Abstract
Proper transport and distribution of mitochondria in axons and at synapses are critical for the normal physiology of neurons. Mitochondria in axons display distinct motility patterns and undergo saltatory and bidirectional movement, where mitochondria frequently stop, start moving again, and change direction. While approximately one-third of axonal mitochondria are mobile in mature neurons, a large proportion remains stationary. Their net movement is significantly influenced by recruitment to stationary or motile states. In response to the diverse physiological states of axons and synapses, the mitochondrial balance between motile and stationary phases is a possible target of regulation by intracellular signals and synaptic activity. Efficient control of mitochondrial retention (docking) at particular stations, where energy production and calcium homeostasis capacity are highly demanded, is likely essential for neuronal development and function. In this review, we introduce the molecular and cellular mechanisms underlying the complex mobility patterns of axonal mitochondria and discuss how motor-adaptor complexes and docking machinery contribute to mitochondrial transport and distribution in axons and at synapses. In addition, we briefly discuss the physiological evidence how axonal mitochondrial mobility impacts synaptic function.
Keywords: mitochondria, axonal transport, docking, synaptic plasticity, kinesin, motor adaptor, anterograde transport, retrograde transport, stationary mitochondria, mitochondrial mobility
Introduction
Neurons in the central nervous system are extremely polarized and consist of three distinct functional and subcellular domains: a cell body (or soma); a long axon with uniform diameter; and thick dendrites with many branches. While the soma and dendrites receive and process information, the axon transfers it by generating an action potential. Essential materials and mitochondria are generally synthesized or generated within the soma and delivered through the neuronal processes to their final destination, i.e., synaptic terminals.
Mitochondria in the neuronal cell bodies are transported down processes in response to changes in the local energy state and metabolic demand (Hollenbeck, 1996). Like many other neuronal organelles, mitochondria exhibit dynamic and bidirectional movements along neuronal processes. Compared with the slow bulk flow of axoplasmic transport (1 mm/day), the maximal rate of mitochondrial movement is generally quoted to be ~20–70 mm/day (Morris and Hollenbeck, 1993; Ligon and Steward, 2000). Since individual mitochondria display frequent pauses and reversal, the average velocity of mitochondrial movement falls between that of fast-moving vesicles and slow-moving cytoskeletal proteins (Blaker et al., 1981).
Because of their extreme polarity, neurons require specialized mechanisms to regulate the transport and retention of mitochondria at specific subcellular locations. Mitochondria accumulate in the vicinity of active growth cones of developing neurons (Morris and Hollenbeck, 1993) and are present at some synaptic terminals (Shepherd and Harris, 1998; Rowland et al., 2000). Mitochondria are thought to produce more than 90% of the cellular ATP in neurons, which supports many neuronal functions including mobilization of synaptic vesicles during intensive neuronal activity and assembly of the actin cytoskeleton among synapses (Verstreken et al., 2006; Lee and Peng 2008). In addition to aerobic ATP production, mitochondria have been associated with certain forms of short-term synaptic plasticity by buffering Ca2+ at nerve terminals (Tang and Zucker, 1997; Billups and Forsythe, 2002; Levy et al., 2003; Yang et al., 2003). Loss of mitochondria from axonal terminals in Drosophila results in impaired synaptic transmission (Stowers et al., 2002; Guo et al., 2005; Verstreken et al., 2005). Defective transport of axonal mitochondria is implicated in human neurological disorders and neurodegenerative diseases (see reviews by Hirokawa and Takemura, 2004; Chan, 2006; Stokin and Goldstein, 2006).
Synaptic structure and function are highly plastic and undergo spontaneous and activity-dependent remodeling, thereby changing the demand for mitochondria at nerve terminals. While mechanisms coordinating mitochondrial energy state and motility are yet to be determined, recent findings raise the possibility that anterograde movement transports mitochondria to the sites of action in neurons while retrograde movement transports mitochondria to the cell body for degradation and recycling (Miller and Sheetz, 2004). Efficient control of mitochondrial docking or retention at particular sites of axons and synapses is likely essential for the diverse physiological states of axons and synapses. Thus, mitochondrial mobility and stationary docking are possible targets of regulation by intracellular signals and synaptic activity (Hollenbeck and Saxton, 2005; Chan, 2006).
The axonal cytoskeleton is required for mitochondrial transport
Microtubules
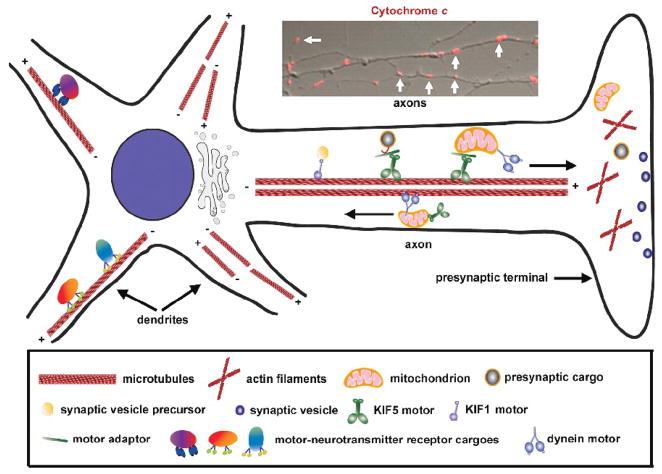
The cytoskeleton provides neurons with support not only to maintain their highly specialized structure, but also to allow the robust transport and stable docking events. Although microtubules, actin, and intermediate filaments are three major components of the axonal cytoskeleton, only microtubules and actin filaments play critical roles in mitochondrial transport. Neurons contain an elaborate network of microtubules radiating from the soma to the terminal regions. Axonal microtubules are organized in a polar array, with ‘plus’ ends directed toward the terminals and ‘minus’ ends directed toward the cell soma although in dendritic microtubules the polarity is mixed (Hirokawa and Takemura, 2004). Microtubule polarity and organization in axons are critical for the targeted transport of mitochondria from the soma to distal axons and synapses by the microtubule-associated motor proteins, which include members of the kinesin superfamily and cytoplasm dynein. While kinesin motors are mostly plus-end directed, dynein travels toward the minus ends of microtubules. Therefore, kinesin motors generally mediate anterograde axonal transport and dynein drives retrograde axonal transport (Figure 1).
Fig. 1.
Axonal transport of mitochondria from the soma to synapses. In axons, microtubules are uniformly organized with the plus (+) ends facing toward the axonal terminals and the minus (−) ends toward the cell body. However, the organization of microtubules in dendrites shows mixed orientation. Polarity and organization of microtubules in axons are critical for the targeted transport of synaptic cargoes and organelles by microtubule-associated motor proteins. While kinesin motors are mostly plus-end directed, dynein travels toward the minus ends of microtubules. Kinesin KIF5 is the major motor responsible for anterograde transport of axonal mitochondria and dynein drives retrograde axonal transport of mitochondria. Inset: Representative imaging showing Cytochrome c-labeled mitochondria (marked by white arrows) along axonal processes (The image is adapted with permission from Qian Cai, Claudia Gerwin, and Zu-Hang Sheng. Syntabulin-mediated anterograde transport of mitochondria along the neuronal processes. Journal of Cell Biology 170, 959–969. 2005).
Actin filaments
The actin cytoskeleton is relatively enriched in the cellular compartments that are essential for synaptic function, such as presynaptic terminals and dendritic spines. Actin monomers assemble into a flexible helical polymer with two distinct ends, one growing fast and one growing slower. In neurons, actin filaments are also bundled into a network. However, the organization of actin cytoskeleton in the nerve terminals has not been fully elucidated. The orientation of actin filaments can directly affect the activity of actin-based motor myosin (Bridgman, 2004). The “dual transport” model was proposed in which microtubule-based motors ensure long-range axonal transport, whereas short-range movement of vesicles and organelles at nerve terminals, growth cones, and subcortical plasma membrane regions depends primarily on actin-based myosins (Langford, 2002). The identified physical interaction between microtubule- and actin-based motors suggests that mitochondrial transport can be coordinated along both cytoskeletal systems (Bridgman, 2004).
