Abstract
Mitochondrial ion channels are involved in numerous cellular processes. Membrane pores and transporters regulate the influx and efflux of calcium, sodium, potassium, zinc and determine the membrane compartmentalization of numerous cytosolic metabolites. The permeability of the inner membrane to ions and solutes helps determine the membrane potential of the inner membrane, but the permeability of the outer membrane, controlled in part by VDAC and the BCL-2 family proteins, regulates the release of important signaling molecules that determine the onset of programmed cell death. BCL-2 family proteins have properties of ion channels and perform specialized physiological functions, for example, regulating the strength and pattern of synaptic transmission, in addition to their well known role in cell death. The ion channels of the inner and outer membranes may come together in a complex of proteins during programmed cell death, particularly during neuronal ischemia, where elevated levels of the divalents calcium and zinc activate inner membrane ion channel conductances. The variety of possible molecular participants within the ion channel complex may be matched only by the variety of different types of programmed cell death.
Introduction: BCL-2 family proteins as regulators of mitochondrial cell death channel formation
Mitochondrial ion channels are intimately involved in numerous cellular processes. Regulated mitochondrial membrane pores such as the voltage dependent anion channel (VDAC) release energy (in the form of ATP) out of mitochondria and conduct ADP into mitochondria (Colombini et al., 1996; Rostovtseva and Colombini, 1997; Lemasters and Holmuhamedov, 2006). Mitochondrial channels and transporters regulate tightly the influx and efflux of calcium, sodium, potassium and zinc (Kirichok et al., 2004; Nicholls and Chalmers, 2004; O’Rourke, 2007). The permeability of the inner membrane to different ions helps determine the membrane potential of the inner membrane, and regulates matrix volume (Bernardi, 1999; Crompton, 1999; Reynolds, 1999). In addition, the BCL-2 family proteins, which are known to play a role in producing or inhibiting the release of pro-apoptotic factors from mitochondria, have properties of ion channels (Schendel et al., 1998; Kroemer and Reed, 2000; Green and Kroemer, 2004; Polster and Fiskum, 2004; Adams and Cory, 2007). These specialized proteins perform physiological functions, for example, regulating the strength and pattern of synaptic transmission, as well as participating in cell death (Jonas, 2006).
The inner mitochondrial membrane is a barrier similar to the plasma membrane that allows the separation of ions to produce an electrochemical gradient (Mitchell, 1961). The potential energy produced by charge separation can be used for work and for signaling, through the activity of transporters and ion channels (Hackenbrock et al., 1971; Skulachev, 1972; Gunter and Pfeiffer, 1990). Energy generated by the reduction of NAD(P) to NAD(P)H and FAD to FADH in the tricarboxylic acid cycle is used (by oxidation of the reduced species) for the transport of electrons from high to low energy electron acceptors while H+ ions are pumped out across the inner membrane through transporters, thereby producing a large electrochemical (voltage and H+ concentration) gradient. Movement of H+ ions back down the gradient across the ATP synthase H+ transporter phosphorylates ADP to produce ATP (phosphorylation).
The gating of outer membrane channels regulates the release of metabolites and ions into the cytosol. These outer membrane channels may function in isolation from the inner membrane or in complexes with channels of the inner membrane. It has become clear that outer membrane permeability is tightly controlled, in part by the voltage dependence of outer membrane conductances. Control of outer membrane permeability compartmentalizes the intermembrane space with implications for metabolic regulation and cell fate determination (Rostovtseva et al., 2005; Lemasters and Holmuhamedov, 2006).
An important mode of cell death occurs when mitochondrial outer membranes are permeabilized by the regulated actions of the BCL-2 family proteins (Green and Kroemer, 2004; Jonas, 2004; Martinez-Caballero et al., 2005). These proteins exhibit two important features: They produce ion channel activity in intracellular organelle membranes (Schendel et al., 1998), and they are regulated by interactions with binding partners, including BCL-2 family members and other cellular proteins (Galonek and Hardwick, 2006). As a result, the BCL-2 family proteins control the release of death-inducing mitochondrial factors such as cytochrome c into the cytosol (Antonsson et al., 2000; Ow et al., 2008). Given the importance and complexity of mitochondria in the context of cellular function, it is possible and indeed likely that the formation of the “cell death channel” involves both inner and outer membrane protein components, most of which probably serve physiological ion conducting and metabolic functions during times when the cell is not undergoing death. In this review, we describe a hypothetical model that implicates the BCL-2 family proteins in controlling the formation of the cell death channel, by binding to and organizing disparate mitochondrial proteins into a large complex. We will attempt to summarize the evidence that exists in support of this model.
Why is a membrane pore needed during cell death?
Programmed cell death or apoptosis is the genetic predisposition of cells to die (Adams and Cory, 2007). Cells undergo this type of death during development and throughout life, when unnecessary or damaged cells require removal (Kroemer and Reed, 2000). During brain insults such as ischemia, infection, or trauma, some brain cells die immediately, but others die a delayed death that necessitates turning on programmed death pathways (Banasiak et al., 2000). BCL-2 family proteins help determine the fate of neurons exposed to many different pro-death signals (Adams and Cory, 2007).
During the process of programmed cell death, mitochondria receive a signal to undergo permeabilization of their outer membranes (Green and Kroemer, 2004; Adams and Cory, 2007). The opening of the outer mitochondrial membrane is also sometimes referred to as activation of the apoptosis channel (Dejean et al., 2005). This event occurs suddenly and rapidly results in release of important intermembrane space proteins such as cytochrome c (Green and Kroemer, 2004; Martinez-Caballero et al., 2005). Release of cytochrome c produces two events. On the one hand, loss of cytochrome c compromises the ability of mitochondria to produce ATP and eventually to maintain the mitochondrial membrane potential. On the other hand, cytochrome c and other factors released from mitochondria serve as signals that activate downstream enzyme pathways that chew up hundreds of cellular proteins (Youle and Strasser, 2008).
BCL-2 family proteins play a major role in either regulating or directly producing the permeabilization of the outer membrane. Three categories of BCL-2 proteins are important in determining cell fate. These are the anti-apoptotic members (such as BCL-xL, BCL-2, and MCL-1), the pro-apoptotic members such as BAX and BAK, and a large group of BH-3 only proteins such as BID, BAD, PUMA and NOXA (Galonek and Hardwick, 2006). The anti-apoptotic members of the group are similar in structure and sequence to BAX and BAK, but in addition to the BH1, 2 and 3 domains, contain a BH4 domain that appears to provide anti-apoptotic features to the molecules. (Schlesinger et al., 1997; Schendel et al., 1998)
Actions of BCL-2 family proteins are regulated by binding to other BCL-2 family members
The mechanisms by which anti-apoptotic proteins such as BCL-2 and BCL-xL prevent cell death are poorly understood. Recent studies have focused on the ability of anti-apoptotic proteins such as BCL-xL to bind to and sequester pro-apoptotic members of the BCL-2 family (Kim et al., 2006). BCL-2 and BCL-xL bind to BAX and to BH3 peptides, preventing the pro-apoptotic actions of these proteins. The binding partners of the different BCL-2 family proteins within the family are known, but the order of binding events is crucial to understanding how BCL-2 family members work together to bring about, or protect from, cell death (Galonek and Hardwick, 2006; Kim et al., 2006). Under resting conditions, BCL-xL and BCL-2 bind to activator BH3 molecules, perhaps keeping them in a quiescent state. After a death signal, activation of inactivator BH3-only proteins such as BAD initiates the permeabilization of the mitochondrial membrane by sequestering the anti-apoptotic BCL-2 proteins BCL-xL and BCL-2, preventing them from binding to BAX or to activator BH3 peptides such as BID. The activator BH3 peptides are free to bind to and activate BAX, or bind to inhibitors of BAK, such as VDAC2 (Cheng et al., 2003). The end result is that BAX or BAK homo-oligomerize within the outer mitochondrial membrane thereby permeabilizing it (Galonek and Hardwick, 2006; Kim et al., 2006).
