Abstract
BACKGROUND
Intracranial artery stenosis is assumed to represent atherosclerotic plaque. Catheter cerebral arteriography shows that intracranial stenosis may progress, regress, or remain unchanged. It is counterintuitive that atherosclerotic plaque should spontaneously regress, raising questions about the composition of intracranial stenoses. Little is known about this disease entity in vivo. We provide the first demonstration of in vivo atherosclerotic plaque with intraplaque hemorrhage using intravascular ultrasound (IVUS).
CASE DESCRIPTION
A 35-year-old man with multiple vascular risk factors presented with recurrent stroke failing medical therapy. Imaging demonstrated left internal carotid artery occlusion, severe intracranial right internal carotid artery stenosis, and cerebral perfusion failure. Cerebral arteriography with IVUS confirmed 85% stenosis of the petrous right carotid artery due to atherosclerotic plaque with intraplaque hemorrhage. Intracranial stent-supported angioplasty was performed with IRB approval. The patient recovered without complication.
CONCLUSIONS
This case supports the premise that symptomatic intracranial stenosis can be caused by atherosclerotic plaque complicated by intraplaque hemorrhage similar to coronary artery plaque. IVUS provides additional characteristics that define intracranial atherosclerosis and high-risk features. To our knowledge, this is the first report of stroke due to unstable atherosclerotic plaque with intraplaque hemorrhage in vivo.
Keywords: Intracranial atherosclerosis, IVUS, atheroma, intraplaque hemorrhage
Introduction
Intracranial artery stenosis accounts for 8–10% of all ischemic strokes.1–3 It is estimated that 40,000–60,000 strokes occur annually in the United States primarily due to narrowing of the cerebral arteries. Depending on the population studied, intracranial atherosclerosis may account for up to 29% of all ischemic events.4–6 Persons of Asian (Japanese, Chinese, Korean)7–9, African,3 and Hispanic1 descent seem to be at higher risk for developing intracranial atherosclerosis than persons of Northern European descent. Besides race and ethnicity, risk factors associated with intracranial atherosclerosis include insulin-dependent diabetes mellitus, hypercholesterolemia, cigarette smoking, and hypertension.1,10,11
However, we know relatively little about the composition of these cerebral artery stenoses apparent on noninvasive and invasive imaging studies. It is generally believed to be due to intracranial atherosclerotic disease, which appears to occur in the setting of widespread atherosclerotic disease.12,13 Most published data on the natural history of intracranial atherosclerosis are from patients who present with acute stroke and are subsequently evaluated using either noninvasive imaging (transcranial Doppler or Duplex ultrasonography, computed or magnetic resonance angiography) or catheter arteriography. Yet, the natural history of this disease remains variable. Cerebral artery stenoses may undergo progression, regression, or remain stable during follow-up. Evidently, intracranial stenosis can be a dynamic process.14,15 Current noninvasive imaging does not readily establish the pathology of a stenosis, whether due to local thrombosis, atherosclerosis, or a combination thereof.
Intravascular ultrasound (IVUS) is a widely used imaging technique in cardiology and has recently been utilized for evaluation of iatrogenic lesions of intracranial arteries.16,17 We report the first case in which IVUS has been used to evaluate a symptomatic atherosclerotic stenosis secondary to intraplaque hemorrhage of the intracranial carotid artery, the most frequently identified and reported location for development of intracranial stenosis,18 prior to any form of instrumentation.
Case Report
A 35-year-old Caucasian man with hyperlipidemia, alcohol and tobacco abuse, hypertension, and poorly controlled, juvenile-onset diabetes mellitus treated with insulin was evaluated at his local hospital for acute aphasia. He was found to have multiple bilateral strokes involving the left thalamus and both hemispheres in the deep white matter territories. He also was found to have left internal carotid artery occlusion and intracranial right internal carotid artery stenosis. He had no evidence of a cardioembolic source on transesophageal echocardiography and telemetry. He was then referred to our medical center and subsequently placed on aspirin 25 mg and dipyridimole 200 mg twice per day and atorvastatin 60 mg daily in addition to his insulin and antihypertensive medications. A month later, he was readmitted to our medical center with worsening dysarthia, imbalance, and lethargy. MRI brain scan with diffusion-weighted imaging showed new right anterior choroidal artery and bilateral white matter strokes. Repeat MRI brain scan with cervical and intracranial MRA showed severe stenosis of the petrous right internal carotid artery and persistent occlusion of the left internal carotid artery (Fig 1A). Transcranial Doppler ultrasonography (TCD) showed decreased mean flow velocities in the left middle cerebral arteries and increased flow velocities in the right middle cerebral artery with right to left collateral flow through the anterior communicating artery. There was also a loss of vasomotor reactivity with carbon dioxide challenge in the left cerebral hemisphere, a sensitive indicator of cerebral perfusion failure.19 After careful consideration, he was offered endovascular revascularization using stent-supported angioplasty under IRB-approved protocol.
