Abstract
Objective
The objective of this study was to quantify hormones that regulate energy and glucose homeostasis in order to establish possible mechanisms for the greater efficacy of Roux-en-Y gastric bypass (RYGB) compared with laparascopic adjustable gastric banding (LAGB) in achieving weight loss and improved insulin sensitivity.
Design
Longitudinal study of patients undergoing LAGB (n=15) and RYGB (n=28) who were studied prior to surgery and at 2, 12, 26 and 52 wks afterwards.
Measurements
Fasting blood samples were drawn at each visit. Postprandial blood samples were also obtained prior to surgery and at 26 and 52 wks. Samples were assayed for peptide YY (PYY), ghrelin, glucagon-like peptide-1 (GLP-1), glucose, insulin, leptin, thyrotropic hormone (TSH), free T4 and free T3.
Results
At one year there was greater weight loss in RYGB compared with LAGB patients (30% vs 15%), but final body mass index was similar (34 vs 33 kg/m2). At wk 52, area under the curve (AUC) for PYY in RYGB subjects was greater than LAGB (P<0.01). GLP-1 levels at 30 min post-meal were three-fold greater after RYGB compared with LAGB (P<0.001). Conversely, ghrelin AUC increased after LAGB at wk 52 (P<0.05) but tended to decrease after RYGB. Fasting glucose, insulin, and leptin, and HOMA-IR decreased in both groups over time but were significantly lower at wk 52 after RYGB compared with LAGB. The change in leptin correlated significantly with weight loss in LAGB (r=0.86) and RYGB (r=0.77), however, HOMA-IR correlated significantly with weight loss only in LAGB (r=0.78), and not RYGB (r=0.15). There was a significant decrease in free T3 (P<0.01) after RYGB.
Conclusions
Differences in levels of gut hormones may play a role in promoting greater weight loss and insulin sensitivity after RYGB compared with LAGB, however, weight loss may be limited by decreases in free T3 and leptin.
Keywords: PYY, ghrelin, GLP-1, leptin, insulin secretion, bariatric surgery
INTRODUCTION
The absence of effective pharmacotherapy for morbidly obese individuals, together with the rise in the prevalence of extreme obesity (BMI ≥ 40 kg/m2) have resulted in a surge in the number of surgical procedures performed for weight loss (1, 2). The average reduction in body weight is approximately 28% after laparoscopic adjustable gastric banding (LAGB) and 40% after Roux-en-Y gastric bypass (RYGB) and metabolic benefits such as remission of type 2 diabetes occurs in 48% and 84% in LAGB and RYGB patients, respectively (3, 4). Both LAGB and RYGB produce weight loss by restricting nutrient flow; the former, via an adjustable band placed around the upper stomach, and the latter by surgical division of the stomach to create a small proximal pouch. RYGB also causes a redirection of nutrient flow from the upper portion of the stomach directly into the mid- to distal jejunum that may cause micronutrient deficiencies, but malabsorption of macronutrients does not typically occur. Recently there has been greater recognition that the modality of weight loss, not just the degree, may have independent effects on neurohormonal regulators of energy balance and glucose homeostasis (5, 6). Changes in hormone secretion may then contribute to the efficacy of the particular procedure in producing long-term weight loss and improvement in obesity-related co-morbidities.
A panoply of hormones is secreted from the gastrointestinal tract that contributes to the regulation of energy balance and glucose homeostasis via interaction with peripheral tissues and communication with the central nervous system as part of the “gut-brain axis” (7). The gut hormones of interest in this report are peptide YY (PYY), glucagon-like peptide-1 (GLP-1) and ghrelin. Secretion of PYY and GLP-1 occurs shortly after food intake from L cells that are located predominantly along the distal small bowel and colon. Both peptides decrease appetite, increase satiety, slow gut motility and improve insulin sensitivity (7). As part of the “enteroinsular axis,” GLP-1 also functions as an incretin to potentiate glucose-stimulated insulin release. In contrast, ghrelin stimulates appetite, increases food intake and gut motility and decreases insulin sensitivity (8). Secretion of ghrelin from A cells in the oxyntic glands of the stomach fundus is suppressed after a meal and is down-regulated in obese individuals. Weight loss by caloric restriction is associated with an increase in circulating concentrations of ghrelin (9).
In previous cross-sectional studies we have demonstrated that circulating levels of several gut hormones differ in patients who had undergone LAGB compared with RYGB (10, 11). The most striking difference between procedures was an exaggerated postprandial increase in PYY, GLP-1 and insulin concentrations in the RYGB group. Fasting ghrelin levels were not different, but the large inter-individual variability and absence of pre-operative data make interpretation of the ghrelin data difficult. A number of other studies have characterized changes in gut hormones after bariatric surgery but prospective studies of a side-by-side comparison of fasting and postprandial hormonal changes after LAGB and RYGB are lacking (12). Given the therapeutic implications that may arise from an understanding of how different modalities of weight loss affect gut hormone secretion and glucose homeostasis, we performed a prospective study of individuals undergoing LAGB and RYGB. Levels of gut peptides prior to surgery and at 2, 12, 26 and 52 weeks after surgery were assessed in addition to insulin, leptin and thyroid hormones that also influence metabolism.
MATERIALS and METHODS
Study Subjects
Subjects older than 21 years of age who were scheduled to undergo LAGB (n=15; 11F/4M) or RYGB (n=28; 24F/4M) were recruited. Individuals who reported the use of weight loss medications within 90 days prior to enrollment were excluded from the study. Criterion for a diagnosis of type 2 diabetes mellitus was based on a fasting glucose ≥ 126 mg/dl in individuals not taking diabetes medications. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The study was approved by the Columbia University Institutional Review Board and written informed consent was obtained from all subjects.
