Abstract
Glial fibrillary acidic protein (GFAP) is the major intermediate filament protein of astrocytes, and its expression changes dramatically during development and following injury. To facilitate study of the regulation of GFAP expression, we have generated dual transgenic mice expressing both firefly luciferase under the control of a 2.2 kb human GFAP promoter and Renilla luciferase under the control of a 0.5 kb human GAPDH promoter for normalization of the GFAP signal. The GFAP-fLuc was highly expressed in brain compared to other tissues, and was limited to astrocytes, whereas the GAPDH-RLuc was more widely expressed. Normalization of the GFAP signal to the GAPDH signal reduced the inter-individual variability compared to using the GFAP signal alone. The GFAP/GAPDH ratio correctly reflected the up-regulation of GFAP that occurs following retinal degeneration in FVB/N mice due to the rd mutation. Following kainic-acid induced seizures, changes in the GFAP/GAPDH ratio precede those in total GFAP protein. In knock-in mice expressing the R236H Alexander disease mutant, GFAP promoter activity is only transiently elevated and may not entirely account for the accumulation of GFAP protein that takes place.
INTRODUCTION
GFAP is the major intermediate filament protein in astrocytes of the vertebrate central nervous system (Eng et al. 2000). Its expression is tightly regulated both during development and following injury. Increased expression of GFAP is also one of the hallmark features of gliosis, the consistent reactive response of astrocytes to injury or dysfunction in surrounding tissues or cell types (Eng and Ghirnikar 1994). What function GFAP provides to astrocytes is not well understood. However, dominant mutations in the coding region of GFAP account for nearly all cases of Alexander disease, a fatal neurogenerative disorder that usually presents as a leukodystrophy of childhood, with symptoms such as seizures, spasticity, and developmental delays (Brenner et al. 2001;Li et al. 2005). The GFAP mutations in Alexander disease appear to act in a gain-of-function fashion, and it is likely that elevated levels of GFAP, apart from expression of mutant protein per se, is central to the pathogenesis of this disorder (Hagemann et al. 2006).
Transgenic animals expressing reporter genes under the control of cell-specific promoters greatly facilitate the study of transcriptional regulation of gene expression in vivo (Cui et al. 1994). Early generation reporters included genes such as herpes thymidine kinase and human growth hormone, selected largely based on the ease with which their expression could be measured at the molecular or physiological levels. Transgenic models employing GFAP regulatory elements utilized second generation reporters that were uniquely valuable for morphological studies, such as the bacterial lacZ or jellyfish green fluorescent protein genes (Mucke et al. 1991;Brenner et al. 1994;Zhuo et al. 1997).
More recently, luciferase reporter genes have been developed that offer marked improvements over previous reporters in terms of sensitivity and dynamic range (Contag and Bachmann 2002). Zhu et al. (2004) have created transgenic mice expressing firefly luciferase under the control of the mouse GFAP promoter for the purpose of monitoring astrocyte response to injury in vivo. One can also imagine using transgenic animals as a source of cells for primary cultures. In applications of such cultures for high-throughput screening in drug discovery, a simple means for normalizing the luciferase signal would be highly desirable (Fan and Wood 2007). The availability of multiple luciferases with distinct substrate specificities allows one to introduce two transgenes at once, whereby the signal from one luciferase that is under the control of a cell-specific promoter can be normalized to the signal from a second luciferase that is under the control of a housekeeping promoter (Ullah et al. 2006). We now report the development of dual luciferase transgenic mice, using the human GFAP and GAPDH promoters to control expression of firefly and Renilla luciferase, respectively, with a goal towards creating better tools for studies of GFAP regulation both in vivo and in vitro.
METHODS
Construction of transgenes
To construct a transgene expressing firefly luciferase regulated by the GFAP promoter (GFAP-fLuc), we utilized the 2.2 kb human GFAP promoter derived from the GFAP-lacZ transgene previously described by Brenner et al. (1994). The GFAP promoter fragment (-2163 to +47) was subcloned into the Bgl II site in the pGL-3 basic vector (Promega) containing the firefly luciferase gene. In parallel, a transgene expressing the Renilla luciferase regulated by the human GAPDH promoter (Alexander et al. 1988) was prepared (GAPDH-RLuc), using a 0.5 kb fragment (-488 to +21) isolated from genomic DNA obtained from a human blood sample. Oligonucleotide primers were designed to generate XhoI and EcoRI restriction sites in the GAPDH PCR fragment (527 bp) that was amplified from the genomic DNA. The fragment was subcloned into XhoI and EcoRI restriction sites in the phRL-null vector (Promega) containing the Renilla luciferase gene. Both transgenes were sequenced to confirm correct orientation and sequence. Transgene fragments were isolated free of plasmid sequence and purified for microinjection using the Gene Clean Turbo kit (Q-Biogene).
Generation of dual transgenic reporter mice
The two transgenes were co-injected at equimolar ratios into the pronuclei of fertilized eggs on either FVB/N or FB6F1 hybrid backgrounds, using a combined concentration of 3 ng/ul. Genotyping was performed by PCR on genomic DNA isolated from tail biopsies at weaning, using the following conditions: one cycle at 95°C 3min, 32 cycles of 95°C 40s, 58°C 30s, 72°C 1min and a final extension step at 72°C for 5 min. The pr imers used for detection of the GFAP-fLuc transgene were 5’-TCTCTAAGGAAGTCGGGGAAGC-3’ (forward) and 5’-CAGCGGGAGCCACCTGATAGCCTT-3’ (reverse), and for detection of the GAPDH-RLuc transgene were 5’-CTCCCATCGGGCCAATCTCAGTCC– 3’ (forward) and 5’-GCGTTTGCGTTGCTCGGGGTCGTA-3’ (reverse). The primer locations in each transgene are shown in Figure 1A, B. The predicted sizes for the PCR fragments are 403 bp for the GFAP-fLuc transgene, and 551 for the GAPDH-RLuc transgene. Nine founder mice were identified that contained both transgenes, and were mated to FVB/N mice to establish lines. Two of the lines were used for the studies reported here, one derived from an FVB/N egg (Tg172-1) and one from an FB6F1 egg (Tg172-9). Transmission analysis from the founders to their offspring confirmed that the two genes segregated together and behaved as a single genetic locus. The Tg172-9 line of mice has been donated to the Jackson Laboratory for unrestricted distribution (stock #JR9638).
