Abstract
Objective
Emerging evidence suggests that exposures during fetal life affect adult metabolism. We assessed the relation between recalled maternal pre-pregnancy body mass, gestational weight gain (GWG), and adiposity in the daughter.
Design
Retrospective cohort study among mother-nurse daughter dyads in the Nurses’ Health Study II and the Nurses’ Mothers’ Cohort. Mothers of participants completed questionnaires regarding their nurse-daughter in 2001.
Participants
26,506 mother-nurse daughter dyads born between 1946 and 1964.
Main outcome measures
Body mass index of the nurse-daughter at age 18 and in 2001.
Results
At age 18, 561 (2.1%) daughters were obese (BMI greater than 30), and in 2001, 5,442 (22.0%) were obese. Adjusting for covariates, women whose mothers had a recalled pre-pregnancy BMI of 29 had a 6.1-fold increased risk of obesity at age 18 and a 3.4-fold risk of obesity in 2001, compared with women whose mothers had a pre-pregnancy BMI of 21. We found a U-shaped association between recalled GWG and offspring obesity. Compared with a maternal weight gain of 15–19 lb, GWG <10 lbs was associated with a significant increase in obesity risk at age 18 (odds ratio[OR] 1.54, 95% confidence interval[CI] 1.02–2.34) and in 2001 (OR 1.27, 95%CI 1.05–1.53). High weight gain (40+ lbs) was also associated with obesity risk at age 18 (OR 1.81, 95%CI 1.22–2.69) and in 2001 (OR 1.74, 95%CI 1.48–2.04). These associations were stronger among mothers who were overweight prior to pregnancy (p for interaction = 0.03), and they persisted with adjustment for birth weight.
Conclusion
A high recalled pre-pregnancy BMI and extremes of recalled GWG are associated with an increased risk of adolescent and adult obesity in offspring, particularly when the mother is overweight. Pre-pregnancy weight and GWG may be modifiable fetal origins of overweight and obesity in women.
Keywords: pregnancy, obesity, gestational weight gain, prenatal programming, birth weight
Introduction
Associations between fetal growth and risk of metabolic disease later in life have been suggested in multiple studies (1–7). Birth weight has been used as a proxy for in utero nutrition; infants that are born small have restricted access to nutrients during development, leading to a thrifty phenotype. In later life, catch up growth among individuals who were small at birth is associated with greater fat, compared with lean, tissue accumulation, reduced insulin sensitivity in muscle, and central adiposity (8). At the other extreme, high birth-weight (> 4000g) has also been associated with an increased risk for obesity and metabolic disease, attributed to overnutrition in utero (4).
In 1990, the Institute of Medicine’ Subcommittee on Nutritional Status and Weight Gain During Pregnancy concluded that low maternal gain was a significant risk factor for low birth weight(9). The subcommittee examined observational data and identified the mean weight gain associated with birth weights of 3000 to 4000g. These findings formed the basis for the current recommendations of 25 to 35 lbs of gain for normal BMI women, 28 to 40 lbs of gain for low BMI women, and 15 to 25 lbs of gain for overweight women. At the time, evidence regarding appropriate gain for obese women was limited. The subcommittee recommended at least 15 lbs of gain for this group, because “it seems prudent to recommend that obese women gain a minimum equivalent to the weight of the products of conception.” In 2006, the Institute of Medicine convened a workshop on the Influence of Pre-pregnancy Weight on Maternal and Child Health to review more recent data (10). Many participants suggested a revision of the 1990 guidelines given more recent demographic trends, including rising rates of obesity and declining rates of low birth weight. Revised recommendations on appropriate gain have not been made.
Both maternal pre-pregnancy body mass index (BMI) and gestational weight gain affect fetal growth (11), but few long-term studies are available regarding the role of these anthropometric characteristics in predicting the offspring’s long-term disease risk. Studies suggest that, with provider guidance, women are more likely to gain appropriately during pregnancy (12, 13). Understanding how maternal BMI and gestational weight gain influences long-term risk of obesity in offspring may therefore allow expectant mothers and their care providers to reduce risks for obesity in the next generation. We therefore analyzed the relation between recalled maternal pre-pregnancy BMI and gestational weight gain and adolescent (age 18) and adult adiposity in the nurse daughters who are participants of the Nurses Health Study II (NHS II).
Materials and Methods
Population
The NHS II is a large, prospective cohort study of 116,609 female nurses from 14 U.S. states. Enrollment began in 1989, when the nurses were 25–44 years old. In 2001, mothers of women in the cohort were invited to complete a questionnaire regarding their nurse-daughter. The Nurses’ Mothers’ Cohort included nurses who were free of cancer and whose mothers were alive in 2001. Data on pregnancy and early life exposures were obtained from 35,826 mother-daughter dyads. Ninety-seven percent of participants are non-Hispanic whites.
Our cohort was limited to nurses whose mothers were alive and able to respond to our questionnaire in 2001, potentially enriching our study group with healthier mothers and daughters. Compared to nurses whose mothers did not complete the 2001 questionnaire, our study population had a slightly lower BMI at age 18 (mean ± SD: 21.1 ± 3.2 vs. 21.3 ± 3.4, t test with equal variance p <0.001). Women in our study group were also less likely to have a mother with diabetes (4.2% vs. 8.7%, Chi Square p<0.001). This was expected, because mothers with diabetes would be at higher risk of dying earlier than mothers without diabetes.
Assessment of exposures
Mothers reported weight gain during the pregnancy with their nurse daughter as a categorical variable in the following categories: Less than 10 pounds, 10–14 pounds, 15–19 pounds, 20–29 pounds, 30–40 pounds, More than 40 pounds, or Don’t remember. They also reported their height and usual weight prior to the index pregnancy and responded to a variety of questions regarding pregnancy complications and outcome. The following data reported by the mothers were included in our study: the nurse daughter’s birth weight, due date for the pregnancy, whether the nurse daughter was a twin and whether she was adopted; severity, duration, and treatment of pregnancy-associated nausea and vomiting, pregnancy complications including high blood pressure and pre-eclampsia, use of tobacco and level of physical activity during the pregnancy, infant feeding practices (any breastfeeding vs. no breastfeeding), and birth order of the daughter. Socioeconomic data included home ownership at the time of the nurse daughter’s birth, cohabitation with the father, father’s educational attainment, and father’s occupation. The mothers also reported the father’s weight and height at the time of the daughter’s birth. Daughters reported parental history of diabetes on questionnaires in 1989 and 1997.