Surgery
The choice of bariatric procedure was based on the preference of surgeon and patient. The surgical procedure for the LAGB consisted of placement of an adjustable silastic band (Lap-BandTM, INAMED Health, Santa Barbara, CA, USA) around the proximal stomach attached by tubing to a port sutured to the anterior rectus fascia. The inner diameter of the band, and thereby the size of the opening through which nutrients must pass, was adjusted post-surgically through the access port by removal or addition of saline within the band system. The surgical procedure for RYGB consisted of creation of a 15–30 ml pouch that was divided from the proximal lesser curvature of the stomach and excluded the fundus. The pouch was anastomosed to a Roux limb of jejunum created by division of the jejunum 50 to 100 cm distal to the ligament of Treitz and anastomosing the afferent biliopancreatic limb to the jejunum 100 to 150 cm distally. Division of the vagus nerve and its branches was avoided.
Protocol
Subjects were studied at a mean of 5.8 weeks prior to surgery, and at 2, 12, 26 and 52 weeks post-surgery. Body weight was measured and fasting venous blood samples were drawn at all visits. A liquid meal challenge (Optifast, Novartis, Minneapolis, MN, USA; 474 ml, 320 kcal, 50% carbohydrate, 35% protein, 15% fat) consumed within a 15 minute period was administered at the pre-operative visit and at 26 and 52 weeks after the surgical procedure. The choice of the meal challenge was based on our previous studies demonstrating that the macronutrient content and volume load does not illicit dumping syndrome or abdominal discomfort in post-surgery patients (11, 13). Venous blood was drawn in the fasted state and 30, 60, 90 and 120 minutes after meal.
Hormone Assays
Plasma hormone measurements were performed on blood samples collected in EDTA tubes that were centrifuged for 15 min at 4° C and stored at −80° C until assayed in duplicate. Plasma leptin and total ghrelin (acyl- and des-acyl forms) were measured as previously described (13). Total plasma levels of PYY were measured using a commercial enzyme-linked immunosorbent assay (ELISA; Diagnostic Systems Laboratories, Webster, TX, USA) that measures PYY(1–36) and PYY(3–36). The lower limit of detection was 12 pg/ml and the coefficients of variation were 10.1% within and 10.3% between assays. Due to discontinued production of the DSL assay during this study, approximately 15% of samples were assayed for PYY using an ELISA from LINCO Research (St. Charles, MO, USA). There was significant correlation between assays (r = 0.9, P < 0.001), however, absolute values were lower in the LINCO assay. The following equation was found by an ordinary least squares solution to calculate comparable DSL value: −58.3 + 2.8(LINCO value). The number of subjects from whom test meal samples were analyzed with the LINCO PYY assay were as follows: for LAGB, 1, 1, and 6 subjects for wks 0, 26, and 52, respectively; for RYGB, 3, 5, and 7 subjects for wks 0, 26, and 52, respectively. Total GLP-1 was measured by RIA (LINCO). Due to the large number of samples and requirement of alcohol extraction, only plasma collected pre-meal and at 30 min was analyzed. Serum insulin (lower limit of detection of 2 µIU/ml), free T3, free T4, and TSH were measured with the Immulite Analyzer (Siemens, Los Angeles, CA, USA). Plasma glucose was measured by the hexokinase method.
Statistical Analysis
SAS version 9.1software (Cary, NC, USA) was used for statistical analysis. Insulin resistance was calculated using the (homeostasis model of assessment (HOMA-IR): fasting insulin in microunits per ml × fasting glucose in mmoles per liter/22.5 (14). Differences in the distribution of continuous variables at baseline were tested with Student’s independent T-test. Longitudinal changes from baseline were tested with linear mixed models with fixed effects for surgical group, week and group by week interaction with an autoregressive(1) covariance structure for the repeated measures. Postprandial excursion differences between surgical groups were assessed with linear mixed models with fixed effect of group, week, group by week interaction, week by sample time interaction, and group by week by sample time interaction with a spatial power covariance structure for repeated measures. The association between variables was estimated using Pearson correlations. All tests were two-tailed, with P-values less than 0.05 considered statistically significant. No adjustment of the critical value of the test statistic was made for the separate tests of different hormones or for HOMA-IR, although a significant F-test was required for the fixed effect for post hoc comparisons of between group differences at specific times, or within-group differences between times using 95% confidence intervals and the model estimated means and standard errors. The area-under-the-curve (AUC) was calculated using the trapezoidal rule. Statistical model estimated means and standard errors are presented.
