SUMMARY
In the present study, we determined the role of hypertension, oxidative stress and inflammation on kidney damage in a rodent model of obesity and diabetes. Hypertension was induced in male obese (db/db) mice and lean (db/m) mice by implantation of deoxycorticosterone acetate (DOCA) pellets and mice were allowed to drink water containing 1% salt. Mice were divided into six groups as follows: obese and lean control, obese and lean 1% salt (salt) and obese and lean DOCA plus 1% salt (DOCA-salt).
Blood pressure was significantly increased in lean and obese DOCA-salt groups relative to their respective control; however, there was no difference in blood pressure between the lean and obese control and salt groups. Urinary 8-isoprostane was increased in obese control compared with lean control mice (1464 ± 267 vs 493 ± 53 pg/μmol creatinine, respectively) and this elevation was further increased in the obese DOCA-salt mice (2430 ± 312 pg/μmol creatinine). Urinary monocyte chemoattractant protein-1 excretion and CD68-positive cells were also increased in both obese and lean DOCA-salt groups compared with their respective controls. Furthermore, DOCA-salt administration increased collagen IV excretion in both obese and lean mice compared with controls, but there was no difference between obese and lean DOCA-salt groups. Urinary albumin excretion was significantly increased in the obese compared with the lean DOCA-salt mice (507 ± 160 vs 202 ± 48 μg/day, respectively).
These data suggest that obese DOCA-salt hypertensive mice exhibit greater renal injury than lean DOCA-salt hypertensive mice in a manner independent of blood pressure and that this renal injury is associated with obesity related pre-existing renal oxidative stress.
Keywords: oxidative stress, obesity, hypertension, inflammation, db/db mice
INTRODUCTION
The incidence of obesity is reaching epidemic levels in developing nations.1–3 Obesity is a disease that commonly clusters with hypertension and Type II diabetes and, together, these are referred to as metabolic syndrome.4 Each of these components are independent risk factors for chronic kidney disease and progression to end-stage renal disease (ESRD).4,5 Prevention and medical treatment of hypertension as a component of metabolic syndrome will reduce mortality and health care costs.6 Renal inflammation and oxidative stress are known contributors to renal injury in hypertension and thus represent potential therapeutic targets in addition to traditional antihypertensive treatment in patients who have metabolic syndrome.7–9 However, the development and use of such treatments will require a better understanding of metabolic syndrome-induced renal disease.
The pathophysiology of renal injury has been studied extensively in hypertension, but further research is needed to discern how the components of metabolic syndrome interact with each other and damage the kidney.4 Thus, the aim of the present study was to examine the role of hypertension, oxidative stress and inflammation in a rodent model of obesity and diabetes. The leptin receptor-deficient mouse has been a commonly studied model; these mice become insulin resistant and develop diabetic nephropathy that is characterized by glomerular hypertrophy and albuminuria.10 In contrast with obesity related hypertension in humans, obese mice are not hypertensive on their own.11,12 We hypothesized that obese mice would be more susceptible to experimentally induced hypertensive renal injury than lean mice. To test this hypothesis, we used an established model of hypertension, the deoxycorticosterone acetate (DOCA)-salt model, in obese and lean mice.13
METHODS
Animals
The Institutional Animal Care and Use Committee at the Medical College of Georgia approved all animal protocols. Male obese (db/db) mice and lean (db/m) littermates, 19 weeks of age on a C57BL6 background, were obtained from an in-house breeding colony and randomly assigned to the control, salt or DOCA-salt group. Mice were fed normal rodent diet ad libitum (Harlan Teklad, Madison, USA) and control mice had ad libitum access to tap water. Salt and DOCA-salt animals had ad libitum access to 1.0% NaCl/0.2% KCl-supplemented water for the 21 days of the experiment. The DOCA-salt mice were anaesthetized with isoflurane gas and were implanted subcutaneously with a DOCA, 21 day release, 50 mg pellet (Innovative Research of America, Sarasota, FL, USA). Body weight and tail-cuff systolic blood pressure (SBP) were determined on Days 1 and 21 of the experiment. Tail-cuff blood pressure (IITC Life Science, Woodland Hills, CA, USA) was monitored in conscious, restrained mice after acclimation to a warming chamber temperature of 32–34°C prior to data collection, as recommended by the manufacturer. After 21 days of DOCA-salt administration, mice were anaesthetized with pentobarbital sodium (50 mg/kg, i.p.) and plasma and tissues were collected, frozen on liquid nitrogen and then stored at −80°C for later use.
