Abstract
Biological timekeeping in birds is a fundamental feature of avian physiology, behavior and ecology. The physiological basis for avian circadian rhythmicity has pointed to a multi-oscillator system of mutually coupled pacemakers in the pineal gland, eyes and hypothalamic suprachiasmatic nuclei (SCN). In passerines, the role of the pineal gland and its hormone melatonin is particularly important. More recent molecular biological studies have pointed to a highly conserved mechanism involving rhythmic transcription and translation of “clock genes”. However, studies attempting to reconcile the physiological role of pineal melatonin with molecular studies have largely failed. Recent work in our laboratory has suggested that melatonin-sensitive physiological processes are only loosely coupled to transcriptional oscillations. Similarly, although the pineal gland has been shown to be critical for overt circadian behaviors, its role in annual cycles of reproductive function appears to be minimal. Recent work on the seasonal control of birdsong, however, suggests that, although the pineal gland does not directly affect gonadal cycles, it is important for seasonal changes in song. Experimental analyses that address these paradoxes will shed light on the roles the biological clock play in birds and in vertebrates in general.
Introduction
The concept of time’s arrow derives at its earliest from one of the 40 paradoxes posited by the pre-Socratic philosopher Zeno of Elea set forth to support his claim that all change and time itself were illusions (Russell, 1996). In this particular paradox, “The Arrow”, the arrow is considered at rest, occupying space. However, when in flight the arrow must still be occupying the same amount of space at every moment during its flight. Thus, at every moment, the arrow is actually at rest within progressively different places, supporting the paradoxical view that change, motion and the time in which they are embedded are illusions. That is, “instantaneous velocity”, as we now conceive it, is not possible. Of course, instantaneous velocity is an important concept in mathematics, chemistry and physics, and everything in biology, it seems, is constantly in motion and embedded in time (cf. Russell, 1996). Thus, the paradox itself is an illusion.
The concept was revived by British astrophysicist Arthur Eddington who coined the phrase to describe the direction of time on the four-dimensional relativistic map of the universe (Eddington, 1922). Beyond sub-atomic dimensions, where time is believed to be “time-symmetric”, time’s arrow at macroscopic scales has an asymmetric, irreversible flow that is reflected at all levels ranging from thermodynamics to cosmology to logic and perception. Biologically, time’s arrow is reflected at many levels of organization as well. Irreversible progression of geologic time sees the appearance and extinction of countless species, and the dynamics of populations changes as climate and other environmental features are in flux (Darwin, 1859). At the level of individuals, organisms develop, mature, reproduce and ultimately die on species-specific timetables that are genetically and physiologically pre-programmed, such that there is little doubt the flow of time washes over all of us like an irreversible ebbing tide.
Still, there is another aspect of time that inundates all of life at every organizational level. This is the cyclical nature of time on Earth. The annual cycle exposes high latitude and temperate zone organisms to cold winters and warm summers elicited by annual changes in photoperiod (Pianka, 1973; Pittendrigh, 1993). Even in subtropical and tropical zones, the annual cycle impinges predictable changes in rainfall, barometric pressures and wind (Pianka, 1973). Further, the daily cycle imposes rhythmic changes in abiotic aspects of the environment as well; daily changes in visible light carry with them changes in photosynthetic activity as well as deleterious aspects of the electromagnetic spectrum, including teratogenic and carcinogenic ultraviolet and gamma radiation as well as the desiccating effects of infrared radiation. Compounding these rhythmic selective pressures, biotic aspects of each species’ niche vary cyclically as well; the presence of predators, prey, competitors and mates all change depending upon the time of day and the time of year.
Circadian Timing Systems
It should therefore come as no real surprise that organisms in every Domain and nearly every Phylum, ranging from eubacteria to humans, have evolved endogenous timing systems that enable them to predict temporal changes and to coordinate complex internal processes (Pittendrigh, 1993; Bell-Pedersen et al. 2005). The cellular and molecular bases of these systems are remarkably conserved, especially among animals (Panda et al. 2002). In vertebrates (Figure 1), the central clock mechanism entails the interaction of “positive elements” CLOCK and BMAL1, which heterodimerize and enter the nucleus, where they stimulate transcription of genes whose promoter regions contain “E-boxes” during the night. Among the genes activated by CLOCK: BMAL1 are the “negative elements” period 1,2 and 3 (per1, per2 and per3) and the two cryptochromes, cryptochrome 1 and cryptochrome 2 (cry1 and cry2). The transcripts of these genes are translated in the cytoplasm and form protein oligomers that in turn re-enter the nucleus during the day, where they inhibit CLOCK: BMAL1 activity, repressing their own transcription (Bell-Pedersen et al. 2005). An interlocking feedback loop regulating Bmal1 transcription also contributes to rhythmic transcription and translation of these clock genes and proteins involving RevErb α and retinoic acid orphan-like receptor A (Rora). These loops have been reviewed in detail elsewhere (eg. Panda et al. 2002; Bell-Pedersen et al. 2005) and have been well-documented in birds (Yoshimura et al. 2000; Bailey et al. 2003; 2004; Karaganis et al. 2008), although some details appear to be distinct in birds and other non-mammalian vertebrates.
Figure 1.
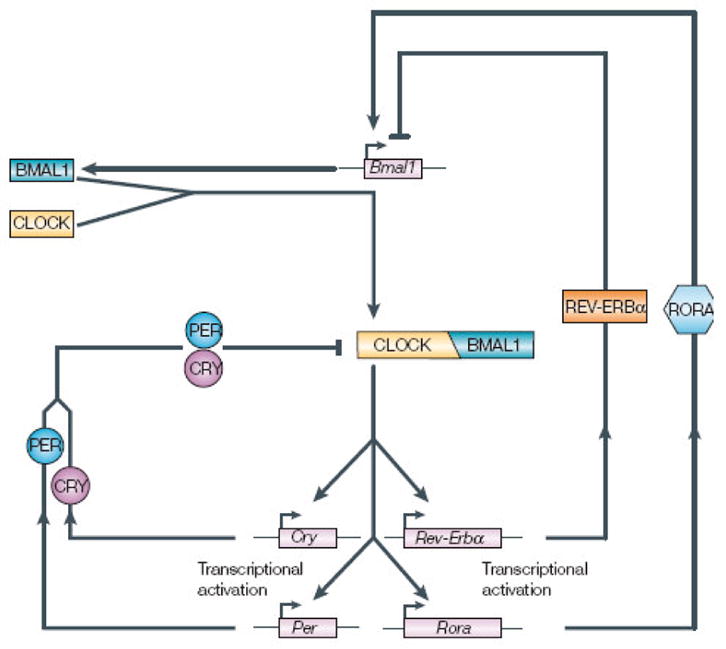
Schematic of the regulatory loops of clock genes expression in vertebrates (with permission from Bell-Pedersen et al. 2005). Positive elements CLOCK and BMAL1 dimerize and bind to E-boxes on promoter regions of clock-controlled genes and on negative element genes, period (Per) and cryptochrome (Cry), stimulating their transcription. These, in turn are translated, oligomerize and reenter the nucleus, where they interfere with CLOCK/BMAL1 activity. Similarly, the positive elements also stimulate Rev-Erb α and Rora (retinoic acid-like orphan receptor a), which feed cak to regulate rhythmic Bmal1 transcription.