RESULTS
Baseline Characteristics and Weight Change
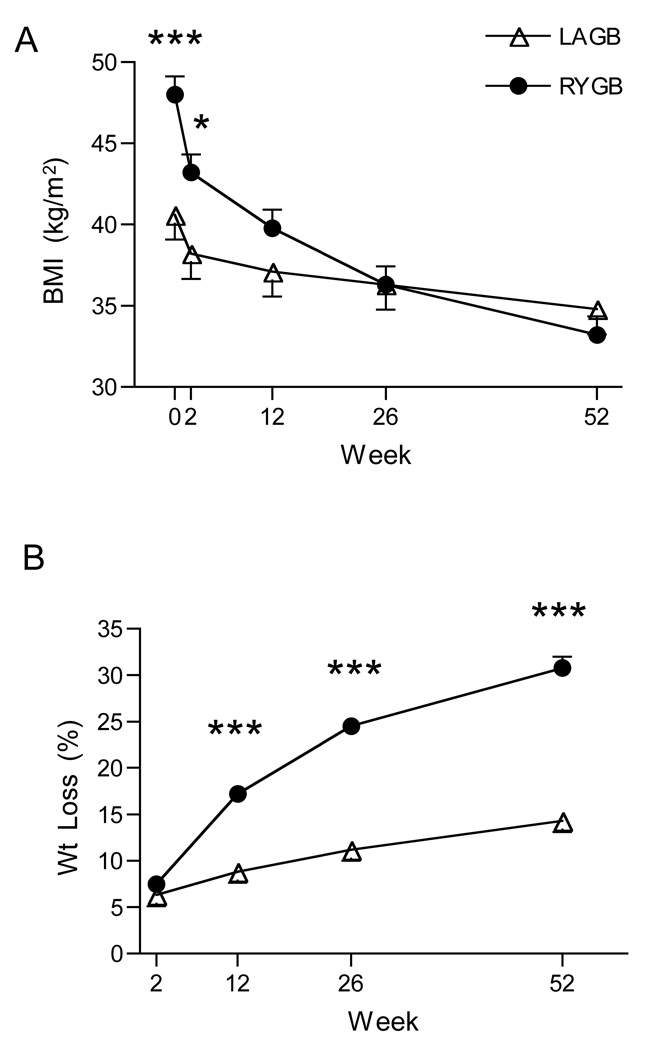
Age was similar between groups (LAGB, 47.1 ± 2.5; RYGB, 45.0 ± 2.0y), while body weight (112 ± 5 vs 128 ± 5 kg; P = 0.056) and BMI (41 ± 1 vs 48 ± 1 kg/m2; P < 0.001) were less in LAGB compared with RYGB. Changes in BMI and body weight are depicted in Fig. 1. At one year, 13/15 LAGB and 25/28 RYGB subjects were available for follow-up measurements. Percent decrease in total body weight at one year was two-fold greater in RYGB compared with LAGB (−15 ± 2.3 vs −30 ± 1.2%; P < 0.001). There was a wide variation in the range of weight change with both procedures (LAGB, +5 to −27; RYGB, −18 to −41%). At one year both groups were of similar weight (LAGB, 94 ± 4.5; RYGB 88 ± 3.6 kg; P = 0.221) and BMI (LAGB, 34 ± 1.1; RYGB, 33 ± 1.0 kg/m2; P = 0.438).
FIG. 1.
Changes in BMI (A) and percent weight loss (B).
PYY
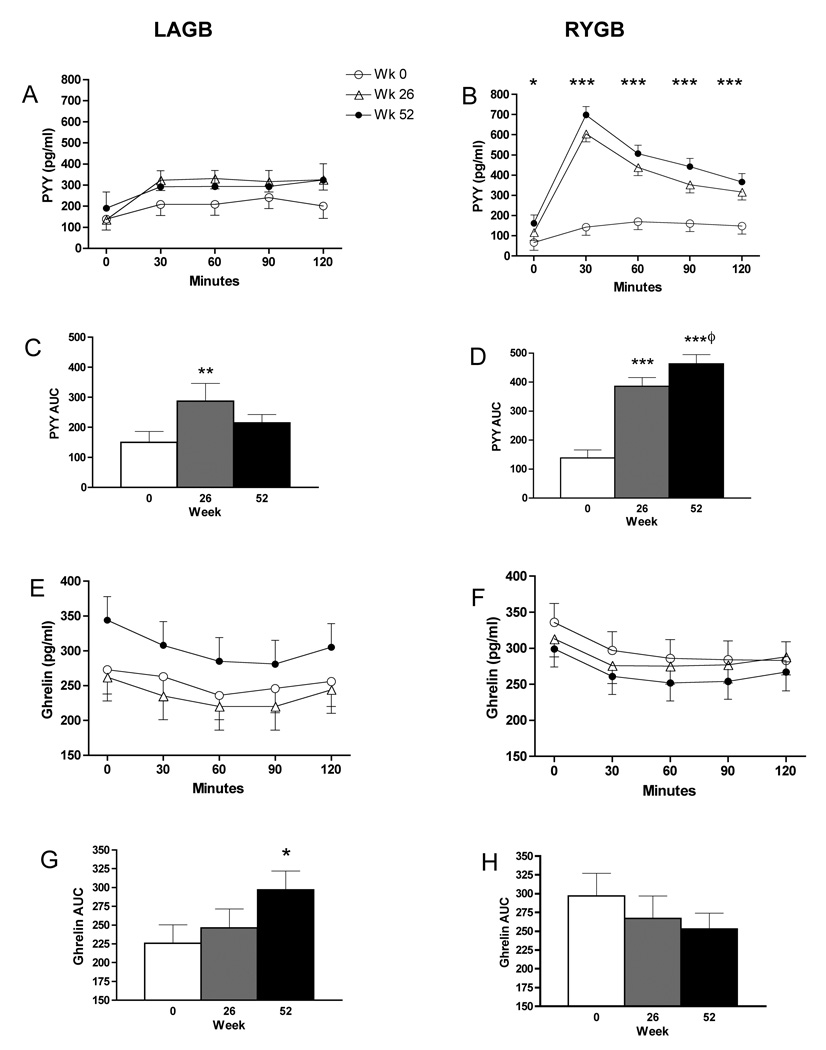
Fasting levels of PYY were not significantly different between groups at any time point (Table 1). There was a progressive increase in fasting PYY in the RYGB group such that at wk 52 levels were significantly greater than baseline (P = 0.014). The most striking differences in PYY levels were noted post-meal in RYGB subjects. At 26 and 52 wks, PYY levels at 30 minutes were approximately 3.5-fold greater than levels prior to surgery and were significantly greater than in LAGB subjects at the same time points (Fig. 2, P < 0.001). There was a corresponding 3-fold increase in AUC for PYY in the RYGB group at wk 26 (P < 0.001) and a continued increase in PYY levels such that AUC at wk 52 was significantly greater than at wk 26 (P = 0.025). AUC in the LAGB subjects was also increased after surgery but the difference was statistically significant only at wk 26 (P = 0.003). The non-significant increase in AUC for PYY at wk 52 may be due to a change in assay (see Methods). PYY did not correlate with percent weight loss in either group.
TABLE 1.