Enzyme-linked immunosorbent assay
On Day 20, mice were placed in metabolic cages for 24 h urine collection. Urine was then centrifuged at 4°C for 20 minutes at 2800g and stored at −80°C until later use. Enzyme-linked immunosorbent assays were used to measure urinary microalbumin and collagen type IV (Exocell, Philadelphia, PA, USA), 8-epi PGF2α (8-isoprostane; Cayman Chemical, Ann Arbor, MI, USA) and monocyte chemoattractant protein (MCP)-1 (BD Biosciences, San Jose, CA, USA) according to the manufacturers’ instructions. For the measurement of nuclear factor (NF)-κB p65 activation, 100 mg frozen kidney was homogenized with the Nuclear Extract Kit (Active Motif, Carlsbad, CA, USA) using the whole cell extract protocol. Protein concentrations were determined by BCA Protein Assay (Pierce, Rockford, IL, USA). Protein (20 μg) was added to each well of the TransAM NF-κB p65 Kit (Active Motif), which was developed according to the manufacturer’s instructions. Relative NF-κB p65 activation was determined using optical density at 450 nm.
CD68 immunohistochemical staining (ED-1 stain)
Half a kidney from each mouse was frozen in OCT compound (Sakura Finetek, Torrance, CA, USA) and stored at −80°C until use. Frozen kidneys were sectioned at 5 μm and incubated with rat anti-mouse CD68 primary antibody (1 : 100; Serotec, Raleigh, NC, USA) for 16 h at room temperature. A secondary antibody, namely goat anti-rat IgG horseradish peroxidase (HRP; 1 : 50; Serotec), was then added for 1 h at room temperature. Slides were then rinsed with water, incubated with AEC substrate chromogen (DAKO, Carpinteria, CA, USA) for 20 min, rinsed with water again and counterstained with haematoxylin for 30 s. Stained sections were visualized by light microscopy at ×400 magnification and digital images of the stained kidney cortex were taken for analysis. Macrophage/monocyte infiltration was determined by point counting of CD68-positive cells by an experienced reviewer blinded to the sample group. The number of positive cells per picture was divided by the metric area of the image, established by micrometer slide image, to obtain the number of positive cells per mm2.
Masson trichrome staining
Frozen kidneys were sectioned at 5 μm and placed on slides. The sections were stained using a Masson Trichrome Kit (Richard-Allan Scientific, Kalamazoo, MI, USA) according to the manufacturer’s instructions. Stained sections were visualized by light microscopy at ×100 and representative digital images of renal vessels and glomeruli were taken.
Statistical analysis
Results are presented as the mean±SEM. Data were analysed using GraphPad Prism software version 4.03 (GraphPad Software, San Diego, CA, USA). All statistical tests were performed using two-way ANOVA followed by Bonferroni’s post hoc test for between-group comparisons. Differences were considered statistically significant when P < 0.05.
RESULTS
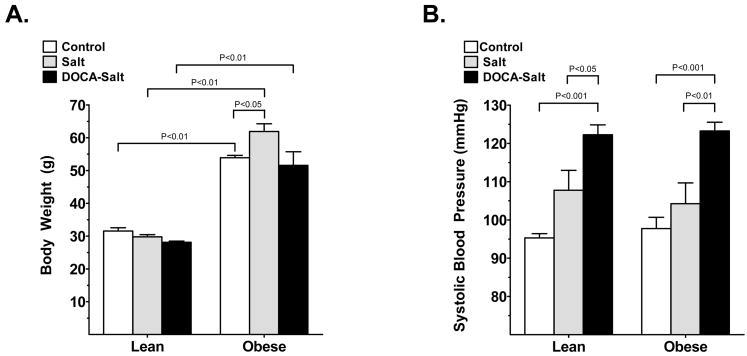
On Day 21, lean-control mice weighed 32 ± 1 g and bodyweight did not change significantly with salt (30 ± 1 g) or DOCA-salt (28 ± 0 g) administration (Fig. 1a). Within the same treatment group, obese mice weighed significantly more than their lean littermates: obese control mice weighed 54 ± 1 g (P < 0.05 vs lean control), obese mice in the salt group weighed 62 ± 3 g (P < 0.001 vs lean salt mice) and obese DOCA-salt mice weighed 52 ± 5 g (P < 0.001 vs lean DOCA-salt mice). Although there was no significant difference in SBP between obese and lean mice in any of the treatment groups, SBP was increased with DOCA-salt administration in both obese and lean mice (Fig. 1b) compared with control or salt administered obese and lean mice, respectively. Plasma glucose levels were significantly elevated in db/db obese compared with lean mice (189 ± 2 vs 118 ± 4 mg/dL, respectively) and plasma insulin levels were higher in obese compared with lean mice, although the difference was not significant (8.8 ± 0.4 vs 7.3 ± 2.2 μg/L, respectively). Salt and/or DOCA-salt administration did not have a significant effect on plasma glucose and insulin levels in lean and obese mice.