First and foremost of these is the fact that, unlike mammals, non-mammalian vertebrates possess extraocular photoreceptors in the pineal gland and brain, primarily within circumventricular structures (Bellingham and Foster, 2002; Okano and Fukada, 2003). In birds, several specialized photopigments have been associated with clock function, including pinopsin (Okano et al. 1994; Max et al. 1995) and melanopsin (Bailey and Cassone, 2005; Chaurasia et al. 2005), among several others (Bellingham and Foster, 2002). Thus, unlike mammals, where retinal photoreceptors are required for entrainment, birds’ circadian clocks may be synchronized to the LD cycle directly within the same cells, at least within the pineal gland (Okano and Fukada, 2003). At this stage, it is not clear how phototransduction pathways of these invertebrate-like photopigments interact with the molecular clockworks within pineal or retinal cells. Secondly, while there is no evidence that cryptochromes are directly photoreceptive in mammals, structural analyses of chick CRY2 predicts photoreceptive capabilities (Bailey et al. 2002), and levels of both cry1 and cry2 respond to lighting conditions (Bailey et al. 2002; Haque et al. 2002; Nagy and Csernus, 2007; Karaganis et al. 2008). Intriguingly, there is some evidence that retinal cryptochromes may be involved in magnetoreception (Moller et al. 2004), critical for navigation, and that magnetic sensitivity of one of the cryptochromes depends upon blue light (Liedvogel et al. 2007). Thus, it is an open question whether cryptochromes play a photoreceptive role in avian circadian clocks. Thirdly, avian molecular clockworks differ from mammalian clocks in that there is no period 1 in the chick genome (Bailey et al. 2003; Okano and Fukada, 2003). Thus, avian clocks appear to bear several important distinctions from those described in mammals; in rodents at least (Bell-Pedersen et al. 2005).
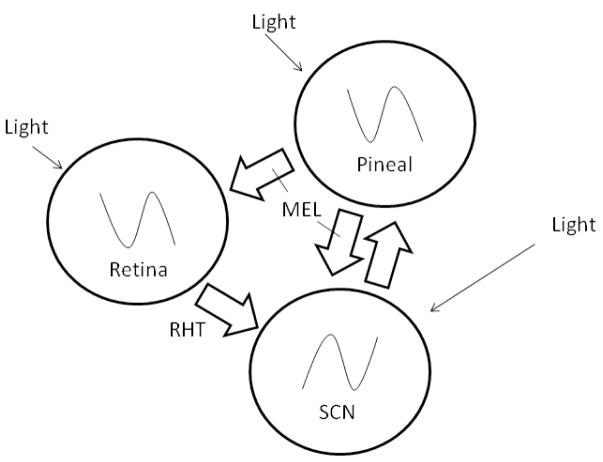
One of the remarkable features of these molecular clocks is the observation that rhythmic expression of these “clock genes” is observed in many tissues and in cultured cells (cf. Cuninkova and Brown, 2008), even in birds (Karaganis et al. in press). Yet, in higher vertebrates, at least, another layer of circadian organization coordinates the rhythmic expression of these genes and overt circadian rhythmicity. In birds, this “circadian system” comprises at least three sets of circadian pacemakers and specialized photoreceptors whose mutual interactions are critical for precision and rhythmic amplitude (Cassone and Menaker, 1984; Gwinner and Brandstatter, 2001). The pacemakers include cells in the pineal gland, retina and hypothalamic suprachiasmatic nuclei (SCN), and there are photoreceptors associated with biological timing in the retina, pineal gland, lateral septum and tuberal hypothalamus (Figure 2).
Figure 2.
Schematic of the interactions of circadian pacemakers within the avian circadian system. The pineal gland and retina, each directly photosensitive, release melatonin during the night, which inhibits SCN activity. Conversely, as day approaches, oscillators in the pineal gland and retina wane in their outputs, disinhibiting the SCN, which become active during subjective day, inhibiting pineal output via the sympathetic autonomic nervous system. It is not known whether the SCN directly affect retinal function.
In mammals, the SCN is the “master pacemaker” that coordinates all downstream rhythmicity (Moore 2007). The SCN receive direct retinohypothalamic input (RHT) that is responsible for entraining endogenous oscillations to external time. Destruction of the SCN abolishes the expression of overt circadian rhythms in many physiological and behavioral processes, and transplantation of SCN tissue or cells into arrhythmic SCN-lesioned rodents restores rhythmicity. Further, the SCN themselves are circadian oscillators in vitro. While it is true that rhythms of clock gene expression may persist in peripheral tissues in vitro, the SCN is responsible for synchronizing those rhythms (Stratman and Schibler, 2006).
In birds, two sets of structures have been associated with SCN function: the medial suprachiasmatic nuclei (mSCN) and the visual suprachiasmatic nuclei (vSCN) (Cantwell and Cassone, 2006a, b). These structures are connected via neuronal projections and are contiguous in terms of their cellular populations, especially in the distribution of astrocytes (Figure 3). The vSCN, but not the mSCN, expresses metabolic and electrical rhythmicity (Lu and Cassone, 1993a, b; Cantwell and Cassone, 2002) and receives RHT input (Cassone and Moore, 1987; Cantwell and Cassone, 2006a, b). Further, the vSCN, but not the mSCN, contains melatonin receptor binding (Cassone et al. 1995), and exogenous melatonin inhibits metabolic activity in the vSCN, but not the mSCN (Cassone and Brooks, 1991; Lu and Cassone, 1993b; Canwell and Cassone, 2002). In quail, only the mSCN expresses clock gene rhythmicity (Yoshimura et al. 2000; Yasuo et al. 2002), but in the house sparrow, both structures rhythmically express per2 rhythms (Abraham et al. 2002; 2003).
Figure 3.