Figure 1.
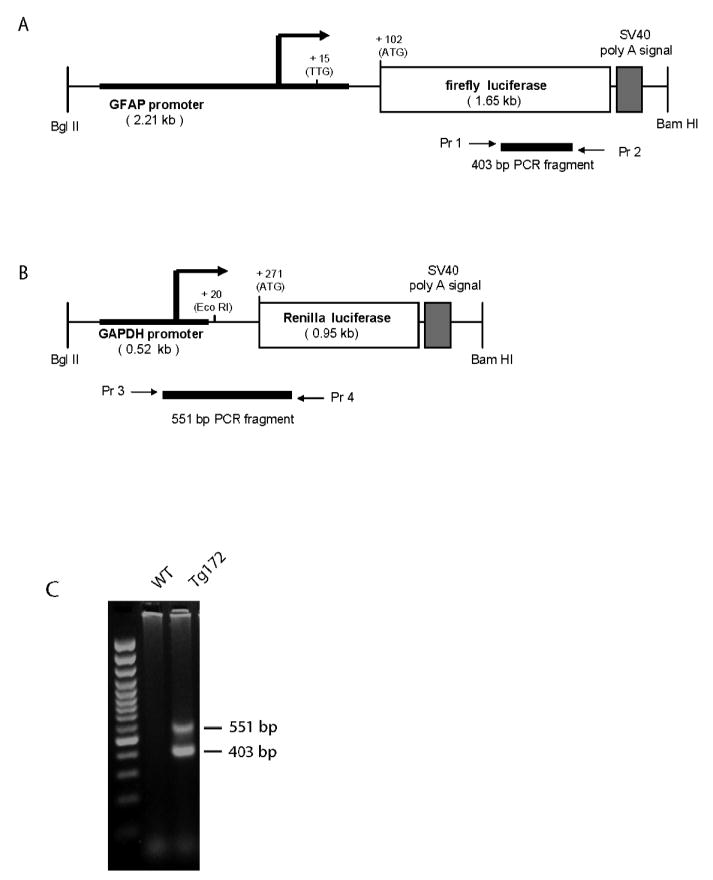
Transgene constructs and PCR genotyping. A, B) The GFAP and GAPDH promoters (bold) were fused with the coding sequences for firefly luciferase and Renilla luciferase, respectively. The locations of the primers used for genotyping are indicated (Pr 1-2 for the GFAP-fLuc and Pr 3-4 for GAPDH-RLuc). C) Genotyping by multiplex PCR for both transgenes in a dual transgenic mouse indicated by the presence of the 403 bp (GFAP-fLuc) and the 551 bp (GAPDH-RLuc) bands. Left lane, 100 bp DNA ladder; middle lane, non-transgenic mouse; right lane, bi-transgenic mouse.
Luciferase reporter expression assay
The dual luciferase reporter assay was performed according to the manufacturer’s recommendations (E1910, Promega), with minor modifications for use on extracts prepared from mouse tissues. The tissues were homogenized in the passive lysis buffer and spun at 17,500 g for 20 min at 4°C. An equal volume of the reagent including the substrate for firefly luciferase was added to 50-100 μg protein in 50 μl supernatant, and the signal was measured using a GloRunner Microplate Luminometer (Tuner Biosystems). Subsequently, the substrate for Renilla luciferase was mixed with the signal quenching solution in a 1:50 (v/v) ratio and 50 μl of this reconstituted reagent was added to each well. The luminescent signal by Renilla luciferase was measured immediately.
Immunofluorescence Microscopy
Frozen sections of brain (50 μm) were treated with blocking solution (10% donkey serum, 0.4% Triton-X 100, 0.02% Na-Azide in PBS) for 1 hr at RT. Sections were incubated with anti-firefly luciferase (Luc-17) mouse monoclonal antibody (1:100, Abcam) and anti-GFAP rabbit polyclonal antiserum (1:1000, DAKO) for 48 hrs at 4 °C. The sections were rinsed three times in PBS and incubated with Alexa-488 and Alexa-594 secondary antibodies for 1 hr at RT. The sections were mounted on slides using VectaShield Mounting Medium (Vector Labs). Immunofluorescent images were obtained from a Nikon C1 confocal microscope at the Waisman Center’s Cellular and Molecular Neuroscience Core facility.
ELISA for GFAP protein quantitation
Brains were divided in half down the midline and each sample homogenized in 2 ml lysis buffer containing 2% SDS, 50 mM Tris-HCl, 5 mM EDTA, pH 7.4, along with 1 mM PMSF and Complete Proteinase Inhibitor Cocktail (cat. #11836145001, Roche). The lysate was boiled for 30-40 minutes and the protein extract was prepared for GFAP quantification using a sandwich ELISA as previously described (Hagemann et al. 2006). The capture antibodies were the SMI-26 mouse anti-GFAP monoclonal cocktail (Covance Research Products), and the detection antibody was a polyclonal rabbit anti-GFAP (DAKO).
Kainic acid-induced seizures
Kainic acid (A.G. Scientific, San Diego) was administered intraperitoneally (15 mg/kg) to male mice from the Tg172-9 line at 10-14 weeks (n = 20). Control mice (n = 4) were from the same transgenic line but injected with vehicle only (PBS). Seizures were evaluated at 5 minute intervals over a 2 hr period based on a clinical scoring system modified from that of Racine (1972): 0, normal; 1, immobile; 2, rigidity/tail extension; 3, repetitive motion/forelimb tremor; 4, rearing/falling; 5, multiple episodes of category 4 within 5 minute period; 6, tonic seizure; 7, death.