Fasting levels of hormones and glucose over time
| Wk 0 | Wk 2 | Wk 12 | Wk 26 | Wk 52 | |
|---|---|---|---|---|---|
| PYY (pg/ml) | |||||
| Band | 130 ± 17 | 95 ± 19 | 124 ± 18 | 135 ± 17 | 151 ± 27 |
| Bypass | 90 ± 19 | 96 ± 14 | 115 ± 13 | 111 ± 14 | 140 ± 15A |
| Ghrelin (pg/ml) | |||||
| Band | 263 ± 34 | 306 ± 35 | 286 ± 34 | 268 ± 34 | 343 ± 35 |
| Bypass | 330 ± 25 | 320 ± 25 | 313 ± 25 | 307 ± 25 | 304 ± 26 |
| GLP-1 (pmol/l) | |||||
| Band | 8.2 ± 1.5 | - | - | 5.4 ± 1.6 | 5.6 ± 1.6 |
| Bypass | 5.8 ± 1.4 | - | - | 5.4 ± 1.3 | 5.9 ± 1.4 |
| Leptin (ng/ml) | |||||
| Band | 36.8 ± 2.7 | 27.0 ± 2.7A | 28.0 ± 2.7A | 26.8 ± 2.8A | 27.3 ± 2.8A |
| Bypass | 38.2 ± 2.0 | 28.1 ± 2.0A | 20.3 ± 2.0AB | 17.2 ± 2.0AB | 14.8 ± 2.0AB |
| Insulin (µIU/ml) | |||||
| Band | 20.2 ± 2.1 | 15.4 ± 2.1A | 18.1 ± 2.1 | 13.4 ± 2.1A | 13.8 ± 2.1A |
| Bypass | 16.6 ± 1.6 | 11.7 ± 1.7 | 11.7 ± 1.6AB | 8.6 ± 1.6AB | 7.5 ± 1.7AB |
| Glucose (mg/dl) | |||||
| Band | 104 ± 2.5 | 100 ± 2.5A | 100 ± 2.5 | 95 ± 2.5A | 97 ± 2.6A |
| Bypass | 103 ± 1.9 | 98 ± 2.0A | 96 ± 1.9A | 91 ± 1.9A | 90 ± 2.0AB |
| HOMA-IR | |||||
| Band | 5.3 ± 0.6 | 3.8 ± 0.6A | 4.4 ± 0.6 | 3.1 ± 0.6A | 3.2 ± 0.6A |
| Bypass | 4.4 ± 0.4 | 3.8 ± 0.5 | 2.7 ± 0.5AB | 1.9 ± 0.5A | 1.6 ± 0.5AB |
Values presented are mixed linear model estimated within-subject mean ± SEM.
P < 0.05 compared to week 0 within group
P < 0.05 compared to Band at same time-point. Analyses for GLP-1, insulin, glucose, and HOMA-IR are restricted to subjects without type 2 diabetes.
FIG. 2.
Plasma PYY and ghrelin levels in response to a test meal before surgery and at weeks 26 and 52 after LAGB (left panel) and RYGB (right panel). Fasting and postprandial levels of PYY (A,B) and ghrelin (E,F): *P < 0.05, **P < 0.01, ***P < 0.001, week 52 compared with week 0. Area under the curve of PYY (C,D) and ghrelin (G,H): *P < 0.05, **P < 0.01, ***P < 0.001, compared with week 0; φP < 0.05, compared with week 26.
Ghrelin
Absolute levels of fasting ghrelin in LAGB subjects at wk 52 did not increase significantly compared with baseline (Table 1, P = 0.073), although the change in ghrelin was significant (43 ± 12%, P = 0.001), as was the increase in AUC (Fig. 2, P = 0.044). Conversely, there was a downward trend in fasting ghrelin levels in RYGB, but the difference in fasting values at wk 52 compared to baseline was not statistically significant (P = 0.422). The degree of suppression of ghrelin levels post-meal did not differ significantly pre-surgery to wk 52 in either the LAGB (16% ± 3.5 vs 19% ± 3.2) or the RYGB (21% ± 3.4 vs 22% ± 1.8) and did not differ between groups. Changes in fasting ghrelin or AUC did not correlate with percent weight loss in either group.
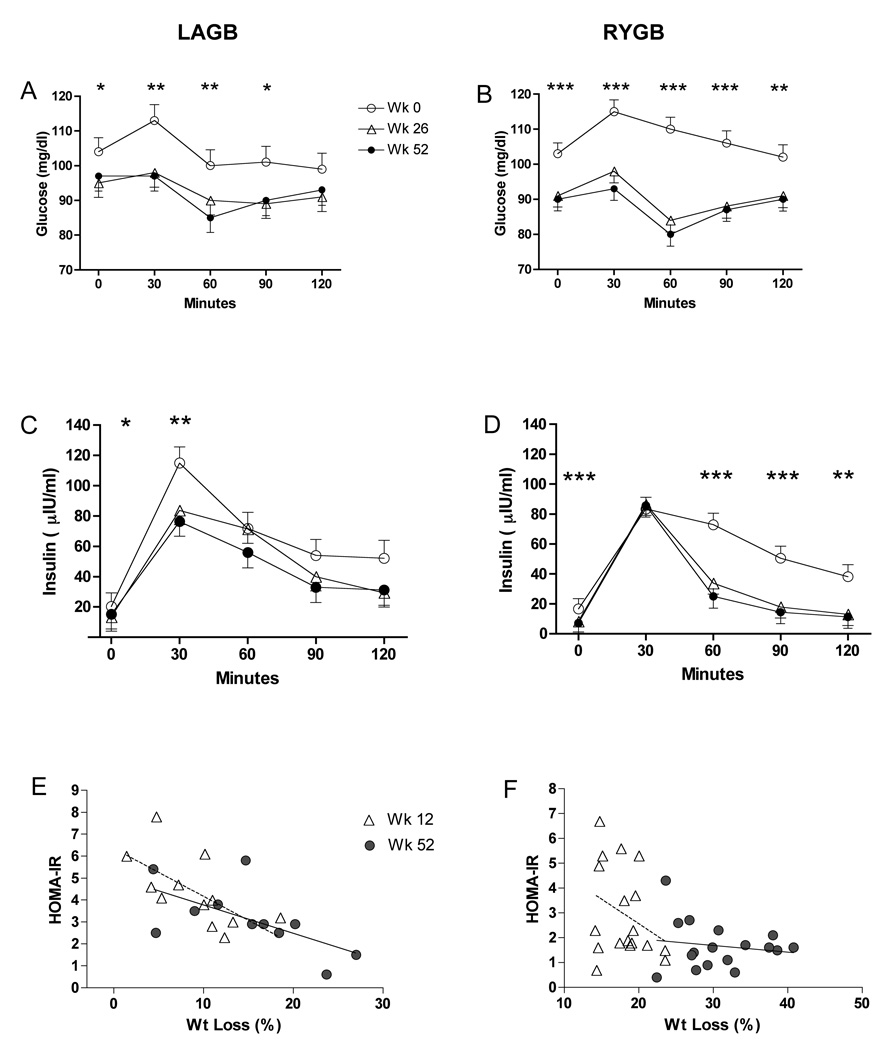
Glucose, Insulin, HOMA-IR