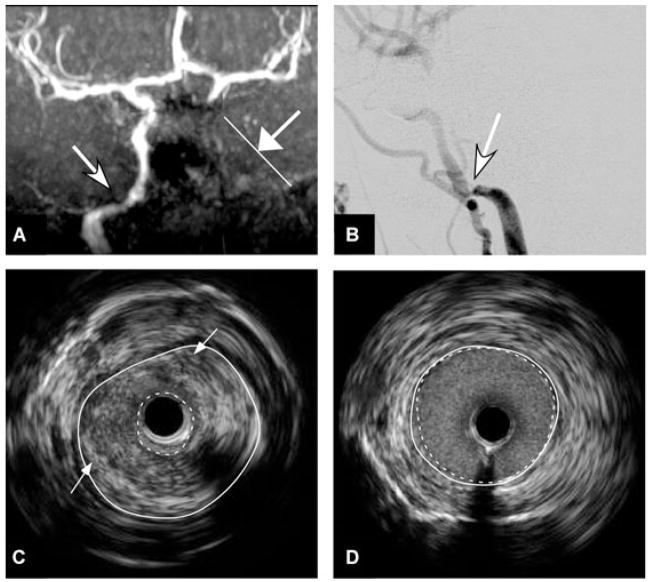
Fig 1.
(A) Time of flight MRA shows narrowing of flow signal in the petrous segment of the right internal carotid artery (arrow) and absence of flow signal in the left internal carotid artery in the neck and skull base (arrow with line marker). (B) Catheter cerebral arteriography of the right common carotid artery, in the lateral projection, during the arterial phase, shows 80–85% stenosis in the petrous segment of the right internal carotid artery. (C and D) IVUS confirms 85% narrowing (dotted white circumferential lines in Image C) in the petrous segment of the right internal carotid artery over a 16 mm segment with heterogeneous echogenicity atherosclerotic plaque with intraplaque hemorrhage (arrows) compared with relatively normal adjacent vessel with minimal narrowing (dotted white circumferential lines in Image D).
Under general anesthesia, catheter arteriography confirmed origin occlusion of the left internal carotid artery and 85% stenosis of the petrous right internal carotid artery (Fig 1B). An IVUS evaluation using a 6F guide-compatible rapid-exchange catheter with a 40 mHz rotating-crystal device (Atlantis™ SR Plus Catheter, Boston Scientific Corporation, Natick, MA) showed severe narrowing of the petrous right internal carotid artery due to a heterogeneously echogenic circumferential stenosis most compatible with atheromatous plaque complicated by intraplaque hemorrhage (Fig 1C and D). Intramural hematomas appear in IVUS of coronary arteries as crescent-shaped, homogeneous and hyperechoic and especially on static images visually continuous with the intima (or plaque) and adventitia.20 The IVUS examination was performed with a standard 0.5 mm/sec automated pullback allowing estimation of lesion length (~16 mm). TCD was performed to monitor any potential emboli generation as a result of the IVUS rotation. No increase in the baseline high intensity transient signal (HITS) rate during IVUS occurred. Following stent-supported angioplasty using a 4.0 × 15 mm Gateway™ balloon catheter, stent revascularization was performed using a 4.5 × 20 mm Wingspan™ self-expanding nitinol stent (Boston Scientific, Fremont, CA). Excellent revascularization was accomplished with 20% residual stenosis (Fig 2A and B). TCD showed a 25% transient increase in mean flow velocity in the right middle cerebral artery trending toward hyperperfusion (Fig 2C and D), which was controlled with systemic reduction in blood pressure. The procedure was well tolerated by the patient, and was without complication. He was monitored in the Neurological Intensive Care Unit primarily for ongoing blood pressure control. He was discharged home on the second postoperative day on oral antihypertensive medications, insulin, aspirin, and Plavix. No further TIA or stroke during 12-month follow-up.