RESULTS
Generation of transgenic mice expressing Dual-Glo reporters
To generate Dual-Glo transgenic mice for the purpose of monitoring expression of GFAP, two transgenes were constructed separately, with the firefly luciferase placed under the control of the human GFAP promoter and the Renilla luciferase placed under the control of the human GAPDH promoter (Figure 1A, B). The transgenes were co-injected into the pronuclei of fertilized eggs, and integration was detected by observation of the predicted fragments from PCR of tail DNA (Figure 1C). Nine founders were identified that carried both transgenes, and lines were established from 7 of these founders. Firefly luciferase activity was measured in brains taken from mice of the F1 generation. Five of the lines exhibited relatively high levels of expression for both transgenes (Figure 2). Subsequent studies reported here utilized mice from the Tg172-1and Tg172-9 lines.
Figure 2.
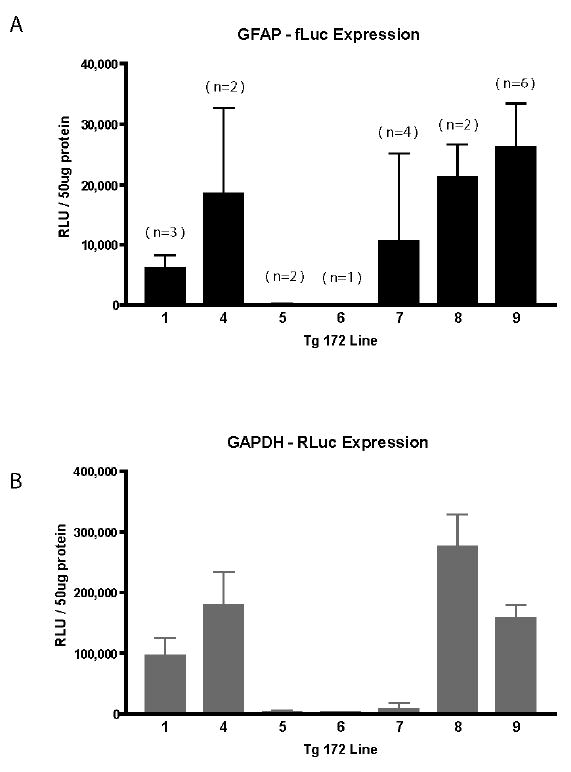
GFAP-fLuc expression in brain for dual transgenic lines. A, B) Transgenic founders were identified by PCR and expression evaluated in brains of F1 offspring at 6 weeks of age (both sexes). Bars denote the mean and SD of multiple animals in each line (n for each group shown in parentheses above each bar). No dual transgenic offspring were obtained from the Tg172.2 and Tg172.3 founders. RLU: relative light unit
Tissue and cell-specificity of reporter gene activity
To investigate tissue specificity of expression of both transgenes, firefly and Renilla luciferase activities were measured in the same samples from mice of the Tg172-9 line. For the GFAP-fLuc transgene, activity was highest in brain, very low but detectable in heart, and undetectable in kidney, muscle, lung, and liver (Figure 3A). In contrast, the GAPDH-RLuc transgene exhibited comparable high levels of activity in brain and heart, and low but detectable levels in the other tissues (Figure 3B). Non-transgenic littermates yield values that are typically < 35 in raw units (or < 1% of the transgenic signal)(data not shown). These results indicate that the GFAP promoter is relatively brain-specific, and that the GAPDH promoter is variable but active in multiple tissues.
Figure 3.
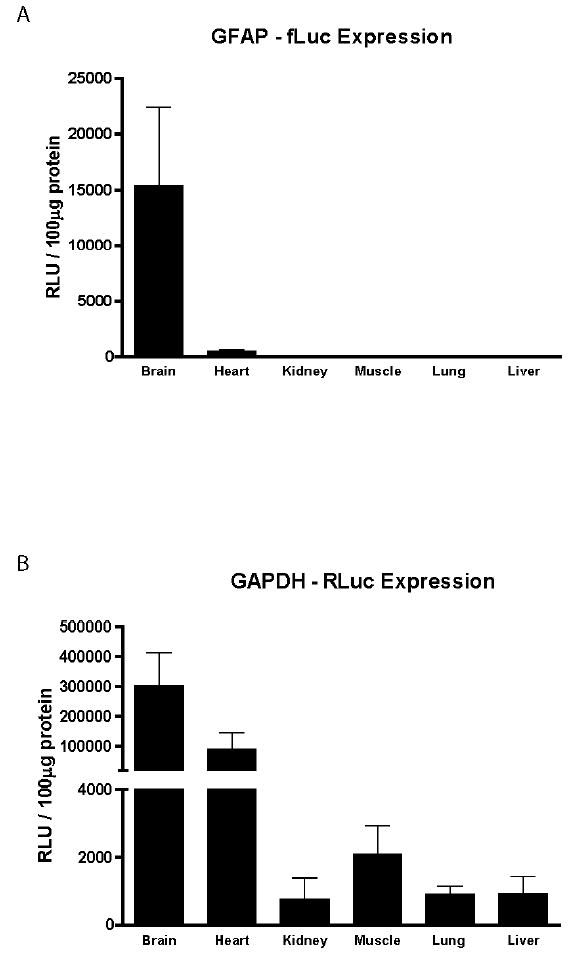
Tissue-specific expression of GFAP and GAPDH reporter transgenes. A) Firefly luciferase and B) Renilla luciferase activities were sequentially measured in the same samples. Tissues were obtained from male mice of the Tg172-9 line at 6 weeks of age. Each bar represents the mean and SD (n=8). RLU: relative light unit
To determine whether the brain-specificity of GFAP-fLuc reflects expression in astrocytes, we attempted immunofluorescent localization of luciferase protein. The level of expression in un-injured mice was below the limit of detection by this method (data not shown). However, promoter activity is increased following exposure to the convulsant kainic acid (see below). Firefly luciferase protein becomes readily detectable under these conditions, and displays extensive co-localization with GFAP (Figure 4).
Figure 4.