Subjects with type 2 diabetes (2 LAGB, 6 RYGB) at baseline were excluded from analyses of glucose, insulin and HOMA-IR. Fasting glucose, insulin and HOMA-IR decreased to a greater extent after RYGB (Table 1). Postprandial insulin levels peaked at 30 minutes and were not different between LAGB and RYGB, however, there was a more rapid decrease in the RYGB group such that insulin levels at 60 minutes were significantly lower at wk 26 (P = 0.002) and wk 52 (P = 0.016) compared with the same time points in the LAGB subjects (Fig. 3). Although baseline HOMA-IR levels were not predictive of the amount of weight loss at 1 year (r = −0.22, P = 0.5), HOMA-IR correlated significantly with percent weight loss at wk 12 (r = 0.64, P = 0.024) and wk 52 (r = 0.78, P = 0.005), (Fig. 3). Significant correlation of HOMA-IR and percent weight loss was not observed in RYGB subjects at week 12 when the range of loss was comparable to LAGB at 1 year (r = 0.21; P = 0.37), or at wk 52 (r = 0.15, P = 0.56) (Fig.3).
FIG. 3.
Glucose and insulin levels in response to a test meal before and after LAGB (A,C) and RYGB (B,D). *P < 0.05, **P < 0.01, ***P < 0.001, week 52 compared with week 0. Relationship between HOMA-IR and percent weight loss at 12 wks (dotted line) and 52 wks (solid line) after LAGB (E) and RYGB (F).
GLP-1
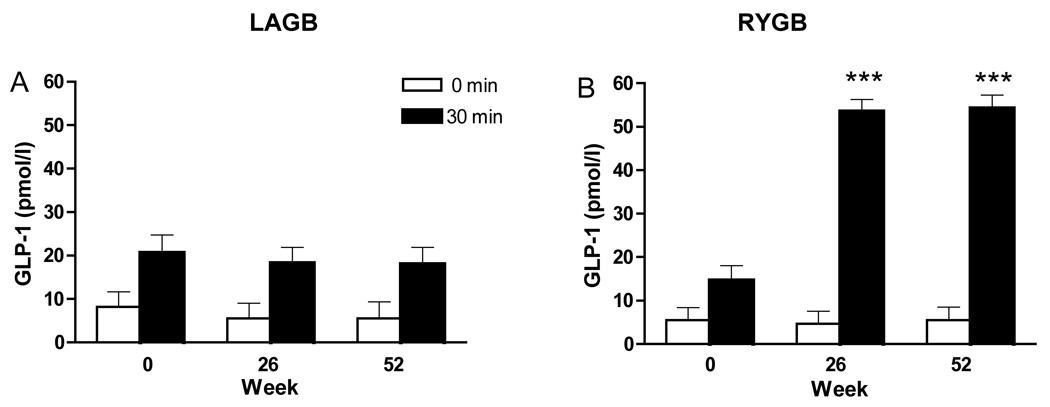
To more fully understand the pattern of insulin secretion, levels of the incretin hormone, GLP-1, were examined. Fasting levels of GLP-1 did not change significantly over time in either group (Table 1). In LAGB patients the postprandial rise of GLP-1 measured at 30 minutes did not change after surgery (Fig. 4). In contrast, postprandial GLP-1 levels after RYGB were significantly greater (P < 0.001) compared with 30 minute values before surgery and three-fold higher compared the same time points after LAGB (P < 0.001).
FIG. 4.
Plasma GLP-1 levels before surgery and at weeks 26 and 52 after surgery. GLP-1 levels in the fasted state and 30 minutes after a test meal before and after LAGB (A) and RYGB (B). *P < 0.05, **P < 0.01, ***P < 0.001 compared with week 0 within group at 30 minutes post-meal.
Leptin
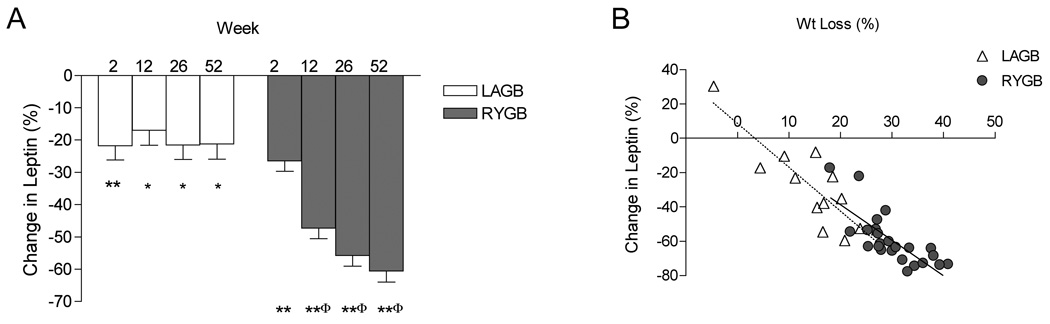
Fasting leptin levels did not differ at baseline between groups (Table 1). There was a significant decrease at wk 2 in LAGB subjects (P < 0.001), but levels did not decrease further despite continued weight loss. Leptin levels at wk 2 were also significantly decreased in RYGB subjects (P < 0.001). The percent change from baseline after RYGB (Fig. 5) continued to decrease from wk 2 to 12 (P < 0.001) and wk 12 to 26 (P = 0.019), showing stabilization from wk 26 to 52 (P = 0.294). The percent change in leptin correlated with percent weight loss at wk 52 in LAGB (r = 0.86, P < 0.001) and RYGB (r=0.77, P < 0.001) (Fig. 5).