Fig. 1.
(a) Bodyweight and (b) systolic blood pressure (SBP) in control (□), salt-treated (&bsl00023;) and DOCA-salt administered (▪) lean and obese mice after 3 weeks of the experiment. Values are the mean±SEM (n = 5–6 per group).
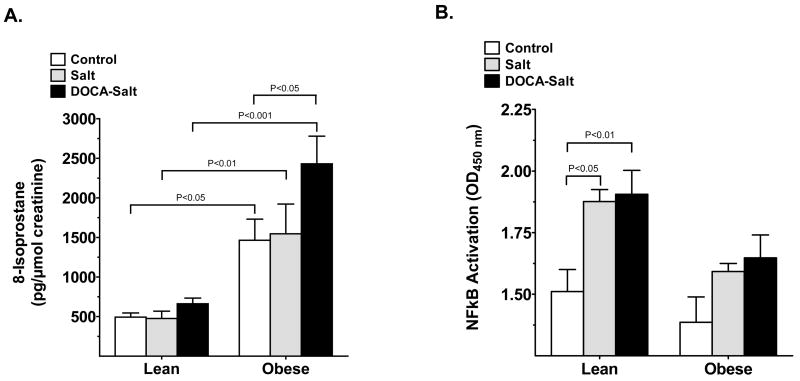
Renal oxidative stress, as determined by urinary 8-isoprostane, was increased in obese compared with lean mice in all experimental groups (Fig. 2a). 8-Isoprostane was also further increased in obese DOCA-salt mice compared with obese control (P < 0.05). Interestingly, DOCA-salt did not increase urinary 8-isoprostane in the lean DOCA-salt mice. Thus, renal oxidative stress thus appears to be closely related to obesity and is increased by obesity and obesity plus hypertension, but not by hypertension alone.
Fig. 2.
(a) Urinary 8-isoprostane, a measure of renal oxidative stress, and (b) renal nuclear factor (NF)- κB activity, a measure of renal inflammation, in lean and obese mice after either control (□), salt (&bsl00023;) or DOCA-salt (▪) administered for 3 weeks. Values are the mean±SEM (n = 5–6 per group).
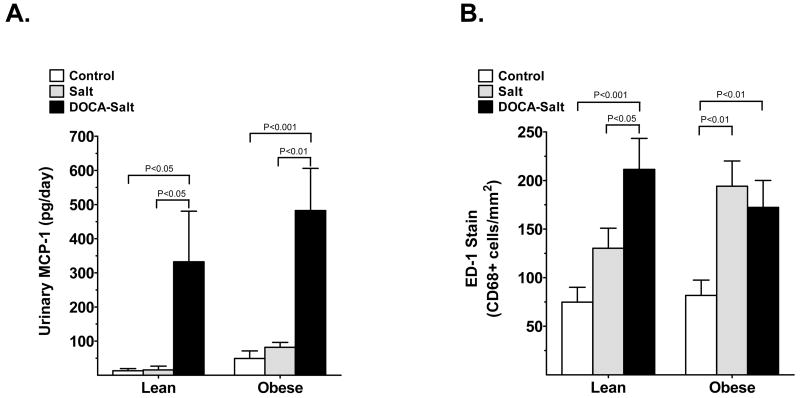
We next assessed renal inflammation in obese, lean and hypertensive groups. Detection of renal NF-κB activation was used as one indicator of renal inflammation. We found that NF-κB activation was significantly elevated following salt and DOCA-salt treatment of lean mice compared with control (Fig. 2b). Surprisingly, NF-κB activation showed some tendency to increase in salt-and DOCA-salt obese mice, although this increase was not significant and was much less pronounced compared with salt- and DOCA-salt lean mice. There were no differences in macrophage/monocyte infiltration between lean and obese control mice (74.8 ± 14.3 and 81.8 ± 15.7 cells/mm2, respectively), but was increased in the obese salt group and with DOCA-salt treatment in both lean and obese mice (Fig. 3b). With salt-treatment, the number of infiltrating cells in the lean and obese salt groups were 130.3 ± 20.6 and 194.1 ± 26.0 cells/mm2, respectively (P < 0.01 vs obese control). In the DOCA-salt hypertension group, further increases in macrophage infiltration were observed in the lean mice (to 211.4 ± 31.9 cells/mm2; P < 0.05 vs lean salt), but infiltration in the obese DOCA-salt mice was not changed any further (172.3 ± 27.7 cells/mm2). Urinary MCP-1 excretion was significantly increased by DOCA-salt in both lean (P < 0.05 vs lean control) and obese DOCA-salt (P < 0.001 vs obese control) mice (Fig. 3a). There was also a slight increase in MCP-1 excretion in the obese control mice compared with the lean control mice, but the difference did not reach statistical significance.
