Abstract
Dietary restraint is heavily influenced by affect, which has been independently related to asymmetrical activation in the prefrontal cortex (prefrontal asymmetry) in electroencephalograph (EEG) studies. In normal weight individuals, dietary restraint has been related to prefrontal asymmetry; however, this relationship was not mediated by affect. This study was designed to test the hypotheses that, in an overweight and obese sample, dietary restraint as well as binge eating, disinhibition, hunger, and appetitive responsivity would be related to prefrontal asymmetry independent of affect at the time of assessment. Resting EEG recordings and self-report measures of overeating and affect were collected in 28 overweight and obese adults. Linear regression analyses were used to predict prefrontal asymmetry from appetitive measures while controlling for affect. Cognitive restraint and binge eating were not associated with prefrontal asymmetry. However, disinhibition, hunger, and appetitive responsivity predicted left-, greater than right-, sided prefrontal cortex activation independent of affect. Findings in this study add to a growing literature implicating the prefrontal cortex in the cognitive control of dietary intake. Further research to specify the precise role of prefrontal asymmetry in the motivation toward, and cessation of, feeding in obese individuals is encouraged.
Keywords: frontal asymmetry, dietary restraint, disinhibition, binge eating, appetitive responsivity
Introduction
Chronic overeating has reached pandemic proportions (CDC, 2006). Such overeating ranges from chronic passive overconsumption (Blundell & MacDiarmid, 1997), to recurrent binge episodes reported in up to 40% of individuals seeking weight loss treatment (Spitzer, Devlin, Walsh & Hasin, 1992). Theories explaining the propensity to overeat have been primarily based on behavioral studies and have not yielded effective long term behavioral interventions. The need for improved methods of examining and conceptualizing the appetitive vulnerabilities that lead to overeating in obese individuals may be, in part, fulfilled by examining the neurobiological correlates of appetitive drive.
Although the investigation of the neural activity associated with appetitive drive remains in its infancy (Chowdhury & Lask, 2001), a relationship between ingestive behavior and activation in the prefrontal cortex (PFC) has emerged (Alonso-Alonso & Pascual-Leone, 2007; Le, Pannacciulli, Chen, Del Parigi, Salbe, Reiman, et al., 2006). Several authors suggest a prominent role of the PFC in the cognitive regulation of food intake (Tataranni & DelParigi, 2003; Le et al., 2006) and further evidence indicates that the (a)symmetry of PFC activation (activation in one, relative to the other, hemisphere of the PFC) may be integral in identifying the specific role of the PFC in appetitive behavior (Andreason, Altemus, Zametkin, King, Lucinio & Cohen, 1992 ; Karhunen, Vanninen, Kuikka, Lappalainen, Tiihonen & Uusitupa, 2000; Silva, Pizzagalli, Larson, Jackson & Davidson, 2002). At rest, individuals typically display relatively symmetrical activation in the PFC (Murphy, Nimmo-Smith & Lawrence, 2003); however, recent research suggests that individuals reporting disordered eating patterns may experience asymmetry in activation of the PFC or “prefrontal asymmetry” (Karhunen et al., 2000; Andreason et al., 1992; Silva et al., 2002). Obese binge eaters, for example, display greater increases in left-, relative to right-, sided prefrontal asymmetry as compared to lean and obese non-binge eaters following exposure to palatable food (Karhunen et al., 2000). Strong linear correlations were also observed in obese binge eaters between increases in hunger and left, greater than right-, sided (left-sided) prefrontal asymmetry (Karhunen et al., 2000).
The PFC is proposed to be responsible for instantiating the experience and execution of affect-related behavior (Davidson, Jackson & Kalin, 2000; Miller & Cohen, 2001). According to the affective theory (Davidson, 2000; 2003), emotion results from neural signals in the PFC, separated into two systems: the approach-related positive affect, and withdrawal-related negative affect, systems. Accordingly, the positive affect system is activated as a person moves toward an appetitive goal, while the negative affect system facilitates withdrawal from sources of aversive stimulation (Davidson, 2003; Tomarken, Davidson, Wheeler & Doss, 1992). Several neuroimaging studies have related positive affect to left-sided prefrontal asymmetry (Davidson, 2000; Sutton & Davidson, 2000; Tomarken et al., 1992) and negative affect to right-sided prefrontal asymmetry (Davidson et al., 2000; Davidson, 2003; Wheeler, Davidson & Tomarken, 1993).