Motor proteins required for mitochondrial transport in axons
Kinesin motors
Long-distance fast axonal transport of mitochondria depends on motor proteins, the specialized enzymes that use the energy of ATP hydrolysis to generate movement along the microtubule-based cytoskeleton (Hollenbeck, 1996). While cytoplasmic dynein motors are the driving force behind retrograde movement (Pilling et al., 2006), kinesin motors are responsible for anterograde transport of axonal mitochondria (Figure 1) (Tanaka et al., 1998; Ligon and Steward, 2000; Stowers et al., 2002; Gorska-Andrzejak et al., 2003; Cai et al., 2005; Guo et al., 2005; Glater et al., 2006). Kinesin-1 was the first identified axonal transport motor which drives plus end-directed transport along microtubules in vitro (Vale et al., 1985; Hirokawa et al., 1991). Kinesin-1 contains two heavy chains and two light chains. The heavy chains of kinesin-1, known as KIF5, can function as a motor and form homo- or heterodimers among themselves through the coiled-coil region in the stalk domains. Each KIF5 heavy chain contains an N-terminal motor domain that binds directly to microtubules, whereas its C-terminal domain mediates the association of KIF5 with kinesin light chains or direct interaction with the intracellular cargo vesicles. Therefore, attachment of kinesin-1 can be directly through the specific cargo-binding region at the C-terminal domain, or indirectly via the light chains, indicating two distinct forms of motor-cargo coupling.
It is likely that kinesin KIF5 is the major motor responsible for anterograde transport of axonal mitochondria (Hurd and Saxton, 1996; Stowers et al., 2002; Cai et al., 2005; Glater et al., 2006). Targeted disruption of kif5b resulted in abnormal perinuclear clustering of mitochondria instead of spreading throughout the cytoplasm and toward the cell periphery in undifferentiated extra-embryonic cells (Tanaka et al., 1998). Moreover, Drosophila mutants of kif5 demonstrated impaired mitochondrial transport and reduced distribution in larval motor axons (Hurd and Saxton, 1996). In addition to KIF5, KIF1Bα, a member of the kinesin-3 family, was also shown to interact directly with mitochondria. KIF1Bα can transport purified mitochondria along microtubules at a velocity of 0.5 μm/s, suggesting that it might be another kinesin motor protein involved in mitochondrial transport (Nangaku et al., 1994). Although the mutation of kif1B leads to peripheral neuropathies in these animals, a detailed role for KIF1Bα in anterograde neuronal mitochondrial transport still remains unclear.
Dynein motors
Cytoplasmic dynein is the major motor driving retrograde transport along microtubules in axons. Dynein is a large and complex molecule and comprises two heavy chains as well as several intermediate, light intermediate and light chains. These polypeptides are thought to function primarily in mediating the association of dynein motor with cargoes or in regulating motility. Dynactin is also assembled as a large complex composed of eleven different subunits and can directly bind to cytoplasmic dynein and microtubules through the p150Glued component. The dynactin complex is not essential but can enhance the processivity of the dynein motor, and also mediates some interactions with cargo (Waterman-Storer et al., 1995; Karki and Holzbaur, 1999; King and Schroer, 2000), or coordinates bi-directional axonal transport (Haghnia et al., 2007). The studies on single dynein-dynactin motor complexes revealed that the complex could move bi-directionally although the overall bias in direction is towards the microtubule minus end (Mallik et al., 2005; Ross et al., 2006). This ability might enable dynein motor to bypass obstacles during transport within the cells. The function of the dynein-dynactin complex is extremely important in motor neurons that are most vulnerable to defects in dynein function (Hafezparast et al., 2003; Puls et al., 2003).
Cytoplasmic dynein is essential for axonal transport of mitochondria in neurons. In Drosophila, cytoplasmic dynein is the primary motor for mediating mitochondrial retrograde transport (Pilling et al., 2006). In addition, mutations of dynein heavy chain and dynactin p150Glued disrupt fast organelle transport in both directions, and consequently result in axonal swellings composed of retrograde and anterograde cargoes, including mitochondria, a phenotype similar to those caused by kinesin mutations (Martin et al., 1999). Moreover, expression of a mutant form of the dynactin subunit p150Glued causes mitochondrial accumulation within the cell body (Levy et al., 2006). Given that neuronal mitochondria display distinct saltatory and bidirectional movements, it is likely that two opposing kinesin and dynein motor proteins coordinate mitochondrial transport in axons although the mechanism for such coordination remains to be elucidated. Thus, it will be interesting to determine if there is a regulatory linkage between the two opposing motors and how these motors coordinately control the overall movement and distribution of axonal mitochondria in correlating with synaptic activity and neuronal function.
Myosin motors
While the microtubule-based motor proteins kinesin and dynein quickly transport cargoes and organelles through lengthy axons, myosins drive short-distance trafficking along actin filaments at presynaptic terminals and growth cones. Even though there is no direct evidence for a role of myosin in actin-based mitochondrial transport in neurons, in the systems of budding yeast, Aspergillus and plant cells, actin-dependent mitochondrial motility is well characterized. Movement of mitochondria along actin filaments supports the idea that myosin motors may also drive short-range mitochondrial transport within certain regions of axons, particularly at nerve terminals where actin filaments are relatively enriched (Figure 1). Although myosins I, II, V and VI were reported to be involved in the transport of organelles and vesicles in neurons, with the exception of myosin V, little is known about the localization and function of other unconventional myosins and their roles in axonal mitochondrial transport (Hollenbeck, 1996; Bridgman, 2004).
Myosin V is a two-headed motor containing a unique globular tail domain and undergoes multiple steps before dissociating from an actin filament (Langford, 2002). Since the motility of myosin V-associated cargoes exhibit a rate similar to mitochondrial movement along actin filaments (Morris and Hollenbeck, 1995), but slower than other axonal vesicles and organelles (Cheney et al., 1993; Wolenski et al., 1995), myosin V is a reasonable candidate for mediating mitochondrial transport along axons. In addition, myosin V can interact directly with either a kinesin motor to form a hetero-motor complex or with the 8-kDa light chain of dynein (Naisbitt et al., 2000), raising the possibility that the dual-motor complexes may facilitate coordination of mitochondrial long-range transport along microtubules and short-range movement on actin filaments. Thus, myosin V is a good candidate for driving actin-based mitochondrial movement, although it remains to be investigated whether myosin V is attached to axonal mitochondria and to determine how much or to what degree synapse-directed transport of mitochondria is required for myosin V.
Motor-adaptor complex essential for mitochondrial transport
Linkage of cargoes with the appropriate transport motors must occur with a high degree of specificity to preserve organelle identity and the proper targeting and progression within cells. In order to accommodate the specific delivery of mitochondria to the axonal domain, neurons must employ mechanisms that attach the organelles to various molecular motors and transport them by the microtubule-based trafficking machinery. There are at least two mechanisms through which motors connect with their cargoes: direct linkage through cargo receptors and indirect linkage via linker/adaptor molecules. Emerging evidence indicates that the indirect coupling of motors with transport cargoes through their specific adaptors is a significant mechanism in neurons (Goldstein and Yang, 2000). While it is unclear whether lipid interactions contribute to recruiting motors to mitochondrial membranes, several adaptor complexes have been identified for specifically linking mitochondria with transport machinery.