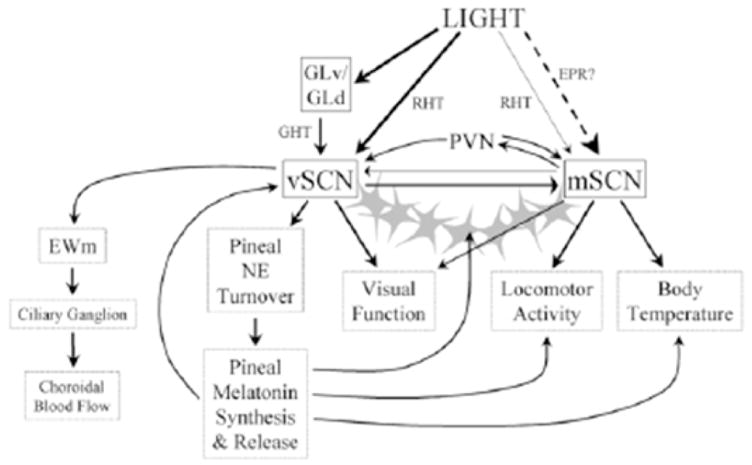
Schematic of the interactions of the medial suprachiasmatic nucleus (mSCN), visual suprachiasmatic nucleus (vSCN) and other components of the circadian system. The mSCN and vSCN are connected via neuronal as well as astrocytic process (stars), These structures are regulated by different processes. The mSCN receives extraocular photoreceptive input (EPR), and the vSCN receives retinal input via the retinohypothalamic tract (RHT). In turn, these structures may regulate different processes downstream (from Cantwell and Cassone, 2006b with permission).
Role of the Pineal Gland in Avian Circadian Organization
The evidence for the pineal gland’s involvement in avian circadian organization is abundant. First, the pineal gland contains circadian oscillators capable of generating rhythms of biosynthesis and release of the indoleamine hormone melatonin in vivo and in vitro in both light: dark (LD) and constant darkness (DD) in many species of birds, such that melatonin is released during the subjective night (Binkley et al. 1978; Takahashi et al. 1980; Csernus et al. 1998; Brandstatter et al. 2000). Secondly, surgical removal of the pineal gland from oscine passerine birds, such as the house sparrow, Passer domesticus, abolishes the expression of circadian locomotor rhythms when birds are placed in DD (Gaston and Menaker, 1968). The effects of pinealectomy (PINX) are variable in other avian taxa, however. For example, in Japanese quail, Coturnix coturnix japonica, PINX has little effect on locomotor rhythms, but removal of the eyes (EX), which also secrete melatonin rhythmically, abolishes rhythmicity (Underwood and Siopes, 1984). In pigeons, Columba livia, neither removal of the pineal (PINX) nor the eyes (EX), both of which secrete melatonin in this species, abolishes locomotor and body temperature rhythms, but combination of PINX and EX does (Oshima et al. 1989). The picture that emerges is that rhythmic melatonin is critical for overt circadian rhythms in birds, irrespective of its source. Thirdly, transplantation of a pineal gland into the anterior chamber of the eye of an arrhythmic, PINX house sparrow restores rhythmicity and confers the circadian phase of the donor to the recipient (Zimmerman and Menaker, 1979). Restoration of rhythmicity occurs almost immediately, before re-innervation of the gland could occur, punctuating the role of a humoral substance, presumably melatonin, in the effect. Finally, rhythmic administration of melatonin to European starlings, house sparrows, domestic pigeons, and Japanese quail rendered arrhythmic by either constant light or removal of endogenous melatonin sources (pineal and/or eyes) synchronizes behavioral rhythms in locomotion and feeding behavior (Gwinner and Benzinger, 1978; Chabot and Menaker, 1994; Lu and Cassone, 1993b; Heigl and Gwinner, 1995; Lumineau et al. 2002).
Paradox 1: Does the Pineal Gland Regulate Clock Gene Expression?
With the discovery and characterization of clock genes in avian pacemaker and peripheral tissues (Yoshimura et al. 2000; Okano and Fukada, 2003; Bailey et al., 2003, 2004; Karaganis et al. 2008; in press), it seemed logical to postulate that pineal (and retinal) melatonin influenced the activity of other pacemakers within the SCN and within peripheral tissues via regulation of clock gene expression. However, in Japanese quail, where constant melatonin administration abolishes and/or otherwise disrupts the expression of thermoregulatory and behavioral rhythmicity (Underwood and Edmonds, 1995) and where rhythmic melatonin administration entrains feeding rhythms (Lumineau et al. 2002), no effect of the hormone can be found on the expression of clock genes in the mSCN of the quail (Yasuo et al. 2002). Rhythms of mRNA expression for per2, per3 and Clock were identified in the mSCN, but not the vSCN, in LD and DD but were abolished in constant light (LL). These rhythms were not affected in birds that had implants of crystalline melatonin that elevated blood melatonin levels to constantly high levels. Locomotor or other measures of behavioral rhythmicity were not measured in this study, so it is possible that behavioral rhythmicity was maintained, but the melatonin levels were consistent with previous studies (Underwood and Edmonds, 1995) that had abolished locomotor rhythms.
Similarly, Abraham et al. (2002) have observed per2 expression in both the mSCN and vSCN of the house sparrow. However, PINX of these birds, which abolishes overt rhythms (Gaston and Menaker, 1968; Lu and Cassone, 1993a), does not abolish rhythms in per2 expression. Instead, the surgery decreases the amplitude and causes small changes in phase within these structures. As with the study in quail, however, the locomotor activity patterns of these birds were not monitored. Further, birds were sacrificed within 3 days of being placed in DD, a time at which PINX sparrows retain lower amplitude but significant behavioral rhythmicity and rhythmic 2DG uptake within the vSCN (Gaston and Menaker, 1968; Lu and Cassone, 1993a, b).
So, in some sense, it remains an open question whether clock gene expression in other components of the clock or in the periphery is dependent on and/or entrained by pineal melatonin in birds. Perhaps, as with Zeno’s paradox, the question may be resolved with more data in which behavioral and/or physiological rhythmicity is linked to rhythms of clock gene expression. Clearly, more experiments are in order.
Even so, there is the following quandary: In vivo, administration of melatonin decreases 2DG uptake in the vSCN of 2 species of bird (Cassone and Brooks, 1991; Lu and Cassone, 1993b; Cantwell and Cassone, 2002) within 1 hr of administration. Yet, as stated above, there is little effect of the hormone or its apparent absence in SCN clock gene expression (Yasuo et al. 2002; Abraham et al. 2003). Interestingly, this appears also to be the case in mammals, where injections of physiological concentrations of melatonin in rats inhibit SCN 2DG uptake immediately (Cassone et al. 1988), coincidentally with a phase-shift in locomotor activity (Cassone et al. 1986) and SCN electrical activity (McArthur et al. 1991). However, injection of melatonin has no effect on SCN clock gene expression (Poirel et al. 2003).