Based on the proposed relationship between negative affect and right-sided prefrontal asymmetry (Davidson, 2000), and the relationship between negative affect and dietary restraint in normal weight individuals (Sheppard-Sawyer, McNalley & Fischer, 2000), Silva and colleagues (Silva et al., 2002) hypothesized that restrained eating would be related to right-sided prefrontal asymmetry in a normal weight sample. Dietary restraint and prefrontal asymmetry were assessed using the Restraint Scale (Herman & Polivy, 1980) and EEG imaging (respectively), and results confirmed the proposed hypothesis. However, affect was not found to mediate the relationship between prefrontal asymmetry and Restraint Scale scores, suggesting a relationship between dietary restraint in lean individuals and prefrontal asymmetry independent of affect (Silva et al., 2002).
Silva and colleagues (Siva et al., 2002) additionally suggest that right-sided prefrontal asymmetry may be related to other indicators of disordered eating, such as bulimia. However, bulimic individuals have been shown to display more left-sided PFC activation relative to normal individuals (Andreason et al., 1992) despite the strong association between bulimia and depression (Hinz & Williamson, 1987). Noting other findings inconsistent with the affective model, particularly the relationship between anger (a negative, but approach-related emotion) and left-sided prefrontal asymmetry (Harmon-Jones & Allen, 1997), Harmon-Jones (2003; 2004) proposed that affective valence (positive-negative) and approach-withdrawal tendencies were two related but distinct constructs. He suggests that left- and right- sided prefrontal asymmetry reflect motivational direction (approach vs. withdrawal respectively) irrespective of associated affect (Harmon-Jones, 2003; 2004).
The present study was designed to test, in an overweight and obese sample, the primary hypotheses that prefrontal asymmetry would be related to dietary restraint as well as binge eating, disinhibition, hunger and appetitive responsivity and that these relationships would be found independent of affect at the time of assessment. The two competing models of prefrontal asymmetry predicted different outcomes in terms of the directionality of the asymmetry. The affective model would have predicted that dietary restraint, binge eating and disinhibition (appetitive behaviors associated with negative affect; Wardle, Waller & Rapoport, 2001; Sheppard-Sawyer et al., 2000) would be related to right-sided prefrontal asymmetry. The motivation direction model would also have predicted that dietary restraint (reflecting a withdrawal-like tendency in the absence of disinhibiting stimuli; Herman & Polivy, 1980; Silva et al., 2002) would be associated with right-sided prefrontal asymmetry, but that binge eating, disinhibition, hunger and appetitive responsivity (reflecting approach-like tendencies) would be related to left-sided prefrontal asymmetry. Being the preeminent theory of prefrontal asymmetry, secondary hypotheses regarding the directionality of asymmetry were based on the affective model.
Methods
Participants
Forty participants were recruited through physician referral to a weight loss intervention study being conducted at Drexel University in Philadelphia, PA. Participants were told they were being recruited for an unrelated study of brain activity and all participants completed this study prior to any weight loss intervention. Nine female and 3 male participants either failed to arrive at their scheduled appointment, produced unusable EEG data due to equipment failure, or were eliminated from the study due to hair styles (e.g., weaves) that precluded the ability to establish a clean EEG connection, yielding 28 (26F; 2M) completers. All participants were right-handed, overweight or obese, were not participating in a weight control program and reported they were not currently dieting to lose weight. Baseline characteristics are shown in Table 1. Participants were not taking medications and had no physical or psychological conditions that may have affected body weight (e.g., pregnancy, depression) or brain activity (e.g., open head wound, learning disability). All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research and approval for this study was granted from the Drexel University Medical Institutional Review Board.
Table 1.
Baseline Sample Characteristics
| Male | Female | ||||
|---|---|---|---|---|---|
| Gender (n) | 2 | 26 | |||
| Range | Mean | SD | |||
| Age (y) | 29 – 70 | 49.2 | 12.3 | ||
| BMI (kg/m2) | 29.1–61.5 | 39.2 | 6.7 | ||
| AA1 | Cauc2 | Latino | > 1 | Unknown | |
| Ethnicity (%) | 79 | 7 | 4 | 7 | 4 |
African American
Caucasian
Appetitive Measures
Dietary Restraint, Disinhibition & Hunger
The Three Factor Eating Questionnaire (TFEQ; Stunkard & Messick, 1985) has demonstrated good reliability and validity (Laessle, Tuschl, Kotthaus & Pirke, 1989; Stunkard & Messick, 1985). The Cognitive Restraint, Disinhibition and Hunger subscales have also demonstrated adequate internal consistency (Laessle et al., 1989; Stunkard & Messick, 1985).
Binge Eating
The Binge Eating Scale (BES; Gormally, Black, Datson & Rardin, 1982) was designed specifically to assess binge eating severity within an obese population. The BES displays adequate psychometric properties (Timmerman, 1999) and successfully discriminates between individuals no, moderate, or severe binge eating problems (Gormally et al., 1982).
