded in the SEER database.
Throughout the period covered by our study (1990–2004), breast cancer mortality rates declined considerably for both black and white women, although the decline was slower in black women. During the same period, black-to-white incidence rate ratios remained relatively constant, and the age-specific incidence rates in black women were typically lower. Therefore, incidence trends alone cannot account for the widening disparity in breast cancer mortality.
In contrast, the hazard of breast cancer death was statistically significantly higher in black women compared with white women, irrespective of ER expression, and it persisted after adjustment for multiple tumor and demographic characteristics. Our counterfactual IBM analysis revealed that these differences in breast cancer prognosis could explain most of the disparity in breast cancer mortality in the United States. Perhaps surprisingly, however, a more modest contribution to the racial disparity can be attributed to the tumor characteristics, which included a disproportionately higher incidence of ER− tumors (which are associated with a less favorable prognosis) among black women, and also a larger fraction for whom the ER status of the tumor was not determined or not documented in the medical record.
In several clinical trials, it was shown that black and white patients derived a similar benefit from adjuvant systemic therapy administered in accordance with tumor characteristics, such as ER status (24). Unfortunately, when equal efficacy can be demonstrated in the trial setting, this does not guarantee equal effectiveness in the population. Our study suggests that racial disparity in breast cancer mortality can only be eliminated when hazard rates of breast cancer death in the entire population (which partly reflect access to therapy) are matched in black and white women irrespective of ER expression.
Various factors have been suggested to contribute to increased hazard rates observed among black women diagnosed with breast cancer. Black women are less likely to have adequate insurance coverage, which may limit their access to therapies (25,26). A study that examined breast cancer survival trends in an equal-access health-care system suggests that access to health care alone cannot account for the widening racial disparity (27). In our study, even after adjusting for socioeconomic variables available in SEER, the hazard RRBW remained greater than 1.0 (ie, higher hazard of breast cancer death among black women vs white women) in both ER− and ER+ diseases. Other socioeconomic factors such as education and cultural and behavioral characteristics have also been implicated as risk factors for breast cancer (8,28) and may also affect survival from this disease. Health-related factors that differ between the two ethnic groups, such as obesity, may also play a role (29,30). Unfortunately, most of these factors are not yet captured by the SEER program at the individual level.
Our study has the usual limitations of descriptive epidemiology (ie, retrospective registry assessment, missing data, nonstandardized ER typing, and lack of individual-level risk factor data). In addition, the IBM calculations in this study pertained to a subcohort of women; essentially, the subcohort of women recently diagnosed with the disease. Thus, it does not include the experience of women who have been living with breast cancer for many years. Furthermore, the counterfactual analysis only illuminates population trends by indicating how much better things could be, but provides no specific information on how to ameliorate the mortality gap.
In conclusion, our results suggest that the widening black-to-white disparity in breast cancer mortality rates in the United States is largely driven by the consistently higher hazard of death among black women living with the disease, irrespective of tumor ER expression at diagnosis. It is also apparent that the greatest absolute difference in the hazard rates occurs during the first several years after diagnosis. These differences in hazard rates may reflect racial differences in response and access to innovations in breast cancer screening and treatment, as well as other biological and nonbiological factors. Hence, greater emphasis should be placed on identifying the reasons for these increased hazards among black women and on developing new therapeutic approaches to address the disparity.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Supplementary Material
Footnotes
The authors take full responsibility for the design, data collection, analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
None of the coauthors has a financial conflict of interest that would have affected this research.
We thank the reviewers for helpful comments, which have greatly improved the content of this article.