Syntabulin
Syntabulin was first reported as an adaptor linking the syntaxin-containing vesicles to the heavy chain of kinesin-1 (KHC, KIF5B) (Su et al., 2004). Our recent study revealed a critical role of syntabulin in the anterograde transport of mitochondria in neuronal processes (Cai et al., 2005). First, we showed that syntabulin is a peripheral membrane-associated protein, relatively enriched in the mitochondrial fraction, and associated with mitochondria via its carboxyl-terminal sequence. Second, immunocytochemical and time-lapse imaging studies in live neurons demonstrated that syntabulin co-localized and co-migrated with mitochondria within axonal processes. Furthermore, knockdown of syntabulin expression or competitive blocking of the syntabulin–KHC interaction with binding-domain transgenes dramatically changed mitochondrial distribution in cultured hippocampal neurons: mitochondria were predominantly clustered in the soma but distributed sparsely in the processes. This distribution profile was consistent with the observation from mobility analysis in live neurons: syntabulin loss-of-function impaired anterograde but not retrograde transport of mitochondria along axonal processes. The loss-of-function phenotype suggests either a failure of mitochondrial loading onto the kinesin motor for anterograde transport or a defect in regulating motor activity. This provides direct evidence that syntabulin is an important component of the mitochondrial trafficking machinery. Based on these findings, we propose that syntabulin functions as a linker molecule or a component of the adaptor complex involved in KIF5-mediated trafficking of mitochondria (Figure 2). However, several important questions remain: How is the function of syntabulin as a linker molecule regulated? Are syntabulin-mediated mobility and distribution of mitochondria modulated in correlating with synapse formation and synaptic activity? Further characterization of syntabulin’s association with both the mitochondrial outer membrane and motor protein will contribute to understanding the molecular and cellular details of how this protein regulates mitochondrial distribution in axons and at synapses.
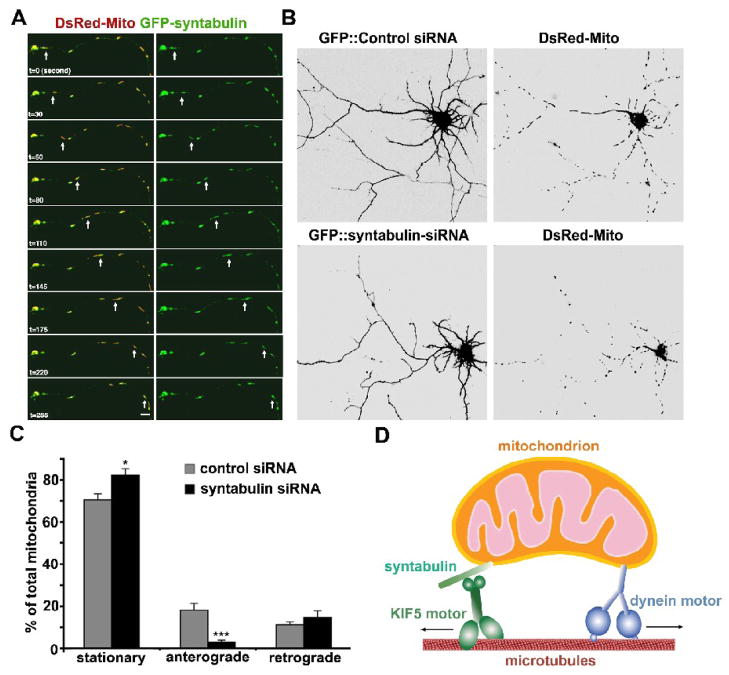
Fig. 2.
Syntabulin serves as a KIF5-adaptor and mediates anterograde transport of mitochondria along neuronal processes. (A) Syntabulin and mitochondria co-localize and co-migrate along axonal process. Cultured hippocampal neurons (DIV 6) were co-transfected with GFP-syntabulin (green) and DsRed-mito (DsRed2-tagged mitochondrial targeting sequence of cytochrome c oxidase, red) and the selected axonal process was time-lapse imaged 18 hours after transfection. Syntabulin-associated mitochondria move along the axonal processes. The arrows point at a moving mitochondrion associated with EGFP-syntabulin, which migrated anterogradely along the axon toward the growth cone at an average velocity of approximately 0.3–0.4 μm/sec. Images were collected every 5 seconds. Scale bar in panel B equals 10 μm. (B) Knockdown of syntabulin expresson reduces mitochondrial density in the neuronal processes of cultured hippocampal neurons. Hippocampal neurons (DIV4 or 5) were co-transfected with DsRed-mito and syntabulin-targeted siRNA or control siRNA. Mitochondrial distribution was examined by imaging GFP fluorescence (left panels) and DsRed-mito (right panels) in the neurons 4 or 5 days after transfection. Note that in the processes of neurons expressing syntabulin-siRNA, an abnormally lower density of mitochondria was observed relative to that of the neurons transfected with control siRNA. Scale bars, 10 μm. (C) Syntabulin loss-of-function impairs anterograde movement of mitochondria along axonal processes. The motility of DsRed-mito-labeled mitochondria was examined in live hippocampal neurons at DIV9-10 after tranfection with siRNAs. The direction of net movement for each mitochondrial organelle along axonal processes was determined during the same time window (15 min) of time-lapse imaging and the relative percentages of stationary, net anterograde, or net retrograde events were calculated. Knockdown of syntabulin inhibits anterograde but not retrograde movement of mitochondria along axonal processes. Of a total of 259 mitochondrial clusters from 11 cells transfected with the control-siRNA, one-third of the mitochondrial organelles in the axons are mobile (29.6 ± 3.0%), which moved either anterogradely (18.4 ± 2.9%) or retrogradely (11.2 ± 1.6%). Knockdown of syntabulin (total 383 mitochondria from 17 cells) exhibited a marked reduction in anterograde transport (***p<0.001), a slight but significant increase in stationary mitochondria (*p<0.02), and no significant change in retrograde movement of mitochondria (p=0.4). (D) Schematic diagram for the proposed role of syntabulin. Syntabulin serves as an adaptor in linking the KIF5 motor to mitochondria and in mediating anterograde transport of mitochondria in axons. (The images and quantification data are adapted with permission from Qian Cai, Claudia Gerwin, and Zu-Hang Sheng. Syntabulin-mediated anterograde transport of mitochondria along the neuronal processes. Journal of Cell Biology 170, 959–969. 2005).
Milton and Miro
The Drosophila protein milton is another well-studied adaptor component responsible for axonal transport of mitochondria. The milton mutation in Drosophila results in mitochondrial loss at synaptic terminals and in axons (Stowers et al., 2002). In addition, Miro, the mitochondrial Rho-like GTPase, locates at the mitochondrial outer membrane and is required for the association of milton and kinesin heavy chain KIF5 with mitochondria (Figure 3A)(Glater et al., 2006). Mutation of dMiro gene disrupts anterograde mitochondrial transport, thus preventing mitochondria from entering the axon and synapses, and in turn, impairing neurotransmitter release and acute Ca2+ buffering during prolonged stimulation (Guo et al., 2005). Furthermore, biochemical and genetic evidence demonstrates that recruitment of kinesin heavy chain KIF5 and mitochondrial transport are independent of kinesin light chains, which instead inhibit KIF5 binding to milton (Glater et al., 2006).