Appetitive Responsivity
The Power of Food Scale (PFS; Lowe, Butryn, Didie, Annunziato, Crerand, Ochner, et al., 2009) was designed to assess individual psychological reactions to the food environment. The PFS demonstrates good internal consistency, temporal stability, convergent validity, and discriminant validity (Annunziato, Lee & Lowe, 2007; Lowe et al., 2009). Validation studies suggest that the PFS reflects global levels of appetitive responsivity and latent potential for overeating (Lowe, 2006; Forman, Hoffman, McGrath, Herbert, Brandsma & Lowe, 2007).
Affective Measures
Anxiety and Depression
The Mood and Anxiety Symptom Questionnaire (MASQ; Clark & Watson, 1991) Anhedonic Depression and Anxious Arousal subscales were used to measure state affect in the aforementioned (Silva et al., 2002) study of prefrontal asymmetry. The Anxious Arousal and Anhedonic Depression sub-scales display adequate levels of reliability and validity (Reidy & Keogh, 1997).
Positive and Negative Affect
The state version of the Positive and Negative Affect Schedule (PANAS; Watson, Clark & Tellegen, 1988) consists of positive and negative mood scales designed to assess affect “at the present moment.” These scales have shown to be highly internally consistent, uncorrelated, and stable over time (Watson et al., 1988), and both scales demonstrate good convergent and discriminant validity (Crawford & Henry, 2004).
General Measures
Handedness
The Edinburgh Handedness Inventory (EHI; Oldfield, 1971) demonstrates good reliability and validity in the assessment of handedness, and has been used in previous prefrontal asymmetry research (e.g., Tomarken et al., 1992).
Height and Weight
A standard physician stadiometer was used to measure height. Weight was measured in street clothes, without shoes, using a standardized Seca®644 scale accurate to 0.1 kg.
Procedure
One week prior to EEG assessments, participants filled out appetitive measures, as a part of the parent study from which they were recruited. Dietary restraint was measured using the TFEQ Cognitive Restraint subscale as it has been shown to be more reliable than the Restraint Scale (Herman & Polivy, 1980) in assessing dietary restraint in overweight and obese individuals (Ruderman, 1986; van Strien, Herman, Engels, Larsen & van Leeuwe, 2007). Binge eating, disinhibition, hunger, and appetitive responsivity were assessed using the BES, TFEQ Disinhibition subscale, TFEQ Hunger subscale and PFS respectively. Immediately preceding EEG recordings, participants filled out the EHI and affective measures (MASQ, and PANAS state version). The specific research hypotheses of this study were withheld from participants so EEG recordings would not be affected. Resting-state EEG was then recorded.
All participants were instructed to consume a ~ 500 kcal breakfast (2 eggs, 2 slice toast, and 1 glass orange juice suggested) and to not consume any caffeine in the morning before reporting for EEG assessments at 11am. EEG was recorded using a stretchable lycra cap with 128 embedded electrodes (Electro-Cap International, Inc.). Electrodes were applied according to the extended International 10–20 System (digitally linked mastoid reference). Data were collected during 8 60-second trials, 4 with eyes open and 4 with eyes closed, presented in counterbalanced alternating order. Electrode impedances were kept below 20,000 Ohms (per manufacturer recommendation). All EEG data were collected using a sample rate of 256 Hz and bandpass filtered at 0.02 – 100 Hz. EEG was amplified 20,000 times using the MICROAMPS™ data acquisition system (SAM Technology, Inc.). EEG signals were then digitized using the MANSCAN® RECORDER system (SAM Technology, Inc.).
Automatic artifact detection, followed by visual inspection was used to remove artifacts due to eye blinks, gross muscle activity, and movement. Artifact-free epochs of data were extracted through a Hanning window. Fast Fourier Transform was applied to all extracted epochs that were four seconds in duration (ranging from 129–228 epochs per condition), with epochs overlapping 50 percent. Power density was then computed for the alpha band by summing power values across each 1-Hz bin within a band and dividing by the number of bins. Mean alpha power was computed separately for eyes-open and eyes-closed trials, weighted by the number of available artifact-free epochs. A mean of alpha power for eyes open and closed was then computed. Finally, all power density values were log transformed to normalize the distribution of the data.
Log-transformed EEG power values in the alpha band (8–13 Hz) were computed for all electrodes. Frontal asymmetry scores were computed by subtracting the value obtained at the left-frontal electrode F3 from the corresponding value at the homologous right-frontal electrode F4 (log F4 – log F3). Because alpha-band EEG power is inversely proportional to magnitude of neural activity, positive asymmetry scores reflect greater left-sided neural activity (i.e., greater alpha band power density on the right than on the left). Conversely, negative asymmetry scores reflect greater right-sided activity.