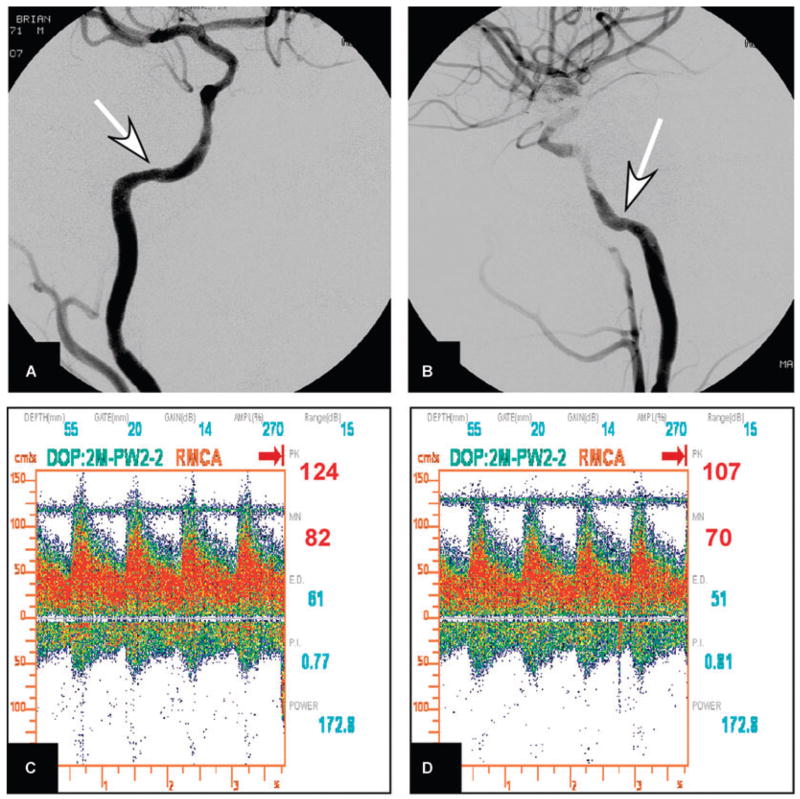
Fig 2.
(A and B) Catheter cerebral arteriography of the right internal carotid artery after stent-supported angioplasty, in the frontal and lateral projections, during the arterial phase, shows 20% residual stenosis in the stented segment (arrows). (C and D) transcranial Doppler ultrasonography (TCD) of the right middle cerebral artery immediately and 5 minutes following stent-angioplasty shows a 50% increase in peak velocity (83 cm/sec to 124 cm/sec) that was reduced to 25% (124 cm/sec to 107 cm/sec) using systemic blood pressure reduction with intravenous nicardipine infusion.
Discussion
Atherosclerotic lesions show a complex composition consisting of variable amounts of fibrosis, necrotic lipid core, and/or intraplaque hemorrhage. Based on experience from the coronary and carotid artery literature, plaque composition is critical for predicting future ischemic events. For example, patients with symptomatic extracranial internal carotid artery stenosis >70% and a lipid core in the stenotic atheroma as defined by multimodal MRI had more frequent ipsilateral infarcts than patients without a lipid core (68% vs. 31%),21 paralleling findings of surgical specimens from patients with symptomatic carotid stenosis.22 Plaque characterization using multimodal MRI seems promising to predict future ipsilateral ischemic strokes.23 The predictors for future events include presence of a thin or ruptured fibrous cap, intraplaque hemorrhage, larger mean intraplaque hemorrhage area, larger maximum percentage of lipid-rich/necrotic core, and larger maximum wall thickness.23
The exact nature of a given stenotic lesion in an intracranial artery remains obscure when using current noninvasive (transcranial ultrasound, computed or magnetic resonance angiography) or invasive neuroimaging (conventional digital subtraction cerebral angiography). These lesions are typically assumed to be of an atherosclerotic nature when no other obvious disorder, like vasculitis or dissection, is found on diagnostic work-up. Most patients with intracranial stenosis are diagnosed in the setting of an acute event. An intracranial atherosclerotic stenotic lesion may represent any one of the following:
Arterial embolus without an underlying atherosclerotic lesion: an initial occluding embolus may undergo partial lysis and vascular imaging may be interpreted erroneously as a stenotic atherosclerotic lesion.
Atheromatous, nonstenotic but ulcerated lesion with superimposed thrombosis leading to significant stenosis.
Atheromatous stenotic lesion without ulceration or superimposed thrombosis.