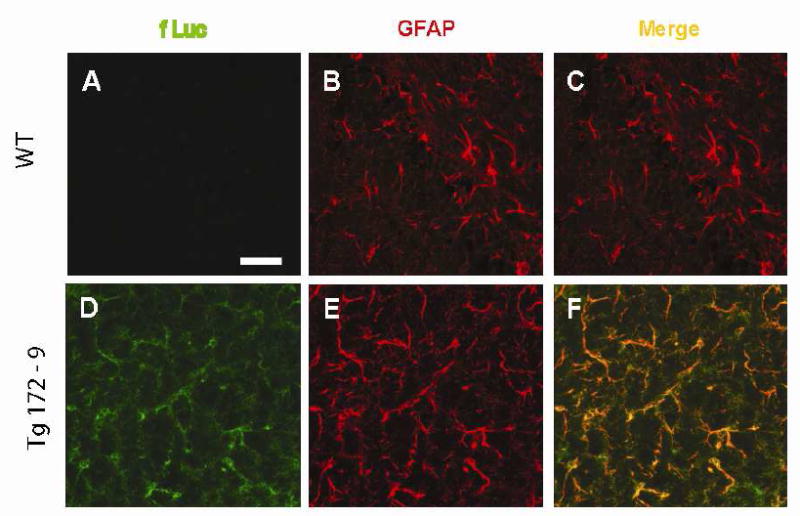
Cell-specific expression of GFAP-fLuc transgene. Co-localization of fLuc and GFAP was performed in sections of hippocampus (CA3 region) obtained from male Tg172-9 mice 6 hr after exposure to kainic acid (15 mg/kg). A-C) non-transgenic controls. D-F) Tg172-9. A,D) fLuc; B,E) GFAP; C,F) merged images. Scale bars = 50 μm.
Normalization of GFAP to GAPDH signal reduces measurement variability
Initial measurements of firefly luciferase activities in brain revealed significant inter-individual variability (see Figure 3A). Co-integration and co-expression of the firefly and Renilla luciferase transgenes allowed us to test whether normalization of the GFAP signal to the corresponding GAPDH signal in the same sample would reduce this variability. We performed sequential measurements of the two luciferases in brains of mice from both the Tg172-1 and Tg172-9 lines. The individual values for each animal are shown for the GFAP-fLuc (Figure 5A) and GAPDH-RLuc (Figure 5B) activities. Normalization of the GFAP signal to the GAPDH signal dramatically reduced the variability (Figure 5C). To further assess the improvement in precision offered by this normalization, we calculated means, standard deviations, and coefficients of variation before and after normalization (Table 1). The coefficients of variation were reduced approximately 3-fold in both transgenic lines by normalization.
Figure 5.
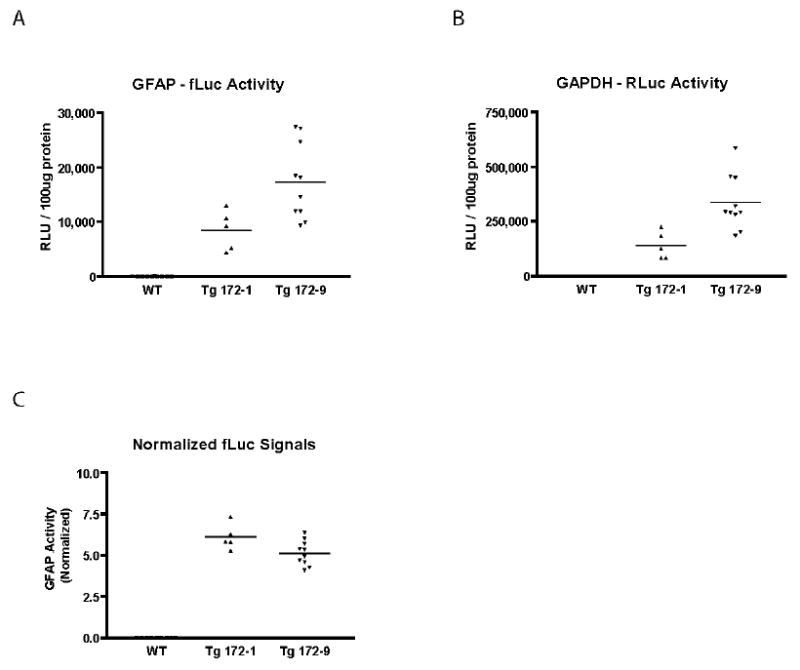
Measurement variability of GFAP promoter activity after normalization to GAPDH. A, B) Raw measurements of firefly and Renilla luciferase activities in the same brain samples from male Tg172-1 and Tg172-9 mice at 6 weeks of age. The signal in non-transgenic animals was indistinguishable from background. C) GFAP-fLuc signals in brains from Tg172-1 and Tg172-9 mice after normalization to GAPDH-RLuc signal (expressed as [fLuc/RLuc] × 100). RLU: relative light unit
Table 1.
Measurement variability before and after normalization of firefly luciferase (fLuc) activity to Renilla luciferase activity. Dual-Glo reporter assay was performed on brains of male mice, 6 weeks old, from two transgenic lines. CU: coefficient of variation, σ: standard deviation, μ: mean.
| transgenic line | Tg172-1 (n = 5) | Tg172-9 (n = 10) | |
|---|---|---|---|
| fLuc signal – raw | μ | 8435 | 17300 |
| σ | 3640 | 7004 | |
| CU | 0.43 | 0.4 | |
| fLuc signal - normalized | μ | 6.1 | 5.14 |
| σ | 0.77 | 0.75 | |
| CU | 0.13 | 0.15 |
GFAP-fLuc reporter responds to rd-induced gliosis
To determine whether the GFAP-fLuc transgene is responsive to a genetically-induced stimulus for gliosis, we took advantage of the fact that the FVB/N strain which was used for generating the lines is homozygous for the rd mutation. This mutation induces post-natal loss of photoreceptors in the retina and up-regulation of GFAP in Muller cells (Sarthy and Fu 1989). Simple F1 crosses to strains such as C57BL/6J will introduce one wild type allele at the rd locus and preserve normal retinal architecture, thus preventing gliosis. We therefore compared luciferase activities in mice carrying the transgenes in either the parent FVB/N background vs. FB6F1 hybrids. Previously the same strain comparison was used to document responsiveness of a GFAP-GFP transgene to genetic injury (Zhuo et al. 1997).