Fig. 3.
(a) Urinary monocyte chemoattractant protein (MCP)-1 excretion and (b) CD68 positive cells (ED-1 staining) in lean and obese mice after either control (□), salt (&bsl00023;) or DOCA-salt (▪) administration for 3 weeks. Values are the mean±SEM (n = 5–6 per group).
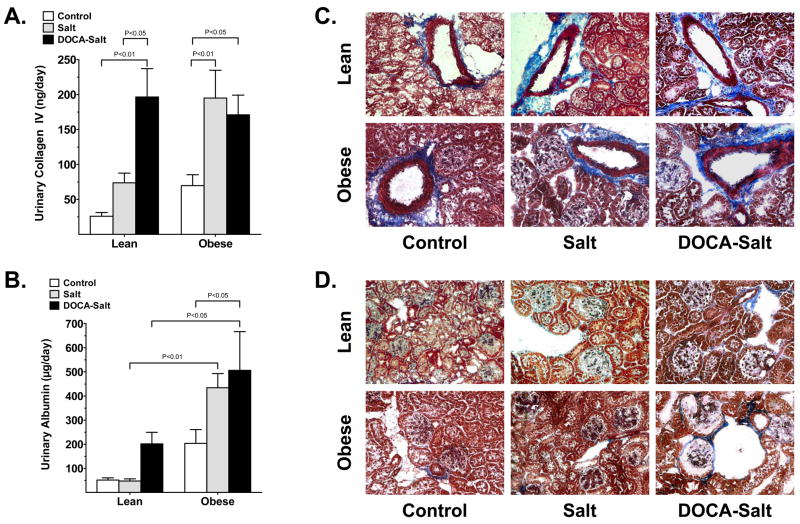
Renal fibrosis and albumin excretion were used as indicators of renal injury. Urinary collagen type IV excretion was increased significantly by both salt and DOCA-salt administration of obese mice and by DOCA-salt administration only of lean mice (Fig. 4a). The DOCA-salt obese mice did not exhibit further increases in collagen type IV excretion above those observed in obese salt mice. Representative images of Masson trichrome-stained kidney cortex (Fig. 4b, d) show increased collagen type IV staining (blue) of renal vessels of all groups compared with the lean control: staining was more prevalent in obese control, lean salt, lean DOCA-salt, obese salt and obese DOCA-salt mice. As shown in the representative images, collagen type IV deposition is increased in the glomerular basement membrane of lean and obese DOCA-salt mice compared with control lean and obese mice (Fig. 4d).
Fig. 4.
Urinary (a) collagen and (c) albumin excretion in lean and obese mice after either control (□), salt (&bsl00023;) or DOCA-salt (▪) administration for 3 weeks. Values are the mean±SEM (n = 5–6 per group). (b, d) Representative images (original magnification ×100) of Masson trichrome-stained kidney sections for collagen deposition in the b) tunica adventitia of arteries and d) glomerular capillaries and Bowman’s capsule in control, salt-administered and DOCA-salt administered lean and obese mice.
Urinary microalbumin excretion was significantly elevated by DOCA-salt administration in obese mice (Fig. 4c) and showed some tendency to increase in DOCA-salt lean mice. In lean and obese DOCA-salt mice, microalbumin excretion was 202 ± 48 and 507 ± 160 μg/day, respectively. Lean control mice excreted 51 ± 9 μg microalbumin/day and albuminuria was significantly higher in obese control mice (204 ± 57 μg/day). Salt treatment did not alter microalbumin excretion in the lean mice, but seemed to have a tendency to increase it in the obese salt group compared with the obese control, but this difference did not reach statistical significance.
