Abstract
Multiple sclerosis (MS) is a devastating CNS disease of unknown origin. Multiple factors including genetic background, infection, and psychological stress affect the onset or progression of MS. Theiler’s murine encephalomyelitis virus (TMEV) infection is an animal model of MS in which aberrant immunity leads to viral persistence and subsequently results in demyelination that resembles MS. Here, we examined how stress during acute TMEV infection altered virus-specific cell mediated responses. Using immunodominant viral peptides specific for either CD4+ or CD8+ T cells, we found that stress reduced IFN-γ producing virus-specific CD4+ and CD8+ T cells in the spleen and CD8+ T cells CNS. Cytokine production by cells isolated from the CNS or spleens following stimulation with virus or viral peptides, indicated that stress decreased both type 1 and type 2 responses. Glucocorticoids were implicated in the decreased T cell function as the effects of stress were partially reversed by concurrent RU486 administration but mimicked by dexamethasone. As T cells mediate viral clearance in this model, our data support the hypothesis that stress-induced immunosuppression may provide a mechanism for enhanced viral persistence within the CNS.
Keywords: Theiler’s virus, Restraint Stress, T cell, Multiple Sclerosis
1. Introduction
MS is a demyelinating and neurodegenerative disorder that targets the central nervous system (CNS) and is autoimmune in nature. While the cause of MS is not known, multiple factors are likely to contribute to the pathogenesis of this disease, including, gender, genetic background, and environment (Noseworthy et al., 2000). No single known pathogen has been associated exclusively with the development of MS, but many different pathogens have been isolated from MS plaques (Fazakerly and Walker, 2003). Other evidence for the involvement of an infectious agent in MS development are epidemiological (Kurtze and Heltberg, 2001), migrational (Gale and Martyn, 1995), and the pathological characterization of MS plaques (Lucchinetti et al., 2000).
Another environmental factor that may contribute to both the onset and exacerbation of MS is psychological stress (Ackerman et al., 2003; Mohr et al., 2004). Stress, acting through the hypothalamic-pituitary-adrenal (HPA) axis as well as the autonomic nervous system, has been shown to modulate immune responses, with acute stress stimulating leukocyte trafficking and chronic stress being immunosuppressive (Dhabhar and McEwen, 1997). We have recently been interested in the mechanisms by which restraint stress can alter the pathogenesis of Theiler’s murine encephalomyelitis virus (TMEV) infection, a commonly used animal model of human MS (Lipton, 1975; Oleszack et al., 2004). In this model, intracranial injection of TMEV into susceptible strains of mice can lead to a biphasic disease consisting of a polioencephalitic phase followed by a chronic demyelinating phase that is perpetuated by T cell and B cell autoimmune responses (Oleszack et al., 2004). Conversely, viral inoculation of genetically-resistant strains of mice does not result in demyelination.
After infection with TMEV susceptible mice fail to generate a well-orchestrated immune response, which allows the virus to persist, primarily in macrophages/monocytes as well as astrocytes, for the duration of the animal’s life time (Theiler, 1937; Clatch et al., 1990; Zheng et al., 2001). Susceptibility to TMEV-induced demyelination is attributed to several factors (Brahic et al., 2005) including the H-2D locus (Lipton and Melvold. 1984; Rodriguez and David, 1985), indicating a role of virus-specific CD8+ T cells in the clearance of TMEV. Findings in support of this hypothesis are: (i) deletion of CD8+ cells by antibody administration exacerbates disease (Borrow et al., 1992), (ii) β-2 microglobulin deficient mice (Begolka et al., 2001; Fiette et al., 1993; Miller et al., 1995; Pullen et al., 1993) as well as (iii), perforin-deficient (Palma et., 2001; Rossi et al., 1998; Murray et al., 1998) mice generated on a Theiler’s virus-induced demyelination resistant background, are rendered susceptible. The generation of TMEV-specific antibodies has also been shown to contribute to viral clearance (Welsh et al., 1989; Kang et al., 2005).
While Th1 type immunity is essential for early TMEV infection clearance by the generation of both T and B cell responses; in the later phase of TMEV Th1 mediated immunity is damaging to the CNS. Nevertheless, pro-inflammatory responses early following infection are beneficial. In fact, in CBA mice, deletion of CD4+ T cells during the time of infection was shown to cause mortality (Welsh et al., 1987). Similarly, in CD4+ T cell deficient SJL mice infected with the DA strain of TMEV the disease was exacerbated (Lin et al., 2004).
Restraint stress (RS) has been shown to be immunosuppressive in several viral infections including herpes simplex virus (Bonneau et al., 1991) and influenza virus (Feng et al., 1991). We have demonstrated that RS can dramatically influence the disease course of TMEV. Particularly, SJL mice that received 4 weeks of 8 hour nightly RS develop an exacerbated disease course (Sieve et al., 2004) with similarities to that generated following depletion of CD8+ T cells via monoclonal antibody administration (Borrow et al., 1992). Moreover, infected CBA mice that received nightly RS of 12 hours produced a survival curve that was almost identical of that generated by the depletion of CD4+ T cells (Welsh et al., 1987; Campbell et al., 2001). Because of these similarities, we hypothesized that restraint stress during acute infection with the BeAn strain of TMEV, alters the generation of T cell mediated adaptive immune responses. Therefore, in this particular study we set out to determine the effects of restraint stress on the generation of virus-specific CD4+ T cell and CD8+ T cell effector functions.
2. Methods and Materials
2.1. Mice
Three to four week old female SJL mice were purchased from Harlan (Indianapolis, IL). Mice were assigned to either infected/restrained (I/R), infected/non-restrained (I/NR), non-infected/restrained (NI/R) or non-infected/non-restrained (NI/NR) groups. In the restrained groups, restraint commenced 24 hrs prior to infection. For each individual experiment mice were housed two animals per cage per group. All mice were given one week to acclimate to their housing environment (light dark cycle 0500– 1700 hr) prior to the onset of the experiment. Mice were kept on a diet of mouse chow containing 9% fat and 20.5% protein. Food and water was provided ad libitum. All animal care protocols were in accordance with NIH Guidelines for Care and Use of Laboratory Animals and were approved by the Texas A&M University Laboratory Animal Care and Use Committee.
2.2. Infection and restraint stress
Following anesthesia with isoflurane (MWI, Meridian, ID), mice were either injected with 5.0 × 105 plaque forming units (PFU) of BeAn strain of TMEV in 20 μl of DMEM media or sham-infected with DMEM into the right mid-parietal cortex at a depth of approximately 1.5mm (Campbell et al., 2001). Restraint stress was carried out as described previously (Campbell et al., 2001). Restraint sessions began the night prior to infection and continued each night until day 8 p.i. All mice were allowed 2 h to recover prior to injection. Mice were weighed daily.
2.3. Experimental design
2.3.1. Experiments to assess the effects of stress on the T cell response to Theiler’s virus
A 2 (Infected vs Non-infected) × 2 (Restrained vs Non-restrained) factorial design was used to determine the effects of 8 nights of restraint stress on the immune response to Theiler’s virus. Mice were sacrificed on day 8 by overdose of ketamine/xylazine, spleens, brain and spinal cords collected for: CD4+ and CD8+ T cell responses by ELISPOT; % CD4+ and CD8+ T cells by flow cytometry; cytokine levels by Bioplex assay; T-bet and GATA-3 expression by Q RT-PCR and western blots. Serum was also collected from these animals for the determination of serum cytokine levels measured by Bioplex assay.
2.3.2. Experiments to determine whether the immune response to Theiler’s virus recovers after stress cessation
A 2 (Infected vs Non-infected) × 2 (Restrained vs Non-restrained) factorial design was used to determine the effects of 8 nights of restraint stress, followed by 8 nights of recovery, on the immune response to Theiler’s virus. Mice were sacrificed on day 16 by overdose of ketamine/xylazine and the serum, spleens, brain and spinal cords collected for determination of CD4+ and CD8+ T cell responses by ELISPOT.
2.3.3. Experiments to determine whether glucocorticoids are the main mediators of stress-induced immunosuppression
In order to assess whether a glucocorticoid agonist (dexamethasone) would mimic the effects of restraint stress and the glucocorticoid antagonist RU486 would reverse the effect of stress, mice were assigned to the following groups: infected/PBS treated (I/PBS), infected/dexamethasone treated (I/DEX), infected/restrained/PEG400 treated (I/R/PEG) or infected/restrained/RU486 treated (I/R/RU486) groups. For each individual experiment mice were housed two animals per cage per group. Mice were treated nightly with dexamethasone (DEX) (Sigma Chemical, St. Louis, MO) at a dose of 1.0 mg/kg or with vehicle control (PBS) by intraperitoneal (i.p.) injection given 1 h prior to restraint. RU486 (Sigma Chemical, St. Louis, MO) treatment was administered according to Tseng et al., (Tseng et al., 2005) such that mice were given nightly subcutaneous (s.c.) injections of RU486 (25mg/kg) or vehicle control (PEG400). Both DEX and RU486 treatments were administered 1 h prior to restraint, began the night prior to infection, and lasted until day 8 p.i. All injection volumes were administered in a total volume of 100 μl.