Atheromatous stenotic or nonstenotic lesion with intraplaque hemorrhage leading to acute vessel stenosis with or without superimposed local thrombosis.
The prognosis for future ischemic events may vary across the different lesion types. Our knowledge on the exact nature of intracranial stenotic lesions is based on autopsy studies.24–27 These pathological data cannot provide sufficient information to predict the future stroke risk of a newly diagnosed intracranial artery stenosis in a given patient. While intracranial stenoses can remain stable, in a significant number of cases they show a variable degree of regression or progression, a process that is unpredictable at the time of diagnosis.28 Symptomatic intracranial atherosclerosis carries a significant risk for stroke. In WASID, the overall stroke rate for patients for intracranial stenosis grades of 70 to 99% was 23% within 1 year and 25% within 2 years while for intracranial stenosis grades of 50 to 69% the corresponding rates were 8% and 11%, respectively.29 This clearly establishes stenosis grade as an important stroke predictor but does not answer the question why only 1 out of 4 patients developed a subsequent stroke distal to the highly stenotic intracranial artery within 2 years. We hypothesize that stroke rates are dependent on the composition of the stenotic lesion as has been shown for coronary arteries,30 vessels of similar size to cerebral arteries but of different vessel wall composition.31
IVUS provides transmural imaging of the entire arterial wall and permits the accurate cross-sectional and even 3-dimensional quantification of plaques.32 Validation studies of in vivo virtual histology using IVUS compared with in vitro histopathology report high correlation of >80–90% for fibrous, fibro-fatty, necrotic core, and for dense calcium regions in extracranial carotid and coronary arteries.33,34 Similar data for intracranial arteries are not reported. Patient inclusion in WASID required conventional angiography to estimate stenosis grades by a newly developed grading system for intracranial stenoses. Advances in noninvasive imaging techniques may replace conventional angiography; but, currently, conventional angiography remains the gold standard for clinical trials, including the planned trial comparing intracranial stenting to medical treatment for high-risk intracranial atherosclerosis patients.35 IVUS technology allows relatively easy deployment of the ultrasound catheter into the intracranial internal carotid artery, a common site of intracranial atherosclerosis. IVUS can be used for evaluation of ICA atheromas in trials comparing stenting to medical treatment prior to randomization, thus providing important data on the natural history of intracranial ICA atheromas depending on plaque composition beyond stenosis grade alone. Invasive imaging of intracranial arteries using current IVUS probes must be balanced against possible complications due to the deployment of the probe. There are few case reports using IVUS for intracranial arteries, and none report complications of its use. The current IVUS probes only allow relatively safe deployment into the intracranial internal carotid artery.
Further, intracranial IVUS can assist in treatment decisions. Wehman et al. identified the exact location of an internal carotid artery dissection over its entire length using IVUS and successfully covered its entire length with stents.16 In the second case of occlusive basilar stenosis, IVUS demonstrated fibrous plaque that led to the decision for stent-assisted angioplasty instead of angioplasty alone,16 similar to a case reported by Takayama et al.17 In our patient, we were able to identify a mostly fibrotic atherosclerotic plaque with intraplaque hemorrhage using IVUS. The frequency of intraplaque hemorrhage in symptomatic intracranial atherosclerosis is unknown. Intraplaque hemorrhage with superimposed occlusive thrombus was identified in 1 out of 8 autopsy cases of strokes secondary to intracranial atherosclerosis. However, another series did not find intraplaque hemorrhage among 11 autopsy cases of intracranial carotid artery occlusions. We hypothesize that in our patient, intraplaque hemorrhage may have triggered or at least contributed to the acute symptoms compatible with perfusion failure distal to the stenosis. In this situation, we decided to use stent-assisted angioplasty instead of angioplasty alone possibly to lower the risk for continuous vessel dissection thus leading to further volume increase of the intraplaque hemorrhage.
In summary, IVUS using current equipment is feasible and provides important information on the morphology of stenotic intracranial ICA lesions. This new technique may prove valuable for risk stratification in patients with symptomatic intracranial stenoses.