BCL-2 family proteins function as ion channels
Both BAX and BCL-xL produce ion channel activity when inserted into artificial lipid membranes (Schlesinger et al., 1997; Schendel et al., 1998). BCL-xL and BAX are both either localized to the outer mitochondrial membrane, or may translocate into the outer membrane upon a death stimulus (Wolter et al., 1997; Kaufmann et al., 2003). At first glance, the ion channel activities of BAX and BCL-xL seem quite similar (Schlesinger et al., 1997) (Tsujimoto and Shimizu, 2000; Sugioka et al., 2003).
The three dimensional structure of BCL-xL is comprised of 7 alpha helices (Muchmore et al., 1996; Schendel et al., 1998). Two outer layers of amphipathic helices serve to screen the long hydrophobic alpha helices from the aqueous domain. A long proline-rich loop found between the first and second helices is absent in the pro-apoptotic members of the family. The loop may be vulnerable to protease digestion, and contains phosphorylation sites. The BH1, 2, and 3 domains of BCL-xL fold together to give a hydrophobic region involved in homo and heterodimerization, and contain subdomains important for interaction with the BH3 only proteins. The structure of BCL-xL is strikingly similar to that of the diphtheria toxin membrane translocation domain and the pore-forming domains of bacterial colicins that kill sensitive cells via the formation of a highly conductive ion channel in the target cell’s plasma membrane. The overall organization of the colicin-like channels is that of a hydrophobic region containing the pore, shielded by amphipathic helices that keep the molecule soluble in the cytoplasm until insertion into a membrane activates the pore function. Two helices of BAX and BCL-xL are insufficient to form a pore (Schendel et al., 1998) but their ability to homo and heterodimerize may provide for interactions critical for their pore-forming ability.
It is possible that channel activity observed in artificial lipid membranes is not the same as activities in vivo. Both BCL-xL and BAX have demonstrated avid pore-forming capability in lipid bilayers if lipid composition of the bilayer is carefully regulated (Minn et al., 1997; Schlesinger et al., 1997; Schendel et al., 1998; Antonsson et al., 2000). Both BAX and BCL-xL display similar channel activity with multiple conductances, but BCL-xL has a linear conductance, whereas BAX appears to be more voltage dependent, is more anion selective than BCL-xL, and has larger peak conductances (Schlesinger et al., 1997)
The similar channel activities of these anti- and pro-apoptotic proteins raised several questions, the most important of which was whether the channel activities of the anti- and pro-apoptotic molecules were important at all for their anti- and pro-apoptotic functions, and if so, why were the channel activities so similar and could they prove more dissimilar in vivo?
Recordings of BCL-2 family proteins in vivo
The giant synapse of the squid stellate ganglion proved to be a good model to study the activity of recombinant BCL-2 proteins in mitochondrial membranes inside a living neuron (Jonas et al., 2003; Jonas et al., 2004; Jonas et al., 2005a). Employing a concentric electrode arrangement, a clean patch pipette tip of small internal diameter was exposed to mitochondria inside the neuronal presynaptic ending, and channel activity was recorded from a patch of outer membrane of a mitochondrion exposed to the inside of the pipette. When such patches were exposed to recombinant anti-apoptotic full length BCL-xL protein (FL BCL-xL) multiple conductance activity of approximately 100 pS to 750 pS was observed (Jonas et al., 2003), and was correlated with an enhancement of synaptic transmission in the squid (Jonas et al., 2003).
Channel activity of recombinant BAX recorded in presynaptic mitochondrial membranes shared some but not all features with that of recombinant full length BCL-xL. BAX, like BCL-xL, forms ion channels in artificial lipid membranes (Schlesinger et al., 1997), but in order to do so readily in vitro, it must be activated by treatment with detergent, which causes the protein to oligomerize (Hsu and Youle, 1998; Antonsson et al., 2000; Green and Kroemer, 2004). In isolated mitochondria or isolated outer mitochondrial membranes, however, this activation of BAX is apparently mediated by endogenous tBID (N-terminally cleaved/truncated BID) (Roucou et al., 2002) or another outer mitochondrial membrane component (Polster et al., 2001) In mitochondria within the squid presynaptic terminal, however, purified recombinant BAX protein lacking the C terminus (BAX C) or full length BAX produced discrete ion channel conductances of 100 pS and 750 pS similar to those of BCL-xL (Jonas et al., 2005a).
In contrast to BCL-xL, however, in 5–10% of the channels observed on mitochondria in the presence of recombinant BAX, a number of large openings with conductances >750 pS were detected, similar to the large conductances reported for purified BAX in artificial lipid membranes (Dejean et al., 2005). Thus, it appears that BAX induces channel activity with two distinct properties, a large conductance state and a less conductive state that shares some properties with that induced by full-length BCL-xL.
The findings suggest that the smaller conductance channel activity of BAX could represent the activity of BAX in a form prior to its exposure to a death stimulus or to activator BH3 peptides. However, this “inactive” form of BAX, like BCL-xL, could have additional physiological functions in the neuronal synapse, such as participating in the release of metabolites from mitochondria during high frequency synaptic events (Fannjiang et al., 2003; Jonas et al., 2005a).
If the smaller conductance activity represents the activity of BAX in healthy cells, then the large conductance activity could be important for its death-promoting actions. Indeed, proteolytic cleavage of BAX accelerates the onset of its pro-death function (Wood and Newcomb, 2000), and it is possible that in some of the recorded synapses, damage to the synapse produced BAX cleavage. As we will see below, BCL-xL also has an alternative (large) channel conductance that can be activated after a death stimulus, in particular after hypoxia in squid synapse, or ischemic injury in brain.
Endogenous death channels produced by BAX-containing protein complexes
The first patch clamp recordings of endogenous death channel activity were performed on mitochondria isolated from cultured cells undergoing apoptosis (Pavlov et al., 2001). Pavlov et al. detected an ion channel (mitochondrial apoptosis-induced channel, MAC) whose pore diameter was estimated to be of sufficient size (~4 nm) to allow the passage of cytochrome c and larger proteins. This channel displays multiple conductances, the largest of which is about 2.5 nS. The channel activity is expressed in mitochondrial outer membranes, inhibited in cells over-expressing BCL-2, and is similar to the activity of pure BAX expressed in artificial lipid membranes. The timing of cytochrome c release in apoptotic cells correlates well with the onset of MAC activity and with the translocation of BAX to mitochondrial membranes, further suggesting that, in these dying cultured cells, channel complexes include BAX protein. Moreover, MAC activity can be immunodepleted from mitochondrial membranes treated with anti-BAX antibodies.
BCL-xL activity in hypoxic neurons: ΔN BCL-xL and VDAC as part of the death channel complex
In adult or post-mitotic neurons, as opposed to many other cell types, BAX is down-regulated, but levels of BCL-xL are high (Krajewska et al., 2002). It is therefore possible that BCL-xL could participate in the acute formation of a cell death channel after a death stimulus. Indeed, BCL-xL is known to exist in two forms, a full length anti-apoptotic form, and an N-truncated version (ΔN BCL-xL) that appears after death stimuli and acts as a cell killer protein in cultured cell lines (Clem et al., 1998). In mitochondria of squid neuronal synapses exposed to the death stimulus hypoxia, large channel activity appeared that had biophysical features of the activity produced by application of recombinant ΔN BCL-xL protein to control mitochondria (Clem et al., 1998; Jonas et al., 2004; Bonanni et al., 2006).
Further studies of the activity in squid neurons also suggested that BCL-xL or ΔN BCL-xL formed a complex with the outer mitochondrial ion channel VDAC (Figure 1). VDAC is perhaps the most prevalent ion channel in the mitochondrial outer membrane (Colombini et al., 1996; Shoshan-Barmatz et al., 2006). The main function of VDAC appears to be its ability to conduct metabolites such as ATP, ADP, NADH, and pyruvate, in addition to other metabolites whose molecular weight can reach almost 1000. VDAC therefore has unusual biophysical characteristics compared to plasma membrane voltage gated ion channels. In keeping with its overwhelming importance in cellular function, VDAC is highly conserved in its tertiary structure from yeast to man (Colombini et al., 1996).
