Abstract
Although wound healing is generally a successful, carefully orchestrated and evolutionary sound process, it can be disregulated by extrinsic factors such as psychological stress. In the SKH-1 restraint stress model of cutaneous wound healing, the rate of wound closure is approximately 30% slower in stressed mice. Delay in healing is associated with exaggerated acute inflammation and deficient bacterial clearance at the wound site. It has been suggested that wound hypoxia may contribute to the mechanisms of impaired cutaneous wound healing in the mouse SKH-1 model.
Optimal healing of a cutaneous wound is a stepwise repair program. In its early phase, an inflammatory oxidative burst generated by neutrophils is observed. 40% of neutrophils cytosolic protein weight is comprised of two calcium binding proteins S100A8 and S100A9. Our previous work has shown that S100A8 act as an oxidation sensitive repellent of human neutrophils in-vitro. Ala42S100A8, a site-directed mutant protein is resistant to oxidative inhibition and inhibits neutrophil recruitment in-vivo.
Accordingly, we tested the hypothesis that S100A8 may ameliorate wound healing in this model. We examined the effect of wild type and ala42S100A8 for their ability to ameliorate wound closure rates. The data indicated that a single local application of ala42S100A8 ameliorated the decreased rate of wound closure resulting from stress. This occurred without significantly affecting wound bacterial clearance. Wild type S100A8 only had a partial beneficial effect on the rate of wound closure. Those findings support further translational studies of S100 based intervention to ameliorate impaired wound healing.
Keywords: Wound healing, calprotectin, S100A8, oxidation, psychological stress, neutrophils, bacterial clearance, cutaneous, translational
1. Introduction
Psychological stress affects physiological functioning both directly via somatic pathways, and indirectly by triggering maladaptive behaviors. Studies have suggested that psychological factors appear to interfere directly with wound healing and closure (Godbout and Glaser, 2006). Marital stress, manifested as marital strife, was associated with a 40% delay in wound healing and a defective immune response (Glaser et al., 1999). Caregivers of patients with Alzheimer’s disease who reported emotional conflicts, compared with age-matched non-caregiver controls, required 9 additional days to heal a full-thickness skin wound (Kiecolt-Glaser et al., 1995), with the widest disparities in healing rates observed in the first 2 weeks after wounding. A study of oral wounds in dental students revealed that healing proceeded at a 40% slower average rate in oral wounds placed 3 days before examinations, compared with identical wounds placed in the same students during their summer vacations (Marucha et al., 1998).
Cutaneous wound healing is a multi-step process prone to hypoxia. Conceptually, wound healing can be divided into three sequential but overlapping phases: inflammation, proliferation and remodeling (Thomas et al., 1995). Oxygen metabolism and redox homeostasis are critical in all phases of the healing process. Initially, wounding produces damage to blood vessels and reduces oxygen availability. Furthermore, the early post-wounding acute inflammation phase is characterized by the rapid recruitment and activation of peripheral neutrophils. During their activation neutrophils consume large amount of oxygen to produce antimicrobial reactive oxygen species which profoundly alter the biology of the inflamed tissue. Consequently the healing process combines an early reduction in blood supply with a substantial increase in oxygen demand further contributing to potential wound hypoxia.
In the established mouse SKH-1 restraint stress model, psychological stress induces delays in wound closure from day 1 post-wounding. Wound closure is slowed by approximately 30% in mice subjected to stress (Padgett et al., 1998). Stress also results in more than four fold higher cortiscosterone plasma levels with a peak after the 4th and 5th stress cycles (Padgett et al., 1998). Delay in wound closure is associated with disregulated inflammation and defective bacterial clearance. Oxygen metabolism and hypoxia appear to be involved in impaired healing seen in stressed animals. Significantly higher inducible nitric oxide synthase (iNOS) levels are present in wounds of stressed compared with control mice (Gajendrareddy et al., 2005). Moreover, hyperoxia produced with hyperbaric oxygen therapy (HBOT) returns iNOS expression to control levels, and partially or wholly reverses the impairment of wound closure associated with psychological stress (Gajendrareddy et al., 2005).
S100A8 is an oxidation-sensitive anti-inflammatory protein which combines with S100A9 to form the heterocomplex calprotectin. By weight, calprotectin represents 40% of total protein in the neutrophil cytosolic fraction. S100A8 and S100A9 are oxidation sensitive repellent of neutrophils which also inhibit neutrophil chemotaxis toward bacterial products (i.e. formylated peptides) in-vitro (Sroussi et al., 2006; Sroussi et al., 2007). Ala42S100A8, an oxidation-resistant analog of S100A8 engineered using site-directed mutagenesis, retains its chemo-repulsive activity under oxidative conditions, which would otherwise inhibit the wild type S100A8 protein. In the rat air-pouch model of acute inflammation, ala42S100A8 inhibits the recruitment of neutrophils stimulated by bacterial endotoxins (Sroussi et al., 2006).