Mean (M) and standard deviation (SD) values were calculated for scores on all measures, as well as asymmetry scores in the PFC (Table 2). Scores on the MASQ and the state version of the PANAS were used to remove the variance in asymmetry accounted for by depressive or anxious symptomatology and affective valence at the time of measurement. The relationships between all self-report measures were calculated using Pearson correlations. Individual linear regression analyses were then used to test the relationships between affective measures, appetitive measures and prefrontal asymmetry, both with and without controlling for BMI. Finally, a stepwise regression analysis was performed to determine the best model for predicting prefrontal asymmetry in this sample. All analyses were additionally repeated controlling for age, and gender.
Table 2.
Descriptive Statistics across Measures
| Appetitive Measures | N | Range | Mean | SD |
|---|---|---|---|---|
| TFEQ Cog Restraint | 24 | 7– 30 | 18.9 | 5.9 |
| BES | 26 | 16 – 40 | 25.4 | 6.4 |
| TFEQ Disinhibition | 26 | 1 – 12 | 5.1 | 2.8 |
| TFEQ Hunger | 21 | 1 – 12 | 3.7 | 2.6 |
| PFS | 21 | 21 – 89 | 36.8 | 15.8 |
| Affective Measures | ||||
| MASQ Anhed Depression | 28 | 32 – 81 | 53.0 | 11.3 |
| MASQ Anxious Arousal | 28 | 18 – 35 | 21.9 | 3.8 |
| PANAS Positive Affect | 28 | 15 – 49 | 34.2 | 7.8 |
| PANAS Negative Affect | 28 | 10 – 16 | 11.5 | 1.6 |
| Asymmetrical Activation | ||||
| Prefrontal Asymmetry1 | 28 | −0.34 | 0.039 | 0.082 |
Positive asymmetry scores reflect left-, greater than right-, sided PFC activation
Note: differences in N’s between measures reflect both incomplete questionnaire data provided by participants, as well as the addition of certain measures (TFEQ Hunger, PFS) after several participants had completed the study
Results
Relationships between appetitive and affective measures
Pearson correlations between all self-report measures are shown in Table 3. Unsurprisingly, Anhedonic Depression [MASQ subscale] was inversely related to Positive Affect [PANAS subscale], and positively related to Negative Affect (p < 0.001 and p = 0.043 respectively). Binge Eating [BES] was positively related to Disinhibition [TFEQ subscale] (p = 0.002) and Appetitive Responsivity [PFS] (p = 0.005). Disinhibition was also positively related to Hunger (p = 0.01) and both Disinhibition and Hunger were positively related to Appetitive Responsivity (p < 0.0005 and p = 0.014 respectively). The only significant relationships between affective, and appetitive, measures were the inverse relationship between Positive Affect and Appetitive Responsivity (p = 0.025) and positive relationship between Anxiety and Disinhibition (p = 0.014). All analyses were repeated controlling for BMI with no significant change in results (not shown).
Table 3.
Pearson Correlations (r) between Appetitive and Affective Measures
| Dietary Restraint(TFEQ) | Binge Eating | Disinhibit | Hunger | Appetitive Respons | Depress | Anxiety | Pos Affect | |
|---|---|---|---|---|---|---|---|---|
| Binge Eating(BES) | 0.11 | |||||||
| Disinhibition(TFEQ) | −0.20 | 0.60** | ||||||
| Hunger(TFEQ) | −0.40 | 0.40 | 0.58** | |||||
| Appetitive Respons (PFS) | −0.33 | 0.60** | 0.78** | 0.55* | ||||
| Depression(MASQ) | 0.07 | 0.21 | −0.02 | −0.05 | 0.27 | |||
| Anxiety(MASQ) | 0.02 | 0.21 | 0.48* | −0.05 | 0.20 | −0.13 | ||
| Pos Affect(PANAS) | 0.38 | −0.31 | −0.25 | −0.19 | −0.49* | −0.66** | −0.10 | |
| Neg Affect(PANAS) | −0.06 | 0.00 | 0.08 | 0.04 | 0.23 | 0.39* | −0.02 | −0.42* |
significant at p < 0.05
significant at p < 0.01
BMI was not related to any measure (all p’s > 0.2).
Relationships between appetitive and affective measures and prefrontal asymmetry
Dietary Restraint
Dietary Restraint did not predict prefrontal asymmetry in this sample with or without controlling for BMI and/or affective measures.
Binge Eating
Binge Eating did not predict prefrontal asymmetry in this sample with, or without, controlling for BMI and/or affective measures.