How can this be, that the outward expressions of circadian clock function are affected immediately by melatonin but that the presumed molecular bases for these rhythms are not? One clue to this paradox is our observation that diencephalic astrocytes from chick express two of the three melatonin receptors (Mel1A or MT1, and Mel1C) and that rhythmic application of melatonin entrains rhythms of release of two glycolytic products, lactate and pyruvate, such that uptake and release of these substances is inhibited by melatonin (Adachi et al. 2002). The interesting thing about these data is that the effect of melatonin did not occur until 4 full days of rhythmic administration and increased in its effect for several days thereafter. Similarly, we have also observed that rhythmic melatonin administration indeed entrains rhythms of per2 and per3 expression in these cells (Paulose et al. in press; Figure 4). As with the rhythms of glycolytic metabolites, the effects of melatonin were not apparent until 6 cycles of administration.
Figure 4.
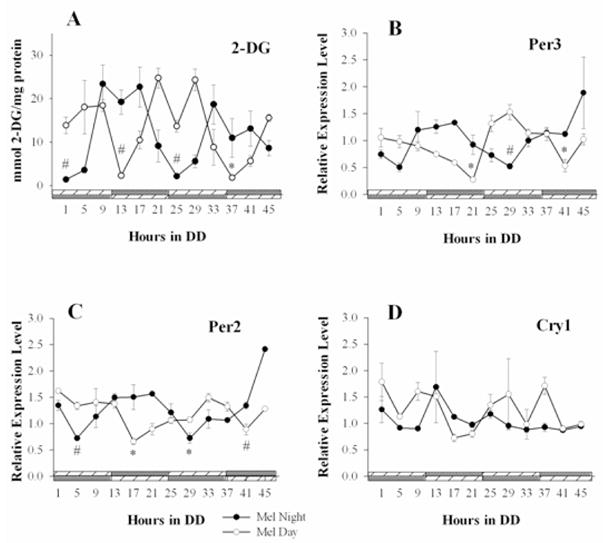
Exogenous melatonin imposes metabolic and clock gene rhythms in diencephalic astrocytes. A) Under constant conditions, opposing cycles of rhythmic melatonin (nM) administration, either during the night or during the day, elicited rhythmic uptake of glucose in the cultures, such that 2DG uptake was higher during the time which melatonin was not present (n=4). B) The gPer3 mRNA rhythm under the MN cycle (n=3 sample replicates) was 180° antiphase with respect to the rhythm generated under the MD cycle (n=3 sample replicates). C) The gPer2 mRNA rhythm under the MN cycle (n=3 sample replicates) was 180° antiphase with respect to the rhythm generated under the MD cycle (n=3 sample replicates). D) No clear pattern of gCry1 expression was evident under either condition (n=3 sample replicates each). For determined rhythmic cycles, significant differences between peak-to-trough values are indicated by # (p<0.05) or * (p<0.001). Comparisons were made between the first observed peak and trough for each day for each treatment (from Paulose et al. in press).
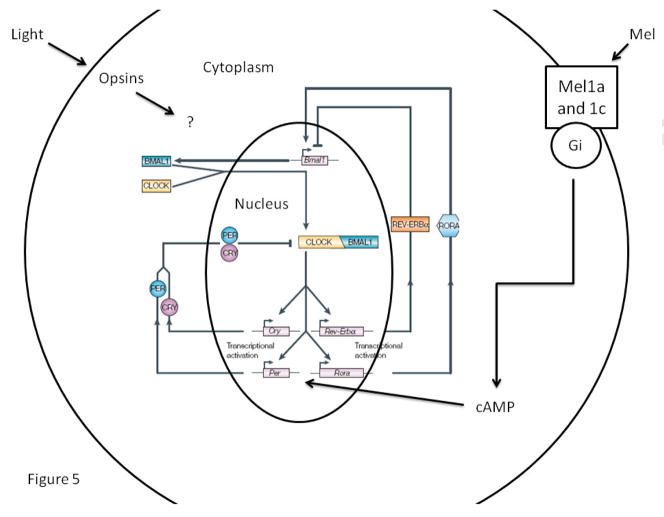
We hypothesize that melatonin does indeed affect the molecular clockworks within pacemaker and peripheral tissues (Figure 5). As stated above, the promoter regions of the period and cryptochrome genes contain multiple E-boxes, which serve as the principle locus of rhythmic regulation via the CLOCK/BMAL1 dimer’s activity (Bell-Pedersen et al. 2005). However, the promoter of per2 of mice contains a cyclic nucleotide response element (CRE), which responds to cAMP signaling (O’Neill et al. 2008) and cAMP activity does affect clock gene expression. It is possible (and purely speculative) that melatonin influences clock gene expression through the Gi GTP binding protein activity of its three receptors (Reppert, 1997). Perhaps, melatonin’s actions, which reduce cAMP levels, act indirectly on transcriptional activity by decreasing 1 but not all regulatory elements on the clock gene promoter. Since these effects may conflict with E-box activity, several cycles may be necessary before coupling of these two signals is accomplished. In this scenario, inputs such as melatonin may directly and immediately affect clock-controlled output but take several cycles to synchronize the transcriptional machinery underlying it. Alternatively, but not exclusively, the dissonance between transcriptional rhythms and metabolic, physiological and behavioral rhythms may be due to different cell groups controlling them. For example, melatonin directly affects glucose utilization and release of lactate and pyruvate in astrocytes (Adachi et al. 2002), which are known to perform more than 80% of the glycolysis in the brain and in turn release lactate and pyruvate to neurons in an activity-dependent fashion (Magistretti, 2006). Perhaps, melatonin influences neuronal rhythmicity through regulation of metabolic activity within neuronal oscillators. Clearly, these are testable hypotheses, which our lab is currently pursuing.
Figure 5.
Schematic of the regulatory loops of clock genes in the vertebrate cell. Perhaps, melatonin influences this process through its receptors that are linked to Gi GTP proteins. By decreasing cellular cAMP levels, melatonin may modestly alter period expression, whose promoter contains a CRE but several “E-boxes” (modified from Bell-Pedersen et al. 2005 with permission).
Paradox 2: Is the Avian Pineal Gland Involved in Annual Reproductive Cycles?
One of the earliest demonstrations that changes in photoperiod mediate seasonal changes in reproductive, metabolic and migratory physiology was the work of Rowan in the 1920s on the Oregon junco, Junco hyemalis (Rowan, 1925). Later work by many authors studying many species of temperate zone birds (cf. Murton and Westwood, 1977; Kumar, 1997; Gwinner and Brandstatter, 2001) clearly demonstrated that as photoperiod increases, reproductive function in long-day breeding birds is induced, that as long day continues, most bird species become insensitive or “photorefractory” to the stimulatory effects of long days (Dawson and Sharp, 2007), and that as photoperiod decreases as winter approaches, gonadal activity is inhibited and ultimately sensitized to the increasing photoperiods of the succeeding spring.