DISCUSSION
The present study investigated renal injury in obese and lean mice that were subjected to DOCA-salt hypertension. Although obesity did not potentiate the elevation in blood pressure upon DOCA-salt administration, renal oxidative stress increased in obese, but not lean, mice and this increase in 8-isoprostane was further elevated in obese mice with DOCA-salt administration. Renal NF-κB activation and macrophage infiltration were increased but more pronounced in DOCA-salt lean mice compared with DOCA-salt obese mice. Intriguingly, albuminuria, a marker of renal injury, was significantly higher in obese compared with lean mice after DOCA-salt administration. These data suggest that increased oxidative stress could be the potential mechanisms for increased renal damage in obese compared with lean hypertensive mice.
While the mechanisms of obesity related kidney damage are not fully understood, but increases in blood pressure, sympathetic nervous activity and sodium reabsorption have been suggested to contribute to the higher prevalence of renal injury in obese populations.14 Recent animal studies indicate that leptin is involved in the regulation of blood pressure through leptin receptor activation of the central sympathetic nervous system.15,16 For example, infusion of leptin significantly increases blood pressure in rats.17 Furthermore, overexpression of leptin in mice results in an elevation in blood pressure and this effect is reduced by α-adrenoceptor blockade.18 However, obese Zucker rats, possessing mutant leptin receptors, do not demonstrate increased sympathetic nervous system activity, despite their elevated leptin and insulin levels,19 suggesting that the increase in blood pressure is absent with a defective leptin receptor. Rosmond and Chagnon found that polymorphisms of the human leptin receptor gene (LEPR) are associated with lower blood pressure and decreased leptin signalling in obese men,20 previous reports have noted normal mean blood pressure in 16-week-old db/db mice and similar observations in obese Zucker rats,11 where both db/db mice and Zucker rats are deficient in leptin signalling.21 In the present study, obese mice at baseline were not hypertensive compared with their lean littermates, despite weighing almost twice as much. Thus, the present study suggests that leptin receptor deficiency causes obesity witout significantly elevating blood pressure in 19-week-old db/db mice, but does result in obesity.
Studies have shown that oxidative stress plays an important role in the pathogenesis of renal damage associated with cardiovascular diseases via increased vascular inflammation.22 In the present study, renal oxidative stress increased in obese compared with lean mice and DOCA-salt administration further exacerbated the increase in renal oxidative stress in obese mice. Oxidative stress has been shown to activate the inflammatory cytokine NF-κB, which then translocates from the cytoplasm to nucleus to activate transcription of downstream inflammatory cytokines and chemokines, such as MCP-1.22,23 Through chemokine signaling, monocytes infiltrate tissue, differentiate into macrophages and release additional chemokines, perpetuating the inflammatory cycle. We found that renal NF-κB activation was increased in both lean and obese mice upon salt and/or DOCA-salt administration; the increase was more pronounced in lean compared with obese mice. Renal MCP-1 excretion also increased significantly in both DOCA-salt lean and obese mice compared with their corresponding control or the salt-treated groups. However, macrophage/monocyte infiltration was increased in salt-treated lean and obese mice and DOCA-salt administration exacerbated this increase in lean but not obese mice. While hypertension is probably the cause of vascular inflammation in both the lean and obese mice; however, in db/db mice obesity seems to be the central factor in increased oxidative stress, the presence of which is exaggerated by the addition of DOCA-salt hypertension. We found that DOCA-salt induced an increase in blood pressure and renal injury in both lean and obese mice. Urinary collagen IV excretion and Masson trichrome stain were also used as indicators of renal damage and fibrosis. In the obese mice, collagen IV excretion was increased significantly by both salt and DOCA-salt administration compared with obese control mice. Conversely, salt alone did not have a significant effect on urinary collagen excretion in lean mice, but DOCA-salt hypertension did increase collagen excretion. These data suggest that fibrosis in obese mice may be due primarily to increased salt insult. Albuminuria is a marker of glomerular injury and is used clinically to diagnose chronic kidney disease; it also predicts progression to ESRD. Albuminuria has been noted previously in DOCA-salt hypertensive animals and obese mice are also known to have increased urinary albumin excretion.10,24 Interestingly, the coexistence of obesity and hypertension further exacerbated the increase in albuminuria. These data suggest that increased salt intake is sufficient to accelerate renal injury in obese mice and the presence of hypertension exacerbates this injury, whereas hypertension is required to cause renal injury in lean mice.