Assessment of outcome
Participants of the Nurses’ Health Study II reported their current height and their weight at age 18 on the 1989 questionnaire. Self-reported weight at age 18 in this cohort has been previously validated (14). Participants reported current weight on the 2001 questionnaire, when participants were 36 to 56 years old. We defined overweight as BMI greater than or equal to 25 and less than 30, and we defined obese as BMI greater than or equal to 30.
Exclusions
We excluded from our study population nurses who were adopted (n=88), or were missing data on: BMI at age 18 (n=274), maternal gestational weight gain (n=2811), or maternal pre-pregnancy BMI (n=1533). In addition, we excluded subjects who were members of a twin pregnancy (n=543), whose mother’s pregnancies were complicated by preeclampsia (n=1002), or for whom gestational age at delivery was not known (n=3069). Subjects with missing data on BMI in 2001 (n=1755) were excluded from the analysis of adult BMI. We used a missing indicator variable for participants missing covariate data.
Statistical Analysis
We performed univariate logistic regression to assess the relation between individual predictors and outcome. We used ANOVA and chi-square tests to assess the relation between gestational weight gain and continuous and categorical covariates, respectively. To assess whether any associations between gestational weight gain or pre-pregnancy weight and obesity in the daughter persisted beyond adolescence, we also modeled these associations with daughters’ obesity in 2001, when the Mothers’ Questionnaire was administered. We used multinomial logistic regression to model the relation between gestational weight gain and the endpoints of overweight or obesity at age 18 in the daughter, using daughters with a BMI < 25 as the comparison group. To prevent over fitting of the model, only variables that were considered a priori predictors of offspring’s obesity were tested for inclusion in our model.
We hypothesized that gestational weight gain would have a U-shaped relation with offspring’s risk of obesity, because both low and high birth weights have been associated with metabolic disease in adulthood(2). We therefore chose the gestational weight gain category associated with the lowest mean BMI at age 18 in the daughter as the reference group for the analysis of obesity at age 18, and we used the same referent category for the analysis of obesity in 2001. Maternal pre-pregnancy BMI, maternal age, and paternal BMI were treated as continuous variables. For the analysis of obesity in 2001 we adjusted for the nurses’ age at the time of the 2001 questionnaire. Maternal and paternal history of diabetes, smoking status, pregnancy-associated nausea and vomiting, maternal physical activity, birth order, and demographic variables were modeled as categorical variables. Only those covariates that added significantly to the model (Likelihood ratio test p < 0.05) or changed the parameter estimates for the exposure by > 10% remained in the models. We tested linearity of associations with continuous variables by adding a quadratic term to the model. Quadratic terms were retained if they were statistically significant.
Some authors have reported differential under-reporting of weight among higher-BMI women (15). We therefore performed a sensitivity analysis to test whether such underreporting would alter any association between gestational weight gain and offspring obesity. We similarly tested whether differential under-reporting of gestational weight gain category by overweight mothers would alter observed associations.
We hypothesized that birth weight might mediate the association between gestational weight gain, maternal pre-pregnancy BMI, and obesity in the daughter. To test its role as an intermediate variable, we added birth weight to the multivariable model. Because birth weight has been shown to have a U-shaped association with adult obesity, birth weight was treated as a categorical variable (<2500g, 2500 to < 3000g, 3000 to <3500g, 3500 to <4000g, 4000 to <4500g, and 4500g or more).
We hypothesized that the association between gestational weight gain and adiposity in the daughter would be modified by maternal pre-pregnancy body mass index. To test this hypothesis, we stratified our population by maternal pre-pregnancy body mass index (<25 kg/m2 vs. >=25 kg/m2) and performed multinomial regression in the two groups to assess the association between gestational weight gain category and obesity in the daughter. We further used multivariable linear regression to model body mass index at age 18 and in 2001, assessing the interaction between body mass index, body mass index squared, and gestational weight gain treated categorically.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The Institutional Review Boards of the Brigham and Women’s Hospital and the National Cancer Institute approved the study.
Results
Among a total of 26,506 mother-daughter dyads, 561 daughters were obese at age 18 (2.1%), and 1802 were overweight (6.8%). BMI in 2001 was available for 24,751 nurse daughters, among whom 5442 (22.0%) were obese and 6576 (26.6%) were overweight. Among the nurses’ mothers, 6.2% were overweight and 0.8% were obese prior to pregnancy.
Sixty-four percent of mothers gained between 15 and 29 pounds during their pregnancy with their nurse daughter, while 3.5 percent gained less than 10 pounds and 5.0 percent gained more than 40 pounds. Maternal body mass index prior to pregnancy, gestational age at delivery, and infant birth weight were linearly associated with gestational weight gain (Table 1a). Both high and low extremes of weight gain were associated with parental history of diabetes, smoking during pregnancy, first pregnancy, and indicators of lower socio-economic status. Maternal prepregnancy BMI was linearly related to maternal and paternal diabetes history, paternal BMI, birth weight of the nurse daughter, and low gestational weight gain (Table 1b). Mothers with higher BMIs prior to pregnancy were less likely to be primiparous, less likely to report nausea and vomiting or high physical activity during pregnancy, and less likely to have breastfed their nurse daughter. Partners of women with higher BMIs were less likely to have a professional job or a college degree.