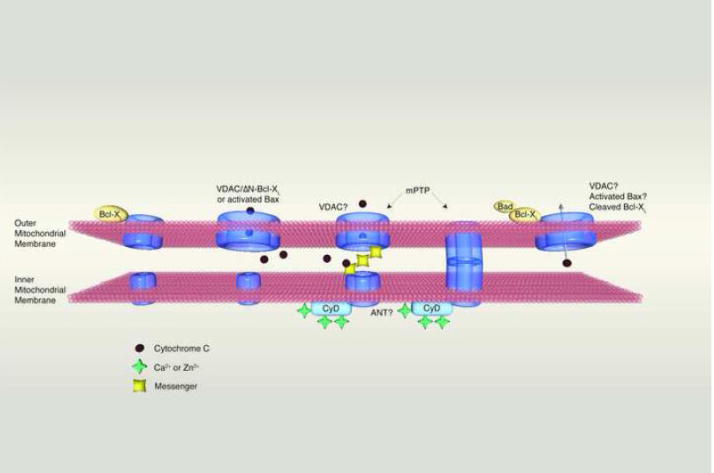
Figure 1.
Interaction of inner and outer mitochondrial membrane ion channels in different scenarios. From left to right: 1. During physiological functioning of mitochondria VDAC opens to allow the release of ATP. This action is assisted by full length BCL-xL protein during the prevention of cell death or during synaptic transmission. 2. During cell death, cytochrome c is released through an outer membrane channel formed by BAX or ΔN BCL-xL working in conjunction with VDAC. 3. In certain types of neuronal death such as occur during ischemia, both inner and outer membrane channels participate. Ca2+ binds to Cyclophilin to activate the inner membrane permeability transition pore, which then either a) sends a messenger to the outer membrane to cause the opening of VDAC or b) contacts the outer membrane directly, forming a two-membrane spanning pore. 4. In certain types of brain ischemia, BAD participates in the formation of the outer membrane component of the death channel.
Supporting a role for VDAC in “cell death channel” formation in the hypoxic squid synapse, both the mitochondrial ion channels produced in the hypoxic squid synapse and the channels activated by the application of ΔN BCL-xL to control neuronal mitochondria were found to be strongly inhibited by mM concentrations of NADH. NADH binds with low affinity to VDAC but in large concentrations can block its channel activity (Wunder and Colombini, 1991; Lee et al., 1994). Although NADH blocked the activity of ΔN BCL-xL in mitochondrial membranes, it was ineffective when exposed to ΔN BCL-xL-induced channel activity in artificial lipid membranes, where VDAC was absent (Basanez et al., 2001; Basanez et al., 2002; Jonas et al., 2004), suggesting that the large channel activity of mitochondria that have undergone a death stimulus (hypoxia) in squid presynaptic neurons is produced by a channel made up of a protein complex of VDAC and ΔN BCL-xL. Whether BCL-xL and VDAC actually interact biochemically or merely biophysically is still in question, although previous studies favor a biochemical as well as a biophysical interaction between VDAC and the death channel-forming protein BAX (Tsujimoto and Shimizu, 2000).
VDAC2 inhibits apoptosis
In mammals, three different VDAC genes encoding distinct isoforms have been reported (Shoshan-Barmatz et al., 2006). More complex multicellular organisms have all three isoforms, suggesting that the different isoforms have specialized functions. VDAC1 and 3 are the predominant pore forming isomers of VDAC in mammalian cells, but a role for VDAC2 has recently been suggested (Cheng et al., 2003). Cells deficient in VDAC2, but not VDAC1, are more susceptible to BAK oligomerization and apoptotic cell death, and these events are prevented by overexpression of VDAC2. In the model to explain these findings, the authors suggested that the pro-apoptotic molecules tBID, BIM or BAD could displace VDAC2 from BAK enabling homo-oligomerization of BAK and release of cytochrome c through the mitochondrial outer membrane.
Cell death channel activity and mitochondrial permeability transition-the mitochondrial permeability transition pore (mPTP)
Another protein complex participating in death channel formation during ischemia may be comprised of the inner and outer membrane protein components of the mitochondrial permeability transition pore (mPTP). The mPTP is activated during ischemic cell death when calcium enters the cytosol and mitochondria (Nicholls and Chalmers, 2004). Mitochondria take up calcium up via the calcium uniporter channel (Kirichok et al., 2004; Nicholls and Chalmers, 2004) but if calcium rises too rapidly, the buffering capacity of the mitochondrial matrix becomes overwhelmed and the mitochondrial inner membrane depolarizes suddenly, severely compromising the ability to make ATP (Crompton, 1999). The cause of the sudden depolarization is most likely the opening of a channel, the mPTP, which plays an important role in cell death (Figure 2).
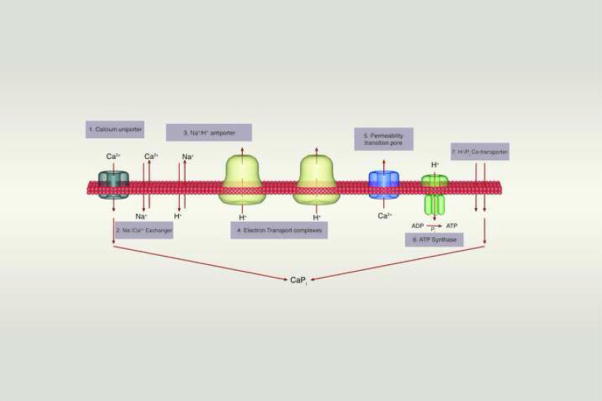
Figure 2.
The inner membrane forms a barrier to ions and solutes that can be breached by transporters and channel pores. When cytosolic calcium levels are low, calcium cycles into and out of the matrix using channels and transporters (1–4). When calcium levels rise, mitochondria buffer calcium through the exchange of H+ ions for calcium, compromising the ability of mitochondria to make ATP (6). The buffering of calcium is aided by the formation of a calcium-phosphate complex in the matrix (7). Calcium can be re-released from the matrix by transporters (2–4), or through a calcium activated pore (5).
The permeability transition is characterized by an increase in permeability of the inner membrane to a large number of unrelated solutes (Gunter and Pfeiffer, 1990). Physiologically relevant molecules and those for which there is no known influx pathway may enter by diffusion down their concentration gradients, because all energy-dependent processes of the inner membrane are arrested by the dissipation of the mitochondrial inner membrane potential gradient. Because permeability transition is induced in part by elevated calcium on the matrix side, transition serves as a calcium efflux pathway from the matrix to the cytosol. Swelling of the matrix occurs after permeability transition because of the rapid influx of ions and small molecules followed by water. Swelling can be measured optically as a decrease in light scattering by the dissolved solutes. Optically measured permeability transition can be correlated with channel activity as measured by patch clamp recording of purified inner mitochondrial membrane remnants called “mitoplasts”.
“MCC” (Kinnally and Tedeschi, 1994), or mitochondrial megachannel (MMC) (Szabo and Zoratti, 1991), is a multi-conductance, 1.0–1.5 nS, voltage dependent channel recorded in mitoplast preparations. The activity of this channel is more prominent at negative potentials with closings to lower conductances at positive potentials. Channels of the MCC are activated by Ca2+. MCC has also been termed mPTP by other investigators. Studies of mPTP reveal that it is inhibited by cyclosporine A (CSA) (a known inhibitor of the prolyl isomerase cyclophilin), and also by Mg2+, Ba2+, Sr2+, Mn2+, ADP, ATP, and pH changes (Szabo and Zoratti, 1992) (Bernardi et al., 1992). Similarly to MCC, this channel is activated by calcium and voltage changes (Gunter and Pfeiffer, 1990; Kinnally et al., 1991; Bernardi, 1992; Bernardi et al., 1992; Scorrano et al., 1997; Crompton, 1999) (Petronilli et al., 1989). (Szabo et al., 1992) (Szabo and Zoratti, 1991). Cyclophilin itself can also inhibit permeability transition measured optically.