Disinhibition
Disinhibition predicted prefrontal asymmetry (t(25) = 2.5, p = 0.018), such that higher Disinhibition scores were associated with greater left-sided PFC activation. This relationship remained unchanged when controlling for BMI (t(25) = 2.6, p= 0.017) or affective measures (t(25) = 2.7, p = 0.016) but showed a modest but nonsignificant increase in strength when controlling BMI and affective measures simultaneously (t(25) = 3.1, p = 0.007).
Hunger
Hunger predicted prefrontal asymmetry (t(20) = 3.4, p = 0.003), such that higher Hunger scores were associated with greater left-sided PFC activation. This relationship remained significant when controlling for BMI (t(20) = 3.0, p = 0.008), affective measures (t(20) = 3.4, p = 0.004) or BMI and affective measures simultaneously (t(20) = 3.0, p = 0.01).
Appetitive Responsivity
Appetitive Responsivity predicted prefrontal asymmetry scores (t(20) = 2.3, p = 0.011), such that higher Appetitive Responsivity scores were associated with greater left-sided PFC activation. The strength of this relationship showed a nonsignificant increase when controlling for BMI (t(20) = 3.2, p = 0.005), affective measures (t(20) = 3.3, p = 0.005), and BMI and affective measures simultaneously (t(20) = 3.4, p = 0.004).
Affect
No scores on any affective measure were related to prefrontal asymmetry in this sample.
A stepwise regression analysis with all self-report measures and BMI entered revealed that the single best predictor of prefrontal asymmetry was Appetitive Responsivity (t(17) = 2.7, p = 0.013, beta = 0.6), accounting for 32% of the variance (Figure 1). The tolerance and variance inflation factor (VIF) values for this model were both 1.00, indicating little coliniarity. All regression analyses were repeated with age and gender entered as covariates with no change in results. Analyses were additionally repeated with only female (n = 26) participants with no change in results.
Figure 1.
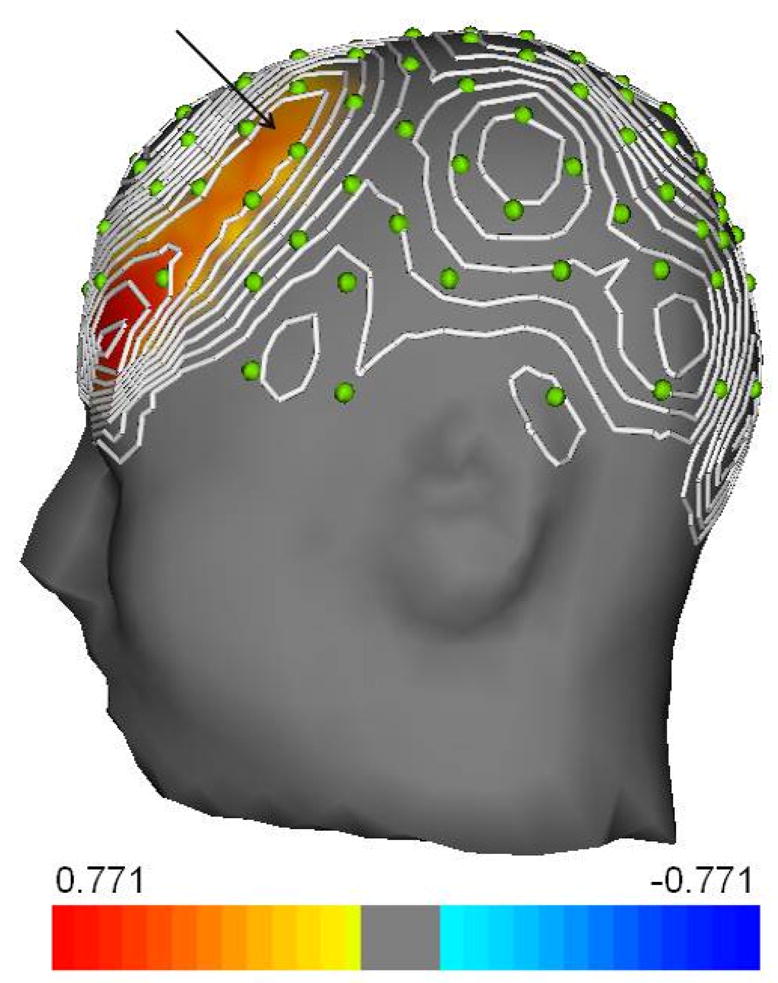
Topographic map of the relationship (standardized coefficient [Beta]) between log-transformed scalp alpha-power hemispheric asymmetry scores across all electrode sites, and scores on the Power of Food Scale (PFS). The map was created by computing Beta for homologous pairs of electrodes. These coefficients were then used to generate a spline-interpolated map on a lateral view of the head. Each green dot represents the location of an electrode, and the arrow denotes the region of the prefrontal cortex. Orange and red regions on the map (Beta coefficients) reflect the positive relationship between Appetitive Responsivity and alpha asymmetry. That is, more neural activity (lower alpha power) measured at left-hemisphere electrodes for each corresponding (hemispheric) pair of electrodes in relation to PFS scores. Only Beta coefficients significant at p < 0.05 are shown.