Table 1.
| Table 1a. Parental characteristics in relation to recalled gestational weight gain. | ||||||
|---|---|---|---|---|---|---|
| Recalled Gestational Weight Gain Category | ||||||
| < 10 | 10–14 | 15–19 | 20–29 | 30–39 | 40+ | |
| N | 939 | 2985 | 5643 | 11,194 | 4432 | 1319 |
| Mother of subject | ||||||
| BMI prior to index pregnancy1 | 22.3 (3.9) | 21.1 (2.7) | 21.1 (2.5) | 21.2 (2.4) | 21.3 (2.7) | 20.9 (2.8) |
| Age at subject’s birth (yr)1 | 26.2 (5.0) | 26.7 (4.9) | 26.5 (4.8) | 26.1 (4.7) | 25.3 (4.7) | 23.9 (4.3) |
| Lifetime diabetes history (%) | 6.3 | 4.0 | 2.8 | 3.5 | 4.4 | 4.6 |
| Pregnancy | ||||||
| Birth weight of daughter (g) 1 | 3050 (607) | 3106 (510) | 3212 (483) | 3338 (463) | 3459 (475) | 3504 (514) |
| Year of birth [median, IQR] | 1955 [1951–59] | 1955 [1951–58] | 1955 [1952–59] | 1956 [1952–59] | 1955 [1952–59] | 1955 [1951–59] |
| First pregnancy (%) | 48.0 | 43.0 | 40.4 | 41.2 | 50.5 | 66.9 |
| Nausea/vomiting 1st trimester (%) | 63.3 | 60.3 | 64.0 | 65.5 | 67.6 | 68.3 |
| High activity level2 (%) | 26.0 | 30.2 | 29.7 | 28.0 | 23.3 | 21.0 |
| Smoked during pregnancy (%) | 28.4 | 24.9 | 24.1 | 24.4 | 26.4 | 28.1 |
| Gestational age at delivery (wks) 1 | 38.9 (2.6) | 39.1 (2.3) | 39.3 (2.1) | 39.6 (1.9) | 39.7 (1.9) | 39.8 (2.0) |
| Breastfed daughter in infancy (%) | 44.6 | 45.2 | 45.5 | 46.1 | 48.3 | 47.9 |
| Father of subject | ||||||
| BMI at time of subject’s birtha | 23.7 (3.2) | 23.5 (2.8) | 23.5 (2.8) | 23.6 (2.8) | 23.6 (2.9) | 23.4 (2.9) |
| Lifetime history of diabetes (%) | 9.8 | 8.2 | 7.4 | 7.3 | 8.2 | 8.0 |
| Socioeconomic Status | ||||||
| Mother not living with father (%) | 1.2 | 1.0 | 1.1 | 0.9 | 1.2 | 2.1 |
| Father professional (%) | 26.9 | 29.0 | 32.2 | 31.7 | 25.9 | 22.7 |
| Father has college degree (%) | 19.8 | 23.1 | 25.1 | 24.0 | 19.3 | 14.4 |
| Family did not own home at time of subject’s birth (%) | 55.3 | 50.5 | 50.5 | 50.8 | 58.0 | 64.7 |
| Table 1b. Parental characteristics in relation to recalled maternal pre-pregnancy BMI. | |||||
|---|---|---|---|---|---|
| Recalled maternal Pre-pregnancy BMI | |||||
| < 22 | 22 to <24 | 24 to <26 | 26 to <28 | 28+ | |
| N | 18,416 | 4,792 | 2,213 | 597 | 488 |
| Mother of subject | |||||
| Age at subject’s birth (yr)1 | 25.6 | 26.7 | 27.1 | 27.4 | 27.2 |
| Lifetime diabetes history (%) | 2.7 | 4.1 | 7.7 | 10.2 | 12.5 |
| Pregnancy | |||||
| Gestational weight gain (lbs) | |||||
| < 10 | 2.9 | 3.3 | 5.2 | 7.7 | 17.8 |
| 10 to 14 | 11.5 | 10.7 | 10.3 | 11.9 | 10.9 |
| 15 to 19 | 22.1 | 19.5 | 20.0 | 18.6 | 16.2 |
| 20 to 29 | 42.2 | 43.9 | 41.6 | 38.7 | 32.6 |
| 30 to 39 | 16.2 | 17.7 | 18.2 | 20.1 | 17.4 |
| 40+ | 5.0 | 5.0 | 4.8 | 3.0 | 5.1 |
| Birth weight of daughter (g) 1 | 3268.7 | 3358.0 | 3404.3 | 3451.3 | 3454.6 |
| Year of birth [median, IQR] | 1955 [1952–1959] | 1955 [1952–1959] | 1956 [1952–1959] | 1956 [1953–1960] | 1956 [1953–1960] |
| First pregnancy (%) | 48.2 | 37.1 | 33.9 | 30.7 | 32 |
| Nausea/vomiting 1st trimester | 65.7 | 64.4 | 62.9 | 62.1 | 59.4 |
| High activity level2 (%) | 28.0 | 26.2 | 27.5 | 22.9 | 22.7 |
| Smoked during pregnancy (%) | 26.7 | 22.4 | 20.7 | 17.9 | 19.1 |
| Gestational age at delivery (wks) 1 | 39.4 | 39.5 | 39.6 | 39.7 | 39.7 |
| Breastfed daughter in infancy (%) | 46.8 | 45.5 | 45.0 | 45.3 | 41.2 |
| Father of subject | |||||
| BMI at time of subject’s birth1 | 23.4 | 23.8 | 24.3 | 24.4 | 24.9 |
| Lifetime history of diabetes (%) | 7.0 | 8.4 | 9.6 | 12.7 | 12.3 |
| Socioeconomic Status | |||||
| Mother not living with father (%) | 1.2 | 0.8 | 0.8 | 0.7 | 1.6 |
| Father professional (%) | 32.0 | 28.3 | 22.4 | 17.8 | 13.6 |
| Father has college degree (%) | 24.9 | 20.1 | 16.8 | 11.9 | 6.4 |
| Family did not own home at time | |||||
| of subject’s birth (%) | 55.1 | 47.8 | 47.7 | 43.7 | 47.3 |
mean (SD)
Upper tertile of physical activity during pregnancy with nurse daughter.