2.4. Virus, viral purification and inactivation, and viral peptides
The BeAn 8683 strain of Theiler’s virus (kindly provided by Dr. H. L. Lipton, Department of Microbiology-Immunology, University of Illinois at Chicago, IL) was initially propagated in lung tumor (L2) cells (Welsh et al., 1987). Virus was semi-purified by ultracentrifugation according to Rueckert and Pallansch, (Rueckert and Pallansch, 1981) by pelletting through 30% sucrose cushion at 80,000 × g for 3 hours in a Optima L-80 XP ultracentrifuge (Beckman Coulter) using an SW-28 rotor. Pellets were resuspended in 1M sodium phosphate buffer (pH 7.4). UV-inactivated virus was generated by exposing previously pelleted virus to UV-light (1330 μW/cm3 at 13 cm distance) for 1.5 h. The virus was determined to be inactivated by plaque assay.
The immunodominant CD4+ T cell peptide QEAFSHIRIPLPH corresponding to TMEV VP274-86 was used to determine CD4+ T cell specific responses (Gerety et al., 1991 & 1994). Additionally, the immunodominant CD8+ T cell peptide FNFTAPFI corresponding to VP3159-166 was used to determine CD8+ T cell specific responses to TMEV (Kang et al., 2002). The non-specific peptide sequence RLNRITKDSYPNS was used as a control peptide to determine non-specific immune responses. All peptides were purchased from Sigma at ≥ 95% purity by HPLC.
2.5. Tissue isolation
At the termination of each experiment (Day 8 or 16 p.i.) all mice were injected with a lethal dose of Beuthanasia special 150mg/kg (Schering-Plough Animal Health) (Welsh et al., 2004). Blood was collected via cardiac puncture, and then mice were perfused through the left ventricle with cold Hanks balanced salts solution containing heparin (10U/mL) buffered at pH 7.2. Serum was collected as described (Welsh et al., 2004). After perfusion, spleens, brains and spinal cords were aseptically removed. Single cell suspensions were prepared as described previously (Welsh et al., 2004). CNS infiltrating lymphocytes (CNS-ILs) were prepared from the CNS tissue using nylon mesh and incubating the tissue with RPMI 1640 containing 250μg/ml of collagenase type IV (Worthington Inc., Lakewood, NJ) for 45 minutes at 37°C and 5% CO2 (Kang et al., 2002). Following incubation, the lymphocytes were isolated by 35/70% percoll gradient centrifugation, then re-suspended in complete RPMI-1640 containing L-glutamine (1.0%), penicillin and streptomycin (1.0%), and 10% FBS.
2.6. Plaque assay
UV-inactivated virus was determined to be inactivated by plaque assay as described previously (Mi et al., 2006a).
2.7. Preparation of feeder cells
Spleens were aseptically removed from aged-matched NI/NR mice and single cell suspensions were prepared. Feeder cells were irradiated with 3000 rads (Co60 source, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University).
2.8. ELISPOT assays
The effects of restraint stress on T cell effector function to TMEV were assessed, in part, by the ability of CNS-ILs to generate IFN-γ in response to either the immunodominant CD4+ or CD8+ T cell specific peptide (VP274-86 or VP3159-166, respectively). In this assay, 96-well filtration plates (MAIPS4510) containing PVDF membranes (Millipore, Corp, Bedford, MA) were coated with 1.0 μg (in 100μl sterile PBS) of anti-mouse IFN-γ capture antibody (AN-18; eBioscience) overnight at 4°C. The plates were blocked with 200μl complete RPMI-1640 (supplemented with 10% FBS) for 2 h at room temperature. Then 2.0 × 104 CNS-ILs were mixed with 1.0 × 106 irradiated feeders, in 150 μl of complete RPMI-1640 with a final concentration of 2.0 μM peptide (CD4+, CD8+ or non-specific) and then added to the plate. Following an incubation at 37° C and 5.0% CO2 for 24 h the plates were washed with PBS containing 0.05% Tween-20 (5X) and rinsed once with water purified by reverse osmosis (RO) H2O. 100 μl assay diluent (PBS with 10% FBS) containing 0.1μg of the biotin labeled anti-IFN-γ detection antibody (R4-6A2; eBioscience) was added to each well, and the plates were incubated at room temperature for 2 h. After the incubation the plates were washed 6X with PBS containing Tween-20 (0.05%). Then, 100μl of avidin-HRP (horseradish peroxidase) (eBioscience) diluted 1/1000 in assay diluent was added to each well and the plates were incubated for 30 min. at room temperature. After washing 6X as described above, spots were developed using 100 μl of 3-amino-9-ethyl-carbazole (AEC) substrate solution (1.0mg AEC, 1.0ml dimethylformamide, 14ml 0.1M citrate-phosphate buffer pH 5.0, and 10.0 μl and H2O2). The plates were rinsed 3X with 200μl of RO H2O, and read with an ELISPOT plate reader (AID EliSpot Reader System, Straberg, Germany). The effects of RS on splenic T cell effector function were determined using the methods described above. However, for these assays, 1.0 × 106 isolated spleen cells were used in the absence of feeders. All samples were run in duplicate. Background was determined as the generation of spots to the non-specific peptide and was subtracted from CD8+ and CD4+ T cell virus-specific peptide responses.
2.9. Flow cytometry
Flow cytometry was used to test the effects of RS on splenic T cell percentages, at days 8 and 16 p.i. Briefly, 1.0 × 106 splenocytes were blocked with 1.0 μg of anti-mouse CD16/CD32 (93; eBioscience) for 10 minutes at 4°C then labeled with PE conjugated anti-CD3e (145-2C11; eBioscience), and FITC conjugated anti-CD4 (RM4-5; eBioscience) or FITC conjugated anti-CD8a (53–6.7; eBioscience) for 20 min. at 4°C. Cells were then washed 3X with staining buffer (eBioscience) then resuspended with 300μl EM grade paraformaldehyde (Electron Microscopy Science; Ft Washington, PA). T cell percentages were then determined using a FACSCaliber (Becton Dickson) and FlowJo software (TreeStar, Inc., Ashland, OR).
2.10. Cytokine secretion
At day 8 p.i. 5 × 104 CNS-IL and 1.0 × 106 irradiated splenocytes or 1.0 × 106 splenocytes were cultured with complete RPMI 1640, and stimulated with either plate bound anti-CD3 (10 μg/ml) and anti-CD28 (2.0 μg/ml), purified UV-irradiated BeAn, or control media for 72 h at 5.0% CO2 and 37°C. The supernatants were then collected and concentrations of Th1, Th2 and Th17 cytokines measured using Bio-plex kits (Th1/Th2) or ELISA (Th17) according to the manufacturers’ instructions. For some experiments, the effects of RS on virus-specific IFN-γ secretion was measured by ELISA (eBioscience) after stimulating 1.0 × 106 splenocytes (isolated at day 8 p.i.) for 24 hours with 2μM of VP274-86, VP3159-166, or non-sense (NS) peptide in complete RPMI 1640 at 5.0% CO2 and 37°C as indicated in the text.
2.11. Serum cytokine profile
At day 8 p.i., the effects of RS on serum cytokine concentrations were determined using a Bio-Plex 23 plex profiling kit according to the manufacturer’s instructions (Bio-Rad, Inc; Hercules, CA). This kit gave the concentrations of 23 serum cytokines including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17, Eotaxin, G-CSF, GM-CSF, IFN-γ, KC, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α.
2.12. Quantitative RT-PCR for T-bet and GATA-3
Total RNA was isolated from 1.0 × 107 spleen cells by QIAshredders (Qiagen), and purified by column and DNase digestion using a Qiagen RNeasy mini kit according to the manufacturer’s instructions (Qiagen). cDNA was generated as described previously, albeit using random primers (Promega) (Mi et al., 2006b). Real-time PCR was used to test for IL-4, IL-5, IFN-γ, T-bet, and GATA-3, expression and normalized to β-actin using methods described previously (Mi et al., 2006b). All primers were purchased from Integrated DNA Technologies (Coralville, IA), sequences used were T-bet: sense, CAACAACCCCTTTGCCCAAAG, antisense, TCCCCCAAGCAGTTGACAGT, (Yamamoto et al., 2005), IFN-γ: sense, CAGCAACAGCAAGGCGAAA, antisense, CTGGACCTGTGGGTTGTTGAC, (Mi et al., 2006), GATA-3: sense, AGAACCGGCCCCTTATCA, antisense, AGTTCGCGCAGGATGTCC, (Yamamoto et al., 2005), IL-4: sense, ACAGGAGAAGGGACGCCAT, antisense, GAAGCCCTACAGACGAGCTCA, (Wei et al., 2005), IL-5: sense, CCGAGCTCTGTTGACAAGCA, antisense, CAGTATGTCTAGCCCCTGAAAGATTT, (Wei et al., 2005), β-actin: sense, GCAACGAGCGGTTCCG, antisense, CCCAAGAAGGAAGGC (Mi et al., 2006b).
2.13. Western blot
To determine the effects of RS on T-bet and GATA-3 protein levels, 1.0 × 106 splenocytes were cultured with plate bound anti-CD3 (10 μg/ml) and anti-CD28 (2 μg/ml) antibodies, or 2 μM peptides VP274-86, VP3159-166, or NS in 150 μl of complete RPMI-1640 for 5 or 24 hours at 37°C and 5% CO2. Nuclear protein was extracted from stimulated splenocytes using NU-PER kits (Pierce), quantified by BCA protein assay (Pierce), separated on 10% polyacrylamide gels, transferred to nitrocellulose, blocked with 5% NFDM, probed with anti-mouse T-bet (4B10; Santa Cruz) or GATA-3 (HG3-31; Santa Cruz) followed by ImmunoPure anti-mouse IgG-HRP (Pierce) and developed with SuperSignal west pico chemiluminescent substrate (Pierce). Blots were then stripped and re-probed with anti-mouse β-actin (a kind gift from Dr. Weston Porter; Texas A&M University). Film (CL-XPosure; Pierce) was developed using an autoprocessor, and densitometry analysis was performed with using a Bio-Rad Analyzer gel 2K system and quantity-one software.