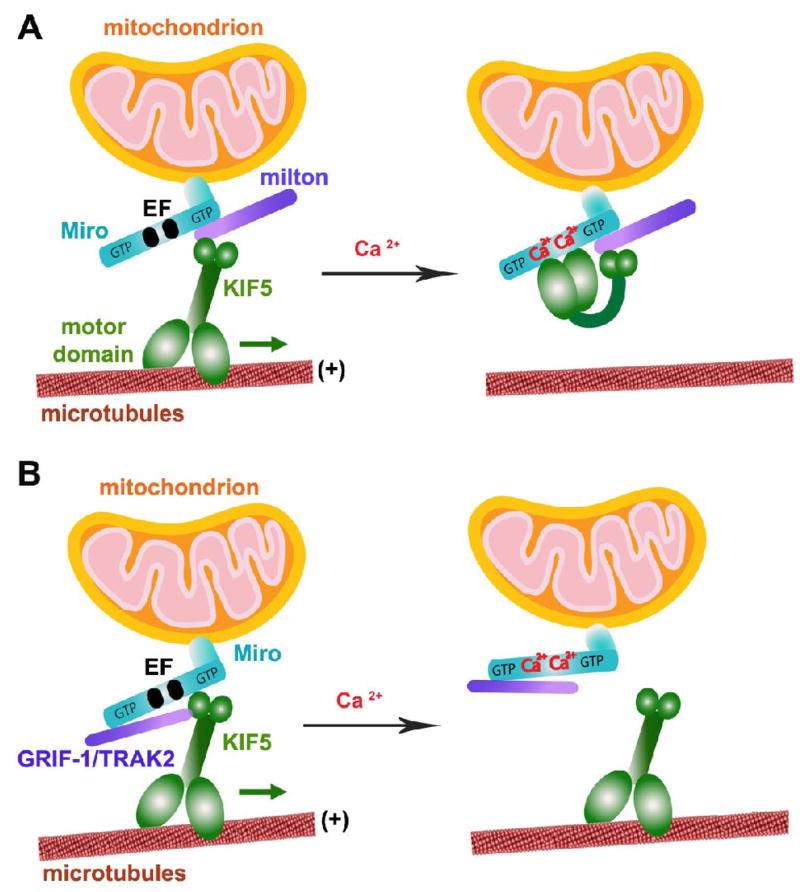
Fig. 3.
Schematic diagram for two proposed models of Miro as a Ca2+ sensor in regulating mitochondrial mobility. Miro contains two GTPase domains and calcium-binding EF hand motifs, thus regulates axonal mitochondrial motility by either GTP hydrolysis or calcium binding in response to calcium signals and synaptic activity. (A) Model 1: Ca2+-binding turns “off” KIF5 engagement with MTs. The tail of KIF5 is linked to Miro via milton in a Ca2+-independent manner, thus leaving its motor domain to engage with MTs. Ca2+-binding to the EF hands triggers the direct interaction of the motor domain with Miro, thus preventing the motor from engaging with MTs (Wang and Schwarz, 2009). (B) Model 2: Ca2+-binding detaches KIF5 from mitochondria. Mitochondrial transport is mediated by linking Miro to KIF5. Ca2+-binding to the EF hands dissociates Miro from KIF5 while KIF5-binding protein GRIF-1/TRAK2 (a mammalian homologue of milton) remains bound to Miro1 (MacAskill et al., 2008; 2009). (The model diagram is adapted with permission from Qian Cai and Zu-Hang Sheng. Moving or stopping mitochondria: Miro as a traffic cop by sensing calcium. Neuron 61, 493–496. 2009).
Although Milton and syntabulin are crucial motor adaptors specific for mitochondria, it is unlikely that both proteins regulate anterograde mitochondrial transport via the same mechanism. First, syntabulin directly binds to the cargo-binding domain of KIF5B (Su et al., 2004). While milton is co-immunoprecipitated with KIF5B, milton’s mammalian orthologues, γ-Aminobutyric acid type A (GABAA) receptor interacting factor-1 (GRIF-1) and β-O-linked N-acetylglucosamine transferase (OGT) interacting protein (OIP) (also termed TRAK) were shown to interact with KIF5C using yeast two-hybrid assays (Stowers et al., 2002; Smith et al., 2006). Second, syntabulin is a peripheral membrane-associated protein and associates with mitochondria via its carboxyl-terminal tail, which has the predicted hydrophobic anchor flanked by three positively charged residues at both ends, a similar structure conserved in most outer mitochondrial membrane proteins. In contrast, milton is thought to attach to mitochondria indirectly via interacting with the mitochondrial outer membrane protein Miro. Certain milton splice variants differ in their kinesin-associating properties, which may exert isoform-specific regulation of mitochondrial transport (Glater et al., 2006). Third, since Miro contains two GTPase domains and calcium-binding EF hand motifs, GTP hydrolysis or changes in calcium binding by the EF hands may provide molecular mechanisms underlying the regulation of milton-Miro-Kinesin complex, thus controlling axonal mitochondrial motility in response to calcium signals and synaptic activity. In contrast, syntabulin lacks these GTPase domains and the EF motifs but consists of multiple kinase consensus sites, providing potential regulation via signal-dependent protein phosphorylation. Identifying mitochondrial receptors or motor-adaptor complexes represents a major advance in understanding the mechanisms underlying mitochondrial transport. These newly identified mitochondrial motor-adaptor complexes provide molecular targets for further investigating the molecular and cellular details of how mitochondrial trafficking and distribution are regulated in neurons.
Neuronal signals that control mitochondrial mobility in axons
Mitochondria accumulate in the vicinity of active growth cones and branches in developing neurons, nodes of Ranvier, myelination boundaries, sites of axonal protein synthesis, and synaptic terminals (Hollenbeck and Saxton, 2005). Mitochondria in axons undergo anterograde transport toward the distal portions of axons and retrograde movement in the opposite direction. These transport events are tightly regulated in response to changes in the local energy state and metabolic demand. There is emerging evidence that mitochondrial transport can be actively regulated during certain biological responses. First, mitochondria are efficiently transported into nascent axons and recruited into stationary pools at the active growth cones of cultured neurons, whereas they switch to retrograde movement or mitochondrial density is significantly reduced during the suppression of axonal growth (Morris and Hollenbeck, 1993; Ruthel and Hollenbeck, 2003). In developing fly larval neurons, mitochondria exhibit greater anterograde movement and increased density along elongated axon (Pilling et al., 2006; O’Toole et al., 2008).
Second, mitochondrial motility can also be modulated by neuronal activity in response to altered intracellular Ca2+ or released neurotransmitters (Yi et al., 2004). Ca2+ influx occurs at presynaptic terminals and postsynaptic dendritic spines, where mitochondria are often retained to maintain Ca2+ homeostasis by providing ATP to pump Ca2+ across the plasma membrane or by direct up-take of Ca2+ into mitochondria. In cultured neurons, mitochondrial movement was increased by the action potential blocker tetrodotoxin, but reduced by KCl depolarization (Li et al., 2004) and Ca2+ influx through NMDA receptors (Rintoul et al., 2003). In addition, serotonin (5-HT) was reported to promote axonal transport of mitochondria, acting through the 5-HT1A receptor subtype by increasing Akt activity, and consequently decreasing glycogen synthase kinase (GSK3β) activity (Chen et al., 2007). In contrast, dopamine inhibits mitochondrial motility in cultured hippocampal neurons through the same signaling cascade (Chen et al., 2008).
Recently, three groups (Saotome et al., 2008; Wang and Schwarz, 2009; MacAskill et al., 2009;) independently identified Miro as a Ca2+ sensor in regulating mitochondrial mobility. The KIF5-milton-Miro or KIF5-Miro complex (probably linked to GRIF-1/TRAK2) allowed mitochondria to move along axons and dendrites of hippocampal neurons. In neurons expressing the Miro EF hand mutated to prevent Ca2+ binding, Ca2+-induced arrest of mitochondrial mobility was efficiently blocked. Thus, the new studies highlight the mechanisms regarding how the mitochondrial motor-adaptor coupling is regulated in response to elevated intracellular Ca2+. Although these studies converge on a similar final conclusion, the proposed mechanisms underlying Ca2+-dependent regulation of motor-adaptor coupling are different (Cai and Sheng, 2009). Wang and Schwarz (2009) proposed a motor-adaptor switch model. They showed that in the absence of Ca2+, the C-terminal tail of KIF5 was bound to the mitochondrion by interacting with milton-Miro complex, thus leaving its N-terminal motor domain free to engage with microtubules allowing anterograde transport. Upon Ca2+ binding to the EF hands, a conformational change was induced, resulting in direct interaction of the motor domain with Miro and preventing KIF5–microtubule interaction (Figure 3A). Thus, through a Ca2+-binding switch mechanism to turn “on” or “off” the motor-microtubule engagement, mitochondrial mobility is tightly regulated in response to synaptic activity. In contrast, MacAskill et al. (2009) proposed that Miro1 mediates mitochondrial transport by linking mitochondria to KIF5. Ca2+ binding to the EF hands dissociated Miro from KIF5 while GRIF-1/TRAK2 (a mammalian homologue of milton) remains bound to Miro1 (Figure 3B). Thus, Ca2+ influx, by activating glutamate receptors, can recruit mobile mitochondria targeting to and being retained at synapses.