Previous work has shown that neutrophil depletion results in an accelerated wound closure (Dovi et al., 2003). While neutrophils play an important function in controlling and eliminating bacterial contamination of the wound, reduced neutrophil recruitment and activation seems to be beneficial for wound closure rates possibly by reducing oxygen demand and wound hypoxia. Accordingly, we hypothesized that S100A8 protein can ameliorate wound healing in a psychological stressed induced model of impaired wounds. Because of the anti-inflammatory nature of S100A8, a secondary aim of this work was to ascertain that S100A8 would not cause a clinically significant defect in wound bacterial clearance. We tested the effect of wild type and ala42S100A8 on wound closure in stressed and non-stressed animals. We found that ala42S100A8 introduced locally immediately after wounding ameliorated the delay in wound closure rates caused by restraint stress. This beneficial effect occurred without negatively impacting in a clinically significant manner bacterial clearance in the wounds.
2. Material and Methods
Animals
This protocol was approved by the Committee on Animal Research at the University of Illinois at Chicago. Virus antibody-free, female SKH-1 mice, 5–6 weeks of age, were purchased from Charles River, Inc (Wilmington, MA). The animals were allowed to acclimate to the animal facility for at least one week prior to experimental procedures.
Restraint Stress
The restraint stress protocol is based on established procedures (Gajendrareddy et al., 2005; Mercado et al., 2002; Padgett et al., 1998). Restraint was initiated 3 days before wounding. Restraint stress (RST) in this model consists of confinement of mice within loosely fitting, well-ventilated 50 ml conical tubes for a period of 12–14 hours during their active nocturnal cycle. Animals were subjected to restraint for an additional five cycles on the day of wounding and for four days thereafter, then stress was discontinued. Mice do not feed during restraint; hence controls were deprived of food and water (FWD) during the restraint period.
Excisional Wounds
Mice were anesthetized with a single 0.25 ml i.p. injection of Ketaset solution (Aveco, Fort Dodge, IA) at a concentration of 7.8 mg/ml, plus Rompum (Haver-Lockhart, Shawnee, KS) at 0.44 mg/ml. Each animal received two standardized circular cutaneous wounds with 3.5mm punch (Miltex Instrument Company), placed just behind the shoulder blades with a biopsy punch.
Wound treatment
S100A8 and mutant ala42S100A8 were produced, cleaved and purified from a GST fusion construct previously described (Tugizov et al., 2005) (Sroussi et al., 2006). The endotoxin level in the recombinant proteins were below 1 ng/μg of proteins as measured by limulus amoebocyte lysate assay (LAL) (Associates of Cap Cod, Falmouth, MA). Solution of S100 proteins at a concentration of 1 μg/ml were used for those experiments. Introduction of S100A8 protein or control saline vehicle were conducted through local injection intradermally of 25 μl solution in the tissue surrounding the wounds with the help of Micro-Fine IV syringes 28G1/2 (Becton Dickinson, Franklin Lakes, NJ). Careful wound manipulation ensured no direct disruption of the wound site. An additional 10 μl solution was applied directly to the wounds.
Wound Biopsy
Wound biopsies were performed to evaluate bacterial clearance. Mice were anesthetized as described above. The dorsal area was cleaned with Betadine and a uniform, full-thickness wound located on the dorsum just below the shoulder blades was created with a sterile 6.0-mm punch (Miltex Instrument Company).
Measurement of wound infection
Wound infection was assessed with measurement of bacterial load at the wound site by methods adapted from Rojas (Rojas et al., 2002). At day 1 and day 5 post-wounding, mice were euthanized; wounds were collected with a 6-mm punch (see above) and homogenized in 1 ml of sterile PBS. Homogenates were serially diluted (1:10); plated in duplicate on BHI agar, and incubated for 15 h. Bacterial colonies were counted.
Measurement of Wound Size
Beginning on the day of wounding (day 0), each animal’s wounds were photographed daily until day 5 post-wounding. Photographs of the wound were obtained with a standard-sized dot placed next to the site. Digitized photographs were analyzed using photoplanimetry (Marucha et al., 1998). An investigator blinded to treatment group and day of photograph measured wound size. Wound size was expressed as the percentage of the wound area determined on every post-wounding day, compared with the original wound area.