FIG. 5.
Percent change in leptin over time (A). *P < 0.01,**P < 0.001; change from baseline within group; φP < 0.001; compared with LAGB at same time-point. Relationship between percent change in leptin levels and percent weight loss at wk 52 (B). LAGB (dotted line) and RYGB (solid line) subjects.
Thyroid function
Free T4, free T3 and TSH did not change significantly over time in the LAGB group (Table 2), although the percent change in free T3 correlated with the percent change in weight at wk 52 (r = 0.57, P = 0.041). In RYGB subjects there was a significant decrease in free T3 at wk 26 (P = 0.017) and wk 52 (P = 0.002). Change in free T3 did not correlate with weight loss. Leptin regulates the thyroid axis, however, correlation of the percent change in free T3 with percent change in leptin at wk 52 did not reach statistical significance in LAGB (r = 0.46, P = 0.115) or RYGB (r = 0.38, P =0.101). Absolute levels of TSH were not significantly different at wk 52 after RYGB.
TABLE 2.
Levels of thyroid hormones.
| Wk 0 | Wk 26 | Wk 52 | |
|---|---|---|---|
| Free T4 (ng/dl) | |||
| Band | 1.1 ± 0.05 | 1.1 ± 0.05 | 1.2 ± 0.05 |
| Bypass | 1.2 ± 0.04 | 1.2 ± 0.04 | 1.2 ± 0.04 |
| Free T3 (pg/ml) | |||
| Band | 3.5 ± 0.15 | 3.2 ± 0.15 | 3.2 ± 0.16 |
| Bypass | 3.5 ± 0.12 | 3.2 ± 0.12A | 3.0 ± 0.12A |
| TSH (µIU/ml) | |||
| Band | 2.10 ± 0.24 | 1.79 ± 0.24 | 1.74 ± 0.25 |
| Bypass | 1.55 ± 0.19 | 1.40 ± 0.20 | 1.62 ± 0.20 |
Values presented are mixed linear model estimated within-subject mean ± SEM.
P < 0.05 compared to week 0 within group. Five bypass subjects on thyroid hormone replacement were excluded from analysis.
DISCUSSION
In this report we show that LAGB and RYGB have different effects on fasting and postprandial concentrations of several hormones that have been shown in animal and human studies to modulate appetite, food intake, energy partitioning, energy expenditure and insulin sensitivity (Table 3). As discussed below, some of these changes were mostly related to weight loss regardless of the type of surgery, yet some were due mostly to the surgical intervention itself
TABLE 3.
Summary of changes after surgery at wk 52 compared with baseline
| Band | Bypass | |
|---|---|---|
| PYY (AUC) | ↔↑ | ↑↑ |
| Ghrelin (AUC) | ↑ | ↔ |
| GLP-1 (30 min) | ↔ | ↑↑ |
| HOMA-IR | ↓ | ↓↓ |
| Leptin | ↓ | ↓↓ |
| Free T3 | ↔ | ↓ |
Single arrows represent a significant increase (↑), decrease (↓), or no significant change (↔) compared with wk 0 within group. Double arrows represent significant increase (↑↑) or decrease (↓↓) compared with wk 0 within group and with LAGB at wk 52.
Several prospective studies of RYGB have also shown an increase in postprandial plasma levels of PYY and/or GLP-1 (12). This increase has been reported to occur as early as 2 days after bypass suggesting that the exaggerated response is secondary to the intervention per se and not to weight loss and is likely related to more rapid delivery of glucose to the distal small intestine (15). We have also shown that over time after RYGB there was a further increase in both fasting and postprandial PYY levels that may reflect adaptive changes within the gut. Biliopancreatic diversion in rats is associated with increased GLP-1 and PYY levels together with hypertrophy and increased mitoses within crypts of the distal small intestine (16). Similarly, hyperplasia of enteroglucagon-containing cells has been demonstrated in the ileum in humans after jejuno-ileal bypass (17). Consistent with other reports (18–20), we did not detect an increase in fasting GLP-1. Others have shown that glucose tolerance and obesity impair the incretin effect independently of one another (21) and that a diet-induced weight loss of 14.8% increases the GLP-1 response curve over a 180 minute period (22). In our study, we did not observe an increase in postprandial GLP-1 levels in LAGB patients after a similar amount of weight loss, however, a single postprandial measurement at 30 minutes may not have been adequate to detect subtle changes. Unfortunately, due to the limitation in sample volume we were unable to obtain GLP-1 measurements for the entire postprandial period. It is unlikely though, that major changes were missed given that in an earlier cross-sectional study we demonstrated that at 60 minutes post-meal there still is a significant difference in GLP-1 levels between LAGB and RYGB subjects (10).
Several studies have shown that PYY and GLP-1 levels may be associated weight loss after RYGB. AUC of PYY was shown to increase at a mean of 32.5 months in subjects in the upper quartile of weight loss relative to the evaluation performed at 52 weeks after surgery (23). In a cross-sectional analysis of patients who had undergone bypass, AUC of PYY and GLP-1 was greater in “good” compared with “poor” responders (24). Although these associations do not prove causality, inhibition of gut hormone release by administration of somatostatin increased appetite and food intake in bypass but not in band subjects, suggesting that gut hormones are more likely to mediate reduced appetite and food intake after RYGB (24). Since most of our subjects were unlikely to have achieved their nadir weight at 1 year, it may be too early to detect significant correlations between levels of L cell peptides and ultimate response to surgery.