The finding that channel activity of mPTP is inhibited by the binding of CSA to cyclophilin establishes that cyclophilin is in a complex with the channel pore protein, whose molecular nature is still not completely known. Studies of cyclophilin D knockout mice by two independent groups (Baines et al., 2005; Nakagawa et al., 2005) suggest that cyclophilin D, and by inference the mPTP, is involved in ischemic necrosis, because mitochondria isolated from the knockout mice are resistant to calcium-induced and CSA-inhibited permeability transition. Correlated with the resistance to permeability transition is resistance to necrotic death induced by reactive oxygen species (ROS) and calcium overload and to cardiac ischemia/reperfusion injury (Nakagawa et al., 2005). In addition, tissues from animals overexpressing cyclophilin D have abnormal swollen mitochondria and a propensity toward spontaneous cell death (Baines et al., 2005). Despite these findings, developmental apoptotic death appears normal in the cyclophilin knockouts, raising suspicions that developmental apoptosis does not require cyclophilin-regulated inner membrane channel activity.
The prevalent hypothesis is that the mPTP contains three components, the ANT which normally transports ATP out of the inner membrane, VDAC, and cyclophilin D (Crompton et al., 1998). The role of the ANT remains unproven, because in hepatocyte mitochondria isolated from knockout animals lacking both liver-expressed isoforms of ANT, calcium-induced permeability transition still occurs, and is non-responsive to inhibitors of ANT such as bongkrekic acid (Kokoszka et al., 2004). In addition, recordings of MCC from yeast mitoplasts lacking any ANT protein were biophysically and pharmacologically similar to those of control, ANT-containing mitoplasts, and not affected by the ANT inhibitor, carboxyatractyloside (Lohret et al., 1996). The role of VDAC also remains unproven, since permeability transition and cell death can take place in the absence of this molecule (Lohret and Kinnally, 1995; Baines et al., 2007), although differentiated hippocampal neurons may be dependent on VDAC for cell death (Akanda et al., 2008).
Complex of channels exists at contact points between outer and inner membrane
It is tempting to speculate that VDAC forms a molecular complex with an inner membrane channel for the purpose of metabolite and ion exchange directly between the matrix and the cytosol. Co-immunoprecipitation studies support this hypothesis suggesting that VDAC, ANT and cyclophilin D are biochemically linked (Crompton et al., 1998). VDAC clusters at contact sites where mPTP-like activity is present by patch recording (Brdiczka et al., 1986; Sandri et al., 1988; Moran et al., 1990). (Figure 1).
There are estimated to be about 37 contact sites per square micron of mitochondrial membrane, making it likely that there are 7 contact sites in a recording of a membrane patch of 0.5 microns. Because of the importance of communication between the matrix and the cytosol, it is possible that a functional mitochondrial channel could require the presence of both inner and outer membrane components at contact sites in series (Halestrap, 2005). This would provide for streamlined flow of ions and metabolites from the matrix bypassing the intermembrane space to the cytosol. If this were to be the only type of conduit, however, there would be no way for intermembrane space components to be taken up or released through a channel. For example, cytochrome c, which resides in the intermembrane space, could not get released in the absence of outer membrane rupture (Brustovetsky et al., 2002; Green and Kroemer, 2004) Opposing the idea that channels of the two membranes exist in a fixed complex is the finding that recordings of MCC in yeast mitoplasts (with contact points) derived from cells lacking VDAC have channel activity that is similar to that of MCC recorded in mitoplasts from wild type cells (Lohret and Kinnally, 1995), and that normal developmental apoptosis (with cytochrome c release) occurs in cyclophilin D knockout animals presumably in the absence of high conductance inner membrane channel activity (Baines et al., 2005; Nakagawa et al., 2005).
Contact point formation may be regulated by cell metabolic needs. Contact between the membranes may occur depending on the need to release ATP, to permit the entry of ADP into the matrix, or to support the entry of calcium. Contact sites may form when oxidative phosphorylation is taking place (Knoll and Brdiczka, 1983). In keeping with this idea, ADP, atractyloside and succinate, a substrate for mitochondrial respiration, appear to induce contacts, whereas glycerol and uncouplers like DNP or the electron transport inhibitor antimycin A decrease their formation (Hackenbrock, 1972; Bucheler et al., 1991). Bonanni et al. have observed a decrease in electron density of contact sites after ischemia, suggesting that channel regulatory proteins may have dispersed from the complex perhaps as a result of damage to the electron transport chain during the ischemic event (Bonanni et al., 2006). One possible explanation for the multiconductance state of many mitochondrial channels could be the ability to increase conductance depending on an increasing interaction between the two membranes (Kinnally and Tedeschi, 1994).
Fundamental mitochondrial events during ischemia
We have seen that mitochondrial ion channel activity of VDAC, the calcium uniporter, and mPTP, either working in coordination or as part of a unified complex, may regulate the functional state of neurons by changing levels of ATP and intracellular calcium. Members of the BCL-2 family may also regulate this complex of proteins, including the mPTP itself.
Brain ischemia is a pathological condition that serves to exemplify the complexity that underlies decisions of cell life and death in the adult nervous system. Human brain ischemia arises as a result of low oxygen tension in the tissues after obstruction of an artery that supplies part of the brain with blood (focal ischemia) or a cardio-pulmonary event that significantly lowers systemic blood pressure for an extended period of time (global ischemia) (Zukin et al., 2004). Models of these human disease states have been developed in rodents. Focal ischemia in rodents most closely resembles human stroke where complete blockage of an artery causes rapid necrosis of tissue dependent on that artery for blood supply. Cells in the penumbra, or area that receives only partial supply from the damaged artery, are at risk for delayed cell death that has many of the biochemical and some of the morphological features of programmed cell death. Transient forebrain or global ischemia, in contrast, induces delayed, cell-specific death only of vulnerable hippocampal CA1 pyramidal neurons, whereas the normal function of other brain areas is restored after the ischemic period (Zukin et al., 2004;(Banasiak et al., 2000) (Lee et al., 1999; Lipton, 1999). In rodents, this latter experimental paradigm serves as a good model for the study of programmed cell death in the vulnerable neurons.
The events during and after global ischemia can be described as follows: During the ischemic episode, mitochondria in the neurons serve as “oxygen-sensing organelles” and rapidly decrease ATP production (Sugawara et al., 1999; Wang et al., 2003). Cells throughout the brain exhibit energy depletion and altered energy dynamics (Howard et al., 1998; Tian and Baker, 2000; Fleidervish et al., 2001) (Bolay et al., 2002). ATP depletion induces neuronal membrane depolarization and promotes release of synaptic glutamate, a rise in cytosolic Ca2+, reverse operation of glutamate transporters and swelling of cells (Choi, 1994; Sattler and Tymianski, 2000; Nishizawa, 2001) (Zukin et al., 2004). After reperfusion, ATP levels and membrane polarization are restored (Zukin et al., 2004). Although histologically detectable neuronal death does not occur until 48 h, apoptotic cascades are activated within 3 h (Alkayed et al., 2001; Northington et al., 2001). An early event is disruption of the integrity of the mitochondrial outer membrane enabling release of cytochrome c from the inter-membrane space (Ouyang et al., 1999; Sugawara et al., 1999).
Cellular events during ischemia-excitotoxicity
Several events occur during ischemia and afterwards in the phase of reperfusion that greatly influence the behavior of mitochondrial ion channels, and it is clear that regulation of these channels helps determine the fate of a neuron undergoing ischemic injury (Crompton, 1999). The first important phenomenon is that cytosolic ATP levels decline. This occurs as the high energy bonds of ATP and ADP are used to provide energy for cellular kinases and pumps, in particular the Na+/K+ ATPase and Ca++ ATPases at the plasma membrane and endoplasmic reticulum. The direct effect on mitochondria of low ATP and high phosphate in the setting of a lack of oxygen is complex, but Pi is known to activate the mPTP when free calcium levels rise rapidly in the matrix. Although adenine nucleotides prevent pore opening, continued hypoxia leads eventually to complete loss of adenine nucleotides as they are degraded to the nucleosides and bases (Jennings and Steenbergen, 1985).