Data analysis
All statistics were performed using SPSS 15.0 (Chicago, IL). Main effect statistical significance was determined at p < 0.05.
Repeated Measure ANOVAs were performed with the ‘days since wounding’ as the Within-Subjects factor and “stress/no stress” and “treatment group” as the Between-Subjects factors. Bonferroni post-hoc analyses were performed for all statistically significant main effects.
Two-Way ANOVAs were performed with ‘colony count’ as the dependent variable and “stress/no stress” and “treatment group” as the Between-Subjects factors.
3. Results
Amelioration of wound closure rates in psychologically stressed animals treated with ala42S100A8
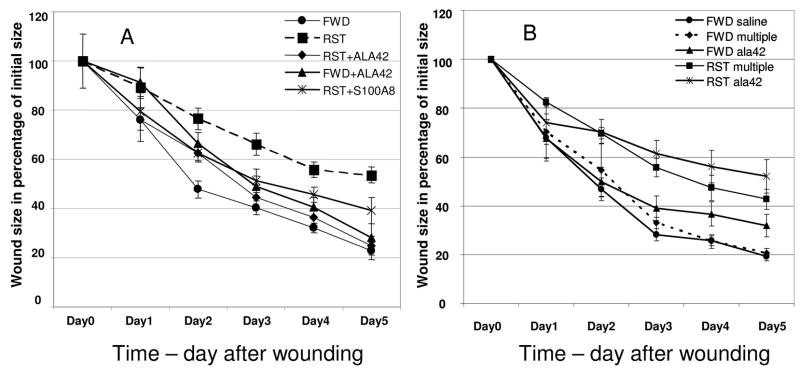
Photoplanometric analysis of wound size indicated that, across the days since wounding, there was a significant effect on wound closure for both the “stress (RST)/no stress (FWD)” and “treatment group”. For the “stress/no stress” condition, restraint animals experienced a delay in wound closure when compared to control animals (F = 6.971, p = 0.001). As soon as day 1 post-wounding, average wound size (in percentage of their original size) were significantly larger in restraint stressed animals (RST) when compared to control animals (FWD). Additionally, the three treatment groups; no treatment (saline control), ala42S100A8 and WTS100A8 had statistically significant wound closure rates across time across time (F = 10.29, p = 0.000) with the control group exhibiting the most closure followed by ala42S100A8 treated wounds (Figure 1A). From day 2 on, average wound size in stressed animals treated with ala42S100A8 was significantly smaller than the average wound size in stressed animals treated with saline (t18 ranging 3.162 to 6.080 with all p = .000 except day 2 where p = .01). Wound closure in stressed animals treated with ala42 S100A8 displayed a closure rate similar to the closure rates observed in the non stressed food and water deprived (FWD) group.
Figure 1.
Wound closure over time in percentage of initial wound size. A. Restraint stress (RST) causes a delay in wound closure form day 1 post wounding when compared to control food and water deprived mice (FWD). Ala42S100A8 (RST-ALA42) reverses the delay in closure caused by restraint stress (RST). # p = 0.01, *p = 0.000 comparing restraint stress group (RST) to RST+ala42S100A8. B. FWD and RST multiple referred to groups with daily injection of saline. FWD saline group was injected with saline once only post- wounding. ALA42 referred to groups injected daily with ala42S100A8. Daily application of ala42S100A8 results in no sustained amelioration in wound closure rates but in slower healing in FWD animals. # p = 0.012, *p = 0.006 comparing FWD multiple (daily saline) to ALA42 (daily injection of ala42S100A8). The data represent mean +/− SEM (n=5 per group) of four (a) and two (B) experiments with similar results.
A similar treatment of the wounds, immediately after wounding with wild type S100A8 (WTS100A8) resulted in a partial acceleration in wound closure rate in stressed animals. This effect did not reach statistical significance when compared to the closure rate in the stressed animals treated with saline (Figure 1A). In additional experiments, daily administration of ala42S100A8 to the wounds was tested for its ability to accelerate wound closure (Figure 1B). Mice treated with repeated daily injection of saline (FWD multiple) displayed wound closure rates similar to that of single saline treatment mice (FWD saline). Daily injections of ala42S100A8 did not result in the amelioration of wound closure rates observed with a single application post-wounding. Instead, daily treatment with ala42S100A8 resulted in a significant delay in wound closure starting at day 4 post-wounding in non-stressed animals (FWD multiple v. FWD ALA42 (t18 = −2.799, p =.012; t18 = −3.152, p = .006 for day 4 and day 5 respectively).