2.14. Statistical analyses
Data are presented as means ± SEM. Data were analyzed using multiple level analysis of variance (ANOVA) and Student’s t-tests. Because pilot studies indicated that the non-infected groups did not respond to viral peptides or UV-BeAn stimulation in ex vivo culture, planned students t-tests between I/NR and I/R groups as well as NI/NR and NI/R groups were used to determine differences in cytokine secretion as well as ELISPOT assays. Significance of correlations was determined by simple regression analysis. In all cases significance was set at p ≤ 0.05.
3. Results
3.1. Theiler’s virus infection and restraint stress decrease body weight
As in our previous studies (Cambell et al., 2001; Sieve et al., 2004) both RS, and infection were found to cause an overall decrease in body weight (p’s < 0.001; data not shown). Moreover, these effects were dependent on time. Specifically, the effects of infection on change in body weight percentage were transient, reaching maximum at day 1 p.i at which time infection exacerbated the RS induced weight loss, p < 0.01, but resolved thereafter. However, RS caused continual weight loss, which was sustained throughout the course of the experiment (data not shown).
3.2. Stress has a profound influence on T cell responses in the CNS and spleens at days 8 p.i
Cellular immune responses, particularly CD8+ T cell responses, are imperative for the clearance of TMEV from the CNS and thus may play an intricate role in the prevention of immune-mediated demyelination in this model of human MS. The benefit of IFN-γ in the clearance of TMEV is illustrated by the fact that IFN-γ−/− mice on a normally resistant background succumb to viral persistence and eventually demyelination (Rodriguez et al., 2003). In order to determine the effects of RS on IFN-γ producing T cells in both the CNS and the periphery, we utilized known immunodominant CD4+ (VP274-86), and CD8+ (VP3159-166) T cell epitopes to stimulate these cells in an ELISPOT assay. Within the CNS, very few cells responded to the CD4+ immunodominant peptide (VP274-86), confirming the results of others suggesting that almost no capsid specific Th1 cells are present in the CNS of TMEV infected SJL mice at this time point (Fig. 1A; (Mohindru et al., 2006). In sharp contrast, infection caused an influx of CNS-infiltrating lymphocytes (CNS-IL) that were specific for the CD8+ immunodominant epitope (VP3159-166) (p < 0.001) at day 8 p.i. (Fig. 1B). Within infected animals, RS decreased the number of VP3159-166 reactive CD8+ T cells by approximately 58% (p < 0.05). Additionally, at this same time point, infection increased the number of both VP274-86 (CD4+) and VP3159-166 (CD8+) specific IFN-γ producing T cells in the spleens of infected mice (ps < 0.01) (Fig. 1C–D). While CD4+ T cell responses were different between the periphery and the CNS, it is worth noting that the CD8+ T cell responses were very similar and in fact significantly correlated (r = 0.6481; p < 0.01). Within infected animals restraint stress decreased both of these responses in the spleens of infected mice by approximately 50 and 60% respectively (ps < 0.01) (Fig. 1B&D). Taken together these data indicate that RS drastically reduces the number of virus-specific IFN-γ producing CD4+ and CD8+ T cells at a time when these T cell responses normally peak.
Figure 1.
Restraint stress alters the numbers of virus-specific IFN-γ producing cells in the CNS and spleens of SJL mice at days 8 and 16 p.i. Female SJL mice aged 4–5 weeks were assigned to either infected/non-restrained (I/NR), infected/restrained (I/R), non-infected/non-restrained (NI/NR) or non-infected/restrained (NI/R) groups. Beginning at day -1 p.i. mice in the restrained groups were subjected to 8 h nightly restraint sessions which lasted until day 8 p.i. At day 8 or 16 p.i. isolated cells were stimulated with either CD4 (VP274-86) or CD8 (VP3159-166) or non-specific (NS) peptides in the context of an IFN-γ ELISPOT assay. (A) IFN-γ producing cells specific for VP274-86 in the CNS of infected mice at day 8 p.i. (B) VP3159-166 specific IFN-γ producing cells in the CNS at day 8 p.i. (C) Numbers of VP274-86 specific IFN-γ producing cells in the spleens of infected mice at day 8 p.i. (D) The effect of infection and stress on the numbers of VP3159-166 reactive cells in the spleens at day 8 p.i. (E) VP274-86 specific IFN-γ producing cells were isolated from the CNS of mice at day 16 p.i. (F). Similar numbers of VP3159-166 specific IFN-γ producing cells were isolated from the CNS of both I/NR and I/R mice, a response mimicked in the spleens (H). (G) I/R mice had significantly higher numbers of VP274-86 specific IFN-γ producing cells in their spleens than I/NR mice at day 16 p.i. All samples were run in duplicate. Background spots were determined for each sample using NS peptide as a stimulus and were subtracted from results obtained with stimulation of viral peptides. Results are combined means ± SEM of 2–3 separate experiments, and are representative of 5–7 mice per group. * P < .05, ** P < .01, *** P < .001.
3.3. Recovery from stress is evident by day 16 p.i
Because body weight rapidly returns to levels comparable to those of non-stressed mice following the secession of RS (data not shown), we questioned if the immune response would be as resilient, or if the observed suppression would be sustained. Therefore, in repeat experiments, we subjected mice to 9 sessions of RS as previously. At day 8 p.i. stress sessions ceased and mice were given until day 16 to recover, at which time the number of IFN-γ producing cells were measured as indicated above. Again, stimulation of CNS-IL with the CD4+ T cell immunodominant peptide, VP274-86, did not induce the production of IFN-γ indicating a lack of virus-specific Th1 response in the CNS of these mice at day 16 p.i. (Fig. 1E). As occurred at day 8 p.i., stimulation of CNS-IL with VP3159-166 demonstrated high numbers of virus-specific IFN-γ producing CD8+ T cells only in infected mice (Fig. 1F) at day 16 (p < 0.001). Unlike at day 8, the effects of RS on VP3159-166 specific cells from the CNS were nearly identical between non-stressed (I/NR) and stressed (I/R) groups (Fig. 1F). In the spleen, infected mice had increased numbers of VP274-86 specific IFN-γ producing CD4+ T cells (p < 0.01) at day 16 p.i. (Fig. 1G). Surprisingly however, the effect of stress was reversed to almost the exact opposite of what occurred at day 8 p.i. such that non-stressed mice (I/NR) had significantly fewer VP274-86 reactive CD4+ T cells compared to stressed mice (I/R) (p < 0.05; Fig. 1C vs. 1G). Similar to the CNS, the numbers of VP3159-166 specific IFN-γ producing CD8+ T cells in the spleens were increased by infection (p < 0.0001) and nearly identical between non-stressed and stressed groups (Fig. 1H). As on day 8 p.i., this response was highly correlative between the spleen and the CNS at day 16 p.i. (r = 0.7582; p < 0.001).
3.4. Restraint stress decreases virus-specific Th1 and Th2 cytokine secretion at day 8 p.i
As our ELISPOT assays were designed to enumerate IFN-γ specific T cells, it could have been possible that stress altered virus-specific T cell responses by inducing a shift from Th1 toward Th2 immunity (Elenkov, 2004). To ascertain if such a shift occurred, we first measured serum cytokines. RS caused a significant decrease in the concentrations of circulating type 1 and type 2 cytokines including IFN-γ, IL-12(p40), IL-12(p70) (type 1), IL-4, and IL-5 (type 2; ps < 0.05). However, infection was only found to increase circulating concentrations of IL-12(p40) at this time point (p < 0.05) (Supp. Table 1).
Stress also caused a decrease in the circulating concentrations of the chemokines RANTES and MCP-1, but increased serum levels of IL-6, G-CSF and KC (Supp. Table 2). As observed for type 1 and type 2 cytokines these concentrations were not significantly altered by infection with TMEV, except in the case of KC, in which infection decreased stress-induced serum concentrations (Supp. Table 2). Isolation of mRNA from unstimulated splenocytes yielded similar results as those obtained with cytokines, insofar as stress appeared to decrease levels of both IFN-γ as well as IL-5, and did not support the occurrence of a Th1 to Th2 shift (ps > 0.05; data not shown).
Therefore, to more specifically address the effects of RS on helper T cell responses, we repeated the above experiments and isolated cells from the CNS and spleens of mice at day 8 p.i. CNS-IL as well as spleen cells were stimulated either with plate bound anti-CD3 and anti-CD28, whole UV-inactivated TMEV (UV-BeAn), or media for 72 hours and supernatants were measured for Th1, Th2 and Th17 cytokines. As seen in figure 2A, when stimulated with UV-BeAn, CNS-IL from I/NR mice produced more IFN-γ, IL-17A and IL-2 than CNS-IL isolated from I/R mice (Student’s t-tests; ps < 0.05; Fig. 2A–C). Generally, the same trend existed for the Th2 cytokines IL-5 and IL-10, when stimulated with either anti-CD3 and anti-CD28 antibodies or UV-BeAn, but these effects were not statistically significant (data not shown). These results indicated that at day 8 p.i., infected mice possess increased levels of virus-specific pro-inflammatory T cells within the CNS, and that stress decreases these responses.