The other important event in ischemia is a rise in cytosolic calcium (Figure 2). Calcium rises as a result of several events. Depletion of intracellular ATP slows the activity of intracellular membrane and plasma membrane calcium ATPases which are used to extrude calcium from the cell. In addition, depolarization of the plasma membrane occurs because of a lack of ATP for use by the Na+/K+ exchanger. This activates voltage-gated calcium channels and the release of glutamate, which further acts on calcium-permeable glutamate receptors postsynaptically to allow the influx of calcium (and zinc) into the cells. Additional glutamate release occurs after reversal of the glutamate uptake transporters upon energy failure in both neurons and glia. High sodium influx through NMDA receptors may also prevent neuronal re-uptake of glutamate even after reperfusion (Nicholls, 2004). Intracellular acidification caused by lactic acidosis also contributes to excitotoxicity by leading to increased intracellular Na+ through Na+/H+ exchange, with resultant further impairment of Na+/Ca++ exchange. The latter is particularly prominent upon reperfusion, when extracellular acidity is rectified, leading to a high pH gradient across the plasma membrane and cell swelling.
A key element in the activation of calcium influx is uptake of calcium by mitochondria. Mitochondria take up calcium via the activity of the calcium uniporter channel across the inner membrane. In the presence of inorganic phosphate, mitochondria load with large amounts of calcium without altering levels of matrix free calcium, since calcium and phosphate precipitate. The calcium buffering capacities of mitochondria enable mitochondria to buffer cytosolic calcium whenever the cytoplasmic calcium level rises above the “set-point” for the balance of mitochondrial influx and efflux of calcium (Nicholls and Chalmers, 2004). However, acutely massive elevations in cytosolic calcium, or chronically elevated cytoplasmic calcium above the setpoint lead to calcium overload in the mitochondria and permeability transition, via the calcium-activated pore (mPTP) in the inner membrane. It is not clear exactly how or if the mPTP always gets activated during ischemia (Reynolds, 1999), but it is clear that continued calcium accumulation by mitochondria causes a drain on cellular energy, as the mitochondria need to constantly re-establish the proton gradient during long-term calcium uptake. It is possible that the energy requirements in the presence of plasma membrane depolarization after glutamate exposure plus the requirements to re-establish the mitochondrial membrane potential require more energy than the mitochondria can produce during ischemia, and therefore the membrane potential of the mitochondria depolarizes, leading to further activation of the voltage dependent mPTP.
The central importance of mitochondrial calcium uptake in the promotion of cell death from excitotoxicity was demonstrated by showing that prior depolarization of mitochondria slowed calcium uptake by decreasing the electrochemical gradient (Budd and Nicholls, 1996b, a) and protecting cells from death. In addition, loss of contractile function in heart on reperfusion of ischemic tissue appears to be related to increased mitochondrial, not cytoplasmic calcium levels (Miyamae et al., 1996).
BCL-2 family proteins in ischemic synaptic rundown
BCL-2 family members are critical to breakdown of the functional integrity of the mitochondrial outer membrane in response to ischemia and other insults (Hengartner, 2000; Kroemer and Reed, 2000). As described, injurious stimuli such as hypoxia promote the N-terminal proteolytic cleavage of BCL-xL, converting it into a BAX-like killer protein ΔN-BCL-xL (Cheng et al., 1997). Pro-apoptotic BCL-2 family members such as BAX and ΔN-BCL-xL are known to promote the formation of large conductance channels in the mitochondrial outer membrane after ischemia (Jonas et al., 2004; Jonas et al., 2005a). Large mitochondrial channels promote cytochrome c release (Clem et al., 1998; Fujita et al., 1998) and synaptic failure (Jonas et al., 2003). If the mPTP, VDAC, and other proteins are involved in excitotoxity, perhaps the BCL-2 family proteins also come together in a protein complex with these mitochondrial players during ischemic injury.
Patch clamp studies revealed that ischemic insults in neurons quickly produce changes in synaptic efficacy coincident with the onset of large mitochondrial ion channel activity (Jonas et al., 2003; Bonanni et al., 2006). The function of the synapses of a neuron could determine whether that neuron will survive or die, and therefore, mitochondrial “death channel” activity within the synaptic terminal could induce synaptic and eventually cellular apoptosis. The presynaptic terminal of the squid giant stellate ganglion is very sensitive to hypoxia, which attenuates synaptic transmission over 10–30 minutes (Jonas et al., 2005b). Thus, the squid synapse provides a good model in which to study the effects of hypoxia on mitochondrial ion channels (Jonas et al., 2005b).
As described above, the channel activity produced by hypoxic conditions in squid synapse closely resembles that produced by applying ΔN BCL-xL protein to control mitochondria during patch clamp recordings, and involves VDAC in the outer mitochondria membrane. Although the possible physiological role of BCL-2 family proteins in regulation of synaptic transmission had not been previously reported, evidence had already indicated that hypoxia-induced neuronal death can be attributed, at least in part, to activation of BCL-2 family proteins (Sugawara et al., 1999; Northington et al., 2001)(Martinou et al., 1994; Kitagawa et al., 1998; Lindsten et al., 2000) (Alkayed et al., 2001) (Cheng et al., 1997); (Condorelli et al., 2001) (Li et al., 1998; Kirsch et al., 1999) (Luo et al., 1998; Wood and Newcomb, 2000), and therefore it seemed that synaptic rundown during hypoxia might also likely involve these proteins. A pro-death role for BCL-xL during hypoxia could be due to early caspase or calpain-like proteolytic cleavage of the full length molecule to produce its death-inducing partner molecule, ΔN BCL-xL.
Proof of the hypothesis that the ion channel activity of the ischemic squid mitochondria was regulated by BCL-xL came from studies with the small molecule ABT-737, a mimetic of the BH3-only protein BAD that binds to BCL-xL with high affinity within a pocket of the three-dimensional structure that usually binds BH3-only proteins. The binding of ABT-737 displaces GFP-tagged BH3-only proteins from BCL-xL at mitochondrial surfaces in intact tumor cells and in cancer cell lines, ABT-737 effectively induces cell death possibly via its ability, as a BAD mimetic, to displace from BCL-xL the pre-bound pro-apoptotic proteins BAX and BAK (Oltersdorf et al., 2005). When injected prior to a hypoxic insult of the squid synapse, however, ABT-737 seemingly has the opposite effect to that in the tumor cells. It prevents the appearance of the large conductance death-like channel activity of mitochondrial membranes associated with declining synaptic function (Hickman et al., 2008). Furthermore, ABT-737 attenuates synaptic dysfunction produced by hypoxia or by exogenous injection of ΔN BCL-xL into the synaptic terminal. These results suggested that BCL-xL, in its pro-apoptotic truncated form, is contributing to large conductance mitochondrial death channel activity in hypoxic neuronal synapses.
Large channels in mitochondria of post-ischemic hippocampal CA1 neurons
In mammalian brain after global ischemia, neurons from the CA1 region of the hippocampus are vulnerable to a type of delayed death with characteristics of programmed cell death, in particular the release of pro-apoptotic molecules such as cytochrome c from mitochondria into the cytososl, the activation of downstream caspases, and the eventual enzymatic destruction of cellular components including DNA (Polster and Fiskum, 2004). Just as in hypoxic squid neurons, the mitochondria isolated from brains of ischemic rats at 50 min after reperfusion demonstrate large channels with multiple levels of conductance. In keeping with the early activation of proteases in ischemic models, global ischemia produces pronounced protease activity in synaptosomes, as assessed by zVAD-FMK fluorescence at 50 min after insult. Control mitochondria from hippocampus exhibit high levels of BCL-xL, with little or no evidence of Δ N-BCL-xL. In contrast, global ischemia rapidly induces the appearance of Δ N-BCL-xL by Western blotting, suggesting that early caspase or calpain cleavage of BCL-xL to the pro-apoptotic N-BCL-xL may cause early changes in mitochondrial conductances after global ischemia. Furthermore, the ischemic channel activity is mimicked by application of recombinant ΔN BCL-xL protein to control mitochondria, and inhibited by a specific antibody against BCL-xL (Bonanni et al., 2006).