The direction of mitochondrial transport is closely correlated with mitochondrial membrane potential. Throughout the cell, mitochondrial membrane potential is heterogeneous and varies in time and space in response to changes in metabolic demand (Overly et al., 1996). Mitochondria in an anterograde movement toward the axon terminal display higher membrane potential while ones with low membrane potential are transported toward the cell body, suggesting that old or damaged mitochondria are recruited to the soma for repair (Miller and Sheetz, 2004). These findings support the notion that in the cell soma, mitochondrial fission generates healthy mitochondria that are transported down along axons toward the distal region or synapses, while dysfunctional mitochondria are delivered toward the soma and repaired by either fusion with healthy mitochondria or degraded through mitophagy (Chang and Reynold, 2006). However, recent study from the Hollenbeck laboratory demonstrated that anterograde and retrograde moving, and stationary mitochondria show no difference in membrane potential (Verburg and Hollenbeck, 2008). Moreover, they further identified that nerve growth factor (NGF) and semaphorin signaling can effectively elicit local receptor-mediated increases in mitochondrial membrane potential at the growth cones of chick sensory neurons. These data argue that the membrane potential of axonal mitochondria, like their distribution, can be regulated locally, highlighting an important role for cellular signaling in regulating mitochondrial transmembrane potential and respiratory status in neurons. In addition, NGF was also shown to serve as a docking signal for immobilizing axonal mitochondria in dorsal root ganglion (DRG) neurons in cultures. This docking process is likely based on F-actin filaments and depends on a P13K phosphorylation pathway (Chada and Hollenbeck, 2004; Reynolds and Rintoul, 2004).
Molecular regulation of mitochondrial movement can also be achieved by altering mitochondrial dynamic fusion/fission events. Mitochondrial dynamics consists of movements at two levels: organelle transport and membrane fusion-fission. The mitochondrial fusion process requires apposition of two adjacent mitochondria associated with efficient transport. Moreover, organelle size likely affects mitochondrial motility as well. Thus, coordination between mitochondrial transport and fusion/fission events probably exists in neurons. Mutation of mitochondrial protein Drp1, a dynamin-like GTPase, impaired axonal transport and synaptic targeting of mitochondria by interfering with the fission process (Verstreken et al., 2005). Because Miro also contains two atypical GTPase domains flanking the two Ca2+-binding EF hand motifs (Fransson et al., 2003), Ca2+-regulated transport is likely coordinated with GTP hydrolysis-mediated fission. Saotome et al (2008) reported that Miro enhances mitochondrial fragmentation (or fission) by coordinating Ca2+ sensing and GTPase activity, thus controlling their mobility and density in dendrites. This duel role of Miro is quite interesting, but needs to be reconciled with the phenotype of Drp1 mutant neurons, where mitochondrial transport in axons and localization in dendritic spines is severely impaired (Li et al., 2004; Verstreken et al., 2005). It is presumed that fission defects lead to mitochondrial connections forming long tubular structures, which would be less mobile than ones with short tubular or vesicular structures. Further investigation is needed to learn the role of the complex set of molecular motors involved in modulating mitochondrial fusion and fission processes.
Isolated mitochondria from N2A cells were shown to associate with both KIF5B and dynein motors, suggesting retention of their ability to move bidirectionally in vitro. This result provides an attractive model for a complex mechanism by regulating mitochondrial motility rather than directly by affecting the attachment of transport motors to mitochondria (De Vos et al., 2003). Thus, the adaptor for motor protein can be the reasonable candidate to modulate motor activity and consequently affect mitochondrial motility in axons. In addition, dynactin complex can also be a candidate to regulate bidirectional movements since the complex associates with dynein, and also plays a critical role in kinesin-mediated anterograde trafficking events (Pilling et al., 2006; Haghnia et al., 2007). Bidirectional axonal transport is dramatically impaired in Drosophila mutants with disrupted dynactin complexes (Haghnia et al., 2007). However, the mechanisms underlying the linkage of the dynactin complex to the anterograde transport machinery and related regulation of mitochondrial movement remain unclear. Finally, to achieve the desired balance between mobile and stationary mitochondria, static anchors are efficient factors for docking mitochondria on the cytoskeleton.
Syntaphilin acting as a “static anchor” for docking axonal mitochondria
Mitochondria in axons display distinct motility patterns and undergo saltatory bidirectional movements. While approximately one-third of axonal mitochondria are mobile in mature neurons, a large proportion remains stationary. Their net movement is significantly influenced by recruitment to stationary or motile states (Hollenbeck, 1996). Such complex mobility patterns suggest that axonal mitochondria might be coupled to two opposing motors (kinesin and dynein) and docking or anchoring machinery. The mechanisms as to how motile mitochondria are recruited to stationary pool in response to neuronal activity and synaptic modification remain unknown. Identifying the proteins mediating mitochondrial docking and retention within axons and at synapses provides a molecular target for such regulation.
Syntaphilin specifically targets to axonal mitochondria
We recently revealed that syntaphilin (SNPH), a neuron-specific and axon-targeted protein, can dock mitochondria and regulate their density within axons (Kang et al., 2008). Localization analysis of GFP-SNPH truncated mutants demonstrated that the middle domain (residues 381–469) of SNPH is an axon-sorting sequence and its carboxyl terminal (CT) tail is necessary for mitochondrial association. The sequence of the CT tail shares similar signal structures specific for mitochondrial outer-membrane-targeting (Rapaport, 2003), which are moderately hydrophobic and relatively short (16–20 residues) with net positive charges flanking both sides. Expression of the truncated mutant of SNPH lacking the axon-sorting sequence results in its distribution into all mitochondria including those in the soma and dendrites, indicating that SNPH may use this axon-sorting sequence for axonal localization independent of its mitochondrial targeting.
Syntaphilin-mediated docking of axonal mitochondria
SNPH is required for maintaining a large number of axonal mitochondria in a stationary state (Kang et al., 2008) (Figure 4). Three lines of evidence support this view. First, the mitochondria that associate with exogenously expressed GFP-SNPH were nearly immobile. This appears to occur through interactions with the cytoskeleton because SNPH contains a microtubule-binding domain, which is necessary and sufficient for SNPH-mediated immobilization of axonal mitochondria. SNPH likely acts as a docking receptor specific for axonal mitochondria through a unique interaction with the microtubule-based cytoskeleton. These findings provide a molecular explanation for the previously identified biochemical interactions between neuronal mitochondria and microtubules (Linden et al., 1989; Jung et al., 1993; Leterrier et al., 1994), and morphological observations of axonal ultrastructures, which revealed cross-bridges between mitochondria and microtubules in vivo (Smith et al., 1977; Hirokawa, 1982; Benshalom and Reese, 1985; Pannese et al., 1986; Price et al., 1991). These cross-bridges may represent both dynamic (motor proteins) and static links (docking or anchoring proteins) between axonal mitochondria and the microtubule-based cytoskeleton.