In the unadjusted multinomial regression model, maternal weight gain was significantly associated with both overweight and obesity at age 18, with increased odds of each outcome at both low and high extremes of gestational weight gain (Table 2). We observed the lowest risk of the daughter’s obesity among women who gained 15 to 19 pounds. Maternal pre-pregnancy BMI attenuated this association (Table 2). Adjusting for maternal pre-pregnancy BMI, less than 10 pounds of gestational gain was associated with a 1.73-fold increase in the odds of obesity in the daughter compared with 15–19 pounds of gain. At the other extreme, more than 40 pounds of weight gain was associated with a maternal BMI-adjusted 2.13-fold increase in odds of obesity in the daughter. Adjustment for other covariates modestly attenuated the association between extremes of gestational weight gain and the odds of obesity at age 18. The relation differed for overweight. After adjustment for maternal BMI, low weight gain was no longer significantly associated with increased odds of overweight at age 18. Weight gain greater than 40 pounds remained significantly associated, with a 1.55-fold increase in odds of overweight at age 18, compared with subjects whose mothers gained 15 to 19 pounds. Further adjustment for other covariates did not materially change the pattern of association.
Table 2.
Odds Ratios for obesity and overweight of 26,512 participants of the Nurses’ Health Study II at age 18 by recalled gestational weight gain of the mother.
| Gestational Weight Gain (pounds) | ||||||
|---|---|---|---|---|---|---|
| < 10 | 10–14 | 15–19 | 20–29 | 30–39 | 40+ | |
| N | 939 | 2983 | 5643 | 11,190 | 4432 | 1319 |
| Obesity of nurse daughter at age 18 (BMI=30+) | ||||||
| No of Cases | 37 | 72 | 85 | 221 | 107 | 39 |
| OR and 95% CI | ||||||
| Unadjusted | 2.77 (1.87–4.11) | 1.62 (1.18–2.22) | 1.0 (ref) | 1.32 (1.03–1.70) | 1.64 (1.23–2.19) | 2.05 (1.40–3.02) |
| Adjusted for maternal pre-pregnancy | ||||||
| BMI1 | 1.73 (1.15–2.60) | 1.59 (1.15–2.19) | 1.0 (ref) | 1.27 (0.99–1.64) | 1.53 (1.14–2.04) | 2.13 (1.44–3.14) |
| Covariate-adjusted2 | 1.54 (1.02–2.34) | 1.58 (1.14–2.18) | 1.0 (ref) | 1.28 (0.99–1.65) | 1.39 (1.03–1.86) | 1.81 (1.22–2.69) |
| Covariate-adjusted2, including birth weight | 1.51 (1.00–2.30) | 1.56 (1.13–2.16) | 1.0 (ref) | 1.27 (0.98–1.64) | 1.34 (0.99–1.80) | 1.68 (1.13–2.52) |
| Overweight (BMI 25 to < 30) | ||||||
| No of Cases | 85 | 183 | 354 | 720 | 342 | 118 |
| OR and 95% CI | ||||||
| Unadjusted | 1.53 (1.19–1.96) | 0.99 (0.82–1.19) | 1.0 (ref) | 1.03 (0.91–1.18) | 1.26 (1.08–1.47) | 1.49 (1.20–1.86) |
| Adjusted for maternal pre-pregnancy | ||||||
| BMI1 | 1.19 (0.92–1.54) | 0.98 (0.81–1.18) | 1.0 (ref) | 1.00 (0.87–1.14) | 1.21 (1.03–1.41) | 1.55 (1.25–1.94) |
| Covariate-adjusted2 | 1.11 (0.85–1.43) | 0.97 (0.8–1.17) | 1.0 (ref) | 0.99 (0.87–1.13) | 1.14 (0.97–1.33) | 1.41 (1.13–1.77) |
| Covariate-adjusted2, including birth weight | 1.12 (0.87–1.46) | 0.99 (0.82–1.19) | 1.0 (ref) | 0.96 (0.84–1.10) | 1.06 (0.90–1.25) | 1.29 (1.02–1.62) |
Maternal BMI modeled as maternal BMI + maternal BMI squared
Maternal BMI (linear + bmi squared), maternal smoking during index pregnancy, nausea during pregnancy, maternal age at time of daughter’s birth (linear + age squared), birth order of daughter, maternal history of diabetes, paternal BMI, paternal history of diabetes, mother living with father at time of nurse’s birth, paternal level of education
When we added birth weight to our model, the odds ratio for obesity at high and low extremes of gestational gain was slightly attenuated, but remained statistically significant. Similarly, high weight gain remained a significant predictor of overweight at age 18 with adjustment for birth weight (Table 2).
The association between gestational weight gain category and obesity in the daughter differed depending on maternal pre-pregnancy BMI (Likelihood ratio test p for mother overweight × gestational weight gain category interaction=0.03). Among daughters of normal weight mothers, we found increased odds of obesity at age 18 with 10–14 lbs of gain (OR 1.54, 95% CI 1.08–2.20) compared with 15–19 lbs of gain. Point estimates suggested an increased risk for weight gain less than 10 lbs or greater than 30 lbs, but confidence intervals were wide. Among daughters of overweight women, the association between extremes of maternal gestational weight gain and obesity was stronger: for < 10 lbs, OR 2.42, 95% CI 1.14–5.16, and for 40+ lbs, OR 3.56, 95% CI 1.47–8.59, compared with 15–19 lbs of weight gain.
In our sensitivity analysis, we tested whether systematic under-reporting of BMI among women in the highest quartile of pre-pregnancy body mass index would affect our results. Incrementing body mass index by 2 kg/m2 in this group did not change the observed association between gestational weight gain and obesity in the daughter. We similarly tested whether differential under-reporting of weight gain category by mothers who were overweight at the time of their pregnancy would affect our results. In our differentially reclassified models, the strength of the association between extremes of weight gain and offspring obesity was attenuated; however, we continued to find an increased odds of obesity in the daughter with 10 to 14 or more than 30 lbs of maternal gestational weight gain when we modeled the effect of 20 percent of overweight mothers under-reporting their weight gain category.
We found similar results for the association between maternal gestational weight gain and offspring’s obesity in adulthood. In the age-adjusted multinomial model, a U-shaped association between gestational weight gain and both obesity and overweight appeared (Table 3). These associations were confounded by maternal pre-pregnancy BMI. In the fully adjusted model, maternal gain of less than 10 pounds was associated with a 1.27-fold higher odds of obesity in adulthood. Daughters of mothers who gained more than 40 pounds had a 1.74-fold odds of obesity and a 1.27-fold odds of overweight in adulthood. Adjustment for birth weight of the daughter did not appreciably modify this association.