Role of VDAC in ischemia
Because the channel recordings on mammalian brain mitochondria are performed directly on predominantly intact mitochondria (by electron micrographic studies, (Bonanni et al., 2006), it is likely that the membrane contacted by the patch pipette is the outer mitochondrial membrane. The conductance of this membrane is known to be reduced by millimolar concentrations of NADH (Lee et al., 1994). In lipid bilayers, NADH reduces the conductance of VDAC (Colombini et al., 1996) and in squid mitochondria, NADH reduces the conductance of both ΔN BCL-xL and the hypoxia channel (Jonas et al., 2004; Jonas et al., 2005b). In keeping with these findings, recordings of channel activity in post-ischemic mammalian brain mitochondria in the presence of NADH (2 mM) applied via the bath perfusate and the patch pipette contain a significantly lower frequency of large conductance activity than recordings of ischemic mitochondria performed in the absence of NADH, suggesting that the ischemia-induced channel requires VDAC.
Building the death channel: Role of the inner membrane in outer mitochondrial membrane activity
Recordings of outer membranes reconstituted into liposomes or of intact mitochondria within living cells reveal that the permeability of the outer membrane is tightly controlled (Tedeschi and Kinnally, 1987; Tedeschi et al., 1989; Jonas et al., 1999; Pavlov et al., 2001). Thus, outer membrane channel regulation could occur at the cytosolic or intermembrane face by ligands, by second messengers, or by contact with inner membrane proteins, particularly ion channels, at contact sites between the two membranes (Marzo et al., 1998; Brenner et al., 2000). Introduction of Ca2+ or other divalent ions to the matrix side of the patch in preparations of isolated inner membranes (“mitoplasts”), (Kinnally et al., 1989; Petronilli et al., 1989) and/or application of Zn2+ via the bath perfusion (Sensi et al., 2003) activates a nS channel in the inner membrane. This inner membrane channel is blocked by CSA (Szabo and Zoratti, 1991) and the Zn2+ chelator TPEN (Sensi et al., 2003) suggesting that the channel is the mPTP.
In ischemic brain, Zn2+ localizes to the inside of ischemic mitochondria by microscopic imaging of fluorescent zinc-specific indicators. Large conductance activity present in the outer membranes of such post-ischemic mitochondria is exquisitely sensitive to bath application of the specific Zn2+ chelator, TPEN (Bonanni et al., 2006). The findings suggest that Zn2+ activates a divalent-sensitive channel on the inner membrane that would in turn activate a VDAC/ΔN BCL-xL complex on the outer membrane.
Ischemic tolerance and mitochondrial ion channel activity: Role of BAD
One set of proofs that the mitochondrial death channel complex activated by ischemia is necessary for cell death comes from studies suggesting that such channel activity is attenuated in mitochondria of neurons that recover from ischemia. Ischemic tolerance is a well-established phenomenon in which a brief ischemic insult (or preconditioning) protects CA1 neurons against a subsequent more prolonged ischemic challenge (Gidday, 2006). The protective action of ischemic tolerance involves inhibition of activated caspase-3 (Tanaka et al., 2004), Akt phosphorylation at Ser473 and phosphorylation/inactivation of downstream targets of Akt. PI3K/Akt signaling, which regulates cell growth and survival (Plas et al., 2001; Plas and Thompson, 2005) is required for preconditioning-induced neuroprotection (Yano et al., 2001). In vitro studies show that the phosphorylated pro-apoptotic molecule BAD normally resides in a complex with the cytosolic chaperone 14-3-3. During ischemia and after other cell death signals, BAD becomes dephosphorylated, dissociates from 14-3-3, and translocates to the outer membrane of the mitochondria, where it binds to BCL-xL and promotes mitochondrial cytochrome c release (Chan, 2004). Dephosphorylated BAD is the only form of BAD that can bind to BCL-xL or can be found in the mitochondrial membrane (Zha et al., 1996), suggesting that the binding of dephosphorylated BAD to BCL-xL at mitochondrial membranes is necessary for formation of the death channel complex and for cell death.
The complete mechanism of the control of cell death by BAD translocation to the mitochondrion is still not completely understood. When BAD binds to BCL-xL, it may release BAX, or release activator pro-apoptotic molecules such as BID (Galonek and Hardwick, 2006; Kim et al., 2006). BID activates the oligomerization of BAX, which then forms pores in the mitochondrial outer membrane to release pro-apoptotic factors from the mitochondria (Luo et al., 1998; Plesnila et al., 2001; Polster et al., 2001; Shangary and Johnson, 2002; Polster et al., 2003; Polster and Fiskum, 2004). Evidence that BCL-xL might also contribute directly to cell death channel formation after BAD translocation comes from findings that the large channel activity recorded in post-ischemic mitochondria is correlated with the co-precipitation of BAD with BCL-xL. BAD binding to BCL-xL after ischemia is also correlated with the proteolytic cleavage of BCL-xL to form ΔN BCL-xL, which, in addition to, or in lieu of BAX, could be responsible for the change in outer membraine permeability and cytochrome c release from mitochondria (Miyawaki et al., 2008). Preconditioning of the animals prior to the ischemic episode prevents BAD interaction with BCL-xL, and prevents large channel formation at mitochondrial membranes. The absence of channel formation is in turn associated with the prevention of cytochrome c release, inhibition of caspase activation and finally with a marked attenuation of cell death.
Conclusions
We have seen that the regulation of programmed cell death is a complex and varied process. In this review we have discussed the participation of mitochondrial ion channel activity in cell death, in particular that activity that represents formation or putative formation of the cytochrome c releasing cell death channel. Formation of the death channel may, under some conditions, involve solely the outer membrane. In this scenario, BAX or BAK homo-oligomerize to form a large pore. In contrast, in some forms of neuronal death such as that produced by brain ischemia, ΔN BCL-xL forms a complex with BAD and/or VDAC in the outer membrane and then may contact the inner membrane divalent-activated mPTP to form a two-membrane spanning channel. Alternatively, opening of the inner membrane mPTP could trigger binding of a ligand to the outer membrane to activate the outer membrane complex. In some scenarios, swelling of the matrix and eventual bursting apart of the outer membrane may accompany channel formation. Further understanding of inner membrane involvement in death channel activation during ischemic neuronal death will be aided by molecular knowledge of the inner membrane pore-foming proteins. Finally, there exist some molecular similarities between the channel complex that forms in mitochondrial membranes during ischemic cell death in neurons, and the complex that forms in mitochondrial membranes of synapses that are undergoing an acute decline in the release of neurotransmitter, suggesting that the cell death machinery may be used in the mitochondrial membrane to help produce certain forms of synaptic plasticity.