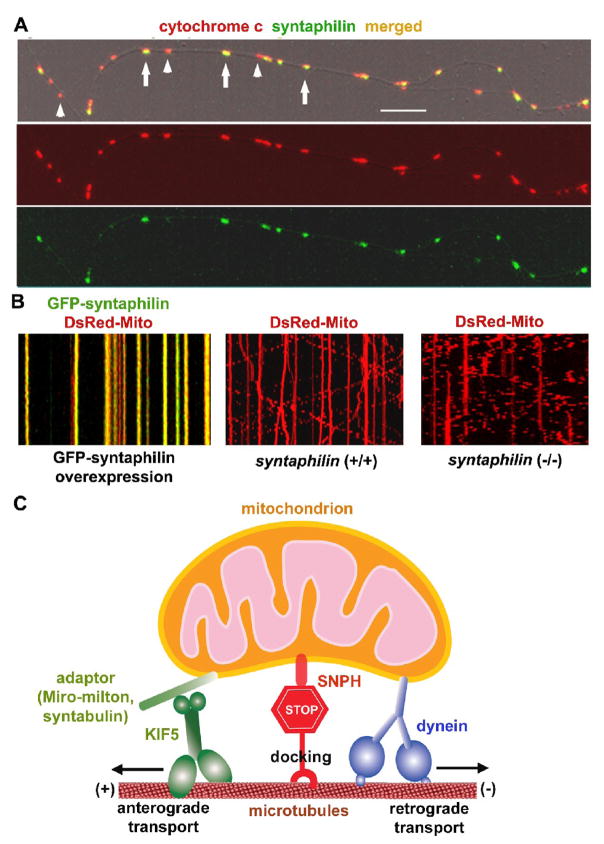
Fig. 4.
SNPH acts as a receptor for docking/anchoring mitochondria at microtubules in axons. (A) Axonal mitochondrial targeting of SNPH in cultured hippocampal neurons. Hippocampal neurons at DIV14 were co-immunostained for SNPH and mitochondrial marker cytochrome c. Arrows point to SNPH-associated mitochondria and arrowheads indicate SNPH-negative mitochondria within an axonal process. Scale bars, 10 μm. (B) SNPH immobilizes axonal mitochondria and deletion of snph gene in mice robustly increases mobility of axonal mitochondria. Mobility of axonal mitochondria was observed in live neurons one week after transfection. Motion data are presented in kymograph, in which vertical lines represent stationary mitochondria and slant lines or curves indicate motile ones. Left panel: wild-type neurons co-transfected at DIV6 with DsRed-mito (red) and GFP-SNPH (green); middle panel: wild-type neurons transfected with DsRed-Mito alone; right panel: snaph (−/−) neurons transfected with DsRed-Mito alone. (C) Schematic diagram for the proposed role of SNPH. SNPH acts as a receptor for docking/anchoring mitochondria in axons and is required for maintaining a large number of axonal mitochondria in a stationary state by interacting with the microtubule-based cytoskeleton. Such a mechanism may enable neurons to maintain proper densities of stationary mitochondria within axons and in the proximity of synapses. (Imaging in Fig. 4A is adapted with permission from Jian-Sheng Kang, Jin-Hua Tian, Philip Zald, Ping-Yue Pan, Cuiling Li, Chuxia Deng, and Zu-Hang Sheng. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132, 137–148, 2008).
Second, by recording mitochondrial movement in living neurons followed by retrospective immunostaining for endogenous SNPH, the immobility of axonal mitochondria depends on their association with endogenous SNPH. Statistical analysis reveals a strong correlation (r=0.98) between SNPH-positive mitochondria and stationary mitochondria. Axonal mitochondria appear as two populations: one associated with SNPH and the other not, demonstrating a binominal B(n, p) distribution. The mean percentage of endogenous SNPH-tagged axonal mitochondria (65±14%, mean±SD) is consistent with the mean percentage of stationary mitochondria (62±15%, mean±SD). These results suggest a complementary relationship between SNPH-tagged mitochondria and motile mitochondria (38±15%, mean±SD), indicating that SNPH is associated with stationary mitochondria.
Third, deletion of the snph gene in mice dramatically increases mitochondrial motility and results in a substantially reduced density of mitochondria within axons. Time-lapse imaging analysis of living hippocampal neurons in vitro demonstrated that in axons of wild-type neurons, approximately one-third (36±15%) of mitochondria were in motion. In contrast, deletion of the snph gene resulted in a robust increase in the percentage (76±20%) of motile axonal mitochondria. In addition, deleting the snph gene decreased mitochondrial density within axons (1.0±0.2/10μm) compared to those of snph (+/+) neurons (1.7±0.4/10μm), suggesting that the SNPH-mediated docking/retention contributes to maintaining proper mitochondrial density in axons. In contrast, deletion of the snph gene has no apparent effect on the velocities of motile mitochondria. Furthermore, the proportion of presynaptic boutons with proximal mitochondria (within the vicinity of 0.5 μm) is significantly reduced in the snph (−/−) neurons (32.80±4.15%) relative to the snph (+/+) controls (57.02±4.00%). The reduced density of axonal mitochondria also reflects a diminished inter-bouton density of axonal mitochondria in the snph (−/−) neurons relative to that of wild-type controls.
This study elucidates SNPH as an anchoring receptor for docking mitochondria in axons. Such a mechanism may enable neurons to maintain proper densities of stationary mitochondria within axons and in the proximity of synapses. The coordination of mitochondrial motility with axonal physiology plays a crucial role in maintaining mitochondrial density at appropriate sites in axons. Mitochondria within nerve fibers are relatively enriched at the nodes of Ranvier (Berthold et al., 1993) where ATP production is in high demand (Aiello et al., 2000). Considering the limited diffusion of ATP in an intracellular environment (Belles et al., 1987; Hubley et al., 1996), docked mitochondria ideally serve as local energy stations, providing ATP to maintain the high activity of Na+-K+ ATPase and fast spike propagation. It is expected that defective docking/anchoring machinery could affect normal neuronal functions, particularly for cells with a long axonal process such as motor neurons.
The impact of syntaphilin-mediated mitochondrial docking on synaptic function
Synaptic mitochondria play important roles in the ATP-dependent mobilization of synaptic vesicles from the reserve pool and in calcium homeostasis (Stowers et al., 2002; Verstreken et al., 2005; Guo et al., 2005). Mitochondria maintain calcium homeostasis at some synapses by buffering extra intracellular [Ca2+] during tetanic stimulation and releasing calcium after stimulation to prolong the tail of residual [Ca2+] (Jonas, 2006). However, whether changes in mitochondrial mobility and density in axons have an impact on synaptic transmission and plasticity remains elusive. The snph mutant neurons provide a unique model for studying the physiological impact of axonal mitochondrial docking on synaptic transmission.