Table 3.
Odds Ratios for obesity and overweight in 2001 of participants of the Nurses’ Health Study, by recalled gestational weight gain of the mother. All models adjusted for the age of the daughter in 2001.
| Gestational Weight Gain (pounds) | ||||||
|---|---|---|---|---|---|---|
| < 10 | 10–14 | 15–19 | 20–29 | 30–39 | 40+ | |
| N | 876 | 2768 | 5291 | 10,451 | 4155 | 1210 |
| Obesity of nurse daughter in 2001 (BMI=30+) | ||||||
| No of Cases | 239 | 591 | 1031 | 2159 | 1052 | 370 |
| OR and 95% CI | ||||||
| Unadjusted | 1.70 (1.42–2.02) | 1.14 (1.01–1.29) | 1.0 (ref) | 1.12 (1.02–1.22) | 1.47 (1.33–1.63) | 2.03 (1.74–2.36) |
| Adjusted for maternal pre-pregnancy | ||||||
| BMI1 | 1.39 (1.16–1.67) | 1.13 (1.00–1.28) | 1.0 (ref) | 1.09 (1.00–1.19) | 1.44 (1.30–1.60) | 2.15 (1.84–2.5) |
| Covariate-adjusted2 | 1.27 (1.05–1.53) | 1.12 (0.99–1.27) | 1.0 (ref) | 1.06 (0.97–1.17) | 1.29 (1.15–1.43) | 1.74 (1.48–2.04) |
| Covariate-adjusted2, including birth weight | 1.26 (1.04–1.52) | 1.12 (0.99–1.27) | 1.0 (ref) | 1.05 (0.96–1.15) | 1.25 (1.12–1.39) | 1.66 (1.42–1.95) |
| Overweight (BMI 25 to < 30) | ||||||
| No of Cases | 243 | 745 | 1366 | 2806 | 1088 | 328 |
| OR and 95% CI | ||||||
| Unadjusted | 1.30 (1.10–1.55) | 1.09 (0.97–1.21) | 1.0 (ref) | 1.09 (1.01–1.18) | 1.15 (1.04–1.27) | 1.36 (1.16–1.58) |
| Adjusted for maternal pre-pregnancy | ||||||
| BMI1 | 1.20 (1.01–1.43) | 1.09 (0.97–1.21) | 1.0 (ref) | 1.08 (0.99–1.17) | 1.14 (1.03–1.26) | 1.41 (1.21–1.65) |
| Covariate-adjusted2 | 1.17 (0.98–1.39) | 1.08 (0.97–1.21) | 1.0 (ref) | 1.07 (0.98–1.16) | 1.08 (0.98–1.19) | 1.27 (1.09–1.49) |
| Covariate-adjusted2, including birth weight | 1.16 (0.97–1.38) | 1.08 (0.97–1.21) | 1.0 (ref) | 1.06 (0.98–1.15) | 1.07 (0.97–1.19) | 1.26 (1.07–1.47) |
Maternal BMI modeled as maternal BMI + maternal BMI squared
Maternal BMI (linear + bmi squared), maternal smoking during index pregnancy, nausea during pregnancy, birth order of daughter, maternal age at time of daughter’s birth (linear + age squared), maternal history of diabetes, paternal BMI, paternal history of diabetes, mother living with father at time of nurse’s birth, paternal level of education, nurse-daughter’s age in 2001
Both maternal pre-pregnancy BMI and the square of maternal pre-pregnancy BMI were significantly related to offspring adiposity both at age 18 and in 2001. We used our multinomial model to predict the odds ratios for obesity for mothers with pre-pregnancy BMIs between 21 and 29. Small increments in BMI were associated with substantially increased odds of obesity and overweight. Compared with daughters of mothers with a pre-pregnancy BMI of 21, those whose mothers had a pre-pregnancy BMI of 23 had a 1.72-fold odds of obesity at age 18 and a 1.42-odds risk of obesity in 2001. Those participants whose mothers had a pre-pregnancy BMI of 29 had a 6.12-fold increased odds of obesity at age 18 and a 3.41-fold odds of obesity in 2001 (Table 4), compared to those whose mothers had a pre-pregnancy BMI of 21. Paternal BMI was also associated with odds of overweight and obesity in the daughter, but the association was not as strong. Compared with daughters of fathers with a BMI of 21, those whose fathers had a BMI of 29 had a 3.6-fold risk of obesity at age 18 and a 2.5-fold risk of obesity in 2001.
Table 4.
Predicted Odds Ratios for obesity and overweight at age 18 for participants of the Nurses’ Health Study II, by recalled pre-pregnancy BMI1 of the mother.