Further, there is abundant evidence that a circadian oscillator or group of oscillators is critical for the generation of annual cycles of reproductive function (cf. Kumar, 1997; Dawson et al. 2001; Gwinner and Brandstatter, 2001). Beginning with the work of Hamner (1963), many studies have indicated that photoperiodic responses in birds entail a circadian rhythm of “photoinducible phase”, in which, as photoperiod increases, light coincides with the internally derived phase, stimulating gonadal activity. This process has been termed an “external coincidence” model. Alternatively, some authors have suggested that instead there are multiple circadian oscillators that track dusk and dawn differentially, and that the relative phase relationship among them changes as photoperiod changes. This process has been termed an “internal coincidence” model (Dawson et al. 2001). In any case, there is a tremendous amount of evidence implicating circadian oscillators in annual cycles, no matter which model is employed.
It was therefore surprising that, even though circadian clocks are critical to photoperiodic time measurement, and even though pineal melatonin is critical for the expression of overt circadian rhythms in oscine passerine birds, there is little or no evidence for a role of the pineal gland or its hormone on photoperiodic regulation of reproduction and gonadal activity (Kumar, 1997; Bentley, 2001). This is especially enigmatic since both in vivo and in vitro, the pineal gland’s rhythmic output of melatonin tracks the length of the night; the duration is longer in short days of winter than in the long days of summer (Brandstatter et al. 2000), so that even though the avian pineal gland does not appear to regulate seasonal cycles of gonad function, it contains the information to do so.
One clue to this paradox arose in the description of melatonin binding sites in birds using the melatonin agonist 2[125I]-iodomelatonin (IMEL) (Reppert 1997). Using IMEL and autoradiography of 14 species of birds in 5 Orders, we found that IMEL binding predominates in retinorecipient and integrative structures involved in vision (Cassone et al. 1995). However, in male house sparrows but not females, significant high affinity IMEL binding was observed in brain structures associated with song control (Whitfield-Rucker and Cassone, 1996). This observation was corroborated the same year in zebra finch (Gahr and Kosar, 1996). In house sparrows, as with many temperate zone oscines (cf. Dawson et al. 2001), the size and complexity of the brain structures associated with song control, Area X, the high vocal center (HVC), and the robust nucleus of the archipallium (RA), as well as others, increase during the long days of spring and summer and decrease in the short days of winter (Whitfield-Rucker and Cassone, 2000). This process is partially dependent on gonadal steroids, since exogenous testosterone increases song behavior and song control structure size (Gahr, 2007). However, the seasonal changes in song control nuclei are only partially blocked by castration in house sparrows (Whitfield-Rucker and Cassone, 2000) and other species (cf. Dawson et al. 2001), suggesting that a gonad-independent process also regulates song control in oscine passerine birds.
One of these signals is likely the seasonal changes in melatonin secretion by the pineal gland. First, castration has little effect on IMEL binding in song control nuclei (Whitfield-Rucker and Cassone, 1996). Second, chronic melatonin administration of melatonin attenuates the seasonal changes in the sizes of song control nuclei in European starlings (Bentley et al. 1999), supporting the view that melatonin regulates song directly. However, since this treatment also abolishes and/or disrupts circadian rhythms in this species (Gwinner and Brandstatter, 2001), it isn’t clear whether these effects are specific to song control.
In seasonally breeding mammals, the duration of melatonin titers transduces seasonal information to the hypothalamo-hypophysial system controlling seasonal regression and recrudescence of the gonads and secondary sexual characteristics (Malpaux et al. 1999; Goldman, 2001). Simulation of long durations of melatonin, indicative of short days of winter, or short durations of the hormone, indicative of long days of summer, transduce seasonally relevant activity in PINX mammals. Thus, in long day breeding Djungarian hamsters, long duration melatonin cycles induces gonadal regression, while in short day breeding sheep, long duration melatonin cycles enable reproduction (Malpaux et al. 1999; Goldman, 2001). Conversely, short duration melatonin cycles induce summer-appropriate reproductive activity in each (regression in sheep; recrudescence in hamsters).
To address this question in birds, we recently asked whether imposition of long duration cycles of melatonin by periodically administering melatonin in drinking water, could 1) entrain circadian patterns of activity in PINX male house sparrows in DD or intact birds in LL, 2) affect gonad size and/or 3) affect song control nucleus size in male house sparrows (Cassone et al. 2008). Both PINX birds in DD and intact birds in LL entrained to the melatonin regime. However, the gonads and song control nuclei responded differentially (Figure 6). The testes of PINX sparrows in DD were small, whether they received the long duration, short duration or no melatonin at all, while the intact birds in LL had large testes irrespective of treatment. The song control nuclei of PINX birds in DD were also small irrespective of treatment. Interestingly, while these structures were large in intact birds in LL if they received a short duration of melatonin or no melatonin at all, the long duration of melatonin inhibited song control nucleus growth, even though the testes were large. This clearly shows that melatonin directly affects song control structures in a fashion that is independent of gonads and independent of their effect of circadian activity patterns.
Figure 6.
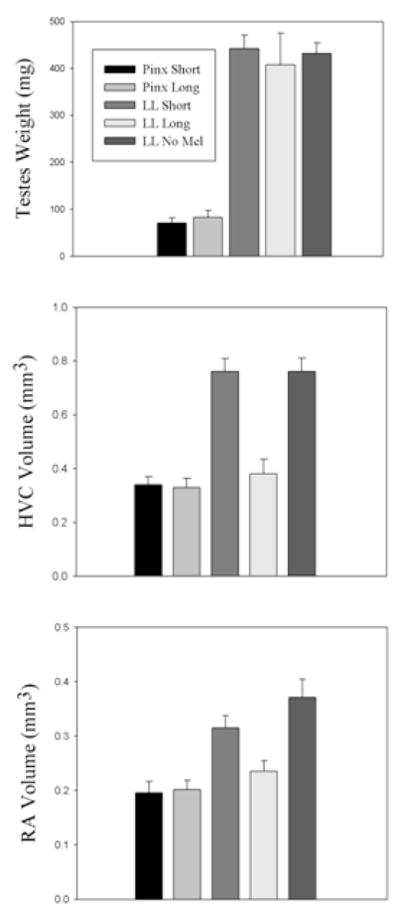
Effects of melatonin cycles on pinealectomized (PINX) house sparrows maintained in constant darkness (DD) and on intact sparrows in constant light (LL). PINX birds exhibited small testes (A), small high vocal center (HVC) (B), and small robust nuclei of the archipallium (RA) (C) irrespective of melatonin treatment. Intact sparrows in LL exhibited large testes (A). However, while birds receiving no melatonin or a short duration of melatonin exhibited large HVC and RA (B, C), birds receiving the long duration melatonin showed small HVC and RA even though their testes were large (from Cassone et al. 2008 with permission).