Taken together, the results of the present study suggest that DOCA-salt hypertension has variable consequences, because it is sufficient to further damage the kidneys of obese mice, but has a mild effect in lean mice. The increase in renal damage in obese DOCA-salt hypertensive mice is due mainly to the elevation in oxidative stress; the mild increase in renal injury in DOCA-salt mice is mainly due to increased inflammation. This conclusion does not rule out the possible role of other lean inflammatory cytokines in obese hypertensive mice. Although previous studies have shown that hypertension accelerates diabetic nephropathy, 3,14 the results of the present study reiterate that even a moderate increase in blood pressure is dangerous for people with metabolic syndrome. Inflammation and oxidative stress are clearly associated with renal injury in obesity, but cause of their presence remains somewhat unclear and will require further elucidation. The importance of blood pressure cannot be overstated, yet hypertension afflicts a large percentage of the world’s population and the impact of this will increase along with the obesity epidemic. Thus, anti-oxidant and anti-inflammatory strategies, whether achieved with lifestyle changes or supplement/drug therapy, could prove beneficial in addition to antihypertensive medications and weight loss.
References
- 1.Caballero BL. The global epidemic of obesity. An overview. Epidemiol Rev. 2007;29:1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Lastra G, Manrique C, McFarlane SI, Sowers JR. Cardiometabolic syndrome and chronic kidney disease. Curr Diab Rep. 2006;6:207–12. doi: 10.1007/s11892-006-0036-5. [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F, Pozzoni P, Del Vecchio L. Renal manifestations in the metabolic syndrome. J Am Soc Nephrol. 2006;17:S81–5. doi: 10.1681/ASN.2005121332. [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 6.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension. 2006;47:557–62. doi: 10.1161/01.HYP.0000198545.01860.90. [DOI] [PubMed] [Google Scholar]
- 8.Elmarakby AA, Williams JM, Imig JD, Pollock JS, Pollock DM. Synergistic actions of enalapril and tempol during chronic angiotensin II-induced hypertension. Vasc Pharmacol. 2007;46:44–51. doi: 10.1016/j.vph.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manhiani MM, Quigley JE, Socha MJ, Motamed K, Imig JD. IL6 suppression provides renal protection independent of blood pressure in a murine model of salt-sensitive hypertension. Kidney Blood Press Res. 2007;30:195–202. doi: 10.1159/000104094. [DOI] [PubMed] [Google Scholar]
- 10.Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J Anat. 2005;207:813–21. doi: 10.1111/j.1469-7580.2005.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koya D, Haneda M, Nakagawa H, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439–47. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 12.Chrostowska M, Szczech R, Narkiewicz K. Antihypertensive therapy in the obese hypertensive patient. Curr Opin Nephrol Hypertens. 2006;15:487–92. doi: 10.1097/01.mnh.0000242173.14082.dc. [DOI] [PubMed] [Google Scholar]
- 13.Elmarakby AA, Morsing P, Pollock JS, Pollock DM. Omapatrilat increases renal endothelin in deoxycorticosterone acetate-salt hypertensive rats. Vasc Pharmacol. 2003;40:253–9. doi: 10.1016/j.vph.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Davy KP, Hall JE. Obesity and hypertension: Two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–13. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 15.Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 16.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–8. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shek EW, Brands MW, Hall JE. Chronic leptin infusion increases arterial pressure. Hypertension. 1998;31:409–14. doi: 10.1161/01.hyp.31.1.409. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa Y, Masuzaki H, Aizawa M, et al. Blood pressure elevation in transgenic mice over-expressing leptin, the obese gene product. J Hypertens. 1998;16:S7. (Abstract) [Google Scholar]
- 19.Koletsky RJ, Ernsberger P. Phenotypic characterization of a genetically obese and hypertensive rat. Rat Genome. 1996;2:10–22. [Google Scholar]
- 20.Rosmond R, Chagnon YC. Hypertension in obesity and the leptin receptor gene locus. J Clin Endocrinol Metab. 2000;85:3126–31. doi: 10.1210/jcem.85.9.6781. [DOI] [PubMed] [Google Scholar]
- 21.Kumano S, Matsumoto H, Takatsu Y, Noguchi J, Kitada C, Ohtaki T. Changes in hypothalamic expression levels of galanin-like peptide in rat and mouse models support that it is a leptin-target peptide. Endocrinology. 2003;144:2634–43. doi: 10.1210/en.2002-221113. [DOI] [PubMed] [Google Scholar]
- 22.Tian N, Moore RS, Braddy S, et al. Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H3388–95. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 23.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008;28:158–67. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 24.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]