Retinal GFAP promoter activity in FVB mice was elevated compared to their F1 counterparts, before and after normalization (Figure 6A and C). Similar results were found in males (data not shown). To determine whether this difference is specific to the retina, we also measured GFAP promoter activity in samples of brain from mice in the two genetic backgrounds. The signal in brains of FVB mice was indeed increased compared to the F1 hybrids (1.4-fold), but not to the same degree as found in the retina. Interestingly, GFAP protein levels in whole brain are also elevated 1.7-fold in FVB mice compared to F1 hybrids (data not shown). Considering the GAPDH promoter alone, we found a 6-fold difference in the levels of Renilla luciferase activity in brain between FVB/N and FB6F1 mice (Figure 6B), but the basis for this difference is not known. However, the GAPDH promoter activity was not increased in response to photoreceptor degeneration (Figure 6B).
Figure 6.
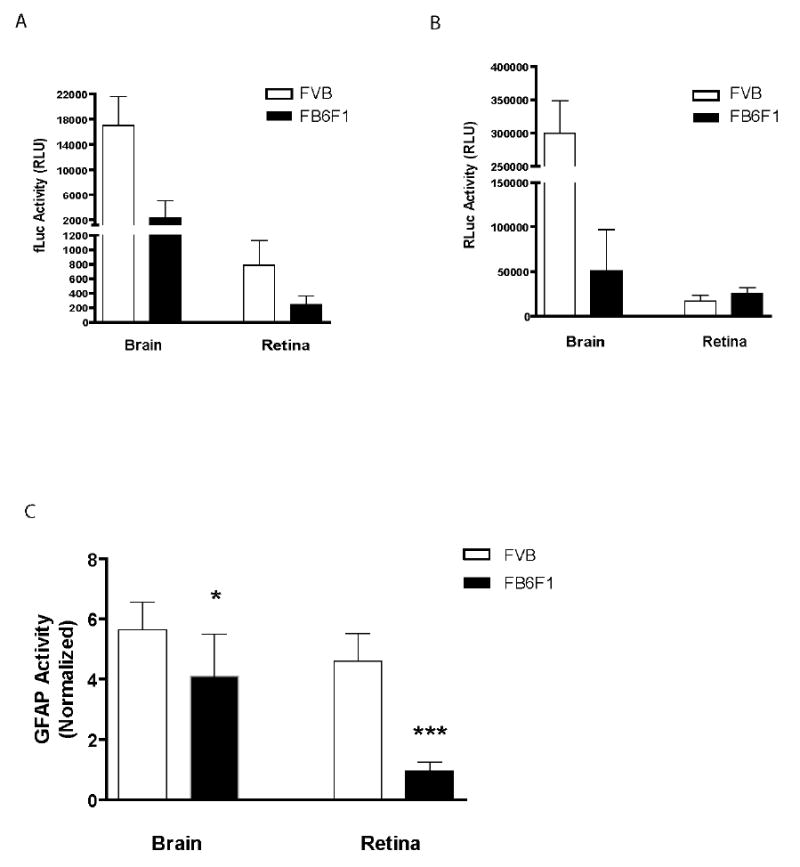
GFAP expression in brain and retina as a function of genetic background and gliosis. A) GFAP-fLuc and B) GAPDH-RLuc activity were measured in brains and retinae from 6 week old female FVB/N (n=10) and FB6F1 (n=7) mice. C) GFAP-f Luc signal normalized to the GAPDH-RLuc signal. GFAP promoter activity in retina (after normalization) is increased 4.9-fold in FVB/N mice (with gliosis) compared to FB6F1 mice (without gliosis). GFAP promoter activity is also slightly increased in brains of FVB/N mice. GAPDH promoter activity is not increased in response to gliosis. Bars represent the mean and SD (* p<0.05, *** p<0.001, unpaired t-test). RLU: relative light unit
GFAP-fLuc reporter increase precedes protein increase in seizure-induced gliosis
To determine whether the GFAP-fLuc transgene is responsive to chemical injury of the brain, and the temporal relation between changes in promoter activity and protein levels, we utilized kainic acid-induced seizures as a means to acutely activate astrocytes in the hippocampus. Adult mice from the Tg172-9 line were injected IP with kainic acid to induce seizures, and then luciferase activities and GFAP protein levels were measured during the subsequent two day period. Mice reliably began to show clinical signs of seizure activity at 10-15 min. after injection, reached maximum clinical scores at 75 min. post-injection, and then gradually recovered (Figure 7A). As expected, kainic acid injection resulted in increases in both GFAP promoter activity (normalized to the GAPDH signal) and GFAP protein levels (Figure 7B, one-way ANOVA, p<0.001). To evaluate the time course of these changes, we used t-tests as post-hoc assessments of promoter activity or protein levels at each time point in reference to the values at the 0 hr. time point. The GFAP promoter activity was significantly increased at 6 hrs after injection (9.86 ± 1.14 vs 6.39 ± 0.86, unpaired t-test, p<0.01) and then declined to baseline levels by 24 hrs. In contrast, GFAP protein accumulated more slowly, reaching a significant increase at 24 hours (762.3 ± 72.4 ng, unpaired t-test, p<0.001) and remaining elevated at 48 hours (535.8 ± 39.1 ng, unpaired t-test, p<0.01), compared to initial levels (289.1 ± 13.5 ng)(Figure 7B). Again, the GAPDH promoter activity showed no significant change at any of the time points measured (Figure 7C). These results indicate that the GFAP-fLuc transgene is responsive to chemically-induced gliosis, and correctly anticipates subsequent changes in protein levels.
Figure 7.