While it is generally accepted that fasting ghrelin levels increase after weight loss induced by a calorie restricted diet (9) changes in ghrelin after bariatric surgery are more varied. As in this report, most studies have shown an increase in ghrelin after LAGB, however, results from prospective studies of RYGB are particularly inconsistent (12). The increase in fasting ghrelin after LAGB appears to occur over time indicating that such change is related to weight loss as opposed to restriction of nutrient flow. In contrast, the relative decrease in fasting ghrelin after RYGB occurs as early as 2–6 weeks post-surgery as also reported by Morinigo et al (25) suggesting that the decrease in ghrelin is mostly a result of altered anatomy (26). In this study, we have found that after RYGB there was inter-individual variation in ghrelin levels over time: fasting and AUC ghrelin remained the same or decreased in most individuals, yet approximately one-fourth of subjects exhibited an increase that was not dependent on weight loss or change in insulin levels. It is possible that variable treatment of vagal fibers explains inter-individual variation in fasting ghrelin levels (27).
HOMA-IR decreased to a greater extent in RYGB subjects and did not correlate with the degree of weight loss as was observed in LAGB subjects. In a large prospective study by Lee et al similar reductions in HOMA-IR were observed in LAGB and RYGB subjects at equivalent amounts of weight loss, however, it was not reported if changes in HOMA-IR correlated statistically with weight reduction; it is, therefore, unclear if the improvement in insulin resistance was driven mainly by weight reduction in both procedures (28). As suggested from human and animal studies, neurohormonal modulators of insulin sensitivity affected by bypass of the proximal intestine, and/or early enhanced nutrient delivery more distally, may play a more prominent role in mediating the beneficial effects on glucose homeostasis after bypass (6), whereas, weight loss is likely to be the predominant factor inducing remission of diabetes after gastric banding (29). Increased GLP-1 and PYY levels together with an absence of a compensatory rise in ghrelin in most RYGB patients would be expected to favor improved insulin sensitivity as these hormone affect glucose homeostasis in addition to appetitive behavior (8, 30–32).
After diet-induced weight loss reductions in bioactive thyroid hormones and sympathetic nervous system tone, and a reduction in energy expenditure beyond that predicted by the loss of body mass have been observed (33, 34). These changes are believed to be an adaptive response to protect an organism from semi-starvation that is mediated in part by the decline in circulating leptin concentrations. Restoration of leptin concentrations to levels measured prior to calorie restriction normalizes some of these neuroendocrine changes (34, 35). Similarly, after RYGB resting energy expenditure is also lower than that expected for the reduction in fat-free mass (36, 37). We have shown that after RYGB there was a profound decrease in leptin levels and a decrease in free T3 without a sufficient compensatory rise in TSH. This pattern mimics a euthyroid sick syndrome observed in low leptin states such as anorexia nervosa (38). Leptin regulates the thyroid axis at multiple levels (39), however, the correlation coefficients relating the percent change in free T3 with the percent change in leptin did not reach statistical significance in either surgical group. It is possible that more subjects would be required to demonstrate an interaction with free T3 and leptin in this setting. Furthermore, measurement of peripheral levels may not accurately reflect leptin levels reaching TRH neurons with the central nervous system. Other metabolic factors, in addition to possible changes in peripheral deiodinase activity, may have also contributed to the decrease in T3 after RYGB. For example, TRH neurons are regulated by the hypothalamic melanocortin system (39), which in turn, is regulated by both insulin and leptin (40, 41). Thus, leptin insufficiency together with decreased insulin levels may act in conjunction to suppress melanocortin tone and limit weight loss via a reduction in T3 levels and energy expenditure.
The decrease in leptin levels at one year was proportional to the degree of weight loss after both procedures. A limitation of this study is that body composition analysis was not performed. However, our findings from a cross-sectional study using whole body MRI in which we have shown that plasma leptin levels adjusted for total adipose tissue and weight loss were similar in weight stable individuals at a mean of 2 years after LAGB and RYGB (42) suggesting that the regulation of leptin secretion does not differ between procedures. While the majority of circulating leptin is produced in fat cells, leptin is also secreted into the systemic circulation from endocrine cells present in the gastric mucosa (43). However, it is unlikely that the early decline in leptin levels after RYGB was due to bypassing the stomach since changes in circulating leptin concentrations have not been detected in the early postoperative period following gastrectomy (26).
Certainly there are variables that play critical roles in weight loss such as physical activity and eating patterns, together with environmental and psychological factors that were not addressed in this study. As an observational study we are only able to draw inferences regarding the physiological effects that result from the hormonal changes described, but these observations form a foundation from which one may proceed with interventional studies in animals and humans. While bariatric surgery is usually quite effective, weight loss is less than optimal in some individuals and significant weight regain may occur in others after having achieved a satisfactory plateau. In such individuals, a combination of lifestyle modifications, surgery and pharmacotherapy may be necessary to maximize results, particularly as new reagents are likely to become available in the future. In theory, one might consider GLP-1 or PYY analogs, or ghrelin antagonists after LAGB, or replacement dose of leptin after RYGB in individuals for whom leptin insufficiency may be limiting further weight reduction. There may be a myriad of other possibilities to optimize non-surgical and surgical treatments for obesity as more gut hormones are studied and as the drug pipeline begins to catch up with our growing understanding of energy homeostasis.
ACKNOWLEDGMENTS
We would like to thank the participants in this study. This work was supported by NIH grants DK072011 (to J.K.) and RR00645 (to the General Clinical Research Center).