Figure 2.
Restraint stress decreases virus-specific proinflammatory responses occurring in the CNS at day 8 p.i. with TMEV. CNS-IL were extracted from mice at day 8 p.i. CNS-IL (5.0 × 104) were combined with 1.0 × 106 sygeneic irradiated feeders and cultured with plate-bound α-CD3 (10μg/ml) and α-CD28 (2μg/ml), purified UV-inactivated BeAn, or media for 72 h. Supernatants were used to measure cytokine production by luminex or ELISA. (A–C). I/NR mice produced increased amounts of the pro-inflammatory cytokines IFN-γ (A), IL-17A (B) and IL-2 (C) when re-stimulated with whole UV-BeAn compared to media controls. I/R mice produced significantly less of these cytokines than infected/non-stressed mice. Significant effects are shown. Results are combined means ± SEM of 3 separate experiments, and are representative of 4–6 mice per group. * P < 0.05, ** P < 0.01, *** P < 0.001.
In the spleen, infected animals produced more IFN-γ, Il-17A, IL-4 and IL-10 following stimulation with UV-BeAn than non-infected mice (ps < 0.05; Fig. 3). With the exception of IL-17A, comparisons between I/NR and I/R mice groups confirmed that stress decreased these virus-specific immune responses (Student’s t-tests; ps < 0.05). Taken together these results suggest that restraint stress administered during acute infection with TMEV inhibits virus-specific Th1 and Th2 cytokine production from cells isolated from both the periphery as well as from the CNS (Figs. 2 and 3). The same tendencies for decreased Th1 and Th2 profiles were observed in the cells stimulated with crosslinking antibodies directed toward CD3/CD28 in so far as cytokine production was lower in stressed groups. However, these effects failed to reach statistical significance.
Figure 3.
Restraint stress decreases both virus specific pro-inflammatory and anti-inflammatory responses occurring in the spleen at day 8 p.i. with TMEV. Splenocytes (1.0 × 106) were isolated from I/NR, I/R, NI/NR, NI/R mice at day 8 p.i. and cultured with plate-bound α-CD3 (10μg/ml) and α-CD28 (2μg/ml), purified UV-inactivated BeAn (1.0 μg), or media for 72 h at 37 C and 5.0% CO2. Supernatants were used to measure cytokine production by luminex or ELISA. (A–D). Splenocytes from infected mice produced increased amounts of the pro-inflammatory cytokines IFN-γ (A), IL-17A (C) and IL-4 (B) and IL-10 (D) when re-stimulated with whole UV-BeAn compared to media controls.
3.5. Restraint stress reduces T-bet mRNA and protein expression and GATA-3 mRNA expression
To explore the effects of stress on type 1 and type 2 responses further, we questioned whether stress altered the polarization of T cells. We hypothesized that if RS caused Th1 and Th2 immunosuppression, rather than just decreased cytokine secretion, then T-bet and GATA-3, the transcription factors for Th1 and Th2 polarization respectively, would also be decreased. Therefore, we first measured mRNA levels of both T-bet and GATA-3 by quantitative RT-PCR from RNA isolated from unstimulated splenocytes. We found that at day 8 p.i., mice in the I/NR group had significantly increased mRNA levels of T-bet (p < 0.05), when compared to other groups, and that RS significantly decreased mRNA levels of T-bet (Fig 4A; p < 0.01). Although not as robust, the same effects of infection and stress were demonstrated for GATA-3 mRNA levels, such that the I/NR group possessed higher expression levels of GATA-3 than any other group, and that RS significantly decreased GATA-3 expression (Fig 4A; p’s < 0.05). To determine if these effects translated into decreased protein levels, and to ensure the validity of our findings, we repeated the experiment, but measured T-bet and GATA-3 protein levels by immunoblots from nuclear extracts of splenocytes at day 8 p.i., following stimulation plate-bound anti-CD3 and anti-CD28 antibodies, VP274-86, VP3159-166 or NS peptides for 5 and 24 hours. Regardless of the stimulus, prior infection with TMEV increased T-bet relative to β-actin controls in non-stressed mice after 5 hours of stimulation (Fig. 4B). Contrary to the results demonstrated by RT-PCR, GATA-3 nuclear protein levels were not affected by RS (Fig 4B). While not as pronounced, the same trend existed following 24 hours of stimulation (Fig. 4B). Densitometry quantification of protein levels at 5 hours of stimulation resembled results obtained from mRNA fold induction by RT-PCR, insofar as stress appeared to decrease T-bet nuclear protein levels, but did not appear to have influenced GATA-3 protein in the same fashion (Fig. 4C).
Figure 4.
Restraint stress decreased T-bet, and causes decreased virus-specific IFN-γ secretion from stimulated splenocytes isolated at day 8 p.i. (A) Total RNA isolated from splenocytes (1.0 × 107) of I/NR, I/R, NI/NR, NI/R mice at day 8 p.i. was reversed transcribed into cDNA using random primers, and mRNA expression of T-bet (top) and GATA-3 (bottom) tested by RT-PCR. Data are indicative of fold induction relative to β-actin and normalize to NI/NR group. (B) In separate experiments splenocytes (1.0 × 106) were cultured with plate-bound α-CD3 (10μg/ml) and α-CD28 (2μg/ml), VP274-86 (2.0 μM), VP3159-166 (2.0 μM), or non-specific peptide (NS; 2.0 μM) for 5 (upper portion) or 24 (lower portion) h at 37°C and 5.0% CO2. Nuclear extracts were subjected to immunoblots specific for T-bet (B; top row); or GATA-3 (B; middle row) or β-actin (B; bottom row). (C) Densitometric analysis at of T-bet relative to β-actin was normalized to the NI/NR group at 5 h stimulation is shown. (D) Supernatants from the above experiment were used to measure IFN-γ production by ELISA. Both (A) and (D) results are representative means ± SEM of 3 separate experiments comprising 5–7 mice per group. Results in (B) represent pooled samples from 2 mice per group; this experiment was repeated with similar results (not shown). Results in (C) are mean densitometric analysis of results obtained of both experiments in (B) at 5 h stimulation and represent 4 mice per group. * P < .05.
In order to determine if these effects of stress correlated with decreases in IFN-γ, a known target of T-bet, we measured IFN-γ secretion from previously stimulated splenocyte cultures taken from infected (I/NR, I/R), or non-infected (NI/NR, NI/R) groups at day 8 p.i. Figure 4D shows that infection caused in increased IFN-γ production by 24 hours following stimulation anti-CD3 and anti-CD28, or viral peptides (VP274-86, VP3159-166), but not the NS peptide. Restraint stress was found to significantly decrease both virus-specific CD4+ T cell (VP274-86) and CD8+ T cell (VP3159-166) responses by approximately 21% and 66% respectively (Students t-test; ps < 0.05). These results are consistent with our ELISPOT data at this time, indicating that RS results in a decrease in IFN-γ secretion as well as the number of IFN-γ producing T cells (Fig. 1).
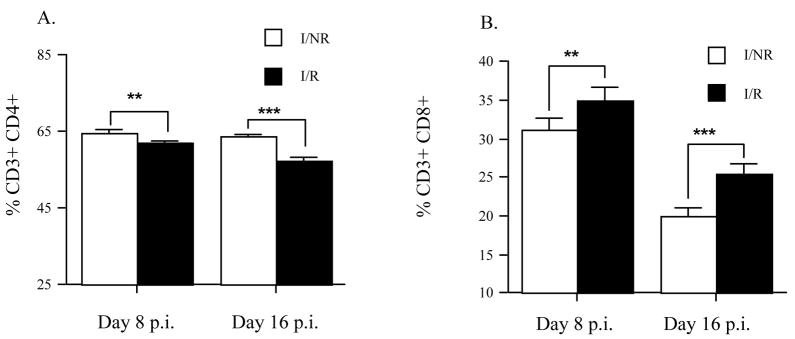
3.6. Restraint stress modestly alters CD4 and CD8 percentages at days 8 and 16 p.i
The possibility that RS was mediating its actions in the periphery (spleen) by decreasing the total percentages of CD4+ and CD8+ T cells, either by trafficking or cell death, or proliferation could account for our observed reductions in T cell responses. In order to investigate this possibility, we measured T cell percentages in isolated splenocytes from I/NR and I/R mice by flow cytometry at days 8 and 16 p.i. At day 8 p.i. we found that RS resulted in a very slight (64.5% vs. 62%), but significant (Student’s t test; p < 0.01), decrease in the percentage of CD4+ T cells (Fig 5A). The effect of RS on CD4+ T cell percentage was found to persist until day 16 p.i. (63.5% vs. 57.5%) despite the secession of RS (Student’s t test; p < 0.001).
Figure 5.
Restraint stress differentially alters CD4+ and CD8+ T cell percentages at days 8 and 16 p.i. Female SJL mice aged 4–5 weeks were assigned to either infected/non-restrained (I/NR), or infected/restrained (I/R), groups. Beginning at day -1 post infection (p.i.) mice in the restrained groups were subjected to 8 h nightly restraint sessions which lasted until day 8 p.i. At day 8 or 16 p.i. percentages of CD4+ (A) and CD8+ (B) T cells from isolated splenocytes (1.0 × 106) was determined by flow cytometry. For each time point n = 7 per group.