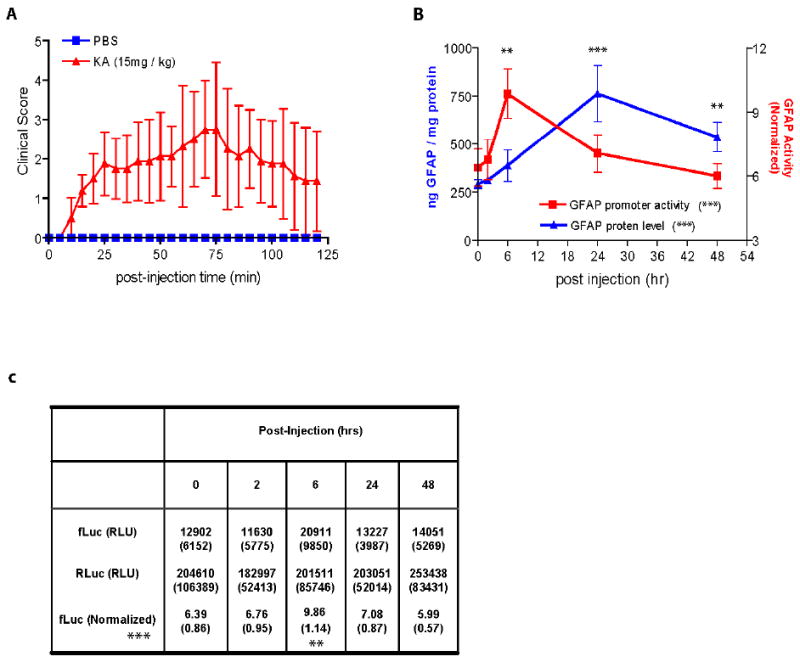
GFAP expression in brain following kainic acid-induced seizures. A) Time course of seizures following IP injection of kainic acid in 10-14 week-old male mice from the Tg172-9 line (n = 20). Controls were transgenics injected with a similar volume of PBS (n = 4). Error bars denote ± 1 SD. B) Time-course of changes in GFAP protein levels (measured by ELISA, left Y-axis) and GFAP promoter activity (measured by Dual-Glo, right Y-axis) following kainic acid-induced seizures. Brains were collected at various time points post-injection (0, 2, 6, 24, 48 hrs, n = 4 at each time point). Error bars denote 1 ± SD. Both protein levels and promoter activity changed over time (one-way ANOVA, *** p<0.001). Promoter activity was significantly increased at 6 hours post-injection, whereas protein levels were increased at 24-48 hours post-injection (post-hoc t-tests of each point in relation to the initial values at time 0, ** p<0.01 *** p<0.001). C) Mean and SD (in parentheses) were calculated for the raw fLuc and RLuc signals from all of the mice in each group (top two rows). For each mouse the fLuc value was then normalized to the RLuc value as described in Methods, and the mean and SD for these normalized values were calculated (bottom row). GFAP promoter increased after kainic acid injection (one-way ANOVA, *** p<0.001), but subsequent analysis of each time point in relation to the initial value revealed a significant change only at the 6 hr. time point, after normalization (unpaired t-test, ** p<0.01). RLU: relative light unit
GFAP-fLuc reporter indicates transient increase in promoter activity in R236H/+ GFAP mutant mice
Heterozygous mutations in the coding region of GFAP cause Alexander disease, yet a key factor in pathogenesis is thought to be elevated levels of the GFAP protein itself (Quinlan et al. 2007). To determine whether activation of transcription at the level of the GFAP promoter contributes to this increase, we crossed the Tg172-9 mice with a mouse strain that carries a common Alexander disease-associated mutation as a knock-in at the endogenous Gfap locus (R236H, equivalent to the human R239H mutation). Previous studies of the R236H mice have shown 6-fold elevations of GFAP mRNA, and 3.7-fold elevations of GFAP protein, at 8 weeks of age (Hagemann et al. 2006). However, as shown in Figure 8, the GFAP promoter activity (normalized again to the GAPDH signal) was only modestly increased in 2-3 week-old mice, and declined to normal levels by 4 weeks. These results suggest that promoter activation only partially accounts for the excessive accumulation of GFAP that occurs in these mice, and that other post-transcriptional mechanisms also play a role.
Figure 8.
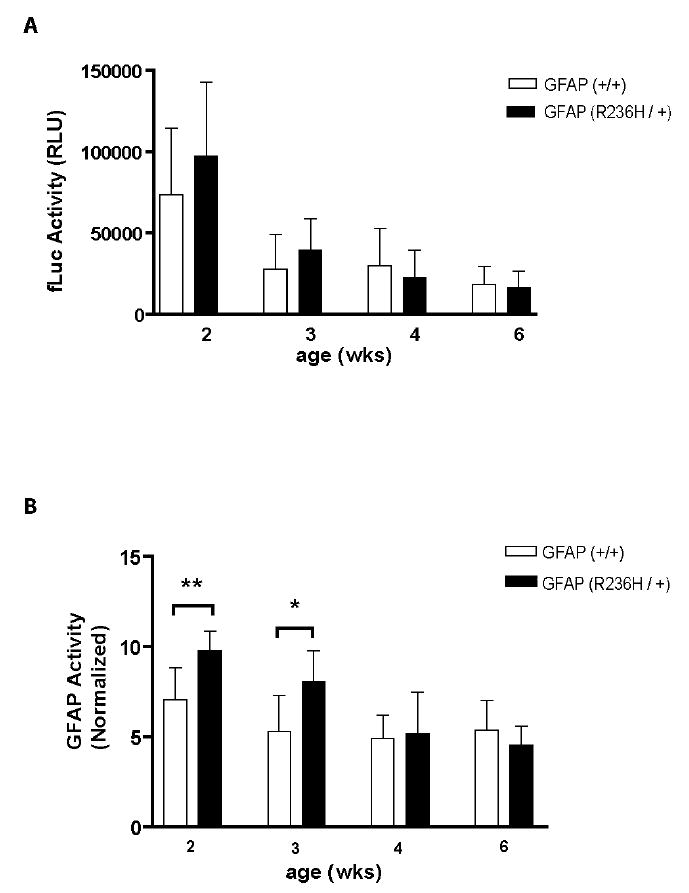
GFAP promoter up-regulation in R236H/+ GFAP mutant mice. Tg172-9 mice (FVB background) were crossed to heterozygous R236H mice (129S6 background), and extracts prepared from brains of F1 female offspring at 2-6 weeks for the dual-luciferase reporter assay. A) GFAP promoter activity, expressed as raw values of the fLuc signal. B) GFAP promoter activity, expressed after normalization to the GAPDH-RLuc signal. Statistical significance was evaluated at each age comparing mice with or without the R236H mutation (* p<0.05 ** p<0.01, unpaired t-test). Error bars denote 1 SD (n = 5–10 mice). RLU: relative light unit
DISCUSSION
We describe generation of transgenic mice expressing both firefly and Renilla luciferase reporter genes under the control of the astrocyte-specific GFAP and housekeeping GAPDH promoters, respectively. The dual reporter assay in these mice reduces inter-animal variability and correctly reflects the direction and time course of gliosis-related changes in activity of the GFAP promoter. Previously, Zhu et al. (2004) described the generation of a transgenic line expressing just the firefly luciferase under the control of the mouse GFAP promoter. This line has subsequently been used for studying changes in GFAP promoter activity in live animals following injection of kainic acid (Zhu et al. 2004), Streptococcus pneumoniae infection (Kadurugamuwa et al. 2005), ischemia (Cordeau et al. 2008), and EAE (Luo et al. 2008). However, direct comparisons between the mouse of Zhu et al. (2004) and the ones reported here are difficult due to differences in methods of injury and quantitation of the reporter.