References
- 1.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25(13):1683–1690. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
- 2.Chevarley F, White E. Recent trends in breast cancer mortality among white and black US women. Am J Public Health. 1997;87(5):775–781. doi: 10.2105/ajph.87.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jatoi I, Miller AB. Why is breast-cancer mortality declining? Lancet Oncol. 2003;4(4):251–254. doi: 10.1016/s1470-2045(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 4.Blackman DJ, Masi CM. Racial and ethnic disparities in breast cancer mortality: are we doing enough to address the root causes? J Clin Oncol. 2006;24(14):2170–2178. doi: 10.1200/JCO.2005.05.4734. [DOI] [PubMed] [Google Scholar]
- 5.Jatoi I, Anderson WF, Rao SR, Devesa SS. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23(31):7836–7841. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 6.Li CI. Racial and ethnic disparities in breast cancer stage, treatment, and survival in the United States. Ethn Dis. 2005;15(2 suppl 2):S5–S9. [PubMed] [Google Scholar]
- 7.Sarker M, Jatoi I, Becher H. Racial differences in breast cancer survival in women under age 60. Breast Cancer Res Treat. 2007;106(1):135–141. doi: 10.1007/s10549-006-9478-3. [DOI] [PubMed] [Google Scholar]
- 8.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 9.Field TS, Buist DS, Doubeni C, et al. Disparities and survival among breast cancer patients. J Natl Cancer Inst Monogr. 2005;35:88–95. doi: 10.1093/jncimonographs/lgi044. [DOI] [PubMed] [Google Scholar]
- 10.Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003;97(11):2853–2860. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 11.Vastag B. Breast cancer racial gap examined: no easy answers to explain disparities in survival. JAMA. 2003;290(14):1838–1842. doi: 10.1001/jama.290.14.1838. [DOI] [PubMed] [Google Scholar]
- 12.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 13.Hofler M. Causal inference based on counterfactuals. BMC Med Res Methodol. 2005;5:28. doi: 10.1186/1471-2288-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao RS, Graubard BI, Breen N, Gastwirth JL. Understanding the factors underlying disparities in cancer screening rates using the Peters-Belson approach: results from the 1998 National Health Interview Survey. Med Care. 2004;42(8):789–800. doi: 10.1097/01.mlr.0000132838.29236.7e. [DOI] [PubMed] [Google Scholar]
- 15.SEER-9. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence-SEER 9 Regs Limited-Use, November 2006 submission (1973–2003)—Linked to County Attributes—Total US, 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007. Underlying mortality data provided by NCHS. 2007 www.cdc.gov/nchs. Accessed February 19, 2009. [Google Scholar]
- 16.SEER. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Mortality—All COD, Aggregated With State, Total U.S. (1969–2004), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007. Underlying mortality data provided by NCHS .2007. www.cdc.gov/nchs. Accessed February 19, 2009. [Google Scholar]
- 17.American Joint Committee on Cancer. Breast. In: Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Handbook. 6th ed. New York, NY: Springer; 2002. pp. 255–281. [Google Scholar]
- 18.Singh GK, Miller BA, Hankey BF, Edwards BK. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. NCI Cancer surveillance Monograph Series, Number 4. Bethesda, MD: National Cancer Institute, 2003. NIH publication No. 03-0000. [Google Scholar]
- 19.Gustafsson JA, Warner M. Estrogen receptor beta in the breast: role in estrogen responsiveness and development of breast cancer. J Steroid Biochem Mol Biol. 2000;74(5):245–248. doi: 10.1016/s0960-0760(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 20.Oehlert GW. A note on the delta method. Am Stat. 1992;46(1):27–29. [Google Scholar]
- 21.Rosenberg PS. Hazard function estimation using B-splines. Biometrics. 1995;51(3):874–887. [PubMed] [Google Scholar]
- 22.Cox D. Regression models and life-tables. J R Stat Soc. 1972;34(2):187–220. [Google Scholar]
- 23.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47(12):1451–1461. doi: 10.1016/0895-4356(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 24.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001;(30):36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 25.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 26.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 27.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U.S. Department of Defense Healthcare system. Cancer. 2003;98(5):894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 28.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer—epidemiology, risk factors, and genetics. BMJ. 2000;321(7261):624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dignam JJ, Wieand K, Johnson KA, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245–254. doi: 10.1007/s10549-005-9118-3. [DOI] [PubMed] [Google Scholar]
- 30.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89(6):2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.