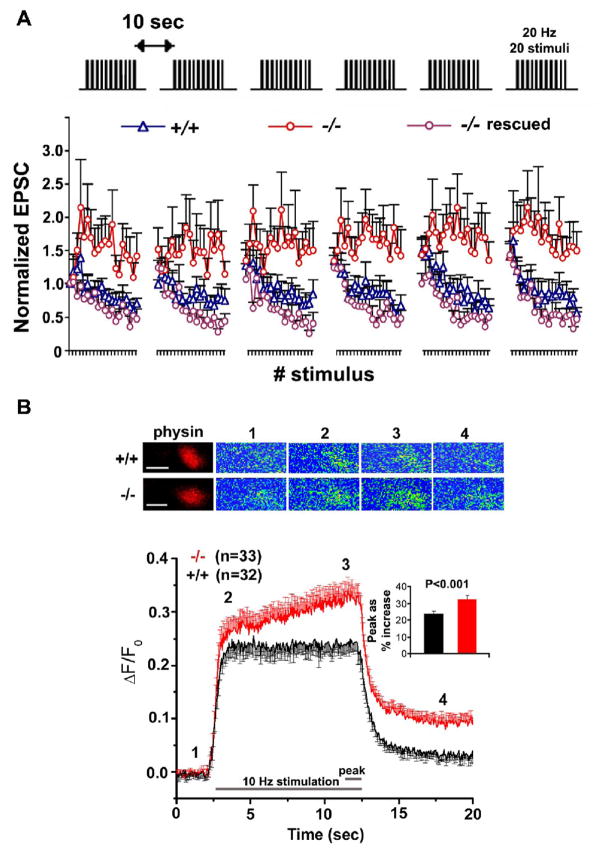
By taking advantage of the observed phenotypes in snph (−/−) neurons, Kang et al (2008) further looked at synaptic physiology by dual whole-cell patch-clamp recording on paired hippocampal neurons in culture. The increased mobility and reduced density of axonal mitochondria by deleting the snph gene in mice does not significantly impact basal synaptic transmission including both frequency and amplitude of mini AMPA events and averaged amplitude and kinetics of EPSCs. However, when applying short stimulus trains (20 Hz, 1 s) to the presynaptic neuron at 10-second intervals, persistent enhanced facilitation was reproduced in the snph (−/−) but not in (+/+) neurons under repetitive pulse train stimulation, suggesting the possible role of residual [Ca2+] in maintaining short-term facilitation of synaptic response (Figure 5A). The observed phenotype could be fully rescued by expressing full-length SNPH in the mutant neurons, further confirming that the enhanced short-term facilitation was due to deleting the snph gene. One possible explanation for this observation is a rapid buildup of intracellular [Ca2+] at presynaptic terminals during intensive stimulation. Kang et al (2008) further showed that disrupting the docking/anchoring mechanism changes the global [Ca2+] dynamics at presynaptic boutons during intensive and prolonged stimulation, probably through reduced [Ca2+] buffering capacity by mitochondria or impaired ability of the terminals to pump [Ca2+] out due to an insufficient supply of ATP (Figure 5B). These results suggest that the inter-bouton axonal mitochondria might play a comparable role in buffering calcium at nerve terminals during high-frequency stimulation. Altogether, these electrophysiological and calcium imaging studies combined with genetic and cell biology analysis provide direct evidence that controlling axonal mitochondrial docking/anchoring has a physiological impact on synaptic function by affecting calcium dynamics at nerve terminals.
Fig. 5.
The snph mutant neurons exhibit enhanced short-term facilitation during prolonged stimulation by affecting calcium signaling at presynaptic boutons. (A) Deletion of the snph gene results in sustained synaptic short-term facilitation. 20 Hz, 1 sec stimulus train was delivered repetitively six times at 10-second intervals (upper panel). Normalized EPSC amplitudes were plotted against stimulus number (lower panel). Note that persistent facilitation in synaptic responses was shown only in the snph (−/−) neurons (red circles). Reintroducing the snph gene into the mutant presynaptic neuron (purple circles) eliminates the short-term facilitation and fully rescues the (+/+) phenotype (blue triangles). (B) Presynaptic calcium dynamics in the snph (+/+) and snph (−/−) neurons. Upper panel: representative calcium images at presynaptic boutons of the snph (+/+) and (−/−) neurons before stimulation (1), at the beginning (2) and the end (3), and after stimulation (4). Calcium transients within presynaptic boutons labeled with DsRed-monomer-synaptophysin were imaged using Fluo-4NW at 50-ms intervals upon stimulation at 10 Hz for 10 seconds. The images are pseudocolored, with blue representing low [Ca2+] concentration and red representing high [Ca2+] concentration. Scale bars: 1 mm. Lower panel: time course of changes in presynaptic fluorescent intensity over baseline (ΔF/F0) from the snph (+/+) (black, n=32) and (−/−) (red, n=33) neurons. Inset: peak values of intracellular [Ca2+] levels within boutons were averaged from last 10 stimuli (20 frames of calcium imaging), expressed as % increase of fluorescence intensity over baseline (ΔF/F0), and are significantly different (p<0.001, t test) between the snph (+/+) (24%±2%) and (−/−) (33%±2%) neurons. Error bars: s.e.m. (Adapted with permission from Jian-Sheng Kang, Jin-Hua Tian, Philip Zald, Ping-Yue Pan, Cuiling Li, Chuxia Deng, and Zu-Hang Sheng. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132, 137–148, 2008).
Conclusions
Mitochondrial balance between the motile and stationary phases is a possible target of regulation by intracellular signals and synaptic activity. How are motile mitochondria recruited to the stationary pool in response to neuronal activity and synaptic modification? Recent advances in identifying motor-adaptor transport complexes and docking machinery specific for axonal mitochondria provide molecular targets for such regulation. Future studies using genetic mouse models combined with live cell tracking of mitochondrial mobility in vivo will provide molecular and cellular mechanisms regulating mitochondrial motility and distribution and impacting their turnover in axons and at synapses. Since defective trafficking and dysfunction of axonal mitochondria is implicated in the pathogenesis of axonal degeneration (Stamer et al., 2002; Pigino et al., 2003; Chan, 2006; Chevalier-Larsen and Holzbaur, 2006), these studies will shed light on fundamental neuronal processes that may affect our understanding human neurodegenerative disorders.
Acknowledgments
We thank the members of the Sheng laboratory for helpful discussion and D. Schoenberg for critical reading of the manuscript. The authors are supported by the Intramural Research Program of NINDS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello GL, Bach-y-Rita P. The cost of an action potential. J Neurosci Methods. 2000;103:145–149. doi: 10.1016/s0165-0270(00)00308-3. [DOI] [PubMed] [Google Scholar]
- Belles B, Hescheler J, Trube G. Changes of membrane currents in cardiac cells induced by long whole-cell recordings and tolbutamide. Pflugers Arch. 1987;409:582–588. doi: 10.1007/BF00584657. [DOI] [PubMed] [Google Scholar]
- Benshalom G, Reese TS. Ultrastructural observations on the cytoarchitecture of axons processed by rapid-freezing and freeze-substitution. J Neurocytol. 1985;14:943–960. doi: 10.1007/BF01224806. [DOI] [PubMed] [Google Scholar]
- Berthold CH, Fabricius C, Rydmark M, Andersén B. Axoplasmic organelles at nodes of Ranvier. I. Occurrence and distribution in large myelinated spinal root axons of the adult cat. J Neurocytol. 1993;22:925–940. doi: 10.1007/BF01218351. [DOI] [PubMed] [Google Scholar]
- Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaker WD, Goodrum JF, Morell P. Axonal transport of the mitochondria-specific lipid, diphosphatidylglycerol, in the rat visual system. J Cell Biol. 1981;89:579–584. doi: 10.1083/jcb.89.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC. Myosin-dependent transport in neurons. J Neurobiol. 2004;58:164–174. doi: 10.1002/neu.10320. [DOI] [PubMed] [Google Scholar]
- Cai Q, Gerwin C, Sheng ZH. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol. 2005;170:959–969. doi: 10.1083/jcb.200506042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Sheng ZH. Moving or stopping mitochondria: Miro as a traffic cop by sensing calcium. Neuron. 2009;61:493–496. doi: 10.1016/j.neuron.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Chen S, Owens GC, Crossin KL, Edelman DB. Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol Cell Neurosci. 2007;36:472–483. doi: 10.1016/j.mcn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Chen S, Owens GC, Edelman DB. Dopamine inhibits mitochondrial motility in hippocampal neurons. PLoS ONE. 2008;30:e2804. doi: 10.1371/journal.pone.0002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney RE, O’Shea MK, Heuser JE, Coelho MV, Wolenski JS, Espreafico EM, Forscher P, Larson RE, Mooseker MS. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. doi: 10.1016/S0092-8674(05)80080-7. [DOI] [PubMed] [Google Scholar]
- Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Sable J, Miller KE, Sheetz MP. Expression of phosphatidylinositol (4,5) bisphosphate-specific pleckstrin homology domains alters direction but not the level of axonal transport of mitochondria. Mol Biol Cell. 2003;14:3636–3649. doi: 10.1091/mbc.E02-10-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson A, Ruusala A, Aspenström P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- Górska-Andrzejak J, Stowers RS, Borycz J, Kostyleva R, Schwarz TL, Meinertzhagen IA. Mitochondria are redistributed in Drosophila photoreceptors lacking milton, a kinesin-associated protein. J Comp Neurol. 2003;463:372–388. doi: 10.1002/cne.10750. [DOI] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, Morgan PJ, Oozageer R, Priestley JV, Averill S, King VR, Ball S, Peters J, Toda T, Yamamoto A, Hiraoka Y, Augustin M, Korthaus D, Wattler S, Wabnitz P, Dickneite C, Lampel S, Boehme F, Peraus G, Popp A, Rudelius M, Schlegel J, Fuchs H, Hrabe de Angelis M, Schiavo G, Shima DT, Russ AP, Stumm G, Martin JE, Fisher EM. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- Haghnia M, Cavalli V, Shah SB, Schimmelpfeng K, Brusch R, Yang G, Herrera C, Pilling A, Goldstein LS. Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment. Mol Biol Cell. 2007;18:2081–2089. doi: 10.1091/mbc.E06-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982;94:129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Kinesin superfamily proteins and their various functions and dynamics. Exp Cell Res. 2004;301:50–59. doi: 10.1016/j.yexcr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1:d91–102. doi: 10.2741/a118. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubley MJ, Locke BR, Moerland TS. The effects of temperature, pH, and magnesium on the diffusion coefficient of ATP in solutions of physiological ionic strength. Biochim Biophys Acta. 1996;1291:115–121. doi: 10.1016/0304-4165(96)00053-0. [DOI] [PubMed] [Google Scholar]
- Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144:1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E. BCL-xL regulates synaptic plasticity. Mol Interv. 2006;6:208–222. doi: 10.1124/mi.6.4.7. [DOI] [PubMed] [Google Scholar]
- Jung D, Filliol D, Miehe M, Rendon A. Interaction of brain mitochondria with microtubules reconstituted from brain tubulin and MAP2 or TAU. Cell Motil Cytoskeleton. 1993;24:245–255. doi: 10.1002/cm.970240405. [DOI] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Holzbaur EL. cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Langford GM. Myosin-V, a versatile motor for short-range vesicle transport. Traffic. 2002;3:859–865. doi: 10.1034/j.1600-0854.2002.31202.x. [DOI] [PubMed] [Google Scholar]
- Lee CW, Peng HB. The function of mitochondria in presynaptic development at the neuromuscular junction. Mol Biol Cell. 2008;19:150–158. doi: 10.1091/mbc.E07-05-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier JF, Rusakov DA, Nelson BD, Linden M. Interactions between brain mitochondria and cytoskeleton: evidence for specialized outer membrane domains involved in the association of cytoskeleton-associated proteins to mitochondria in situ and in vitro. Microsc Res Tech. 1994;27:233–261. doi: 10.1002/jemt.1070270305. [DOI] [PubMed] [Google Scholar]
- Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD. Mitochondrial regulation of synaptic plasticity in the hippocampus. J Biol Chem. 2003;278:17727–17734. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- Levy JR, Sumner CJ, Caviston JP, Tokito MK, Ranganathan S, Ligon LA, Wallace KE, LaMonte BH, Harmison GG, Puls I, Fischbeck KH, Holzbaur EL. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J Cell Biol. 2006;172:733–745. doi: 10.1083/jcb.200511068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Steward O. Movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427:340–350. doi: 10.1002/1096-9861(20001120)427:3<340::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lindén M, Nelson BD, Loncar D, Leterrier JF. Studies on the interaction between mitochondria and the cytoskeleton. J Bioenerg Biomembr. 1989;21:507–518. doi: 10.1007/BF00762522. [DOI] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik R, Petrov D, Lex SA, King SJ, Gross SP. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr Biol. 2005;15:2075–2085. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Jr, Hays TS, Saxton WM. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, Weinberg RJ, Sheng M. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J Neurosci. 2000;20:4524–4534. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole M, Latham R, Baqri RM, Miller KE. Modeling mitochondrial dynamics during in vivo axonal elongation. J Theor Biol. 2008;255:369–377. doi: 10.1016/j.jtbi.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Overly CC, Rieff HI, Hollenbeck PJ. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J Cell Sci. 1996;109:971–980. doi: 10.1242/jcs.109.5.971. [DOI] [PubMed] [Google Scholar]
- Pannese E, Procacci P, Ledda M, Arcidiacono G, Frattola D, Rigamonti L. Association between microtubules and mitochondria in myelinated axons of Lacerta muralis. A quantitative analysis. Cell Tissue Res. 1986;245:1–8. doi: 10.1007/BF00218080. [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RL, Lasek RJ, Katz MJ. Microtubules have special physical associations with smooth endoplasmic reticula and mitochondria in axons. Brain Res. 1991;540:209–216. doi: 10.1016/0006-8993(91)90509-t. [DOI] [PubMed] [Google Scholar]
- Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, Brown RH, Jr, Ludlow CL, Fischbeck KH. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- Rapaport D. Finding the right organelle. Targeting signals in mitochondrial outer-membrane proteins. EMBO Rep. 2003;4:948–952. doi: 10.1038/sj.embor.embor937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds IJ, Rintoul GL. Mitochondrial stop and go: signals that regulate organelle movement. Sci STKE. 2004;21:PE46. doi: 10.1126/stke.2512004pe46. [DOI] [PubMed] [Google Scholar]
- Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Wallace K, Shuman H, Goldman YE, Holzbaur EL. Processive bidirectional motion of dynein-dynactin complexes in vitro. Nat Cell Biol. 2006;8:562–570. doi: 10.1038/ncb1421. [DOI] [PubMed] [Google Scholar]
- Rowland KC, Irby NK, Spirou GA. Specialized synapse-associated structures within the calyx of Held. J Neurosci. 2000;20:9135–9144. doi: 10.1523/JNEUROSCI.20-24-09135.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnóczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3-->CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Järlfors U, Cayer ML. Structural cross-bridges between microtubules and mitochondria in central axons of an insect (Periplaneta americana) J Cell Sci. 1977;27:255–272. doi: 10.1242/jcs.27.1.255. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Pozo K, Brickley K, Stephenson FA. Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J Biol Chem. 2006;281:27216–27228. doi: 10.1074/jbc.M600522200. [DOI] [PubMed] [Google Scholar]
- Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Goldstein LS. Axonal transport and Alzheimer’s disease. Annu Rev Biochem. 2006;75:607–627. doi: 10.1146/annurev.biochem.75.103004.142637. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Górska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Su Q, Cai Q, Gerwin C, Smith CL, Sheng ZH. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat, Cell Biol. 2004;6:941–953. doi: 10.1038/ncb1169. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg J, Hollenbeck PJ. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci. 2008;28:8306–8315. doi: 10.1523/JNEUROSCI.2614-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur EL. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci U S A. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolenski JS, Cheney RE, Mooseker MS, Forscher P. In vitro motility of immunoadsorbed brain myosin-V using a Limulus acrosomal process and optical tweezer-based assay. J Cell Sci. 1995;108:1489–1496. doi: 10.1242/jcs.108.4.1489. [DOI] [PubMed] [Google Scholar]
- Yang F, He XP, Russell J, Lu B. Ca2+ influx-independent synaptic potentiation mediated by mitochondrial Na(+)-Ca2+ exchanger and protein kinase C. J Cell Biol. 2003;163:511–523. doi: 10.1083/jcb.200307027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnóczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]