In principle, expressing Renilla luciferase under the control of a housekeeping promoter in the Dual-Glo assay should prove especially valuable in the context of assays where significant changes in global gene expression of astrocytes may take place. In the case of screening for compounds that reduce expression of GFAP, focusing on changes in the firefly luciferase signal alone might be mis-interpreted when non-specific toxicity causes down-regulation of many genes and perhaps even cell death. Unfortunately, there is considerable disagreement over which RNA/protein serves as the best internal control to use in normalizing expression data (Suzuki et al. 2000). In addition, while housekeeping promoters are widely used in cell culture to achieve uniform and high-level expression, they have proved problematic in transgenic animals with highly variable patterns of expression (Kisseberth et al. 1999). One luciferase transgenic line has been made utilizing a CMV-chicken β-actin promoter, but was noted to express the reporter only in a selected group of tissues that did not include brain (Cao et al. 2005). We chose the GAPDH promoter for control of the Renilla luciferase based on its frequent use in normalizing expression data, and recent studies indicate its utility for studies of chronic neurodegenerative disease in the mouse (Calvo et al. 2008). We utilized a relatively short fragment (~0.5 kb) of the human GAPDH promoter to control expression of Renilla luciferase, and found considerable variation between tissues, with brain expression being the highest. However, this variability is unlikely to be due to the length of the promoter, as non-uniform expression was also found in a mouse expressing firefly luciferase under the control of an 11.8 kb fragment from the human gene (Xenogen, unpublished observations). With the exception of skeletal muscle, our comparison of multiple tissues displayed a similar ranking to that found in a survey of GAPDH mRNA levels in human (Barber et al. 2005).
We employed three distinct injury models by which to induce gliosis in astrocytes and/or retinal Muller cells and cause up-regulation of GFAP expression. While the dual luciferase reporter mice described here detect increased levels of GFAP promoter activity in all three models, it is important to consider more closely how faithfully they report the precise time courses and levels of induction. For instance, we found using kainic acid that GFAP promoter activity was significantly increased at 6 hours after injection, but had declined to normal levels by 24 hours. Mucke et al. (1991), using lacZ as a reporter, also found rapid induction of the GFAP promoter following a stab wound injury. However, two other studies of kainic acid injury in mice found significant elevations of GFAP mRNA, and in one case GFAP driven luciferase activity, with slower and more sustained time courses (Kaasinen et al. 2000;Zhu et al. 2004), although the doses were also higher and genetic background may play a role. Secondly, the level of induction achieved by our dual luciferase reporter mice may be lower than that obtained with the mouse of Zhu et al. (2004). Using 30 mg/kg of kainic acid for injury, these investigators found approximately 10-fold elevations of both GFAP and luciferase mRNA levels at 48 hrs after injection, and ~40-fold elevation of luciferase signal as detected by imaging. In contrast, we observed a 2-fold change in luciferase activity, and only at 6 hours post-injection (though our dose of kainic acid was lower and we used tissue extracts for measuring luciferase activity). Similarly, in the rd model of gliosis we observed a 4.9-fold increase in GFAP promoter activity, whereas others have reported a 10-fold elevation of GFAP mRNA (although the age of the mice in this study was not specified) (Sarthy and Fu 1989).
In the case of a mouse expressing the R236H mutant GFAP that is a model of Alexander disease, we found that GFAP promoter activity is only modestly elevated, and only at early developmental time points, in contrast to the higher and more sustained elevations of GFAP mRNA and protein found in earlier studies of the same mice (Hagemann et al. 2006). One explanation for these discrepant results is that post-transcriptional mechanisms account for much of the elevated expression that takes place in the context of this disease. GFAP is a long-lived protein in vivo, with a half-life of approximately 8-9 weeks in spinal cord (DeArmond et al. 1986), and therefore may persist much longer than the luciferase reporter. Other studies in cell lines indicate that the accumulation of GFAP that occurs in Alexander disease in part reflects inhibition of proteasomal function (Tang et al. 2006). However, an alternative possibility to explain our results with the luciferase reporter is that the 2.2 kb human GFAP promoter, while capable of detecting up-regulation of GFAP expression in gliosis (Brenner et al. 1994;Zhuo et al. 1997), does not contain all the regulatory elements that contribute to up-regulation of the endogenous gene.
Finally, one appeal of luciferase transgenic mice is the opportunity to visualize reporter gene expression in living animals, as originally demonstrated using the firefly luciferase gene (Contag et al. 1998). With suitable substrates it may prove possible to perform dual imaging of firefly and Renilla luciferase activities in the same mouse (Bhaumik and Gambhir 2002).