Results are means ± SEM. **P < 0.01, ***P < 0.001.
In contrast to that observed for CD4+ T cells, RS resulted in a moderate, but significant increase in the percentages of CD8+ T cells at both day 8 (31% vs. 35%) and day 16 (20% vs. 25%) (Fig. 5B; Student’s t test; ps < 0.01). These results indicate that RS is capable of modifying peripheral CD4+ and CD8+ T cell percentages. However, while statistically significant, these effects accounted for only a modest alteration, and thus are unlikely to fully account for our observed RS-induced immunosuppression of virus-specific CD4+ T cell (VP274-86) effector function. On the other hand, as stress resulted in an increased percentage of CD8+ T cells, the observed decrease in virus-specific CD8+ T cell restricted (VP3159-166) responses becomes more evident.
3.7. Glucocorticoids are partially responsible for the effects of RS on virus-specific T cell responses to TMEV at day 8 p.i
As restraint stress has been previously demonstrated to increase plasma corticosterone in SJL mice infected with TMEV (Sieve et al., 2004; Young et al., 2008), glucocorticoids could be involved in this observed immunosuppression. To test this hypothesis, we infected mice and treated them with either dexamethasone (DEX), or the glucocorticoid receptor antagonist RU486, or respective vehicle controls, and measured virus-specific immune responses during their peak at day 8 p.i. Analysis of change in body weight percentages indicated that our treatments were successful because as seen before, (not shown) stress caused a decrease in body weight which was reversed by concurrent administration of RU486 (Fig. 6B). At the same time administration of DEX mimicked the effect of RS (Fig. 6A). Additionally, the effects of RS on CD4+ (Fig. 7A–B) and CD8+ (Fig. 7C–D) T cell percentages in the spleens appeared to be entirely mediated by glucocorticoids as these effects were completely reversed when RU486 was administered to I/R mice, but were reestablished in I/NR mice when administered DEX. As before, (Fig. 1) stress decreased the number of VP274-86 and VP3159-166 specific IFN-γ producing T cells in the spleen, and DEX treatment mimicked these effects (Fig. 7E–H). However, unlike T cell percentages, RU486 treatment during concurrent RS did not appear to completely alleviate the effects of RS (Fig. 7G–H). In agreement with our previous data, almost no VP274-86 reactive IFN-γ producing T cells were detected in the CNS of infected animals, while a robust VP3159-166 response was evident (Fig 7I–L). Compared to the spleen, the responses in the CNS exhibited similar effects of RU486 and DEX treatments on the number of VP3159-166 reactive IFN-γ producing T cells although the effects failed to reach statistical significance; possibly attributable to a low sample number (n = 4 per group; Fig. 7K–L).
Figure 6.
Restraint stress induced weight loss is mediated by the actions of glucocorticoids. Female SJL mice aged 4–5 weeks were assigned to either infected/non-restrained PBS treated (I/NR-PBS), infected/non-restrained DEX treated (I/NR-DEX), infected/restrained PEG treated (I/R-PEG), or infected/restrained RU486 treated (I/R-RU486). Beginning at day -1 post infection (p.i.) mice in the restrained groups were subjected to 8 h nightly restraint sessions which lasted until day 8 p.i. Treatments with DEX (1.0 mg/kg) and RU486 (25mg/kg) or vehicle control (PBS or PEG400 respectively) were administered nightly 1 h prior to each stress session by i.p. or s.c. injection in a total volume of 100 μl. (A) Effects of DEX on body weight. (B) Effects of RS and RU486 on body weight. Results are combined means ± SEM, of at least 2 separate experiments representing 5–10 mice per group. * All Ps < 0.05.
Figure 7.
Effects of RS on T cell number and virus-specific effector function are partly due to the actions of glucocorticoids. Female SJL mice aged 4–5 weeks were assigned to either infected/non-restrained PBS treated (I/NR-PBS), infected/non-restrained DEX treated (I/NR-DEX), infected/restrained PEG treated (I/R-PEG), or infected/restrained RU486 treated (I/R-RU486). Beginning at day -1 post infection (p.i.), mice in the restrained groups were subjected to 8 h nightly restraint sessions which lasted until day 8 p.i. Treatments with DEX (1.0 mg/kg) and RU486 (25mg/kg) or vehicle control (PBS or PEG400 respectively) were administered nightly 1 h prior to each stress session by i.p. or s.c. injection in a total volume of 100 μl. (A–D) Effects of glucocorticoids on percentage of CD4+ (A–B) and CD8+ (C–D) T cells in the spleens of infected mice. (E–H) The effects of DEX, RS and RU486 on the number of IFN-γ producing CD4+ restricted (VP274-86) cells (E–F) and CD8+ restricted (VP3159-166) cells (G-H) in the spleens. (I-L) The effects of DEX, RS and RU486 treatment on the number of IFN-γ producing CD4+ restricted (VP274-86) cells (I–J) and CD8+ restricted (VP3159-166) IFN-γ producing cells (K–L) in the CNS. Results are combined means ± SEM of 2–5 independent experiments with 8 mice per group.
4. Discussion
4.1. Summary of results
The current study demonstrated that restraint stress had a profound impact on the T cell responses to Theiler’s virus. Lymphocytes isolated from stressed mice and then re-stimulated in culture with either the immunodominant CD4+ (VP274-86) or CD8+ (VP3159-166) T cell peptides had significantly reduced numbers of virus-specific IFN-γ producing T cells in the periphery, as well as the number of virus-specific IFN-γ producing CD8+ T cells in the CNS compared to unstressed mice. Moreover, the absence of the type 1 immune response in the serum, periphery, and CNS was coupled with reductions in virus-specific type 2 responses. In isolated splenocytes Th1 and Th2 cytokine reduction was mirrored by decreases in T-bet at the mRNA and protein levels, as well as a slight decrease in GATA-3 mRNA, suggesting a globalized suppressive response to stress rather than a Th1 to Th2 shift. As such, our results support those of Dobbs et al., (Dobbs et al., 1996) which show that RS causes a globalized immunosuppression during acute viral infection. Additionally, we have, to the best of our knowledge demonstrated for the first time, a suppressive effect of stress on virus-specific IL-17 production within the CNS; which interestingly was not duplicated in the periphery. In contrast, the numbers of virus-specific CD8+ T cells as determined by IFN-γ ELISPOTs were correlated between the CNS and spleens of infected mice, and cytokine profiles exhibited following stimulation of CNS-ILs or splenocytes with UV-inactivated virus were in agreement, which may indicate the development of virus-specific immunity in the periphery and subsequent trafficking to the CNS. Alternatively, reductions in virus-specific responses by stress could have been brought about by reductions in lymphocyte proliferation, increased cell death within both the spleen and CNS, or enhanced efflux of cells from the CNS back into the periphery.
4.2. Glucocorticoids are the main mediators of the stress-induced T cell suppression
Glucocorticoids were found to contribute to this RS-induced suppression as the antagonist RU486 resulted in partial restoration of T cell effector function in both the CNS and the spleen, whereas administration of the synthetic glucococorticoid, dexamethasone mimicked the effects of stress. The observed suppressive effects of stress and glucocorticoids on T cell effector function, particularly virus-specific IFN-γ producing cells, could have been a byproduct of suppressive effects directed toward antigen presenting cells, (macrophages and dendritic cells), resulting in decreased numbers of virus-specific T cell clones. This possibility is supported by the fact that corticosterone can inhibit processing and presentation of MHC class I molecules on DCs (Trukenmiller et al., 2005), as well as decrease CD80/CD86 transcription, cause MHC class II retention and reduce IL-6, IL-12 and TNF-α production in DCs activated with LPS (Elftman et al., 2007). In support of this mechanism of immunosuppression, T cells from stressed mice stimulated with α-CD3/α-CD28 antibodies tended to produce less type 1 and type 2 cytokines than T cells from non-stressed mice, a finding that may indicate an overall reduction on the number of Th1/Tc1 and Th2/Tc2 clones present within the CNS and the spleen. Alternatively, the stress-induced increases in corticosterone observed in our model (Campbell et al., 2001; Sieve et al., 2004; Young et al., 2008) could also act directly at the level of the T cell by inhibiting the functions of the transcription factors responsible for mediating effector function. To date, glucocorticoids have been shown to inhibit the actions of AP-1, NFκB and GATA-3 (Webster et al., 2002; Jee et al., 2005). Recently, the actions of glucocorticoids, specifically dexamethasone, have been demonstrated to inhibit the binding of T-bet to the IFN-γ promoter, possibly providing a mechanism for defective IFN-γ transcription and production in previously polarized Th1 or Tc1 effector T cells (Liberman et al., 2007).