References
- 1.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The northern Manhattan stroke study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Segura T, Serena J, Castellanos M, Teruel J, Vilar C, Davalos A. Embolism in acute middle cerebral artery stenosis. Neurology. 2001;56:497–501. doi: 10.1212/wnl.56.4.497. [DOI] [PubMed] [Google Scholar]
- 3.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974–1980. doi: 10.1161/01.str.27.11.1974. [DOI] [PubMed] [Google Scholar]
- 4.Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, Pessin MS, Weichel E, Sila CA, Furlan AJ. The warfarin-aspirin symptomatic intracranial disease study. Neurology. 1995;45:1488–1493. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 5.Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490–497. doi: 10.1212/wnl.55.4.490. [DOI] [PubMed] [Google Scholar]
- 6.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 7.Inzitari D, Hachinski VC, Taylor DW, Barnett HJ. Racial differences in the anterior circulation in cerebrovascular disease. How much can be explained by risk factors? Arch Neurol. 1990;47:1080–1084. doi: 10.1001/archneur.1990.00530100042012. [DOI] [PubMed] [Google Scholar]
- 8.Resch JA, Okabe N, Loewenson RB, Kimoto K, Katsuki S, Baker AB. Pattern of vessel involvement in cerebral atherosclerosis. A comparative study between a Japanese and Minnesota population. J Atheroscler Res. 1969;9:239–250. doi: 10.1016/s0368-1319(69)80019-0. [DOI] [PubMed] [Google Scholar]
- 9.Wong KS, Huang YN, Gao S, Lam WW, Chan YL, Kay R. Intracranial stenosis in Chinese patients with acute stroke. Neurology. 1998;50:812–813. doi: 10.1212/wnl.50.3.812. [DOI] [PubMed] [Google Scholar]
- 10.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648–655. doi: 10.1161/01.str.17.4.648. [DOI] [PubMed] [Google Scholar]
- 11.Ingal TJ, Horner D, Baker HLJ, Kottke BA, O’Fallon WM. Predictors of intracranial carotid atherosclerosis: duration of cigarette smoking and hypertension are more powerful than serum lipid levels. Arch Neurol. 1991;48:687–691. doi: 10.1001/archneur.1991.00530190033011. [DOI] [PubMed] [Google Scholar]
- 12.Craig DR, Meguro K, Watridge C, Robertson JT, Barnett HJ, Fox AJ. Intracranial internal carotid artery stenosis. Stroke. 1982;13:825–828. doi: 10.1161/01.str.13.6.825. [DOI] [PubMed] [Google Scholar]
- 13.Fisher CM, Gore I, Okabe N, White PD. Atherosclerosis of the carotid and vertebral arteries: extracranial and intracranial. J Neuropathol Exp Neurol. 1965;24:455–476. [Google Scholar]
- 14.Akins PT, Pilgram TK, Cross DT, III, Moran CJ. Natural history of stenosis from intracranial atherosclerosis by serial angiography. Stroke. 1998;29:433–438. doi: 10.1161/01.str.29.2.433. [DOI] [PubMed] [Google Scholar]
- 15.Hinton RC, Mohr JP, Ackerman RH, Adair LB, Fisher CM. Symptomatic middle cerebral artery stenosis. Ann Neurol. 1979;5:152–157. doi: 10.1002/ana.410050208. [DOI] [PubMed] [Google Scholar]
- 16.Wehman JC, Holmes DR, Jr, Hanel RA, Levy EI, Hopkins LN. Intravascular ultrasound for intracranial angioplasty and stent placement: technical case report. Neurosurgery. 2006;59:ONSE481–483. doi: 10.1227/01.NEU.0000222825.92929.0C. discussion ONSE483. [DOI] [PubMed] [Google Scholar]
- 17.Takayama K, Taoka T, Nakagawa H, Myouchin K, Wada T, Sakamoto M, Fukusumi A, Iwasaki S, Kurokawa S, Kichikawa K. Successful percutaneous transluminal angioplasty and stenting for symptomatic intracranial vertebral artery stenosis using intravascular ultrasound virtual histology. Radiat Med. 2007;25:243–246. doi: 10.1007/s11604-007-0127-5. [DOI] [PubMed] [Google Scholar]
- 18.Schumacher HC, Khaw AV, Meyers PM, Gupta R, Higashida RT. Intracranial angioplasty and stent placement for cerebral atherosclerosis. J Vasc Interv Radiol. 2004;15:S123–132. doi: 10.1097/01.rvi.0000107488.61085.8f. [DOI] [PubMed] [Google Scholar]
- 19.Marshall RS, Rundek T, Sproule DM, Fitzsimmons BF, Schwartz S, Lazar RM. Monitoring of cerebral vasodilatory capacity with transcranial doppler carbon dioxide inhalation in patients with severe carotid artery disease. Stroke. 2003;34:945–949. doi: 10.1161/01.STR.0000062351.66804.1C. [DOI] [PubMed] [Google Scholar]