Acknowledgments
We thank Denice Springman and Channi Kaur for technical assistance, and Michael Brenner for comments on the manuscript. This work was supported by NIH grants HD046599, NS060120, and NS042803 (to A.M.), and HD03352 (to the Waisman Center).
Footnotes
Publisher's Disclaimer: This is an Accepted Article that has been peer-reviewed and approved for publication in the Journal of Neurochemistry, but has yet to undergo copy-editing and proof correction. Please cite this article as an “Accepted Article”
References
- Alexander MC, Lomanto M, Nasrin N, Ramaika C. Insulin stimulates glyceraldehyde-3-phosphate dehydrogenase gene expression through cis-acting DNA sequences. Proc Natl Acad Sci USA. 1988;85:5092–5096. doi: 10.1073/pnas.85.14.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–395. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- Bhaumik S, Gambhir SS. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci USA. 2002;99:377–382. doi: 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Johnson AB, Boespflug-Tanguy O, Rodriguez D, Goldman JE, Messing A. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nature Genet. 2001;27:117–120. doi: 10.1038/83679. [DOI] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo AC, Moreno-Igoa M, Manzano R, Ordovás L, Yagüe G, Oliván S, Muñoz MJ, Zaragoza P, Osta R. Determination of protein and RNA expression levels of common housekeeping genes in a mouse model of neurodegeneration. Proteomics. 2008;8:4338–4343. doi: 10.1002/pmic.200701091. [DOI] [PubMed] [Google Scholar]
- Cao YA, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijnenburg RJ, Reeves R, Taylor-Edwards C, Schulz S, Doyle TC, Fathman CG, Robbins RC, Herzenberg LA, Negrin RS, Contag CH. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation. 2005;80:134–139. doi: 10.1097/01.tp.0000164347.50559.a3. [DOI] [PubMed] [Google Scholar]
- Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annual Review of Biomedical Engineering. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- Contag PR, Olomu IN, Stevenson DK, Contag CH. Bioluminescent indicators in living mammals. Nature Med. 1998;4:245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- Cordeau P, Lalancette-Herbert M, Weng YC, Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke. 2008;39:935–942. doi: 10.1161/STROKEAHA.107.501460. [DOI] [PubMed] [Google Scholar]
- Cui C, Wani MA, Wight D, Kopchick J, Stambrook PJ. Reporter genes in transgenic mice. Transgenic Res. 1994;3:182–194. doi: 10.1007/BF01973986. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Lee Y-L, Kretzschmar HA, Eng LF. Turnover of glial filaments in mouse spinal cord. J Neurochem. 1986;47:1749–1753. doi: 10.1111/j.1471-4159.1986.tb13084.x. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Fan F, Wood KV. Bioluminescent assays for high-throughput screening. Assay Drug Development Technology. 2007;5:127–136. doi: 10.1089/adt.2006.053. [DOI] [PubMed] [Google Scholar]
- Hagemann TL, Connor JX, Messing A. Alexander disease-associated glial fibrillary acidic protein mutations in mice induce Rosenthal fiber formation and a white matter stress response. J Neurosci. 2006;26:11162–11173. doi: 10.1523/JNEUROSCI.3260-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen K, Koistinaho J, Alhonen L, Jänne J. Overexpression of spermidine/spermine N1-acetyltransferase in transgenic mice protects the animals from kainate-induced toxicity. Eur J Neurosci. 2000;12:540–548. doi: 10.1046/j.1460-9568.2000.00940.x. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Modi K, Coquoz O, Rice B, Smith S, Contag PR, Purchio T. Reduction of astrogliosis by early treatment of pneumococcal meningitis measured by simultaneous imaging, in vivo, of the pathogen and host response. Infect Immun. 2005;73:7836–7843. doi: 10.1128/IAI.73.12.7836-7843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- Li R, Johnson AB, Salomons G, Goldman JE, Naidu S, Quinlan R, Cree B, Ruyle SZ, Banwell B, D’Hooghe M, Siebert JR, Rolf CM, Cox H, Reddy A, Gutiérrez-Solana LG, Collins A, Weller RO, Messing A, van der Knaap MS, Brenner M. GFAP mutations in infantile, juvenile, and adult forms of Alexander disease. Ann Neurol. 2005;57:310–326. doi: 10.1002/ana.20406. [DOI] [PubMed] [Google Scholar]
- Luo J, Ho P, Steinman L, Wyss-Coray T. Bioluminescence in vivo imaging of autoimmune encephalomyelitis predicts disease. Journal of Neuroinflammation. 2008;5:6. doi: 10.1186/1742-2094-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Oldstone MBA, Morris JC, Nerenberg MI. Rapid activation of astrocyte-specific expression of GFAP-lacZ transgene by focal injury. New Biol. 1991;3:465–474. [PubMed] [Google Scholar]
- Quinlan RA, Brenner M, Goldman J, Messing A. GFAP and its role in Alexander disease. Exp Cell Res. 2007;313:2077–2087. doi: 10.1016/j.yexcr.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Fu M. Transcriptional activation of an intermediate filament protein gene in mice with retinal dystrophy. DNA. 1989;8:437–446. doi: 10.1089/dna.1.1989.8.437. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Higgins PJ, Crawford DR. Control selection for RNA quantitation. bt. 2000;29:332–337. doi: 10.2144/00292rv02. [DOI] [PubMed] [Google Scholar]
- Tang G, Xu Z, Goldman JE. Synergistic effects of the SAPK/JNK and the proteasome pathway on glial fibrillary acidic protein (GFAP) accumulation in Alexander disease. J Biol Chem. 2006;281:38634–38643. doi: 10.1074/jbc.M604942200. [DOI] [PubMed] [Google Scholar]
- Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- Zhu LY, Ramboz S, Hewitt D, Boring L, Grass DS, Purchio AF. Non-invasive imaging of GFAP expression after neuronal damage in mice. Neurosci Lett. 2004;367:210–212. doi: 10.1016/j.neulet.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Sun B, Zhang CL, Fine A, Chiu SY, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev Biol. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]