Discussion
In this overweight and obese sample, measures of dietary restraint, binge eating, disinhibition, hunger, and appetitive responsivity were examined in relation to prefrontal asymmetry and affect at the time of assessment. Dietary restraint was not related to other appetitive measures; however, consistent with previous literature (Lowe et al., 2009; Marcus, Wing & Lamparski, 1985), individuals reporting more binge eating also reported greater levels of disinhibition and appetitive responsivity. Affect at the time of assessment was generally unrelated to appetitive measures in this study. The only exceptions were an inverse relationship between scores on the PFS and PANAS Positive Affect subscale and positive relationship between scores on the TFEQ Disinhibition and MASQ Anxious Arousal subscales, indicating that individuals higher in appetitive responsivity reported less positive affect and individuals with a greater tendency to become disinhibited reported higher levels of anxiety at the time of assessment.
Dietary restraint, as measured by the TFEQ, was not related to prefrontal asymmetry in this overweight and obese sample. Evidence of restraint theory (Herman & Mack, 1975) has not been consistent with measures of dietary restraint other than the Restraint Scale (Lowe & Kleifield, 1988; Westenhoefer, Broeckmann, Munch & Pudel, 1994), previously shown to correlate with prefrontal asymmetry in lean individuals (Silva et al., 2002). It has also been suggested that the Restraint Scale, used in the Silva et al. (2002) study, actually measures disinhibition more so than dietary restraint (Stunkard & Messick, 1985; Westenhoefer et al., 1994). “The restraint subscales of the DEBQ and TFEQ measure the tendency of actually restricted caloric intake in everyday eating behavior (Laessle et al., 1989), whereas the restraint scale identifies dieters who have a tendency to get disinhibited” (Westenhoefer et al., 1994; p. 28). This assertion would suggest a relationship between disinhibition and right-sided PFC activation in lean individuals (Silva et al., 2002), and a relationship between disinhibition and left-sided PFC activation in obese individuals found in the present study. The finding that binge eating was not related to prefrontal asymmetry in this sample was somewhat surprising, given correlations between BES scores and scores on the PFS and TFEQ Disinhibition subscales (see Table 3). Although past literature has demonstrated increased left-sided prefrontal asymmetry in obese binge eaters (Karhunen et al., 2000), this relationship was only found during exposure to highly palatable food images, which may have elicited positive affect and/or approach motivation.
No relationship was found between affect at the time of assessment and prefrontal asymmetry, indicating that affect did not mediate the relationship between appetitive measures and prefrontal asymmetry (Baron & Kenny, 1986). Paired with the findings that disinhibition, hunger, and appetitive responsivity were related to prefrontal asymmetry after controlling for affect at the time of assessment, outcomes from this study suggest that there may be a relationship between prefrontal asymmetry and the propensity to overeat independent of affect. These results are also consistent with previous studies of behavioral and psychological measures in relation to prefrontal asymmetry, found to be independent of affect (Davidson et al., 2000; Harmon-Jones & Allen, 1997; Karhunen et al., 2000; Silva et al., 2002; Sutton & Davidson, 2000; Wheeler et al., 1993). The use of state affect measurements in this study leaves open the possibility that trait affect may mediate such relationships; however, Karhunen et al. (2000) found that depressive symptomatology, assessed by the BDI (Beck, Ward, Mendelson, Mock & Erbaugh, 1961), were not associated with prefrontal asymmetry; “The observed differences in the asymmetry of the hemispheric blood flow between the binge and non-binge eating subjects could thus be suggested to be associated with the core features of eating behavior, rather than with depression.” (p. 40). In addition, the directionality of results (i.e. measures of overeating related to left-sided PFC activation), reduce the likelihood that trait affect could have mediated the relationships between prefrontal asymmetry and appetitive measures found in the present study.
According to the affective hypothesis of prefrontal asymmetry (Davidson, 2000; Davidson, 2003), individuals higher in appetitive responsivity and disinhibition should be prone to experience more positive affect, due to the relationships between both PFS and TFEQ Disinhibition subscale scores and left-sided PFC activation. The inverse relationship between PFS and PANAS Positive Affect Scale scores, as well as the relationship between TFEQ disinhibition subscale and MASQ Anxious Arousal subscale scores, seem to contradict this theory. The relationship between left-sided PFC activation and disinhibition may be particularly disconcerting for proponents of the affective theory, as disinhibition has frequently been associated with negative affect (Sheppard-Sawyer et al., 2000; Stunkard et al., 1991). Although not allowing for direct comparison across models, results in this study appear more consistent with the approach-withdrawal (Harmon-Jones, 2003; 2004) model. That is, increased disinhibition, hunger, and appetitive responsivity may reflect more “approach” tendencies, rather than reflecting positive affect. This contention is also consistent with a meta-analysis of 106 studies (Murphy et al., 2003) revealing that left-sided frontal asymmetry was associated with approach, but not necessarily positive, emotions.