4.3. Restraint stress effects on T-bet and GATA-3 expression
The transcription factor T-box expressed in T cells (T-bet) brings about polarization of naive Th0 cells to the functional Th1 phenotype, and is required for optimal trafficking of this subtype into inflamed tissue (Szabo et al., 2000; Lord et al., 2005), the development of functionally active NK cells (Szabo et al., 2002) and NKT cells (Townsend et al., 2004), the optimal production of IFN-γ from dendritic cells (Lugo-Villarino et al., 2003) and is also reported to play a role in class switching in B cells (Peng et al., 2002; Xu et al., 2005). In addition, recent studies indicate that T-bet is important for antigen-specific cytotoxicity in CD8+ T cells (Sullivan et al., 2003), and the secretion of IFN-γ from CD8+ T cells during infection (Mayer et al., 2008). Therefore, it is not surprising that T-bet−/− mice are more susceptible to HSV-2 and vaccinia virus infections than wild-type controls (Svensson et al., 2005; Matsui et al., 2005). Interestingly, the immunologic phenotype of restraint stressed mice infected with Theiler’s virus, compares with that of the T-bet−/− mice, as RS mice are known to display reduced NK cell function (Welsh et al., 2004; Tseng et al., 2005) decreased CD4+ and CD8+ T cell responses to viral infections (Dobbs et al., 1996; Sheridan et al., 1991; Freeman et al., 2007; current study), decreased T cell trafficking (Zhang et al., 1998; Ottaway and Husband 1994; Mizobe et al., 1997) and display greater susceptibility to TMEV (Campbell et al., 2001), and HSV-1 infection (DeLano and Mallery 1998; Anglen et al., 2003).
To explore a possible connection between the immunosuppressive effects of RS and T-bet, we first demonstrated that RS reduced mRNA and protein levels of the transcription factor T-bet isolated from primary splenocyte cultures. These stress effects were not entirely attributable to altered T cell populations, as stress only moderately affected CD4+ and CD8+ T cell percentages. Glucocorticoids have been shown to inhibit IFN-γ dependent activation of STAT1 (Hu et al., 2003) that is an inducer of T-bet (Afkarian et al., 2002). Alternatively, the polarization of Th1 cells is dependent on the binding of IL-12(p70) to its receptor and the subsequent phosphorylation of STAT4 (Usui et al., 2006; Wurster et al., 2000). As mentioned previously, glucocorticoids can inhibit IL-12 production and secretion at the DC level, which would reduce the number of T-bet positive cells. Supporting this hypothesis is the fact that RS caused significant decreases in both circulating serum IL-12(p40) and IL-12(p70) concentrations (Supp. Table 1). This process could also be ablated downstream of the signaling pathway, as dexamethasone has also been shown to inhibit the phosphorylation of STAT4 by a mechanism that was independent of IL-12R activation (Franchimont et al., 2000).
Restraint stress reduced Th2 cytokines, GATA-3 mRNA expression but not protein levels as detected by immunoblots. However, dexamethasone has recently been shown to reduce the expression of GATA-3 protein in Con-A stimulated splenocytes (Liberman et al., 2009). The reason for the difference may be due to technical differences since our studies employed nuclear extracts whereas Liberman et al., incorporated whole cell lysates.
4.4. Pro-inflammatory cytokines IL-17 and IFN-γ in MS and models of MS
MS patients given interferon-gamma developed an increased frequency of relapses and therefore IFN-γ is considered to be a pathogenic cytokine in the pathogenesis of MS (Panitch et al., 1987). Another proinflammatory cytokine that has recently received considerable attention in the realm of autoimmune diseases is IL-17. While T cells and neutrophils are capable of producing IL-17, the major source of IL-17 is the Th17 cell, which in humans have been shown to also be capable of producing IFN-γ (Annunziato et al., 2007). Indeed, recent evidence strongly suggests that these cells are actively involved in the pathogenesis of MS (Witowski et al., 2004; Vaknin-Dembinsky et al., 2006; Kebir et al., 2007; Ifergan et al., 2008).
The role of proinflammatory cytokines in models of MS is complex. In some animal models proinflammatory cytokine secretion can have devastating effects within the CNS, whereas in other models proinflammatory cytokine secretion can potentiate some protection. For instance, EAE is exacerbated in IFN-γ−/− mice (Krakowski, et al., 1996; Ferber et al., 1996; Willenborg, 1996). In Theiler’s virus infection, IFN-γ is thought to assume a protective role. During early infection it is beneficial as illustrated by the fact that it has potent in vitro anti-viral activity against TMEV (Welsh et al., 1995) and may be required for neuronal viral clearance from the CNS, as IFN-γ−/− mice display decreased survival rates following infection with TMEV (Murray et al., 2002; Rodriguez et al., 2003). Additionally, both IFN-γ−/− and IFN-γR−/− mice on a normally demyelination-resistant background, develop severe demyelination (Rodriguez et al., 1995; Johnson et al., 2001). While many studies have investigated the role of IFN-γ in TMEV infection, to date, virtually nothing is known about the involvement of Th17 cells in the pathogenesis of Theiler’s virus infection. However, some studies indirectly implicated these cells in the disease process. For instance, deletion of IL-12(p40), a subunit required for the formation of both active IL-12 and IL-23 heterodimers, by antibody administration given during early infection with TMEV delays the onset of demyelination in susceptible mice (Inoue et al., 1998). In contrast, deletion of STAT4, a transcription factor responsible for IFN-γ transcription, in mice on a normally TMEV-IDD resistant background, renders them susceptible to demyelination (Rodriguez et al., 2006). Also, the TMEV susceptibility gene tmevp3 is suspected of coding for IL-22, an additional product of Th17 cells (Liang et al 2006), and that this loci limits viral titers within the CNS controlling mortality due to encephalitis in SJL mice during early infection (Levillayer et al., 2007).
4.5. Recovery of the immune response following removal of the stressor
The immune system appears to recover from the stress-induced immunosuppression in the current study. Interestingly, at day 16p.i. after 8 days of RS and then 8 days of recovery, both the T and B cell response in stressed mice increases when compared to the non-stressed mice. This effect maybe due to the fact that there is more viral replication in the stressed animals and, once the immune system recovers, the increased viral titers stimulate the immune system.
4.6. Relevance of our findings with regard to MS
If MS adheres strictly to an autoimmune pathogenesis, then the immunosuppressive effects of stress on the natural history of the disease are paradoxical. This is best illustrated by the effects of RS on the progression of EAE (Levine et al., 1962; Griffin et al., 1993; Correa et al., 1998). However, if an infectious agent is involved in the initiation and progression of MS, then the immunosuppressive effects of stress, as occurs in Theiler’s virus infection, will favor the pathogen and, after the resolution of the stressful stimuli, allow for the establishment of persistent infection. This scenario would also expedite the autoimmune process, possibly through the attraction of naturally occurring autoreactive cells into the CNS from the periphery or by molecular mimicry and subsequent epitope spreading. The recent finding that bone marrow reconstitution in a chronic myelogenous leukemia patient with MS was unable to alter disease course may support the above concept (Jeffery, 2007).
Alternatively, it is known that viral infections, particularly upper respiratory infections, precipitate MS exacerbation (Buljevac et al., 2002). Through immunosuppressive properties, stress may increase the risk of MS exacerbation by indirectly increasing the risk of acquiring an infection. Supporting this hypothesis is the fact that experimental stress in humans followed by rhinovirus infection resulted in increased disease severity (Cohen et al., 1991). Nevertheless, our results indicate that stress results in immunosuppression of both TMEV-specific T cell responses, particularly protective IFN-γ, and provides a sound mechanism for observed increased CNS viral titers and dissemination during early Theiler’s virus infection (Mi et al., 2004 & 2006b).
Acknowledgments
The authors would like to thank Dr. Mwangi (Department of Veterinary Pathobiology, Texas A&M University) for the use of his ELISPOT reader, Dr. Porter (Department of Veterinary Integrative Biosciences, Texas A&M University) for the use of his spectrophotometer, and Dr. Boudreau for her technical support. This research was funded by grants to C.J.R.W. and M.W.M. from the National Multiple Sclerosis Society RG 3128, NIH/NINDS R01 NS39569 and NIH/NINDS R01-NS060822 awarded to M.W.M and C.J.R.W. A.J.S. received support from the Recovery of Function Graduate Training Program, NIH/NRSA 5T32AI052 “Mechanistic studies at the host-pathogen interface” and performed this work in - partial fulfillment of the requirements for the Ph.D. degree at Texas A&M University, College Station, Texas.