| 21 | 23 | 25 | 27 | 29 | |
|---|---|---|---|---|---|
| Obesity of nurse daughter at age 18 (BMI=30+) | |||||
| OR and 95% CI | |||||
| Unadjusted | 1.0 (ref) | 1.80 (1.68–1.94) | 3.08 (2.69–3.51) | 4.95 (4.13–5.94) | 7.53 (6.00–9.45) |
| Adjusted for gestational weight gain | 1.0 (ref) | 1.79 (1.66–1.93) | 3.03 (2.65–3.45) | 4.04 (4.83–5.77) | 7.28 (5.84–9.09) |
| Covariate-adjusted2 | 1.0 (ref) | 1.72 (1.61–1.85) | 2.79 (2.47–3.15) | 4.26 (3.64–4.99) | 6.12 (5.07–7.39) |
| Covariate-adjusted2, including birth weight | 1.0 (ref) | 1.72 (1.59–1.86) | 2.77 (2.40–3.20) | 4.21 (3.46–5.11) | 5.99 (4.69–7.66) |
| Overweight (BMI 25 to < 30) | |||||
| OR and 95% CI | |||||
| Unadjusted | 1.0 (ref) | 1.54 (1.48–1.60) | 2.22 (2.08–2.38) | 3.03 (2.76–3.32) | 3.88 (3.42–4.40) |
| Adjusted for gestational weight gain | 1.0 (ref) | 1.54 (1.48–1.60) | 2.22 (2.08–2.37) | 3.00 (2.75–3.28) | 3.82 (3.39–4.32) |
| Covariate-adjusted2 | 1.0 (ref) | 1.51 (1.46–1.57) | 2.14 (2.01–2.27) | 2.82 (2.60–3.07) | 3.49 (3.10–3.92) |
| Covariate-adjusted2, including birth weight | 1.0 (ref) | 1.40 (1.44–1.56) | 2.09 (1.96–2.24) | 2.73 (2.50–2.99) | 3.34 (2.95–3.79) |
Maternal BMI modeled as maternal BMI + maternal BMI squared
Gestational weight gain category, maternal smoking during index pregnancy, nausea during pregnancy, birth order of daughter, maternal age at time of daughter’s birth (linear + age squared), maternal history of diabetes, paternal BMI, paternal history of diabetes, mother living with father at time of nurse’s birth, paternal level of education
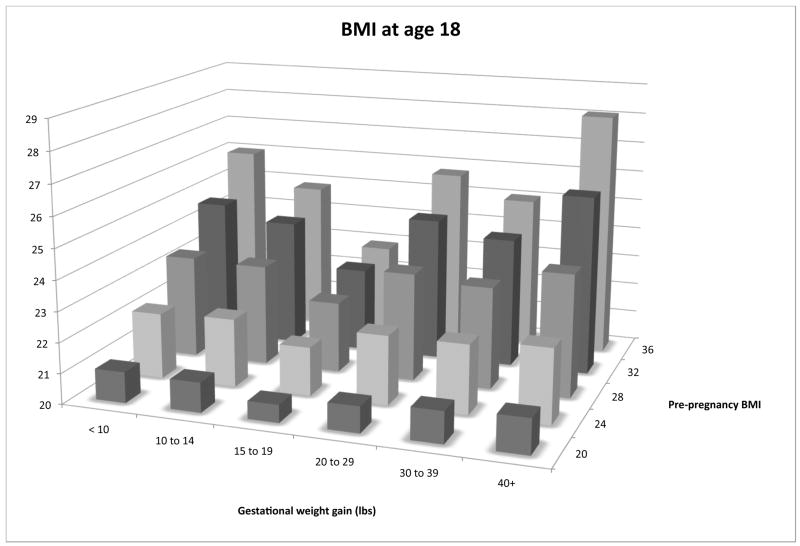
In multivariable linear regression models of BMI at age 18 and in 2001, we found significant interactions between maternal pre-pregnancy BMI and gestational weight gain category (partial F test p < 0.01 for interactions in both models). Daughters whose mothers gained 15 to 19 lbs had the lowest body mass index at age 18 and in 2001. Higher maternal pre-pregnancy body mass index was associated with a greater increase in the daughter’s body mass index with both low and high gestational weight gain (Figure 1).
Figure 1. Recalled maternal BMI, gestational weight gain, and predicted BMI1 in the Daughter.
1Predicted body mass index for first-born daughter of a non-smoker who experienced first trimester nausea, was of mean maternal age, has no history of diabetes, and was married to a father of mean BMI with no history of diabetes and a high-school education.
Discussion
In this cohort of 35,826 mother-daughter pairs, maternal recalled gestational weight gain above or below 15–19 lbs in the index pregnancy was associated with an increased risk of adolescent and adult obesity in the daughter. This association was modest among normal weight mothers and stronger among mothers with higher pre-pregnancy body mass indices. This association persisted with adjustment for parental BMI, maternal smoking during pregnancy, family history of diabetes, and socio-economic factors, as well as nurse’s age in 2001. Mothers with a high recalled pre-pregnancy BMI were more likely to have daughters with a high BMI at age 18 and in 2001. To our knowledge, this is the first study to document an association between maternal gestational weight gain, maternal pre-pregnancy weight and obesity in adult offspring–observations of acute relevance given the current obesity epidemic among childbearing women.
Our findings must be interpreted within the context of the study design. In this retrospective cohort study, data on pregnancy weight gain and associated exposures were collected 36 to 56 years after the daughter’s birth. It is likely that mothers were aware of their weight gain at the time of the index pregnancy, because obstetrical texts during this time period emphasized the importance of both measuring weight at each prenatal visit and limiting maternal gain to prevent pre-eclampsia and other pregnancy complications (16, 17). Random misclassification of recalled weight gain would likely bias our results toward the null, but differential misclassification is a concern. In a study of short-term recalled gestational weight gain, overweight or obese women were more likely to underreport weight at delivery. When self-reported delivery weight was used to calculate gestational weight gain, the resulting misclassification attenuated associations between weight gain and obstetrical outcomes (15). Therefore we undertook our sensitivity analysis; our results remained fairly robust, even when we assumed that 20 percent of overweight mothers had under-reported their gestational weight gain.
Recalled pre-pregnancy body mass index is also subject to measurement error. In our sensitivity analysis, we explored the effects of systematic under-reporting of pre-pregnancy BMI; we found that even underreporting of 2 kg/m2 for maternal pre-pregnancy BMI among heavier women would not materially alter the association between gestational weight gain and obesity in the daughter. Moreover, in our study, the nurse daughters were asked about their body weight independent of maternal data collection, and their recall interval was limited to age 18 years or to reporting current weight. Any misclassification of gestational weight gain or maternal BMI would likely be non-differential with respect to the daughter’s BMI. As a result, misclassification would underestimate, rather than overestimate, any true association.
Secular trends may limit the generalizability of our findings to a contemporary population. Of note, the lowest risk weight gain category, 15–19 pounds, was the “recommended” weight gain category during the era when these births occurred. Weight gain within these limits may be a marker for health-conscious behavior and as such, unmeasured confounders may have contributed to the association between weight gain and offspring obesity. Other changes in obstetrical practice limited our analysis. Because women were not routinely screened for gestational diabetes (GDM) during the study period, we could not determine the role of GDM in this cohort. However, approximately 50% of women with GDM go on to develop type 2 diabetes, and adjustment for maternal history of type 2 diabetes did not alter the observed associations. In addition, our study population is limited to mothers of registered nurses, which comprise a limited socio-economic stratum, and this may further limit our ability to generalize our results.