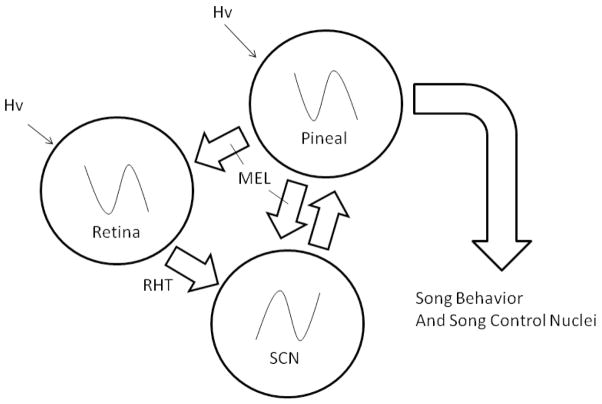
Thus, it appears that recrudescence and regression of primary sexual characteristics in male house sparrows have been functionally segregated from the ebbs and flows of male courtship behavior and the structures underlying them (Figure 7). What selective pressures might favor this separation? Unlike seasonally breeding rodents, from whom most research regarding a role of melatonin on seasonal cycles derives (Malpaux et al. 1999), birds exhibit a complex set of courtship and territorial behaviors before reproductive behavior can begin (Murton and Westwood, 1977). Moreover, the growth of large testes and ovarian follicles must present an aerodynamic challenge, especially in small passerines. Thus, it may be that parallel regulatory systems involved in courtship and primary reproductive function may have been functionally segregated. The presence of a separate circadian oscillator in the medial basal hypothalamus involved in gonadal cycles supports this contention (Yoshimura, 2006).
Figure 7.
Schematic of the avian circadian system showing that the output of melatonin may independently affect downstream processes, such as birdsong.
Conclusion and Perspective
The circadian system is a fundamental property of most living organisms on Earth, integrally linked with Time’s Arrow at every level of biological organization. Indeed, one could think of the biological Time’s Arrow as a spiral, progressively and rhythmically flying from birth to death. Underlying this system, highly conserved molecular processes have evolved that keep the time within the cells of many tissues and cell-types. However, selective pressures on organisms’ lifestyles reveal diverse ways by which these organisms employ their clock, and it is important for biologists to recognize that basic functions may be employed by organisms differentially. In birds, as with all organisms, it is the outward expression of these adaptations that determine their success or failure.
Acknowledgments
Research in the Cassone laboratory is supported by NIH P01 NS39546 and NSF UBM 0436308.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Abraham U, Albrecht U, Gwinner E, Brandstätter R. Spatial and temporal variation of passer Per2 gene expression in two distinct cell groups of the suprachiasmatic hypothalamus in the house sparrow (Passer domesticus) Eur J Neurosci. 2002;16(3):429–36. doi: 10.1046/j.1460-9568.2002.02102.x. [DOI] [PubMed] [Google Scholar]
- Abraham U, Albrecht U, Brandstätter R. Hypothalamic circadian organization in birds. II. Clock gene expression. Chronobiol Int. 2003;20(4):657–69. doi: 10.1081/cbi-120022414. [DOI] [PubMed] [Google Scholar]
- Adachi A, Natesan AK, Whitfield-Rucker MG, Weigum SE, Cassone VM. Functional melatonin receptors and metabolic coupling in cultured chick astrocytes. Glia. 2002;39(3):268–78. doi: 10.1002/glia.10109. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Cassone VM. Melanopsin expression in the chick retina and pineal gland. Brain Res Mol Brain Res. 2005;134(2):345–8. doi: 10.1016/j.molbrainres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Chong NW, Xiong J, Cassone VM. Chickens’ Cry2: molecular analysis of an avian cryptochrome in retinal and pineal photoreceptors. FEBS Lett. 2002;513(2–3):169–74. doi: 10.1016/s0014-5793(02)02276-7. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Beremand PD, Hammer R, Bell-Pedersen D, Thomas TL, Cassone VM. Transcriptional profiling of the chick pineal gland, a photoreceptive circadian oscillator and pacemaker. Mol Endocrinol. 2003;17(10):2084–95. doi: 10.1210/me.2003-0121. [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Beremand PD, Hammer R, Reidel E, Thomas TL, Cassone VM. Transcriptional profiling of circadian patterns of mRNA expression in the chick retina. J Biol Chem. 2004;279(50):52247–54. doi: 10.1074/jbc.M405679200. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6(7):544–566. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham J, Foster RG. Opsins and mammalian photoentrainment. Cell Tissue Res. 2002;309(1):57–71. doi: 10.1007/s00441-002-0573-4. [DOI] [PubMed] [Google Scholar]
- Bentley GE. Unraveling the enigma: the role of melatonin in seasonal processes in birds. Microsc Res Tech. 2001;53(1):63–71. doi: 10.1002/jemt.1069. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Van’t Hof TJ, Ball GF. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc Natl Acad Sci U S A. 1999;96(8):4674–9. doi: 10.1073/pnas.96.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolucci C, Foà A. Extraocular photoreception and circadian entrainment in nonmammalian vertebrates. Chronobiol Int. 2004;21(4–5):501–19. doi: 10.1081/cbi-120039813. [DOI] [PubMed] [Google Scholar]
- Binkley SA, Riebman JB, Reilly KB. The pineal gland: a biological clock in vitro. Science. 1978;202(4373):1198–20. doi: 10.1126/science.214852. [DOI] [PubMed] [Google Scholar]
- Brandstätter R, Kumar V, Abraham U, Gwinner E. Photoperiodic information acquired and stored in vivo is retained in vitro by a circadian oscillator, the avian pineal gland. Proc Natl Acad Sci U S A. 2000;97(22):12324–8. doi: 10.1073/pnas.200354997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell EL, Cassone VM. Daily and circadian fluctuation in 2-deoxy[(14)C] glucose uptake in circadian and visual system structures of the chick brain: effects of exogenous melatonin. Brain Res Bull. 2002;57(5):603–11. doi: 10.1016/s0361-9230(01)00753-5. [DOI] [PubMed] [Google Scholar]
- Cantwell EL, Cassone VM. Chicken suprachiasmatic nuclei: I. Efferent and afferent connections. J Comp Neurol. 2006a;496(1):97–120. doi: 10.1002/cne.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantwell EL, Cassone VM. Chicken suprachiasmatic nuclei: II. Autoradiographic and immunohistochemical analysis. J Comp Neurol. 2006b;499(3):442–57. doi: 10.1002/cne.21124. [DOI] [PubMed] [Google Scholar]
- Cassone VM. Melatonin: time in a bottle. Oxf Rev Reprod Biol. 1990;12:319–67. [PubMed] [Google Scholar]