Acknowledgments
I gratefully acknowledge the artwork by Kambiz Alavian.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanda N, Tofighi R, Brask J, Tamm C, Elinder F, Ceccatelli S. Voltage-dependent anion channels (VDAC) in the plasma membrane play a critical role in apoptosis in differentiated hippocampal neurons but not in neural stem cells. Cell Cycle. 2008;7:3225–3234. doi: 10.4161/cc.7.20.6831. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, Bernard O, Traystman RJ, Hurn PD. Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke. Journal of Neuroscience. 2001;21:7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochemical Journal. 2000;345(Pt 2):271–278. [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death.[see comment] Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death.[see comment] Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Progress in Neurobiology. 2000;62:215–249. doi: 10.1016/s0301-0082(00)00011-3. [DOI] [PubMed] [Google Scholar]
- Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem. 2002;277:49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- Basanez G, Zhang J, Chau BN, Maksaev GI, Frolov VA, Brandt TA, Burch J, Hardwick JM, Zimmerberg J. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J Biol Chem. 2001;276:31083–31091. doi: 10.1074/jbc.M103879200. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992;267:8834–8839. [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiological Reviews. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabo I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992;267:2934–2939. [PubMed] [Google Scholar]
- Bolay H, Gursoy-Ozdemir Y, Sara Y, Onur R, Can A, Dalkara T. Persistent defect in transmitter release and synapsin phosphorylation in cerebral cortex after transient moderate ischemic injury.[see comment] Stroke. 2002;33:1369–1375. doi: 10.1161/01.str.0000013708.54623.de. [DOI] [PubMed] [Google Scholar]
- Bonanni L, Chachar M, Jover-Mengual T, Li H, Jones A, Yokota H, Ofengeim D, Flannery RJ, Miyawaki T, Cho CH, Polster BM, Pypaert M, Hardwick JM, Sensi SL, Zukin RS, Jonas EA. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. Journal of Neuroscience. 2006;26:6851–6862. doi: 10.1523/JNEUROSCI.5444-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdiczka D, Knoll G, Riesinger I, Weiler U, Klug G, Benz R, Krause J. Microcompartmentation at the mitochondrial surface: its function in metabolic regulation. Advances in Experimental Medicine & Biology. 1986;194:55–69. doi: 10.1007/978-1-4684-5107-8_5. [DOI] [PubMed] [Google Scholar]
- Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, Leber B, Andrews D, Duclohier H, Reed JC, Kroemer G. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene. 2000;19:329–336. doi: 10.1038/sj.onc.1203298. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. Journal of Neurochemistry. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- Bucheler K, Adams V, Brdiczka D. Localization of the ATP/ADP translocator in the inner membrane and regulation of contact sites between mitochondrial envelope membranes by ADP. A study on freeze-fractured isolated liver mitochondria. Biochimica et Biophysica Acta. 1991;1056:233–242. doi: 10.1016/s0005-2728(05)80054-4. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. Journal of Neurochemistry. 1996a;66:403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. Journal of Neurochemistry. 1996b;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochemical Research. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium and excitotoxic neuronal injury. Annals of the New York Academy of Sciences. 1994;747:162–171. doi: 10.1111/j.1749-6632.1994.tb44407.x. [DOI] [PubMed] [Google Scholar]
- Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci U S A. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini M, Blachly-Dyson E, Forte M. VDAC, a channel in the outer mitochondrial membrane. Ion Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- Condorelli F, Salomoni P, Cotteret S, Cesi V, Srinivasula SM, Alnemri ES, Calabretta B. Caspase cleavage enhances the apoptosis-inducing effects of BAD. Molecular & Cellular Biology. 2001;21:3025–3036. doi: 10.1128/MCB.21.9.3025-3036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochemical Journal. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. European Journal of Biochemistry. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA, Kinnally KW. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannjiang Y, Kim CH, Huganir RL, Zou S, Lindsten T, Thompson CB, Mito T, Traystman RJ, Larsen T, Griffin DE, Mandir AS, Dawson TM, Dike S, Sappington AL, Kerr DA, Jonas EA, Kaczmarek LK, Hardwick JM. BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development. Developmental Cell. 2003;4:575–585. doi: 10.1016/s1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Gebhardt C, Astman N, Gutnick MJ, Heinemann U. Enhanced spontaneous transmitter release is the earliest consequence of neocortical hypoxia that can explain the disruption of normal circuit function. Journal of Neuroscience. 2001;21:4600–4608. doi: 10.1523/JNEUROSCI.21-13-04600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T. Acceleration of apoptotic cell death after the cleavage of Bcl-XL protein by caspase-3-like proteases. Oncogene. 1998;17:1295–1304. doi: 10.1038/sj.onc.1202065. [DOI] [PubMed] [Google Scholar]
- Galonek HL, Hardwick JM. Upgrading the BCL-2 network.[comment] Nat Cell Biol. 2006;8:1317–1319. doi: 10.1038/ncb1206-1317. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. American Journal of Physiology. 1990;258:C755–786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Hackenbrock CR. States of activity and structure in mitochondrial membranes. Annals of the New York Academy of Sciences. 1972;195:492–505. [PubMed] [Google Scholar]
- Hackenbrock CR, Rehn TG, Weinbach EC, Lemasters JJ. Oxidative phosphorylation and ultrastructural transformation in mitochondria in the intact ascites tumor cell. J Cell Biol. 1971;51:123–137. doi: 10.1083/jcb.51.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. Biochemistry: a pore way to die.[comment] Nature. 2005;434:578–579. doi: 10.1038/434578a. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis.[see comment] Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hickman JA, Hardwick JM, Kaczmarek LK, Jonas EA. Bcl-xL inhibitor ABT-737 reveals a dual role for Bcl-xL in synaptic transmission. J Neurophysiol. 2008;99:1515–1522. doi: 10.1152/jn.00598.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EM, Gao TM, Pulsinelli WA, Xu ZC. Electrophysiological changes of CA3 neurons and dentate granule cells following transient forebrain ischemia. Brain Research. 1998;798:109–118. doi: 10.1016/s0006-8993(98)00403-x. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Jennings RB, Steenbergen C., Jr Nucleotide metabolism and cellular damage in myocardial ischemia. Annual Review of Physiology. 1985;47:727–749. doi: 10.1146/annurev.ph.47.030185.003455. [DOI] [PubMed] [Google Scholar]
- Jonas E. Regulation of synaptic transmission by mitochondrial ion channels. J Bioenerg Biomembr. 2004;36:357–361. doi: 10.1023/B:JOBB.0000041768.11006.90. [DOI] [PubMed] [Google Scholar]
- Jonas E. BCL-xL regulates synaptic plasticity. Molecular Interventions. 2006;6:208–222. doi: 10.1124/mi.6.4.7. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Buchanan J, Kaczmarek LK. Prolonged activation of mitochondrial conductances during synaptic transmission. Science. 1999;286:1347–1350. doi: 10.1126/science.286.5443.1347. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Hardwick JM, Kaczmarek LK. Actions of BAX on mitochondrial channel activity and on synaptic transmission. Antioxidants & Redox Signaling. 2005a;7:1092–1100. doi: 10.1089/ars.2005.7.1092. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Hickman JA, Hardwick JM, Kaczmarek LK. Exposure to hypoxia rapidly induces mitochondrial channel activity within a living synapse. J Biol Chem. 2005b;280:4491–4497. doi: 10.1074/jbc.M410661200. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Hoit D, Hickman JA, Brandt TA, Polster BM, Fannjiang Y, McCarthy E, Montanez MK, Hardwick JM, Kaczmarek LK. Modulation of synaptic transmission by the BCL-2 family protein BCL-xL. Journal of Neuroscience. 2003;23:8423–8431. doi: 10.1523/JNEUROSCI.23-23-08423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA, Hickman JA, Chachar M, Polster BM, Brandt TA, Fannjiang Y, Ivanovska I, Basanez G, Kinnally KW, Zimmerberg J, Hardwick JM, Kaczmarek LK. Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proc Natl Acad Sci U S A. 2004;101:13590–13595. doi: 10.1073/pnas.0401372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies.[see comment] Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kinnally KW, Campo ML, Tedeschi H. Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr. 1989;21:497–506. doi: 10.1007/BF00762521. [DOI] [PubMed] [Google Scholar]