Abbreviations
- CNS-IL
Central nervous system infiltrating lymphocytes
- RS
Restraint stress
- T-bet
T box expressed in T cells
- DEX
Dexamethasone
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman KD, Heyman R, Rabin BS, Anderson BP, Houck PR, Frank E, Baum A. Stressful life events precede exacerbations of multiple sclerosis. Psychosomo Med. 2003;64:916–920. doi: 10.1097/01.psy.0000038941.33335.40. [DOI] [PubMed] [Google Scholar]
- Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy TM. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Anglen CS, Truckenmiller ME, Schell TD, Bonneau RH. The dual role of CD8+ T lymphocytes in the development of stress-induced herpes simplex encephalitis. J Neuroimmunol. 2003;140:13–27. doi: 10.1016/s0165-5728(03)00159-0. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begolka WS, Haynes LM, Olson JK, Padilla J, Neville KL, Dal Canto M, Palma J, Kim BS, Miller SD. CD8-deficient SJL mice display enhanced susceptibility to Theiler’s virus infection and increased demyelinating pathology. J NeuroVirol. 2001;7:409–420. doi: 10.1080/135502801753170264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau RH, Sheridan JF, Feng NG, Glaser R. Stress induced suppression of herpes simplex virus (HSV)-specific cytotoxic T lymphocyte and natural killer cell activity and enhancement of acute pathogenesis following local HSV infection. Brain Behav Immun. 1991;5:170–92. doi: 10.1016/0889-1591(91)90015-3. [DOI] [PubMed] [Google Scholar]
- Borrow P, Tonks P, Welsh CJ, Nash AA. The role of CD8+ T cells in the acute and chronic phases of Theiler’s murine encephalomyelitis virus-induced disease in mice. J Gen Virol. 1992;73:1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- Borrow P, Welsh CJ, Nash AA. Study of the mechanisms by which CD4+ T cells contribute to protection in Theiler’s murine encephalomyelitis. Immunol. 1993;80:502–506. [PMC free article] [PubMed] [Google Scholar]
- Brahic M, Bureau JF, Michiels T. The genetics of the persistent infection and demyelinating disease caused by Theiler’s virus. Ann Rev Microb. 2005;59:279–298. doi: 10.1146/annurev.micro.59.030804.121242. [DOI] [PubMed] [Google Scholar]
- Buljevac D, Flach HZ, Hop WC, Hijdra D, Laman JD, Savelkoul HF, van Der Meché FG, van Doorn PA, Hintzen RQ. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125:952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, Welsh CJR. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection: I. Acute disease. Brain Behav Immun. 2001;15:235–254. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- Clatch RJ, Miller SD, Metzner R, Dal Canto MC, Lipton HL. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler’s murine encephalomyelitis virus (TMEV) Virol. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Correa SG, Rodriguez-Galán MC, Rivero VE, Riera CM. Chronic varied stress modulates experimental autoimmune encephalomyelitis in Wistar rats. Brain Behav Immun. 1998;12:134–148. doi: 10.1006/brbi.1998.0519. [DOI] [PubMed] [Google Scholar]
- DeLano RM, Mallery SR. Stress-related modulation of central nervous system immunity in a murine model of herpes simplex encephalitis. J Neuroimmunol. 1998;89:51–58. doi: 10.1016/s0165-5728(98)00087-3. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses immune function in vivo: A potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dobbs CM, Feng N, Beck FM, Sheridan JF. Neuroendocrine regulation of cytokine production during experimental influenza viral infection: Effects of restraint stress-induced elevation in endogenous corticosterone. J Immunol. 1996;157:1870–1877. [PubMed] [Google Scholar]
- Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann NY Acad Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- Elftman MD, Norbury CC, Bonneau RH, Truckenmiller ME. Corticosterone impairs dendritic cell maturation and function. Immunology. 2007;122:279–290. doi: 10.1111/j.1365-2567.2007.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley JK, Walker R. Virus demyelination. J Neurovirol. 2003;9:148–164. doi: 10.1080/13550280390194046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Fiette L, Aubert C, Brahic M, Rossi CP. Theiler’s virus infection of β-2 microglobulin-deficient mice. J Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O’Shea JJ. Inhibition of Th1 immune response by glucocorticoids: Dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol. 2000;164:1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol. 2007;179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol. 1995;47:425–448. [PubMed] [Google Scholar]
- Gerety SJ, Clatch RJ, Lipton HL, Goswami RG, Rundell MK, Miller SD. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease IV. Identification of an immunodominant T cell determinant on the N-terminal end of the VP2 capsid protein in susceptible SJL/J mice. J Immunol. 1991;146:2401–2408. [PubMed] [Google Scholar]
- Gerety S, Karpus W, Cubbon AR, Goswami R, Randell MK, Peterson J, Miller SD. Class II-restricted T cell responses in Theieler’s murine encephalomyelitis virus-induced demyelinating disease V. Mapping of a dominant immunopathological VP2 T cell epitope in susceptible mice. J Immunol. 1994;152:908–918. [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Chronic stress modulates the virus specific immune response to latent herpes simplex virus type 1. Annal Behav Med. 1997;19:78–82. doi: 10.1007/BF02883323. [DOI] [PubMed] [Google Scholar]
- Griffin AC, Lo WD, Wolny AC, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis by restraint stress: Sex differences. J Neuroimmunol. 1993;44:103–116. doi: 10.1016/0165-5728(93)90273-2. [DOI] [PubMed] [Google Scholar]
- Hu X, Li WP, Meng C, Ivashkiv LB. Inhibition of IFN-γ signaling by glucocorticoids. J Immunol. 2003;170:4833–4839. doi: 10.4049/jimmunol.170.9.4833. [DOI] [PubMed] [Google Scholar]
- Ifergan I, Kébir H, Bernard M, Wosik K, Dodelet-Devillers A, Cayrol R, Arbour N, Prat A. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain. 2008;131:785–799. doi: 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- Inoue A, Koh CS, Yamazaki M, Yahikozawa H, Ichikawa M, Yagita H, Kim BS. Suppressive effects on Theiler’s murine encephalomyelitis virus-induced demyelinating disease by the administration of anti-IL-12 antibody. J Immunol. 1998;161:5586–5593. [PubMed] [Google Scholar]
- Jee YK, Gilmour J, Kelly A, Bowen H, Richards D, Soh C, Smith P, Hawrylowiez C, Cousins D, Lee T, Lavender P. Repression of interleukin-5 transcription by glucocorticoid receptor targets GATA3 signaling and involves histone deacetylase recruitment. J Biol Chem. 2005;280:23243–23250. doi: 10.1074/jbc.M503659200. [DOI] [PubMed] [Google Scholar]
- Jeffery DR. Failure of allogeneic bone marrow transplantation to arrest disease activity in multiple sclerosis. Mult Scler. 2007;13:1071–1075. doi: 10.1177/1352458507076981. [DOI] [PubMed] [Google Scholar]
- Johnson AJ, Upshaw J, Pavelko KD, Rodriguez M, Pease LR. Preservation of motor function by inhibition of CD8+ virus peptide-specific T cells in Theiler’s virus infection. FASEB J. 2001;15:2760–2762. doi: 10.1096/fj.01-0373fje. [DOI] [PubMed] [Google Scholar]
- Kang BS, Lyman MA, Kim BS. The majority of infiltrating CD8 cells in the central nervous system of susceptible SJL/J mice infected with Theiler’s virus are virus specific and fully functional. J Virol. 2002;76:6577–6585. doi: 10.1128/JVI.76.13.6577-6585.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BS, Palma JP, Lyman MA, Dal Canto M, Kim BS. Antibody response is required for protection from Theiler’s virus induced encephalitis in C57BL/6 mice in the absence of CD8+ cells. Virol. 2005;340:84–94. doi: 10.1016/j.virol.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;10:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF, Heltberg A. Multiple sclerosis in the Faroe Islands: An epitome. J Clin Epidemiol. 2001;54:1–22. doi: 10.1016/s0895-4356(00)00268-7. [DOI] [PubMed] [Google Scholar]
- Levillayer E, Mas M, Levi-Acobas F, Brahic M, Bureau JE. Interleukin 22 is a candidate gene for Tmevp3, a locus controlling Theiler’s virus-induced neurological disease. Genetics. 2007;176:1835–1844. doi: 10.1534/genetics.107.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Strebel R, Wenk EJ, Harman PJ. Suppression of experimental allergic encephalomyelitis by stress. Proc Soc Exp Biol Med. 1962;109:294–298. doi: 10.3181/00379727-109-27183. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman AC, Refojo D, Jimena D, Toscano M, Rein T, Holsboer F, Arzt E. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB J. 2007;21:1177–1188. doi: 10.1096/fj.06-7452com. [DOI] [PubMed] [Google Scholar]
- Liberman AC, Druker J, Refojo D, Holsboer F, Arzt E. Glucocorticoids inhibit GATA-3 phosphorylation and activity in T cells. FASEB J. 2009 doi: 10.1096/fj.08-121236. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lin X, Ma X, Rodriguez M, Roos RP. CD4+ T cells are important for clearance of DA strain of TMEV from the central nervous system of SJL/J mice. Int Immunol. 2004;16:1237–1240. doi: 10.1093/intimm/dxh125. [DOI] [PubMed] [Google Scholar]
- Lipton HL. Theiler’s virus infection in mice: An unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Melvold R. Genetic analysis of susceptibility to Theiler’s virus induced demyelinating disease in mice. J Immunol. 1984;132:1821–1825. [PubMed] [Google Scholar]
- Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-γ and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci USA. 2003;100:7749–7754. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Moriya O, Yoshimoto T, Akatsuka T. T-bet is required for protection against vaccinia virus infection. J Virol. 2005;79:12798–12806. doi: 10.1128/JVI.79.20.12798-12806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KD, Mohrs K, Reiley W, Wittmer S, Kohlmeier JE, Pearl JE, Cooper AM, Johnson LL, Woodland DL, Mohrs M. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol. 2008;180:693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- Mi W, Belyavskyi M, Johnson RR, Sieve AN, Storts R, Meagher MW, Welsh CJR. Alterations in chemokine expression following Theiler’s virus infection and restraint stress. J Neuroimmunol. 2004;151:103–115. doi: 10.1016/j.jneuroim.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Mi W, Prentice TW, Young CR, Johnson RR, Sieve AN, Meagher MW, Welsh CJR. Restraint stress decreases virus-induced pro-inflammatory cytokine expression during acute Theiler’s virus infection. J Neuroimmunol. 2006a;178:49–61. doi: 10.1016/j.jneuroim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Mi W, Young CR, Storts R, Steelman A, Meagher MW, Welsh CJR. Stress alters pathogenecity and facilitates systemic dissemination of Theiler’s virus. Microb Pathog. 2006b;41:133–143. doi: 10.1016/j.micpath.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Rivera-Quiñones C, Njenga MK, Leibowitz J, Rodriguez M. Spontaneous CNS remyelination in beta 2 microglobulin-deficient mice following virus-induced demyelination. J Neurosci. 1995;15:8345–8352. doi: 10.1523/JNEUROSCI.15-12-08345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobe K, Kishihara K, Ezz-Din El-Naggar R, Madkour GA, Kubo C, Nomoto K. Restraint stress-induced elevation of endogenous glucocorticoid suppresses migration of granulocytes and macrophages to an inflammatory locus. J Neuroimmunol. 1997;73:81–89. doi: 10.1016/s0165-5728(96)00169-5. [DOI] [PubMed] [Google Scholar]
- Mohindru M, Kang B, Kim BS. Initial capsid-specific CD4+ T cell responses protect against Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Eur J Immunol. 2006;36:1–10. doi: 10.1002/eji.200535785. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: A meta-analysis. BMJ. 2004;328:731–735. doi: 10.1136/bmj.38041.724421.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PD, McGavern DB, Lin X, Njenga MK, Leibowitz J, Pease LR, Rodriguez M. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J Neurosci. 1998;18:7306–7314. doi: 10.1523/JNEUROSCI.18-18-07306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PD, McGavern DB, Pease LR, Rodriguez M. Cellular sources and targets of IFN-γ-mediated protection against viral demyelination and neurological deficits. Eur J Immunol. 2002;32:606–615. doi: 10.1002/1521-4141(200203)32:3<606::aid-immu606>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. New Eng J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler’s virus infection: A model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway CA, Husband AJ. The influence of neuroendocrine pathways on lymphocyte migration. Immunol Today. 1994;15:511–517. doi: 10.1016/0167-5699(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Palma JP, Lee HG, Mohindru M, Kang BS, Dal Canto M, Miller SD, Kim BS. Enhanced susceptibility to Theiler’s virus-induced demyelinating disease in perforin-deficient mice. J Neuroimmunol. 2001;116:125–135. doi: 10.1016/s0165-5728(01)00293-4. [DOI] [PubMed] [Google Scholar]
- Pachner AR, Li L, Narayan K. Intrathecal antibody production in an animal model of multiple sclerosis. J Neuroimmunol. 2007;185:57–63. doi: 10.1016/j.jneuroim.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG2a class switching and pathogenic autoantibody production. Proc Natl Acad Sci. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen LC, Miller SD, Dal Canto MC, Kim BS. Class I-deficient resistant mice intracerebrally inoculated with Theiler’s virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur J Immunol. 1993;23:2287–2293. doi: 10.1002/eji.1830230935. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, David CS. Demyelination induced by Theiler’s virus: Influence of the H-2 haplotype. J Immunol. 1985;135:2145–2148. [PubMed] [Google Scholar]
- Rodriguez M, Kenny JJ, Thiemann RL, Woloschak GE. Theiler’s virus induced demyelination in mice immunosuppressed with anti-IgM and in mice expression the xid gene. Microb Pathog. 1990;8:23–35. doi: 10.1016/0882-4010(90)90005-b. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Pavelko KD, McKinney CW, Leibowitz JL. Recombinant human IL-6 suppresses demyelination in a viral model of multiple sclerosis. J Immunol. 1994;153:3811–3821. [PubMed] [Google Scholar]
- Rodriguez M, Pavelko K, Coffman RL. Gamma interferon is critical for resistance to Theiler’s virus-induced demyelination. J Virol. 1995;69:7286–7290. doi: 10.1128/jvi.69.11.7286-7290.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Zoecklein LJ, Howe CL, Pavelko KD, Gamez J, Nakane S, Papke LM. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler’s murine encephalomyelitis virus infection. J Virol. 2003;77:12252–12265. doi: 10.1128/JVI.77.22.12252-12265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Zoecklein L, Gamez JD, Pavelko KD, Papke LM, Nakane S, Howe C, Radhakrishnan S, Hansen MJ, David CS, Warrington AE, Pease LR. STAT4- and STAT6-signaling molecules in a murine model of multiple sclerosis. FASEB J. 2006;20:343–345. doi: 10.1096/fj.05-4650fje. [DOI] [PubMed] [Google Scholar]
- Rossi CP, McAllister A, Tanguy M, Kagi D, Brahic M. Theiler’s virus infection of perforin-deficient mice. J Virol. 1998;72:4515–4519. doi: 10.1128/jvi.72.5.4515-4519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert RR, Pallansch MA. Preparation and characterization of Encephalomyocarditis (EMC) virus. Meth Enzymol. 1981;78:315–325. [PubMed] [Google Scholar]
- Sapatino BV, Petrescu AD, Rosenbaum BA, Smith R, 3rd, Piedrahita JA, Welsh CJ. Characteristics of cloned cerebrovascular endothelial cells following infection with Theiler’s virus. II Persistent infection. J Neuroimmunol. 1995;62:127–135. doi: 10.1016/0165-5728(95)00094-4. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Feng N, Bonneau RH, Allen CM, Huneycutt BS, Glaser R. Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J Neuroimmunol. 1991;3:245–255. doi: 10.1016/0165-5728(91)90046-a. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJR, Meagher MW. Chronic restraint stress during early Theiler’s virus infection exacerbates the subsequent demyelinating disease in SJL mice. J Neuroimmunol. 2004;155:103–118. doi: 10.1016/j.jneuroim.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Sullivan BM, Juedes A, Szabo SJ, von Herranth M, Glimcher LH. Antigen driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci USA. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson A, Nordstrom I, Sun JB, Eriksson K. Protective immunity to genital herpes simplex virus type 2 infection is mediated by T-bet. J Immunol. 2005;174:6266–6273. doi: 10.4049/jimmunol.174.10.6266. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in Th1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- Theiler M. Spontaneous encephalomyelitis of mice, a new virus disease. J Exp Med. 1937;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LM. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- Truckenmiller ME, Princiotta MF, Norbury CC, Bonneau RH. Corticosterone impairs MHC class I antigen presentation by dendritic cells via reduction of peptide generation. J Neuroimmunol. 2005;160:48–60. doi: 10.1016/j.jneuroim.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Tseng RJ, Padgett DA, Dhabhar FS, Engler H, Sheridan JF. Stress induced modulation of NK activity during influenza viral infection: role of glucocorticoids and opioids. Brain Behav Immun. 2005;19:153–164. doi: 10.1016/j.bbi.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O’Shea JJ, Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Welsh CJ, Tonks P, Nash AA, Blakemore WF. The effect of L3T4 T cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68:1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- Welsh CJR, Blakemore WF, Tonks P, Borrow P, Nash AA. Theiler’s murine encephalomyelitis virus infection in mice: A persistent viral infection of the central nervous system which induces demyelination. In: Dimmock N, editor. Immune responses, virus infections and disease. Oxford University Press; Oxford: 1989. pp. 125–147. [Google Scholar]
- Welsh CJR, Sapatino B, Rosenbaum B, Smith R, Linthicum DS. Correlation between susceptibility to demyelination and interferon gamma induction of major histocompatibility complex class II antigens on murine cerebrovascular endothelial cells. J Neuroimmunol. 1993;48:91–98. doi: 10.1016/0165-5728(93)90062-4. [DOI] [PubMed] [Google Scholar]
- Welsh CJR, Bustamante L, Nayak M, Welsh TH, Dean DD, Meagher MW. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection II: NK cell function and cytokine levels in acute disease. Brain Behav Immun. 2004;18:166–174. doi: 10.1016/S0889-1591(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3327. [PubMed] [Google Scholar]
- Witowski J, Ksiazek A, Jorres A. Interleukin-17: A mediator of inflammatory responses. Cell Mol Life Sci. 2004;61:567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000;19:2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhang JJ. Stat1-dependent synergistic activation of T bet for IgG2a production during early stage of B cell activation. J Immunol. 2005;175:7419–7424. doi: 10.4049/jimmunol.175.11.7419. [DOI] [PubMed] [Google Scholar]
- Yahikozawa HA, Inoue A, Koh CS, Choe YK, Kim BS. Major linear antibody epitopes and capsid proteins differentially induce protective immunity against Theiler’s virus-induced demyelinating disease. J Virol. 1997;71:3105–3113. doi: 10.1128/jvi.71.4.3105-3113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EE, Prentice TW, Satterlee D, McCullough H, Sieve AN, Johnson RR, Welsh TH, Welsh CJR, Meagher MW. Glucocorticoid exposure alters the pathogenesis of Theiler’s murine encephalomyelitis virus during acute infection. Physiology and Behavior. 2008;95:63–71. doi: 10.1016/j.physbeh.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kishihara K, Wang B, Mizobe K, Kubo C, Nomoto K. Restraint stress-induced immunosuppression by inhibiting leukocyte migration and Th1 cytokine expression during the intraperitoneal infection of Listeria monocytogenes. J Neuroimmunol. 1998;92:139–151. doi: 10.1016/s0165-5728(98)00197-0. [DOI] [PubMed] [Google Scholar]
- Zheng L, Calenoff MA, Dal Canto MC. Astrocytes not microglia are the main cells responsible for viral persistence in Theiler’s murine encephalomyelitis virus infection leading to demyelination. J Neuroimmunol. 2001;118:256–267. doi: 10.1016/s0165-5728(01)00338-1. [DOI] [PubMed] [Google Scholar]