Effect of ala42S100A8 on wound bacterial clearance
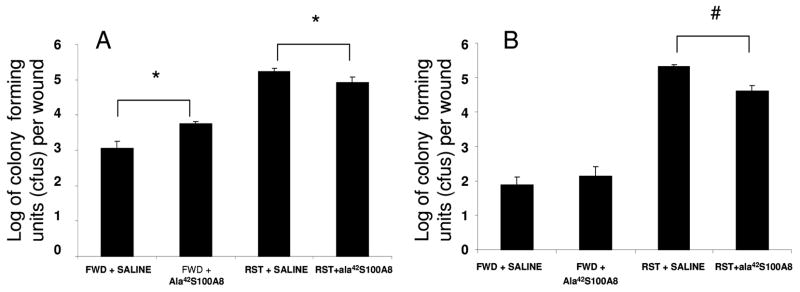
To assess the effect of anti-inflammatory ala42S100A8 on bacterial clearance, we next tested the effect of a single local administration of ala42S100A8 on wound bacterial counts. Our previous work demonstrated that, ala42S100A8 inhibited neutrophil recruitment in-vivo (Sroussi et al., 2006). We tested the possibility that ala42S100A8 treatment, applied immediately post-wounding, would cause a decrease in bacterial clearance secondary to its anti-inflammatory effect. Accordingly bacterial counts in the wound of ala42S100A8 and vehicle (saline) treated were measured at day-1 and day-5 post wounding in stressed and control mice. Firstly, the data indicated that restraint stress in this model resulted in a dramatic increase in wound bacterial counts both at day 1 (Figure 2A) and day 5 (Figure 2B) post-wounding. This finding was in accordance with data published by others (Rojas et al., 2002). Secondly, ala42S100A8 treatment resulted in a statistically significant increase in wound bacterial counts in FWD mice on day 1 post wounding (t16 = −6.179, p = .000) (Figure 2A). By day 5 post wounding, bacterial counts had reverted to control values in ala42S100A8-treated FWD mice (Figure 2B). In contrast, statistically significant reduction in bacterial loads were recorded on both day 1 and 5 (t18 = 2.896, p = .01 and t16 = 7.492, p = .000 respectively) in the wounds of stressed mice treated with ala42S100A8 when compared to saline treated wounds in stressed mice (Figure 2B).
Figure 2.
Effects of restraint stress on bacterial clearance in control food and water deprived mice (FWD) and restraint stress mice (RST) at day 1 (A) and day 5 (B) post-wounding. Ala42S100A8 significantly increases bacterial counts in FWD mice at day 1 and decreases bacterial counts at day 1 and 5 in RST mice. Mean +/− SEM. n=5 per group. # P<0.01, * P<0.001 comparing CFU counts from ala42S100A8 treated v. control vehicle (saline) treated wounds.
4. Discussion
The data collected in this study indicate that, in the SKH-1 mouse model, psychological stress caused a delay in dermal wound closure associated with defective bacterial clearance. The delay in wound closure was observed as early as one day after wounding and the defective bacterial clearance was measured throughout the stress cycles at day 1 and day 5 post wounding. Those observations are in accordance with data reported in previous studies (Padgett et al., 1998; Rojas et al., 2002).
The data also indicated that a single local application of ala42S100A8 post-wounding ameliorated the rate of wound closure in wounds impaired by restraint stress. In effect, the rate of wound closure in stressed mice is restored to control levels with ala42S100A8 treatment of the wound. A similar application of wild type S100A8 (WTS100A8) partially ameliorated delayed wound closure caused by stress but to a much lesser extent than ala42S100A8. Ala42S100A8 is a molecule engineered to inhibit neutrophil recruitment in-vivo in an oxidative condition, which would inhibit the function of WTS100A8 (Sroussi et al., 2006). Since stress impaired wound closure was shown to be reversed by hyperbaric oxygen therapy (HBOT) (Gajendrareddy et al., 2005) in the SKH-1 model, the greater effect of ala42S100A8 over WTS100A8 on wound closure rates offers further support to redox homeostasis as a mechanism for the dysregulation of wound closure in psychologically stressed animals. Alternatively, while not directly involved in the mechanism of wound healing impairment, redox signaling and its manipulation may represent practical therapeutic targets to ameliorate impaired wound healing.