Our results confirm and extend earlier work on the association between in utero exposure and obesity in the offspring (5). Barker and colleagues (18, 19) have proposed the developmental origins hypothesis, describing the relation between low birth weight, catch-up growth, and adult cardiovascular disease. In epidemiologic studies, low birth weight is associated with higher odds of central adiposity (20, 21). Maternal undernutrition during pregnancy is similarly associated with obesity. Ravelli et al reported an increased obesity risk among both adolescents and middle-aged adults who were exposed in utero to nutritional deprivation during the Dutch Famine (22, 23). These data suggest that maternal nutritional constraint may increase the offspring’s risk of obesity.
At the other extreme, high birth weight is associated with higher body mass index in childhood (24), early adolescence (1) and adulthood (7, 25, 26). Birth weight is influenced by gestational weight gain (27), although this relationship appears to be modified by maternal pre-pregnancy body mass index (11). The relation between gestational weight gain and neonatal body composition also appears to vary by maternal BMI. Among normal weight women, Sewell et al (28) reported that maternal weight gain is associated with lean body mass, whereas among overweight women, gestational gain is associated with neonatal fat mass. These observations are consistent with our finding that the impact of gestational weight gain on offspring obesity is modified by maternal BMI.
Oken et al recently reported that high gestational weight gain is associated with increased risk of adiposity in children (29) and adolescents (30), after adjusting for maternal BMI and other pregnancy parameters. By contrast, Whitaker (3) did not observe a consistent association between quartiles of weight gain rate (total weight gain – birth weight/gestational age) and offspring’s adiposity at ages 2 to 4. No studies to our knowledge have related maternal gestational weight gain to adiposity risk of the offspring in adulthood.
Our findings linking maternal BMI to offspring obesity confirm and extend earlier studies of parental BMI and adult adiposity (31–33). Clearly, the biologic underpinning here may include genetic components, shared environment, as well as intrauterine metabolic programming, potentially through epigenetic mechanisms. Our observation that maternal BMI was more strongly associated than paternal BMI with obesity in the daughter suggests that the intrauterine environment acts synergistically with genetic factors to influence obesity risk. Lawlor et al reported a stronger effect of maternal BMI than paternal on offspring adiposity at age 14 (34), but two other studies have not confirmed a stronger maternal contribution to offspring obesity risk (35, 36). While our study did not provide the opportunity to separate these factors explicitly, our observations would encourage avoidance of obesity at the time of conception.
We found stronger associations between maternal adiposity and offspring obesity at age 18 than in adulthood. This difference likely reflects the greater time between exposure in utero and the outcome of measured weight. Other predictors of adiposity, including diet, physical activity, and parity, are likely to have influenced weight change between age 18 and 2001, and these differences would attenuate the strength of the association between maternal and offspring adiposity.
Our results may inform current debate regarding gestational weight gain recommendations. As obesity rates among childbearing women continue to rise, clinicians have questioned whether obese women should be advised not to gain any weight during pregnancy (37). Such advice may not be appropriate, given the increased adiposity in daughters that we observed among mothers with less than 15 lbs of weight gain. At the same time, we found that high gestational weight gain increases the odds of adiposity in the daughter, and this effect is greater with higher maternal BMI. This finding underscores the need for effective clinical interventions to prevent excessive weight gain during pregnancy and break the cycle of intergenerational obesity (27).
In conclusion, our data suggest that both constrained and excessive maternal weight gain during pregnancy, as well as a high pre-pregnancy BMI, are associated with adolescent and adult adiposity in the daughter. These associations are stronger when the mother was overweight prior to pregnancy, and they are independent of other parental and childhood risk factors. These findings suggest that maternal pre-pregnancy obesity and maternal weight gain during pregnancy may be modifiable risk factors for offspring metabolic disease.
Figure 2.
Table 5.
Predicted Odds Ratios for obesity and overweight in 2001 for participants of the Nurses’ Health Study II, by recalled pre-pregnancy BMI1 of the mother. All models adjusted for the age of the daughter in 2001.
| 21 | 23 | 25 | 27 | 29 | |
|---|---|---|---|---|---|
| Obesity of nurse daughter in 2001 (BMI=30+) | |||||
| OR and 95% CI | |||||
| Adjusted for age in 2001 | 1.0 (ref) | 1.41 (1.37–1.45) | 1.98 (1.87–2.09) | 2.75 (2.51–3.01) | 3.79 (3.29–4.36) |
| Adjusted for gestational weight gain | 1.0 (ref) | 1.41 (1.37–1.45) | 1.97 (1.87–2.08) | 2.72 (2.50–2.96) | 3.69 (3.24–4.21) |
| Covariate-adjusted2 | 1.0 (ref) | 1.42 (1.38–1.46) | 1.95 (1.85–2.06) | 2.62 (2.41–2.85) | 3.41 (3.01–3.87) |
| Covariate-adjusted2, including birth weight | 1.0 (ref) | 1.41 (1.37–1.45) | 1.93 (1.83–2.05) | 2.58 (2.36–2.82) | 3.34 (2.93–3.81) |
| Overweight (BMI 25 to < 30) | |||||
| OR and 95% CI | |||||
| Adjusted for age in 2001 | 1.0 (ref) | 1.22 (1.19–1.25) | 1.47 (1.40–1.54) | 1.74 (1.61–1.89) | 2.04 (1.78–2.34) |
| Adjusted for gestational weight gain | 1.0 (ref) | 1.22 (1.19–1.25) | 1.47 (1.40–1.53) | 1.73 (1.60–1.87) | 2.01 (1.76–2.29) |
| Covariate-adjusted2 | 1.0 (ref) | 1.23 (1.20–1.26) | 1.46 (1.39–1.53) | 1.70 (1.56–1.84) | 1.92 (1.68–2.19) |
| Covariate-adjusted2, including birth weight | 1.0 (ref) | 1.22 (1.19–1.26) | 1.46 (1.38–1.55) | 1.69 (1.53–1.86) | 1.91 (1.64–2.21) |
Maternal BMI modeled as maternal BMI + maternal BMI squared
Gestational weight gain category, maternal smoking during index pregnancy, nausea during pregnancy, birth order of daughter, maternal age at time of daughter’s birth (linear + age squared), maternal history of diabetes, paternal BMI, paternal history of diabetes, mother living with father at time of nurse’s birth, paternal level of education, nurse-daughter’s age in 2001
Acknowledgments
Financial Support
The Nurses’ Health Study II is supported by Public Health Service grant CA50385 from the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. The Nurses’ Mothers’ Cohort Study was funded by the Intramural Research Program of the National Cancer Institute (to MRF) – Research Contract N02-RC-17027 from the National Cancer Institute, and by P.O. 263 MQ 411027 from the National Cancer Institute (to KBM).