- 20.Maehara A, Mintz GS, Bui AB, Castagna MT, Walter OR, Pappas C, Pinnow EE, Pichard AD, Satler LF, Waksman R, Suddath WO, Laird JR, Jr, Kent KM, Weissman NJ. Incidence, morphology, angiographic findings, and outcomes of intramural hematomas after percutaneous coronary interventions: an intravascular ultrasound study. Circulation. 2002;105:2037–2042. doi: 10.1161/01.cir.0000015503.04751.bd. [DOI] [PubMed] [Google Scholar]
- 21.Ouhlous M, Flach HZ, de Weert TT, Hendriks JM, van Sambeek MR, Dippel DW, Pattynama PM, Van Der Lugt A. Carotid plaque composition and cerebral infarction: MR imaging study. AJNR Am J Neuroradiol. 2005;26:1044–1049. [PMC free article] [PubMed] [Google Scholar]
- 22.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the oxford plaque study. Circulation. 2006;113:2320–2328. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 23.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI–initial results. Stroke. 2006;37:818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 24.Castaigne P, Lhermitte F, Gautier JC, Escourolle R, Derouesne C, Der AP, Popa C. Arterial occlusions in the vertebro-basilar system. A study of 44 patients with post-mortem data. Brain. 1973;96:133–154. doi: 10.1093/brain/96.1.133. [DOI] [PubMed] [Google Scholar]
- 25.Ogata J, Masuda J, Yutani C, Yamaguchi T. Mechanisms of cerebral artery thrombosis: a histopathological analysis on eight necropsy cases. J Neurol Neurosurg Psychiatry. 1994;57:17–21. doi: 10.1136/jnnp.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lammie GA, Sandercock PA, Dennis MS. Recently occluded intracranial and extracranial carotid arteries. Relevance of the unstable atherosclerotic plaque. Stroke. 1999;30:1319–1325. doi: 10.1161/01.str.30.7.1319. [DOI] [PubMed] [Google Scholar]
- 27.Schumacher HC, Tanji K, Mangla S, Meyers P, Pile-Spellman J, Hays AP, Mohr JP. Histopathological evaluation of middle cerebral artery after percutaneous intracranial transluminal angioplasty. Stroke. 2003;34:170–173. doi: 10.1161/01.STR.0000086764.86787.9C. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher HC, Meyers PM, Mohr JP, Higashida RT. Elective angioplasty and stenting for intracranial atherosclerosis. In: Al-Mubarak N, Rubin GS, Iyer SS, Vitek JJ, editors. Carotid Artery Stenting: Current Practice and Techniques. New York: Lippincott Williams Wilkins; 2004. pp. 255–289. [Google Scholar]
- 29.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG, Sila CA, Jovin TG, Romano JG, Cloft HJ. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 30.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part i: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 31.Meyers PM, Schumacher HC, Tanji K, Higashida RT, Caplan LR. Use of stents to treat intracranial cerebrovascular disease. Annu Rev Med. 2007;58:107–122. doi: 10.1146/annurev.med.58.121205.100631. [DOI] [PubMed] [Google Scholar]
- 32.Erbel R, Ge J, Gorge G, Baumgart D, Haude M, Jeremias A, von Birgelen C, Jollet N, Schwedtmann J. Intravascular ultrasound classification of atherosclerotic lesions according to American Heart Association recommendation. Coron Artery Dis. 1999;10:489–499. doi: 10.1097/00019501-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, Murata A, Takeda Y, Ito T, Ehara M, Matsubara T, Terashima M, Suzuki T. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 34.Diethrich EB, Pauliina Margolis M, Reid DB, Burke A, Ramaiah V, Rodriguez-Lopez JA, Wheatley G, Olsen D, Virmani R. Virtual histology intravascular ultrasound assessment of carotid artery disease: the carotid artery plaque virtual histology evaluation (capital) study. J Endovasc Ther. 2007;14:676–686. doi: 10.1177/152660280701400512. [DOI] [PubMed] [Google Scholar]
- 35.Chimowitz MI. Stenting vs. Aggressive medical management for preventing recurrent stroke in intracranial stenosis (sammpris), (r01ns058728-01a1, r01ns058728-01a1; identifier no. Nct00576693). 2007;2007:Service of the U.S. National Institutes of Health.