Footnotes
DISCLOSURES
J. Korner has received lecture fees from Merck and is on the Scientific Advisory Board of Nutrisystem. M. Bessler has received lecture fees from Ethicon and Inamed. W. Inabnet has received consulting fees from the Surgical Review Corporation and research support from Covidien.
REFERENCES
- 1.Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288:1758–1761. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 2.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–1079. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 3.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 5.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Overduin J, Foster-Schubert KE, Carlson MJ. Role of the bypassed proximal intestine in the anti-diabetic effects of bariatric surgery. Surg Obes Relat Dis. 2007;3:109–115. doi: 10.1016/j.soard.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent RP, Ashrafian H, le Roux CW. Mechanisms of Disease: the role of gastrointestinal hormones in appetite and obesity. Nature clinical practice. 2008;5:268–277. doi: 10.1038/ncpgasthep1118. [DOI] [PubMed] [Google Scholar]
- 8.Wiedmer P, Nogueiras R, Broglio F, D'Alessio D, Tschöp MH. Ghrelin, obesity and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3:705–712. doi: 10.1038/ncpendmet0625. [DOI] [PubMed] [Google Scholar]
- 9.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 10.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated GLP-1 and blunted GIP secretion are associated with gastric bypass but not gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity. 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 12.Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf) 2008;69:173–179. doi: 10.1111/j.1365-2265.2007.03164.x. [DOI] [PubMed] [Google Scholar]
- 13.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Chaikomin R, Doran S, Jones KL, Feinle-Bisset C, O'Donovan D, Rayner CK, et al. Initially more rapid small intestinal glucose delivery increases plasma insulin, GIP, and GLP-1 but does not improve overall glycemia in healthy subjects. Am J Physiol Endocrinol Metab. 2005;289:E504–E507. doi: 10.1152/ajpendo.00099.2005. [DOI] [PubMed] [Google Scholar]
- 16.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG. Biliopancreatic diversion in rats is associated with intestinal hypertrophy and with increased GLP-1, GLP-2 and PYY levels. Obes Surg. 2007;17:1193–1198. doi: 10.1007/s11695-007-9211-2. [DOI] [PubMed] [Google Scholar]
- 17.Buchan AM, Pederson RA, Koop I, Gourlay RH, Cleator IG. Morphological and functional alterations to a sub-group of regulatory peptides in human pancreas and intestine after jejuno-ileal bypass. Int J Obes Relat Metab Disord. 1993;17:109–113. [PubMed] [Google Scholar]
- 18.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–215. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 19.Kellum JM, Kuemmerle JF, O'Dorisio TM, Rayford P, Martin D, Engle K, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–770. doi: 10.1097/00000658-199006000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–1348. doi: 10.2337/db07-1315. [DOI] [PubMed] [Google Scholar]
- 22.Verdich C, Toubro S, Buemann B, Lysgard Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety–effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–1214. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 23.Morinigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–275. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 24.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 25.Morinigo R, Casamitjana R, Moize VV, Lacy AM, Delgado S, Gomis R, et al. Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res. 2004;12:1108–1116. doi: 10.1038/oby.2004.139. [DOI] [PubMed] [Google Scholar]
- 26.Jeon TY, Lee S, Kim HH, Kim YJ, Son HC, Kim DH, et al. Changes in plasma ghrelin concentration immediately after gastrectomy in patients with early gastric cancer. J Clin Endocrinol Metab. 2004;89:5392–5396. doi: 10.1210/jc.2004-0872. [DOI] [PubMed] [Google Scholar]
- 27.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–5187. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 28.Lee WJ, Lee YC, Ser KH, Chen JC, Chen SC. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–1125. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- 29.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 30.Boey D, Sainsbury A, Herzog H. The role of peptide YY in regulating glucose homeostasis. Peptides. 2007;28:390–395. doi: 10.1016/j.peptides.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 32.Aulinger B, D'Alessio D. Glucagon-like peptide 1: continued advances, new targets and expanding promise as a model therapeutic. Current opinion in endocrinology, diabetes, and obesity. 2007;14:68–73. doi: 10.1097/MED.0b013e328013e79e. [DOI] [PubMed] [Google Scholar]
- 33.Kok P, Roelfsema F, Langendonk JG, Frolich M, Burggraaf J, Meinders AE, et al. High circulating thyrotropin levels in obese women are reduced after body weight loss induced by caloric restriction. J Clin Endocrinol Metab. 2005;90:4659–4663. doi: 10.1210/jc.2005-0920. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest. 2003;111:1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrasco F, Papapietro K, Csendes A, Salazar G, Echenique C, Lisboa C, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 37.Bobbioni-Harsch E, Morel P, Huber O, Assimacopoulos-Jeannet F, Chassot G, Lehmann T, et al. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–4700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 38.Reinehr T, Isa A, de Sousa G, Dieffenbach R, Andler W. Thyroid Hormones and Their Relation to Weight Status. Hormone research. 2008;70:51–57. doi: 10.1159/000129678. [DOI] [PubMed] [Google Scholar]
- 39.Flier JS, Harris M, Hollenberg AN. Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring. J Clin Invest. 2000;105:859–861. doi: 10.1172/JCI9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 41.Lee M, Wardlaw SL. The central melanocortin system and the regulation of energy balance. Front Biosci. 2007;12:3994–4010. doi: 10.2741/2366. [DOI] [PubMed] [Google Scholar]
- 42.Korner J, Punyanita M, Taveras C, McMahon DJ, Kim HJ, Inabnet W, et al. Sex differences in visceral adipose tissue post-bariatric surgery compared to matched non-surgical controls. Int J Body Composition Res. 2008;6:93–99. [PMC free article] [PubMed] [Google Scholar]
- 43.Cinti S, de Matteis R, Ceresi E, Pico C, Oliver J, Oliver P, et al. Leptin in the human stomach. Gut. 2001;49:155. doi: 10.1136/gut.49.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]