Role of the funding source
One of the study authors (MRF) was a member of the Intramural Program at The National Cancer Institute at the time the Nurses’ Mothers’ Cohort study was designed and the data were collected. For this manuscript, the National Cancer Institute had no involvement in the analysis and interpretation of the data, or in the preparation, review, or approval of the manuscript. The authors had full access to the data for the study.
Footnotes
Preliminary findings were presented at the Society for Maternal Fetal Medicine, San Francisco, California, February 2007.
References
- 1.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal Gestational Diabetes, Birth Weight, and Adolescent Obesity. Pediatrics. 2003;111:e221–226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- 2.Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Annals of Internal Medicine. 1999;130:278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29–36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 4.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic Syndrome in Childhood: Association With Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics. 2005;115:e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 5.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 6.Mcmillen IC, Robinson JS. Developmental Origins of the Metabolic Syndrome: Prediction, Plasticity, and Programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 7.Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94:1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- 8.Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab. 2008;22:155–171. doi: 10.1016/j.beem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine. Nutrition during pregnancy. National Academies Press; Washington, DC: 1990. [Google Scholar]
- 10.National Research Council and Institute of Medicine. Workshop Report. The National Academies Press; Washington, DC: 2007. Influence of Pregnancy Weight on Maternal and Child Health. [Google Scholar]
- 11.Abrams BF, Laros RK., Jr Prepregnancy weight, weight gain, and birth weight. Am J Obstet Gynecol. 1986;154:503–509. doi: 10.1016/0002-9378(86)90591-0. [DOI] [PubMed] [Google Scholar]
- 12.Olson C, Strawderman M, Reed R. Efficacy of an intervention to prevent excessive gestational weight gain. American Journal of Obstetrics & Gynecology. 2004;191:530–536. doi: 10.1016/j.ajog.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Cogswell M, Scanlon K, Fein S, Schieve L. Medically advised, mother’s personal target, and actual weight gain during pregnancy. Obstetrics & Gynecology. 1999;94:616–622. doi: 10.1016/s0029-7844(99)00375-0. [DOI] [PubMed] [Google Scholar]
- 14.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- 15.Schieve L, Perry G, Cogswell M, Scanion K, Rosenberg D, Carmichael S, et al. Validity of self-reported pregnancy delivery weight: an analysis of the 1988 National Maternal and Infant Health Survey. NMIHS Collaborative Working Group. American Journal of Epidemiology. 1999;150:947–956. doi: 10.1093/oxfordjournals.aje.a010103. [DOI] [PubMed] [Google Scholar]
- 16.Lull CB, Kimbrough RA. Clinical Obstetrics. J.B. Lippicott Company; Philadelphia, PA: 1953. [Google Scholar]
- 17.Eastman NJ, Hellman LM. Williams Obstetrics. 12. Appleton-Century-Crofts, Inc; New York: 1961. [Google Scholar]
- 18.Barker D, Eriksson J, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. International Journal of Epidemiology. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 19.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okosun IS, Liao Y, Rotimi CN, Dever GE, Cooper RS. Impact of birth weight on ethnic variations in subcutaneous and central adiposity in American children aged 5–11 years. A study from the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord. 2000;24:479–484. doi: 10.1038/sj.ijo.0801182. [DOI] [PubMed] [Google Scholar]
- 21.Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ. Early growth and abdominal fatness in adult life. J Epidemiol Community Health. 1992;46:184–186. doi: 10.1136/jech.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 23.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 24.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes (Lond) 2006;30:610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen HT, Sabroe S, Rothman KJ, Gillman M, Fischer P, Sorensen TI. Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ. 1997;315:1137. doi: 10.1136/bmj.315.7116.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth Weight and Adult Hypertension, Diabetes Mellitus, and Obesity in US Men. Circulation. 1996;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 27.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 28.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322, e321–328. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol. 2008;112:999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terry MB, Wei Y, Esserman D. Maternal, Birth, and Early-Life Influences on Adult Body Size in Women. Am J Epidemiol. 2007;166:5–13. doi: 10.1093/aje/kwm094. [DOI] [PubMed] [Google Scholar]
- 32.Stettler N, Tershakovec AM, Zemel BS, Leonard MB, Boston RC, Katz SH, et al. Early risk factors for increased adiposity: a cohort study of African American subjects followed from birth to young adulthood. Am J Clin Nutr. 2000;72:378–383. doi: 10.1093/ajcn/72.2.378. [DOI] [PubMed] [Google Scholar]
- 33.Laitinen J, Power C, Jarvelin M-R. Family social class, maternal body mass index, childhood body mass index, and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74:287–294. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- 34.Lawlor DA, Smith GD, O’Callaghan M, Alati R, Mamun AA, Williams GM, et al. Epidemiologic Evidence for the Fetal Overnutrition Hypothesis: Findings from the Mater-University Study of Pregnancy and Its Outcomes. Am J Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 35.Kivimaki M, Lawlor DA, Smith GD, Elovainio M, Jokela M, Keltikangas-Jarvinen L, et al. Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am J Clin Nutr. 2007;86:1509–1514. doi: 10.1093/ajcn/86.5.1509. [DOI] [PubMed] [Google Scholar]
- 36.Davey Smith G, Steer C, Leary S, Ness A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC) Arch Dis Child. 2007;92:876–880. doi: 10.1136/adc.2006.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artal R, Catanzaro RB, Gavard JA, Mostello DJ, Friganza JC. A lifestyle intervention of weight-gain restriction: diet and exercise in obese women with gestational diabetes mellitus. Appl Physiol Nutr Metab. 2007;32:596–601. doi: 10.1139/H07-024. [DOI] [PubMed] [Google Scholar]