- Kinnally KW, Zorov D, Antonenko Y, Perini S. Calcium modulation of mitochondrial inner membrane channel activity. Biochemical & Biophysical Research Communications. 1991;176:1183–1188. doi: 10.1016/0006-291x(91)90410-9. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, Yang G, Tanabe H, Martinou JC, Hori M, Yanagihara T. Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice.[see comment][comment] Stroke. 1998;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Knoll G, Brdiczka D. Changes in freeze-fractured mitochondrial membranes correlated to their energetic state. Dynamic interactions of the boundary membranes. Biochimica et Biophysica Acta. 1983;733:102–110. doi: 10.1016/0005-2736(83)90095-0. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore.[see comment] Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska M, Mai JK, Zapata JM, Ashwell KW, Schendel SL, Reed JC, Krajewski S. Dynamics of expression of apoptosis-regulatory proteins Bid, Bcl-2, Bcl-X, Bax and Bak during development of murine nervous system. Cell Death & Differentiation. 2002;9:145–157. doi: 10.1038/sj.cdd.4400934. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nature Medicine. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Lee AC, Zizi M, Colombini M. Beta-NADH decreases the permeability of the mitochondrial outer membrane to ADP by a factor of 6. J Biol Chem. 1994;269:30974–30980. [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box. Biochimica et Biophysica Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Lindsten T, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Molecular Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiological Reviews. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lohret TA, Kinnally KW. Multiple conductance channel activity of wild-type and voltage-dependent anion-selective channel (VDAC)-less yeast mitochondria. Biophysical Journal. 1995;68:2299–2309. doi: 10.1016/S0006-3495(95)80412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohret TA, Murphy RC, Drgon T, Kinnally KW. Activity of the mitochondrial multiple conductance channel is independent of the adenine nucleotide translocator. J Biol Chem. 1996;271:4846–4849. doi: 10.1074/jbc.271.9.4846. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Caballero S, Dejean LM, Jonas EA, Kinnally KW. The role of the mitochondrial apoptosis induced channel MAC in cytochrome c release. J Bioenerg Biomembr. 2005;37:155–164. doi: 10.1007/s10863-005-6570-z. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Miyamae M, Camacho SA, Weiner MW, Figueredo VM. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. American Journal of Physiology. 1996;271:H2145–2153. doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Mashiko T, Ofengeim D, Flannery RJ, Noh KM, Fujisawa S, Bonanni L, Bennett MV, Zukin RS, Jonas EA. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran O, Sandri G, Panfili E, Stuhmer W, Sorgato MC. Electrophysiological characterization of contact sites in brain mitochondria.[erratum appears in J Biol Chem 1990 Jul 5;265(19):11405] J Biol Chem. 1990;265:908–913. [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death.[see comment] Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Current Molecular Medicine. 2004;4:149–177. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Chalmers S. The integration of mitochondrial calcium transport and storage. J Bioenerg Biomembr. 2004;36:277–281. doi: 10.1023/B:JOBB.0000041753.52832.f3. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sciences. 2001;69:369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. Journal of Neuroscience. 2001;21:1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B. Mitochondrial ion channels. Annual Review of Physiology. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Tan Y, Comb M, Liu CL, Martone ME, Siesjo BK, Hu BR. Survival- and death-promoting events after transient cerebral ischemia: phosphorylation of Akt, release of cytochrome C and Activation of caspase-like proteases. Journal of Cerebral Blood Flow & Metabolism. 1999;19:1126–1135. doi: 10.1097/00004647-199910000-00009. [DOI] [PubMed] [Google Scholar]
- Ow YL, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- Pavlov EV, Priault M, Pietkiewicz D, Cheng EH, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA, Kinnally KW. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J Cell Biol. 2001;155:725–731. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronilli V, Szabo I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Letters. 1989;259:137–143. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, Chiarugi A, Thomas SS, Kohane DS, Korsmeyer SJ, Moskowitz MA. BID mediates neuronal cell death after oxygen/glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci U S A. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. Journal of Neurochemistry. 2004;90:1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- Polster BM, Kinnally KW, Fiskum G. BH3 death domain peptide induces cell type-selective mitochondrial outer membrane permeability. J Biol Chem. 2001;276:37887–37894. doi: 10.1074/jbc.M104552200. [DOI] [PubMed] [Google Scholar]
- Polster BM, Basanez G, Young M, Suzuki M, Fiskum G. Inhibition of Bax-induced cytochrome c release from neural cell and brain mitochondria by dibucaine and propranolol. Journal of Neuroscience. 2003;23:2735–2743. doi: 10.1523/JNEUROSCI.23-07-02735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds IJ. Mitochondrial membrane potential and the permeability transition in excitotoxicity. Annals of the New York Academy of Sciences. 1999;893:33–41. doi: 10.1111/j.1749-6632.1999.tb07816.x. [DOI] [PubMed] [Google Scholar]
- Rostovtseva T, Colombini M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophysical Journal. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtseva TK, Tan W, Colombini M. On the role of VDAC in apoptosis: fact and fiction. J Bioenerg Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- Roucou X, Montessuit S, Antonsson B, Martinou JC. Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochemical Journal. 2002;368:915–921. doi: 10.1042/BJ20020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri G, Siagri M, Panfili E. Influence of Ca2+ on the isolation from rat brain mitochondria of a fraction enriched of boundary membrane contact sites. Cell Calcium. 1988;9:159–165. doi: 10.1016/0143-4160(88)90020-6. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. Journal of Molecular Medicine. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Schendel SL, Montal M, Reed JC. Bcl-2 family proteins as ion-channels. Cell Death & Differentiation. 1998;5:372–380. doi: 10.1038/sj.cdd.4400365. [DOI] [PubMed] [Google Scholar]
- Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci U S A. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Petronilli V, Bernardi P. On the voltage dependence of the mitochondrial permeability transition pore. A critical appraisal. Journal of Biological Chemistry. 1997;272:12295–12299. doi: 10.1074/jbc.272.19.12295. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc Natl Acad Sci U S A. 2003;100:6157–6162. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangary S, Johnson DE. Peptides derived from BH3 domains of Bcl-2 family members: a comparative analysis of inhibition of Bcl-2, Bcl-x(L) and Bax oligomerization, induction of cytochrome c release, and activation of cell death. Biochemistry. 2002;41:9485–9495. doi: 10.1021/bi025605h. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Current Pharmaceutical Design. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Solution of the problem of energy coupling in terms of chemiosmotic theory. J Bioenerg. 1972;3:25–38. doi: 10.1007/BF01515994. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. Journal of Neuroscience. 1999;19:RC39. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka R, Shimizu S, Funatsu T, Tamagawa H, Sawa Y, Kawakami T, Tsujimoto Y. BH4-domain peptide from Bcl-xL exerts anti-apoptotic activity in vivo. Oncogene. 2003;22:8432–8440. doi: 10.1038/sj.onc.1207180. [DOI] [PubMed] [Google Scholar]
- Szabo I, Zoratti M. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem. 1991;266:3376–3379. [PubMed] [Google Scholar]
- Szabo I, Zoratti M. The mitochondrial megachannel is the permeability transition pore. J Bioenerg Biomembr. 1992;24:111–117. doi: 10.1007/BF00769537. [DOI] [PubMed] [Google Scholar]
- Szabo I, Bernardi P, Zoratti M. Modulation of the mitochondrial megachannel by divalent cations and protons. J Biol Chem. 1992;267:2940–2946. [PubMed] [Google Scholar]
- Tanaka H, Yokota H, Jover T, Cappuccio I, Calderone A, Simionescu M, Bennett MV, Zukin RS. Ischemic preconditioning: neuronal survival in the face of caspase-3 activation. Journal of Neuroscience. 2004;24:2750–2759. doi: 10.1523/JNEUROSCI.5475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi H, Kinnally KW. Channels in the mitochondrial outer membrane: evidence from patch clamp studies. J Bioenerg Biomembr. 1987;19:321–327. doi: 10.1007/BF00768535. [DOI] [PubMed] [Google Scholar]
- Tedeschi H, Kinnally KW, Mannella CA. Properties of channels in the mitochondrial outer membrane. J Bioenerg Biomembr. 1989;21:451–459. doi: 10.1007/BF00762517. [DOI] [PubMed] [Google Scholar]
- Tian GF, Baker AJ. Glycolysis prevents anoxia-induced synaptic transmission damage in rat hippocampal slices. J Neurophysiol. 2000;83:1830–1839. doi: 10.1152/jn.2000.83.4.1830. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death & Differentiation. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Xiao AY, Sheline C, Hyrc K, Yang A, Goldberg MP, Choi DW, Yu SP. Apoptotic insults impair Na+, K+-ATPase activity as a mechanism of neuronal death mediated by concurrent ATP deficiency and oxidant stress. Journal of Cell Science. 2003;116:2099–2110. doi: 10.1242/jcs.00420. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, Newcomb EW. Cleavage of Bax enhances its cell death function. Experimental Cell Research. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]
- Wunder UR, Colombini M. Patch clamping VDAC in liposomes containing whole mitochondrial membranes. J Membr Biol. 1991;123:83–91. doi: 10.1007/BF01993966. [DOI] [PubMed] [Google Scholar]
- Yano S, Morioka M, Fukunaga K, Kawano T, Hara T, Kai Y, Hamada J, Miyamoto E, Ushio Y. Activation of Akt/protein kinase B contributes to induction of ischemic tolerance in the CA1 subfield of gerbil hippocampus. Journal of Cerebral Blood Flow & Metabolism. 2001;21:351–360. doi: 10.1097/00004647-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L)[see comment] Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]