Limitations of this study include the questionnaire-based assessment of appetitive behavior, limited generalizability to other populations, and the heterogeneity of the sample (large BMI and age ranges). Equipment failure resulted in unusable data for 7 additional patients, and missing self-report data was not interpolated, resulting in unusable outcome measure scores for several patients; however, outcome measures with the lowest sample size (TFEQ Hunger and PFS) yielded significant results in relation to prefrontal asymmetry. It is important to note that functional and anatomical divisions exist within the PFC and that EEG imaging, suggested to reflect mainly dorsolateral regions of the PFC (Davidson, 2004), does not provide the spatial resolution necessary to isolate and examine specific regions within the PFC. Finally, the authors would like to point out that asymmetrical activation was not found exclusively in the PFS (as reflected in Fig. 1), however, a priori predictions involved only the PFC as reliable interpretation of asymmetry in other brain areas is not yet supported by the literature.
Conclusion
Left-sided PFC activation in obese individuals was related to measures of disinhibition, hunger, and appetitive responsivity, but not dietary restraint or binge eating in this overweight and obese sample. In addition, disinhibition was correlated with negative affect (anxiety) and appetitive responsivity was inversely correlated with positive affect; however, affect at the time of assessment was not related to prefrontal asymmetry. These results partially support the proposed relationship between the tendency to overeat and asymmetrical activation in the prefrontal cortex, but do not support the affective model of prefrontal asymmetry. Findings in this study encourage further exploration into the motivational model of prefrontal asymmetry and its relation to overeating in overweight and obese humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. Journal of the American Medical Association. 2007;297(16):1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- Andreason PJ, Altemus M, Zametkin AJ, King AC, Lucinio J, Cohen RM. Regional cerebral glucose metabolism in bulimia nervosa. American Journal of Psychiatry. 1992;149(11):1506–1513. doi: 10.1176/ajp.149.11.1506. [DOI] [PubMed] [Google Scholar]
- Annunziato RA, Lee JN, Lowe MR. A comparison of weight-control behaviors in African American and caucasian women. Ethnicity & Disease. 2007;17:262–267. [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blundell JE, MacDiarmid JI. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. Journal of the American Dietetic Association. 1997;97(7 Suppl):S63–9. doi: 10.1016/s0002-8223(97)00733-5. [DOI] [PubMed] [Google Scholar]
- Bruch H. Eating disorders: Obesity, anorexia nervosa, and the person within. New York: Basic Books.; 1973. [Google Scholar]
- Center for Disease Control and Prevention. National Health and Nutrition Survey (NHANES) 2003–04. National Center for Health Statistics; Bethesda, MD.: 2006. [Google Scholar]
- Chowdhury U, Lask B. Clinical implications of brain imaging in eating disorders. Psychiatric Clinics of North America. 2001;24(2):227–234. doi: 10.1016/s0193-953x(05)70219-7. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Health Psychology. 2004;43(3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126(6):890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. The functional neuroanatomy of affective style. In: Lane Richard D, Nadel Lynn, editors. Cognitive neuroscience of emotion. New York, NY, US: Oxford University Press; 2000. pp. 371–388. [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology. 2003;40(5):655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67:219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Forman EM, Hoffman KL, McGrath KB, Herbert JD, Brandsma LL, Lowe MR. A comparison of acceptance- and control-based strategies for coping with food cravings: An analog study. Behavior Research and Therapy. 2007;45:2372–2386. doi: 10.1016/j.brat.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia. 1986;24:417–422. doi: 10.1016/0028-3932(86)90028-x. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Gluck ME, Hashim SA. Plasma ghrelin concentrations are lower in binge-eating disorder. Journal of Nutrition. 2005;135(5):1326–1330. doi: 10.1093/jn/135.5.1326. [DOI] [PubMed] [Google Scholar]
- Gormally J, Black S, Datson S, Rardin D. The assessment of binge eating severity among obese persons. Addictive Behavior. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106(1):159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40:838–848. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. On the relationship of frontal brain activity and anger: Examining the role of attitude toward anger. Cognition and Emotion. 2004;18(3):337–361. [Google Scholar]
- Herman CP, Polivy J. Obesity. Philadelphia: Saunders; 1980. Restrained eating; pp. 208–225. [Google Scholar]