- Cassone VM, Menaker M. Is the avian circadian system a neuroendocrine loop? J Exp Zool. 1984;232(3):539–49. doi: 10.1002/jez.1402320321. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Moore RY. Retinohypothalamic projection and suprachiasmatic nucleus of the house sparrow, Passer domesticus. J Comp Neurol. 1987;266(2):171–82. doi: 10.1002/cne.902660204. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Brooks DS. Sites of melatonin action in the brain of the house sparrow, Passer domesticus. J Exp Zool. 1991;260:302–309. [Google Scholar]
- Cassone VM, Chesworth MJ, Armstrong SM. Dose-dependent entrainment of rat circadian rhythms by daily injection of melatonin. J Biol Rhythms. 1986;1(3):219–29. doi: 10.1177/074873048600100304. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Roberts MH, Moore RY. Effects of melatonin on 2-deoxy-[1–14C]glucose uptake within rat suprachiasmatic nucleus. Am J Physiol. 1988;255(2 Pt 2):R332–7. doi: 10.1152/ajpregu.1988.255.2.R332. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Brooks DS, Kelm TA. Comparative distribution of 2[125I]iodomelatonin binding in the brains of diurnal birds: outgroup analysis with turtles. Brain Behav Evol. 1995;45(5):241–56. doi: 10.1159/000113553. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Bartell PA, Earnest BJ, Kumar V. Duration of melatonin regulates seasonal changes in song control nuclei of the house sparrow, Passer domesticus: independence from gonads and circadian entrainment. J Biol Rhythms. 2008;23(1):49–58. doi: 10.1177/0748730407311110. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Menaker M. Feeding rhythms in constant light and constant darkness: the role of the eyes and the effect of melatonin infusion. J Comp Physiol [A] 1994;175(1):75–82. doi: 10.1007/BF00217438. [DOI] [PubMed] [Google Scholar]
- Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, Iuvone PM, Provencio I. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem. 2005;92(1):158–70. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- Csernus V, Ghosh M, Mess B. Development and control of the circadian pacemaker for melatonin release in the chicken pineal gland. Gen Comp Endocrinol. 1998;110(1):19–28. doi: 10.1006/gcen.1997.7039. [DOI] [PubMed] [Google Scholar]
- Cuninkova L, Brown SA. Peripheral circadian oscillators: interesting mechanisms and powerful tools. Ann N Y Acad Sci. 2008;1129:358–70. doi: 10.1196/annals.1417.005. [DOI] [PubMed] [Google Scholar]
- Darwin, C (1859) Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life, John Murray, 1873
- Dawson A, Sharp PJ. Photorefractoriness in birds--photoperiodic and non-photoperiodic control. Gen Comp Endocrinol. 2007;153(1–3):378–84. doi: 10.1016/j.ygcen.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. J Biol Rhythms. Photoperiodic control of seasonality in birds. 2001;16(4):365–80. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Eddington AS. Theory of relativity and its Influence on Scientific Thought. Oxford University Press; 1922. [Google Scholar]
- Gahr M. Sexual differentiation of the vocal control system of birds. Adv Genet. 2007;59:67–105. doi: 10.1016/S0065-2660(07)59003-6. [DOI] [PubMed] [Google Scholar]
- Gahr M, Kosar E. Identification, distribution and developmental changes of a melatonin binding site in the song control system of the zebra finch. J Comp Neurol. 1996;367:308–318. doi: 10.1002/(SICI)1096-9861(19960401)367:2<308::AID-CNE11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gaston S, Menaker M. Pineal function: the biological clock in the sparrow? Science. 1968;160(832):1125–7. doi: 10.1126/science.160.3832.1125. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16(4):283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Gwinner E, Benzinger I. Synchronization of pinealectomized European starlings by daily injection of melatonin. J Comp Physiol. 1978;127:209–213. [Google Scholar]
- Gwinner E, Hau M, Heigl S. Melatonin: generation and modulation of avian circadian rhythms. Brain Res Bull. 1997;44(4):439–44. doi: 10.1016/s0361-9230(97)00224-4. [DOI] [PubMed] [Google Scholar]
- Gwinner E, Brandstätter R. Complex bird clocks. Philos Trans R Soc Lond B Biol Sci. 2001;356(1415):1801–10. doi: 10.1098/rstb.2001.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner WM. Diurnal rhythm and photoperiodism in testicular recrudescence of the house finch. Science. 1963;142(3597):1294–1295. doi: 10.1126/science.142.3597.1294. [DOI] [PubMed] [Google Scholar]
- Haque R, Chaurasia SS, Wessel JH, 3rd, Iuvone PM. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. Neuroreport. 2002;13(17):2247–51. doi: 10.1097/00001756-200212030-00016. [DOI] [PubMed] [Google Scholar]
- Heigl S, Gwinner E. Synchronization of circadian rhythms of house sparrows by oral melatonin: effectsof changing period. J Biol Rhythms. 1995;10(3):225–33. doi: 10.1177/074873049501000305. [DOI] [PubMed] [Google Scholar]
- Karaganis SP, Kumar V, Beremand PD, Bailey MJ, Thomas TL, Cassone VM. Circadian genomics of the chick pineal gland in vitro. BMC Genomics. 2008 May 3;9:206. doi: 10.1186/1471-2164-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaganis SP, Bartell PA, Shende V, Cassone VM. Modulation of metabolic and clock gene mRNA rhythms by retinal and pineal circadian oscillators. Gen Comp Endo. doi: 10.1016/j.ygcen.2008.12.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. Photoperiodism in higher vertebrates: an adaptive strategy in temporal environment. Indian J Exp Biol. 1997;35(5):427–37. [PubMed] [Google Scholar]
- Liedvogel M, Maeda K, Henbest K, Schleicher E, Simon T, Timmel CR, Hore PJ, Mouritsen H. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE. 2007;2(10):e1106. doi: 10.1371/journal.pone.0001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Cassone VM. Pineal regulation of circadian rhythms of 2-deoxy[C14]glucose uptake and 2[125I]iodomelatonin binding in the visual system of the house sparrow, Passer domesticus. J Comp Physiol A sens Neur Behav Physiol. 1993a;173:7765–774. [Google Scholar]
- Lu J, Cassone VM. Daily melatonin administration synchronizes circadian patterns of brain metabolism and behavior in pinealectomized house sparrows, Passer domesticus. J Comp Physiol A sens Neur Behav Physiol. 1993b;173:775–782. [Google Scholar]