Treatment with ala42S100A8 caused a significant increase in wound bacterial counts at day 1 in control animals. This increase in bacterial count is unlikely to be clinically significant as supported by normal wound closure rates in non-stressed control animals treated with ala42S100A8 and by restoration of normal bacterial count by day 5 post-wounding. Conversely, in restraint stressed animals, ala42S100A8 caused a decrease in bacterial counts. Whether this decrease in bacterial counts explains the beneficial effect of ala42S100A8 on wound closure rates is unknown. The data on bacterial clearance in stressed animals treated with ala42S100A8 indicated a modest reduction in bacterial counts which remain well above the bacterial counts observed in control food and water deprived control mice. This denoted that ala42S100A8 restored normal wound closure rates in stressed animals without restoring normal bacterial wound clearance. Similar observations were made with glucocorticoid receptor antagonists in the same SKH-1 ameliorates wound healing rates in stressed SKH-1 mice (Padgett et al., 1998) without reestablishing normal wound bacterial clearance (Rojas et al., 2002). The magnitude of the observed improvement in bacterial clearance in stressed mice treated with ala42S100A8 was similar to what was observed with RU486, a glucocorticoid antagonist (Rojas et al., 2002). Altogether, it appears that while restraint stress in this model is causative for delay wound closure and defective bacterial clearance, no clear and simple causality can be established between a defective bacterial clearance and a delay in wound closure rates in this model. The lack of a simple causality between those two outcomes is further supported by observations that bacterial inoculation of wounds in this model can accelerate wound closure rates (Rojas et al., 2002). It is therefore unlikely that ala42S100A8 affected wound closure rates by altering bacterial clearance. The aim of the bacterial studies in this work was to ascertain that ala42S100A8, an anti-inflammatory molecule, would not impair bacterial clearance to the point where it would represent a clinical significant factor. The data we present support this aim.
Finally, daily injection of ala42S100A8 did not ameliorate wound closure rates and may have caused a significant late delay in wound closure in control non-stressed animals. This would imply that the effects of ala42S100A8 while beneficial initially, may compromise healing at later stages of healing possibly at the proliferative stage. It is possible that applications of ala42S100A8 in later phases of wound healing, may also interfere with the beneficial recruitment of macrophage (Savill et al., 1989) or alternatively that ala42S100A8 may exert additional activities with detrimental consequences on the rate of wound closure such as an anti proliferative effect (Yui et al., 2003). The timing of a S100A8 based anti-inflammatory intervention in improving wound healing is therefore critical in establishing its overall effect on wound healing. Understanding the mechanisms of action of ala42S100A8 and its temporal relationship with the well-orchestrated wound healing process may help us design S100A8-based mechanistically and temporally targeted strategy for the treatment of impaired wound healing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–55. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- Gajendrareddy PK, Sen CK, Horan MP, Marucha PT. Hyperbaric oxygen therapy ameliorates stress-impaired dermal wound healing. Brain Behav Immun. 2005;19:217–22. doi: 10.1016/j.bbi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry. 1999;56:450–6. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–7. doi: 10.1007/s11481-006-9036-0. Epub 2006 Aug 10. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–6. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998;60:362–5. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- Mercado AM, Quan N, Padgett DA, Sheridan JF, Marucha PT. Restraint stress alters the expression of interleukin-1 and keratinocyte growth factor at the wound site: an in situ hybridization study. J Neuroimmunol. 2002;129:74–83. doi: 10.1016/s0165-5728(02)00174-1. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- Rojas IG, Padgett DA, Sheridan JF, Marucha PT. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun. 2002;16:74–84. doi: 10.1006/brbi.2000.0619. [DOI] [PubMed] [Google Scholar]
- Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–75. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroussi HY, Berline J, Dazin P, Green P, Palefsky JM. S100A8 Triggers Oxidation-sensitive Repulsion of Neutrophils. J Dent Res. 2006;85:829–33. doi: 10.1177/154405910608500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81:818–24. doi: 10.1189/jlb.0706433. Epub 2006 Nov 30. [DOI] [PubMed] [Google Scholar]
- Thomas DW, O’Neill ID, Harding KG, Shepherd JP. Cutaneous wound healing: a current perspective. J Oral Maxillofac Surg. 1995;53:442–7. doi: 10.1016/0278-2391(95)90721-1. [DOI] [PubMed] [Google Scholar]
- Tugizov S, Berline J, Herrera R, Penaranda ME, Nakagawa M, Palefsky J. Inhibition of human papillomavirus type 16 E7 phosphorylation by the S100 MRP-8/14 protein complex. J Virol. 2005;79:1099–112. doi: 10.1128/JVI.79.2.1099-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull. 2003;26:753–60. doi: 10.1248/bpb.26.753. [DOI] [PubMed] [Google Scholar]