- Herman CP, Mack D. Restrained and unrestrained eating. Journal of Personality. 1975;43(4):647–660. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. European ournal of Neuroscience. 2004;20(5):1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- Hinz L, Williamson D. Bulimia and Depression: A Review of the Affective Variant Hypothesis. Psychological Bulletin. 1987;102(1):150–158. [PubMed] [Google Scholar]
- Karhunen LJ, Vanninen EJ, Kuikka JT, Lappalainen RI, Tiihonen J, Uusitupa MI. Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Research. 2000;99(1):29–42. doi: 10.1016/s0925-4927(00)00053-6. [DOI] [PubMed] [Google Scholar]
- Laessle RG, Tuschl RJ, Kotthaus BC, Pirke KM. Behavioral and biological correlates of dietary restraint in normal life. Appetite. 1989;12(2):83–94. doi: 10.1016/0195-6663(89)90098-6. [DOI] [PubMed] [Google Scholar]
- Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in response to a meal: A feature of obesity. American Journal of Clinical Nutrition. 2006;84:725–731. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Crerand CE, Ochner CN, Coletta M, Lucks D, Halford J. The power of food scale: A new measure of the psychological influence of the food environment. Psychological Assessment. doi: 10.1016/j.appet.2009.05.016. in review. [DOI] [PubMed] [Google Scholar]
- Lowe MR. The power of food: A new dimension of appetite and a new scale to measure it [Abstract] Appetite. 2006;47S:31. [Google Scholar]
- Lowe MR, Kleifield E. Cognitive restraint, weight suppression, and the regulation of eating. Appetite. 1988;10:159–168. doi: 10.1016/0195-6663(88)90009-8. [DOI] [PubMed] [Google Scholar]
- Marcus MD, Wing RR, Lamparski DM. Binge eating and dietary restraint in obese patients. Addictive Behaviors. 1985;10:163–168. doi: 10.1016/0306-4603(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective & Behavioral Neuroscience. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Reidy J, Keogh E. Testing the discriminant and convergent validity of the mood and anxiety symptoms questionnaire using a British sample. Personality and Individual Differences. 1997;23(2):337–344. [Google Scholar]
- Ruderman AJ. Dietary restraint: A theoretical and empirical review. Psychological Bulletin. 1986;99:247–262. [PubMed] [Google Scholar]
- Sheppard-Sawyer C, McNalley R, Fischer JH. Film-induced sadness as a trigger for disinhibited eating. International Journal of Eating Disorders. 2000;28:215–220. doi: 10.1002/1098-108x(200009)28:2<215::aid-eat11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Silva JR, Pizzagalli DA, Larson CL, Jackson DC, Davidson RJ. Frontal brain asymmetry in restrained eaters. Journal of Abnormal Psychology. 2002;111(4):676–681. doi: 10.1037//0021-843x.111.4.676. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Devlin MJ, Walsh BT, Hasin D. Binge eating disorder: A multisite field trial of the diagnostic criteria. International Journal of Eating Disorders. 1992;11(3):191–203. [Google Scholar]
- Stunkard AJ, Fernstrom MH, Price RA, Buss E, Frank E, Kupfer DJ. Weight change in depression: Influence of “disinhibition” is mediated by body mass and other variables. Psychiatry Research. 1991;38(2):197–200. doi: 10.1016/0165-1781(91)90044-p. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia. 2000;38:1723–1733. doi: 10.1016/s0028-3932(00)00076-2. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, DelParigi A. Functional neuroimaging: A new generation of human brain studies in obesity research. Obesity Reviews. 2003;4(4):229–238. doi: 10.1046/j.1467-789x.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- Timmerman GM. Binge eating scale: Further assessment of validity and reliability. Journal of Applied Biobehavioral Research. 1999;4(1):1–12. [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62(4):676–687. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- van Strien T, Herman CP, Engels RCME, Larsen JK, van Leeuwe FJ. Construct validation of the restraint scale in normal-weight and overweight females. Appetite. 2007;49:109–121. doi: 10.1016/j.appet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wardle J, Beinart H. Binge eating: A theoretical review. British Journal of Clinical Psychology. 1981;20(2):97–109. doi: 10.1111/j.2044-8260.1981.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Wardle J, Waller J, Rapoport L. Body dissatisfaction and binge eating in obese women: The role of restraint and depression. Obesity Research. 2001;9(12):778–787. doi: 10.1038/oby.2001.107. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality & Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Westenhoefer J, Broeckmann P, Munch AK, Pudel V. Cognitive control of eating behaviour and the disinhibition effect. Appetite. 1994;23(1):27–41. doi: 10.1006/appe.1994.1032. [DOI] [PubMed] [Google Scholar]
- Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]