- Lumineau S, Guyomarc’h C, Vivien-Roels B, Houdelier C. Cyclic melatonin synchronizes the circadian rhythm of feeding activity in Japanese quail, Coturnix c. japonica. C R Biol. 2002;325(3):205–12. doi: 10.1016/s1631-0691(02)01431-2. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209(Pt 12):2304–11. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Thiéry JC, Chemineau P. Melatonin and the seasonal control of reproduction. Reprod Nutr Dev. 1999;39(3):355–66. doi: 10.1051/rnd:19990308. [DOI] [PubMed] [Google Scholar]
- Max M, McKinnon PJ, Seidenman KJ, Barrett RK, Applebury ML, Takahashi JS, Margolskee RF. Pineal opsin: a nonvisual opsin expressed in chick pineal. Science. 1995;267(5203):1502–6. doi: 10.1126/science.7878470. [DOI] [PubMed] [Google Scholar]
- McArthur AJ, Gillette MU, Prosser RA. Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Res. 1991;565(1):158–61. doi: 10.1016/0006-8993(91)91748-p. [DOI] [PubMed] [Google Scholar]
- Möller A, Sagasser S, Wiltschko W, Schierwater B. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften. 2004;91(12):585–8. doi: 10.1007/s00114-004-0578-9. [DOI] [PubMed] [Google Scholar]
- Moore RY. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Med. 2007;8(Suppl 3):27–33. doi: 10.1016/j.sleep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Murton RK, Westwood NJ. Avian Breeding Cycles. Clarendon; Oxford: 1977. [Google Scholar]
- Nagy AD, Csernus VJ. Cry1 expression in the chicken pineal gland: effects of changes in the light/dark conditions. Gen Comp Endocrinol. 2007;152(2–3):144–7. doi: 10.1016/j.ygcen.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Kawano T, Shiota K, Murakami N. Effects of microinjection of melatonin into various brain regions of Japanese quail on locomotor activity and body temperature. Neurosci Lett. 2003;345(2):117–20. doi: 10.1016/s0304-3940(03)00514-7. [DOI] [PubMed] [Google Scholar]
- Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature. 1994;372(6501):94–7. doi: 10.1038/372094a0. [DOI] [PubMed] [Google Scholar]
- Okano T, Fukada Y. Chicktacking pineal clock. J Biochem. 2003;134(6):791–7. doi: 10.1093/jb/mvg221. [DOI] [PubMed] [Google Scholar]
- O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320(5878):949–53. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima I, Yamada H, Goto M, Sato K, Ebihara S. Pineal and retinal melatonin is involved in the control of circadian locomotor activity and body temperature rhythms in the pigeon. J Comp Physiol A: Neuroethol, Sens, Neur, Behav Phys. 1989;166:217–226. [Google Scholar]
- Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–35. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- Paulose JK, Peters JL, Karaganis SP, Cassone VM. Pineal melatonin acts as a circadian Zeitgeber and growth factor in chick astrocytes. J Pineal Res. doi: 10.1111/j.1600-079X.2008.00659.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka ER. Evolutionary Ecology. Benjamin Cummings; 1973. [Google Scholar]
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Poirel VJ, Boggio V, Dardente H, Pevet P, Masson-Pevet M, Gauer F. Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience. 2003;120(3):745–55. doi: 10.1016/s0306-4522(03)00344-0. [DOI] [PubMed] [Google Scholar]
- Reppert SM. Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J Biol Rhythms. 1997;12(6):528–31. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- Rowan W. Relation of light to bird migration and developmental changes. Nature. 1925;115:494–495. [Google Scholar]
- Russell B. The Principles of Mathematics. W. W. Norton & Company; 1996. Reissue edition. [Google Scholar]
- Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21(6):494–506. doi: 10.1177/0748730406293889. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hamm H, Menaker M. Circadian rhythms of melatonin release from individual superfused chicken pineal glands in vitro. Proc Natl Acad Sci U S A. 1980;77(4):2319–22. doi: 10.1073/pnas.77.4.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Inoue K, Miyabara H, Suzuki S, Ogura Y, Haraguchi S. 7Alpha-hydroxypregnenolone mediates melatonin action underlying diurnal locomotor rhythms. J Neurosci. 2008;28(9):2158–67. doi: 10.1523/JNEUROSCI.3562-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood H, Edmonds K. The circadian rhythm of thermoregulation in Japanese quail: III. Effects of melatonin administration. J Biol Rhythms. 1995;10(4):284–98. doi: 10.1177/074873049501000402. [DOI] [PubMed] [Google Scholar]
- Underwood H, Siopes T. Circadian organization in Japanese quail. J Exp Zool. 1984;232(3):557–66. doi: 10.1002/jez.1402320323. [DOI] [PubMed] [Google Scholar]
- Whitfield-Rucker MG, Cassone VM. Melatonin binding in the house sparrow song control system: sexual dimorphism and the effect of photoperiod. Horm Behav. 1996;30(4):528–37. doi: 10.1006/hbeh.1996.0056. [DOI] [PubMed] [Google Scholar]
- Whitfield-Rucker MG, Cassone VM. Photoperiodic regulation of the male house sparrow song control system: gonadal dependent and independent mechanisms. Gen Comp Endocrinol. 2000;118(1):173–83. doi: 10.1006/gcen.2000.7455. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Yoshimura T, Bartell PA, Iigo M, Makino E, Okabayashi N, Ebihara S. Effect of melatonin administration on qPer2, qPer3, and qClock gene expression in the suprachiasmatic nucleus of Japanese quail. Eur J Neurosci. 2002;16(8):1541–6. doi: 10.1046/j.1460-9568.2002.02222.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura T. Molecular mechanism of the photoperiodic response of gonads in birds and mammals. Comp Biochem Physiol A Mol Integr Physiol. 2006;144(3):345–50. doi: 10.1016/j.cbpa.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Suzuki Y, Makino E, Suzuki T, Kuroiwa A, Matsuda Y, Namikawa T, Ebihara S. Molecular analysis of avian circadian clock genes. Brain Res Mol Brain Res. 2000;78(1–2):207–15. doi: 10.1016/s0169-328x(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Zimmerman NH, Menaker M. The pineal gland: a pacemaker within the circadian system of the house sparrow. Proc Natl Acad Sci U S A. 1979;76(2):999–1003. doi: 10.1073/pnas.76.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]